
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 07 August 2023
Sec. Animal Nutrition and Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1200272
Objective: The purpose of this study was to investigate the effects of different doses of Sophora alopecuroides (SA) on the rumen fermentation and microbial diversity of sheep.
Methods: A total of 32 healthy Dumont crossbred male lambs weighing 25.73 ± 2.17 kg were randomly assigned to 4 treatment groups with 8 replicates each: a control group (CG) fed a basal diet with a concentrate-to-forage ratio of 7:3 and three experimental groups - the 0.1% group(TG1), 0.3% group (TG2), and 0.5% group (TG3), which were fed the same basal diet but supplemented with increasing doses of SA.
Results: (1) Increasing the SA dose led to a significant linear increase (p-< 0.05) in acetate, propionate, butyrate, and total volatile fatty acid (TVFA) concentrations in the rumen, as well as a significant quadratic effect (p-< 0.05) on the propionate concentration. In contrast, there was a significant linear decrease (p-< 0.05) in the NH3-N concentration in the rumen. (2) At the level of rumen bacterial phyla, the abundance of Bacteroidetes in the rumen increased, and that of Firmicutes decreased (p = 0.08). At the genus level, the rumen abundances of Ruminococcus and Phocaeicola of sheep in the three experimental groups were significantly higher than in the control group (p-< 0.05), and the abundances of Clostridiales and Candidatus-Hepatincola were significantly increased in the 0.1% and 0.3% groups (p < 0.05). (3) Regarding rumen anaerobic fungi, the differences between the control group and experimental groups at the phylum level and genus level were not significant (p > 0.05), but the relative abundances of Neocallimastigomycota and Piromyces in the 0.1% group were significantly higher than that in the control group.
Conclusion: SA addition to a high grain diet could increase the VFA concentration and pH in the sheep rumen, reduce the NH3-N concentration in the rumen and improve rumen fermentation function. Although there was no significant change in rumen bacterial or fungal diversity, SA addition increased the rumen abundances of Bacteroidetes, Ruminococcus, Phocaeicola, Clostridiales, Neocallimastigomycota and Piromyces, decreased the rumen abundance of Firmicutes, and had a positive effect on the rumen microbiota to improve sheep health.
The necessary condition to improve ruminant performance and maintain their health of the organism is to maintain the balance of the rumen microecology, while the balance of the rumen microecosystem depends mainly on the diet. In recent years, in order to shorten the feeding cycle, accelerate the turnover and maximize the economic benefits, farmers have started to feed high concentrate diets to fattening sheep and high yielding cows. According to relevant reports, the fermentation of high grain diets in the rumen could easily cause the accumulation of short-chain volatile fatty acids in the rumen, resulting in a decrease in pH, which in turn could lead to changes in the rumen microbiota and subacute rumen acidosis (SARA) in severe cases (1, 2), thus affected the health of the organism. Although feeding antibiotics can provide some relief, they have been banned because of their ability to cause drug resistance in animals and residues in livestock products, which are harmful to human health (3). Therefore, finding substitutes for antibiotics to keep animals healthy has become a research hot spot in recent years.
Sophora alopecuroides, a Chinese herbal medicine, belongs to the genus Sophora of Leguminosae, which is mainly distributed in desert areas such as Inner Mongolia and Ningxia (4). It is rich in alkaloids, flavonoids, amino acids, fatty acids and other components (5). Among them, alkaloids are its main active ingredients, with anti-inflammatory and hypolipidemic, immunomodulatory effects (6). And it is mostly used for the treatment of gastrointestinal bleeding in monogastric animals (7). Some studies have found that alkaloids can effectively inhibit the growth of harmful bacteria, promote the proliferation of beneficial bacteria, improve the microflora in the intestinal tract of broilers, and promote the development of villus structure of small intestine in broilers, and improve the absorption function of the small intestine (8). Jia et al. (9) showed that 75-300 mg/kg SA alkaloids significantly improved the colitis induced by dextran sodium sulfate (DSS) in mice, inhibited expression of IL-1β and TGF-β1 and upregulated IL-10 expression. In addition, in vivo experiments using mice to establish models of Helicobacter pylori gastritis showed that total alkaloids of Sophora alopecuroides (TASA) combined with bismuth pectin or omeprazole could effectively reduce the expression levels of IL-8, COX-2 and NF-κB in gastric mucosa infected with Helicobacter pylori, thus alleviating gastric mucosal inflammation (10). Li et al. (11) used 60 mg/kg TASAs for intraperitoneal perfusion in rats with ulcerative colitis (UC) and found that S. alopecuroides alkaloids may maintain a balance between pro-inflammatory and anti-inflammatory factors by increasing the levels of protective proteins, thereby alleviating the degree of inflammation as well as regulating the local immune response in the intestine. The results of previous studies revealed that the limited studies of SA and its extracts on gastrointestinal health were mostly focused on monogastric animals, while few studies on ruminants were reported. The group’s preliminary in vitro experiments showed that adding 0.1‒0.4% SA to the diet had positive effects on ruminal fermentation in sheep under high concentrate diets (12), but the specific effects, suitable additive amounts and mechanisms of action were unclear and needed further study.
Therefore, we hypothesized that the addition of different doses of SA to high concentrate diets for sheep would mitigate the hazards produced by high concentrate diets on the animal organism through the changing patterns of the rumen environment and microbial diversity (rumen bacteria and fungi). Our aim was to reveal the effect and mechanism of action of SA on rumen fermentation function, and further provide theoretical basis for the prevention and treatment of subacute rumen acidosis and the improvement of body health of ruminants under the condition of high concentrate by using SA as a feed additive.
All experimental procedures were approved by Inner Mongolia Agricultural University Institutional Animal Care and Use Committee and conformed to national animal welfare regulations.
In this experiment, thirty-two 3-months-old Dumont crossbred male lambs (25.73 ± 2.17 kg) were divided into 4 groups of 8 lambs each for immunization and deworming in a one-way completely randomized trial design. The test period was 15 days for the preliminary and 60 days for the main test, in which the control group (CG) was fed the basal diet; the TG1 group was fed the basal diet +0.1% SA; the TG2 group was fed the basal diet +0.3% SA; and the TG3 group was fed the basal diet +0.5% SA. The experimental diet was designed according to the table of common feed ingredients and nutritional value of Chinese sheep (NY/T 816–2004), and the diet formula and nutritional ingredients are shown in Table 1.
Source and application method of SA: It was purchased from Yanchi, Ningxia. After being pulverized and sieved, it was mixed with the concentrate supplement at the corresponding addition ratio for feeding and the addition amount was selected based on the preliminary in vitro test conducted by our research group (12). The content of total alkaloids (main active substances) in SA is 5.75% by acidic dye spectrophotometry, and the recovery rate is 95.95%.
The feeding experiment was carried out in the teaching experimental pasture of Inner Mongolia Agricultural University. The sheep shed was thoroughly disinfected before the experiment. During the experiment, the experimental sheep were randomly assigned to 1.5 × 1 × 1 m iron cages for single cage feeding according to the principle of no significant difference in body weight between groups. The temperature and humidity of the sheep shed were kept at 15–20°C and 50–60%, respectively, with natural ventilation. The experimental sheep were fed twice a day at 08:00 and 18:00, with free access to feed and water.
In the late stage of formal feeding, the rumen fluid was collected 0 h before intake, 2 h, 4 h, 6 h, 8 h and 10 h after intake by using oral sampling method, and the rumen fluid was filtered through four layers of gauze, measured ruminal pH of the rumen fluid, which was then put into a centrifuge tube and stored at −20°C for the determination of volatile fatty acid (VFA) and ammonia nitrogen (NH3-N) contents.
After the main test, five sheep of similar body weight in each group were selected for slaughter and collected the rumen chyme, divided in enzyme-free sterile centrifuge tubes and stored in liquid nitrogen. 16S rDNA and ITS rDNA high-throughput sequencing were analyzed by Shanghai Meiji Biological Technology Co., Ltd., and microbial diversity was detected by MiSeq PE300 sequencing platform to analyze the community species composition and abundance at the phylum and genus levels. We have uploaded the sequencing data of 16S rDNA and ITS rDNA to Sequence Read Archive (SRA) in NCBI (BioProject id: PRJNA963087).
Firstly, sample DNA was extracted, specific primers with barcode in the assay region were synthesized, and the total DNA of the extracted samples was used as template to select specific primers for bacterial 16S rRNA and fungal ITS rRNA high variation regions for PCR amplification using an ABI GeneAmp® 9700 PCR instrument. The PCR products of the same samples were mixed and detected by 2% agarose gel electrophoresis was used to detect the PCR products, and the PCR products were recovered by cutting the gel using the AxyPrepDNA gel recovery kit (AXYGEN) and eluted with Tris–HCl; 2% agarose electrophoresis was used to detect the PCR products. The PCR products were detected and quantified by QuantiFluor™-ST Blue Fluorescence Quantification System (Promega), and then the samples were sequenced using Illumina MiSeq sequencing platform.
The PE reads obtained from Miseq sequencing were firstly spliced according to the overlap relationship, while the raw data were filtered and chimeras were removed, etc. After that, the resulting sequences were OTU clustered using the software platform Usearch, and the sequences were clustered according to 97% similarity for non-repeated sequences (excluding single sequences) were OTU clustered, and sequences with 97% or more similarity to OTU representative sequences were selected to generate OTU tables. The representative sequences from OUT were compared with the microbial reference database, species annotation was performed, α-diversity was analyzed, and dilution curves and Veen plots were plotted using R language tools. The QIIME software was used to analyze the β-diversity and draw the principal coordinate analysis (PCoA) diagram. According to the results of taxonomic analysis, we can know what kind of microorganisms are contained in the samples and the sequence number of each microorganism in the sample, that is, the relative abundance of each microorganism. The data are analyzed and sorted out by Qiime platform.
All the recorded data were entered into an Excel table for preliminary collation. The MIXED PROC in SAS 9.2 statistical software was used for two-factor analysis of variance and regression analysis of rumen fermentation parameters. The two-factor analysis of variance included the dosage of SA, sampling time and interaction (SA × Time). The dose dependence of SA was analyzed by orthogonal polynomial analysis (Linear and Quadratic); The alpha diversity index of rumen bacteria and fungi was a two-tailed difference test using Student’ s t-test; the nonparametric Kruskal–Wallis H-test was used to compare the differences of rumen microbial flora in sheep. p < 0.05 indicated a significant difference or a significant regression relationship, and 0.05 < p < 0.10 indicated an upward or downward trend.
As shown in Table 2, after different doses of SA were added, the rumen pH of TG3 group was significantly higher than that of CG and TG2 groups at 2 h of feeding (p < 0.01), the rumen pH of TG3 group was significantly higher than that of CG and TG1 groups at 4 h of feeding (p < 0.05), the rumen pH of TG1 and TG3 groups were significantly higher than that of CG and TG2 groups at 6 h of feeding (p < 0.05). Compared with the CG, ruminal NH3-N concentrations were significantly or highly significantly lower in the TG1, TG2, and TG3 groups at 4–8 h of feeding (p < 0.05 or p < 0.01), and there were no significant differences in NH3-N concentrations between the groups at the other time points (p > 0.05). With the increase of the dosage of SA, the concentration of NH3-N in the rumen presented a linear significant decrease (p < 0.05), and the quadratic curve showed a trend of decrease (p = 0.07). With the prolongation of sampling time, the concentration of NH3-N in the rumen presented a change rule of increase first and then decrease, and reached the peaked at 2 h.
At 0 h before feeding, the concentrations of acetate and TVFA in TG2 group were significantly lower than those in other groups (p < 0.01), the concentration of propionate and the ratio of acetate to propionate in TG2 group was significantly lower than that in TG1 group (p < 0.05), the concentration of butyrate in TG3 group was significantly higher than that in TG2 group (p < 0.05). At 2 h after feeding, the concentrations of acetate, propionate, butyrate and TVFA in rumen of the three experimental groups were significantly or extremely significantly higher than those of the control group (p < 0.05 or p < 0.01). At 4 h after feeding, the concentrations of rumen acetate, TVFA and the ratio of acetate to propionate in TG1, TG2 and TG3 groups were significantly or extremely significantly higher than CG group (p < 0.05 or p < 0.01), and TG1 group was the highest. At 6 h after feeding, the concentrations of acetate, propionate and TVFA in the rumen of TG3 group were significantly or extremely significantly higher than those of the other three groups (p < 0.05 or p < 0.01), while the concentrations of butyrate in the rumen of TG2 group was significantly or extremely significantly higher than the CG and TG1 group (p < 0.05 or p < 0.01). At 8 h after feeding, the rumen acetate concentration in TG3 group was significantly higher than that in the control group (p < 0.05), while that in TG1 and TG3 groups were significantly higher than that in TG2 group (p < 0.01). The rumen propionate, butyrate and TVFA concentrations in TG3 group were significantly or extremely higher than those in the other three groups (p < 0.05 or p < 0.01). The concentration of butyrate in TG2 group was significantly lower than that in the control group (p < 0.05), and the ratio of acetate to propionate in TG1 group was significantly or extremely significantly higher than that in the other three groups (p < 0.05 or p < 0.01). At 10 h after feeding, there were no significant difference in each index all the groups (p > 0.05).
With the increase of the dosage of SA, the concentrations of acetate, propionate, butyrate and TVFA in rumen all increased linearly (p < 0.05), the concentration of propionate in rumen showed a significant quadratic curve effect (p < 0.05), and the concentration of butyrate in rumen showed a quadratic curve effect (p = 0.07). The sampling time is closely related to various parameters of rumen fermentation. With the extension of sampling time, the concentrations of acetate, propionate, butyrate and TVFA in rumen all showed a trend of increasing first and then decreasing, and the concentrations of acetate and TVFA reached the peak at 2 h, while those of propionate and butyrate reached the peak at 4 h.
Sobs refers to the number of OTU actually observed, Shannon and Simpson mainly reflect species diversity, and Ace and Chao measure species richness. It can be seen from Table 3 that Shannon was TG1 > TG2 > CG > TG3, and Simpson was TG3 > TG1 > CG = TG2, but the difference was not significant (p > 0.05), which showed that the diversity of rumen bacteria with different doses of SA in high-concentrate diet was similar. Sobs, Ace and Chao in the three experimental groups were all higher than those in the control group, and there was no significant difference among the groups (p > 0.05), indicating that the species richness of the four groups were basically the same.
As shown in Figure 1 and Table 4 below, at the phylum level, there were 17 phyla in CG and TG1 groups, 15 phyla in TG2 group and 16 phyla in TG3 group, of which 14 phyla were shared by the control group and the experimental group. Mainly Actinobacteria, Firmicutes, Chloroflexi, etc. The common phylum of CG group and TG1 group were Armatimonadetes and Fusobacteria, the common phylum of CG group and TG1 and TG3 group were Absconditabacteria, and the common phylum of TG2 and TG3 group were Lentisphaerae.
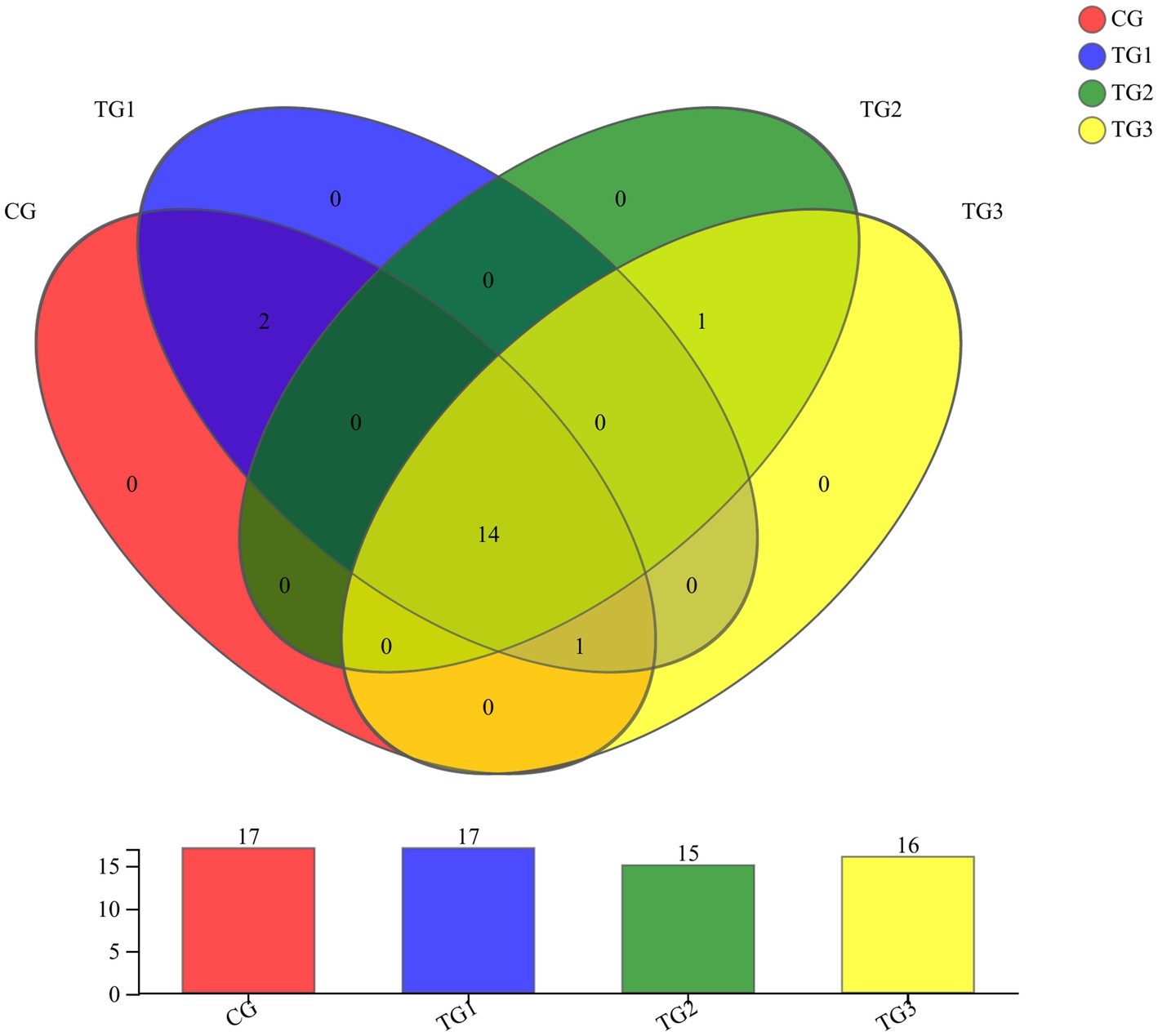
Figure 1. Venn diagram of rumen bacteria at phylum levels. Different colors represent different groups, numbers in overlapping parts represent the number of species common to the plurality of groups, and numbers in non-overlapping parts represent the number of species specific to the corresponding group.
As shown in Figure 2 and Table 5 below, at genus level, there were 186 genera in CG, TG2 and TG3 groups. 194 genera in TG1 group, and 165 genera in common in control group and the experimental groups. Mainly include Paraprevotella, Papillibacter, Terrisporobacter, Succiniclasticum, etc. The endemic genera in CG group was Oscillospira, Marinilabiaceae and Fibrobacteriaceae. The specific genera of TG1 were Syntrophomonas, Rickettsiales, Jeotgalicoccus and Eubacterium; the specific genus in TG2 group was Staphylococcus. The genera endemic to TG3 group were Pseudoscardovia and Dialister. The common genera in TG1, TG2 and TG3 groups were Hydrogenoanaerobacterium, Ruminiclostridium, Erysipelotrichaceae and Mitsuokella.
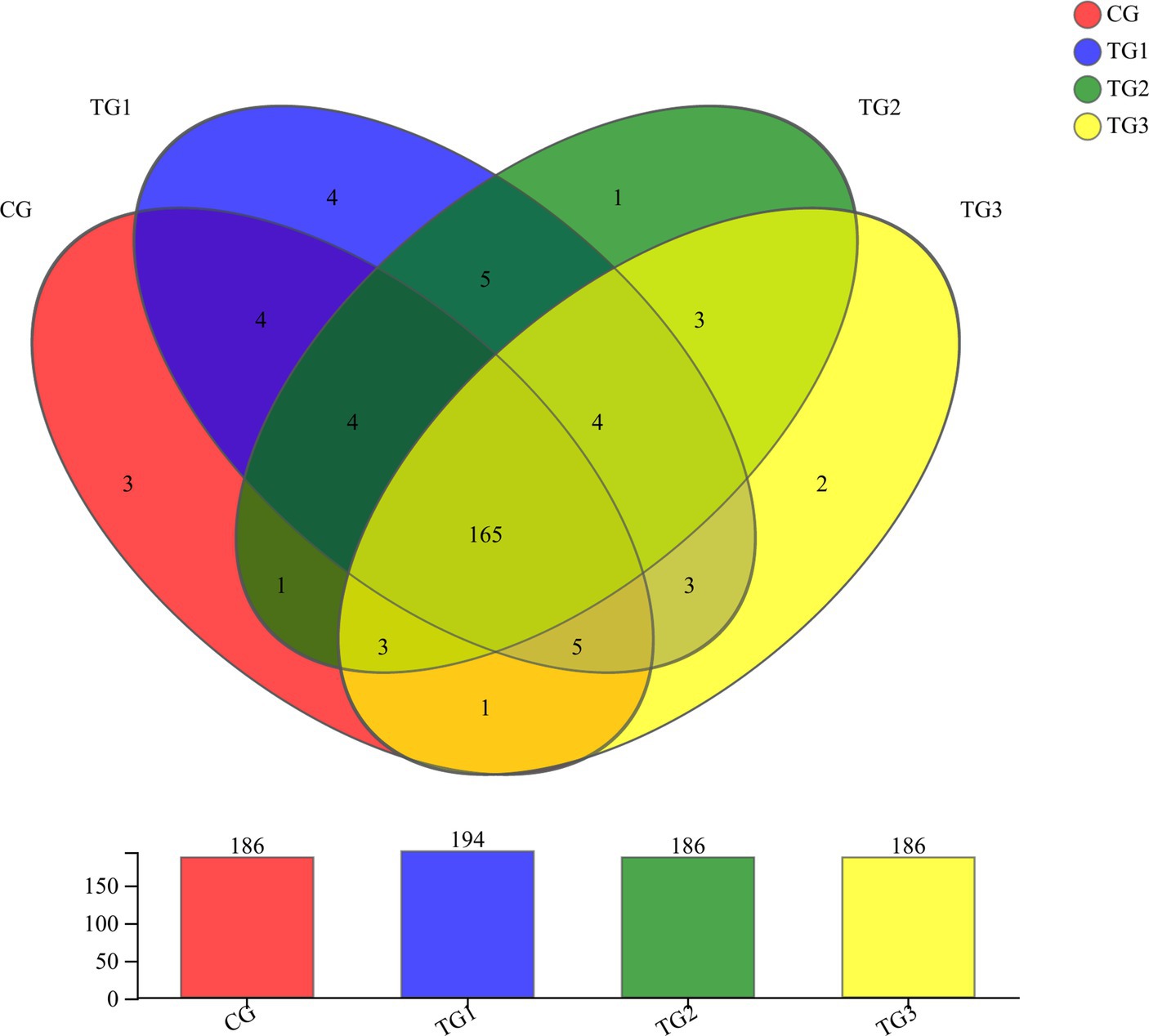
Figure 2. Venn diagram of rumen bacterial at genus levels (Figure 1).
As shown in Table 6 and Figure 3, Bacteroidetes and Firmicutes were the dominant phyla in each group. Compared to the control group, Bacteroidetes had a tendency to increase the percentage of TG3 group (p = 0.08), while Firmicutes had a tendency to decrease in the TG3 group (p = 0.07). There were no significant differences in other phyla between the groups (p > 0.05).
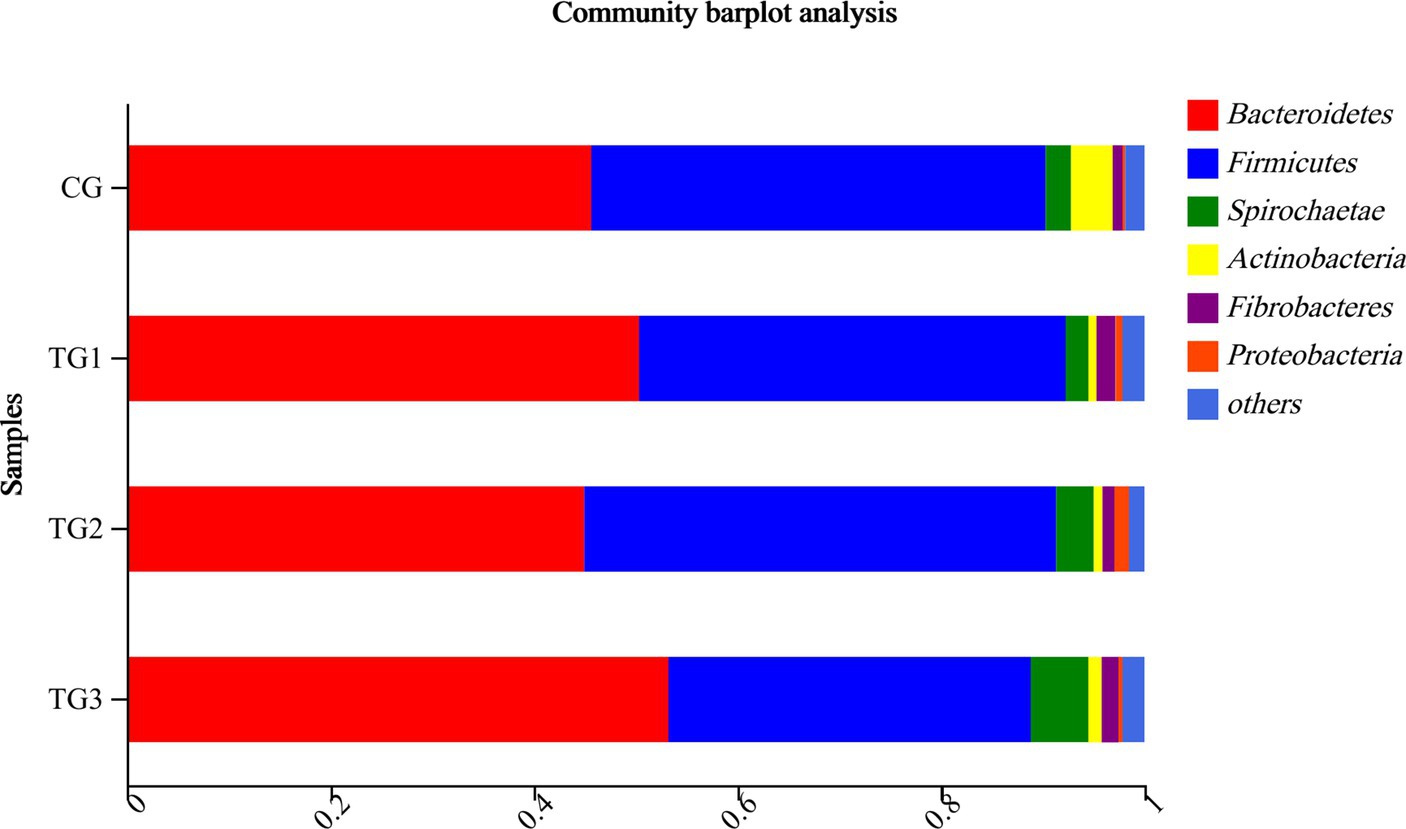
Figure 3. Horizontal column chart of rumen bacteria at phylum levels. The ordinate indicates the grouping, the abscissa is the proportion of species in the group, different colors represent different species, and the length represents the proportion of the species.
As shown in Figure 4 and Table 7 below, rumen bacteria were subjected to taxonomic statistics at the genus level, and 7 kinds of rumen bacteria with significant difference or difference trend were obtained. The proportion of Ruminococcus and Phocaeicola in the experimental groups were significantly higher than that in CG group, especially in TG1 group. The proportion of Sphaerochaeta, Clostridiales and Candidatus-Hepatincola in TG1 and TG2 groups were higher than that in CG group (p = 0.09 or p < 0.05). The proportion of Eubacterium in TG2 group was lower than that in CG group (p = 0.07). The percentages of Asterolemia in TG1, TG2 and TG3 groups were lower than that in CG group (p = 0.09).
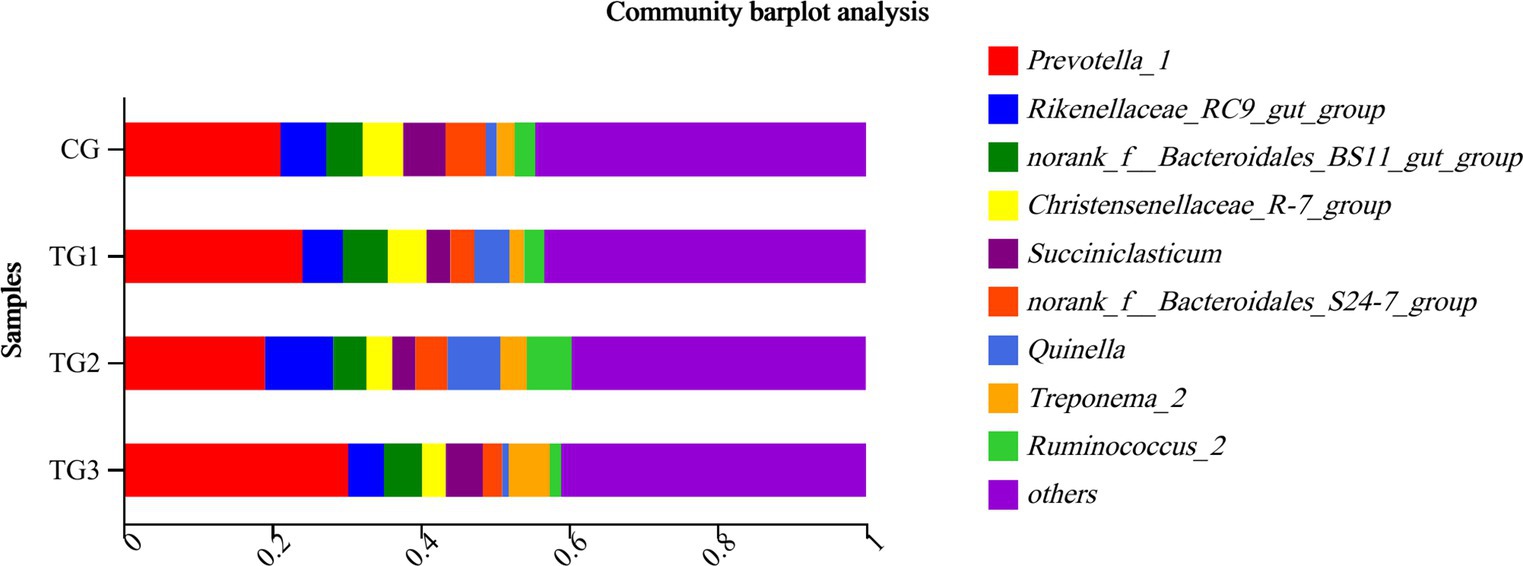
Figure 4. Horizontal column chart of rumen bacteria at genus levels (Figure 3).
As shown in Table 8, Simpson ranked in the order of TG1 > TG2 > TG3 > CG, but the differences were not significant (p > 0.05). The Sobs, Shanon, Ace and Chao indexes of the control group were slightly higher than those of the three experimental groups in numerical value, but the differences were not significant among different groups. These results indicated that the diversity and abundance of rumen fungi when different doses of SA were added into high concentrate diets were similar.
As shown in Figure 5 and Table 9 below, at the phylum level, CG group had 7 phyla, TG1 group had 9 phyla, TG2 and TG3 groups each had 5 phyla, among which 5 phyla were shared by the control group and the three experimental groups. They were Ascomycota, Neocallimastigomycota, etc. The unique anaerobic fungi in CG group were Cercozoa and Glomeromycota, and the unique anaerobic fungi in TG1 group were Chytridiomycota, Mucoromycota, etc.
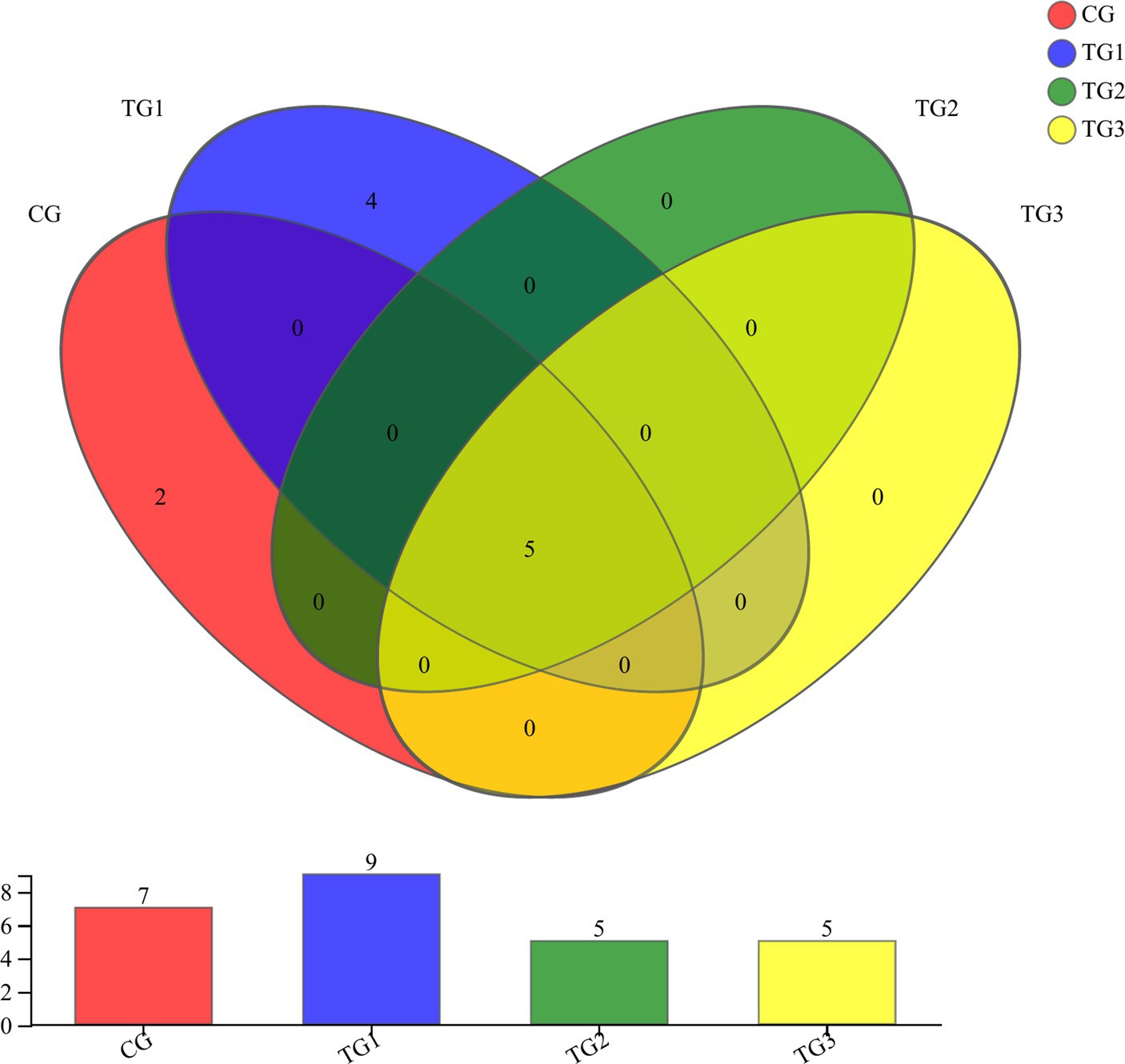
Figure 5. Venn diagram of rumen anaerobic fungi at phylum levels (Figure 1).
As shown in Figure 6 and Table 10 below, at the genus level, there were 128 genera in the CG group, 110 genera in the TG1 group, 70 genera in the TG2 group, 77 genera in the TG3 group, and 34 genera common to the control and experimental groups, mainly including Unclassified-Chaetomiaceae, Filobasidium, Unclassified-Didymellaceae, etc. There were 40 species of fungal genera endemic to TG1 group, mainly Ramularia, Geminibasidium, Cordyceps, etc. There were 15 fungal genera endemic to TG2 group, mainly Phialemonium, Cotylidia, Thanatephorus, etc. There were 10 endemic fungal genera in TG3 group, mainly Mrakia, Uwebraunia, Lectera, etc. The fungal genera shared by TG1, TG2 and TG3 groups were Sarocladium.
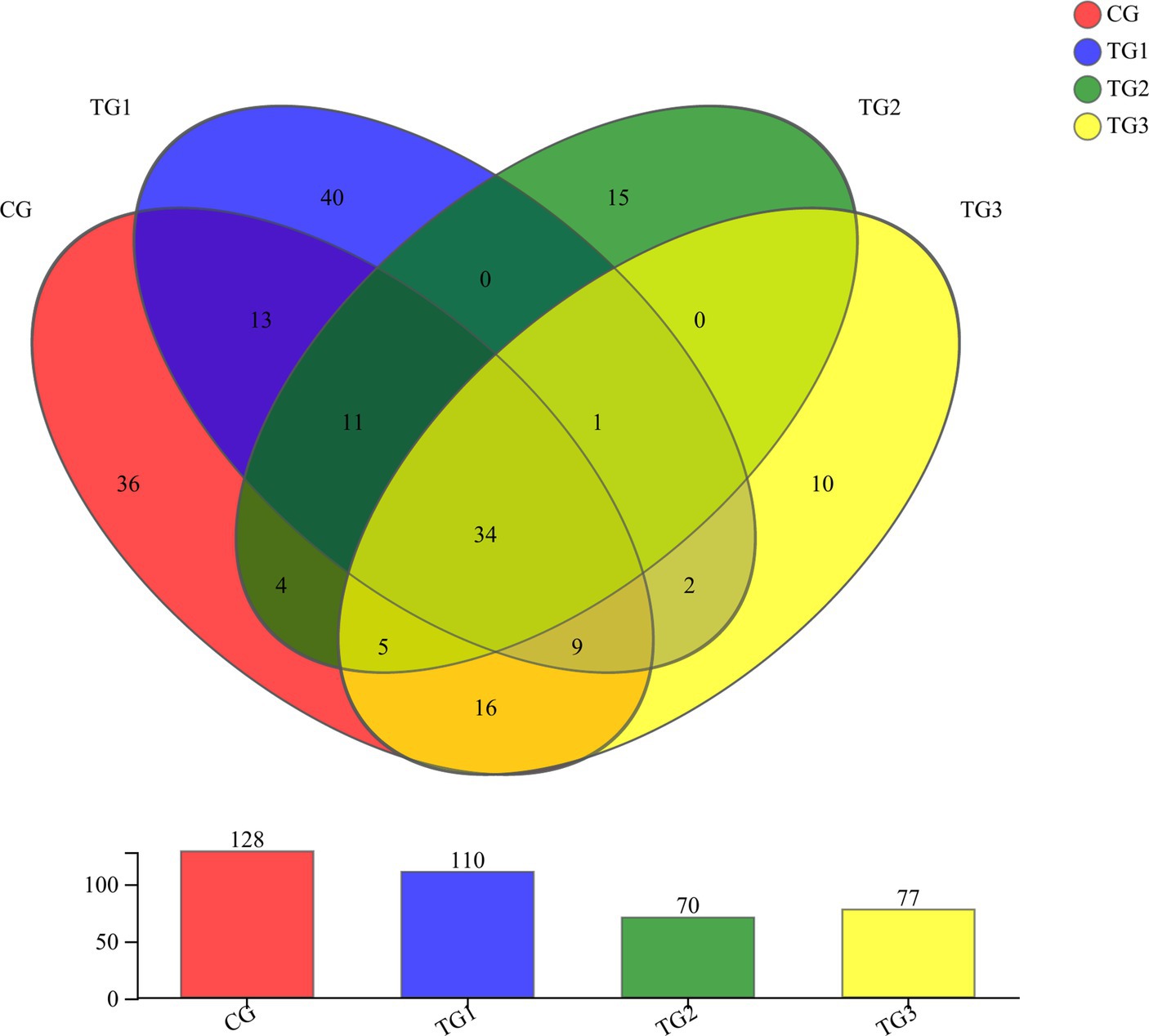
Figure 6. Venn diagram of rumen anaerobic fungi at genus levels (Figure 1).
As can be seen from Table 11 and its Figure 7, the control and experimental groups of Neocallimastigomycota, Ascomycota, Unclassified-Fungi, Basidiomycota, and Mortierellomycota were not significantly different at the phylum level (p > 0.05).
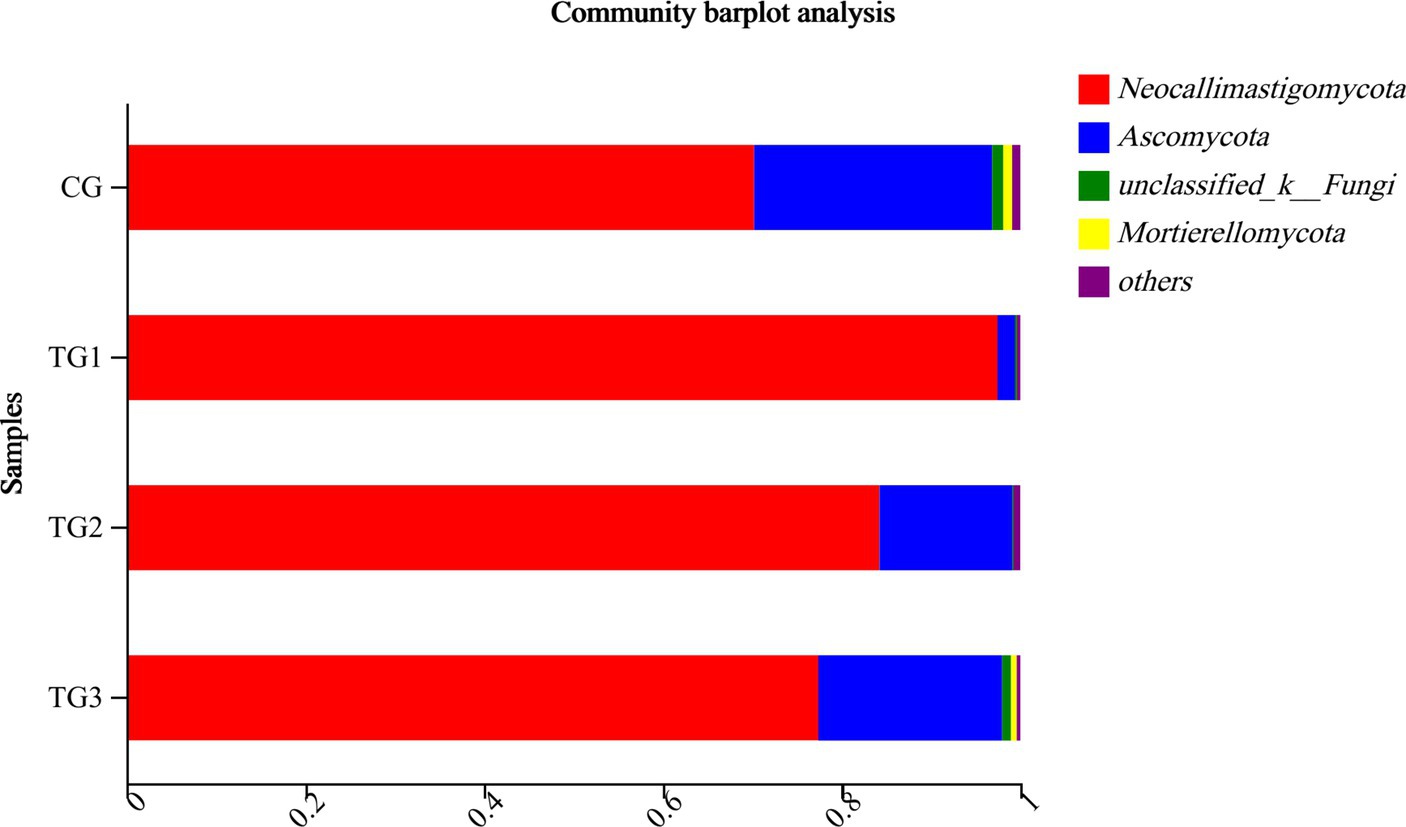
Figure 7. Horizontal column chart of rumen fungi at phylum levels (Figure 3).
As shown in Table 12 and Figure 8, the control and experimental groups of Unclassified-Neocallimastigaceae, Piromyces, Neocallimastix were not significantly different at the genus level (p > 0.05).
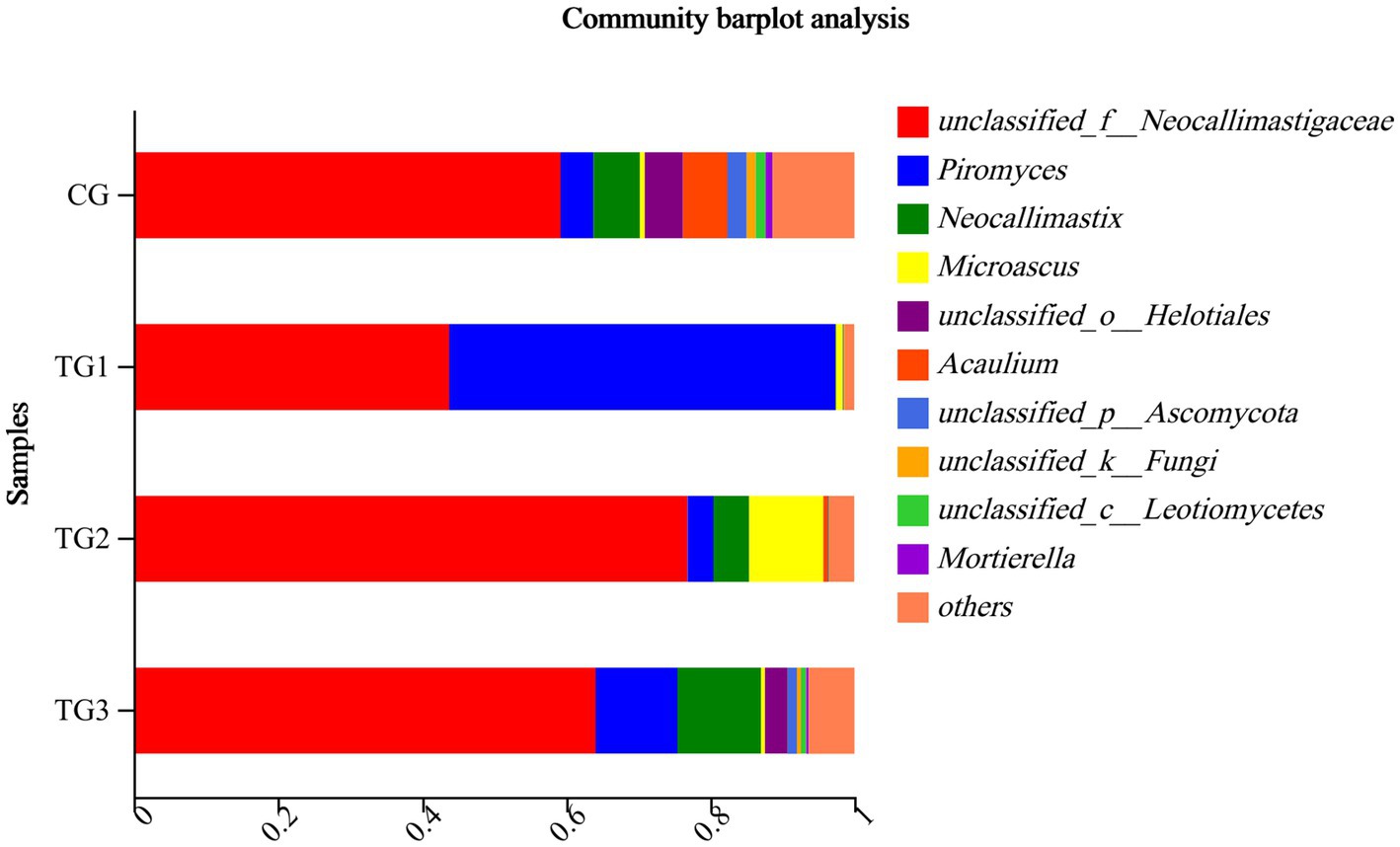
Figure 8. Horizontal column chart of rumen fungi at genus levels (Figure 3).
Rumen pH is an important indicator to maintain normal rumen fermentation of animals. The normal pH range of the rumen is 6.0–7.5. The rumen pH (5.2–5.6) exceeding 3 h is often recognized as the cause of SARA, and acute acidosis occurs when pH is below 5.0 (13). The pH value is affected by the feeding method, concentrate-to-forage ratio and dry matter level. Many studies have found that frequent intake of high-grain diets by animals will lead to a decrease in the pH of the rumen. When the pH falls to 6.0, a slight decrease in the fiber decomposition rate will be presented, but the number of fiber decomposition bacteria is generally not affected. When it falls to 5.5 or 5.0, it will lead to a decrease in both fiber decomposition rate and fiber decomposition bacteria, and may even completely inhibit fiber digestion, thereby changing the rumen fermentation function (14). This study showed that the rumen pH of the control group was 5.4, 5.5, and 5.7 when the animals were fed for 2 h, 4 h, and 6 h, respectively, indicating that there was a trend of SARA in the control group. While the rumen pH of the TG3 group was about 6.0 during this time, significantly higher than that of the control group, and indicating that SA added in the high-concentrate diet was conducive to improving the rumen pH under high concentrate diets, and might play an important role in the utilization of cellulose by microorganisms to change rumen fermentation functions. In addition, the pH values in the rumen of the other three experimental groups were relatively stable, which had a positive effect on the maintenance of the homeostasis of the rumen environment, which was similar to the result of Gu et al. (15) who added Chinese herbal medicine additives into the diet to improve the pH of rumen and maintain the stability of the intragastric environment.
Ammonia nitrogen concentration is a dynamic indicator of the degradation of ammonia and nitrogen sources by rumen microorganisms, which can reflect the rate of microbial degradation and synthesis of nitrogen-containing substances as well as the utilization of ammonia nitrogen by microorganisms (16). Rumen microorganisms can utilize ammoniacal nitrogen to synthesize protein, and the appropriate concentration of NH3-N is beneficial to the synthesis of bacterial protein for use by the animal organism. Pablo et al. (17) found that with the increase of the dosage of the composite herbal medicine, the concentrations of NH3-N and TVFA in the rumen of lambs tended to increase first and then decrease, which might be related to the synergistic effect of the active components in the composite herbal medicine. However, Vera et al. (18) found that both pine bark extract and mignonette extract could reduce the concentration of NH3-N in the rumen, which might be due to the fact that the polyphenol tannin in pine bark and mignonette extract could inhibit the activities of protease and microbial deaminase, and thus forming tannin complex to reduce NH3-N production at rumen pH = 6–7. The results of this experiment showed that the ammonia nitrogen concentrations in the three experimental groups were significantly reduced 4–8 h after intake, and the NH3-N concentration in TG1 group was the lowest. The previous weight gain results of our research group also showed that the average daily weight gain and F/G of the 0.1% SA group were significantly increased (19), indicating that the addition of different doses of SA in the high-precision diet might improve the utilization rate of ammonia nitrogen by rumen microorganisms, promote rumen fermentation and play a role in promoting growth, and the effect of 0.1% addition was better.
VFA produced by ruminant fermentation mainly include acetate, propionate and butyrate, etc. They are the main source of energy for ruminant growth, production and reproduction, and can provide 70 to 80% of total energy for animals to use. Tian et al. (20) showed a significant increase in ruminal propionate, butyrate and isobutyrate levels after the addition of inulin to the rations of fattening beef cattle. Wei et al. (21) found that the concentrations of acetate and TVFA in the astragalus powder group were higher than those in the control and antibiotic groups in an in vitro study. Although the types and doses of additives as well as animal species were different, the test results were similar, indicating that herbal additives facilitate the degradation of feed by rumen microorganisms, promoting rapid fermentation and providing an opportunity to maintain animal metabolism. The results of this study showed that the concentrations of acetate, propionate, and TVFA in the rumen of TG1, TG2, and TG3 groups were higher than those of CG group at 2 h feeding, and the concentrations of acetate rapidly decreased 2–6 h after reaching the peak, and propionate in the experimental group began to decrease slowly after reaching the peak at 4 h feeding. It indicated that the Chinese herbal medicine SA might begin to work 2 h after the sheep ate it. Studies have found that Ruminococcus was the main fiber-degrading bacteria in the rumen, which could participate in the acetate metabolism, resulting in a decrease in the concentration of acetate, which promoted the rumen fermentation to tend to propionate type (22). While propionate was a glycogenic substance, the higher concentration of propionate in rumen fluid indicated the higher energy available for weight gain, which was similar to the results of our following rumen bacterial diversity. These results indicated that the addition of SA might improve the rumen fermentation function under high concentrate condition by the composition and structure of rumen microflora, change the proportion of VFA in the rumen, and further affect the digestion and absorption of dietary nutrients by the host, thereby promoting the growth of healthy animals.
Ruminal microorganisms provide energy to ruminants by converting nutrients contained in the feed into VFA as well as microbial proteins through a series of complex biochemical reactions (23). Microbial diversity studies were conducted mainly based on conserved regions of rRNA nucleic acid sequences and bacteria were mainly based on the 16S region. Shannon and Simpson mainly reflect species diversity, while Ace and Chao measure species richness. We found that shannon, simpson indices were not significantly different between groups by rumen bacterial alpha diversity. This indicates that the rumen bacterial diversity of SA added at different doses in high concentrate diets has some similarity. Ace index and Chao index were greater than the control group in all the three experimental groups, but the difference between the groups was not significant, indicating that the species abundance was also basically the same in the four groups. In this experiment, it was found that Ruminiclostridium is a common genus in TG1, TG2 and TG3 groups, and Clostridium butyricum in Ruminiclostridium has the ability to improve intestinal flora and improve animal immune function and enhance disease resistance by increasing the level of humoral immunity, enhancing the intestinal mucosal barrier, and inhibiting inflammation (24, 25). This was verified by our previous findings that SA could improve immune and antioxidant functions in serum, alleviate rumen damage caused by high concentrate diets through inflammatory signaling pathway-related inflammatory factor production and release, and enhance rumen epithelial barrier function by promoting rumen epithelial tight junction protein expression in lambs (19, 26).
At the phylum level, studies have found that, after goats were injected with LPS, the number of Firmicutes-unclassfied and Spirochaetes-unclassified bacteria in the rumen was significantly increased, while the number of Bacteroidetes-unclassfied, Paraprevotella and Centipeda was significantly decreased (27). Zened et al. (28) found that the dominant bacteria in rumen of dairy cows would not change with the change of diet, and they were still Bacteroidetes and Firmicutes. The results of this study showed that Bacteroidetes and Firmicutes were the dominant phyla in each group, and compared with the control group, the 0.5% SA group increased the abundance of rumen Bacteroidetes and decreased the abundance of Firmicutes. Prevotella was the most dominant species of Bacteroidetes, which can degrade polysaccharides such as starch, xylan and pectin in the rumen, but cannot degrade cellulose (29). It is suggested that the addition of SA to high concentrate diets enhanced the degradation of non-fibrous carbohydrates, which helped to alleviate the harm caused by excess LPS in sheep to rumen microorganisms and positively influenced the community structure of rumen microbial flora in high concentrate diets conditions. In addition, it is reported that the increase of the ratio of Firmicutes to Bacteroidetes could lead to the deposition of fat (30). In this experiment, the ratio of Firmicutes to Bacteroidetes TG1 and TG3 was lower than that of the control group, which just proves that our previous study found that SA group could reduce the concentration of triglyceride in serum (19), thus better explains that the reason for the growth promotion of SA might be that the breakdown of triglyceride provides a large amount of energy for animal growth. However, Wei et al. (21) found in vitro studies yet Astragalus group yaks had a significant decrease Bacteroidetes and a significant increase Firmicutes, which may be due to the ratio of dietary concentrate, the main active ingredients and animal breed that differ from the results of this experiment.
At the genus level, Ruminococcus is a kind of vatal bacteria for ruminant to utilize fiber material in rumen. In this experiment, the number of Ruminococcus in CG group was significantly lower than that in expermental groups, indicating that the addition of SA in the diet increased the number of rumen fiber utilization bacteria and promoted the fermentation of fiber material, which was similar to the above-mentioned change rule of pH value in rumen. At the same time, we also demonstrated by qPCR results that the number of Ruminococcus albicans in experimental groups increased significantly compared with the control group. However, Zhou et al. (31) found that the relative abundance of Ruminococcus was positively correlated with the release of IL-1β in the body, which might increase the risk of organ and tissue damage. Our previous experimental results found that the difference of IL-1β in serum between the experimental group and the control group was not significant, which may be caused by the difference of animal species and detection sites, and its specific mechanism needs further verification. Liu et al. (32) found that Biochanin A significantly increased the abundance of the dominant rumen bacterium Prevotella ruminicola protein hydrolysing bacteria. P. ruminicola synthesizes proteases and deaminases to generate ammonia from dietary protein for using by bodies. Emma et al. (33) found that Clostridiaceae showed a high positive correlation with crude protein content, protein digestibility, and total energy, and a weaker positive correlation with fat digestibility. The percentage of Clostridiaceae was significantly higher in TG1 and TG2 groups in this experiment, indicating that the addition of 0.1–0.3% SA to the diets could improve the number of protein-using bacteria, thus ensuring better nutrient absorption in lambs under high concentrate conditions.
Anaerobic fungi that colonize the digestive tract of ruminants produce and secrete a series of polysaccharide-degrading enzymes, including glucoside hydrolases, cellulases and xylanases, which can better facilitate the utilization of dietary cellulose by the rumen (34). Most studies have shown that feeding practices, feed concentrate-to-forage ratio and diet nutrient levels can affect the bacterial diversity and composition of the dominant flora in the rumen (35, 36), while there have fewer reports on rumen fungi in sheep. Ye et al. (37) found that the addition of fructans to the ration could affect the Ace, Shannon and Simpson index of rumen fungi in Holstein cows, but did not significantly affect the overall diversity and abundance of rumen fungal flora. We found that Simpson showed TG1 > TG2 > TG3 > CG among the groups by sheep rumen fungal Alpha diversity, and Sobs, Shanon, Ace and Chao index control groups were all slightly higher than the three experimental groups, but none of the differences were significant (p > 0.05), which was similar to the results of Wang et al. (38) suggesting that the addition of SA in high-concentrate diet had different degrees of sheep rumen fungal diversity and abundance, but they also had certain similarities. In this experiment, the rumen fungal composition from sheep rumen was found to be more abundant in the TG1 group both at the phylum level and genus level, and the fungal composition at the rumen genus level in this experiment revealed that Sarocladium was a genus unique to the TG1, TG2, and TG3 groups, and Sarocladium can degrade cellulose and xylan (39), which also further indicates that the addition of SA to high concentrate diets might enrich the species of fibrous degrading bacteria.
At the phylum level, Li et al. (40) found that the dominant phyla of rumen fungi in the diets of small-tailed Han sheep consuming diets with at different protein levels was mainly Ascomycota, Basidiomycota and Neocallimastigomycota. Han (41) found in the study of Shaanxi cashmere goats that the dominant phylum of rumen fungi in different concentrate-to-forage ratio diets groups was Ascomycota, and it was mainly involved in the degradation of recalcitrant nutrients in the feed. In contrast, Peng et al. (42) added grape seeds to the sheep diet and found that the dominant rumen fungi of Dorang sheep were Neocallimastigomycota and Ascomycota, which were consistent with the experimental results. It follows that different species and feeding practices, as well as differences in the main active substances could alter the relative abundance of the dominant rumen phylum. Neocallimastigomycota was a functional fungus widely present in the digestive tract of herbivorous ruminants, and played an important role in degrading lignified cellulose (43), and could also provide the host with nutrients required for vital activities by consuming rumen degradable proteins (44). The relative abundance of Neocallimastigomycota in all three experimental groups in this experiment was numerically higher than that of the control group, and the highest in the TG1 group, indicating that the addition of appropriate amounts of SA to high concentrate diets would enhance cellulose degradation and provide energy for animal metabolism. In this experiment, the relative abundance of Neocallimastigomycota was numerically higher in all three test groups than in the control group, and was highest in the TG1 group, indicating that the addition of SA to high concentrate diets would enhances the degradation of cellulose and provides energy for animal metabolism.
At the genus level, Wang et al. (45) showed that Piromyces fungal inoculants increased the in vitro digestibility of dry matter and neutral detergent fiber of silage after 30 days of fermentation. Piromyces, Unclassified-Neocallimastigaceae and Neocallimastix belong to Neocallimastigomycota, which had the ability to digest cellulose efficiently. The results of this experiment showed a relatively high level of these three fungal genera, suggesting that the addition of SA in high concentrate diets would improve the utilization of cellulose, which was also verified in the above results of rumen bacterial diversity. Dietary concentrate-to-forage ratio has a large effect on the characteristics of rumen fungi. Yang et al. (46) found that feeding a high concentrate full-price pelleted diet inhibited the activity of anaerobic fungi and rumen fiber-degrading bacteria in calves. Zhu et al. (47) reported that concentrates inhibited the production of fungal xylan isomerase, which might be related to the induction of crude fiber-like substrates, while Piromyces can produce xylan isomerase and increase the number of cellulose degrading bacteria (48, 49). In this experiment, the relative abundance of Piromyces in TG1 group was much higher in value than the control group, suggesting that the addition of SA to high concentrate diets might increase the activity of xylan isomerase and promote the proliferation of fiber-degrading bacteria in the rumen of sheep, which helped maintain the ruminal microecological balance of lambs under high concentrate conditions.
In this study, the addition of SA to the high grain diet could increase the concentration of acetate, propionate, butyrate, TVFA and pH in the rumen of sheep, and reduce the concentration of NH3-N in the rumen. The dominant rumen bacteria after adding SA to the high grain diet were Bacteroidetes and Firmicutes, which increased the relative abundance of Ruminococcus, Phocaeicola and Clostridiales in the rumen of SA group. And the dominant phylum of sheep rumen fungi was Neocalymastigomycota, and the dominant genus was unclassified-Neocalymastigaceae, which increased the relative abundance of rumen Neocalymastigomycota and Piromyces in the 0.1% SA group to a certain extent. This has a positive effect on promoting rumen fermentation and fine-tuning the rumen microbial ecosystem to improve sheep health.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/bioproject; PRJNA963087.
The animal study was reviewed and approved by Inner Mongolia Agricultural University Institutional Animal Care and Use Committee. Written informed consent was obtained from the owners for the participation of their animals in this study.
All authors participated in study design, data acquisition and analysis, and preparation of the manuscript.
This work was financially supported by National Natural Science Foundation of China (31860658).
The authors are grateful to the staff of Inner Mongolia Agricultural University Experimental Pasture for providing assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Plaizier, JC, Khafipour, E, Li, S, Gozho, GN, and Krause, DO. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim Feed Sci Technol. (2012) 172:9–21. doi: 10.1016/j.anifeedsci.2011.12.004
2. Oetzel, GR. Diagnosis and management of subacute ruminal acidosis in dairy herds. Vet Clin N Am Food Anim Pract. (2017) 33:463–80. doi: 10.1016/j.cvfa.2017.06.004
3. Liu, YH, Espinosa, CD, Abelilla, JJ, Casas, GA, Lagos, LV, Lee, SA, et al. Non-antibiotic feed additives in diets for pigs: a review. Anim Nutr. (2018) 4:113–25. doi: 10.1016/j.aninu.2018.01.007
4. Wang, RZ, Deng, XX, Gao, QX, Wu, XL, Han, L, Gao, XJ, et al. Sophora alopecuroides L.: an ethnopharmacological, phytochemical, and pharmacological review. J Ethnopharmacol. (2020) 248:112172. doi: 10.1016/j.jep.2019.112172
5. You, JJ, Li, YY, Sha, BY, and Yin, XY. Advances in studies on the alkaloid of Sophora alopecuroides L. J Jiangxi Univ Tradit Chin Med. (2015) 27:109–16.
6. Haroon, UR, Shagufta, R, Yousaf, A, Kamin, K, and Marco, AUM. Anti-cancer potential of sophoridine and its derivatives: recent progress and future perspectives. Bioorg Chem. (2020) 99:103863. doi: 10.1016/j.bioorg.2020.103863
7. Rong, ZJ, Hu, GS, Lin, SY, Yan, T, Li, N, Zhao, Y, et al. Constituents from the seeds of Sophora alopecuroides L. Molecules. (2020) 25:1–8. doi: 10.3390/molecules25020411
8. Hu, GL, Liu, J, Tian, S, Song, ZH, Fan, ZY, Zhang, SR, et al. Effects of tea seed polysaccharide and Macleaya cordata alkaloids on intestinal flora of yellow-feathered broilers and antimicrobial activity of Macleaya cordata sanguinarine. Chin J Anim Nutr. (2018) 30:4619–25. doi: 10.3969/j.issn.1006-267x.2018.11.038
9. Jia, YQ, Yuan, ZW, Zhang, XS, Dong, JQ, Liu, XN, Peng, XT, et al. Total alkaloids of Sophora alopecuroides L. ameliorated murine colitis by regulating bile acid metabolism and gut microbiota. J Ethnopharmacol. (2020) 255:112775. doi: 10.1016/j.jep.2020.112775
10. Tian, AP, Xu, T, Liu, KY, Zou, QM, and Yan, X. Anti-helicobacter pylori effect of total alkaloids of Sophora alopecuroides in vivo. Chin Med J. (2014) 127:2484–91. doi: 10.3760/cma.j.issn.0366-6999.20140615
11. Li, H, Luo, WT, Chen, ZW, Zheng, WW, Xie, WY, Long, YQ, et al. Effects of total alkaloids of Sophora alopecuroides on HSP70 in rats with ulcerative colitis. Lab Med Clin. (2018) 15:3428–30. doi: 10.3969/j.issn.1672-9455.2018.22.030
12. Xie, MX. Effect of Sophora alopecuroides L. on Rumen Fermentation Parameters, Ruminal Microbial Population and Blood Physiological-Biochemical Indexes of Mongolian Lamb. Hohhot: Inner Mongolia Agricultural University (2018).
13. Abdela, N. Sub-acute ruminal acidosis (SARA) and its consequence in dairy cattle: a review of past and recent research at global prospective. Achiev Life Sci. (2016) 10:187–96. doi: 10.1016/j.als.2016.11.006
14. Bach, A, Iglesias, C, and Devant, M. Daily rumen pH pattern of loose-housed dairy cattle as affected by feeding pattern and live yeast supplementation. Anim Feed Sci Technol. (2007) 136:146–53. doi: 10.1016/j.anifeedsci.2006.09.011
15. Gu, XW, Zhao, GQ, Jin, XJ, and Guo, P. Effects of feeding herbal additives on dry matter intake and the ruminal environment in dairy cows. China Dairy Cattle. (2010) 4:18–21. doi: 10.3969/j.issn.1004-4264.2010.04.009
16. Yang, DL, Tong, JJ, Zhang, J, Guo, Q, Guo, QH, Jiang, SL, et al. Effects of grape seed procyanidine on rumen fermentation parameters and microflora of dairy cows in vitro. Chin J Anim Nutr. (2018) 30:324–32. doi: 10.3969/j.issn.1006-267x.2018.02.037
17. Pablo, BRO, German, DMM, Gabriela, VS, Amada, IOS, José, FGS, Pedro, AHG, et al. Polyherbal feed additive for lambs: effects on performance, blood biochemistry and biometry. J Appl Anim Res. (2020) 48:419–24. doi: 10.1080/09712119.2020.1814786
18. Vera, N, Gutiérrez-Gómez, C, Williams, P, Allende, R, Fuentealba, C, and Ávila-Stagno, J. Comparing the effects of a pine (Pinus radiata D. Don) bark extract with a Quebracho (Schinopsis balansae Engl.) extract on methane production and in vitro rumen fermentation parameters. Animals. (2022) 12:1080. doi: 10.3390/ani12091080
19. An, YW, Yang, XD, Gao, ZX, Guo, SQ, Gao, AW, Yang, JL, et al. Effects of adding Sophora alopecuroides to high grain diet on growth and serum biochemical indexes of lambs. Acta Agriculturae Zhejiangensis. (2022) 34:908–14. doi: 10.3969/j.issn.1004-1524.2022.05.04
20. Tian, K, Liu, JH, Sun, YW, Wu, YJ, Chen, JC, Zhang, RM, et al. Effects of dietary supplementation of inulin on rumen fermentation and bacterial microbiota, inflammatory response and growth performance in finishing beef steers fed high or low-concentrate diet. Anim Feed Sci Technol. (2019) 258:114299. doi: 10.1016/j.anifeedsci.2019.114299
21. Wei, HY, Wang, XJ, Yan, Q, Jiang, CX, Ding, LM, and Zhao, SN. Effect of Astragalus superfine powder and antibiotics on in vitro rumen fermentation. J Domest Anim Ecol. (2020) 41:23–30. doi: 10.3969/j.issn.1673-1182.2020.07.005
22. Xu, W, Lin, L, Liu, A, Zhang, T, Zhang, S, Li, YH, et al. L-Theanine affects intestinal mucosal immunity by regulating short-chain fatty acid metabolism under dietary fiber feeding. Food Funct. (2020) 11:8369–79. doi: 10.1039/D0FO01069C
23. Caporaso, JG, Lauber, CL, Walters, WA, Berg-Lyons, D, Lozupone, CA, Turnbaugh, PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. (2011) 108:4516–22. doi: 10.1073/pnas.1000080107
24. He, JJ, Wu, HK, Yang, XJ, Li, L, Zhao, GQ, Meng, QX, et al. Effects of Clostridium butyricum on growth performance, serum biochemical, antioxidant, immune indexes and fecal microorganism number of calves. Chin J Anim Nutr. (2021) 33:5076–85. doi: 10.3969/j.issn.1006-267x.2021.09.028
25. Dai, JJ, Liao, CS, Tan, XW, Hu, JP, Huang, X, and Gong, FY. Effects of different feed additives on growth performance, immunity and gut health of white-feathered broilers. China Feed. (2022) 9:146–50. doi: 10.15906/j.cnki.cn11-2975/s.20220928
26. Li, SF, Ma, T, Yang, JL, An, YW, Zhang, Y, Yang, XD, et al. Effects of Sophora alopecuroides L. on c-Jun N-terminal kinase/p38 mitogen-activated protein kinase signaling pathway and tight junction protein expression in rumen epithelium of fattening lambs fed high concentrate diet. Chinese journal of animal. Nutrition. (2022) 6:3940–52. doi: 10.3969/j.issn.1006-267x.2022.06.053
27. Liu, ZL. Regulation of Different Dietary Concentrare-Roughness Ratio and Jugular Vein Perfusion Lipopolysaccharide Treatment on Rumen Microflora of Dairy Goats. Tai’an: Shandong Agricultural University (2020).
28. Zened, A, Combes, S, Cauquil, L, Mariette, J, Klopp, C, Bouchez, O, et al. Microbial ecology of the rumen evaluated by 454 GS FLX pyrosequencing is affected by starch and oil supplementation of diets. FEMS Microbiol Ecol. (2013) 83:504–14. doi: 10.1111/1574-6941.12011
29. Karnati, SKR, Yu, Z, Sylvester, JT, Dehority, BA, Morrison, M, and Firkinset, JL. Technical note: specific PCR amplification of protozoal 18S rDNA sequences from DNA extracted from ruminal samples of cows. J Anim Sci. (2003) 81:812–25. doi: 10.2527/2003.813812x
30. Li, H, Wang, Y, Gao, J, and Qi, ZL. Effects of heat stress on rumen microbiota and its relationship with performance of dairy cows. Chin J Anim Nutr. (2019) 31:4458–63. doi: 10.3969/j.issn.1006-267x.2019.10.007
31. Zhou, Y, Zhang, MH, Liu, QX, and Feng, JH. The alterations of tracheal microbiota and inflammation caused by different levels of ammonia exposure in broiler chickens. Poult Sci. (2020) 100:685–96. doi: 10.1016/j.psj.2020.11.026
32. Liu, SJ, Zhang, ZY, Hailemariam, S, Zheng, N, Wang, M, Zhao, SA, et al. Biochanin a inhibits ruminal nitrogen-metabolizing bacteria and alleviates the decomposition of amino acids and urea in vitro. Animals. (2020) 10:368. doi: 10.3390/ani10030368
33. Bermingham, EN, Maclean, P, Thomas, DG, Cave, NJ, and Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ. (2017) 5:e3019. doi: 10.7717/peerj.3019
34. Wang, TY, Chen, HL, Lu, MY, Chen, YC, Sung, HM, Mao, CT, et al. Functional characterization of cellulases identified from the cow rumen fungus Neocallimastix patriciarum W5 by transcriptomic and secretomic analyses. Biotechnol Biofuels. (2011) 4:24. doi: 10.1186/1754-6834-4-24
35. Liu, H, Xu, T, Xu, S, Mao, L, and Zhao, X. Effect of dietary concentrate to forage ratio on growth performance, rumen fermentation and bacterial diversity of Tibetan sheep under barn feeding on Qinghai-Tibetan plateau. PeerJ. (2019) 7:e7462. doi: 10.7287/peerj.preprints.27807v2
36. Bi, YL, Zeng, SQ, Zhang, R, Diao, QY, and Tu, Y. Effects of dietary energy levels on rumen bacterial community composition in Holstein heifers under the same forage to concentrate ratio condition. BMC Microbiol. (2018) 18:69. doi: 10.1186/s12866-018-1213-9
37. Ye, WX, Zhang, J, Li, N, Zhang, LL, and Xu, XF. Effect of fructooligosaccharide on the rumen fungi flora of dairy cows by ITS high-throughput sequencing technology. J Yunnan Agric Univ (Nat Sci). (2019) 34:965–70. doi: 10.12101/j.issn.1004-390X(n).201903052
38. Wang, XG, Zhang, XL, Xu, TW, Geng, YY, Hu, LY, Zhao, N, et al. Effects of dietary protein levels on ruminal fungal community structure and function in Tibetan sheep. Acta Pratacul Sin. (2022) 31:182–91. doi: 10.11686/cyxb2020545
39. Tarayre, C, Bauwens, J, Brasseur, C, Mattéotti, C, Millet, C, Guiot, PA, et al. Isolation and cultivation of xylanolytic and cellulolytic Sarocladium kiliense and Trichoderma virens from the gut of the termite Reticulitermes santonensis. Environ Sci Pollut Res Int. (2015) 22:4369–82. doi: 10.1007/s11356-014-3681-2
40. Li, HQ, Jia, JL, Hou, SZ, and Wei, T. Effects of different protein levels dietary on diversity and structure of rumen fungal community in small tail Han sheep lambs. Chin J Vet Sci. (2021) 41:2256–62. doi: 10.16303/j.cnki.1005-4545.2021.11.26
41. Han, XF, Li, BB, Wang, XL, Chen, YL, and Yang, YX. Effect of dietary concentrate to forage ratios on ruminal bacterial and anaerobic fungal populations of cashmere goats. Anaerobe. (2019) 59:118–25. doi: 10.1016/j.anaerobe.2019.06.010
42. Peng, WW, Ma, ZJ, Wang, Y, Gao, F, and Xu, GS. Effects of supplements of different proportions grape seeds to diets on rumen Fungi Flora in Duolang sheep. J Tarim Univ. (2020) 32:7–16. doi: 10.3969/j.issn.1009-0568.2020.04.002
43. Gruninger, RJ, Puniya, AK, Callaghan, TM, Edwards, JE, Youssef, N, Dagar, SS, et al. Anaerobic fungi (phylum neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol Ecol (2015) 90: 1–17. doi: 10.1111/1574-6941.12383
44. Belanche, A, Doreau, M, Edwards, JE, Moorby, JM, Pinloche, E, and Newbold, CJ. Shifts in the rumen microbiota due to the type of carbohydrate and level of protein ingested by dairy cattle are associated with changes in rumen fermentation. J Nutr. (2012) 142:1684–92. doi: 10.3945/jn.112.159574
45. Wang, DD, Zhao, CC, Liu, SM, Zhang, T, Yao, JH, and Cao, YC. Effects of Piromyces sp. CN6 CGMCC 14449 on fermentation quality, nutrient composition and the in vitro degradation rate of whole crop maize silage. AMB Express. (2019) 9:121. doi: 10.1186/s13568-019-0846-x
46. Yang, HB, Liu, H, Zhan, JS, Li, M, and Zhao, GQ. Effects of diet pellets with different concentrate-roughage ratios on rumen fermentation parameters and microorganism abundance in weaned bull calves. Acta Pratacul Sin. (2015) 24:131–8. doi: 10.11686/cyxb2015022
47. Zhu, CS, Mao, SY, Sun, YZ, and Zhu, WY. Screening of anaerobic fungi and their medium modification for xylanase production. Microbiol China. (2004) 31:11–5. doi: 10.13344/j.microbiol.china.2004.03.003
48. Dagar, SS, Kumar, S, Mudgil, P, and Puniya, KM. Comparative evaluation of lignocellulolytic activities of filamentous cultures of monocentric and polycentric anaerobic fungi. Anaerobe. (2018) 50:76–9. doi: 10.1016/j.anaerobe.2018.02.004
Keywords: Sophora alopecuroides, high concentrate diet, rumen fermentation, rumen bacteria, rumen fungi, lambs
Citation: An Y, Wang H, Zong Z, Gao Z, Shi C, Li S and Khas-Erdene (2023) Effects of adding Sophora alopecuroides to high concentrate diet on rumen fermentation parameters and microbial diversity of sheep. Front. Vet. Sci. 10:1200272. doi: 10.3389/fvets.2023.1200272
Received: 04 April 2023; Accepted: 21 July 2023;
Published: 07 August 2023.
Edited by:
Vivian Fischer, Federal University of Rio Grande do Sul, BrazilReviewed by:
Zhiyuan Ma, Lanzhou University, ChinaCopyright © 2023 An, Wang, Zong, Gao, Shi, Li and Khas-Erdene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hairong Wang, d2FuZ2hhaXJvbmc5N0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.