- 1Clinic für Small Animal Surgery, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland
- 2Clinic of Diagnostic Imaging, Department of Clinical Services, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland
- 3Kleintierklinik Dennler, Affoltern am Albis, Switzerland
Malignant insulinoma is the most common type of neuroendocrine tumor found in the pancreas of dogs. Canine insulinoma displays malignant behavior with a high rate of metastasis. The most common sites of metastases are the draining lymph nodes, which are also the primary location sites for the recurrence of functional disease. However, identifying metastatic nodes can often be complicated, as the pancreas is drained by numerous lymphatic centers, and clinical enlargement or structural changes may not always be present in metastatic nodes. Additionally, unaltered nodes are frequently small (a few millimeters) and can be hard to distinguish from the surrounding tissues. Therefore, lymphadenectomy is generally recommended for affected dogs. Unlike in human medicine, there are currently no established strategies for lymph node resection in dogs with malignant insulinoma. This report presents a technique for identifying and removing sentinel nodes using indocyanine green and near-infrared lymphography (NIRFL) during surgery. A total of six sentinel nodes were detected and resected with this method. This technique could provide a more structured approach for lymph node resection in affected dogs and potentially in humans in the future. However, its therapeutic benefits must be evaluated in a larger cohort of cases.
1. Introduction
Canine insulinoma is a tumor that develops from the beta cells of the pancreas and is the most common neoplasm affecting the endocrine pancreas in dogs (1–6). Unlike human pancreatic tumors, most canine insulinomas are mostly both malignant and functional (7, 8). Surgical resection is the recommended treatment option for affected dogs with a median survival time (MST) ranging between 372 and 785 days (1–4, 9–11).
Gross metastatic disease is detectable in up to 50% of dogs with insulinoma at the time of initial diagnosis, with lymph nodes and the liver being the most commonly affected sites (3, 4, 10, 11). As insulinoma tumors are usually functional, removal of all potential metastatic lesions is recommended whenever possible, as persistent postoperative hypoglycemia is a major negative prognostic factor. (2–4, 10–12). Unfortunately, clinical and pathological staging do not always correlate well, as lymph nodes with metastases may not display abnormal size or texture (12–15). Consequently, removing only a single altered node is not sufficient for precise staging and carries the risk of leaving functional tissue behind.
Given the challenges associated with identifying metastatic lymph nodes in canine insulinoma, it appears reasonable to focus on the draining lymph nodes of the pancreas region, known as the sentinel nodes, rather than attempting to identify individual nodes. However, the complex drainage pattern of the pancreas and the poor visibility of small nodes make this approach difficult. Based on Baum's anatomy of the canine lymphatic system, numerous lymph nodes potentially drain the pancreas, including 3 left hepatic nodes, 1–5 right hepatic nodes, 1–5 splenic nodes, a duodenal lymph node, and two jejunal nodes, resulting in 6–16 possible sentinel lymph nodes (SLNs) (16).
Sentinel node mapping using indocyanine green (ICG) and near-infrared fluorescence (NIR-F) offers a potential alternative approach for identifying and removing early metastatic lymph nodes in affected dogs. In human medicine, SLN mapping using ICG and NIR-F has been mainly used to detect the intestinal nodes in patients with colonic or gastric cancer, with varying success rates (17–19). To date, no reports of intestinal SLN mapping exist for dogs (20). Previous attempts to map the lymphatics of pancreatic neoplasia in humans using methylene blue have been reported to show insufficient performance (21, 22); however, studies or case reports on NIR-F lymphography in pancreatic tumors is currently lacking in humans and dogs.
This case described the first application of ICG NIRF to map the lymphatics in a dog with naturally occurring malignant insulinomas.
2. Case description
In April 2022, an 11.5-year-old intact female Border Collie was presented after experiencing a single generalized seizure. In May 2022, the dog experienced a second seizure episode during which the owner measured the dog's glucose which was 1.5 mmol/l. Apart from these episodes, the dog was considered healthy and had not shown any signs of illness in the past year. In addition, the dog was also known to have biceps tendinopathy.
2.1. Clinical findings
During the first presentation, the dog weighed 17.4 kg (BCS 5) and appeared clinically unremarkable, except for a slight bradycardia (heart rate: 54/BPM) and a 2/6 left-sided systolic heart murmur. Multiple fractured teeth were also observed.
2.2. Diagnostic assessment
Blood work revealed a moderate increase in liver enzymes (GPT 734 U/L, reference range: 17–78; ALP 157 U/L, reference range: 13–83) and mild thrombocytopenia (PLT 101 E3μl, reference range: 148–484) but was otherwise normal. Blood glucose at presentation was 4.2 mmol/L (reference range: 4.2–7.1). An abdominal ultrasound revealed a mildly enlarged and rounded liver with multiple small nodular areas with mixed echogenicity, sludge accumulation within the gallbladder, a small (19 mm diameter) heterogeneous cavitary lesion at the tail of the spleen, and a 9-mm sharply circumscribed, highly vascularized nodular structure in the left pancreatic branch. A single enlarged (7 mm) mesenteric lymph node with a heterogeneous echotexture in the cranial abdomen was also identified, along with multiple small mural cystic lesions and minimal intraluminal fluid in the uterus. Figure 1 shows the ultrasonographic findings of the pancreas and the enlarged lymph node. The ventrodorsal and right lateral thoracic radiographs were unremarkable.
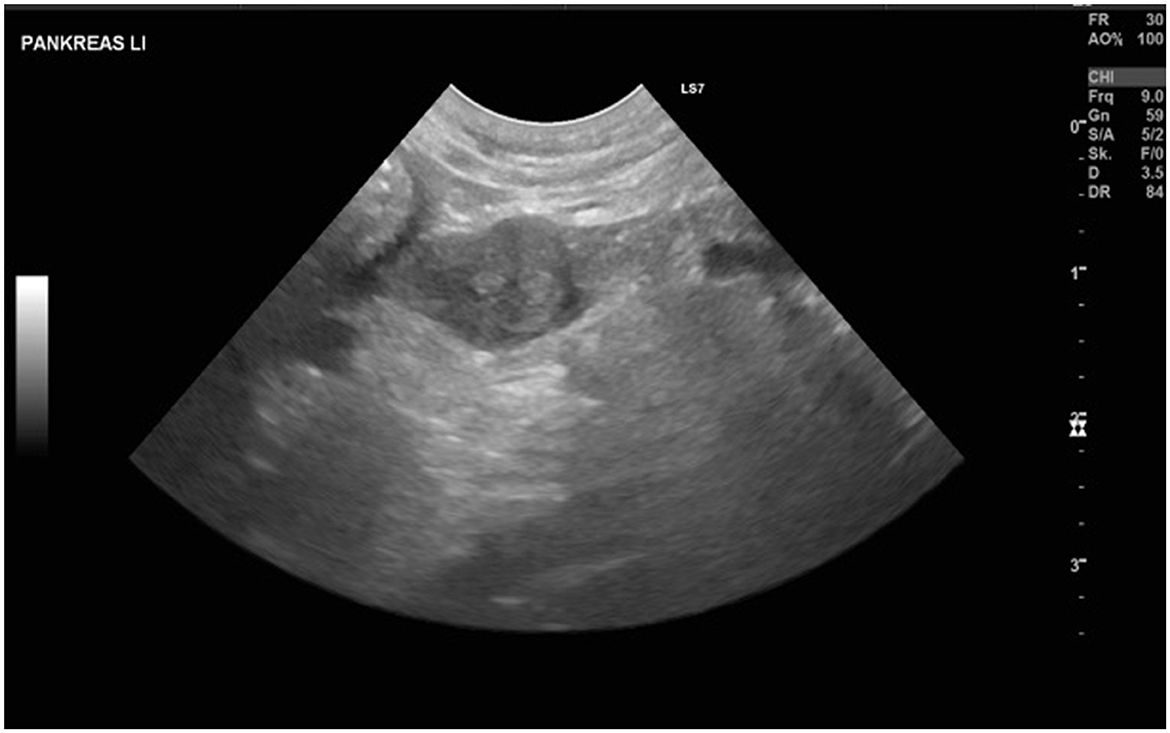
Figure 1. Showing the lesion as well as the enlarged lymph node detected at the initial ultrasound examination.
The dog was then presented for work up at the Clinic for Small Animal Surgery, Vetsuisse Faculty, Zürich University. Helical CT scans were performed using a Philips Brilliance16 scanner (Philips AG, Zurich, Switzerland) for the thorax and abdomen, which were reconstructed using a soft tissue, bone, and lung algorithm for the thorax and a soft tissue and bone algorithm for the abdomen. A triple-phase CT scan of the abdomen was timed with bolus tracking in the descending aorta and reconstructed using a soft tissue algorithm, with 2 ml/kg Accupaque 350 (GE Healthcare, Glattbrugg, Switzerland) administered through a power injector (Accutron CT-D Medtron Injector, SMD Medical Trade GmbH, Salenstein, Switzerland). The images were reviewed on a workstation (IntelliSpace PACS 4.4 Radiology, Philips AG, Zurich, Switzerland), revealing a well-defined focal, round, soft tissue-attenuated nodule (1 cm in diameter) located in the caudal third of the left pancreatic branch. The nodule exhibited marked contrast enhancement during the arterial phase, with attenuation differing from normal pancreatic tissue, which equalized in the venous phase (early wash in late wash out). A lymph node (considered hepatic) was found to be mildly enlarged but showed normal contrast uptake. An intraparenchymal nodule measuring 2 cm with rim enhancement was detected in the body of the spleen. The liver presented with multiple ill-defined, hypoattenuating areas in all stages of contrast distribution. A small amount of mineral attenuating material occurred in the gallbladder. The uterus contained a single oval-shaped lesion in the left horn and minimal fluid.
Based on the imaging results, the suspected diagnoses were malignant insulinoma (with potential differential diagnoses of carcinoma) with concurrent lymphadenopathy (although a metastatic lesion was considered less likely), nodular hepatopathy (unspecific), a splenic lesion (hematoma, hyperplasia, or neoplasia), and an endometrial cyst.
Repeat blood glucose testing revealed a glucose level of 5.6 mmol/L. Despite a high suspicion of insulinoma, no additional insulin analysis was performed since the dog had euglycemia at presentation, and insulin concentration is only reliable during hypoglycemia (< 3.3 mmol/L). Surgery was recommended and scheduled for 10 days after the workup. Initial therapy involved administering 2 mg/kg of oral prednisone one time daily until surgery.
2.3. Intervention
Anesthesia was induced using fentanyl (3 μg/kg IV) and alfaxalone (1 mg/kg IV Alfaxalon Multidose, Dr. Graeub AG, Bern) and maintained after endotracheal intubation using sevoflurane (Sevorane®, AbbVie AG, Cham) delivered in oxygen. During surgery, analgesia was maintained by administering fentanyl (10 μg/kg/h, Fentanyl Sintetica, Sintetica, Mendrisio, CRI). In addition, the dog received lidocaine (30 μg/kg/h, Lidocaine HCL Bichsel, Bichsel AG, Interlaken, CRI) and dobutamine (2.5 μg/kg/min, Dobutrex®, Teva AG, Rapperswil). At induction, the blood glucose level was 3.7 mmol/L.
After performing a standard coeliotomy, the mass in the pancreas was visualized, and a complete inspection of the abdomen was conducted. Apart from the jejunal (1.5 × 1.5 × 0.5 cm) and right colic lymph nodes (1.5 × 1 × 0.5 cm), one additional node was identified between the pancreas and the stomach (the duodenal lymph node, 1.4 × 0.8 × 0.3 cm). Subsequently, 0.2 ml of ICG (2.5 mg/kg, Verdye, Diagnostic Green GmbH, Kirchheim) was injected into the tumor. Lymphatics became visible within seconds (Supplementary Video 1, Visionsense, VS3 Iridium, Medtronic, Switzerland), resulting in the identification of a total of six draining nodes, including the three nodes previously identified prior to injection, as well as the right (0.6 × 0.4 × 0.4 cm) and left hepatic nodes (0.2 × 0.2 × 0.2 cm) and a splenic node (0.5 × 0.5 × 0.5 cm) (please refer to Figure 2).
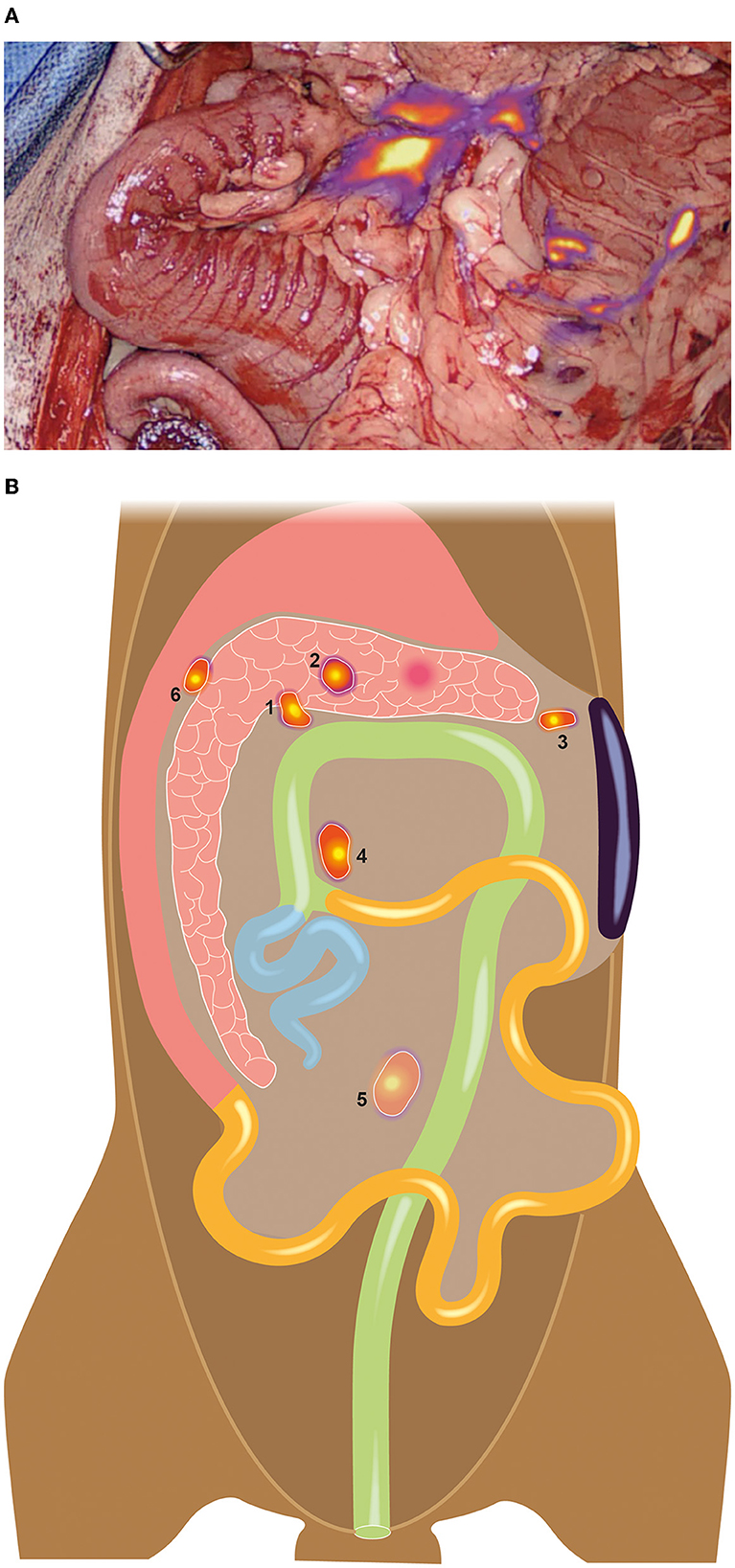
Figure 2. A total of six draining nodes was detected using NIR lymphography (A), three of which were not palpable or visible without NIR guidance during surgery. (B) Schematic illustration of the detected nodes: 1 = Right hepatic lymph node, 2= left hepatic lymph node, 3= splenic lymph node, 4= right colic lymph node, 5= jejunal lymph node, 6= duodenal lymph node.
All nodes were removed using a ligature (LigaSureTM Small Jaw Instrument, Medtronic, Switzerland). The ligature also removed the pancreatic mass through an en-bloc resection of the left branch of the pancreas. After completion of the lymphadenectomies, blood glucose was measured at 4.1 mmol/L. Blood glucose levels were recorded again after partial pancreatectomy and were found to be 5.9 mmol/L and 6 mmol/L at the end of surgery. Liver biopsies were taken from the left and central lobes of the liver, where nodular areas were visible. Finally, a splenectomy and an ovariohysterectomy were performed. The total surgical time was 68 m (the total anesthesia time was 145 m).
Before wound closure, 2 mg/kg ropivacaine (total volume 4.3 ml; Ropivacaine Sintetica, Sintetica, Mendrisio) was administered into the abdomen. Postoperative analgesia was maintained using methadone 0.1 mg/kg IV (Methadon Streuli, Streuli Pharma AG, Uznach) q4 h for 24 h and metamizole (Minalgin, Streuli Pharma AG, Uznach) 25 mg/kg IV q8 h for 24 h, followed by oral administration for 7 days. Prednisone was gradually tapered by administering 0–5 mg/kg every other day for two additional doses. The dog remained hypoglycemic after surgery and was stable enough to be discharged the following day.
Histopathologic examination confirmed the presence of a well-demarcated and densely cellular neoplasm with a diameter of 15 mm, surrounded by a fibrous capsule invaded by neoplastic cells. The mitotic rate was low (1 in 10 HPF), and immunohistochemistry was negative for CAM-5.2 and strongly positive for anti-insulin, leading to a final diagnosis of malignant insulinoma. No signs of lymph node metastasis were detected (Stage I). The splenic lesion was diagnosed as nodular hyperplasia and the liver lesions as vacuolar hepatopathy.
2.4. Follow-up and outcome
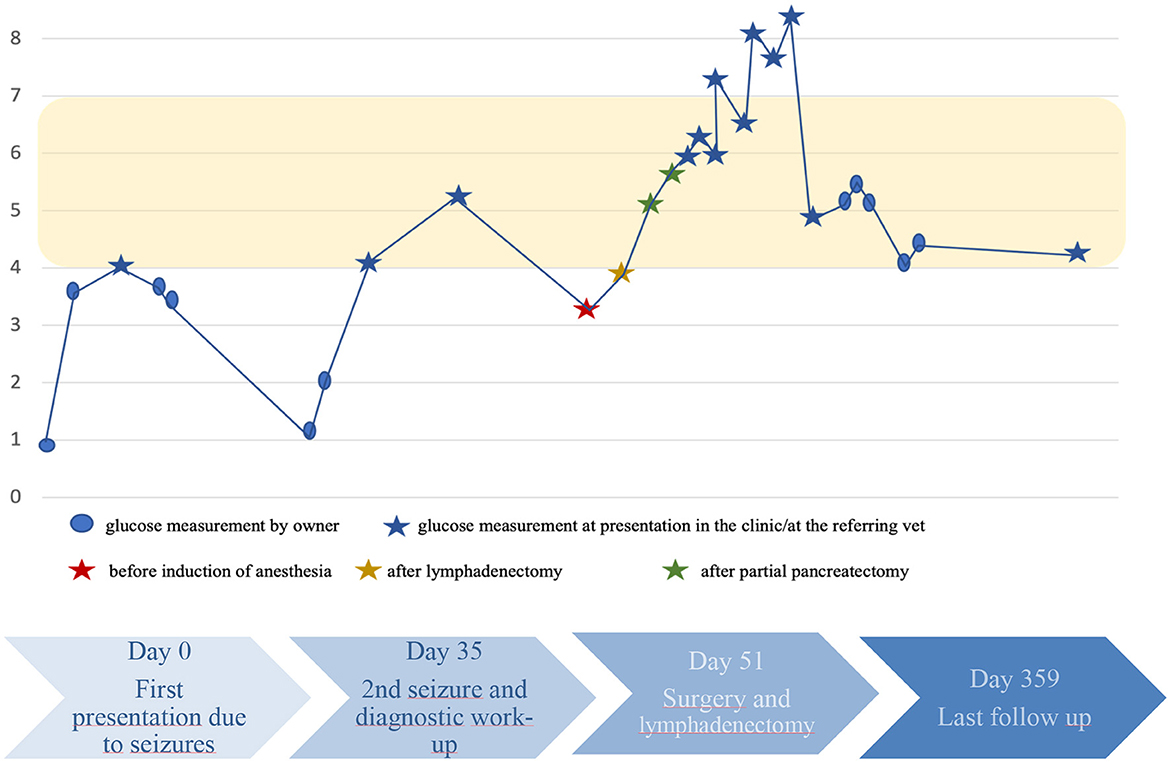
The dog's recovery was unremarkable, and up until the time of drafting the manuscript, no further seizures or hypoglycemic episodes were detected. A timeline with progression of the case is depicted in Figure 3.
3. Discussion
While lymph node resection is standardized in people with pancreatic carcinoma and involves the resection of 12 lymph node stations in the head and three stations in tumors of the tail or body of the pancreas (23–25), there is currently no established approach for lymph node resection in dogs with malignant pancreatic neoplasia.
The studies currently available on dogs with malignant insulinoma mostly documented the resection of grossly enlarged or “visible” nodes (with and without control of the impact on hypoglycemia) but fail to provide information on the number of nodes resected per animal (1–3, 9, 10). This approach appears overly simplistic, given the relatively complex drainage pattern of the canine pancreas and the potentially large number of SLNs.
This is especially true considering the fact that the clinical stage based on imaging (e.g., “enlarged” or structurally altered nodes in CT or ultrasound) does not correspond well to the histopathological stage. Cleland et al. (2) compared the clinical and histopathological stages in affected dogs in 2020 and documented significant discrepancies, with 25% of dogs at each stage 1, 2, 3 or with no imaging of the signs of a tumor, translating to dogs with stage I disease in 45% of cases, stage II in 24% of cases, and stage III in 31% of cases based on histology (2). Notably, nodes were only resected in 55% of cases, leaving the question of how many dogs were in stage I at the end of the study.
In another study performed by Buishand et al. (1), the sensitivity of CT to detect nodal metastasis was evaluated at 67% (1). Unfortunately, the authors failed to provide information on the number of lymph nodes that were considered normal/non-detectable in the CT that were metastatic, as these were not resected. Similar findings have been published for human pancreatic carcinoma. Cesmebasi et al. (26) found that it is problematic to identify metastatic nodes in affected patients. In MRI and surgery, 21% of their metastatic nodes were normal in size and texture (26).
In human medicine, routine lymphadenectomy is an important component in the treatment of pancreatic adenocarcinomas, but the extent of lymphadenectomy is still a topic of debate (23–27). However, a standard protocol has been widely established for pancreatic adenocarcinoma, which involves the resection of 12 or 3 lymph node stations in the head or tail/body of the pancreas, respectively (25). Extended lymphadenectomies involving the removal of more lymph nodes failed to demonstrate superior outcomes but were associated with higher morbidity for the patients (23–27).
In humans with pancreatic neuroendocrine tumors (P-NET), the current recommendation is to resect lymph nodes only in aggressive functional tumors with negative prognostic expectations or tumors that are bigger than 1–3 cm in size (28–32). Several studies have investigated the impact of nodal resections in P-NET and documented that the metastatic rate of tumors was significantly underrated if fewer than six or eight nodes were resected (31, 32). Given the malignant nature of canine insulinoma, it is most likely to fall under the subgroup of aggressive P-Net, where regional lymphadenectomy is recommended. Zhang et al. (32) conducted one of the largest studies on this topic in humans, reporting a lymph node metastatic rate of 21% and the benefit of lymphadenectomy when at least six nodes were removed (32). Wu et al. (31) found that patients with aggressive P-NET (>2 cm, Ki-67 Y3%, located in the pancreatic head) had a higher therapeutic index when at least eight nodes were removed (31). Extended radical lymphadenectomies did not appear to provide any additional value and were thus not recommended (28–32). In summary, for aggressive P-NET, moderate resections of the regional draining nodes are recommended and likely to improve outcomes in humans. As malignant insulinoma in dogs shows aggressive behavior (7), with nodal metastatic rates of 70–95%, a similar approach is likely to be reasonable.
Sentinel node mapping could help identify regional draining nodes and therefore offer a more structured approach than resecting enlarged nodes in dogs. However, early attempts in humans with pancreatic carcinoma to map sentinel nodes using methylene blue were not convincing due to low detection rates and high false-negative rates (21, 22). As methylene blue is one of the least sensitive options for nodal mapping (20), caution should be exercised when interpreting these results, and further validation of the usefulness of other mapping techniques is warranted.
In the presented case, we successfully performed lymphography on a dog with a malignant insulinoma located in the pancreas using ICG. We were able to detect and remove eight sentinel nodes, six of which would not have been detectable through visualization or palpation during surgery. Based on our findings, we concluded that ICG NIRF is generally feasible in dogs with insulinoma and allows for easy identification of small, non-altered nodes. The technique might offer a more structured approach for easy identification of small, non-altered nodes. This technique may offer a more structured approach to nodal resection in dogs with malignant insulinoma in the future. However, further studies are necessary to evaluate the performance and clinical impact of this technique, including the detection rate and false-negative rate.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the animal study because ICG Lymphography is now a routine procedure in our facility, that is routinely used in our cases due to medical/treatment reasons. As this is merely a retrospective description of mapping of a new tumor type in a single dog that was done due to medical reasons it does not require an animal license. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
The original draft of the manuscript was written by MN. MD and RD also contributed to the initial draft and provided revisions for the above manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wish to thank Jeanne Peters for her illustration used in this case.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1178454/full#supplementary-material
Supplementary Video 1. ICG Lymphography.
References
1. Buishand FO. Current trends in diagnosis, treatment and prognosis of canine insulinoma. Vet Sci. (2022) 9:540. doi: 10.3390/vetsci9100540
2. Cleland NT, Morton J, Delisser PJ. Outcome after surgical management of canine insulinoma in 49 cases. Vet Comp Oncol. (2021) 19:428–41. doi: 10.1111/vco.12628
3. Collgros NC, Bray JP. Blood glucose monitoring during surgery in dogs to assess completeness of surgical resection of insulinoma: 11 cases. J Am Vet Med Assoc. (2022) 261:1–8. doi: 10.2460/javma.22.07.0282
4. Goutal CM, Brugmann BL, Ryan KA. Insulinoma in dogs: a review. J Am Anim Hosp Assoc. (2012) 48:151–63. doi: 10.5326/JAAHA-MS-5745
5. Leifer CE, Peterson ME, Matus RE. Insulin-secreting tumor: diagnosis and medical and surgical management in 55 dogs. J Am Vet Med Assoc. (1986) 188:60–4.
6. Trifonidou MA, Kirpensteijn J, Robben JH, A. retrospective evaluation of 51 dogs with insulinoma. Vet Q. (1998) 20:S114–5. doi: 10.1080/01652176.1998.10807459
7. Capodanno Y, Altieri B, Elders R, Colao A, Faggiano A, Schrader J. Canine insulinoma as a model for human malignant insulinoma research: Novel perspectives for translational clinical studies. Transl Oncol. (2022) 15:101269. doi: 10.1016/j.tranon.2021.101269
8. de Vries C, Konukiewitz B, Weichert W, Kloppel G, Aupperle-Lellbach H, Steiger K. Do Canine pancreatic neuroendocrine neoplasms resemble human pancreatic neuroendocrine tumours? a comparative morphological and immunohistochemical investigation. J Comp Pathol. (2020) 181:73–85. doi: 10.1016/j.jcpa.2020.10.001
9. Del Busto I, German AJ, Treggiari E, Romanelli G, O'Connell EM, Batchelor DJ, et al. Incidence of postoperative complications and outcome of 48 dogs undergoing surgical management of insulinoma. J Vet Intern Med. (2020) 34:1135–43. doi: 10.1111/jvim.15751
10. Hixon LP, Grimes JA, Wallace ML, Schmiedt CW. Risk factors for gastrointestinal upset and evaluation of outcome following surgical resection of canine pancreatic beta-cell tumors. Can Vet J. (2019) 60:1312–8.
11. Wouters EG, Buishand FO, Kik M, Kirpensteijn J. Use of a bipolar vessel-sealing device in resection of canine insulinoma. J Small Anim Pract. (2011) 52:139–45. doi: 10.1111/j.1748-5827.2011.01040.x
12. Ryan D, Perez-Accino J, Goncalves R, Czopowicz M, Bertolani C, Tabar MD, et al. Clinical findings, neurological manifestations and survival of dogs with insulinoma: 116 cases (2009-2020). J Small Anim Pract. (2021) 62:531–9. doi: 10.1111/jsap.13318
13. Fukushima K, Fujiwara R, Yamamoto K, Kanemoto H, Ohno K, Tsuboi M, et al. Characterization of triple-phase computed tomography in dogs with pancreatic insulinoma. J Vet Med Sci. (2016) 77:1549–53. doi: 10.1292/jvms.15-0077
14. Lamb CR, Simpson KW, Boswood A, Matthewman LA. Ultrasonography of pancreatic neoplasia in the dog: a retrospective review of 16 cases. Vet Rec. (1995) 137:65–8. doi: 10.1136/vr.137.3.65
15. Mai W, Caceres AV. Dual-phase computed tomographic angiography in three dogs with pancreatic insulinoma. Vet Radiol Ultrasound. (2008) 49:141–8. doi: 10.1111/j.1740-8261.2008.00340.x
17. Tsioulias GJ, Wood TF, Morton DL, Bilchik AJ. Lymphatic mapping and focused analysis of sentinel lymph nodes upstage gastrointestinal neoplasms. Arch Surg. (2000) 135:926–32. doi: 10.1001/archsurg.135.8.926
18. Wexner S, Abu-Gazala M, Boni L, Buxey K, Cahill R, Carus T, et al. Use of fluorescence imaging and indocyanine green during colorectal surgery: results of an intercontinental Delphi survey. Surgery. (2022) 172:S38–45. doi: 10.1016/j.surg.2022.04.016
19. Liao Y, Zhao J, Chen Y, Zhao B, Fang Y, Wang F, et al. Mapping lymph node during indocyanine green fluorescence-imaging guided gastric oncologic surgery: current applications and future directions. Cancers. (2022) 14:5143. doi: 10.3390/cancers14205143
20. Beer P. Chiti. LE, Nolff, MC. The role of sentinel node mapping and lymphadenectomies in veterinary surgical oncology Lymphatics. (2023) 1:2–18. doi: 10.3390/lymphatics1010002
21. Durczynski A, Hogendorf P, Szymanski D, Grzelak P, Strzelczyk J. Sentinel lymph node mapping in tumors of the pancreatic body: preliminary report. Contemp Oncol. (2012) 16:206–9. doi: 10.5114/wo.2012.29285
22. Kocher HM, Sohail M, Benjamin IS, Patel AG. Technical limitations of lymph node mapping in pancreatic cancer. Eur J Surg Oncol. (2007) 33:887–91. doi: 10.1016/j.ejso.2007.02.037
23. Aziz H, Cloyd JM, Spolverato G, Pawlik TM. Does extended lymphadenectomy help in pancreatic cancer? Ann Surg Oncol. (2022) 29:2131–3. doi: 10.1245/s10434-022-11370-1
24. Erdem S, Bolli M, Muller SA, von Flue M, White R, Worni M. Role of lymphadenectomy in resectable pancreatic cancer. Langenbecks Arch Surg. (2020) 405:889–902. doi: 10.1007/s00423-020-01980-2
25. Tol JA, Gouma DJ, Bassi C, Dervenis C, Montorsi M, Adham M, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the international study group on pancreatic surgery (ISGPS). Surgery. (2014) 156:591–600. doi: 10.1016/j.surg.2014.06.016
26. Cesmebasi A, Malefant J, Patel SD, Du Plessis M, Renna S, Tubbs RS, et al. The surgical anatomy of the lymphatic system of the pancreas. Clin Anat. (2015) 28:527–37. doi: 10.1002/ca.22461
27. Kotb A, Hajibandeh S, Hajibandeh S, Satyadas T. Meta-analysis and trial sequential analysis of randomised controlled trials comparing standard versus extended lymphadenectomy in pancreatoduodenectomy for adenocarcinoma of the head of pancreas. Langenbecks Arch Surg. (2021) 406:547–61. doi: 10.1007/s00423-020-01999-5
28. Ausania F, Senra Del Rio P. Lymphadenectomy in pancreatic neuroendocrine neoplasms: Why are we still debating? Pancreatology. (2018) 18:855–61. doi: 10.1016/j.pan.2018.09.005
29. Bolm L, Nebbia M, Wei AC, Zureikat AH, Fernandez-Del Castillo C, Zheng J, et al. Long-term outcomes of parenchyma-sparing and oncologic resections in patients with nonfunctional pancreatic neuroendocrine tumors <3 cm in a large multicenter cohort. Ann Surg. (2022) 276:522–31. doi: 10.1097/SLA.0000000000005559
30. Conrad C, Kutlu OC, Dasari A, Chan JA, Vauthey JN, Adams DB, et al. Prognostic value of lymph node status and extent of lymphadenectomy in pancreatic neuroendocrine tumors confined to and extending beyond the pancreas. J Gastrointest Surg. (2016) 20:1966–74. doi: 10.1007/s11605-016-3243-7
31. Wu L, Sahara K, Tsilimigras DI, Maithel SK, Poultsides GA, Rocha FG, et al. Therapeutic index of lymphadenectomy among patients with pancreatic neuroendocrine tumors: a multi-institutional analysis. J Surg Oncol. (2019) 120:1080–6. doi: 10.1002/jso.25689
Keywords: functional neuroendocrine pancreatic tumor, pancreatic lymph node mapping, sentinel node mapping, staging malignant melanoma, lymphadenectomy
Citation: Nolff MC, Dennler R and Dennler M (2023) Use of indocyanine green near-infrared lymphography to detect sentinel lymph nodes in a dog with a malignant insulinoma: a case report. Front. Vet. Sci. 10:1178454. doi: 10.3389/fvets.2023.1178454
Received: 02 March 2023; Accepted: 03 April 2023;
Published: 27 April 2023.
Edited by:
Gerardo Fatone, University of Naples Federico II, ItalyReviewed by:
Yoshiharu Okamoto, Tottori University, JapanJoy Archer, University of Cambridge, United Kingdom
Copyright © 2023 Nolff, Dennler and Dennler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirja Christine Nolff, mnolff@vetclinics.uzh.ch
†ORCID: Mirja Christine Nolff orcid.org/0000-0001-9317-7769
Matthias Dennler orcid.org/0000-0002-8134-9495
 Mirja Christine Nolff
Mirja Christine Nolff