- 1College of Animal Science and Veterinary Medicine, Shandong Agricultural University, Taian, Shandong, China
- 2Shandong Livestock Product Quality and Safety Center, Shandong, China
- 3Zhongcheng Feed Technology Co., Ltd., Feicheng, Shandong, China
- 4Ciyao Animal Husbandry Station, Ningyang, Shandong, China
- 5Guelph Research and Development Center, Agriculture and Agri-Food Canada (AAFC), Guelph, ON, Canada
- 6Challenge Biotechnology Co., Ltd., Beijing, China
This study aims to investigate the effects of macleaya extract and glucose oxidase combination (MGO) on growth performance, antioxidant capacity, immune function, and cecal microbiota in piglets. A total of 120 healthy 28-day-old weaned piglets were randomly divided into two treatments of six replicates. Piglets were either received a basal diet or a basal diet supplemented with 250 mg/kg MGO (2 g/kg sanguinarine, 1 g/kg chelerythrine, and 1 × 106 U/kg glucose oxidase). The results showed that MGO supplementation increased average daily gain (ADG) and decreased feed:gain ratio (F/G) (p < 0.05). MGO increased serum superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity, and immunoglobulin G (IgG) content (p < 0.05), but decreased malondialdehyde (MDA) and interleukin 1β (IL-1β) content (p < 0.05). The jejunal mRNA expression of nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase 1 (GPX1), and heme oxygenase 1 (HO-1) were increased in MGO group (p < 0.05), while that of kelch like ECH associated protein 1 (Keap1) was decreased (p < 0.05). The Firmicutes was significantly increased at phylum levels in MGO group (p < 0.05). In conclusion, 250 mg/kg MGO improved piglet growth, and regulated intestinal flora of piglets, which provided a theoretical basis for MGO as an alternative additive for antibiotics.
Introduction
Antibiotics have been used as feed additives in piglets to enhance their growth performance, promote intestinal health and improve immunity (1). However, with the ban on the use of antibiotics in feed, piglets are now faced with oxidative stress and inflammation, leading to growth restriction, disease, and even death (2). Hence, there is an urgent need to explore a alternative antibiotic substitutes than can improve the antioxidant capacity and immune function in piglets (3).
The macleaya extract (MCE) contains sanguinarine and chelerythrine, which possess anti-inflammatory, antioxidant, bactericidal, and anti-tumor properties (4). Accordingly, MCE plays roles in improving animal immunity and animal growth, and preserving intestinal health (5). Studies have shown that macleaya extract can improve the growth performance, antioxidant capacity, and immune function of piglets (6, 7) and broiler chickens (8). Previous study showed that 50 mg/kg macleaya extract containing 1.5% sanguinarine increased average daily gain (ADG) of piglets (7). Moreover, supplement 50 mg/kg macleaya extract in piglet’s diet increased ADG and average daily feed intake (ADFI) (Ref). In addition, the content of cecal microbiota, such as Escherichia coli and Salmonella, were significantly reduced (9). Previous study reported that 40 mg/kg macleaya extract could increase the immunoglobulin G (IgG) content in the serum of piglets on the seventh day of growth (10). Dietary supplementation of MCE (50 mg/kg) also increased the superoxide dismutase (SOD) activity, and decreased the malondialdehyde (MDA) content in piglet serum (11).
Glucose oxidase (GOD) is an aerobic dehydrogenase that originates from the fermentation of specific fungal strains, including Aspergillus and Penicillium (12). This enzyme is capable of oxidizing β-D-glucose into gluconic acid, while also producing hydrogen peroxide (HP) through the consumption of oxygen (13). HP can inhibit the growth of harmful gut bacteria and prevent bacterial invasion (14). Therefore, the characteristics of GOD, such as acid production, deoxygenation and sterilization indicate its potential as a substitute additive for antibiotics (15–17). Previous study have shown that 100 U/kg GOD supplementation could improve the growth performance of piglets (18). In addition, dietary supplementation of 3,000 U/kg GOD can increase the total superoxide dismutase (T-SOD) and glutathione peroxidase (GSH-Px) activity in the jejunum of piglets (19). Research shown that 200 mg/kg glucose oxidase reduced the ETEC induced decrease of IgG content and the abundance of Lactobacillaceae and Lactobacillus salivarius in the intestine (13).
Therefore, MCE or GOD can improve piglet growth performance, antioxidant capacity, immune function and gut microbiota structure. However, the effects of the combination of MCE and GOD on piglet physiology have not been reported. The objective of this study was to investigate the impact of MCE, which includes sanguinarine and chelerythrine, combined with GOD on growth performance, antioxidant capacity, immune function, and cecal microbiota in piglets. The findings of this study could serve as a theoretical basis for the efficacy of MCE-GOD compound additives on piglets.
Materials and methods
Experimental design and layers management
Piglets used in this experiment were cared in accordance with the guidelines for the care and use of laboratory animals described by the Guide for the Care and Use of Laboratory Animals and approved by the Committee on the Ethics of Shandong Agricultural University (SDAUA-2020-0710).
A total of 120 healthy 28-day-old weaned piglets (Duroc × Large White × Landrace) were randomly divided into two treatments of six replicates, and with ten piglets per replicate. The control group was fed a basal diet, while the experimental group was fed a basal diet supplemented with 250 mg/kg of MGO (a combination of MCE and GOD). The MGO contained 2 g/kg of sanguinarine, 1 g/kg of chelerythrine, and 1 × 106 U/kg of GOD. The MGO was provided by Shengdao Biological Co., Ltd. (Taian, Shandong, China). The feeding trial lasted for 35 days after 7-d adaptation at a piggery of Shandong Agricultural University. Piglets have free access to feed and water. The piglets were feed with powdered feed, and the treatment group’s feed was mixed with 250 mg/kg of MGO before feeding, ensuring an even distribution of the additive. The experimental diets were formulated according to National Research Council (20). The composition and calculated nutrient levels in the basal diet were shown in Table 1. Before feeding, diets were completed, sampled, and stored in covered containers. The piggery was thoroughly cleaned and disinfected before the experiment, and the pigs were disinfected once a week during the experimental period. During the first week, the ambient temperature was kept at about 30°C, and then maintained between 26°C to 28°C until the end of experiment. The relative humidity of the pig house was approximately 65%.
Sampling collection
At the begining and end of the experiment, the body weight of each piglet was measured. The feeding intake of each replicate was recorded daily. The average daily gain (ADG), average daily feed intake (ADFI), and feed: gain ratio (F/G) were calculated.
At the end of the experiment, 10 mL blood was sampled from the jugular veins into tubes without anticoagulant after a 12-h fasting on the last day of the experiment. After centrifugation at 3,000 × g for 15 min at room temperature, the serum was obtained in 1.5 mL Eppendorf tubes.
One piglet with similar weight was selected from each repeat, and the head was electrocuted (110 V, 60 Hz) for 5 s to cause death. The 3 cm jejunum in the same part were isolated under sterile conditions from piglets randomly selected from each replicate. The removed jejunum was immediately frozen in liquid nitrogen and stored at −80°C for the subsequent analysis of mRNA expression. In addition, about 3 mL of cecal contents were stored at −80°C for microbial sequencing analysis.
Determination of serum antioxidant, immunoglobulin and cytokines
Serum superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity, and malondialdehyde (MDA) content were measured according to the methods of SOD assay kits (A001-3-2, WST-1 method), GSH-Px assay kits (A005-1-2, Colorimetric method), and MDA assay kits (A003-1-1, TBA method) (Jiangsu Nanjing Jiancheng Biotechnology Co., Ltd., Jiangsu, China). The measurement wavelengths of SOD, GSH-Px and MDA were 450 nm, 412 nm, and 532 nm, respectively. Serum immunoglobulin G (IgG, H106-1-1), immunoglobulin M (IgM, H109-1-2), immunoglobulin A (IgA, H108-1-2) and inflammatory factors including interleukin 1β (IL-1β, H002), interleukin 6 (IL-6, H007-1-2), interleukin 10 (IL-10, H009-1), and tumor necrosis factor-α (TNF-α, H052-1) were determined using commercial enzyme linked immunosorbent assay (ELISA) kits (Jiangsu Nanjing Jiancheng Biotechnology Co., Ltd., Jiangsu, China).
Determination of relative mRNA expression in jejunum
The total RNA was extracted from the jejunum samples using RNAiso Plus (D9108B, Takara Bio Inc., Kusatsu, Japan) according to the manufacturer’s instructions. RNA purity and concentration were assessed using an Eppendorf Biophotometer (RS323C, Eppendorf Aktien Gesellschaft, Hamburg, Germany) with an absorbance ratio of 260/280 nm (range 1.8–2.0 represents pure RNA samples). The RNA integrity was verified by agarose gel electrophoresis. Total RNA was reverse transcribed to cDNA using the Reverse Transcription System Kits (Prime-Script RT Master Mix, RR036A, Takara Bio Inc., Kusatsu, Japan). The cDNA is used for polymerase chain reaction (PCR).
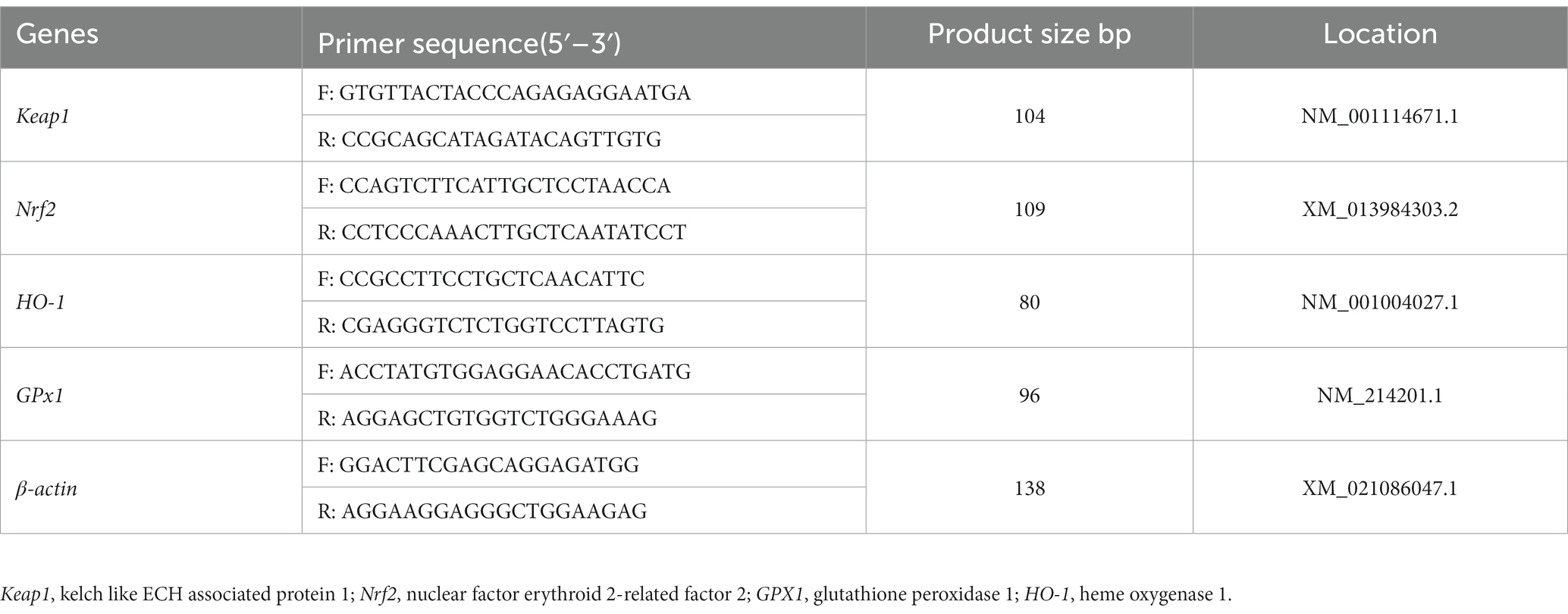
The total volume of the PCR mix was 20 μL, containing 10 μL SYBRY Premix Ex Taq II, 0.4 μL DyeII (SYBRY Premix Ex Taq-TIi RNaseH Plus, DRR420A, Takara Bio Inc., Kusatsu, Japan), 0.4 μL forward primers, 0.4 μL reverse primers, and 2 μL of cDNA (< 100 ng) for quantitative real-time PCR (qRT-PCR) analysis. The optimized qRT-PCR protocol consisted of an initial denaturation step at 95°C for 30 s, followed by 43 cycles of 95°C for 5 s, 60°C for 34 s, 95°C for 15 s, 60°C for 60 s, and 95°C for 15 s. The qRT-PCR was performed using the AB 7500 Real-Time PCR System (Applied Biosystems, Foster City, United States). The relative mRNA expression levels of kelch like ECH associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase 1 (GPX1), and heme oxygenase 1 (HO-1) were calculated by the 2-△△CT method, and each sample was analyzed three times in replicates. The primer sequence and product size are shown in Table 2.
Determination of cecal microbial sequencing
The total genomic DNA from the samples was extracted according to CTAB method. The DNA integrity and purity were determined using 1% agarose gel electrophoresis and a NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, United States). DNA concentration was accurately quantified by a Qubit Fluorometer (Thermo Scientific, Waltham, United States) and then diluted to 1 ng/μL with sterile water. The sequenced region of RCR amplification was V3-V4, and the primer sequences were 341F (CCTACGGGGRBGCASCAG) and 806R (GGACTACNNGGGTATCTAAT). The total volume of the PCR reaction system was 30 μL, consisting of Phusion® High-Fidelity PCR Master Mix with GC Buffer 15 μL, Phusion® High-Fidelity DNA Polymerase 0.5 μL (New England Biolabs, Herts Hitchin, UK), 1 μL upstream, 1 μL downstream, 2 μL of 10 ng/μL genomic DNA, and 10.5 μL of sterile ultrapure water. The amplification program was as follows: pre-denaturation at 98°C for 1 min, denaturation at 98°C for 10 s, annealing at 50°C for 30 s, extension at 72°C for 30 s for a total of 30 cycles, stable extension at 72°C for 5 min, and finally at 4°C was stored (PCR instrument: ABI GeneAmp® Model 9,700, Applied Biosystems, Foster City, Unites States). PCR products were pooled and detected by 2% agarose gel electrophoresis; then quantified by the Quantus™ Fluor-ST Fluorometric Quantitation System (Promega, Madison, United States). The resulting PCR products were concentrated, mixed in equal amounts, and re-electrophoresed on a 2% agarose gel. The target product bands were recovered using the QIAquick gel extraction kit (Axygen, Santa Clara Valley, United States). The library was constructed with TruSeq DNA PCR-free DNA library kit (Illumina, San Diego, United States), and sequenced with HiSeq2500 PE250 after qubit and qPCR quality control. The constructed library was quantified by Qubit, and then NovaSeq6000 was used for on-machine sequencing. After strict filtering and quality control screening, the sequences were clustered into Operational Taxonomic Units (OTUs) with 97% identity and the number of OTUs was calculated. OTUs clustering and species classification analysis were performed on the valid data.
Statistical analyzes
Data analysis was conducted using the general linear model (GLM) in SAS 9.4 (SAS Institute Inc., Cary, NC, United States), with t-test utilized to compare differences among treatments. Mean and standard error of the mean (SEM) were presented as results. The level of p < 0.05 was used to determine differences. GraphPad prism 9.0 (GraphPad Software, San Diego, United States) was used for image production. The petal diagrams for the OTUs data were generated using R software version 3.0.3 and the Venn Diagram package. The Chao1, ACE, Shannon, and Simpson indices were calculated using Qiime software (Version 1.7.0) (3). PyNAST software (Version 1.2) was utilized for rapid multiple sequence alignment with the Core Set data from the SILVA database (21). The phylogenetic tree was constructed using FastTree software and the sequence alignment results were compared using the approximate maximum likelihood algorithm (22).
Results
Growth performance
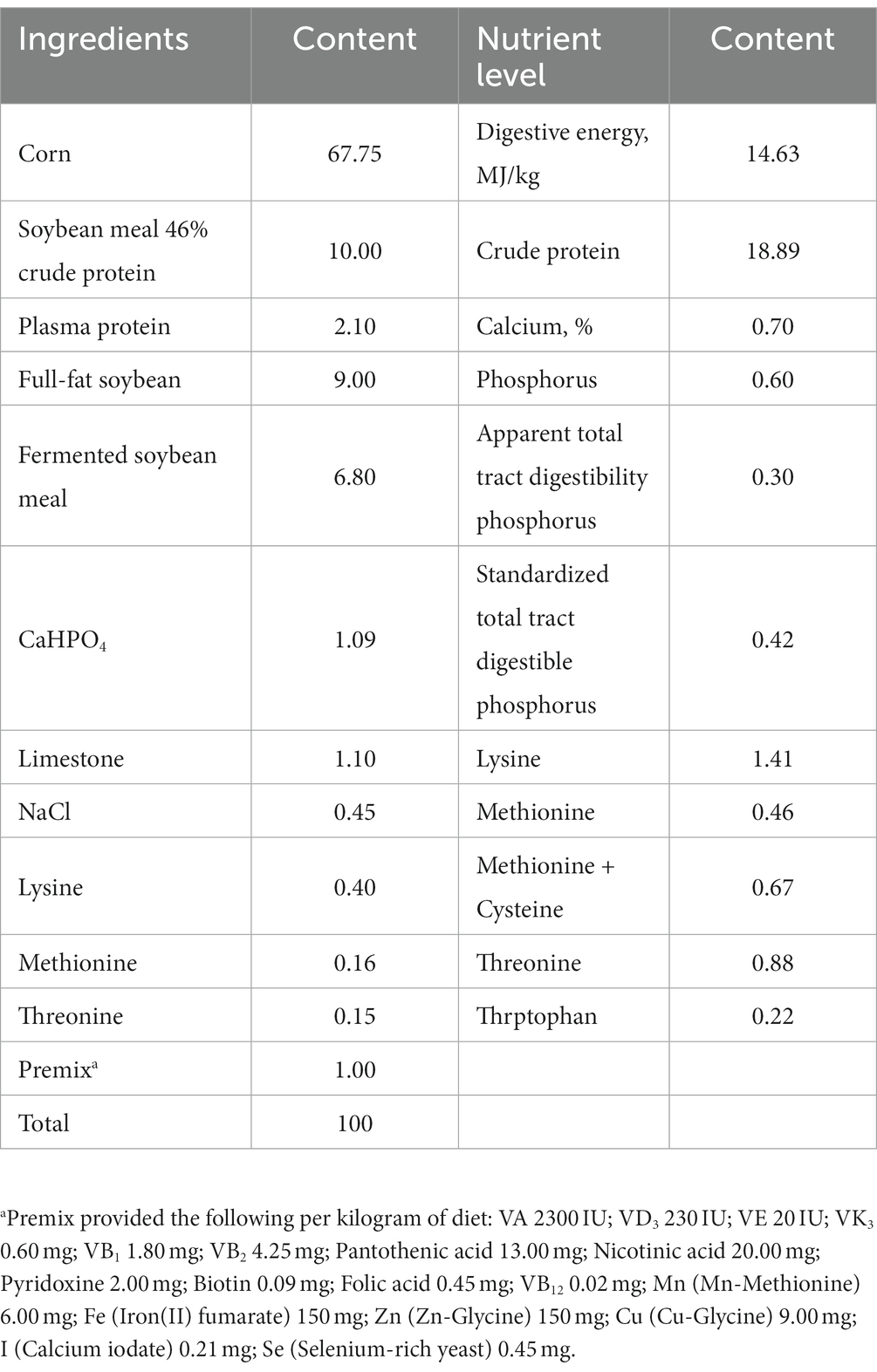
The effect of MGO on growth performance of piglets is shown in Figure 1. Compared with the CON group, MGO group supplementation in the diet increased ADG (Figure 1A) (p < 0.05), and decreased the F/G (Figure 1C) (p < 0.05). However, no significantly were observed in the ADFI (Figure 1B) between the CON and MGO groups (p > 0.05).
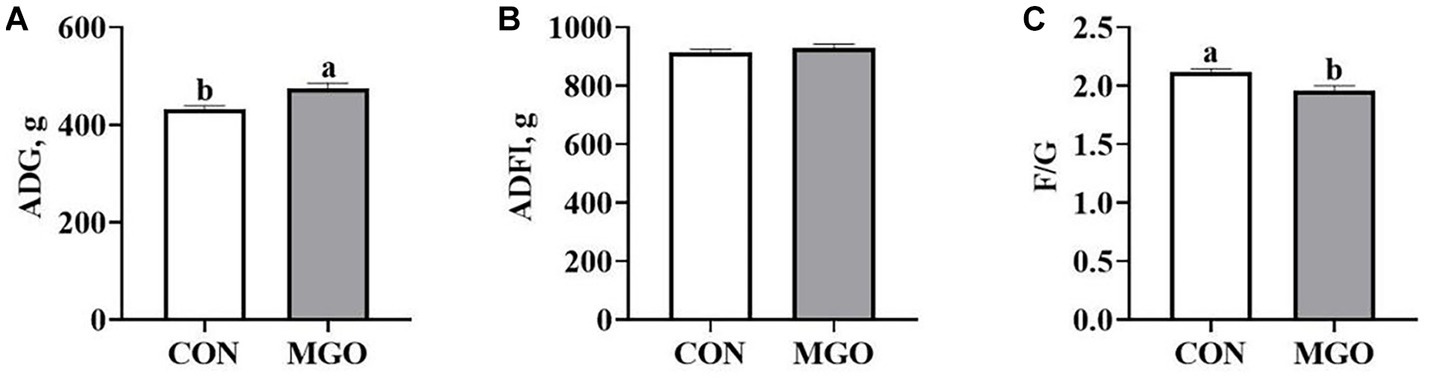
Figure 1. Effects of macleaya extract and glucose oxidase combination on growth performance of piglets. (A–C) Represent the average daily gain (ADG), average daily feed intake (ADFI), and feed:gain ratio (F/G), respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 6). Different lowercase letters in the figure indicate significant differences (p < 0.05).
Serum antioxidant capacity
The effect of MGO on the serum antioxidant capacity of piglets is shown in Figure 2. The increased serum SOD (Figure 2A) and GSH-Px (Figure 2C) activity (p < 0.05), and decreased MDA (Figure 2B) content in MGO group were observed compared with CON group (p < 0.05).
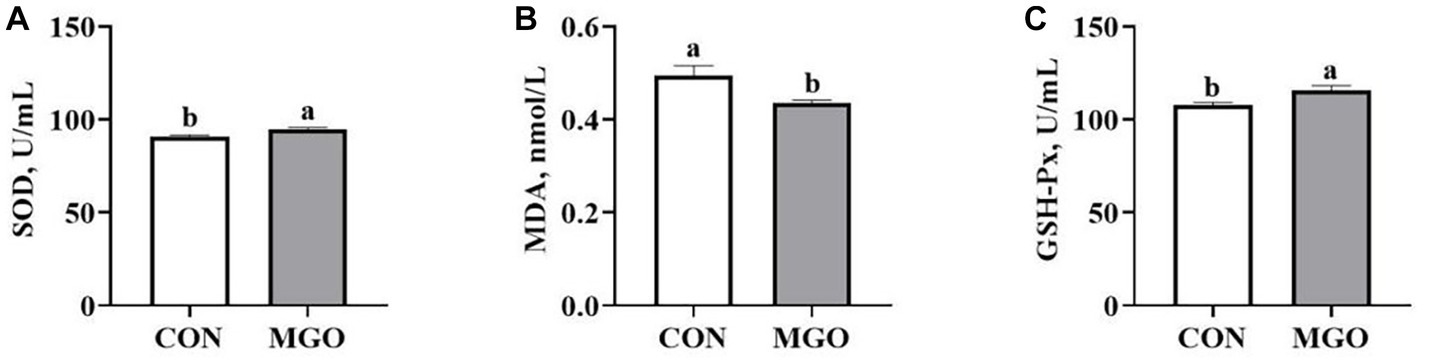
Figure 2. Effects of macleaya extract and glucose oxidase combination on serum antioxidant capacity of piglets. (A–C) Represent the serum superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione peroxidase (GSH-Px), respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 6). Different lowercase letters in the figure indicate significant differences (p < 0.05).
Serum immunoglobulin content
The effect of MGO on the serum immunoglobulin content of piglets is shown in Figure 3. The serum IgG (Figure 3C) content was significantly increased when MGO was added to the diet (p < 0.05). However, there were no significant differences in IgA (Figure 3A) or IgM (Figure 3B) content between CON and MGO groups (p > 0.05).
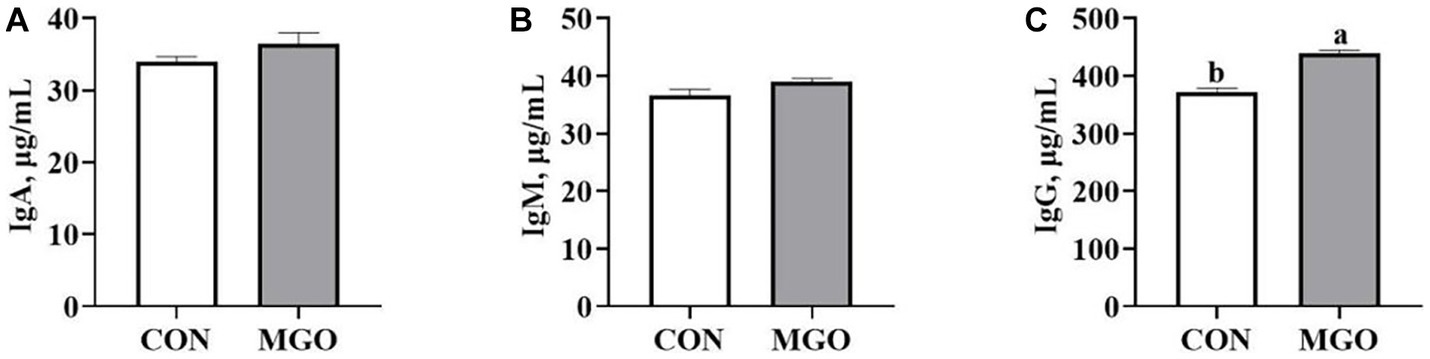
Figure 3. Effects of macleaya extract and glucose oxidase combination on serum immunoglobulin content of piglets. (A–C) Represent the serum immunoglobulin A (IgA), immunoglobulin M (IgM), and immunoglobulin G (IgG), respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 6). Different lowercase letters in the figure indicate significant differences (p < 0.05).
Serum cytokine levels
The Figure 4 showed the effect of MGO on the serum cytokine levels of piglets. Compared with the CON group, the serum IL-1β (Figure 4A) level was significantly decreased in the MGO group (p < 0.05), However, no significant changes were found in IL-6 (Figure 4B), IL-10 (Figure 4C) or TNF-α (Figure 4D) levels between CON and MGO groups (p > 0.05).
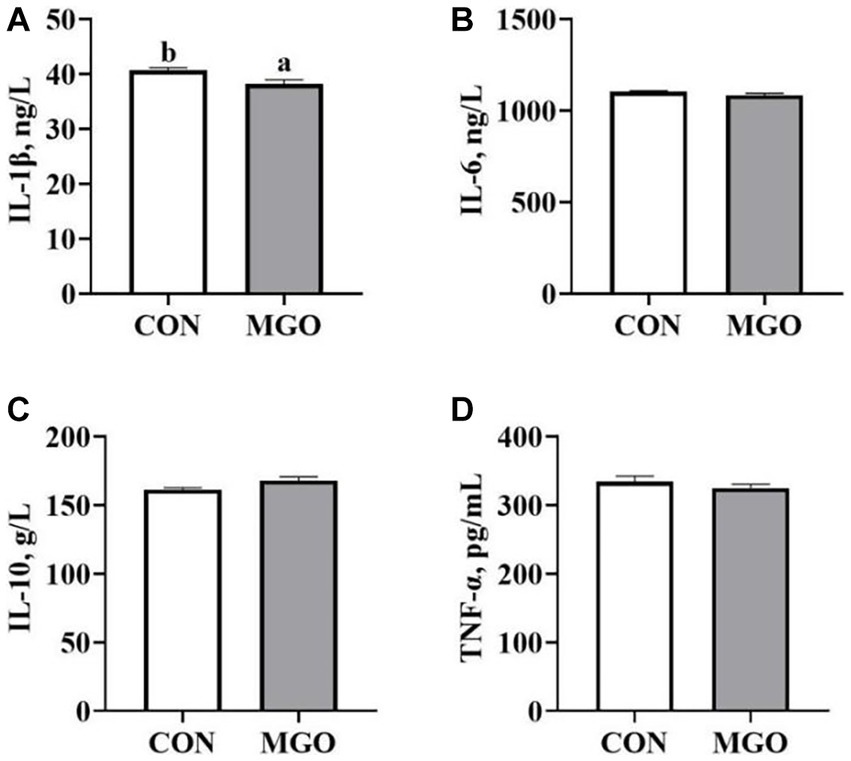
Figure 4. Effects of macleaya extract and glucose oxidase combination on serum cytokine levels of piglets. (A–D) Represent the serum cytokines Interleukin 1β (IL-1β), interleukin 6 (IL-6), interleukin 10 (IL-10), and tumor necrosis factor-α (TNF-α), respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 6). Different lowercase letters in the figure indicate significant differences (p < 0.05).
Antioxidant mRNA expression
As shown in Figure 5, supplementation of MGO in the diet significantly increased the mRNA expression of Nrf2 (Figure 5B), GPX1 (Figure 5C), and HO-1 (Figure 5D) (p < 0.05). However, the mRNA expression of Keap1 (Figure 5A) was decreased in MGO group compared to the CON group (p < 0.05).
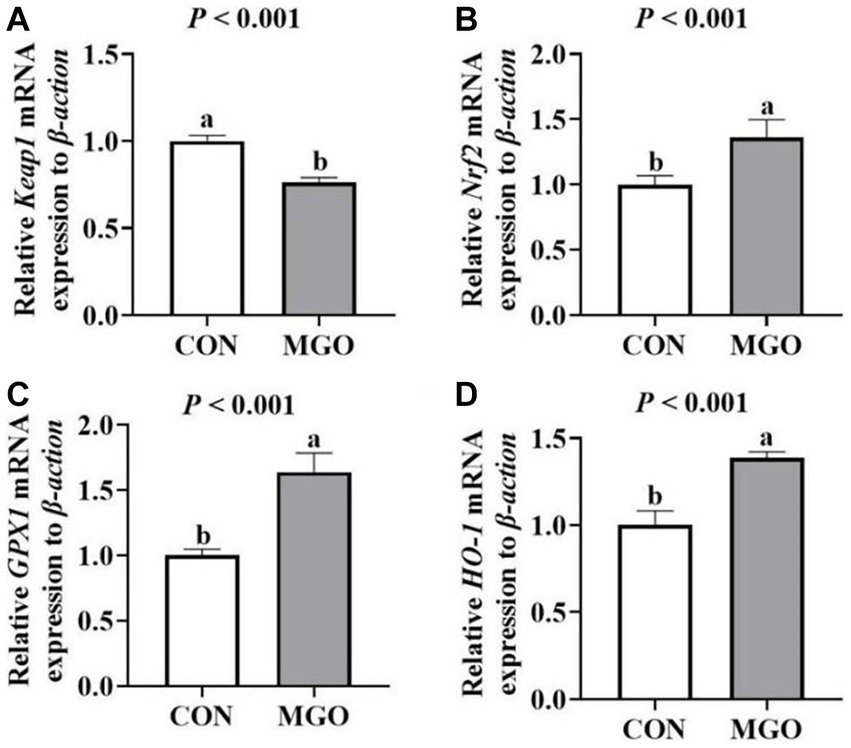
Figure 5. Effects of macleaya extract and glucose oxidase combination on the antioxidant mRNA expression of piglets. (A–D) Represent the jejunum antioxidant mRNA expression of recombinant kelch like ECH associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), glutathione peroxidase 1 (GPX1), and heme oxygenase 1 (HO-1), respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 6). Different lowercase letters in the figure indicate significant differences (p < 0.05).
Microbial diversity analysis
Sequencing data analysis
There were 809 general OTUs (Figure 6A), among which the unique OTUs of CON and MGO groups were 204 and 332, respectively. The cumulative amount of sequencing data is shown as the species accumulation boxplot (Figure 6B) and rarefaction curve (Figure 6C). The results showed that the boxplots flatten out when the sample size reached 8. In addition, the curve tended to be flat when the number of randomly sampled sequences was close to 20,000, and the dilution curve tended to be saturated when the number of sequences was close to 60,000 for all samples. The curve lengths of the samples were similar, indicating that the number of sequenced samples sequenced was reasonable and could truly reflect the information of most microorganisms in the cecal contents.
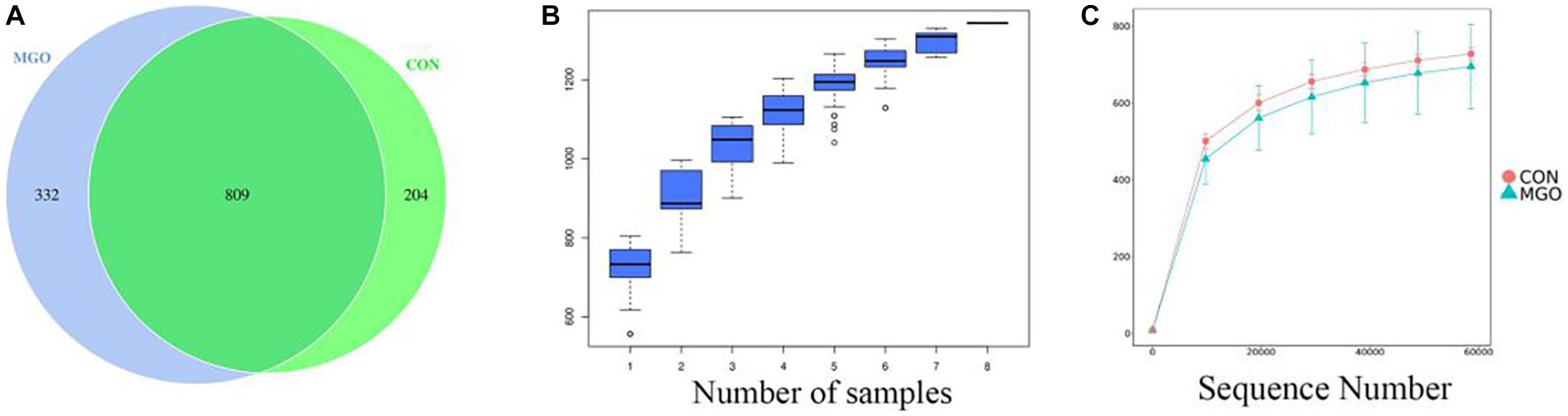
Figure 6. Differences in bacterial community diversity and richness. (A–C) Represent the cecal microbiota venn, species cumulative box, and species diversity curve by OTUs diagram drawn, respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 4).
Alpha diversity index analysis
In the alpha diversity index analysis (Figure 7), Chao1 (Figure 7A) or ACE (Figure 7B) indices represent bacterial abundance, and Simpson (Figure 7C) or Shannon (Figure 7D) indices represent bacterial diversity. In the present study, MGO supplementation significantly increased Chao1 and ACE indices (p < 0.05), but had no significant effect on Simpson and Shannon indices compared to the CON group (p > 0.05).
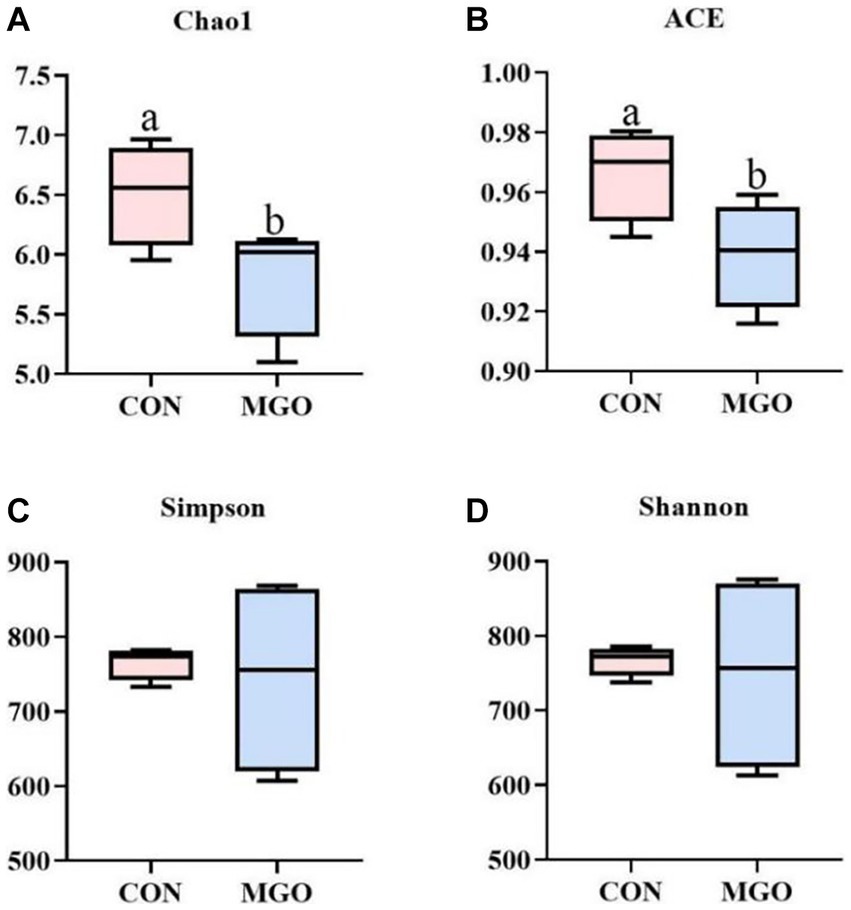
Figure 7. The alpha diversity index analysis. (A–D) Represent the cecal microbiota Chao1, ACE, Simpson, and Shannon index in alpha diversity index, respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 4).
PCA and PCoA analysis
In beta-diversity index analysis (Figure 8), the distance between samples on PCA (Figure 8A) and PCoA (Figure 8B) represents the degree of structural similarity in species composition. In the present study, PCA and PCoA indices were not significantly effect between CON and MGO groups (p > 0.05).
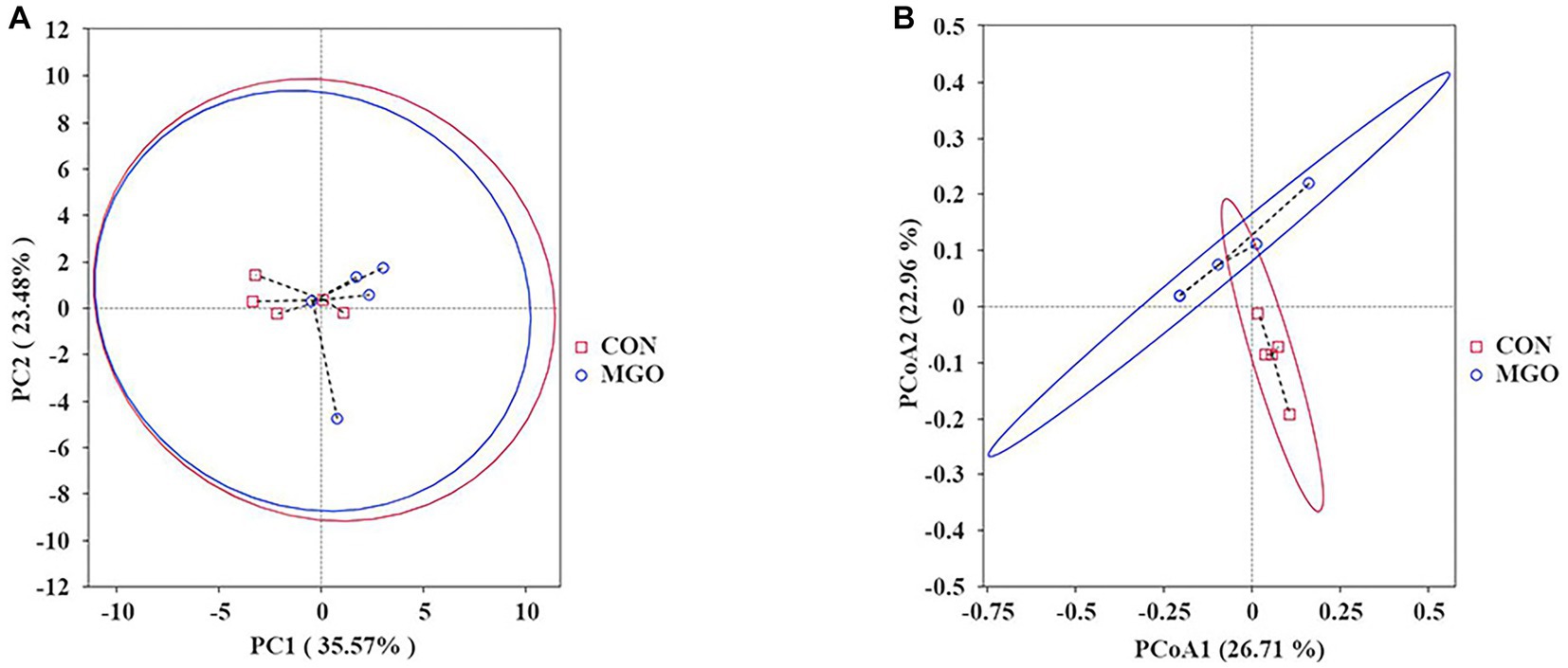
Figure 8. The beta-diversity index analysis. (A,B) Represent the cecal microbiota principal component analysis (PCA) and principal component coordinate analysis (PCOA) in beta-diversity index, respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 4).
Relative abundance of cecal microbiota
The predominant phyla in cecal samples are Bacteroidetes and Firmicutes (Figure 9). The addition of MGO to the diet significantly increased the number of Firmicutes (Figure 9B) compared to the CON group (p < 0.05). However, the Bacteroidota, Proteobacteria, Spirochaetota, unidentified_Bacteria, Actinobacteriota, Campylobacterota, Desulfobacterota, Euryarchaeota, and Halanaerobiaeota (Figure 9A) were not significantly effect between CON and MGO groups (p > 0.05).
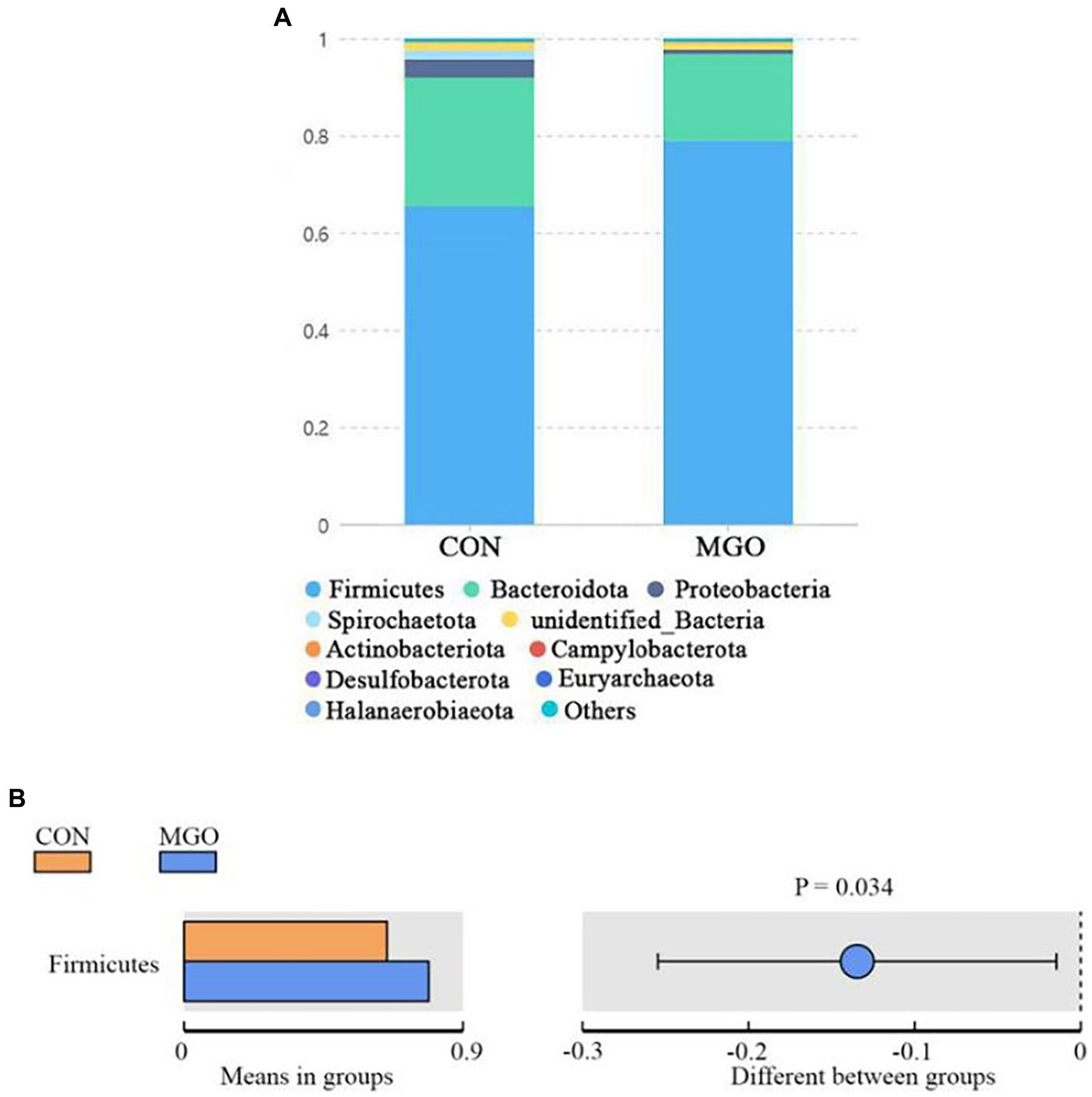
Figure 9. The relative abundance of species at phylum level. (A,B) Represent the phylum level relative abundance and T-test, respectively. CON, basal diet; MGO, basal diet supplemented with 250 mg/kg macleaya extract and glucose oxidase (n = 4).
Discussion
As the global prohibition of antibiotics, many researches paid attention to plant extracts compound additives as an alternative additive for antibiotics to improve animal growth performance. Previous study found that 120 mg/kg macleaya extract (a standardized premixture of MCE) increased weight gain and nutrient digestibility of piglets (23). Sureshkumar et al. reported that the ADG were significantly increased in piglets fed diet supplemented with 400 U/kg glucose oxidase (24). In the present study, MCE and GOD compound additives significantly increased ADG and decreased F/G, but did not change ADFI in piglets. The joint use of these supplements improved piglet growth and development, possibly due to their individual properties such as anti-inflammatory and antibacterial functions. In addition, MCE and GOD combination can improve feed utilization of piglets. However, the synergistic mechanism of the macleaya extract and glucose oxidase needs further confirmation.
It was remarkable that the activity of SOD and GSH-Px, and content of MDA content were the important markers of animal antioxidant capacity (3). MCE have been shown antioxidant effects because of their richness in sanguinarine and chelerythrine (25, 26). Wang et al. reported that 50 mg/kg MCE containing more than 3.75% sanguinarine could significantly increase serum SOD activity and decrease MDA content in piglets (11). GOD catalyzes glucose to produce gluconic acid and H2O2, which can inhibit harmful bacteria (15). Zhang et al. found that GOD (3,000 U/g) improved the T-SOD and GSH-Px activity in the jejunum of piglets (Zhang et al., 2020). We found that the serum SOD and GSH-Px acitvity of piglets were significantly increased in MGO group compared to the CON group, which was similar to previous studies. Moreover, the serum MDA content was significantly decreased. The Keap1-Nrf2 signaling pathway is vital in protecting cells from endogenous and exogenous stress (27, 28). External factors enhanced antioxidant capacity by activating the expression of related antioxidant gene (GPX1 and HO-1) mRNA expression of the Keap1-Nrf2 signaling pathway (29). GOD can significantly inhibit the expression of Keap1, and increase Nrf2 expression (17). In addition, MCE induced Nrf2 accumulation in RAW264.7 cells of rat macrophages (30). It was worth noting that the mRNA expression of Nrf2, Keap1, GPX1, and HO-1 in the jejunum of MGO group increased in accrodance with the activation of SOD and GSH-Px in the present study. The combination of MCE and GOD can improve the antioxidant capacity of piglets, while the mechanism behind this phenomenon still needs further research.
IgA, IgM, and IgG are considered as important markers of immune function, and the improvement of growth performance may be attributed to more balanced immune homeostasis (31). Li et al. found that supplementation of 500 mg/kg MCE containing 0.15% sanguinarine in the diet significantly increased the serum IgG and IgM contents of piglets (32). In addition, Liu et al. reported that dietary supplementation with MCE containing 0.15% sanguinarine significantly reduced the serum IL-1β and IL-6 contents of piglets (33). These results indicated that the immune effects of MCE may be partly attributed to the induction of immunoglobulin production and the repression of inflammatory responses. Previous study has shown that GOD affects immune function by blocking the expression of inflammatory factors and increasing the content of immunoglobulin to enhance humoral immunity (34). In the present study, the MGO group significantly increased the serum IgG level and decreased IL-1β content in piglets, which was consistent with previous studies. The combination of MCE and GOD can improve the immune function of piglets by reducing oxidative stress and inflammatory response.
Microbiota play important roles in in the regulation of intestinal nutrient metabolism, immunity and barrier function, which were considered as regulator of pig intestinal health and growth performance (22, 35). Chao1, ACE, Simpson, and Shannon indexes are important indicators of alpha diversity, which are used to evaluate microbiota community richness and diversity (36). In the present study, we found that MCE and GOD combination significantly decreased Chao1 and ACE index indicating the decrease of intestinal microbial community abundance, which may be caused by the antibacterial effect of MGO (37). Studies have shown that Bacteroides and Firmicutes are the major phyla in piglet cecum, which was consistent with the present study (38–40). In this study, it was found that piglet dietary supplementation of macleaya extract and glucose oxidase combination resulted in significant increase in the abundance of Firmicutes bacteria. Previous studies have shown that increasing the richness of Firmicutes in the cecum could increase the production of short-chain fatty acids, which can reduce the intestinal pH value and inhibit harmful bacteria growth (41–43). The combination of MCE and GOD can improve the composition of the cecal microbiota, thereby promote intestinal health in piglets.
Conclusion
This study proposed that dietary MCE and GOD supplementation can enhance the growth performance, antioxidant capacity, and immune function of piglets. This can be attributed to an increase in the abundance of beneficial microbiota and a decrease in the abundance of harmful bacteria in the intestine. However, the synergistic molecular mechanism odf MCE and GOD in piglet growth, antioxidant capacity, and immune function need to be further confirmed by in vivo and in vitro studies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA890335.
Ethics statement
Piglets used in this experiment were cared in accordance with the guidelines for the care and use of laboratory animals described by the Guide for the Care and Use of Laboratory Animals and approved by the Committee on the Ethics of Shandong Agricultural University (SDAUA-2020-0710).
Author contributions
XC and FZ: conceptualization and verification. FR, LH, XY, and NJ: data management. YJ and YL: formal analysis. WY and SJ: fund acquisition. YJ, CY, and SL: survey and resources. XC and NJ: methods. WY, NJ, and SJ: project management. FZ and YL: software. WY: supervision. NJ and SJ: visualization. XC: writing – manuscript. HL, JL, LH, XY, YL, CY, NJ, and SJ: writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Shandong Provincial Science and Technology SME Innovation Capacity Improvement Project (grant no: 2022TSGC1275) and Shandong Provincial Pig Industry Technology System (grant no: SDAIT-08-05).
Conflict of interest
YJ was employed by Zhongcheng Feed Technology Co., Ltd. SL was employed by Challenge Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Valini, GAC, Duarte, MS, Calderano, AA, Teixeira, LM, Rodrigues, GA, Fernandes, KM, et al. Dietary nucleotide supplementation as an alternative to in-feed antibiotics in weaned piglets. Animal. (2021) 15:100021. doi: 10.1016/j.animal.2020.100021
2. Johnson, JS, and Lay, DC. Evaluating the behavior, growth performance, immune parameters, and intestinal morphology of weaned piglets after simulated transport and heat stress when antibiotics are eliminated from the diet or replaced with L-glutamine. J Anim Sci. (2017) 95:91–102. doi: 10.2527/jas.2016.1070
3. Liu, L, Chen, D, Yu, B, Luo, Y, Huang, Z, Zheng, P, et al. Influences of selenium-enriched yeast on growth performance, immune function, and antioxidant capacity in weaned pigs exposure to oxidative stress. Biomed Res Int. (2021) 2021:1–11. doi: 10.1155/2021/5533210
4. Guo, S, Lei, J, Liu, L, Qu, X, Li, P, Liu, X, et al. Effects of macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poult Sci. (2021) 100:101031. doi: 10.1016/j.psj.2021.101031
5. Ling, H, Xiao, H, Zhang, Z, He, Y, and Zhang, P. Effects of Macleaya Cordata extract on performance, nutrient apparent Digestibilities, Milk composition, and plasma metabolites of dairy goats. Animals (Basel). (2023) 13:566. doi: 10.3390/ani13040566
6. Dávila-Ramírez, JL, Munguía-Acosta, LL, Morales-Coronado, JG, García-Salinas, AD, González-Ríos, H, Celaya-Michel, H, et al. Addition of a mixture of plant extracts to diets for growing-finishing pigs on growth performance, blood metabolites, carcass traits, organ weight as a percentage of live weight, quality and sensorial analysis of meat. Animals. (2020) 10:1229. doi: 10.3390/ani10071229
7. Kantas, D, Papatsiros, VG, Tassis, PD, Athanasiou, LV, and Tzika, ED. Effect of a natural feed additive (macleaya cordata), containing sanguinarine, on the performance and health status of weaning pigs. Anim Sci J. (2015) 86:92–8. doi: 10.1111/asj.12240
8. Guo, S, Liu, L, Lei, J, Qu, X, He, C, Tang, S, et al. Modulation of intestinal morphology and microbiota by dietary macleaya cordata extract supplementation in Xuefeng black-boned chicken. Animal. (2021) 15:100399. doi: 10.1016/j.animal.2021.100399
9. Chen, J, Kang, B, Zhao, Y, Yao, K, and Fu, C. Effects of natural dietary supplementation with macleaya cordata extract containing sanguinarine on growth performance and gut health of early-weaned piglets. J Anim Physiol Anim Nutr (Berl). (2018) 102:1666–74. doi: 10.1111/jpn.12976
10. Liu, G, Guan, G, Fang, J, Martínez, Y, Chen, S, Bin, P, et al. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. Biomed Res Int. (2016) 2016:1069585. doi: 10.1155/2016/1069585
11. Wang, F, Yin, Y, Yang, M, Chen, J, Fu, C, and Huang, K. Effects of combined supplementation of macleaya cordata extract and benzoic acid on the growth performance, immune responses, antioxidant capacity, intestinal morphology, and microbial composition in weaned piglets. Front Vet Sci. (2021) 8:708597. doi: 10.3389/fvets.2021.708597
12. Dang, X, Liu, Y, Chen, N, and Kim, IH. Dietary supplementation of aspergillus Niger-expressed glucose oxidase ameliorates weaning stress and improves growth performance in weaning pigs. J Anim Physiol Anim Nutr (Berl). (2022) 106:258–65. doi: 10.1111/jpn.13576
13. Wang, W, Xie, R, Cao, Q, Ye, H, Zhang, C, Dong, Z, et al. Effects of glucose oxidase on growth performance, clinical symptoms, serum parameters, and intestinal health in piglets challenged by enterotoxigenic Escherichia coli. Front Microbiol. (2022) 13:994151. doi: 10.3389/fmicb.2022.1106265
14. Wu, S, Li, T, Niu, H, Zhu, Y, Liu, Y, Duan, Y, et al. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult Sci. (2019) 98:828–41. doi: 10.3382/ps/pey393
15. Bruen, D, Delaney, C, Florea, L, and Diamond, D. Glucose sensing for diabetes monitoring: recent developments. Sensors (Basel). (2017) 17:1866. doi: 10.3390/s17081866
16. Wu, S, Chen, X, Li, T, Ren, H, Zheng, L, and Yang, X. Changes in the gut microbiota mediate the differential regulatory effects of two glucose oxidases produced by aspergillus Niger and Penicillium amagasakiense on the meat quality and growth performance of broilers. J Anim Sci Biotechnol. (2020) 11:73. doi: 10.1186/s40104-020-00480-z
17. Zhang, J, Liu, Y, Yang, Z, Yang, W, Huang, L, Xu, C, et al. Illicium verum extracts and probiotics with added glucose oxidase promote antioxidant capacity through upregulating hepatic and jejunal Nrf2/Keap1 of weaned piglets. J Anim Sci. (2020) 98:98. doi: 10.1093/jas/skaa077
18. Tang, HB, Yao, X, Gao, P, Yang, Z, and Zhang, G. Effects of glucose oxidase on the growth performance, serum parameters and faecal microflora of piglets. S Afr J Anim Sci. (2016) 46:14–20. doi: 10.4314/sajas.v46i1.2
19. Zhang, S, Wu, Z, Heng, J, Song, H, Tian, M, Chen, F, et al. Combined yeast culture and organic selenium supplementation during late gestation and lactation improve preweaning piglet performance by enhancing the antioxidant capacity and milk content in nutrient-restricted sows. Anim Nutr. (2020) 6:160–7. doi: 10.1016/j.aninu.2020.01.004
20. National Research Council of the National Academies. Nutrient requirements of swine [M].. Washington: the National Academies Press, (2012).
21. Morgan, XC, Tickle, TL, Sokol, H, Gevers, D, Devaney, KL, Ward, DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. (2012) 13:R79. doi: 10.1186/gb-2012-13-9-r79
22. Zhang, Q, Zhang, Z, Zhou, S, Jin, M, Lu, T, Cui, L, et al. Macleaya cordata extract, an antibiotic alternative, does not contribute to antibiotic resistance gene dissemination. J Hazard Mater. (2021) 412:125272. doi: 10.1016/j.jhazmat.2021.125272
23. Goodarzi Boroojeni, F, Männer, K, and Zentek, J. The impacts of macleaya cordata extract and naringin inclusion in post-weaning piglet diets on performance, nutrient digestibility and intestinal histomorphology. Arch Anim Nutr. (2018) 72:178–89. doi: 10.1080/1745039X.2018.1459342
24. Sureshkumar, S, Liu, YJ, Chen, NB, and Kim, IH. Dietary inclusion of glucose oxidase supplementation to corn-wheat-based diet enhance growth performance, nutrient digestibility, blood profile of lactating sows. J Anim Sci Technol. (2021) 63:778–89. doi: 10.5187/jast.2021.e66
25. Meng, YY, Liu, Y, Hu, ZF, Zhang, Y, Ni, J, Ma, ZG, et al. Sanguinarine attenuates lipopolysaccharide-induced inflammation and apoptosis by inhibiting the TLR4/NF-κB pathway in H9c2 cardiomyocytes. Curr Med Sci. (2018) 38:204–11. doi: 10.1007/s11596-018-1867-4
26. Peng, L, Wen, L, Shi, Q, Gao, F, Huang, B, and Wang, C. Chelerythrine ameliorates pulmonary fibrosis via activating the Nrf2/ARE signaling pathway. Cell Biochem Biophys. (2021) 79:337–47. doi: 10.1007/s12013-021-00967-0
27. Teixeira, TM, da Costa, DC, Resende, AC, Soulage, CO, Bezerra, FF, and Daleprane, JB. Activation of Nrf2-antioxidant signaling by 1,25-dihydroxycholecalciferol prevents leptin-induced oxidative stress and inflammation in human endothelial cells. J Nutr. (2017) 147:506–13. doi: 10.3945/jn.116.239475
28. Wu, CC, Huang, YS, Chen, JS, Huang, CF, Su, SL, Lu, KC, et al. Resveratrol ameliorates renal damage, increases expression of heme oxygenase-1, and has anti-complement, anti-oxidative, and anti-apoptotic effects in a murine model of membranous nephropathy. PLoS One. (2015) 10:e0125726. doi: 10.1371/journal.pone.0125726
29. Lin, X, Bai, D, Wei, Z, Zhang, Y, Huang, Y, Deng, H, et al. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS One. (2019) 21:e0216711. doi: 10.1371/journal.pone.0216711
30. Vrba, J, Orolinova, E, and Ulrichova, J. Induction of heme oxygenase-1 by macleaya cordata extract and its constituent sanguinarine in RAW264.7 cells. Fitoterapia. (2012) 83:329–35. doi: 10.1016/j.fitote.2011.11.022
31. Becker, CE, and O'Neill, LA. Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin Immunopathol. (2007) 29:239–48. doi: 10.1007/s00281-007-0081-4
32. Li, Y, Fan, M, Qiu, Q, Wang, Y, Shen, X, and Zhao, K. Nano-selenium and macleaya cordata extracts improved immune function and reduced oxidative damage of sows and IUGR piglets after heat stress of sows in late gestation. Biol Trace Elem Res. (2022) 200:5081–90. doi: 10.1007/s12011-022-03103-y
33. Liu, Y, Li, Y, Niu, J, Liu, H, Jiao, N, Huang, L, et al. Effects of dietary macleaya cordata extract containing isoquinoline alkaloids supplementation as an alternative to antibiotics in the diets on growth performance and liver health of broiler chickens. Front Vet Sci. (2022) 9:950174. doi: 10.3389/fvets.2022.1079580
34. Liu, J, Liu, G, Chen, Z, Zheng, A, Cai, H, Chang, W, et al. Effects of glucose oxidase on growth performance, immune function, and intestinal barrier of ducks infected with Escherichia coli O88. Poult Sci. (2020) 99:6549–58. doi: 10.1016/j.psj.2020.09.038
35. Camara-Lemarroy, CR, Metz, LM, and Yong, VW. Focus on the gut-brain axis: multiple sclerosis, the intestinal barrier and the microbiome. World J Gastroenterol. (2018) 24:4217–23. doi: 10.3748/wjg.v24.i37.4217
36. Chen, J, Li, F, Yang, W, Jiang, S, and Li, Y. Supplementation with exogenous catalase from Penicillium notatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol Spectr. (2021) 9:e0065421. doi: 10.1128/Spectrum.00654-21
37. Zhang, J, Li, W, Ying, Z, Zhao, D, Yi, G, Li, H, et al. Soybean protein-derived peptide nutriment increases negative nitrogen balance in burn injury-induced inflammatory stress response in aged rats through the modulation of white blood cells and immune factors. Food Nutr Res. (2020) 64:64. doi: 10.29219/fnr.v64.3677
38. Beaumont, M, Cauquil, L, Bertide, A, Ahn, I, Barilly, C, Gil, L, et al. Gut microbiota-derived metabolite signature in suckling and weaned piglets. J Proteome Res. (2021) 20:982–94. doi: 10.1021/acs.jproteome.0c00745
39. Gresse, R, Chaucheyras-Durand, F, Fleury, MA, van de Wiele, T, Forano, E, and Blanquet-Diot, S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
40. Yang, Y, Liu, Y, Liu, J, Wang, H, Guo, Y, Du, M, et al. Composition of the fecal microbiota of piglets at various growth stages. Front Vet Sci. (2021) 8:661–71. doi: 10.3389/fvets.2021.661671
41. Qi, M, Cao, Z, Shang, P, Zhang, H, Hussain, R, Mehmood, K, et al. Comparative analysis of fecal microbiota composition diversity in Tibetan piglets suffering from diarrheagenic Escherichia coli (DEC). Microb Pathog. (2021) 158:105106–6. doi: 10.1016/j.micpath.2021.105106
42. Yang, A, Wang, K, Peng, X, Lv, F, Wang, Y, Cui, Y, et al. Effects of different sources of calcium in the diet on growth performance, blood metabolic parameters, and intestinal bacterial community and function of weaned piglets. Front Nutr. (2022) 9:885497. doi: 10.3389/fnut.2022.885497
Keywords: macleaya extract, glucose oxidase, growth performance, cecal microbiota, piglets
Citation: Chen X, Zhang F, Li H, Liu J, Jiang Y, Ren F, Huang L, Yuan X, Li Y, Yang W, Yang C, Li S, Jiao N and Jiang S (2023) The combination of macleaya extract and glucose oxidase improves the growth performance, antioxidant capacity, immune function and cecal microbiota of piglets. Front. Vet. Sci. 10:1173494. doi: 10.3389/fvets.2023.1173494
Edited by:
Jiashun Chen, Hunan Agricultural University, ChinaReviewed by:
Shahid Ali Rajput, Muhammad Nawaz Shareef University of Agriculture, PakistanIn Ho Kim, Dankook University, Republic of Korea
Copyright © 2023 Chen, Zhang, Li, Liu, Jiang, Ren, Huang, Yuan, Li, Yang, Yang, Li, Jiao and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Jiao, amlhb25pbmdAc2RhdS5lZHUuY24=; Shuzhen Jiang, c2h1emhlbjMwNUAxNjMuY29t
 Xing Chen
Xing Chen Fan Zhang1
Fan Zhang1 Libo Huang
Libo Huang Yang Li
Yang Li Weiren Yang
Weiren Yang Ning Jiao
Ning Jiao Shuzhen Jiang
Shuzhen Jiang