- 1Wisconsin Equine Clinic and Hospital, Oconomowoc, WI, United States
- 2Department of Veterinary Clinical Sciences, Washington State University, Pullman, WA, United States
Background: Acetaminophen is utilized in human infants for pain management and fever. Neonatal foals might benefit from administration of acetaminophen but effective and safe dosage regimens for neonatal foals remains to be determined.
Objective: The objective was to determine the plasma pharmacokinetics of acetaminophen following oral administration of a single dose of 20 mg/kg or 40 mg/kg to neonatal foals. A secondary objective was to evaluate any changes in hematology and biochemistry profiles.
Study design: Randomized study.
Methods: Eight clinically healthy 7–9-day old Quarter Horse foals (3 colts and 5 fillies) received a single oral dose of acetaminophen either 20 (n = 4) or 40 (n = 4) mg/kg. Hematology and biochemistry profiles were evaluated before and 7 days after drug administration. Blood samples were collected before and 8 times after acetaminophen administration for 48 h to quantify plasma acetaminophen concentrations. Plasma pharmacokinetic parameters were estimated using non- compartmental analysis.
Results: The median peak plasma concentrations (and range) occurred at 1.5 (0.5–2) hours, and 1.0 (1–2) hours for the 20 and 40 mg/kg doses. The maximum plasma concentration (and range) was 12 (7.9–17.4) μg/mL for the 20 mg/kg dose and 14 (11–18) μg/mL for 40 mg/kg dose. The median AUC0-∞ ranged from 46 to 100 and 79 to 160 h*-μg/mL for the 20 and 40 mg/kg dose, respectively. Hematology and biochemistry profiles remained within normal limits.
Conclusion: Plasma disposition of acetaminophen after oral administration of 20 and 40 mg/kg to neonates is comparable to adult horses. However, safety and the optimal dosage regimen of acetaminophen for treating pain and or pyrexia in neonates in this age group remains to be determined.
1. Introduction
Acetaminophen is a common over-the-counter analgesic and antipyretic drug used in human medicine (1). In human patients, acetaminophen can be administered orally, intravenously or per rectum and can provide analgesia within 40 min with maximal effect at 1 h (1, 2). Aside from being used alone, it has been used in humans in multimodal pain management (3–5).
Acetaminophen is metabolized extensively by the liver. The main metabolic pathways are glucuronidation and sulfation, which in human adult’s accounts for 55 and 30% of acetaminophen metabolism, respectively (6–8). A small fraction (2–5%) of the absorbed dose is excreted unchanged in the urine (8–12). Human neonates have a slower total clearance of acetaminophen with higher sulfation and lower glucuronidation per kg of bodyweight compared to adults (12–16).
Several studies have shown gastric emptying is the rate-limiting the step in the adult horses’ absorptive process of acetaminophen (17, 18). Acetaminophen in adult horses is then rapidly absorbed in the proximal small intestine via passive diffusion (19, 20). In regard to liver function in the equine foal, microsomal enzyme activity increases rapidly by 3–4 weeks of age (21–25) while conjugation takes longer to reach adult levels (24, 25) With respect to acetaminophen in the foal, acetaminophen appears to have linear disposition following a single dose of 10–40 mg/kg in foals (26). However, in adult horses, with repeated dosing of acetaminophen, the disposition is no longer linear, with a decrease in elimination (27–29) suggesting that the disposition of acetaminophen may be age dependent in horses.
In a recent study in 1–3-month-old foals receiving a single dose of acetaminophen, the maximum concentration (Cmax) median and range at the 10 mg/kg dose was 4.4 μg/mL (1.8–5.1) at the 20 mg/kg dose was 6.3 μg/mL (2.6–12.6) and at the 40 mg/kg dose was 14 μg/mL (7.3–18) (26). Further studies are needed to understand the plasma disposition of acetaminophen in neonatal foals.
Acetaminophen can cause dose-dependent liver disease in humans (30, 31). In a study performed on adult horses, acetaminophen administered at a dose of 25 mg/kg orally every 12 h for 30 days caused no detectable renal or hepatic effects as shown by biochemistry profiles (32, 33). Chronically lame adult horses receiving 30 mg/kg orally every 12 h for 21 days had no adverse effects in their liver biopsy histopathology or biochemistry profiles either (28). In foals (1–3-month-old) treated with 10, 20 and 40 mg/kg once orally, no clinically significant changes were noted in the hematology or serum biochemistry parameters, but this is only after a single dose administration (26). Any potential effect of acetaminophen on hematology or serum biochemistry parameters in neonatal foals remains to be determined.
The main objective of this study was to determine the plasma pharmacokinetics of acetaminophen following oral administration of a single dose of 20 mg/kg or 40 mg/kg to neonatal foals. A second objective of this study was to observe any changes in the hematology or biochemistry profiles after oral administration of 20 and 40 mg/kg of acetaminophen.
2. Materials and methods
This study was approved by the Utah State University Institutional Animal Care and Use Committee ASAF #11023. Eight clinically healthy Quarter Horses foals (3 colts and 5 fillies) from Utah State University Animal Sciences Department were studied using a randomized study design where 4 foals received 20 mg/kg and 4 foals received 40 mg/kg of acetaminophen once orally, where the foals were selected for 2 different doses by a random generator.1 The number of foals was selected to obtain the maximum information from the smallest number of animals. Toxicity studies are conducted on small groups of 3–5 rodents of each sex per dose (34, 35). The non-rodent species groups typically utilize 4–6 animals, hence the reason for selected 4 foals per group (34, 35).
The mean (±standard deviation) body weight (kg) of the foals was 51.0 ± 8.0 kg. All the foals were between 7–9 days of age. All foals were housed with their dams in stalls with an outdoor run, which is the typical management scheme of mare and foals on this property. The foals were allowed to nurse without restrictions during the research project. The mares were fed while the project was ongoing in the mornings and evenings.
2.1. Prior to start of the study
The afternoon before the first administration of acetaminophen a Jorvet2 extended-use catheter was placed aseptically in one jugular vein of each foal. The catheters were heparin-locked (2.5 mls normal saline,3 2.5 mls of 100 units/mL heparin4) until the next morning. The catheters were wrapped with roll gauze5 and elastikon tape6 to protect the catheter. Whole blood and plasma were obtained the same afternoon for the initial hematology7 and biochemistry profile.8 These were analyzed at the Utah State Diagnostic Laboratory for confirmation of normal hematology parameters; white cell count (WBC), red cell count (RBC), hemoglobin (Hb), fibrinogen, and total protein (TP), and normal biochemistry parameters including blood urea nitrogen (BUN), creatinine (CR), sorbitol dehydrogenase (SDH), gamma-glutamyl transferase (GGT), and alkaline phosphatase (ALP), triglycerides, aspartate aminotransferase (AST), total bilirubin, and total protein (TP). These biochemistry values were chosen to observe liver and renal values during the study period.
2.2. Study time frame
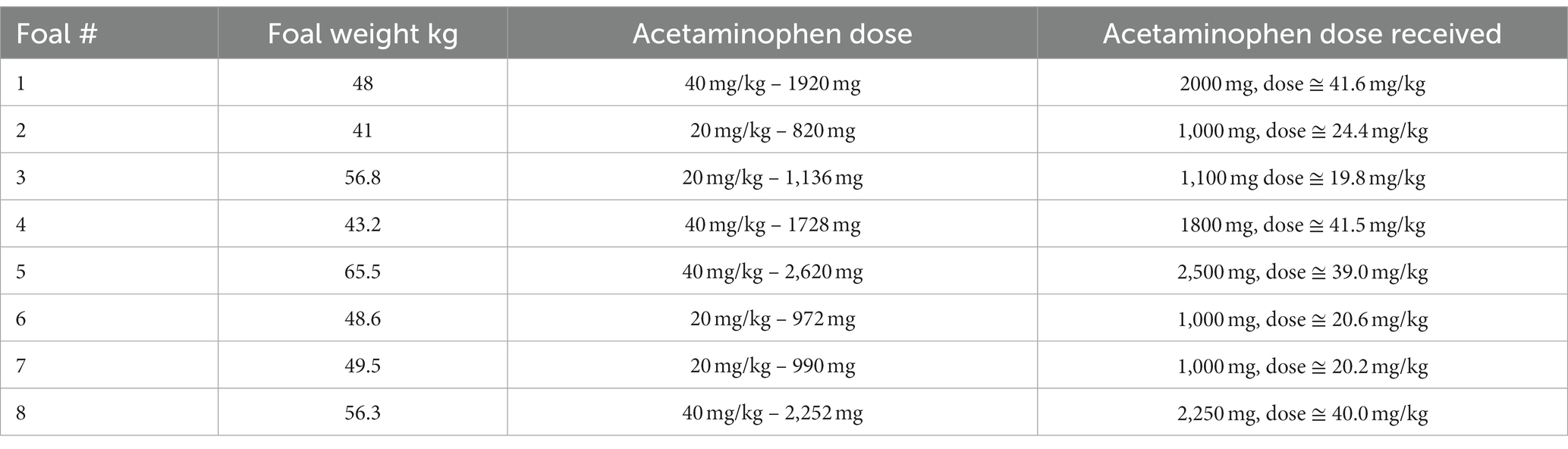
Physical examinations were performed on each foal before drug administration, at the start of the study and three times a day throughout the study.9 The examinations included the determination of heart rate, respiratory rate, body temperature, mucous membrane color, capillary refill time and gastrointestinal borborygmi, and palpation of joints and umbilicus. The 500 mg acetaminophen extra strength regular release tablets10 were ground in a coffee grinder. The dose of acetaminophen administered was rounded to the closet number of whole tablets based on the weight of each foal in each treatment group (Example-a 48 kg foal getting a 40 mg/kg dose would need 1920 mg of acetaminophen, the actual dose the foal got was 2000 mg or 41.6 mg/kg) Table 1. The ground acetaminophen was mixed with water (24 mls) and karo syrup or molasses (1 mL) and administered orally via a catheter tip syringe (water, karo syrup or molasses and ground acetaminophen).
Five mls of blood were withdrawn at each time point from the jugular catheter, after which an additional 5 mls of blood was withdrawn and placed into a plastic heparin blood collection tube. The initial 5 mls of blood was reinjected into the jugular vein through the catheter and the catheters were flushed with normal saline solution. To limit the amount of heparin administered, the catheters were flushed with saline twice a day and twice a day with (50 USP units of heparin/5 mls saline)11 to maintain catheter patency. Blood was collected for the pharmacokinetic analysis once before the administration of acetaminophen (time 0) and a total of 8 times 0.50, 1, 2, 4, 8,16, 24 and 48 h post administration of acetaminophen. Blood was also obtained from each foal for hematology and biochemistry profiles which were performed prior to acetaminophen administration and 7 days after the dosage of acetaminophen. The samples were placed on ice until they were spun down. The heparinized samples were centrifuged for the plasma acetaminophen concentrations at 1800 x g for 5 min and the plasma was removed and stored at -80o C until analysis.
The assays were performed within 6 months of collection. A preliminary study using horse plasma samples from our prior study (26) stored under the same conditions showed good stability of acetaminophen concentrations (105 ± 6% of the original value) more than 12 months after the original assay. This is consistent with stability studies of acetaminophen, acetaminophen glucuronide and acetaminophen sulfate in humans’ plasma, which showed minimal change after 6 months of storage at −800 C (36).
2.3. Drug quantification and analytical method
Acetaminophen concentrations were measured in the plasma samples by high-performance liquid chromatography (HPLC) with mass spectrometry detection using the same method previously validated for horse plasma [26]. Standard curves were linear (R2 > 0.99) over assayed range (0.05–50 μg/mL). The lower and upper limit of quantification was 0.05 μg/mL and 50 μg/mL, respectively.
Assay precision (coefficient of variation) and accuracy (percent deviation from nominal) was evaluated in each run using quality control samples consisting of blank horse plasma spiked with multiple concentrations of acetaminophen. Assay precision was 5, 5, and 11% for 50, 2.5, and 0.05 μg/mL concentrations. Assay accuracy was −6%, +4% and + 16% for 50, 2.5, and 0.05 μg/mL concentrations.
2.4. Estimation of plasma pharmacokinetic parameters
Non-compartmental analysis was used to calculate pharmacokinetic parameters (37) as implemented by Phoenix WinNonlin® v8.0.12 Estimated pharmacokinetic parameters include; area under the plasma concentration-time curve from 0 h to infinity after dosing (AUC0-∞), area under the plasma concentration-time curve from 0 h to the last sampling time (AUC0-last), maximum concentration (Cmax), time to maximum concentration (Tmax), half-life of terminal portion of the curve after oral administration.
2.5. Data analysis
The effect of acetaminophen treatment on hematology and biochemistry profile parameters were assessed statistically using the Wilcoxon matched-pairs signed-rank test. Dose proportionality was evaluated by comparing the dose-normalized AUC0-∞ and dose-normalized Cmax values between dose levels. All statistical comparisons were made using GraphPad Prism v7.4 for Windows.13 The level of significance was set at p < 0.05.
3. Results
The hematology profiles remained within the normal reference range (35, 36). The biochemistry parameters were normal in all but 3 foals. The GGT concentrations were outside the reference range before and after administration of acetaminophen in 1 foal, and alkaline phosphatase concentrations were outside the reference range in 1 foal before acetaminophen administration but were normal post acetaminophen administration. One foal had SDH concentration outside the reference range prior to acetaminophen concentration but was normal 7 days post administration. However, no statistical significance was found (Tables 2, 3). Physical examination parameters also remained within normal limits for all foals throughout the study.
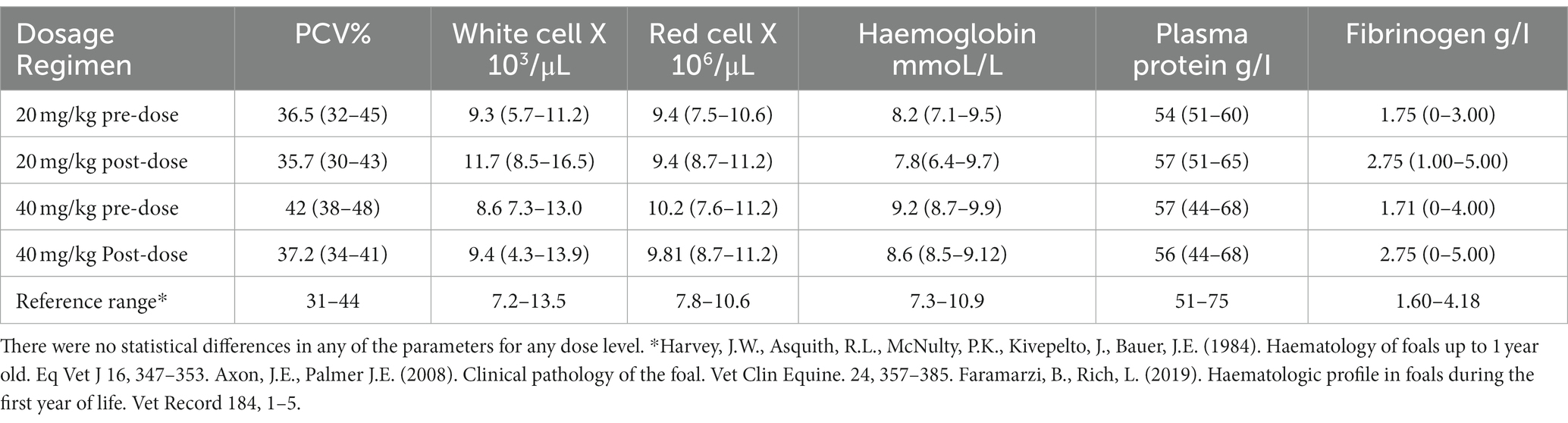
Table 2. Hematology parameters (mean and range) from 7–9-day-old foals before and after single oral administration of acetaminophen at 20 mg/kg (n = 4) and 40 mg/kg (n = 4).
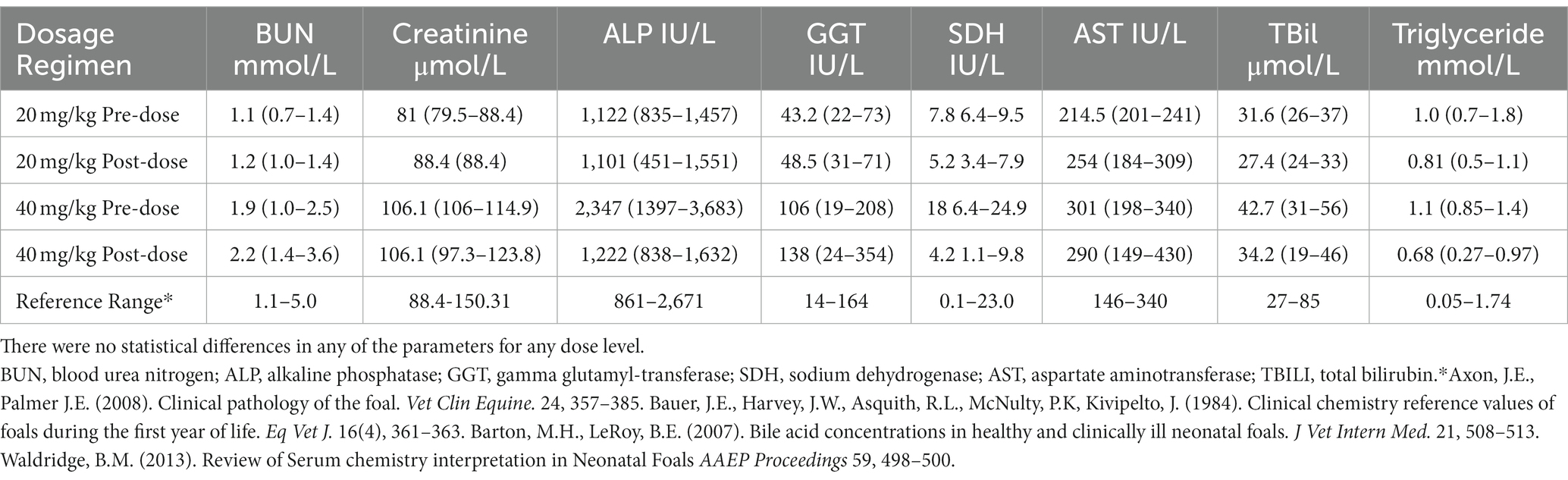
Table 3. Biochemistry profile (mean and range) from 7–9-day-old foals before and 7 days after a single oral administration of acetaminophen at 20 mg/kg (n = 4), and 40 mg/k (n = 4).
Pharmacokinetic parameters determined by non-compartment analysis are presented in Table 4. Acetaminophen was detected in the plasma of all foals (Figure 1). Peak plasma concentrations occurred from 0.5 to 2 h and 1 to 2 h for 20 and 40 mg/kg and maximum plasma concentration ranged from 7.9–17 μg/mL and 11 to 18 μg/mL for 20 and 40 mg/kg dose level, respectively. AUC0-∞ ranged from 46 to 100 h*μg/mL and 79 to 160 h*μg /mL for the 20 and 40 mg/kg dose, respectively. The dose-normalized AUC0-last ranged from 2.2 to 5 and 2 to 4 for the 20 and 40 mg/kg doses, respectively. The dose-normalized Cmax ranged from 0.4–0.9 and 0.3 to 0.5 for the 20 and 40 mg/kg doses, respectively.
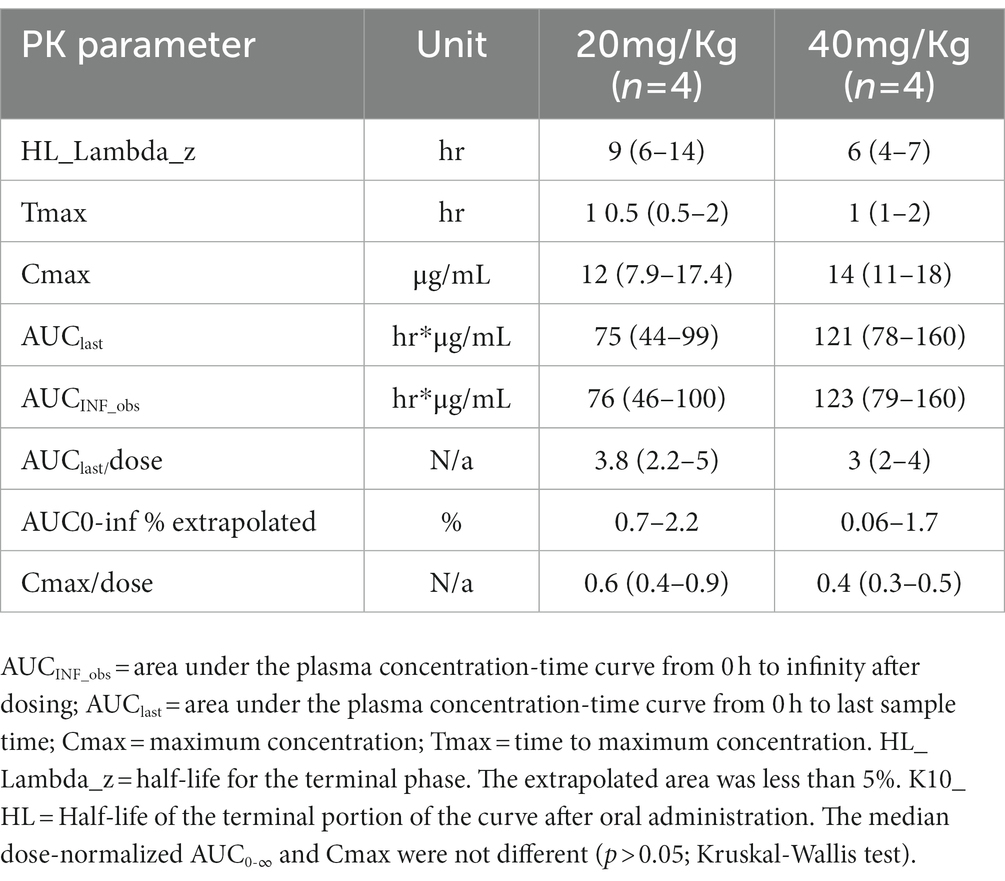
Table 4. Plasma pharmacokinetic parameters (median (range)) of acetaminophen estimated by non-compartmental analysis in 7–9 days-old foals after oral administration at 20 (n = 4 foals) and 40 mg/Kg (n = 4 foals) of body weight.
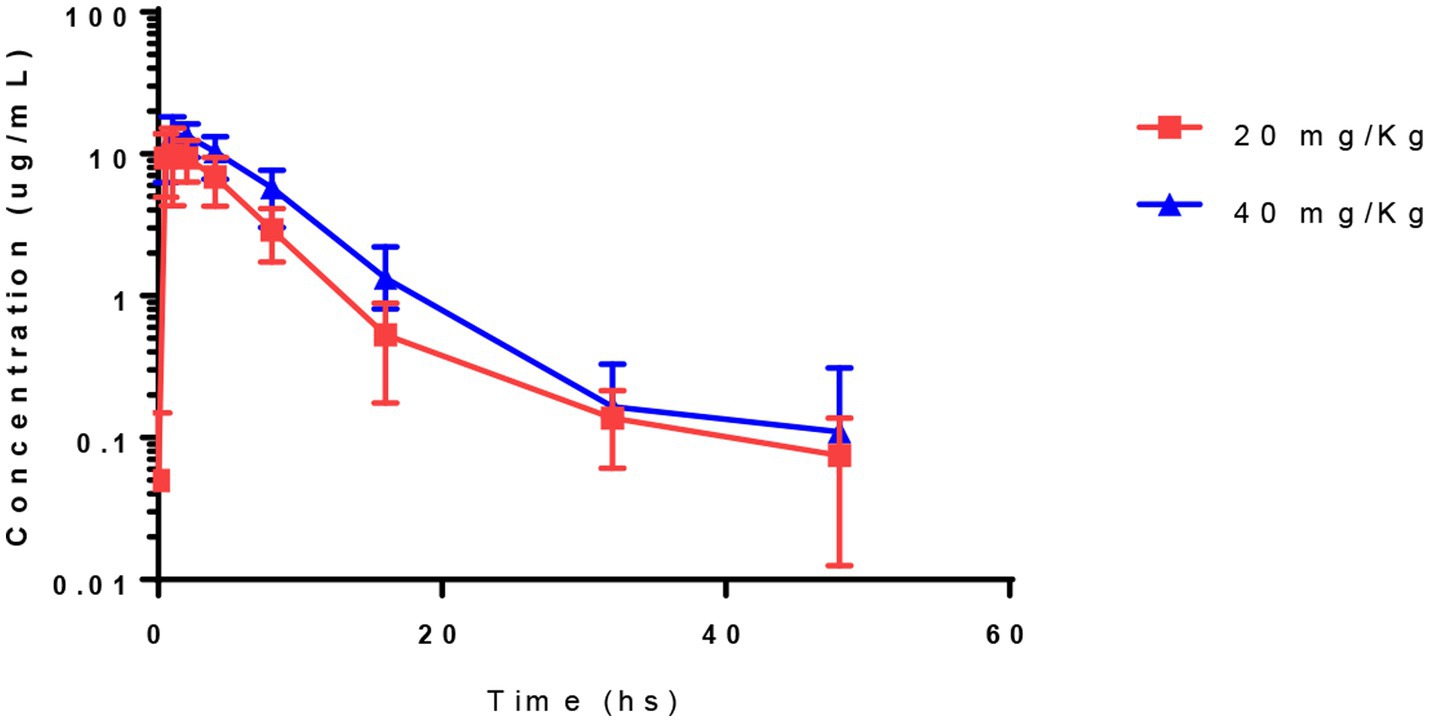
Figure 1. Plasma concentrations of acetaminophen in 7–9 days old foals after a single oral administration at 20 and 40 mg/Kg of body weight (Mean ± SD).
4. Discussion
This is the first study to describe the plasma pharmacokinetics and effect on physical examination, hematology, and biochemical profiles of a single oral dose administration of acetaminophen in 7–9-day-old foals. No adverse reactions were observed in any foal treated with acetaminophen. After a single acetaminophen dose administration, the clinical, hematology, and biochemical profiles remained within the reference range except in 3 foals where one foal had elevations in GGT before and after administration of acetaminophen and 1 foal had an elevated alkaline phosphatase level prior to acetaminophen concentration and one foal had a mild elevation of SDH prior to acetaminophen but was normal 7 days post acetaminophen administration. This may be a normal variation with age since the elevations were noted in the foals prior to the administration of acetaminophen and concentrations were lower but still above the reference range 1 week later in the foal with elevated GGT. The safety profile of acetaminophen in neonatal foals needs to be assessed after repeated dosing of acetaminophen to determine if the long-term administration causes liver damage or other adverse effects.
The plasma disposition of acetaminophen in 7–9-day old neonates is comparable to that in older foals (26). However, a relevant difference in the disposition of acetaminophen between neonates and 1–3-months old foals is that the median terminal half-life was relatively longer in neonatal foals (Table 4). The median terminal half-life (range) in the neonatal foals was 9 (6–14 h) and 6 (4–7) hrs for 20 mg/kg and 40 mg/kg, respectively. This finding is different compared to 1–3-month-old foals where the terminal half-life for a single oral dose at 10 mg/kg, 20 mg/kg and 40 mg/kg (range) was 2.6 (1.4–3.4) hrs, 2.8 (2.05–3.9) hrs and 2.7(2.4–7.4) hrs, respectively.
The AUC /dose at 20 mg/kg (range) was larger at 3.8 (2.2–5) in the 7–9 day-old- foals compared to the same dose 1-3-month-foals which was 2 (1.1–3.7), and at 40 mg/kg in 7-9-old-foals was 3 (2–4) compared to the 1-3-month-foals at 40 mg/kg which was 2.6 (2.1–3.6). This discrepancy is likely attributable to the slower drug clearance of acetaminophen in neonates than in older foals. Neonatal foals have an immature biotransformation system which can explain the slower drug elimination of acetaminophen. Neonatal foals have longer half-lives for some drugs versus older foals, particularly between birth and 5 days of age (26, 27). However, foals seem to develop microsomal-mediated metabolic pathways rapidly in the first 3–4 weeks of life and reach the activity of adults by 6–12 weeks of life (26, 27). Differences in the maturity of enzymatic systems involved in the biotransformation of acetaminophen could explain the interindividual variability in neonates and age-dependent differences in the disposition of this drug. The clinical relevance of the age-dependent disposition of acetaminophen in foals deserves further research.
No evidence for dose non-proportionality in AUC0-last occurred in this study and this agrees with the results of a previous study in 1–3 months old foals treated with acetaminophen at 20 and 40 mg/kg orally once (26). In contrast, the Cmax in the 7-9-day-old foals did not increase in a dose-proportional manner, as the median Cmax of acetaminophen increased from 12 μg/mL to 14 μg/mL, for the 20 and 40 mg/kg dose levels in comparison to the 1-3-month old foals where the median Cmax at 10 mg/kg was 4.4 μg/mL, at 20 mg/kg was 6.3 μg/mL and 40 mg/kg was 14 μg/mL which increased in a dose proportional fashion. It is possible, that this discrepancy between the two cohort of foals is result of age-depended differences in rate and extent of distribution of acetaminophen. A larger study is necessary to confirm these dose proportionality findings. Furthermore, it is not possible to determine if this finding would be clinically relevant as the therapeutic concentration of acetaminophen remains to be determined in the horse.
The effective serum concentration of acetaminophen that elicits 50% of the maximum anti-pyretic drug response (EC50) in adult humans has been estimated to be between 15.2 and 16.5 μg/mL and the minimum therapeutic concentration for pyrexia is established to be 10 μg/mL (7–14). In human neonates, effective concentrations for pyrexia have been established to be 10–11 μg/mL in different studies (38–41). In children that have undergone tonsillectomy, 10 μg/mL was found to alleviate pain, but lower strength may be sufficient with less pain (42). In the present study, the Cmax was higher than 10 μg/mL in all foals treated with 40 mg/kg of acetaminophen. If the human analgesic serum acetaminophen concentration is extrapolated to foals, the administration of 40 mg/Kg of acetaminophen may result in analgesic concentrations, assuming that the maximal plasma concentration of acetaminophen is positively correlated with the drug effect. Two studies in horses have looked at the pharmacodynamics of analgesia in horses (28, 33). However, more studies are needed to determine the optimal dosage regimen for treating pain and pyrexia.
This study generated novel information but has several limitations. One limitation of this study is the number of foals was small, with only 4 foals in each group. Thus, the finding should be interpreted with care and knowledge that further studies are needed before dosing foals of any age.
The main limitation of this project is that the study design does not allow assessment of acetaminophen safety in with multiple doses because the drug was administered once and because the biochemistry and hematology was assessed 7 days after the administration, which may have served to capture only delayed or long-lasting effects. Considering the significant liver toxicity concern in humans, and futures studies should be designed specifically to rule out liver adverse effects of acetaminophen in foals.
Liver toxicity is a significant finding in human studies at elevated doses (30, 31) but has not been noted in multi-dose administration in horses. (27–29, 32, 33) Since neonatal foals have rapidly changing liver function, impairment due to liver toxicity might change the metabolism, prolong the half-life, and alter drug disposition (1, 2, 12–14, 21–25). Thus, further studies are needed to assess safety of multiple dosing of acetaminophen in neonatal and older foals.
In conclusion, this study is the first to describe the disposition of acetaminophen in neonatal foals at 20 mg/kg and 40 mg/kg doses and the resulting pharmacokinetic parameters. The results of this study are encouraging for the potential to use of acetaminophen in foals. Further studies are needed to assess the safety, analgesic, and antipyretic effects and the disposition of acetaminophen after repeated administration of different doses in foals of varying ages.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Study protocols were approved via Institutional Animal Care and Use Committee IACUC Approval ASAF# 11023.
Author contributions
JG wrote the grant and assisted for the project, abstract, and manuscript. TG helped the manuscript and data collection. MC performed the lab HPLC analysis of samples and assisted the manuscript. NV performed the pharmcokinetic analysis, statistical analysis, and assisted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the WSU Intramural Funding-Luella Gottstein Endowment for Equine Research, Stanley L. Alder Research Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Random.org, Dublin. Ireland.
2. ^Jorvet labs, Loveland, CO. USA.
3. ^Sargent Pharmaceuticals, Schaumberg, IL. USA.
4. ^Medex Supply, Passiac, NJ. USA.
5. ^Becton, Dickenson and Company, Franklin Lakes, NJ. USA.
6. ^Johnson and Johnson, New Brunswick, NJ. USA.
7. ^Siemens Advia 120, Siemens Health Care Diagnostics Inc., Tarrytown, NY. USA.
8. ^Siemens Dimension Xpand Plus, Siemens Health Care Diagnostics Inc., Tarrytown, NY. USA.
9. ^Better Living Brands, Pleasanton, CA. USA.
10. ^Sigma Aldrich, St Louis, MO. USA.
11. ^Toronto Research Chemicals, Toronto, Ontario, Canada.
12. ^Certara Princeton, NJ. USA.
13. ^GraphPad Software, San Diego, CA. USA.
References
1. Mazeleuskaya, LL, Sagnkuhl, K, Thorn, CF, Fitzgerald, GA, Altman, RB, and Klein, TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics. (2015) 25:416–26. doi: 10.1097/FPC.0000000000000150
2. Graham, GG, Davies, MJ, Day, RO, Mohamudally, A, and Scott, KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. (2013) 21:201–32. doi: 10.1007/s10787-013-0172-x
3. Elia, N, Lysakowski, C, and Tramer, MR. Does multimodal analgesia with acetaminophen, nonsteroidal anti-inflammatory drugs or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trials. Anesthesiology. (2005) 103:1296–304. doi: 10.1097/00000542-200512000-00025
4. Hybo, KH, Hägi-Pedersen, D, and Dahl, JB. Effect of combination of Paracetamol (acetaminophen) and ibuprofen vs either alone on patient-controlled morphine consumption in the first 24 hours after Total hip Arthroplasty: the PANSAID randomized clinical trial. JAMA. (2019) 321:562–71. doi: 10.1001/jama.2018.22039
5. Prescott, LF. Kinetics and metabolism of paracetamol and phenacetin. Br J Clin Pharmacol. (1980) 10:291S–8S. doi: 10.1111/j.1365-2125.1980.tb01812.x
6. Mazaleuskaya, LL, Sangkul, K, Thorn, CF, FitzGerald, GA, Altman, RB, and Klein, TE. PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic and toxic doses. Pharmacogenet Genomics. (2018) 25:416–26. doi: 10.1097/FPC.0000000000000150
7. Court, MH, Duan, SX, von Moltke, LL, Greenblatt, DL, Patten, CJ, Miners, JO, et al. Individual variability inn acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther. (2001) 299:998–1006. doi: 10.1046/j.1365-2125.2000.00231.x
8. Critchley, JA, Nimmo, GR, Gregson, CA, Woolhouse, NM, and Prescott, LF. Inter-subject and ethnic differences in paracetamol metabolism. Br J Clin Pharmacol. (1986) 22:649–57. doi: 10.1111/j.1365-2125.1986.tb02953.x
9. Clements, JA, Critchley, JA, and Prescott, LF. The role of sulphate conjugation in the metabolism and disposition of oral and intravenous paracetamol in man. Br J Clin Pharmacol. (1984) 18:481–5. doi: 10.1111/j.1365-2125.1984.tb02495.x
10. Sahahwalla, CG, and Ayres, JW. Multiple-dose acetaminophen pharmacokinetics. J Pharm Sci. (1999) 80:855–60. doi: 10.1002/jps.2600800911
11. Gelotte, CK, Auiler, JF, Lynch, JM, Temple, AR, and Slatttery, JT. Disposition of acetaminophen at 4,6 and 8 g/day for 3 days in healthy young adults. Clin Pharm Ther. (2007) 81:840–8. doi: 10.1038/sj.clpt.6100121
12. Cook, SF, Roberts, JK, Samiee-Zafarghandy, S, Stockmann, C, King, AD, Deutch, N, et al. Population pharmacokinetics of intravenous paracetamol (acetaminophen) in preterm and term neonates: model development and external evaluation. Clin Pharmacokinet. (2016) 55:107–19. doi: 10.1007/s40262-015-0301-3
13. Cook, SF, Stockmann, C, Samiee-Zafarghandy, S, King, AD, Deutsch, N, Williams, EF, et al. Neonatal maturation of paracetamol (acetaminophen) glucuronidation, sulfation, and oxidation based on a parent-metabolite population pharmacokinetic model. Clin Pharmacokinet. (2016) 55:1395–411. doi: 10.1007/s40262-016-0408-1
14. Wang, C., Allegaert, K., Tibboel, D., Meindert, D., van der Marel, C.D., and Mathott, R, A.A., Knibbe, C.A. Population pharmacokinetics of paracetamol across the human age-range from (pre)term neonates, infants, children to adults. J Clin Pharmacol (2014). 54:619–629, doi: 10.1002/jcph.259
15. Anderson, BJ, Woollard, GA, and Holford, NHG. A model for size and age changes in the pharmacokinetics of paracetamol in neonates, infants, and children. Br J Clin Pharm. (2008) 50:25–134.
16. Arana, A, Morton, NS, and Hansen, TG. Treatment with paracetamol in infants. Acta Anaesthesiol Scand. (2001) 45:20–9. doi: 10.1034/j.1399-6576.2001.450104.x
17. Doherty, TJ, Andrews, FM, Provenza, MK, and Frazier, DL. (1988) acetaminophen as a marker of gastric emptying in ponies. Equine Vet J. (1988) 10:349–51. doi: 10.1111/j.2042-3306.1998.tb04109.x
18. Lohmann, KL, Bahr, A, Cohen, ND, Boothe, DM, and Roussel, AJ. Evaluation of acetaminophen absorption in horses with experimentally induced delayed gastric emptying. Am J Vet Res. (2002) 63:170–4. doi: 10.2460/ajvr.2002.63.170
19. Neirinckx, E, Vervaet, C, and de Boever, S. Species comparison of oral bioavailability, first-pass metabolism, and pharmacokinetics of acetaminophen. Res Vet Sci. (2010) 89:113–9. doi: 10.1016/j.rvsc.2010.02.002
20. Englking, LR, Blyden, GT, Lofstedt, J, and Greenblatt, DJ. Pharmacokinetics of antipyrine, acetaminophen and lidocaine in fed and fasted horses. J Vet Pharmacol Ther. (1987) 10:73–82. doi: 10.1111/j.1365-2885.1987.tb00079.x
21. Fielding, CL, and Magdesian, KG. Body water and fluid distribution in neonatal foals. J Vet Emerg Crit Care. (2009) 19:A11.
22. Fisher, B, and Clark-Price, S. Anesthesia of the equine neonate in health and disease. Vet Clin North Am Equine Pract. (2015) 31:567–85. doi: 10.1016/j.cveq.2015.09.002
23. Bernard, WV, and Reimer, JM. Examination of the foal. Vet Clin North Am Equine Pract. (1994) 10:37–66. doi: 10.1016/S0749-0739(17)30368-1
24. Baggot, JD, and Short, CR. Drug deposition in the neonate with particular reference to the foal. E Vet J. (1984) 16:364–7. doi: 10.1111/j.2042-3306.1984.tb01945.x
25. Baggot, JD. Drug therapy in the neonatal foal. Vet Clin North Am Equine Pract. (1994) 10:87–107. doi: 10.1016/S0749-0739(17)30370-X
26. Gold, JR, Grubb, T, Court, M, and Villarino, NF. Pharmacokinetics of single dose administration of three increasing doses of acetaminophen per os in 1-3-month-old foals. Equine Vet J. (2022). doi: 10.1111/evj.13903
27. Mercer, MA, McKenzie, HC, Davis, JL, Wilson, KE, Hodgson, DR, Cecere, TE, et al. Pharmacokinetics, and safety of repeated oral dosing of acetaminophen in adult horses. E Vet J. (2020) 52:120–5. doi: 10.1111/evj.13112
28. Mercer, MA, Davis, JL, McKenzie, HC, Byron, CB, Trager-Burns, LR, Kelleher, ME, et al. Pharmacokinetics, pharmacodynamic efficacy, and safety of acetaminophen in adult horses with naturally occurring chronic lameness. Equine Vet J. (2022) 55:524–33. doi: 10.1111/evj.13601
29. Pesko, B, Habershon-Butcher, J, Muir, T, Taylor, P, Fenwick, S, Hincks, P, et al. Pharmacokinetics of paracetamol in the thoroughbred horse following an oral multi-dose administration. J Vet Pharm Ther. (2022) 45:54–62. doi: 10.1111/jvp.13024
30. Yoon, E, Babar, A, Choudhary, M, Kutner, M, and Pyrsopouplos, N. Acetaminophen induced hepatoxicity: a comprehensive update. J Clin Transl Hepatol. (2016) 4:131–42. doi: 10.14218/JCTH.2015.00052
31. Chun, LJ, Tong, MJ, Busuttil, RW, and Hiat, JR. Acetaminophen hepatoxicity and acute liver failure. J Clin Gastroenterol. (2009) 43:342–9. doi: 10.1097/MCG.0b013e31818a3854
32. https://digitalcommons.augustana.edu/cgi/viewcontent.cgi?article=1350&context=celebrationoflearning#:~:text=The%20slightly%20lower%20dosage%20(20,for%20up%20to%2030%20days.&text=There20are20no%20controlled%20studies%20of%20acetaminophen%20toxicity%20in%20horses.
33. Foreman, J, Foreman, C, and Bergstrom, B. Acetaminophen/paracetamol efficacy in a reversible model of equine foot pain In: AAEP annual convention, AAEP. Orlando, FL: AAEP (2016). 295–6.
34. Chinedu, E, Arome, D, and Ameh, FS. A new method for determining acute toxicity in animal models. Toxicol Int. (2013) 20:224–6. doi: 10.4103/0971-6580.121674
35. Prior, H, Haworth, R, Labram, B, Roberts, R, Wolfreys, A, and Sewell, F. Justification for species selection for pharmaceutical toxicity studies. Toxicol Res. (2020) 9:758–70. doi: 10.1093/toxres/tfaa081
36. Cook, SF, King, AD, van den Anker, JN, and Wilkins, DG. Simultaneous quantification of acetaminophen and five acetaminophen metabolites in human plasma and urine by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry: method validation and application to a neonatal pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. (2015) 1007:30–42. doi: 10.1016/j.jchromb.2015.10.013
37. Gabrielsson, J, and Weiner, D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 4th ed Sweden: Swedish Pharmaceutical Press (2007).
38. Tan, E, Braiithwaiite, I, McKinlay, CJD, and Dalziel, SR. Comparison of acetaminophen with ibuprofen for treatment of fever or pain in children younger than 2 years systematic review and meta-analysis. JAMA. (2020) 3:1–15.
39. Sarrrell, EM, Wielunsky, E, and Cohen, HA. Antipyretic treatment in young children with fever, acetaminophen, ibuprofen or both alternating in a randomized, double-blinded study. Arch Pediatr Adolesc Med. (2006) 160:197–202. doi: 10.1001/archpedi.160.2.197
40. Anderson, B, Kanagasundarum, S, and Woollard, G. Analgesic efficacy of paracetamol in children using tonsillectomy as a pain model. Anesthesia Intensive Care. (1996) 24:669–73. doi: 10.1177/0310057X9602400606
41. Bauer, JE, Harvey, JW, Asquith, RL, McNultty, PK, and Kivipelto, J. Clinical chemistry reference values for foals during the first year of life. Equine Vet J. (1984) 16:361–3. doi: 10.1111/j.2042-3306.1984.tb01944.x
Keywords: equine, anti-inflammatory, neonate, plasma disposition, horse
Citation: Gold JR, Grubb T, Court MH and Villarino NF (2023) Pharmacokinetics of acetaminophen after a single Oral administration of 20 or 40 mg/kg to 7–9 Day-old foals. Front. Vet. Sci. 10:1198940. doi: 10.3389/fvets.2023.1198940
Edited by:
Ted Whittem, James Cook University, AustraliaReviewed by:
Heather Knych, University of California, Davis, United States Robert Paul Hunter, One Medicine Consulting, United StatesCopyright © 2023 Gold, Grubb, Court and Villarino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jenifer R. Gold, amdvbGRAd2llcXVpbmUuY29t
 Jenifer R. Gold
Jenifer R. Gold Tamara Grubb
Tamara Grubb Michael H. Court2
Michael H. Court2