- 1Department of Comparative, Diagnostic, and Population Medicine, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
- 2Department of Physiological Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
- 3Florida Fish and Wildlife Conservation Commission, The Marine Mammal Pathobiology Laboratory, St. Petersburg, FL, United States
- 4Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL, United States
- 5Department of Population Medicine and Diagnostic Sciences, Cornell University, College of Veterinary Medicine, Ithaca, NY, United States
Manatees (Antillean-, Amazonian, and African-) and dugongs belong to the Order Sirenia, and when combined with elephants and rock hyraxes, form the Paenungulata. A bilobed mononuclear cell has previously been identified in elephants and rock hyraxes, but not in manatees and dugongs, with cytochemical staining identifying these cells as bilobed monocytes in elephants. The objective of this study was to characterize leukocytes (white blood cells, WBC) and platelets in blood films of Florida manatees (Trichechus manatus latirostris; n = 8) using one routine hematological (Wright-Giemsa) and eight cytochemical stains: alkaline phosphatase (ALP), α-naphthyl butyrate esterase (ANBE), chloroacetate esterase (CAE), Luna, myeloperoxidase (MPx), periodic acid-Schiff (PAS), Sudan black B (SBB), and toluidine blue (TB). Heterophils and lymphocytes comprised most of the WBC, with low numbers of eosinophils, basophils, and monocytes. Additionally, 1–3% of the WBC were bilobed mononuclear cells. Bilobed mononuclear cell proportions were similar to rock hyraxes, but lower than elephants (approximate range 20–60%). Heterophils and eosinophils were positive for MPx, ALP, SBB, and PAS, with heterophils also being positive for CAE. Most of the lymphocytes were positive for ANBE and they were variably positive for CAE. Monocytes and bilobed mononuclear cells had similar cytochemical staining reactions (variably positive for all stains, except Luna and TB), supporting a monocytic origin, like elephants. Platelets were ANBE- and PAS-positive. Luna stain was useful for identifying eosinophils and TB was uninformative. This study provides new information on the morphological features and cytochemical staining characteristics of WBC and platelets and will aid in obtaining accurate hematological data of Florida manatees.
Introduction
The Florida manatee (Trichechus manatus latirostris) is a subspecies of the West Indian manatee (Trichechus manatus) that inhabits Florida coastal waters. The species is listed as threatened by the US Fish and Wildlife Service under the Endangered Species Act (1, 2) and as endangered by the International Union for Conservation of Nature Red Book (3). Manatees, along with dugongs (Dugong dugon) belong to the Order Sirenia. Sirenians also belong to the clade Afrotheria, which is subdivided into Afroinsectiphilia (aardvarks, tenrecs, golden moles, elephant shrews) and Paenungulata (hyraxes, dugongs, manatees, elephants) (4, 5).
Baseline hematological evaluation of blood cell populations are essential in the general health assessment of any species and to monitor trends in individuals with disease. Blood film review is an integral part of the complete blood count (CBC) and relies on accurate blood cell identification. Hematological variables in elephants and manatees have been previously studied with reference data established (6–9). Harvey et al. (7) described the morphological features of mononuclear cells in the Florida manatee and identified small to intermediate lymphocytes with low numbers of granular lymphocytes, and monocytes with similar features to domestic mammals (in the current study referred to as “classic” monocytes). Eosinophils and heterophils can have similar staining properties with Romanowsky-type stains, so Medway et al. (10) applied a Luna stain to blood films of the West Indian Manatee to differentiate these two cell types. Medway et al. (10) confirmed that eosinophil granules are slightly larger, more uniform in size, and more refractile than those of heterophils. Manatee eosinophils cells also have a less lobulated nucleus and deeper blue staining cytoplasm than heterophils (10, 11).
A unique mononuclear cell type with a bi- or tri-lobed nucleus as well as “classic” monocytes have been identified in Asian (Elephas maximus) and African (Loxodonta africana) elephants and rock hyrax (Procavia capensis) within the Afrotheria clade (12, 13). Other species are suspected to have similar bilobed mononuclear cells, including the giant panda (Ailuropoda melanoleuca), flying fox (Pteropus giganteus), guinea pig, rabbit, and diamond python (Morelia spilota) (6, 12, 14–18).
As with elephants, a challenge with hematological evaluation of manatees relies on the proper differentiation of WBC during light microscopical evaluation. Cytochemical staining has been used to identify cellular constituents, such as lipids, carbohydrates, enzymes, and differentiate blood cell types in animals, when standard staining techniques (e.g., Wright-Giemsa, aqueous Romanowsky) are insufficient for cell classification (19, 20). The objective of this study was to characterize WBC and platelets in blood films of Florida manatees using standard and cytochemical staining techniques.
Materials and methods
Animals and blood sampling
This project was approved by the University of Florida’s Institutional Animal Care and Use Committee #202006823 and samples were collected under US Fish and Wildlife Service research permits #MA791721 and #MA067116-2.
Blood was collected from captured wild manatees from the brachial vascular bundle/arteriovenous plexus in the interosseous space between the radius and ulna into EDTA anticoagulant tubes [Becton Dickinson and Company (BD), Sparks, Maryland, United States] from eight clinically normal manatees during an ongoing health assessment study performed by the Florida Fish and Wildlife Conservation Commission and the US Geological Survey, Sirenia Project (21, 22). Blood samples were insulated and placed on ice packs immediately after collection until processing.
Laboratory analysis and cytochemical staining
Blood films were prepared within 1 h after sampling at the field site. Blood was analyzed using an ADVIA 2120i (Siemens Healthcare Diagnostics, Tarrytown, NY) within 6–8 h after sample collection at the Diagnostic Laboratory of the University of Florida College of Veterinary Medicine within 24 h. Blood films were stained with Wright-Giemsa (Harleco ®, EMD Millipore, Billerica, MA, United States) and evaluated with light microscopy via performance of a 200-WBC differential count and morphological evaluation of WBC, erythrocytes, and platelets. WBC were classified as granulocytes (heterophils, eosinophils, basophils) and mononuclear cells (lymphocytes, “classic” monocytes, and bilobed mononuclear cells). Bilobed mononuclear cells were not differentiated from monocytes in a previous hematological study in manatees (7); however, they were differentiated from classic monocytes in this study because of their prominence in elephants. Reticulocytes and Heinz bodies were quantified as a percentage of total RBCs using a new methylene blue-stained blood film (Harleco ®, EMD Millipore, Billerica, MA, United States) (7).
Within 1 week after sampling, blood films were stained with eight cytochemical stains: alkaline phosphatase (ALP), α-naphthyl butyrate esterase (ANBE), naphthol AS-D choroacetate esterase (CAE), Luna, myeloperoxidase (MPx), periodic acid-Schiff (PAS), Sudan black blue (SBB), and toluidine blue (TB) at the Animal Health Diagnostic Center at Cornell University College of Veterinary Medicine. Commercially available kits (ALP, Procedure No. 85; ANBE, Procedure No. 181; CAE, Procedure No. 91; SBB, Procedure No. 380; Sigma-Aldrich, St. Louis, MO, United States) were utilized as previously described (14, 23). The myeloperoxidase assay was done manually by fixing the slides for 30 s in a buffer containing 0.35 mM disodium hydrogen phosphate, 1.4 mM potassium phosphate dibasic, 45% v/v acetone, and 10% formaldehyde (pH 6.6) then allowing them to air-dry. The slides were then incubated with the substrate for 30 min in the dark. The substrate consisted of 10 mg 3-amino-9-ethylcarbazole (Sigma-Aldrich) dissolved in dimethylsulfoxide in 50 mL of a buffer containing 3% v/v of 0.02 M glacial acetic acid and 7% v/v of 0.02 M sodium acetate. Hydrogen peroxide (0.006%) was added to the substrate buffer prior to incubation. The slides were counterstained with Gill’s hematoxylin for 30 s, then rinsed and air-dried. All slides were kept in the dark before and after staining. For the other stains, the slides were fixed with the kit fixative (citrate-acetone-formaldehyde for ANBE, CAE, and Sudan Black B or citrate-acetone for ALP). The Luna, PAS, and TB stains were performed via previously established protocols with histological sections of eosinophilic infiltrates, gastrointestinal tract, and a mast cell tumor as positive controls (14, 24, 25). Cytochemically stained blood films were evaluated by two independent reviewers (TS, NIS), using a 50 and 100x objective, to subjectively grade the staining pattern of each leukocyte type, similar to that described by Kehoe and colleagues for the giant panda (14). Pattern of staining was described as cytoplasm (C) or in granules (G), with degree of staining graded as positive (+), negative (−), equivocal (negative or weakly positive, +/−), weak (+), moderate (++), or strong (+++) positive. The pattern of cytoplasmic staining was further described as punctate (multiple small granular-like staining), focal (distinct foci), multifocal (multiple distinct foci), or diffuse (distributed throughout the cytoplasm). The numbers of each leukocyte observed during scanning varied depending on their frequency in blood, with few basophils being identified conclusively in the cytochemically-stained blood films. The results were then compared to obtain a consensus agreement on the frequency of observed cells by cell type in order to achieve evaluation of a sufficient number of cells. Images of the cells were taken with an Olympus BX53® (Tokyo, Japan) microscope equipped with a calibrated DP74® (Olympus, Tokyo, Japan) camera using the Olympus cellSens™ software (Standard Version 1.18, Tokyo, Japan).
Results
Blood samples from eight wild caught clinically normal manatees (4 adults, 3 large calves, 1 subadult; 3 females, 5 males) were collected. Hematological data are reported in Table 1. The data was within ranges reported for Florida manatees, except for mildly increased nucleated red blood cells (nRBC) in one calf, which may be due to individual variation since calves tend to have higher numbers of nRBC compared to older life stages (7). WBC morphology was similar for all manatees across ages and sexes in the Wright-Giemsa-stained blood films, with exceptions noted below. A low number of bilobed mononuclear cells (1–3%, 0.04–0.36 K/μL) of undetermined origin was identified in Wright-Giemsa-stained blood films (Figure 1). White blood cells and platelets were further characterized by cytochemical staining (Figure 2 and Table 2).
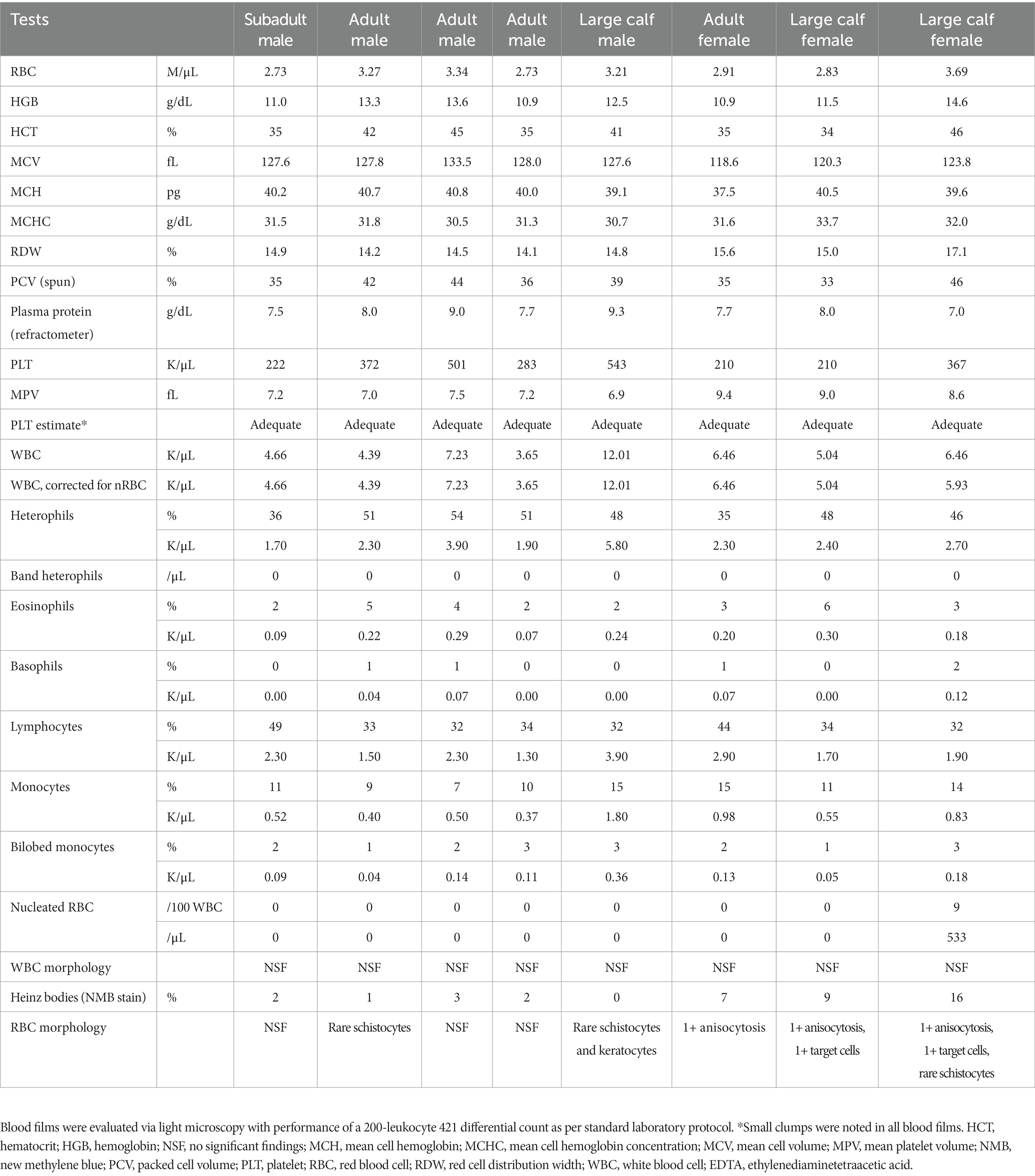
Table 1. Hematological data from eight clinically normal, free-ranging Florida manatees (Trichechus manatus latirostris) obtained from EDTA-anticoagulated blood using an automated ADVIA 2120i hematology analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY) and manual blood film review.
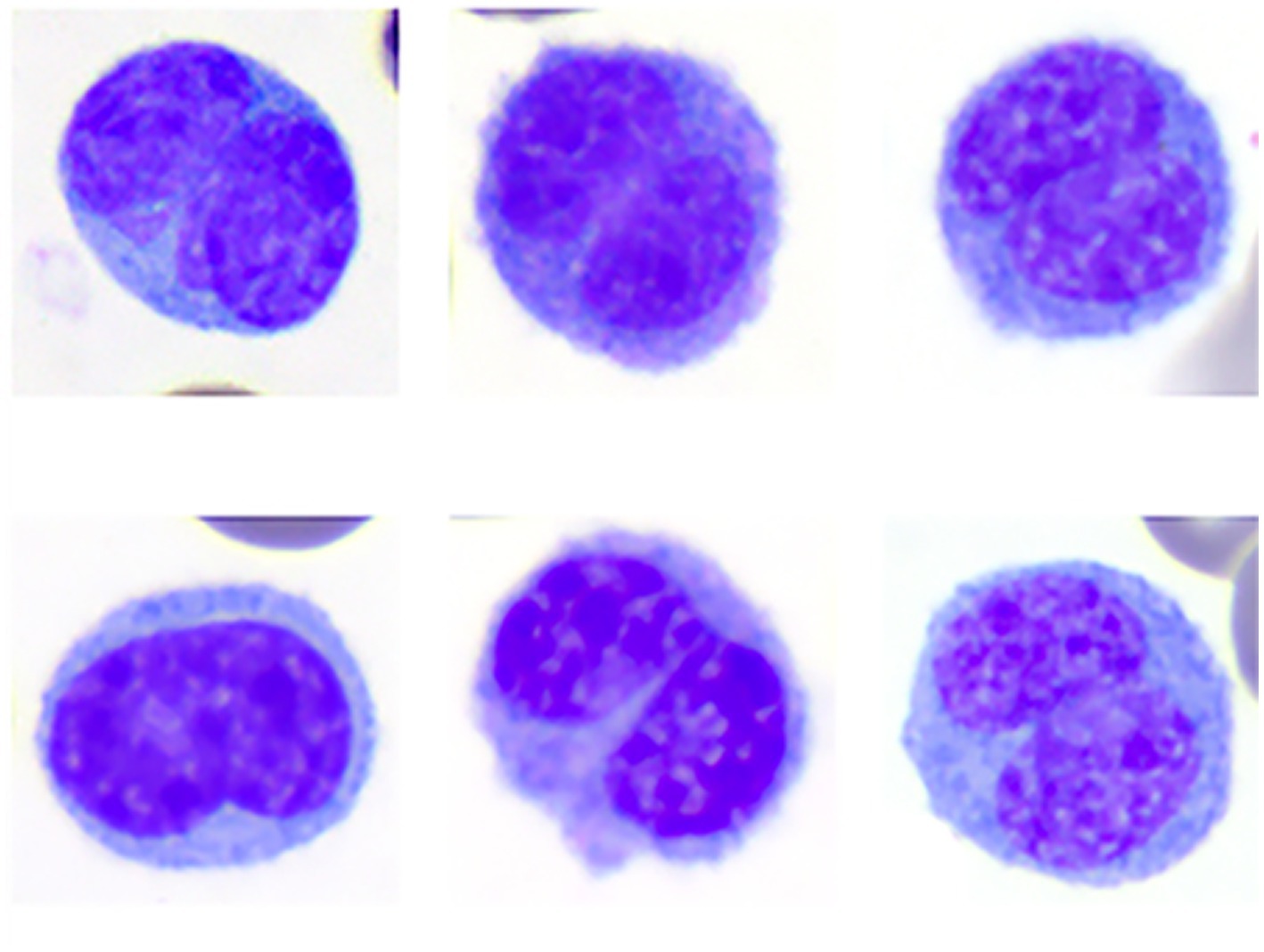
Figure 1. Image composite of monocytes of the Florida manatee (Trichechus manatus latirostris) showing the morphologic variability of bilobed monocytes. Wright-Giemsa-stain.
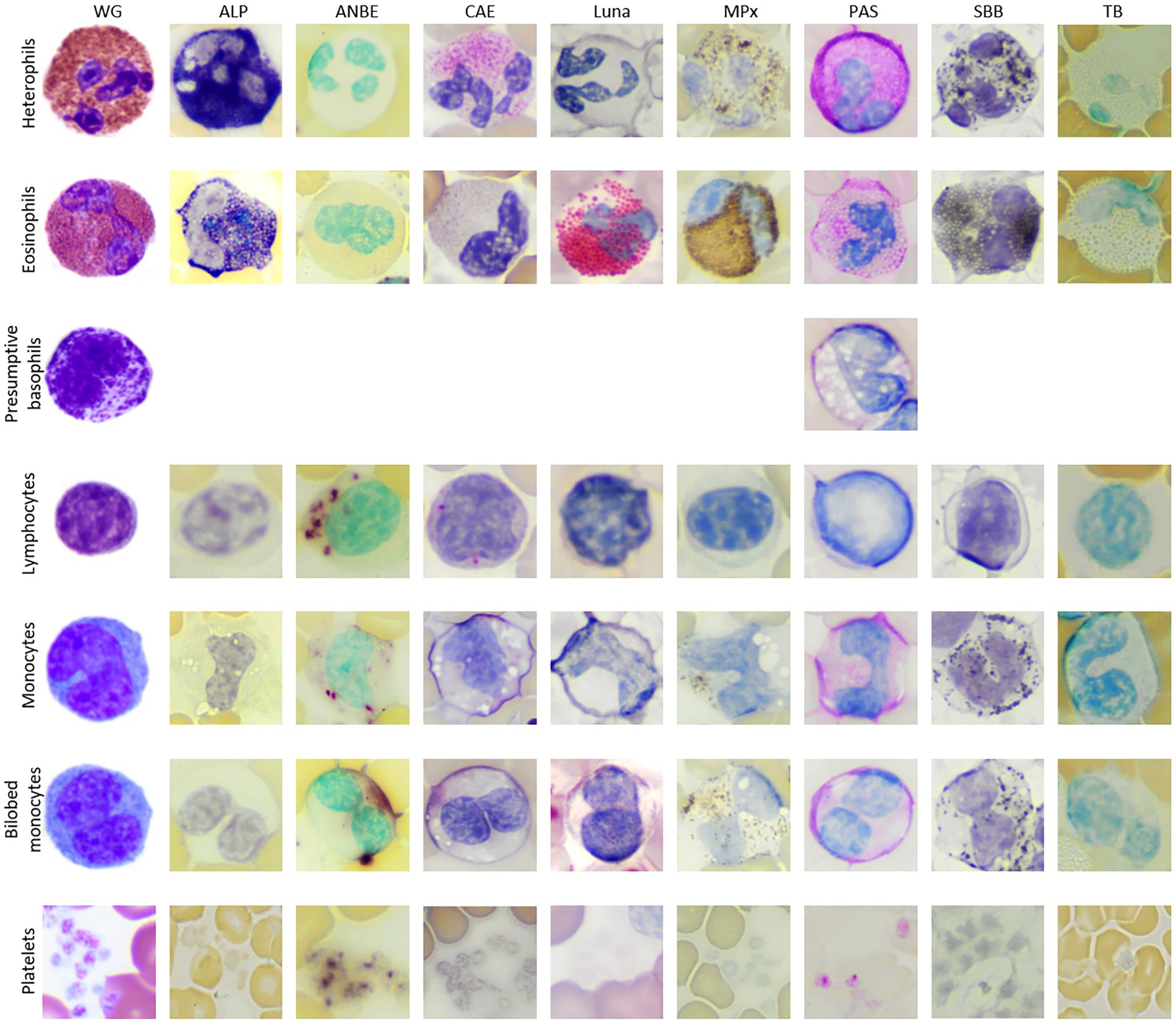
Figure 2. Image composite of leukocytes of the Florida manatee (Trichechus manatus latirostris) from Wright-Giemsa-stained blood films and blood films stained with eight cytochemical stains (ALP, alkaline phosphatase; ANBE, α-naphthyl butyrate esterase; CAE, chloroacetate esterase; Luna; MPx, myeloperoxidase; PAS, periodic acid-schiff, SBB; sudan black B; TB, toluidine blue). Basophils were not conclusively identified with the cytochemical stains, except for PAS.
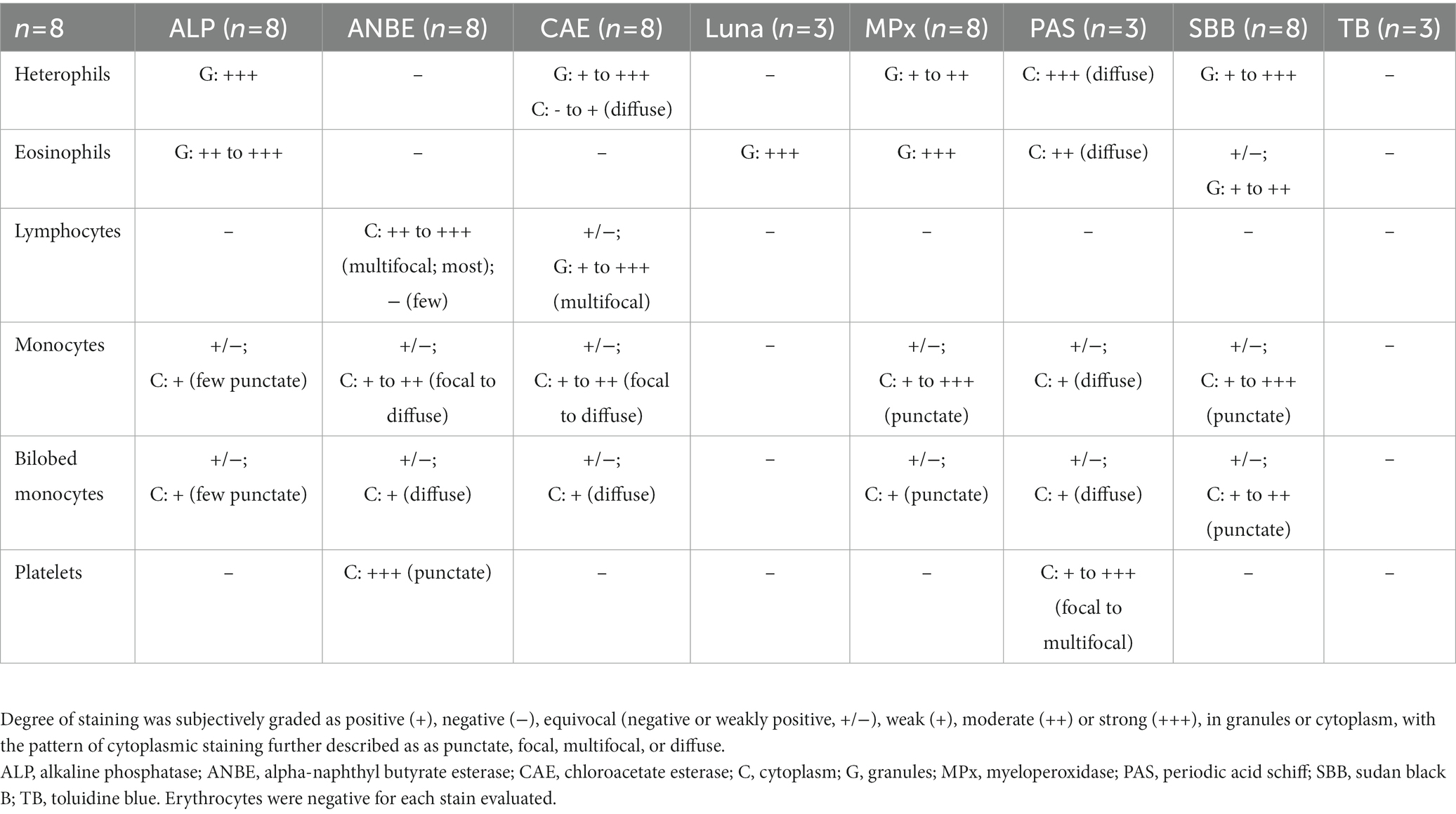
Table 2. Cytochemical staining characteristics of leukocytes and platelets in Florida manatees (Trichechus manatus latirostris).
Heterophils
Heterophils were the predominant WBC in manatees (mean 46%, range 36–54%). Heterophils measured ~12–16 μm in diameter and contained a segmented nucleus with 2–4 segments and clumped chromatin. They were characterized by abundant round, oval, or slightly elongated pink granules in a colorless to light blue cytoplasm in Wright-Giemsa-stained blood films. Cytochemical staining yielded strong ALP staining, variable (weak to strong) staining for CAE and SBB, and weak to moderate staining for MPx in the granules. The cytoplasm of heterophils was negative or weakly diffuse positive for CAE and strongly diffuse positive with slight granular appearance for PAS. Heterophils were negative for ANBE, Luna, and TB stains.
Eosinophils
Eosinophils comprised a low percentage of the WBC population (mean 3%, range 2–6%). Eosinophils measured ~13 to 18 μm in diameter and contained a segmented nucleus with clumped chromatin. They contained round pink cytoplasmic granules in a colorless to pale blue cytoplasm. The granules were slightly larger and brighter pink than heterophil granules in Wright’s-Giemsa-stained blood films. Eosinophil granules had moderate to strong staining for ALP, strong positive staining for Luna and MPx and variable (negative to moderate positive) staining for SBB. They had moderate diffuse cytoplasmic positivity for PAS. MPx and PAS staining was subjectively stronger and weaker, respectively, in eosinophils compared to heterophils. Eosinophils were negative for ANBE, CAE, and TB stains.
Basophils
Basophils were identified in very low numbers (mean 0.6%, range 0–2%). They measured ~13 to 14 μm in diameter and contained an indented nucleus with clumped chromatin. The cytoplasm was colorless and contained variable numbers of irregularly sized, round purple granules in Wright’s-Giemsa-stained blood films. Basophils were not convincingly identified with most of the cytochemical stains, except for PAS, where they appeared negative.
Lymphocytes
Lymphocytes were the second most frequent WBC type following heterophils (mean 36%, 32–49%). Lymphocytes ranged from 9 to 13 μm in diameter (small to intermediate) with rare lymphocytes up to 15 to 16 μm (large lymphocytes). In Wright-Giemsa-stained blood films, the majority of lymphocytes were round to oval with a round to infrequently indented or lobulated nucleus, containing finely granular to clumped chromatin. They had a small rim of pale blue cytoplasm. Rare lymphocytes with red cytoplasmic granules were noted. On cytochemical staining, lymphocytes had moderate to strong chunky multifocal ANBE cytoplasmic staining. Lymphocytes stained negatively or showed multifocal weak positive granular staining in the cytoplasm with CAE. Lymphocytes were negative for ALP, Luna, MPx, PAS, SBB, and TB stains.
“Classic” monocytes
Monocytes were noted in low to moderate numbers (mean 11.5%, range 7–15%). With the Wright-Giemsa stain, monocytes measured ~13 to 18 μm in diameter and had an irregular to reniform to oval nucleus with fine, lacy to slightly condensed chromatin. They had a moderate to abundant amount of pale blue to gray cytoplasm that infrequently contained a few small, punctate, non-staining vacuoles and/or occasional fine, pink granules. Cytochemical staining was variable, with individual cells being variably positive for all stains except negative staining for Luna and TB. Positive cytoplasmic staining reactions ranged from weak to moderate focal to diffuse positivity for ANBE and CAE, weak diffuse positivity for PAS, low numbers of weak positive granules for ALP, and weak to strong granular staining for MPx and SBB.
Bilobed mononuclear cells
Bilobed mononuclear cells constituted about 1–3% (mean 2%) of the WBC differential count. With the Wright-Giemsa stain, bilobed mononuclear cells measured ~14–18 μm in diameter and contained indented to bilobed nuclei with lacy to condensed chromatin. They had a moderate amount of pale blue to gray cytoplasm that rarely contained a few small, punctate, non-staining vacuoles, similar to monocytes. Cytochemical staining resulted in negative to weakly positive staining for ALP, ANBE, CAE, MPx, PAS, and SBB. Cytochemical staining reactions largely mimicked those of monocytes, although reactions were judged weaker for most of the stains. Bilobed mononuclear cells were negative for Luna and TB. The staining characteristics of bilobed mononuclear cells support a monocyte origin for these cells.
Platelets
Platelets were identified in small clumps or were scattered individually throughout the smears. They were round to oval with elongated forms and contained pink to red cytoplasmic granules in blood films stained with Wright-Giemsa. Platelets showed strong granular staining for ANBE and weak to strong granular focal to multifocal staining for PAS in their cytoplasm. They were negative for the other stains.
Discussion
This study used morphological features in a routine hematological stain and a suite of cytochemical stains to characterize WBCs and platelets and classify WBC in manatees. Heterophils were the predominant WBC in manatees, which is in contrast to elephants in which monocytes predominate (12, 13). As in this study, heterophils and lymphocytes comprised the majority of leukocytes in previous studies evaluating free-ranging and captive manatees along with free-ranging dugongs (7, 26). Harvey et al. concluded that heterophils and lymphocytes are typically present in approximately equal numbers, with manatees in managed care (n = 62) having slightly but significantly higher absolute numbers of heterophils than free-ranging manatees (n = 52) (mean of 3.25 × 109/L versus 2.33 × 109/L, respectively) (7). The animals in this study were considered clinically normal based on physical examination and results of blood analyses. Comparing morphological features and cytochemical staining to other species in the same clade, manatee heterophils have similar granule staining compared to dugongs but smaller and less prominent granule staining compared to elephants and rock hyraxes in Wright’s-stained blood films (13). Cytochemical staining reactions in heterophils were more similar comparing manatees to elephants versus the dugong (Table 3).
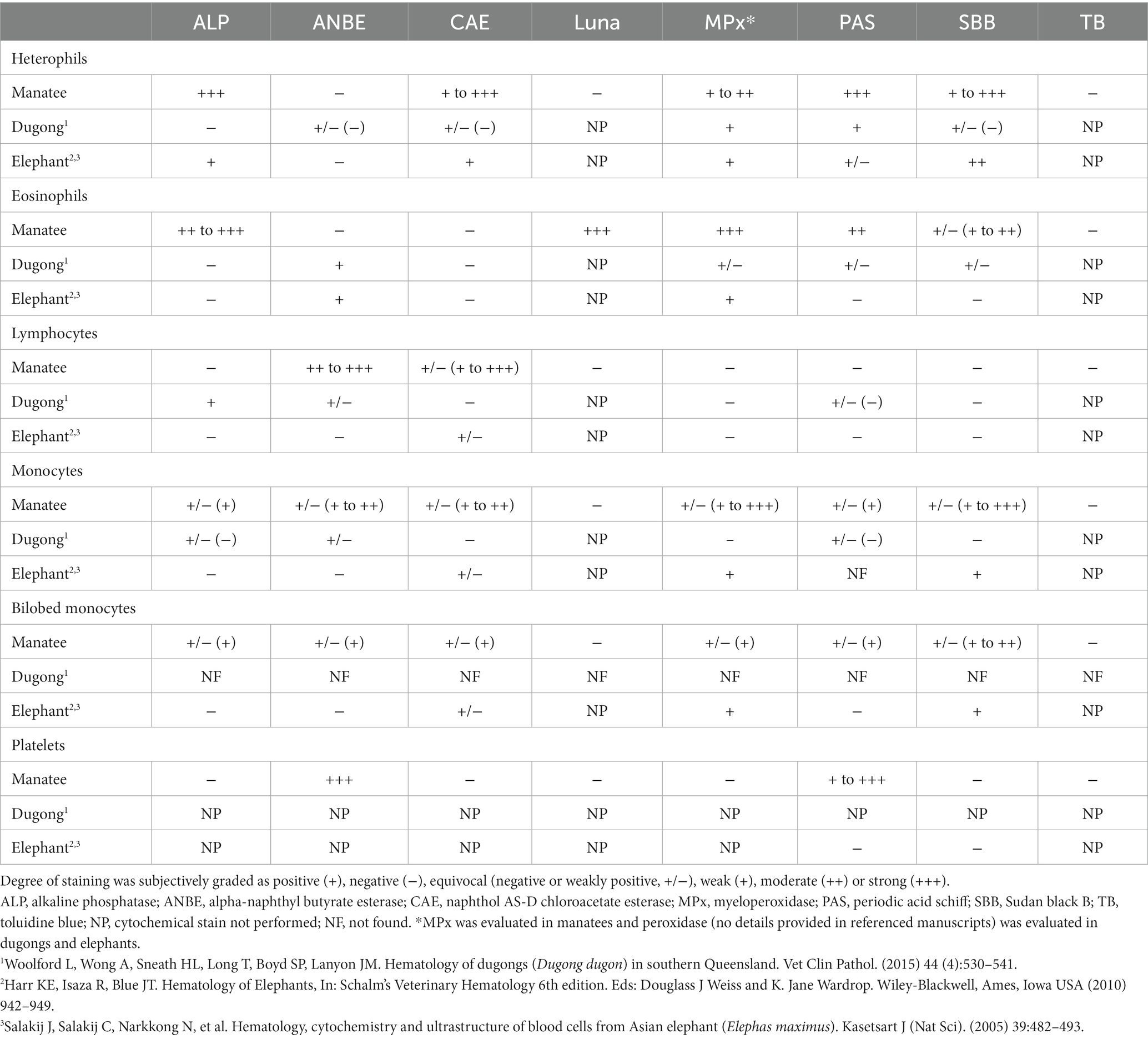
Table 3. Cytochemical staining comparisons between manatees (Trichechus manatus latirostris), dugongs (Dugong dugon), and elephants.
Eosinophils have previously been reported to stain positive for Luna, Luxol fast blue, MPx, and SBB with inconsistent staining with CAE or ANBE in other mammals (18, 24, 25, 27, 28). In this study, manatee eosinophils showed positive staining reactions for ALP, MPx, PAS, and SBB, which differs from that reported in elephants and dugongs (Table 3). They were also the only WBC that was positive with the Luna stain, indicating that this is a useful stain for differentiating eosinophils from heterophils in this species (Luna staining has not yet been evaluated in elephants or dugongs). Basophils were seen in too few numbers to ascertain their characteristic reactions with the cytochemical stains.
Lymphocytes in the manatees of this study were morphologically similar to other animals with a Wright-Giemsa stain, and absolute numbers were comparable to free ranging manatees evaluated in Harvey and colleagues and similar to that reported for elephants and dugongs (7, 12, 13, 26). Lymphocytes could be differentiated from monocytes in this study by negative staining reactions for ALP, MPx, PAS, and SBB, which is similar to elephant and dugong lymphocytes except that dugong lymphocytes stain positively for ALP and have variable positivity with PAS (Table 3).
In Wright-Giemsa-stained blood films, monocytes in the manatees of this study shared morphological characteristics with “classic” monocytes in other mammals although numbers are lower in manatees and dugongs than in elephants (7, 12, 13, 26). Monocyte cytochemical staining reactions largely differed in the manatee versus the elephant and dugong. In this study, monocytes were variably positive for all stains, except Luna and TB, whereas elephant monocytes are positive for MPx and variably positive for CAE and dugong monocytes can be positive for ANBE, ALP and PAS (12, 13, 26) (Table 3).
Bilobed monocytes have been identified in various species, particularly elephants, rock hyraxes, and giant pandas (12, 14, 26). Cytochemical staining for MPx and SBB has been previously used to aid in differentiating bilobed monocytes from lymphocytes in elephants and red pandas with monocytes being positive and lymphocytes being negative for these stains (12, 14) (Table 3). Cytochemical staining of the bilobed mononuclear cell identified in Wright-Giemsa-stained blood films of the manatees in this study yielded similar results to monocytes, indicating that bilobed mononuclear cells in manatees could be classified as a monocyte variant. It is suspected that these bilobed monocytes represent either a morphological variant with a bilobed nucleus that initially develops as a spherical nucleus and that subsequently acquires an indentation resulting in a separation of the lobes (29) or an acquired phenotypic variant (6). Manatees and rock hyraxes have far lower proportions of these cells (1–3%) compared to elephants, which can have between 20 and 60% bilobed monocytes (12, 13, 26). Bilobed monocytes were not described in a recent study of dugongs (26), again demonstrating species differences within the clade. We recommend these bilobed cells are counted as monocytes when performing a differential leukocyte count on Romanowsky-type stained blood films from manatees. Since their numbers are low in clinically normal free-ranging manatees, there are minimal consequences for not correctly identifying bilobed cells as monocytes in Wright-Giemsa-stained blood films. However, it is unknown if and how the proportions of these bilobed monocytes change with various diseases or clinical conditions in manatees. Monocytosis has been documented in manatees with cold-stress syndrome, although it is not known if the monocyte counts in that study included the bilobed variants (30). If the proportions of the latter cells increase with specific diseases, misclassification as lymphocytes may have more clinical relevance in manatees.
Manatee platelets had strong cytoplasmic granular positivity to ANBE and weak to strong positivity with PAS in this study. PAS positivity appears seen in platelets across many animal species, including thrombocytes of birds and reptiles, but is negative in elephants (13, 18, 31, 32) (Table 3). Nonspecific esterase staining has been previously identified in platelets of cats and dogs with positive staining of thrombocytes in green sea turtles and iguanas (19).
Conclusion
In this study, we identified a type of leukocyte not reported in manatees to date, a bilobed mononuclear cell. These cells were classified as monocytes based on morphological and cytochemical staining characteristics, which is similar to other select species within the Paenungula, including Asian and African elephants and, based on morphological features alone, the rock hyrax. This new information will aid in determining accurate WBC differential cell counts in Florida manatees. Differences in cytochemical staining reactions for WBCs and platelets were seen in manatees of this study compared to elephants and dugongs. These differences can potentially be explained by species-specific expression of proteins or enzymes or the type of cytochemical stains used in each study. This study provides new information on the morphological features and cytochemical staining characteristics of Florida manatee WBCs and platelets. This information will aid in obtaining accurate hematological data for this species and is relevant for health assessment studies, diagnosis and monitoring of disease during rehabilitation, and clinical management of these animals.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This project was approved by the University of Florida’s Institutional Animal Care and Use Committee #202006823 and samples were collected under US Fish and Wildlife Service research permits #MA791721 and #MA067116-2.
Author contributions
LC acquired the data, took the photomicrographs, compiled the image composites and tables, and drafted the manuscript. NS designed the study, acquired the samples, performed the Wright-Giemsa staining, analyzed the data, interpreted the cytochemical staining reactions, took photomicrographs, and wrote the manuscript. TS completed the cytochemical staining and interpretation, took photomicrographs, performed the data analysis, interpretation, and wrote the manuscript. JH assisted with the data interpretation and manuscript editing. MdW, LA, MW, and RB supported sample acquisition, provided logistical support, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the Florida Fish and Wildlife Conservation Commission.
Acknowledgments
The authors thank the biologists and veterinarians with the Florida Fish and Wildlife Conservation, the US Geological Survey, and Aquatic Animal Health Program, University of Florida. We also thank the Cornell University, Veterinary Diagnostic Laboratory, for technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kurth, JW. Endangered and threatened wildlife and plants; reclassification of the west indian manatee from endangered to threatened. Fed Regist. (2017) 82:16668–704.
2. Runge, MC, Sanders-Reed, CA, Langtimm, CA, Hostetler, JA, Martin, J, Deutsch, CJ, et al. Status and threats analysis for the Florida manatee (Trichechus manatus latirostris). (2016) U.S. Geological Survey Scientific Investigation Report 2017–5030, 40. doi: 10.3133/sir20175030
3. Deutsch, CJ. Trichechus manatus ssp. latirostris (Florida Manatee). (2008) Available at: https://www.iucnredlist.org/species/22106/9359881
4. Kunter, M, May-Collado, LJ, and Agnarsson, I. Phylogeny and conservation priorities of afrotherian mammals (Afrotheria, Mammalia). Zool Scr. (2011) 40:1–15. doi: 10.1111/j.1463-6409.2010.00452.x
5. Pardini, AT, O’Brien, PC, Fu, B, Bonde, RK, Elder, FFB, Ferguson-Smith, MA, et al. Chromosome painting among proboscidea, hyracoidea and sirenia: support for paenungulata (afrotheria, mammalia) but not tethytheria. Proc Bio Sci. (2007) 274:1333–340. doi: 10.1098/rspb.2007.0088
6. Allen, JL, Jacobson, ER, Harvey, JW, and Boyce, W. Hematologic and serum chemical values for young African elephants (Loxodonto africana) with variations for sex and age. J Zoo Wild Med. (1985) 16:98–101. doi: 10.2307/20094754
7. Harvey, JW, Harr, KE, Murphy, D, Walsh, MT, Nolan, EC, Bonde, RK, et al. Hematology of healthy Florida manatees (Trichechus manatus). Vet Clin Pathol. (2009) 38:183–93. doi: 10.1111/j.1939-165X.2009.00113.x
8. Steyrer, C, Miller, M, Hewlett, J, Buss, P, and Hooijberg, EH. Reference intervals for hematology and clinical chemistry for the African elephant (Loxodonta africana). Front Vet Sci. (2021) 8:599387. doi: 10.3389/fvets.2021.599387
9. Perrin, KL, Kristensen, AT, Gray, C, Nielsen, SS, Bertelsen, MF, and Kjelgaard-Hansen, M. Biological variation of hematology and biochemistry parameters for the Asian elephant (Elephas maximus), and applicability of population-derived reference intervals. J Zoo Wildl Med. (2020) 51:643–51. doi: 10.1638/2020-0007
10. Medway, W, Rathbun, GB, and Black, DJ. Hematology of the West Indian manatee (Trichechus manatus). Vet Clin Pathol. (1982) 11:11–5. doi: 10.1111/j.1939-165x.1982.tb00822.x
11. Schalm, OW, Jain, NC, and Carroll, EJ. Veterinary Hematology, 3rd Edn. Philadelphia, Lea & Febiger. (1975) pp. 219–283.
12. Harr, KE, Isaza, R, and Blue, JT. Hematology of Elephants In: J Douglass, K Weiss, and J Wardrop, editors. Schalm’s Veterinary Hematology. 6th ed. Ames, Iowa USA: Wiley-Blackwell (2010). 942–49.
13. Hooijberg, EH. Hematology of paenungulata: elephants, sirenians, and hyraxes In: MB Brooks, KE Harr, D Seelig, KJ Wardrop, and DJ Weiss, editors. Schalm’s Veterinary Hematology. 7th ed. Ames, Iowa USA: Blackwell (2022). 1090–103.
14. Kehoe, SP, Stacy, NI, Frasca, S, Stokol, T, Wang, C, Leach, KS, et al. Leukocyte and platelet characteristics of the Giant Panda (Ailuropoda melanoleuca): morphological, cytochemical, and ultrastructural features. Front Vet Sci. (2020) 7:156. doi: 10.3389/fvets.2020.00156
15. McLaughlin, AB, Epstein, JH, Prakash, V, Smith, CS, Daszak, P, Field, HE, et al. Plasma biochemistry and hematologic values for wild-caught flying foxes (Pteropus giganteus) in India. J Zoo Wildl Med. (2007) 38:446–52. doi: 10.1638/1042-7260(2007)38[446:PBAHVF]2.0.CO;2
16. Aroch, I, King, R, and Baneth, G. Hematology and serum biochemistry values of trapped, healthy, free-ranging rock hyraxes (Procavia capensis) and their association with age, sex, and gestational status. Vet Clin Pathol. (2007) 36:40–8. doi: 10.1111/j.1939-165x.2007.tb00180.x
17. Stacy, NI, Isaza, R, and Wiedner, E. First report of changes in leukocyte morphology in response to inflammatory conditions in Asian and African elephants (Elephas maximus and Loxodonta africana). PLoS One. (2017) 12:e0185277. doi: 10.1371/journal.pone.0185277
18. Canfield, PJ. Comparative cell morphology in the peripheral blood film from exotic and native animals. Aust Vet J. (1998) 76:793–800. doi: 10.1111/j.1751-0813.1998.tb12328.x
19. Raskin, RE, Santangelo, K, and Polak, K. Cytochemical Staining and Immunocytochemistry In: MB Brooks, KE Harr, D Seelig, KJ Wardrop, and DJ Weiss, editors. Schalm’s Veterinary Hematology. 7th ed. Ames, Iowa USA: Blackwell (2022). 478–99.
20. Li, C-Y, and Yam, LT. Cytochemistry and immunochemistry in hematologic diagnoses. Hematol Oncol Clin N Am. (1994) 8:665–81. doi: 10.1016/S0889-8588(18)30153-9
21. Stamper, MA, and Bonde, RK. Health assessment of captive and wild-caught West Indian manatees (Trichechus manatus). Sirenian Conserv. (2012):139. doi: 10.2307/j.ctvx079z0.22
22. Bonde, RK, Garrett, A, Berlanger, M, Askin, N, Tan, L, and Wittnich, C. Biomedical health assessments of Florida manatee in crystal river – providing opportunities for training during the capture, handling, and processing of this unique aquatic mammal. J Mar Anim Ecol. (2012) 5:17–28.
23. Stokol, T, Schaefer, M, Shuman, M, Belcher, N, and Doug, L. Alkaline phosphatase is a useful cytochemical marker for the diagnosis of acute myelomonocytic and monocytic leukemia in the dog. Vet Clin Path. (2015) 44:79–93. doi: 10.1111/vcp.12227
24. Luna, LG. Luna’s method for erythrocytes and eosinophil granules In: LG Luna, editor. Manual of histologic staining methods of the armed forces institute of pathology. 3rd ed. New York: McGraw Hill (1968). 111–12.
25. MacManus, JF, and Mowry, RW. Staining methods: histologic and histochemical. New York, NY: Hoeber Med Div Harper & Row. (1960) 132–261.
26. Woolford, L, Wong, A, Sneath, HL, Long, T, Boyd, SP, and Lanyon, JM. Hematology of dugongs (Dugong dugon) in southern Queensland. Vet Clin Pathol. (2015) 44:530–41. doi: 10.1111/vcp.12305
27. Work, TM, Raskin, RE, Balazs, GH, and Whittaker, SD. Morphologic and cytochemical characteristics of blood cells from Hawaiian green turtles. Am J Vet Res. (1998) 59:1252–257.
28. Young, KM, Moriello, KA, and Peickert, H. Characterization of eosinophil progenitor cells in feline bone marrow. Am J Vet Res. (1997) 58:348–53.
29. Skinner, BM, and Johnson, EE. Nuclear morphologies: their diversity and functional relevance. Chromosoma. (2017) 126:195–212. doi: 10.1007/s00412-016-0614-5
30. Martony, M, Hernandez, JA, de Wit, M, Leger, JS, Erlacher-Reid, C, Vandenberg, J, et al. Clinicopathological prognostic indicators of survival and pathological findings in cold-stressed Florida manatees Trichechus manatus latirostris. Dis Aquat Org. (2019) 132:85–97. doi: 10.3354/dao03306
31. Mateo, MR, Roberts, ED, and Enright, FM. Morphologic, cytochemical, and functional studies of peripheral blood cells of young healthy American alligators (Alligator mississippiensis). Am J Vet Res. (1984) 45:1046–53.
32. Swayne, DE, Stockham, SL, and Johnson, GS. Cytochemical properties of chicken blood cells resembling both thrombocytes and lymphocytes. Vet Clin Pathol. (1986) 15:17–24. doi: 10.1111/j.1939-165x.1986.tb00639.x
Glossary
Keywords: Sirenia, Afrotheria, cytochemistry, hematology, leukocyte morphology, white blood cells
Citation: Cagle LA, Stacy NI, Harvey JW, de Wit M, Adler L, Walsh M, Bonde R and Stokol T (2023) Cytochemical staining of leukocytes and platelets in the Florida manatee (Trichechus manatus latirostris): identification of a bilobed monocyte similar to other members of the Paenungulata. Front. Vet. Sci. 10:1149000. doi: 10.3389/fvets.2023.1149000
Edited by:
Peter James O'Brien, University College Dublin, IrelandReviewed by:
Ashley Barratclough, National Marine Mammal Foundation, United StatesJennifer Johns, Oregon State University, United States
Copyright © 2023 Cagle, Stacy, Harvey, de Wit, Adler, Walsh, Bonde and Stokol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicole I. Stacy, c3RhY3luQHVmbC5lZHU=
 Laura A. Cagle
Laura A. Cagle Nicole I. Stacy
Nicole I. Stacy John W. Harvey2
John W. Harvey2 Martine de Wit
Martine de Wit Michael Walsh
Michael Walsh Tracy Stokol
Tracy Stokol