- Department of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia
Arsenic (As), lead (Pb), cadmium (Cd), and mercury (Hg) have been recognized as most toxic heavy metals that are continuously released into the environment, both from natural sources and from anthropogenic production of fertilizers, industrial activities, and waste disposal. Therefore, As, Cd, Hg, and Pb are found in increasing concentrations in bodies of water, fodder, feed, and in the tissues of livestock, including poultry, in the surroundings of industrial areas, leading to metabolic, structural, and functional abnormalities in various organs in all animals. In poultry, bioaccumulation of As, Pb, Cd, and Hg occurs in many organs (mainly in the kidneys, liver, reproductive organs, and lungs) as a result of continuous exposure to heavy metals. Consumption of Cd lowers the efficiency of feed conversion, egg production, and growth in poultry. Chronic exposure to As, Pb, Cd, and Hg at low doses can change the microscopic structure of tissues (mainly in the brain, liver, kidneys, and reproductive organs) as a result of the increased content of these heavy metals in these tissues. Histopathological changes occurring in the kidneys, liver, and reproductive organs are reflected in their negative impact on enzyme activity and serum biochemical parameters. Metal toxicity is determined by route of exposure, length of exposure, and absorbed dosage, whether chronic and acute. This review presents a discussion of bioaccumulation of As, Cd, Pb, and Hg in poultry and the associated histopathological changes and toxic concentrations in different tissues.
Introduction
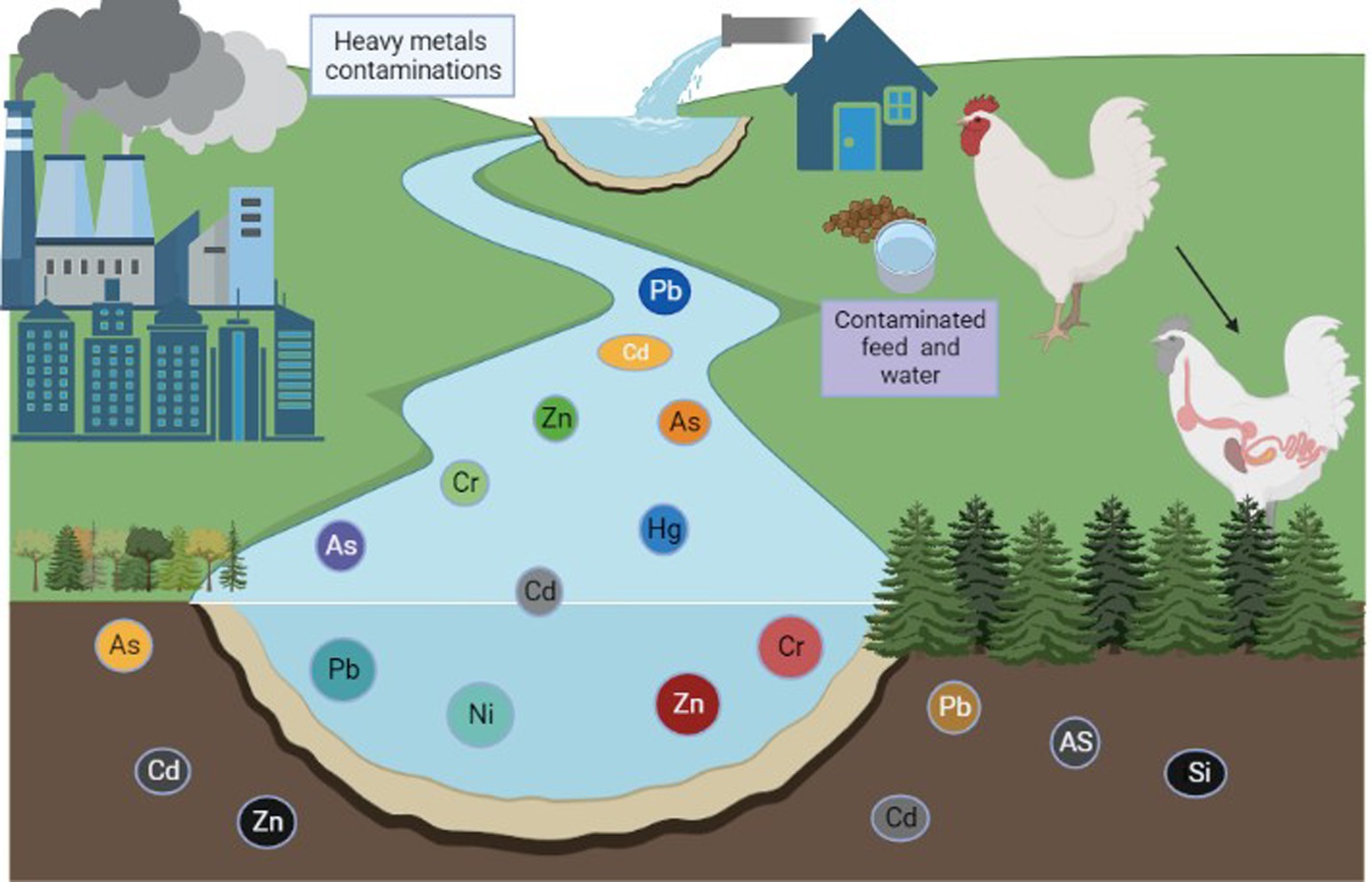
Heavy metals are members of the class of metalloids and metals with an atomic density greater than or equal to 4,000 kg/m3 (1). Animals can absorb environmental elements and metals from the air, water, sediment, and food (2, 3). Heavy metals are among the main contaminants of our food supply, and heavy metal contamination is a serious issue for our ecosystem (4). Heavy metal contamination is pervasive throughout the world, especially in areas close to urban regions and industrial zones (5). Zinc (Zn), iron (Fe), copper (Cu), and selenium (Se) are essential metals that have specific functions in regulating body metabolism (6, 7). In contrast, toxic elements such as lead (Pb), chromium (Cr), mercury (Hg), nickel (Ni), and cadmium (Cd) are typically associated with contamination and can have hazardous effects on living organisms when specific concentrations are exceeded (8, 9). Nonessential elements have no known specific function in the body but are also not assumed to be toxic to any significant degree (9). Trace amounts of some heavy metals, such as Cd, Pb, As, Cr, Hg, and Ni, can be found in water, poultry, fish, and birds (4, 10). Prolonged exposure to these heavy metals, even at low doses, can have severe negative effects on both animal and human health (11), and the buildup of heavy metals in the environment and biosphere is considered to be a biohazard (12, 13). Metal pollutants are already present in the atmosphere, but may become more prevalent as a result of pollution and industrial activity (Figure 1) (14). In particular, expanding patterns of anthropogenic activity (including industrialization, mining, the use of chemical fertilizers and pesticides, unrestricted sewage discharge, and extensive groundwater irrigation) have accelerated the spread of heavy metals (15, 16). A wide range of factors contribute to the presence of toxic metals in agricultural soils, including air deposition, sewage irrigation, agrochemicals, and animal and bird manure (17–19). Agricultural soil contains heavy metals that have a prolonged residence time (often many decades) and sustained bioavailability (Figure 2) (20) due to the toxicity of heavy metals at low levels of exposure. Many of these toxic metals can pose serious ecological threats to animals (21, 22), even threatening the health of poultry and animals through food chain transmission and accumulation (23).
After air deposition, the application of poultry and animal manure is the main source of the majority of heavy metals found in agricultural soil (24–26). The use of poultry and livestock manure in certain ways has contributed to the accumulation of several heavy metals (including Cd and Hg) in cultivated fields over the past decade (27–29).
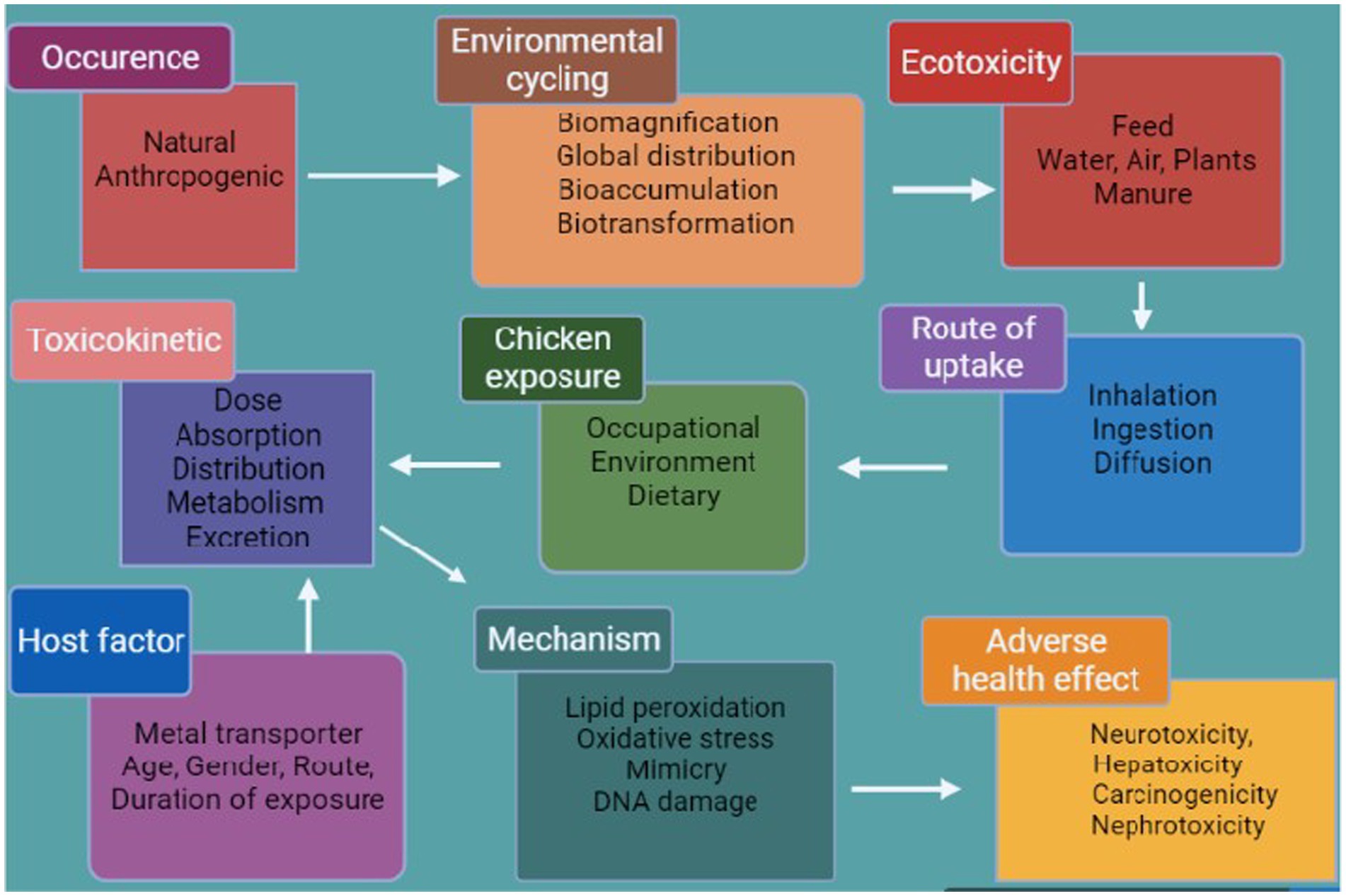
Pb and Cd are the most poisonous of the most common heavy metals to accumulate in the food chain. Following absorption, these are predominantly dispersed across several tissues, mainly the kidneys and liver (30, 31). The accumulation of a high level of heavy metals triggers a variety of deadly symptoms, such as reproductive issues and hepato–renal dysfunction (32). Pb is a neurotoxin that can impair metabolism and exert negative effects on the neurological, gastrointestinal, and renal systems, as well as hemopoiesis and renal function (33). Pb exposure can block heme synthesis and harm the brain and kidney systems (Figure 3) (34). Diet is a source of Cd contamination; this arises from a variety of food sources and from the environment and is passed to animals through the food chain (35), causing hypertension, kidney dysfunction, and damage to the lungs and liver as well as pulmonary and hepatocellular tissue (36).
The liver and kidneys are crucial for detoxification and the excretion of hazardous substances in both humans and animals (37). Organs sustain the most harm when there is an overabundance of poisonous substances in feed (38), and this depends on the type of feed consumed. In terms of specific metals, As is stored in animal tissues and can cause nausea, headache, and severe gut irritation (39, 40). Like other metals, Cu impairs liver, kidney, and brain functions at high doses and can cause hemolytic crisis (41).
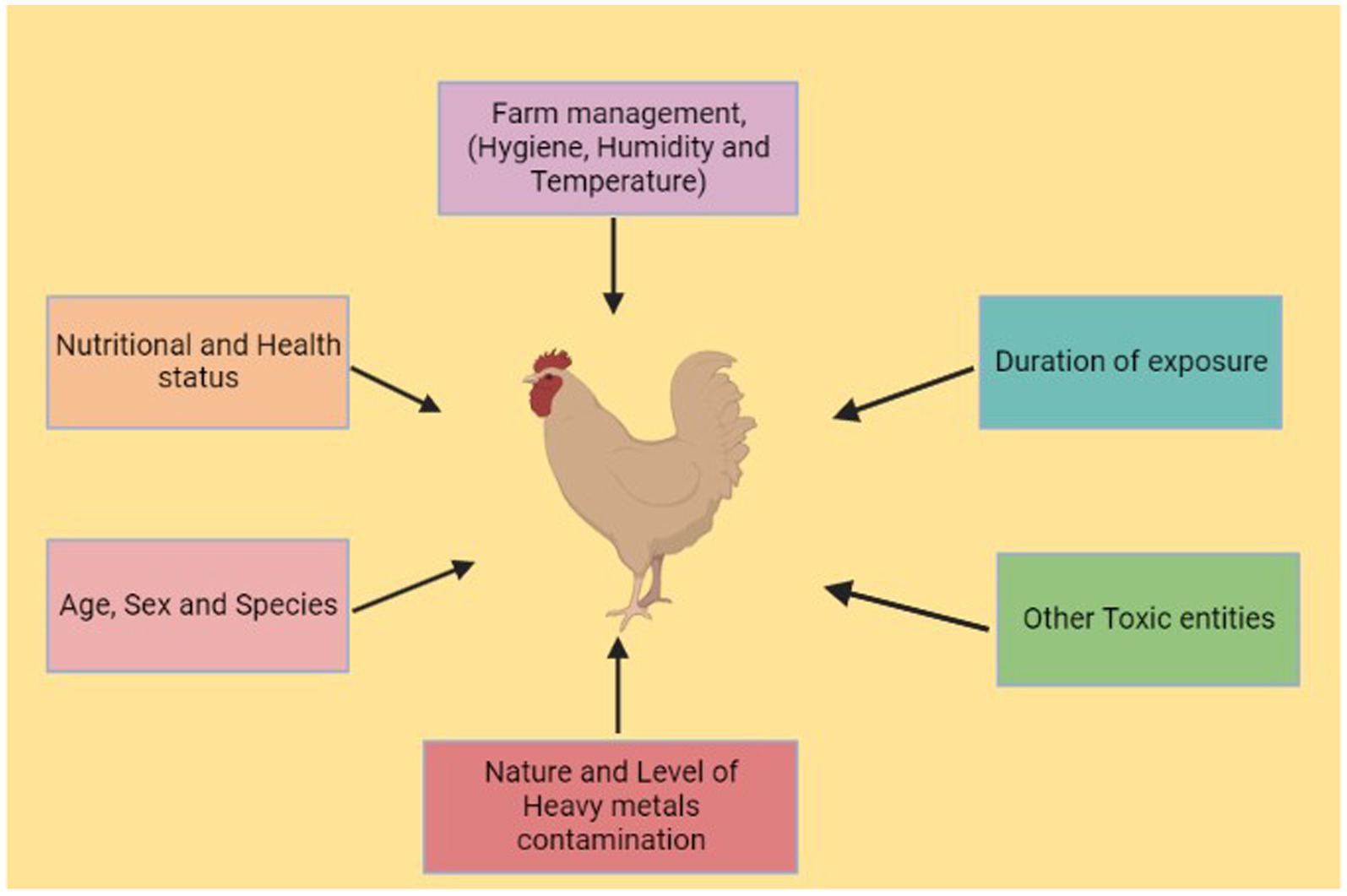
Poultry farming is one of the most important food-producing industries in the world (42), and poultry is the main source of protein for millions of people across the world (43). In 2019, worldwide egg production reached 83 Mt., a 63% increase since 2000, and poultry meat represented approximately 40% of worldwide meat production, highlighting its significance as the most widely produced meat globally (44). Numerous toxic metals are found as trace components and used as feed additives in poultry feed (45). The hazardous effects of heavy metals on poultry include loss of weight, organ failure, and death (46, 47). Metal toxicity is determined by route of exposure, length of exposure, and absorbed dosage, whether chronic or acute. The aim of this review is to present a comprehensive account of the mechanisms of heavy metal toxicity, its effects, and the histopathological changes that occur in different tissues in poultry under exposure to heavy metals.
Sources of heavy metal transmission and their impact on poultry
Sources of cadmium transmission
Cd is a significant environmental contaminant that is continuously released into the environment from industrial and natural sources (48, 49). Along with numerous other pollutants, Cd is a contaminant of the atmosphere with two types of sources, anthropogenic and natural. The contribution of anthropogenic sources is three to 10 times more dangerous than that of natural sources (5, 50). The main natural sources include forest fires, movement via wind-borne soil, and volcanic eruptions (51, 52). The smelting of Cu and Ni, the burning of fossil fuels, the production of phosphate fertilizers from rocks containing different levels of Cd, and the usage of sewage sludge in soil are all examples of anthropogenic sources. Cd is dispersed into soil and water, where it accumulates in biogenic species through food chains and presents a threat to poultry health. Cd can enter the bodies of poultry animals to a small extent via food and drinking water (53).
Effects of cadmium on poultry
Cd is transported to target tissues, where it accumulates, after binding to metallothionine in the bloodstream (54). Cd has teratogenic consequences in various animals, including chickens, such as appendage deformities, ear abnormalities, and gastrointestinal problems (55, 56). Additionally, non-hypertrophic emphysema, osteoporosis, persistent rhinitis, anemia, and eosinophilia can all result from Cd exposure (57, 58). When the amount of Cd in the blood exceeds the metallothionine ability to bind it, free Cd triggers the production of free radicals and lipid peroxidases, which harm the liver and kidneys (59). Ingestion of Cd at a high rate results in a reduction in egg production by poultry as a result of histopathological damage, reducing feed intake and increasing sensitivity to stress (60, 61). Furthermore, absorption of Cd in the digestive tract increases deficiencies of minerals such as Fe and Ca normally obtained via the diet (62). In addition to increasing bioaccumulation in tissues, exposure to Cd in poultry also transfers Cd to eggs. Cd exposure may lower the protein concentration needed for absorption and transport, and thus decreases excretory activity in the oviduct in poultry (56).
Sources of lead transmission
Animals are routinely exposed to Pb, which is one of the greatest environmental poisons in industrialized areas of the world (63). Pb is a naturally occurring element in the inner layer of the earth’s crust; it enters the environment in various ways, including the burning of gasoline (the primary source of Pb exposure), plant fuel, drinking water, recycled material, dust, cosmetics, and lead-based paints (64, 65). Pb poisoning, which is particularly prevalent in animals, can be brought on by a variety of environmental variables, including industrial pollutants, agricultural practices, use of automobiles, and contaminated feed and soil (66, 67). Pb ingested orally is only slightly absorbed by the animals; however, after constant exposure at a low level, due to the relatively slow rate of Pb removal, a hazardous level of Pb can accumulate in tissues (68). When Pb comes into contact with air, food, and drink, it has an impact on all biological systems, including that of poultry (69).
Effects of lead on poultry
Pb has the capacity to cause oxidative stress and serves as a catalyst for oxidative processes of biological molecules by generating free radicals (70). Depending on the degree of exposure, the negative consequences of Pb can range from minor physiological or biochemical abnormalities to significant pathologic illnesses, in which various organs and systems may be harmed or their functions altered (71). Pb acetate in subclinical amounts reduces the sensitivity of chickens to endotoxins. Pb has the potential to deactivate antibodies, thus impairing the resistance of poultry to infectious illness (72). Pb poisoning also reduces lysosome activity and is involved in phagocytic activity of polymorphonuclear leukocytes (73). Finally, Pb obstructs the actions of many antioxidant defenses; low antioxidant levels may damage various organ systems, including the nervous system, the liver, the kidneys, and the reproductive system (74). In severe cases, Pb toxicity has also been shown to cause death in poultry (75).
Sources of arsenic transmission
As is a chemical found in the environment that has a significant impact on the health of animals, including poultry (76). As can be found in trivalent, pentavalent, organic, and inorganic forms and can combine with variety of elements, such as S, H, O, Pb, and Cu (31, 77). Similar to animal exposure more generally, poultry in As-affected areas are exposed to dangerous level of the toxic metal (78). As is a source of toxicity and is typically present in fluids used to spray animals to control ectoparasites (3, 79). Feed ingredients, contaminated drinking water, vegetables, grasses, plants, and atmospheric emissions are sources of As contamination (80), with the first four mentioned being the main sources of As (81).
Effects of arsenic On poultry
The role of arsenic in poultry nutrition is heavily disputed; it is highly hazardous even in very low quantities in food (82). In poultry, acute As poisoning causes circulatory collapse, stomach pain, excessive salivation, hypothermia, watery diarrhea, and death (83, 84). Symptoms of long-term exposure to As at low concentrations in poultry include chronic indigestion, stomach cramps, and skin discoloration (85, 86). Long-term consequences can include gangrene-like sores, carcinoma of the skin, liver, kidneys, and lungs, and cancer (87, 88). The liver is typically thought to be the primary organ involved in the metabolism of As (89). As can block the action of intracellular enzymes and may impact acetyl-CoA synthesis, glutathione (GSH) synthesis, fatty acid oxidation, glucose uptake, and gluconeogenesis (90). One of the most frequently recognized explanations for As-induced toxicity is oxidative stress: oxidative stress brought on by As-induced liver damage results in the production of reactive oxygen species (ROS) (91). Despite the fact that As cannot directly cause DNA damage, it still has an impact on the enzymes involved in DNA repair and the energy pathway of cells. Finally, As causes oxidative damage in the skeletal muscles, liver, and kidneys in chickens (92).
Sources of mercury transmission
Hg is one of the most potent neurotoxins, and it has a range of negative health effects on both humans and animals (93). Hg is considered to be a significant environmental pollutant, along with other non-essential trace metals, because of its high toxicity and capacity for biomagnification and bioaccumulation (94). Methyl mercury is known to be the most dangerous form, but Hg (II) is more frequently and abundantly present in the environment and has the potential to exert extremely negative effects on poultry (95). Hg can exist in environment in the form of metal divalent, monovalent, dimethyl mercury, and methyl mercury. Inorganic mercury salts and organic mercury compounds make up the majority of the mercury found in water, soil, sediments, plants, and animals (96, 97). The main sources of Hg include the paper industry, chemical industry, paint industry, insecticides, and fungicides, as well as geothermal steam used to generate electricity (98). Hg was originally utilized in medicine, but this therapeutic use was halted due to its severe toxic effects in both people and animals (99).
Effects of mercury on poultry
Hg is recognized as a toxic chemical that can cause devastating effects in poultry, such as kidney and liver damage, even at a very low level of exposure (100). Toxic concentrations of Hg are dangerous for poultry, with symptoms including development of anemia and depressed growth rate. Young growing chickens are typically more susceptible to the toxic effects of chronic Hg exposure than adults (101, 102). The production of oxidative stress, suppression of nitric oxide, and the disruption of cytokine profiles are the main mechanisms of Hg-induced toxicity in immune cells (103). To assess the effects of Hg exposure on the immune system in poultry, activated immunity should be considered, as this is more important in vulnerability to diseases. Hg exposure can damage tissues and organs, and it is absorbed and distributed in the liver and kidneys in poultry (104, 105).
Worldwide reports on heavy metal toxicity in poultry
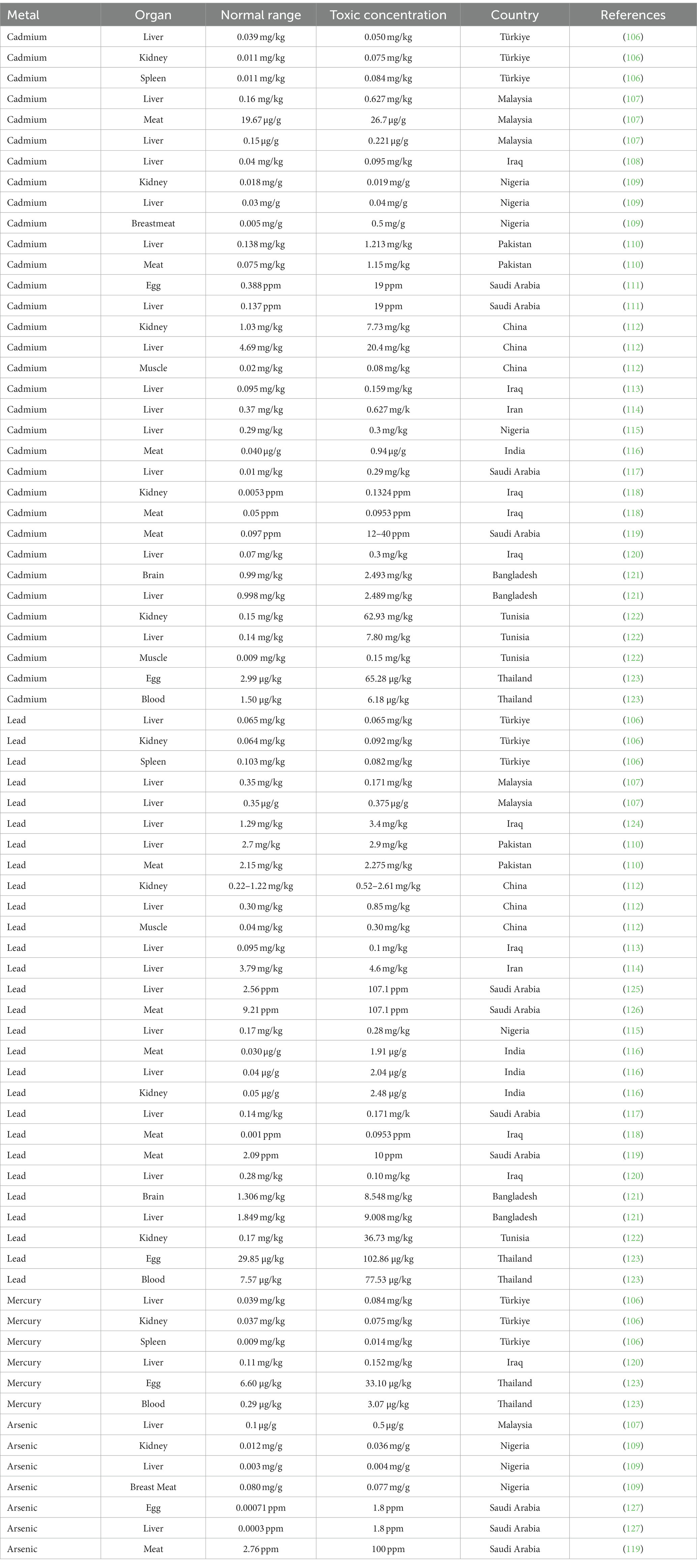
Metal toxicity has been observed in many living organisms, but our main focus here is on poultry. It has been found that metal toxicity is highly prevalent in poultry worldwide, as outlined in Table 1. Various heavy metals have been examined in different studies, among which one study has measured the concentrations of Pb, Cd, Ni, Hg, Fe, Zn, Mg, and Cu in the kidneys, spleen, and liver of poultry from Manisa, Turkiye. Concentrations of heavy metals can be determined using atomic absorption spectrophotometry (106, 128). The highest concentration of Cu was observed in the liver, at 3.7 mg/kg, and the lowest level in the spleen, at 1.99 mg/kg (129). For Pb, the highest concentration was observed in the kidney, at 0.103 mg/kg, and the lowest level in the liver of chickens, at 0.065 mg/kg. The concentrations of Pb and Hg in the liver in chicken were found to be 0.102 and 0.053 mg/kg, respectively (106).
In another study, Cd, Zn, and Pb concentrations in poultry were measured in a mining area of China. In chickens, a low Pb concentration of 0.52 mg/kg was observed in the muscles (130) and a high Pb concentration of 0.63–0.73 mg/kg in the liver. Pb has been responsible for acute poisoning in poultry and has adverse effects on poultry health (131). Descending levels of concentration of Cd in chicken were observed in the liver, kidney, and muscles. In a separate study, a low Cd concentration of 4.64 mg/kg was observed in the kidneys and a high Cd concentration of 9.36 mg/kg was observed in the liver in poultry (112). A kidney: liver Cd ratio greater than 1 is an indicator of acute poisoning, whereas a ratio less than 1 indicates a lower level of poisoning (132). The highest concentrations of Zn and Cd were observed in kidneys and liver in poultry, which are known to be specific target organs for bioaccumulation of toxic metals (112).
The concentrations of several heavy metals (Zn, Cd, and Pb) were assessed in the liver, kidney, heart, and meat of chickens acquired from Kohat market, Pakistan, using a PerkinElmer PinAAcle™ 900 T atomic absorption spectrophotometer (110, 133). Concentrations of Cd in the range of 0.075 ± 0.010 to 15.763 ± 0.012 mg/kg were observed in the kidneys and liver of chickens, while concentrations of Pb in the range of 1.85 ± 0.007 to 11.838 ± 0.005 mg/kg were observed in kidneys and liver (110). It was discovered that chicken meat contained the lowest levels of concentration of these metals, while the kidneys and liver contained the most significant quantities.
In another study, heavy metals Pb, As, and Cd were measured in the liver, kidneys, and breast meat of chicken in Nigeria. An As concentration of 0.0802 ± 0.021 mg/g was observed in the breast meat and 0.0037 ± 0.018 mg/g in the liver. Cd concentrations of 0.019 ± 0.001 mg/g and 0.003 ± 0.001 mg/g were observed in the kidneys and liver, respectively (109). These results indicated that the concentration of As was higher in the breast meat and lower in the liver. The concentration of Cd was higher in the kidneys and lower in the breast meat of chickens, and Pb was not detected in samples of chicken (109).
The concentrations of heavy metals such as Cd and Pb were assessed in the liver, kidneys, and meat of chickens from an industrial area of India. The highest levels of Cd and Pb in tissues and muscles have been determined in the kidneys. Cd concentrations of 2.02 μg/g and 1.86 μg/g were observed in the kidneys and liver, respectively, in poultry (116). The findings showed that chickens in areas with toxic metal exposure may exhibit pathological lesions in various tissues as a result of heavy metal accumulation (116). As a result, eating chicken meat from the commercially exposed area may present a potential health risk.
An additional study was conducted to determine concentrations of the heavy metals Pb, Ni, and Cd in the brain and liver of poultry in Dhaka, Bangladesh, using atomic absorption spectrometry. Zn concentrations of 68.267 mg/kg and 53.778 mg/kg were observed in the liver and brain, respectively, in broiler chickens; concentrations of 348.52 mg/kg and 619.648 mg/kg were observed in liver and brain, respectively, in domestic chickens. Pb concentrations of 2.397 mg/kg in the liver and 4.141 mg/kg in the brain were observed in broiler chickens; 5.190 mg/kg in the liver and 9.008 mg/kg in the brain were observed in domestic chickens. Finally, Cd concentrations of 2.48 mg/kg in the liver and 2.493 mg/kg in the brain were observed in broiler chickens; 2.498 mg/kg in the liver and 0.999 mg/kg in the brain were observed in domestic chickens (121). These concentrations of heavy metals observed in poultry exceeded the recommended values of the WHO/FAO. A high Zn concentration of 619.648 mg/kg was observed in the brain and a low Zn concentration of 32.430 mg/kg in the liver in poultry (134).
In another study, chicken liver samples were obtained from markets in Erbil, Iraq, and inductively coupled plasma optical emission spectrometry was used to determine the presence of heavy metals such as Pb, Hg, Cd, and Ni (120). A low Ni concentration of 0.15 mg/kg was observed, in contrast to the findings of an earlier. In a separate study in Diyala, Iraq, in which a high concentration of 0.414 mg/kg of Ni was found in poultry (117). A lower Zn concentration of 20.72 mg/kg in chicken liver samples has been reported in Saudi Arabia (135), and a higher concentration of 100.87 mg/kg was observed in Turkiye. In previous studies, Cd concentrations of 0.159 mg/kg, 0.29 mg/kg, and 0.37 mg/kg had been observed in the liver in chickens in Iraq (107), Nigeria (115), and Iran (114), respectively. A Pb concentration of 0.28 mg/kg was observed in chicken liver samples, which is more than twice the limit of 0.1 mg/kg permitted by the Codex Alimentarius Commission. According to various studies, a low Pb concentration of 0.14 mg/kg has been observed in Saudi Arabia (117) and a higher Pb concentration of 0.171 mg/kg has been observed in Nigeria (115). In 40% of the samples, an Hg concentration of 0.11 0.08 mg/kg was found, which is above the FAO/WHO acceptable limit; this figure is three times greater than reported in previous Nigerian research (115).
Histopathological changes in the kidneys in poultry
The kidneys, which are responsible for excreting poisonous substances, are the organs second-most severely impacted by Cd poisoning (136). When poultry are given Cd at a concentration of 50 mg/l in the drinking water, their kidneys have been found to develop congestion, with or without pinpoint hemorrhage (56). With administration of Cd to poultry at the same level in the drinking water, microscopic examinations of kidney tissues have revealed congested renal parenchyma, degeneration and desquamation of the tubule lining epithelium, hyaline masses, interstitial nephrosis, mononuclear cell infiltration, necrosis in the renal tubules, hypercellularity of glomeruli, and intracytoplasmic hyaline cast in the lumen (137). Cd-induced toxicity in the kidneys also causes changes in cell adhesion, autophagic responses, and cellular signaling cascades (54).
Additionally, histopathological changes in the kidney indicate necrotic lesions and eosinophilic intranuclear inclusion in epithelium cells of the renal tubules (138). Histopathological changes attributable to Hg accumulation in the kidneys have been found to include enlarged renal tubules, tubular hyalinization, fibrosis, fold increase in nucleosome content, increased levels of malondialdehyde (MDA), and decreased levels of intracellular glutathione (GSH) in the kidneys (139).
Finally, histopathological changes in the kidneys attributable to exposure to As include tubular fibrosis, enlargement of the renal tubules (140), severe hyalinization, increased renal MDA level, decreased renal SOD activity, decreased renal GSH-Px activity, and decreased CAT and GR activity (141).
Histopathological changes in the liver in poultry
Cd is initially supplied to the liver through portal blood circulation, which is primarily associated with albumin, after which it is taken up by hepatocytes from the sinusoidal capillaries of the liver (63, 142). A higher Cd dosage results in increased hepatic cell size, hepatic cell destruction and necrosis, and significant infiltration of macrophages in the liver (143). Lower doses do not cause any notable alterations in poultry (144). Daily administration of Cd at 50 mg/l induces degenerative changes in the lymphocytes, macrophages, plasma cells, and hepatocytes, as well as producing swollen, fragile increases in the sinusoidal spaces and focal necrotic spots in livers. Cd causes primary hepatocellular injury, and thus ischemia is induced due to endothelial cell damage (145). Acute Cd exposure results in secondary liver injury due to the stimulation of Kupffer cells, eliciting a series of inflammatory events involving various types of liver cells and several inflammatory and cytotoxic mediators (146).
Absorbed Pb is accumulated in the liver, and Pb exposure may lead to histological abnormalities in the liver in poultry (147). After exposure to large doses of Pb, the livers of these animals have been found to exhibit abnormalities such as irregularity and dilatation of blood sinusoids, hepatic lipid vacuolization, vacuolization of other cells, hyalinization of the hepatocellular cytoplasm, hepatocyte necrosis, and severe sinusoid congestion (148). Additionally, Pb accumulation in the liver causes pinpoint hemorrhages and small necrotic foci (149).
In terms of histopathological changes in the liver after exposure to Hg in poultry, the sinusoids and central veins are dilated, hepatic cells show hypertrophy, and karyolytic and pycnotic cells are not prominent (150, 151). Finally, histopathological changes in the liver attributable to As accumulation include decreased GSH levels, increased hepatic MDA levels, decreased hepatic SOD activity, and decreased activity of CAT, GR, and GSH-Px (152).
Histopathological changes in brain tissues in poultry
Histopathological changes in the brain after As poisoning in poultry include vacuolization and severe bleeding, which ultimately causes neuronal cell damage (153), lesions in the brain, mitochondrial swelling, and infiltration into glial cells (154).
Histopathological changes in the reproductive system in poultry
The blood–testis barrier, certain seminiferous tubules, and the basement membrane have been found to undergo damage in poultry (155). As a result of Pb deposition, spermatogenic cells have been found to be organized erratically and to produce more spermatogonium, and the spermatogenic tubes are distorted (156).
Conclusion
It can be concluded that worldwide heavy metal toxicity in poultry ranges from 2.1 to 3.4%. Chronic exposure to the heavy metals discussed here (i.e., As, Cd, Pb, and Hg) leads to their accumulation in various organs of the body; however, Cd accumulates at the highest concentrations, followed by As, Pb, and Hg in decreasing order. Various organs in poultry are affected by these heavy metals, with the sequence of impact beginning with the liver and continuing down to the kidneys, brain, and reproductive system. Overproduction of these heavy metals leads to oxidative stress in poultry. As a result of the accumulation of heavy metals, both gross and histopathological changes occur, leading to poor growth and production of multiple organs in poultry.
Author contributions
AA worked on the development of this unique title of review, planned, designed, structured, wrote and reviewed the article.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University, Saudi Arabia for funding the publication of this project.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. El-Hamaky, AM, Hassan, AA, Wahba, AK, and El, MM. Influence of copper and zinc nanoparticles on genotyping characterizations of multiDrug resistance genes for some calf pathogens. Int J Vet Sci. (2023) 12:309–17. doi: 10.47278/journal.ijvs/2022.195
2. Muñoz-Olivas, R, and Cámara, C. Speciation related to human. In L Ebdon, L Pitts, R Cornelis, H Crews, O F X Donard, and Philippe Quevauviller Trace element speciation for environment, food and health Royal Society of Chemistry (2001) 331.
3. Sharaf, R, Khan, A, Khan, MZ, Hussain, I, Abbas, RZ, Gul, S, et al. Arsenic induced toxicity in broiler chicks and its amelioration with ascorbic acid: clinical, hematological and pathological study. Pak Vet J. (2013) 33:277–81.
4. Mehar, S, Anam, I, Masood, Z, Alvi, S, Khan, W, Kabir, M, et al. Bioaccumulation of heavy metals in the different tissues of mackerel scad, Decapterus macarellus (Cuvier, 1833) collected from Karachi and Gwadar coasts of Pakistan. Saudi J Biol Sci. (2023) 30:103540. doi: 10.1016/j.sjbs.2022.103540
5. Zhang, Q, Shi, B, Su, G, Zhao, X, Meng, J, Sun, B, et al. Application of a hybrid GEM-CMB model for source apportionment of PAHs in soil of complex industrial zone. J Hazard Mater. (2023) 445:130565. doi: 10.1016/j.jhazmat.2022.130565
6. Valkova, E, Atanasov, V, Vlaykova, T, Tacheva, T, Zhelyazkova, Y, Dimov, D, et al. The serum levels of the heavy metals Cu, Zn, Cd, and Pb and progression of COPD—A preliminary study. Int J Environ Res Public Health. (2023) 20:1427. doi: 10.3390/ijerph20021427
7. Wu, L, Cui, F, Zhang, S, Ding, X, Gao, W, Chen, L, et al. Associations between multiple heavy metals exposure and neural damage biomarkers in welders: a cross-sectional study. Sci Total Environ. (2023) 869:161812. doi: 10.1016/j.scitotenv.2023.161812
8. Mohajane, C, and Manjoro, M. Sediment-associated heavy metal contamination and potential ecological risk along an urban river in South Africa. Heliyon. (2022):e12499. doi: 10.1016/j.heliyon.2022.e12499
9. Kumar, N, Chandan, NK, Bhushan, S, Singh, DK, and Kumar, S. Health risk assessment and metal contamination in fish, water and soil sediments in the East Kolkata wetlands, India, Ramsar site. Sci Rep. (2023) 13:1546. doi: 10.1038/s41598-023-28801-y
10. Lysenko, Y, Koshchayev, A, Luneva, A, Omarov, R, and Shlykov, S. Organic meat production of broiler chickens Hubbard redbro cross. Int J Vet Sci. (2021) 10:25–30. doi: 10.47278/journal.ijvs/2020.021
11. Saedi, S, Watson, SE, Young, JL, Tan, Y, Wintergerst, KA, and Cai, L. Does maternal low-dose cadmium exposure increase the risk of offspring to develop metabolic syndrome and/or type 2 diabetes? Life Sci. (2023) 315:121385
12. Nagajyoti, PC, Lee, KD, and Sreekanth, T. Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett. (2010) 8:199–216. doi: 10.1007/s10311-010-0297-8
13. Rong, S, Wu, J, Cao, X, and Sun, Y. Comprehensive ecological risk assessment of heavy metals based on species sensitivity distribution in aquatic of coastal areas in Hong Kong. Int J Environ Res Public Health. (2022) 19:13376. doi: 10.3390/ijerph192013376
14. Wang, L, Cui, X, Cheng, H, Chen, F, Wang, J, Zhao, X, et al. A review of soil cadmium contamination in China including a health risk assessment. Environ Sci Pollut Res. (2015) 22:16441–52. doi: 10.1007/s11356-015-5273-1
15. Zahoor, A, Hai-Hua, L, Jie, C, Sohail, H, Noor, I, and Niaz, M. Causes and Effect by Heavy Metals in Urban and Agriculture Soil. J. Pollut. (University of Tehran) (2019) 2:116.
16. Azizullah, A, Taimur, N, Khan, S, and Häder, D-P. Heavy metals pollution in surface waters of Pakistan. Ecosystems. (2021):271–312. doi: 10.1007/978-3-030-75602-4_13
17. Luo, L, Ma, Y, Zhang, S, Wei, D, and Zhu, Y-G. An inventory of trace element inputs to agricultural soils in China. J Environ Manag. (2009) 90:2524–30. doi: 10.1016/j.jenvman.2009.01.011
18. Shi, T, Ma, J, Wu, X, Ju, T, Lin, X, Zhang, Y, et al. Inventories of heavy metal inputs and outputs to and from agricultural soils: a review. Ecotoxicol Environ Saf. (2018) 164:118–24. doi: 10.1016/j.ecoenv.2018.08.016
19. Peng, H, Chen, Y, Weng, L, Ma, J, Ma, Y, Li, Y, et al. Comparisons of heavy metal input inventory in agricultural soils in north and South China: a review. Sci Total Environ. (2019) 660:776–86. doi: 10.1016/j.scitotenv.2019.01.066
20. Ding, F, He, Z, Liu, S, Zhang, S, Zhao, F, Li, Q, et al. Heavy metals in composts of China: historical changes, regional variation, and potential impact on soil quality. Environ Sci Pollut Res. (2017) 24:3194–209. doi: 10.1007/s11356-016-8057-3
21. Ijaz, MU, Ishtiaq, A, Ehsan, N, Imran, M, and Zhu, G-p. Hepatoprotective potential of Genkwanin against aflatoxin B1-induced biochemical, inflammatory and histopathological toxicity in rats. PVJ. (2022) 42:493–8. doi: 10.29261/pakvetj/2022.048
22. Pirhadi, M, Shariatifar, N, Bahmani, M, and Manouchehri, A. Heavy metals in wheat grain and its impact on human health: a mini-review. J. Chem. Health Risks. (2022) 12:421–6. doi: 10.47278/journal.ijvs/2020.021
23. Zhang, J, Mo, L, Li, X, Zhu, Y, Hu, B, and Zhang, L. Distribution, historical variations, and geochemical fractions of toxic trace metals and their ecological risks in sediments of the Nanliu River estuary. South China Ecol Indicators. (2022) 145:109708. doi: 10.1016/j.ecolind.2022.109708
24. Liu, X, Zhu, H, Zhang, B, Xu, C, Li, L, and Xing, W. Heavy metals (HMs) in soils of different land-use types in Zhengzhou, China: occurrence, source and ecological risk. Soil Sediment Contam Int J. (2022) 32:1–21. doi: 10.1080/15320383.2022.2130163
25. Luo, H, Wang, Q, Guan, Q, Ma, Y, Ni, F, Yang, E, et al. Heavy metal pollution levels, source apportionment and risk assessment in dust storms in key cities in Northwest China. J Hazard Mater. (2022) 422:126878. doi: 10.1016/j.jhazmat.2021.126878
26. Aziz, S, Abdullah, S, Anwar, H, and Latif, F. DNA damage and oxidative stress in economically important fish, bighead carp (Hypophthalmichthys nobilis) exposed to engineered copper oxide nanoparticles. Pak Vet J. (2022) 1:42. doi: 10.29261/pakvetj/2022.002
27. Qian, X, Wang, Z, Shen, G, Chen, X, Tang, Z, Guo, C, et al. Heavy metals accumulation in soil after 4 years of continuous land application of swine manure: a field-scale monitoring and modeling estimation. Chemosphere. (2018) 210:1029–34. doi: 10.1016/j.chemosphere.2018.07.107
28. Wu, S, Liu, H, Huang, H, Lei, Q, Wang, H, Zhai, L, et al. Analysis on the amount and utilization of manure in livestock and poultry breeding in China. Strat Study Chin Acad Engin. (2018) 20:103–11. doi: 10.15302/J-SSCAE-2018.05.016
29. Huang, H, Wang, Y, An, Y, Jiao, W, Xu, Y, Han, Q, et al. Selenium alleviates oxidative stress and autophagy in lead-treated chicken testes. Theriogenology. (2019) 131:146–52. doi: 10.1016/j.theriogenology.2019.03.015
30. Singh, AD, Khanna, K, Kour, J, Dhiman, S, Bhardwaj, T, Devi, K, et al. Critical review on biogeochemical dynamics of mercury (hg) and its abatement strategies. Chemosphere. (2023) 319:137917. doi: 10.1016/j.chemosphere.2023.137917
31. Verma, N, Rachamalla, M, Kumar, PS, and Dua, K. Assessment and impact of metal toxicity on wildlife and human health. Metals Water. (2023):93–110. doi: 10.1016/B978-0-323-95919-3.00002-1
32. Mitra, S, Chakraborty, AJ, Tareq, AM, Emran, TB, Nainu, F, Khusro, A, et al. Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci. (2022):101865. doi: 10.1016/j.jksus.2022.101865
33. Wąsik, M, Miśkiewicz-Orczyk, K, Słota, M, Lisowska, G, Kasperczyk, A, Bellanti, F, et al. Relationship between postural stability, Lead content, and selected parameters of oxidative stress. Int J Mol Sci. (2022) 23:12768. doi: 10.3390/ijms232112768
34. Liu, Y, Li, H, Ren, P, Che, Y, Zhou, J, Wang, W, et al. Polysaccharide from Flammulina velutipes residues protects mice from Pb poisoning by activating Akt/GSK3β/Nrf-2/HO-1 signaling pathway and modulating gut microbiota. Int J Biol Macromol. (2023):123154. doi: 10.1016/j.ijbiomac.2023.123154
35. Singh, S, Yadav, R, Sharma, S, and Singh, AN. Arsenic contamination in the food chain: a threat to food security and human health (Cross mark). (2023)
36. Kalisińska, E, Kot, K, and Łanocha-Arendarczyk, N. Red fox as a potential bioindicator of metal contamination in a European environment. Chemosphere. (2023) 319:138037. doi: 10.1016/j.chemosphere.2023.138037
37. Hassan, NH, Mehanna, S, Hussien, AM, Ibrahim, MA, and Hassanen, EI. The potential mechanism underlying the hepatorenal toxicity induced by hymexazol in rats and the role of NF-κB signaling pathway. J Biochem Mol Toxicol. (2023):e23304. doi: 10.1002/jbt.23304
38. Sonone, SS, Jadhav, S, Sankhla, MS, and Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food chain. Lett Appl NanoBioScience. (2020) 10:2148–66. doi: 10.33263/LIANBS102.21482166
39. Ashish, B, Neeti, K, and Himanshu, K. Copper toxicity: a comprehensive study. Res J Recent Sci. (2013) 2277:2502.
40. Mohammad, A-M, Chowdhury, T, Biswas, B, and Absar, N. Food poisoning and intoxication: a global leading concern for human health. Food Safety and Preservation. Elsevier Amsterdam (2018). 307–352.
41. Gupta, UC, and Gupta, SC. Trace element toxicity relationships to crop production and livestock and human health: implications for management. Commun Soil Sci Plant Anal. (1998) 29:1491–522. doi: 10.1080/00103629809370045
42. Shi, X, Guo, J, Hu, C, Zwain, HM, Jalbani, S, Li, Y, et al. Anthropocentric perspective on climatic variability, potentially toxic elements, and health risk assessment in the Mansehra district: a case study of the Kunhar River, Pakistan. J Water Climate Change. (2023) 14:1132–46. doi: 10.2166/wcc.2023.308
43. Ashrafudoulla, M, Na, KW, Byun, K-H, Kim, DH, Yoon, JW, Mizan, MFR, et al. Isolation and characterization of Salmonella spp. from food and food contact surfaces in a chicken processing factory. Poult Sci. (2021) 100:101234. doi: 10.1016/j.psj.2021.101234
44. Emami, MH, Saberi, F, Mohammadzadeh, S, Fahim, A, Abdolvand, M, Dehkordi, SAE, et al. A review of heavy metals accumulation in red meat and meat products in the Middle East. J Food Prot. (2023) 86:100048. doi: 10.1016/j.jfp.2023.100048
45. Zhao, X, Liu, X, Xing, Y, Wang, L, and Wang, Y. Evaluation of water quality using a Takagi-Sugeno fuzzy neural network and determination of heavy metal pollution index in a typical site upstream of the Yellow River. Environ Res. (2022) 211:113058. doi: 10.1016/j.envres.2022.113058
46. Jamil, M, Khatoon, A, Saleemi, M, Gul, A, Imran, M, Majeed, W, et al. An overview of copper toxicity and public health concerns with mitigation strategies In: RZ Abbas, NM Saeed, M Younus, L Aguilar Marcelino, and A Khan, editors, vol. 2. Faisalabad, Pakistan: One Health Triad, Unique Scientific Publishers (2023). 162–7.
47. Taslima, K, Al-Emran, M, Rahman, MS, Hasan, J, Ferdous, Z, Rohani, MF, et al. Impacts of heavy metals on early development, growth and reproduction of fish–a review. Toxicol Rep. (2022) 9:858–868. doi: 10.1016/j.toxrep.2022.04.013
48. Xiang, Y, Jiang, L, Zhou, Y, Luo, Z, Zhi, D, Yang, J, et al. Microplastics and environmental pollutants: key interaction and toxicology in aquatic and soil environments. J Hazard Mater. (2022) 422:126843. doi: 10.1016/j.jhazmat.2021.126843
49. de Oliveira, CPA, Carneiro, AA, Ervilha, LOG, Machado-Neves, M, Souza, ACF, and Carvalho, RPR. Does environmental pollution affect male reproductive system in naturally exposed vertebrates? A systematic review. Theriogenology. (2023) 64:1457–1474. doi: 10.1016/j.theriogenology.2023.01.004
50. Kubra, K, Mondol, AH, Ali, MM, Islam, MS, Akhtar, S, Ahmed, AS, et al. Assessment of as, Cr, cd, and Pb in urban surface water from a subtropical river: contamination, sources, and human health risk. Int J Environ Anal Chem. (2023):1–21. doi: 10.1080/03067319.2023.2170232
51. Irkin, LC, and Öztürk, Ş. Ameliorative effects of Ulva rigida (C. Agardh, 1823) on cadmium-induced nephrotoxicity in Wistar albino rats. Pak Vet J. (2022) 42. doi: 10.29261/pakvetj/2022.051
52. Jafarli, BS. Comparative study of the amount of macro- and microelements in the content of different muscles of lambs and kids. International Journal of Veterinary Science (Unique scientific publishers) (2022) 12:559–565. doi: 10.47278/journal.ijvs/2023.002
53. Zakanova, A, Yerzhanov, N, and Litvinov, Y. The impact of industrial pollution on the populations of small mammals in northern Kazakhstan. Environ Sci Pollut Res. (2023) 30:49980–91. doi: 10.1007/s11356-023-25836-6
54. Hernández-Cruz, EY, Amador-Martínez, I, Aranda-Rivera, AK, Cruz-Gregorio, A, and Chaverri, JP. Renal damage induced by cadmium and its possible therapy by mitochondrial transplantation. Chem Biol Interact. (2022) 361:109961. doi: 10.1016/j.cbi.2022.109961
55. Jabeen, G, Manzoor, F, Arshad, M, and Barbol, B. Effect of cadmium exposure on hematological, nuclear and morphological alterations in erythrocyte of fresh water fish (Labeo rohita). Continen Veterin J. (2021) 1:20–4.
56. Kar, I, and Patra, AK. Tissue bioaccumulation and toxicopathological effects of cadmium and its dietary amelioration in poultry—a review. Biol Trace Elem Res. (2021) 199:3846–68. doi: 10.1007/s12011-020-02503-2
57. Suardana, IBK, Widyastuti, SK, Pradnyadana, K, IBK, K, and Agustina, K. Effect of age and presence of maternal antibodies on success of avian influenza and Newcastle disease vaccinations in broiler. Int. J Vet Sci. (2023) 12:101–6. doi: 10.47278/journal.ijvs/2022.165
58. Bakhshalizadeh, S, Mora-Medina, R, Fazio, F, Parrino, V, and Ayala-Soldado, N. Determination of the heavy metal bioaccumulation patterns in muscles of two species of mullets from the southern Caspian Sea. Animals. (2022) 12:2819. doi: 10.3390/ani12202819
59. Genchi, G, Lauria, G, Catalano, A, Carocci, A, and Sinicropi, MS. Arsenic: a review on a great health issue worldwide. Appl Sci. (2022) 12:6184. doi: 10.3390/app12126184
60. Wang, H, Zhang, R, Song, Y, Li, T, and Ge, M. Protective effect of ganoderma triterpenoids on cadmium-induced testicular toxicity in chickens. Biol Trace Elem Res. (2019) 187:281–90. doi: 10.1007/s12011-018-1364-4
61. Oraby, M, Baraka, T, and Rakha, G. Hazardous effects of lead intoxication on health status, rumen functions, hematological and serum biochemical parameters in egyptian ossimi sheep. Adv Anim Vet Sci. (2021) 9:48–54. doi: 10.1007/s12011-022-03318-z
62. Kosečková, P, Zvěřina, O, Pěchová, M, Krulíková, M, Duborská, E, and Borkovcová, M. Mineral profile of cricket powders, some edible insect species and their implication for gastronomy. J Food Compos Anal. (2022) 107:104340. doi: 10.1016/j.jfca.2021.104340
63. Liu, Q, Zhao, W, Ma, J, Zhou, Y, Wu, Y, Qu, Y, et al. Spatial clustering and source-specific risk of combined pollutants in soils from an industrial area in Shanxi Province. China Environ Pollut. (2022) 299:118925. doi: 10.1016/j.envpol.2022.118925
64. Kumar, A, Kumar, A, Pinto, MMSC, Chaturvedi, AK, Shabnam, AA, Subrahmanyam, G, et al. Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. Int J Environ Res Public Health. (2020) 17:2179. doi: 10.3390/ijerph17072179
65. O’Connor, D, Hou, D, Ok, YS, and Lanphear, BP. The effects of iniquitous lead exposure on health. Nature Sustain. (2020) 3:77–9. doi: 10.1038/s41893-020-0475-z
66. Munir, N, Jahangeer, M, Bouyahya, A, El Omari, N, Ghchime, R, Balahbib, A, et al. Heavy metal contamination of natural foods is a serious health issue: a review. Sustainability. (2022) 14:161. doi: 10.3390/su14010161
67. Ali, AMA, Fahmy, MF, Metwally, MM, Hassanin, O, Azazy, HA, and Mowafy, RE. Ameliorative effects of cholestyramine and Oxihumate on Aflatoxicosis in broiler chickens. Pak Vet J. (2021) 41:51–6. doi: 10.29261/pakvetj/2020.093
68. Ercal, N, Gurer-Orhan, H, and Aykin-Burns, N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. (2001) 1:529–39. doi: 10.2174/1568026013394831
69. Islam, M, Hossain, M, Sobur, M, Punom, SA, Rahman, A, and Rahman, M. A systematic review on the occurrence of antimicrobial-resistant Escherichia coli in poultry and poultry environments in Bangladesh between 2010 and 2021. Biomed Res Int. (2023) 2023. 1:2023:2425564. doi: 10.1155/2023/2425564
70. Mashkoor, J, Khan, A, Khan, MZ, and Hussain, I. Chromium toxicity and oxidative stress in broiler chicks and its amelioration with vitamin E and bentonite. Int J Agric Biol. (2016) 18:1103–8. doi: 10.17957/IJAB/15.0192
71. Mateo, R, Vallverdú-Coll, N, López-Antia, A, Taggart, MA, Martínez-Haro, M, Guitart, R, et al. Reducing Pb poisoning in birds and Pb exposure in game meat consumers: the dual benefit of effective Pb shot regulation. Environ Int. (2014) 63:163–8. doi: 10.1016/j.envint.2013.11.006
72. Naz, D, Rahman, S, Aslam, MA, and Muhammad, F. Immunotherapeutic and immunomodulatory potentials of antigen-antibody complex vaccines. Med Hypotheses. (2023) 170:111001. doi: 10.1016/j.mehy.2022.111001
73. Wolthuis, JC, Magnúsdóttir, S, Stigter, E, Tang, YF, Jans, J, Gilbert, M, et al. Multi-country metabolic signature discovery for chicken health classification. Metabolomics. (2023) 19:9. doi: 10.1007/s11306-023-01973-4
74. Smirnova, E, Moniruzzaman, M, Chin, S, Sureshbabu, A, Karthikeyan, A, Do, K, et al. A review of the role of curcumin in metal induced toxicity. Antioxidants. (2023) 12:243. doi: 10.3390/antiox12020243
75. Martins, N, Marques, M, Vilela, DAR, Resende, J, Carvalhaes, A, Andrade, E, et al. Lead poisoning mortality in wild passeriformes and its detection in free-range chicken eggs in southern Minas Gerais, Brazil. Brazilian. J Poultry Sci. (2010) 12:149–52. doi: 10.1590/S1516-635X2010000300002
76. Younas, Z, Mashwani, ZUR, Ahmad, I, Khan, M, Zaman, S, and Sawati, L. Mechanistic approaches to the application of Nano-zinc in the poultry and biomedical industries: a comprehensive review of future perspectives and challenges. Molecules. (2023) 28:1064. doi: 10.3390/molecules28031064
77. Valskys, V, Hassan, HR, Wołkowicz, S, Satkūnas, J, Kibirkštis, G, and Ignatavičius, G. A review on detection techniques, health hazards and human health risk assessment of arsenic pollution in soil and groundwater. Fortschr Mineral. (2022) 12:1326. doi: 10.3390/min12101326
78. Akhtar, MS, Hameed, A, Aslam, S, Ullah, R, and Kashif, A. Phytoremediation of metal-contaminated soils and water in Pakistan: a review. Water Air Soil Pollut. (2023) 234:11. doi: 10.1007/s11270-022-06023-8
79. Sharma, P, Chaturvedi, P, Chandra, R, and Kumar, S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere. (2022) 295:133823. doi: 10.1016/j.chemosphere.2022.133823
80. Das, A. Nanotheranostics: the toxicological implications. Design Appl. (2023):369. doi: 10.1016/B978-0-323-89953-6.00012-X
81. Murthy, MK, Khandayataray, P, Mohanty, CS, and Pattanayak, R. A review on arsenic pollution, toxicity, health risks, and management strategies using nanoremediation approaches. Rev Environ Health. (2022) 26:1. doi: 10.1515/reveh-2022-0103
82. Suleman, S, Qureshi, JA, Rasheed, M, Farooq, W, and Yasmin, F. Poultry feed contamination and its potential hazards on human health. Biomed Lett. (2022) 8:70–81. doi: 10.37185/266
83. Abu, SM, Monira, N, Asmaul, H, EEA, M, and Hena, KA. Seroprevalence of Newcastle disease in layer chickens and pathology in clinically affected chickens at Gazipur, Bangladesh Continental Vet J. (2022) 2:35–41.
84. Sattar, A, Khan, A, Hussain, HI, He, C, Hussain, R, Zhiqiang, S, et al. Immunosuppressive effects of arsenic in broiler chicks exposed to Newcastle disease virus. J Immunotoxicol. (2016) 13:861–9. doi: 10.1080/1547691X.2016.1217105
85. Khan, A, Hussain, HI, Sattar, A, Khan, MZ, and Abbas, RZ. Toxico-pathological aspects of arsenic in birds and mammals: a review. Int J Agric Biol. (2014) 16:1213–1224. Available at: http://www.fspublishers.org
86. Awuchi, CG, Igwe, VS, and Amagwula, IO. Nutritional diseases and nutrient toxicities: a systematic review of the diets and nutrition for prevention and treatment. Int J Adv Acad Res. (2020) 6:1–46.
87. Khan, A, Sharaf, R, Zargham Khan, M, Kashif Saleemi, M, and Mahmood, F. Arsenic toxicity in broiler chicks and its alleviation with ascorbic acid: a Toxico-patho-biochemical study. Int J Agric Biol. (2013) 15:1105–1111.
88. Mandal, A, Bisht, R, Rupenthal, ID, and Mitra, AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. (2017) 248:96–116. doi: 10.1016/j.jconrel.2017.01.012
89. Medda, N, Patra, R, Ghosh, TK, and Maiti, S. Neurotoxic mechanism of arsenic: synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol Trace Elem Res. (2020) 198:8–15. doi: 10.1007/s12011-020-02044-8
90. Muthumani, M, and Prabu, SM. Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicol Mech Methods. (2012) 22:277–88. doi: 10.3109/15376516.2011.647113
91. Zhong, G, Wan, F, Ning, Z, Wu, S, Jiang, X, Tang, Z, et al. The protective role of autophagy against arsenic trioxide-induced cytotoxicity and ROS-dependent pyroptosis in NCTC-1469 cells. J Inorg Biochem. (2021) 217:111396. doi: 10.1016/j.jinorgbio.2021.111396
92. Ning, Z, Lan, J, Jiang, X, Zhong, G, Zhang, H, Wan, F, et al. Arsenic trioxide-induced autophagy affected the antioxidant capacity and apoptosis rate of chicken hepatocytes. Chem Biol Interact. (2022) 354:109821. doi: 10.1016/j.cbi.2022.109821
93. Kojima, LV, Tuberville, TD, and Parrott, BB. Integrating mercury concentrations in American alligators (Alligator mississippiensis) with hunter consumption surveys to estimate exposure risk. Environ Toxicol Chem. (2023) 42:525–534. doi: 10.1002/etc.5524
94. Beckers, F, and Rinklebe, J. Cycling of mercury in the environment: sources, fate, and human health implications: a review. Crit Rev Environ Sci Technol. (2017) 47:693–794. doi: 10.1080/10643389.2017.1326277
95. Whitney, MC, and Cristol, DA. Impacts of sublethal mercury exposure on birds: a detailed review. Rev Environ Contam Toxicol. (2018) 244:113–63. doi: 10.1007/398_2017_4
96. Agarwal, S, and Darbar, S. Microorganism assisted synthesized metal and metal oxide nanoparticles for removal of heavy metal ions from the wastewater effluents. Environ Appl Microbial Nanotechnol. (2023):127–148. doi: 10.1016/B978-0-323-91744-5.00017-5
97. Hu, H, Gao, Y, Yu, H, Xiao, H, Chen, S, Tan, W, et al. Mechanisms and biological effects of organic amendments on mercury speciation in soil–rice systems: a review. Ecotoxicol Environ Saf. (2023) 251:114516. doi: 10.1016/j.ecoenv.2023.114516
98. Gaines, LG. Historical and current usage of per-and polyfluoroalkyl substances (PFAS): a literature review. Am J Ind Med. (2022) 66:353–78. doi: 10.1002/ajim.23362
99. Mazumder, K, Aktar, A, Roy, P, Biswas, B, Hossain, ME, Sarkar, KK, et al. A review on mechanistic insight of plant derived anticancer bioactive Phytocompounds and their structure activity relationship. Molecules. (2022) 27:3036. doi: 10.3390/molecules27093036
100. Patrick, L. Mercury toxicity and antioxidants: part I: role of glutathione and alpha-lipoic acid in the treatment of mercury toxicity.(mercury toxicity). Altern Med Rev. (2002) 7:456–72.
101. Bersényi, A. Study of toxic metals (cd, Pb, hg and Ni) in rabbits and broiler chickens. (HuVetA Hungarian Veterinary Archive) (2003)
102. Qui, NH. Recent advances of using polyphenols to extenuate oxidative stress in animal production: evidence from poultry. Kafkas Univ. Vet. Fak. Derg. (2022) 28:535–41. doi: 10.9775/kvfd.2022.27381
103. Guzzi, G, and La Porta, CA. Molecular mechanisms triggered by mercury. Toxicology. (2008) 244:1–12. doi: 10.1016/j.tox.2007.11.002
104. Aziz, S, Abdullah, S, Anwar, H, Latif, F, and Mustfa, W. Effect of engineered nickel oxide nanoparticles on antioxidant enzymes in freshwater fish, Labeo rohita. Pak Vet J. (2021) 41:424–8. doi: 10.29261/pakvetj/2021.044
105. Tian, X, Lin, X, Zhao, J, Cui, L, Gao, Y, Yu, Y-L, et al. Gut as the target tissue of mercury and the extraintestinal effects. Toxicology. (2022) 484:153396. doi: 10.1016/j.tox.2022.153396
106. Demirbaş, A. Proximate and heavy metal composition in chicken meat and tissues. Food Chem. (1999) 67:27–31. doi: 10.1016/S0308-8146(99)00103-X
107. Abduljaleel, SA, Shuhaimi-Othman, M, and Babji, A. Assessment of trace metals contents in chicken (Gallus gallus domesticus) and quail (Coturnix coturnix japonica) tissues from Selangor (Malaysia). J Environ Sci Technol. (2012) 5:441–51. doi: 10.3923/jest.2012.441.451
108. Hussein, M, El-Hady, M, Sayed, W, and Hefni, H. Preparation of some chitosan heavy metal complexes and study of its properties. Polymer Sci Series A. (2012) 54:113–24. doi: 10.1134/S0965545X12020046
109. Mohammed, AI, Kolo, B, and Geidam, YA. Heavy metals in selected tissues of adult chicken layers (Gallus spp.). ARPN J Sci Technol. (2013) 3:518–22.
110. Abdel-Salam, N, Ahmed, S, Basir, A, Rais, AK, Bibi, A, Ullah, R, et al. Distribution of heavy metals in the liver, kidney, heart, pancreas and meat of cow, buffalo, goat, sheep and chicken from Kohat market Pakistan. Life Sci J. (2013) 10:937–40.
111. Jaja, N, Mbila, M, Codling, EE, Reddy, SS, and Reddy, CK. Trace metal enrichment and distribution in a poultry litter-amended soil under different tillage practices. Open Agric J. (2013) 7. doi: 10.2174/1874331501307010088
112. Zhuang, P, Zou, B, Lu, H, and Li, Z. Heavy metal concentrations in five tissues of chickens from a mining area. Pol J Environ Stud. (2014) 23:2375–9.
113. Al-Zuhairi, WS, Farhan, MA, and Ahemd, MA. Determine of heavy metals in the heart, kidney and meat of beef, mutton and chicken from Baquba and Howaydir market in Baquba, Diyala Province Iraq. Int J Recent Sci Res. (2015) 6:5965–7.
114. Sadeghi, B, and Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. (2015) 134:310–5. doi: 10.1016/j.saa.2014.06.046
115. Ogbomida, ET, Nakayama, SM, Bortey-Sam, N, Oroszlany, B, Tongo, I, Enuneku, AA, et al. Accumulation patterns and risk assessment of metals and metalloid in muscle and offal of free-range chickens, cattle and goat in Benin City. Nigeria Ecotoxicology and environmental safety. (2018) 151:98–108. doi: 10.1016/j.ecoenv.2017.12.069
116. Kar, I, Mukhopadhayay, SK, Patra, AK, and Pradhan, S. Bioaccumulation of selected heavy metals and histopathological and hematobiochemical alterations in backyard chickens reared in an industrial area. India Environ Sci Pollut Res. (2018) 25:3905–12. doi: 10.1007/s11356-017-0799-z
117. Al Bratty, M, Alhazmi, HA, Ogdi, SJ, Otaiif, J, Al-Rajab, AJ, Alam, MF, et al. Determination of heavy metals in various tissues of locally reared (Baladi) chicken in Jazan region of Saudi Arabia: assessment of potential health risks. Pak J Zool. (2018) 50:1509–17. doi: 10.17582/journal.pjz/2018.50.4.1509.1517
118. Almayahi, W, Saeed Abbas, S, and Abbas, B. Molecular detection and histopathological effect of infectious bronchitis virus circulating in vaccinated broiler flocks in Basrah, Iraq. Iran J War Public Health. (2022) 14:339–45.
119. Korish, MA, and Attia, YA. Evaluation of heavy metal content in feed, litter, meat, meat products, liver, and table eggs of chickens. Animals. (2020) 10:727. doi: 10.3390/ani10040727
120. Ali, HS, Almashhadany, DA, and Khalid, HS. Determination of heavy metals and selenium content in chicken liver at Erbil city, Iraq. Italian. J Food Saf. (2020) 9. doi: 10.4081/ijfs.2020.8659
121. Hossain, S, Farid, FB, Hasan, MNB, Rahman, SA, Muztaba, MA, and Rahman, MM. Assessment of heavy metal contamination in liver, gizzard, and brain of parent, broiler, layer, and domestic poultry chickens in Dhaka, Bangladesh: a threat to Bangladeshi chicken consumers. IJSEI. (2022) 3:159–66. doi: 10.47540/ijsei.v3i2.488
122. Elkribi-Boukhris, S, M’hamdi, N, Boughattas, I, Helaoui, S, Coriou, C, Bussiere, S, et al. Assessment of heavy metal pollution transfer and human exposure risks from the consumption of chicken grown in mining-surrounding areas. Environ Sci Pollut Res. (2022) 29:5661–73. doi: 10.1007/s11356-021-15995-9
123. Aendo, P, De Garine-Wichatitsky, M, Mingkhwan, R, Senachai, K, Santativongchai, P, Krajanglikit, P, et al. Potential health effects of heavy metals and carcinogenic health risk estimation of pb and cd contaminated eggs from a closed gold mine area in northern Thailand. Foods. (2022) 11:2791. doi: 10.3390/foods11182791
124. Hussein, S, Rathi, M, and Kadhim, T. Determination of the levels of lead and cadmium in canned fish and meat, imported to the local markets of Diyala Province, Iraq In:. IOP conference series: Earth and environmental science : IOP Publishing (2021)
125. Okeke, O, Ujah, I, Okoye, P, Ajiwe, V, and Eze, C. Assessment of the heavy metal levels in feeds and litters of chickens rose with in Awka Metropolis and its environs. IOSR J Appl Chem. (2015) 8:60–3.
126. Ravindran, B, Mupambwa, HA, Silwana, S, and Mnkeni, PN. Assessment of nutrient quality, heavy metals and phytotoxic properties of chicken manure on selected commercial vegetable crops. Heliyon. (2017) 3:e00493. doi: 10.1016/j.heliyon.2017.e00493
127. Wang, W, Zhang, W, Wang, X, Lei, C, Tang, R, Zhang, F, et al. Tracing heavy metals in ‘swine manure-maggot-chicken’ production chain. Sci Rep. (2017) 7:8417. doi: 10.1038/s41598-017-07317-2
128. Kraljević Pavelić, S, Simović Medica, J, Gumbarević, D, Filošević, A, Pržulj, N, and Pavelić, K. Critical review on zeolite clinoptilolite safety and medical applications in vivo. Front Pharmacol. (2018) 9:1350. doi: 10.3389/fphar.2018.01350
129. Ye, Q, Feng, Y, Wang, Z, Zhou, A, Xie, S, Zhang, Y, et al. Effects of dietary Gelsemium elegans alkaloids on growth performance, immune responses and disease resistance of Megalobrama amblycephala. Fish Shellfish Immunol. (2019) 91:29–39. doi: 10.1016/j.fsi.2019.05.026
130. Xiao, T, Guha, J, Boyle, D, Liu, C-Q, and Chen, J. Environmental concerns related to high thallium levels in soils and thallium uptake by plants in Southwest Guizhou China. Sci Total Environ. (2004) 318:223–44. doi: 10.1016/S0048-9697(03)00448-0
131. Awad, W, Böhm, J, Razzazi-Fazeli, E, Hulan, H, and Zentek, J. Effects of deoxynivalenol on general performance and electrophysiological properties of intestinal mucosa of broiler chickens. Poult Sci. (2004) 83:1964–72. doi: 10.1093/ps/83.12.1964
132. Nakayama, SM, Nakata, H, Ikenaka, Y, Yabe, J, Oroszlany, B, Yohannes, YB, et al. One year exposure to cd-and Pb-contaminated soil causes metal accumulation and alteration of global DNA methylation in rats. Environ Pollut. (2019) 252:1267–76. doi: 10.1016/j.envpol.2019.05.038
133. Bokori, J, Fekete, S, Glavits, R, Kadar, I, Koncz, J, and Kövári, L. Complex study of the physiological role of cadmium. IV. Effects of prolonged dietary exposure of broiler chickens to cadmium. Acta Vet Hung. (1996) 44:57–74.
134. Mwesigye, AR, Young, SD, Bailey, EH, and Tumwebaze, SB. Population exposure to trace elements in the Kilembe copper mine area, Western Uganda: a pilot study. Sci Total Environ. (2016) 573:366–75. doi: 10.1016/j.scitotenv.2016.08.125
135. Aljaff, P, Rasheed, BO, and Salh, DM. Assessment of heavy metals in livers of cattle and chicken by spectroscopic method. IOSR J Appl Physics. (2014) 6:23–6. doi: 10.9790/4861-06122326
136. Peana, M, Pelucelli, A, Chasapis, CT, Perlepes, SP, Bekiari, V, Medici, S, et al. Biological effects of human exposure to environmental cadmium. Biomol Ther. (2022) 13:36. doi: 10.3390/biom13010036
137. Singh, R, Srivastava, A, Gangwar, N, Giri, D, Singh, R, and Kumar, R. Pathology of sub-chronic cadmium and chlorpyrifos toxicity in broilers (Indian journal of veterinary pathology). (2016)
138. Trujillo-Rangel, WÁ, García-Valdés, L, Méndez-del Villar, M, Castañeda-Arellano, R, Totsuka-Sutto, SE, and García-Benavides, L. Therapeutic targets for regulating oxidative damage induced by ischemia-reperfusion injury: A study from a pharmacological perspective. Oxidative medicine and cellular longevity (2022). 2022 p.
139. Ma, Y, Cheng, B, Li, Y, Wang, Z, Li, X, Ren, A, et al. Protective effect of nanoselenium on renal oxidative damage induced by mercury in laying hens. Biol Trace Elem Res. (2022) 200:3785–97. doi: 10.1007/s12011-021-02956-z.
140. Renu, K, Pureti, LP, Vellingiri, B, and Valsala, GA. Toxic effects and molecular mechanism of doxorubicin on different organs–an update. Toxin Rev. (2022) 41:650–74. doi: 10.1080/15569543.2021.1912099
141. Shalaby, AM, and El Shaer, DF. Lycopene protects against renal cortical damage induced by nandrolone decanoate in adult male rats. Ann Anat Anatomis Anzeiger. (2019) 224:142–52. doi: 10.1016/j.aanat.2019.05.003
142. Zhang, H, Qi, H-Y, Zhang, Y-L, Ran, D-D, Wu, L-Q, Wang, H-F, et al. Effects of sewage sludge pretreatment methods on its use in agricultural applications. J Hazard Mater. (2022) 428:128213. doi: 10.1016/j.jhazmat.2022.128213
143. Knorr, J, Kaufmann, B, Inzaugarat, ME, Holtmann, TM, Geisler, L, Hundertmark, J, et al. Interleukin-18 signaling promotes activation of hepatic stellate cells in mouse liver fibrosis. Hepatology. (2022) 77:1968–82. doi: 10.1002/hep.32776
144. Helmy, YA, Kathayat, D, Deblais, L, Srivastava, V, Closs, G Jr, Tokarski, RJ, et al. Evaluation of novel quorum sensing inhibitors targeting auto-inducer 2 (AI-2) for the control of avian pathogenic Escherichia coli infections in chickens. Microbiology Spectrum. (2022) 10:e00286–22. doi: 10.1128/spectrum.00286-22
145. Kuester, RK, Waalkes, MP, Goering, PL, Fisher, BL, McCuskey, RS, and Sipes, IG. Differential hepatotoxicity induced by cadmium in Fischer 344 and Sprague-Dawley rats. Toxicol Sci. (2002) 65:151–9. doi: 10.1093/toxsci/65.1.151
146. Binatti, E, Gerussi, A, Barisani, D, and Invernizzi, P. The role of macrophages in liver fibrosis: new therapeutic opportunities. Int J Mol Sci. (2022) 23:6649. doi: 10.3390/ijms23126649
147. Zhu, X, He, Y, Zhang, Q, Ma, D, and Zhao, H. Lead induced disorders of lipid metabolism and glycometabolism in the liver of developmental Japanese quails (Coturnix japonica) via inhibiting PI3K/Akt signaling pathway. Compar Biochem Physiol Part C Toxicol Pharmacol. (2023) 263:109489. doi: 10.1016/j.cbpc.2022.109489
148. Kou, H, Ya, J, Gao, X, and Zhao, H. The effects of chronic lead exposure on the liver of female Japanese quail (Coturnix japonica): histopathological damages, oxidative stress and AMP-activated protein kinase based lipid metabolism disorder. Ecotoxicol Environ Saf. (2020) 190:110055. doi: 10.1016/j.ecoenv.2019.110055
149. Milićević, D, Jovanović, M, Matekalo-Sverak, V, Radičević, T, Petrović, MM, and Lilić, S. A survey of spontaneous occurrence of ochratoxin a residues in chicken tissues and concurrence with histopathological changes in liver and kidneys. J Environ Sci Health C. (2011) 29:159–75. doi: 10.1080/10590501.2011.577687
150. Daly, C. Hepato-renal pathology with special reference to aflatoxicosis in chicken (Gallus domesticus): Centre of Excellence in pathology, College of Veterinary and Animal Sciences (Kerala Agricultural university these information and Retrieval) (2010).
151. Badamasi, I, Odong, R, and Masembe, C. Implications of increasing pollution levels on commercially important fishes in Lake Victoria. J Great Lakes Res. (2019) 45:1274–89. doi: 10.1016/j.jglr.2019.09.024
152. Ma, Y, Shi, Y, Wu, Q, and Ma, W. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult Sci. (2021) 100:982–92. doi: 10.1016/j.psj.2020.11.061
153. Huang, L, Guo, X, Liu, P, Zhao, Y, Wu, C, Zhou, C, et al. Correlation between acute brain injury and brain metabonomics in dichlorvos-poisoned broilers. J Hazard Mater. (2022) 422:126849. doi: 10.1016/j.jhazmat.2021.126849
154. Nie, X, Wang, Y, Zhao, H, Guo, M, Liu, Y, and Xing, M. As3+ or/and Cu2+ exposure triggers oxidative stress imbalance, induces inflammatory response and apoptosis in chicken brain. Ecotoxicol Environ Saf. (2020) 203:110993. doi: 10.1016/j.ecoenv.2020.110993
155. Zheng, Y, Zhang, Q, Jing, L, Fei, Y, and Zhao, H. The effects of chronic Lead exposure on testicular development of Japanese quail (Coturnix japonica): histopathological damages, oxidative stress, steroidogenesis disturbance, and hypothalamus-pituitary-testis Axis disruption. Biol Trace Elem Res. (2022) 201:3446–60. doi: 10.1007/s12011-022-03436-8
Keywords: poultry, lead, cadmium, environmental pollution, disease, toxicity
Citation: Aljohani ASM (2023) Heavy metal toxicity in poultry: a comprehensive review. Front. Vet. Sci. 10:1161354. doi: 10.3389/fvets.2023.1161354
Edited by:
Dandan Han, China Agricultural University, ChinaReviewed by:
Ahrar Khan, Shandong Vocational Animal Science and Veterinary College, ChinaRiaz Hussain, Islamia University of Bahawalpur, Pakistan
Hanem Khater, Benha University, Egypt
Copyright © 2023 Aljohani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah S. M. Aljohani, amhueUBxdS5lZHUuc2E=
 Abdullah S. M. Aljohani
Abdullah S. M. Aljohani