- 1Department of Parasitology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 2Department of Veterinary Population Medicine, University of Minnesota, St. Paul, MN, United States
- 3Department of Animal Wealth Development, Biostatistics Section, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 4Department of Health Management, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, PEI, Canada
- 5Department of Microbiology, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia
- 6Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Najran University, Najran, Saudi Arabia
- 7Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Najran University, Najran, Saudi Arabia
- 8Department of Animal Medicine, Division of Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 9Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 10Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
Toxoplasmosis is a global zoonotic disease caused by Toxoplasma gondii (T. gondii). The primary aim of this study was to identify hygienic and cat management practices that could affect the occurrence of T. gondii in cats and their owners in Sharqia Governorate, Egypt. T. gondii infection was evaluated in 80 pregnant women and 29 domestic cats using Real-time PCR. A questionnaire was administered to obtain information regarding the risk factors associated with T. gondii infection. Blood samples were collected from enrolled pregnant women, and fecal samples were collected from their pet cats. Firth logistic regression model complemented with random forest (RF) analysis was used to evaluate the association of different hygiene and cat management practices with T. gondii infection in pregnant women. T. gondii infection was identified in 27.5% of pregnant women and 17% of domestic cats. Drinking raw milk and contacting stray and pet cats were significantly associated with higher odds of T. gondii infection. The proportion of T. gondii-positive women who ate raw meat (60.0%) was higher than those ate heat-cooked meat (25.3%). Moreover, women who did not wash their hands after contact with cats were 6 times (OR 6.12; CI: 3.03–9.21) more likely to experience T. gondii infection than those washed their hands after cat contact. The RF analysis showed that washing hands constitute a serious yet preventable public health concern that requires targeted, community-specific efforts. Cat owners, particularly pregnant women, need to be aware about the risk of T. gondii infection, while handling cat and pet's owner should be advised to take necessary hygienic measures to prevent its infection.
1. Introduction
Toxoplasma gondii is an obligatory intracellular protozoan parasite that causes worldwide toxoplasmosis in all warm-blooded animals and humans. Previous meta-analyses showed pooled prevalence rates of 1.1 and 33.8% for acute and latent Toxoplasma infection in pregnant women, respectively (1, 2). The prevalence of human toxoplasmosis substantially varies from one region to other (3, 4). Previously, reported risk factors included the type and the cooking method of food, adequate treatment for drinking water, contact with cats being immunocompromised (5–7). In Egypt, similar risk factors were previously reported (8–14). The life cycle of T. gondii includes final hosts (i.e., the Felidae family, mainly cats), where the sexual cycle of replication of T. gondii oocytes occurs and intermediate hosts (i.e., non-feline including dogs and humans). The intermediate hosts primarily are infected by consuming undercooked meat or food contaminated with oocytes excreted by cats (15). Clinically, toxoplasmosis is most often in apparent in humans; however, clinical symptoms sometimes become critical particularly when congenital or postnatal infection occurs. The congenital infection mainly occurs in the first trimester, when pregnant women are more susceptible to T. gondii compared to the rest of the pregnancy stages (16, 17). Congenital toxoplasmosis might also affect the central nervous system resulting in neurological complications and ocular lesions (18).
In most cases, toxoplasmosis is a latent infection; however, non-specific clinical signs could also appear. Therefore, diagnosing T. gondii using clinical signs might be infeasible. Alternatively, serological tests such as the direct agglutination test, the latex agglutination test, and ELISA are commonly used to detect humoral antibodies (19). Moreover, molecular identification using PCR has been showing higher sensitivity and specificity than serological assays (20–23). Varying levels of toxoplasmosis seroprevalence in Egyptian pregnant women, ranging from 3.8 to 67.5%, have been reported across different governorates (9, 10, 13, 21, 24–27), with a pooled rate of 45.9% (2). Sharqia Governorate is Egypt's third most populous governorate (28), and it has been experiencing an increasing trend of keeping domestic cats and a growing number of stray cats. Health and hygiene practices have also been challenging, especially in the rural areas of the Governorate. A previous study focused on the molecular identification of T. gondii DNA in the milk samples from goats, sheep and cows in the governate (27). However, no study has addressed the molecular positivity of T. gondii in both pregnant women and cats in this area. The primary aim of this study was to identify hygienic and cat management practices affecting the occurrence of T. gondii in cats and pregnant women in Sharqia Governorate, Egypt.
2. Materials and methods
2.1. Patients and study area
Data on the hygiene and cat management practices associated with T. gondii infection were collected using a structured questionnaire adapted from previous studies (9, 29). The main domains of the questionnaire included socio-demographic characteristics, hygienic practices, and cat management factors. The questionnaire was initially drafted in Arabic; however, a full English-translated version is available in in a Supplementary Table S1. The questionnaire was distributed among 80 pregnant women admitted to Al-Ahrar hospital and Minya-Elqamh hospital in the Sharqia governorate, Egypt. Written consent was obtained from all participating women, and they were also asked about their willingness to enroll their pet cats in the study.
2.2. Collection of human blood and cat fecal samples
Peripheral blood samples were collected from all enrolled women and stored at −20°C for DNA extraction. Blood samples were collected on EDTA as anticoagulant (1 mg/mL). The participants who agreed to enroll their pet cats into the study were advised to collect fresh feces into an air tight plastic bag and store them at 4°C for transportation to the Laboratory of Parasitology, Faculty of Veterinary Medicine, Zagazig University. This study followed the guidance of the Research, Publication, and Ethics Committee of the Faculty of Veterinary Medicine, Zagazig University, Egypt (ethical approval number: ZU-IACUC/2/F/205/2022).
2.3. Fecal samples processing
The oocytes of T. gondii were recovered using the floataion centrifugation technique. Three grams of fecal samples were mixed with distilled water in a centrifuge tube and spun at 3,000 rpm for 5 min. The clear supernatant was discarded, and saturated NaCl solution was added until ¾ of the tube's height; then, the tube was centrifuged again at 3,000 rpm for 5 min. Subsequently, we filled the tubes with saturated NaCl and let them sit for 20 min. We carefully touched a glass slide on the water surface, then covered it with a cover slide and examined the presence of thick-walled unstained oocysts under the microscope (400 ×) (30). The examined positive and negative fecal samples were stored at −20°C for confirmation by molecular identification.
2.4. Real-time PCR of T. gondii
Quantitative molecular identification was used to identify the B1 gene. The QIAamp DNA Mini Kit (Qiagen, Germany, GmbH) was used to extract DNA according to the producer's instructions, followed by ten cycles of freezing (in liquid nitrogen) and thawing (in a water bath at 60°C) to disrupt the oocytes. Briefly, 200 μL from each fecal suspension and blood sample were incubated with 10 μL of proteinase K and 200 μL of lysis buffer at 56°C for 10 min. After incubation, 200 μL of absolute ethanol was added to the lysate. The sample was then washed and centrifuged following the manufacturer's recommendations. The nucleic acid was eluted with 100 μL of elution buffer provided in the kit. The oligonucleotide primer supplied from Metabion, Germany was used. The primer sequences used in this study and PCR cycle conditions carried out in Strata gene MX3005P RT PCR machine (31) are available in a Supplementary Table S2. The extracted DNA samples were examined by Real-time PCR using primers in a 25 μL reaction containing 12.5 μL of 2 × QuantiTect SYBR Green PCR Master Mix (Qiagen, Gmbh), 1 μL of each primer of 20 pmol concentration, 4.5 μL of water, and 6 μL of DNA template. A positive control T. gondii strain was kindly obtained from the biotechnology department, Animal Health Research Institute, Dokki, Giza, and a reaction mixture with no added DNA was run in the PCR reaction as positive and negative controls, respectively.
2.5. Data analysis
Toxoplasma gondii positivity among pregnant women was modeled as a categorical outcome encoded as zero for negative individuals and one for cases. Two complementary analyses were performed on the data. We used the penalized maximum likelihood estimation in a Firth logistic regression framework and random forest supervised machine learning algorithms. Due to the small sample size and few positive cases, estimates from conventional logistic regressions had wide confidence intervals (CI). We ran univariable regression models fitting each risk factor as an independent variable and variables associated with the outcome at a p-value ≤ 0.1 were subsequently included in the multivariable model. Wald's test was used to evaluate the overall significance of categorical variables with more than 2 levels. Results were presented as odds ratios (OR) and 95% CI. We found no evidence of confounding or significant interaction among the tested variables.
A random forest (RF) classification analysis was also applied to complement the statistical model so that inferences would not be solely based on the p-values (32). The RF analysis allows for a multi-way comparison of all independent variables; it uses different metrics to weigh the relative importance of each variable in explaining variability in the outcome (33, 34).
In the RF model, we split the data such that 80% was used for training and parametrising the model, and the remaining 20% was used to make predictions and test model performance. After model tuning using hyperparameters, we ran 500 iterations and evaluated model performance and tuning using accuracy (overall proportion of observations correctly classified as T. gondii positive and negative), sensitivity (proportion of T. gondii positive cases correctly classified), and specificity (proportion of T. gondii negative correctly classified). Additionally, we generated variable importance plots, which ranked specific model covariates based on their influence on model accuracy and the Gini index, which represents when the data was split based on a given predictor, and how homogenous were the T. gondii positive and negative groups.
3. Results
The study recruited 80 pregnant women from two hospitals, with 22 women (27.5%) testing positive for T. gondii. The age of women ranged from 18 to 50 years, and 48 (60%) of the 80 were residing in rural areas. Complete demographic data are presented in Table 1.
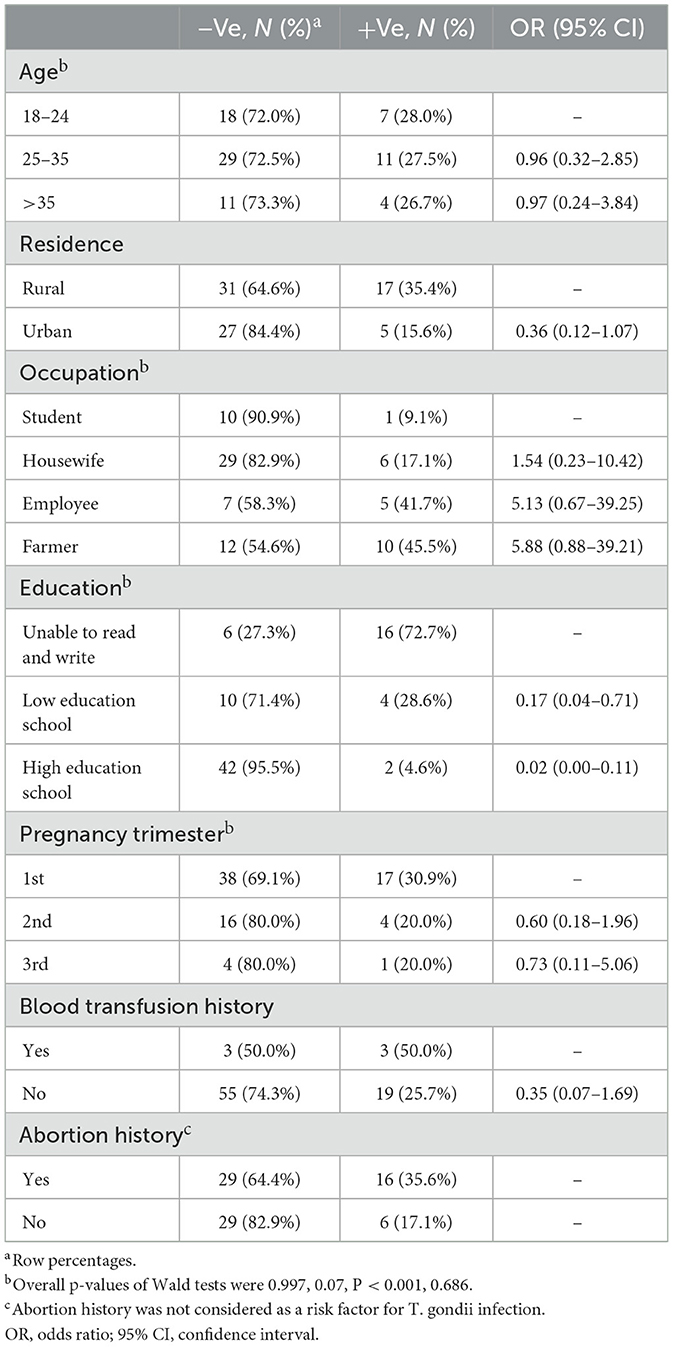
Table 1. Demographic characteristics of the enrolled women and univariable firth logistic regression model.
From the univariable analysis, factors significantly associated with T. gondii infection included education, where the odds of being T. gondii positive decreased by 83 and 98% for low-educated women (i.e., at a lower secondary qualification, at or below International Standard Classification of Education) and high-educated women (i.e., with a Bachelor program, Master degree program, Doctoral degree program at research university), respectively, compared to illiterate women. Employees and farmers showed higher odds (OR: 5.13; 95% CI: 0.67–39.25, and OR: 5.88; 95% CI: 0.88–39.21, respectively) of T. gondii infections compared to students; however, there was no sufficient power to identify significant differences. There was also no evidence of significance that women with a history of blood transfusion were more likely to encounter T. gondii infection.
Drinking raw milk was significantly associated with higher odds of T. gondii infection, such that women drinking raw milk were 5.5 times more likely to experience T. gondii infection than women who used pasteurized or processed milk. Also, getting in contact with stray and pet cats was associated with higher odds of T. gondii infection (OR 47.4; CI: 6.60–342.40). The proportion of T. gondii-positive women who eat raw meat (60.0%) was higher than those who cooked (25.3%). Moreover, women who did not wash their hands after contact with cats were 6 times (OR 6.12; CI: 3.03–9.21) more likely to experience T. gondii infection than those who washed their hands after cat contact (Table 2).
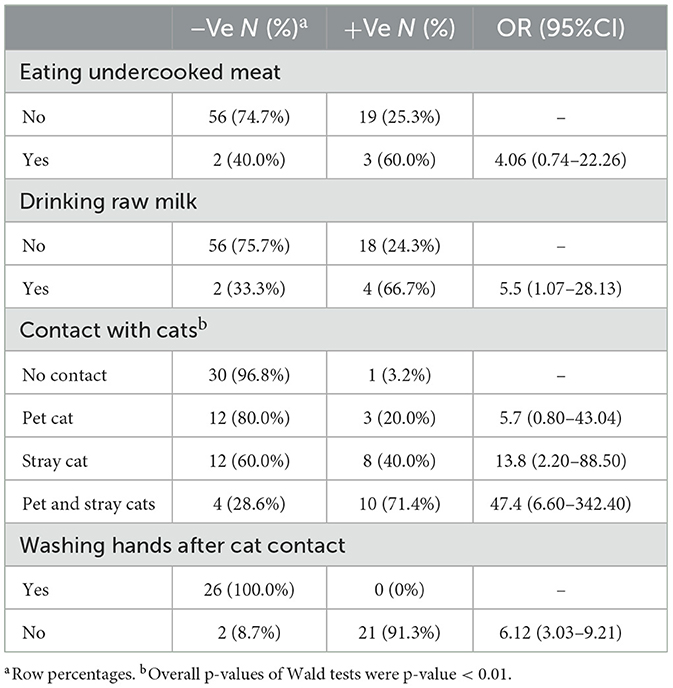
Table 2. A summary of hygienic practices and contact with cats, univariable firth logistic regression model.
The most crucial covariate identified by the RF model was hand washing after contact with cats which was highly ranked both on effect on model accuracy and Gini index. Other highly ranked factors were the levels of education of the women interviewed and their interactions with pets and stray cats, which also had high odds ratios in Firth logistic regression. Interestingly, neither eating undercooked meat nor drinking raw milk came up as highly important in our study population using this analytical approach (Figure 1).
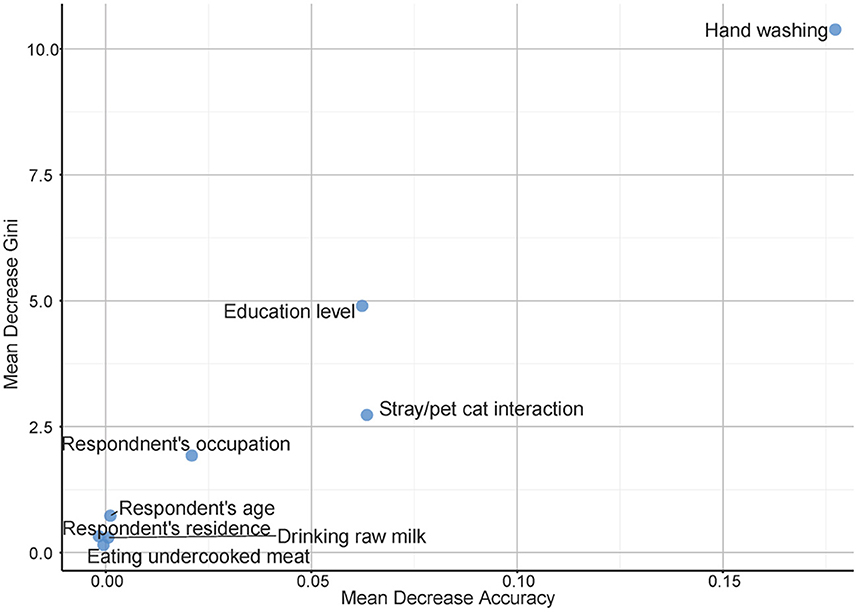
Figure 1. Multi-way variable importance plot from the best random forest model for risk factors associated with toxoplasmosis among pregnant women in Sharqia Governorate, Egypt.
From the partial dependency plots, it was evident that failing to wash hands after contact with cats was the riskiest behavior, with the odds of positive T. gondii cases in those individuals being 1.3 times high compared to almost negligible odds for women who either washed their hands or had no contact with cats (Figure 2). Additionally, the least educated group of our sample population were more likely to be T. gondii positive than any other group. Any contact with cats increased the likelihood of being T. gondii positive irrespective of whether the casts were domestic or stray (Figure 2). Similarly, consumption of raw milk and undercooked meat increased the likelihood of testing positive and women residing in rural parts of the Sharqia governorate were also more likely to be T. gondii positive than those who lived in urban Sharqia (Figure 2).
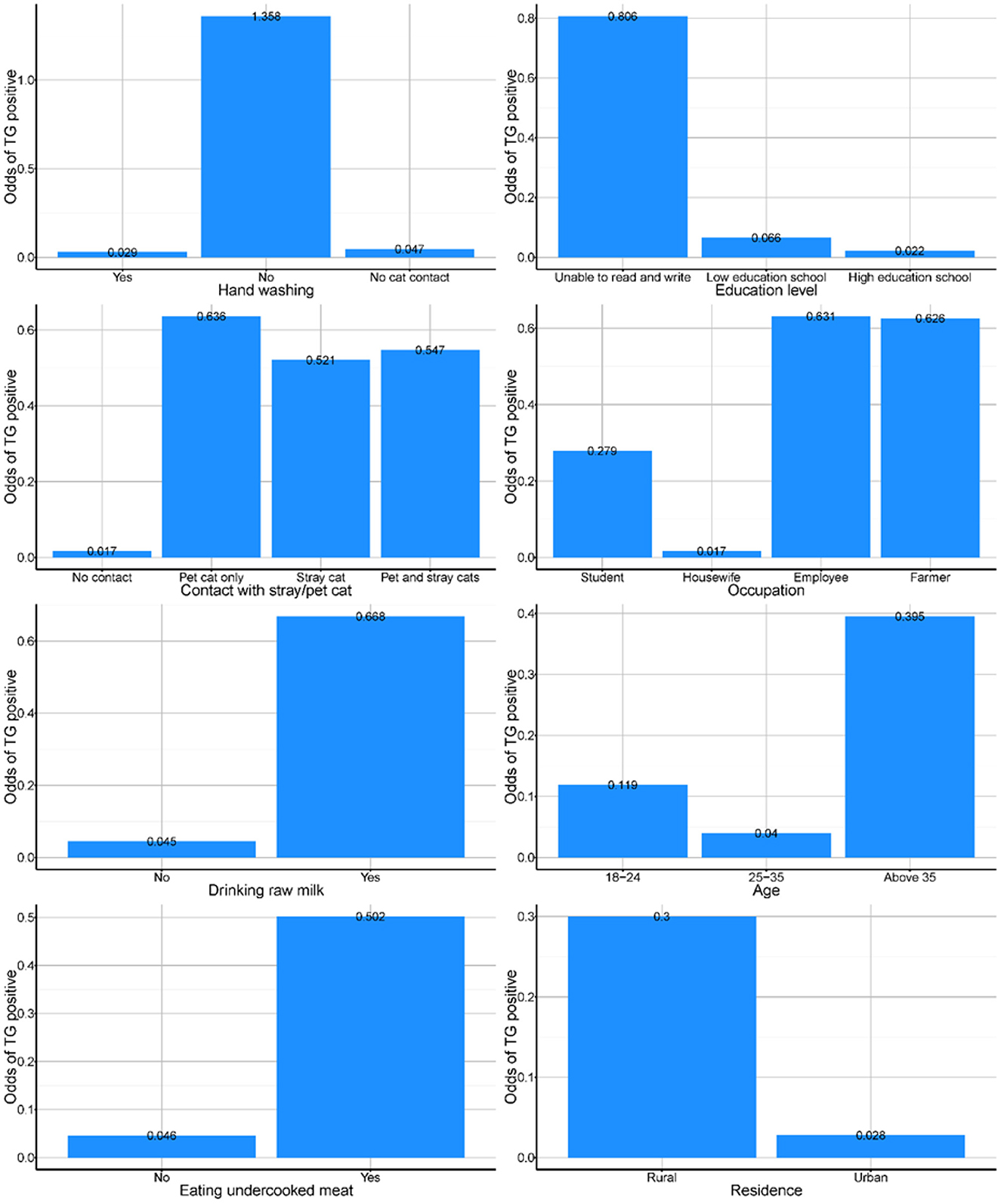
Figure 2. Partial dependency plots from the best random forest model presenting odds of toxoplasmosis among pregnant women exposed to different factors in Sharqia Governorate, Egypt.
Only 29 participants agreed to enroll their domestic cats in the study, of which 5 (17%) were T. gondii positive. Additionally, women letting their pet cats outdoors or allowing them to contact other animals, such as dogs or other stray cats or small ruminants, particularly in rural areas, increased the odds of their cats getting infected with T. gondii. The use of dry food and litter trays, among other factors, was associated with lower odds of T. gondii infection (Table 3).
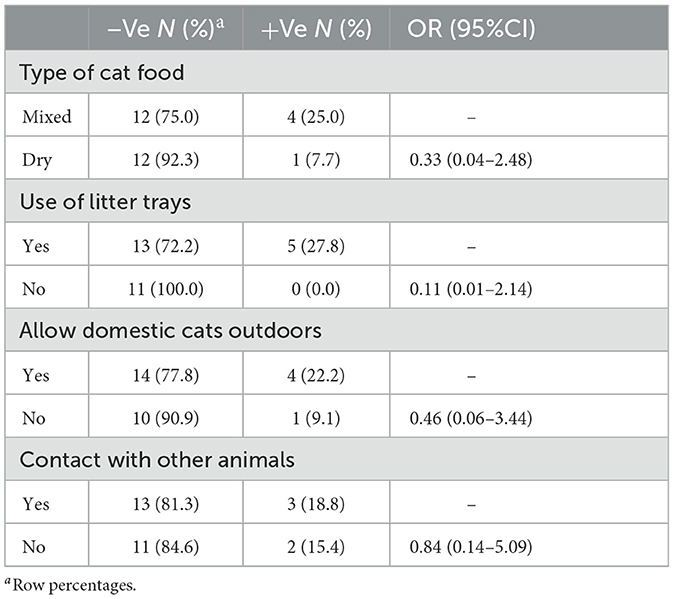
Table 3. A summary of risk factors of pet cats (n = 29), univariable firth logistic regression model.
4. Discussion
Toxoplasmosis, an obligatory intracellular protozoan, is a major global zoonotic disease caused by the protozoan T. gondii (phylum Apicomplexa). Deleterious health effects with severe consequences have been associated with toxoplasmosis in immunocompromised individuals and pregnant women (35). It causes chronic illness and clinical symptoms in neonate cats, geriatric, and immunocompromised animals (36).
Concerning risk factors significantly associated with T. gondii infection include education, where the odds of being T. gondii positive decreased by 83% and 98% for low- and high-educated women, respectively, compared to illiterate women. This finding was similar to Bittencourt et al. (37) and Demiroglu et al. (38) in Brazil Agmas et al. (39), in Ethiopia Alsammani (40), and Olariu et al. (41) who reported that illiterate pregnant women have a higher T. gondii infection in Western Romania. In Egypt, illiterate women cannot read and adopt less hygienic practices and are more likely to live in rural areas where they are more likely to be in close contact with animals as compared to educated ones. However, there were findings that there was no relation between the education level of pregnant women and T. gondii infection in other African countries (40, 42, 43). Regarding occupation, employees and farmers showed higher odds of T. gondii infections compared to students. This result is in line with Mwambe et al. (43), who found that the rate of T. gondii infection was higher among employed pregnant women. This association could, however be influenced by the occupation the women were involved in and the associated occupational hazards/risks.
According to hygienic practices related to pregnant women, the proportion of T. gondii infection in women who ate raw meat was higher than in those ate cooked ones. This finding agreed with Abdelbaset et al. (14), Ibrahim et al. (21), Nassef et al. (23), Demiroglu et al. (38), van Enter et al. (44), Abamecha and Awel (45), and Eroglu and Asgin (46) in Turkey. Drinking raw milk was more likely to experience T. gondii infection than drinking pasteurized or processed milk. This result agreed with Cook et al. (47) while it contrasts with that mentioned by Bahia-Oliveira et al. (48), in Brazil Olariu et al. (41), in Western Romania and Eroglu and Asgin (46) in Turkey.
Considering the contact with cats which are the final host and responsible for spreading of oocysts through feces; our study showed that pregnant women who got in contact with stray and pet cats were associated with higher odds of T. gondii infection. This data concurs with results reported by Agmas et al. (39); Abamecha and Awel (45); Negero et al. (49) in Eithiopia, Abdelbaset et al. (14), Ibrahim et al. (21); in Egypt and Olariu et al. (41) in western Romania. However, studies conducted in Turkey and Sri Lanka showed no relationship between toxoplasmosis and cat ownership (50, 51). The difference between the results may be due to various factors, including the difference in the prevalence of toxoplasmosis in cats living in diverse geographic regions, the difference in contact time, and compliance with hygiene requirements. Washing the hand after contact with cats; our study revealed that women who did not wash their hands after contact with cats were more likely to experience T. gondii infection than those who washed their hands as recorded by Kapperud et al. (29) in Norway. Given the fecal oral transmission route, hand washing reduced the risks of infection to the women. The high prevalence of T. gondii in pregnant women in this study could be the residence of most participants in rural areas, contact with cats and did not follow hygienic measures.
The oocysts of T. gondii are excreted for a short period of about 1–3 weeks from infected cats, mostly once in their lifetime (52) this explains the difficulty in diagnosis during single fecal examination. Applying the PCR technique for the detection of T. gondii DNA in cat feces is appropriate for a high detection sensitivity, specificity and reproducibility. Also, a sensitive PCR test may be used to detect low numbers of shedding oocysts, which are usually undetectable using traditional microscopy (7).
Regarding risk factors contributed to toxoplasmosis in cat, the use of dry food and litter trays were found to be associated with lower odds of T. gondii infection, while the increase of getting an infection due to allowing pet cats outdoors or contact with other animals such as dogs or donkeys with T. gondii (53–55). The high prevalence of T. gondii in domestic cats in this study is attributed to allow domestic cats outdoors and contact with other animals.
Educating pregnant women about the source of Toxoplasma infection, hand hygiene, proper cooking of meat products and hygienic measure for cleaning the cat litter box, keeping cats indoors away from animals and stimulating cats to use the litter box are important as preventive and control strategy.
5. Conclusion
As far as we know, this is the first study to use the molecular detection of T. gondii in both humans and cats in the Sharqia Governorate, Egypt. Education, occupation, drinking raw milk, contact with stray and pet cats and eating under cooked meat were significantly associated with the presence of T. gondii infections. Washing hands constitutes a serious yet preventable public health concern that requires targeted, community-specific efforts. Cat owners, particularly pregnant women, need to be aware about the risk of T. gondii infection while handling cats and pets owner should be advised to take necessary hygienic measure to prevent the infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Committee of the Faculty of Veterinary Medicine, Zagazig University, Egypt (ethical approval number: ZU-IACUC/2/F/205/2022). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Committee of the Faculty of Veterinary Medicine, Zagazig University, Egypt (ethical approval number: ZU-IACUC/2/F/205/2022). Written informed consent was obtained from the owners for the participation of their animals in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors donated to the study's design, methodology, data collection statistical analysis, and manuscript editing and writing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1147614/full#supplementary-material
References
1. Rostami A, Riahi SM, Contopoulos-Ioannidis DG, Gamble HR, Fakhri Y, Shiadeh MN, et al. Acute Toxoplasma infection in pregnant women worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2019) 13:e0007807. doi: 10.1371/journal.pntd.0007807
2. Rostami A, Riahi SM, Gamble HR, Fakhri Y, Nourollahpour Shiadeh M, Danesh M, et al. Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Clin Microbiol Infect. (2020) 26:673–83. doi: 10.1016/j.cmi.2020.01.008
3. Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. (2012) 25:264–96. doi: 10.1128/CMR.05013-11
4. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of T. gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. (2009) 39:1385–94. doi: 10.1016/j.ijpara.2009.04.003
5. Bolais PF, Vignoles P, Pereira PF, Keim R, Aroussi A, Ismail K, et al. Toxoplasma gondii survey in cats from two environments of the city of Rio de Janeiro, Brazil by modified agglutination test on sera and filter-paper. Parasit Vectors. (2017) 10:1–8. doi: 10.1186/s13071-017-2017-8
6. Frimpong C, Makasa M, Sitali L, Michelo C. Seroprevalence and determinants of toxoplasmosis in pregnant women attending antenatal clinic at the university teaching hospital, Lusaka, Zambia. BMC Infect Dis. (2017) 17:10. doi: 10.1186/s12879-016-2133-7
7. Sroka J, Karamon J, Dutkiewicz J, Wójcik Fatla A, Zajac V, Cencek T. Prevalence of T. gondii infection in cats in southwestern Poland. Ann Agric Environ Med. (2018) 25:1–5. doi: 10.26444/aaem/94675
8. Abou Elez RMM, Hassanen EAA, Tolba HMN, Elsohaby I. Seroprevalence and risk factors associated with T. gondii infection in domestic rabbits and humans. Vet Parasitol Reg Stud Rep. (2017) 8:133–7. doi: 10.1016/j.vprsr.2017.02.009
9. Bassiony H, Soliman N, Tawab S, Eissa S, Eissa A. Sero-prevalence and risk factors associated with T. gondii infection among pregnant women in Alexandria, Egypt. Int J Reprod Contracept Obstet Gynecol. (2016) 5:4220–7. doi: 10.18203/2320-1770.ijrcog20164318
10. El Deeb HK, Salah-Eldin H, Khodeer S, Allah AA. Prevalence of T. gondii infection in antenatal population in Menoufia governorate, Egypt. Acta Trop. (2012) 124:185–91. doi: 10.1016/j.actatropica.2012.08.005
11. El-Henawy A, El-Nahas H, Alkhiary MM. Latent toxoplasmosis is not a risk factor for pregnancy-induced hypertension. Br Microbiol Res J. (2016) 12:1–8. doi: 10.9734/BMRJ/2016/23770
12. El-Tantawy N, Taman A, Shalaby H. Toxoplasmosis and female infertility: is there a co-relation. Am J Epidemiol Infect Dis. (2014) 2:29–32. doi: 10.12691/ajeid-2-1-6
13. Kamal AM, Ahmed AK, Abdellatif MZ, Tawfik M, Hassan EE. Seropositivity of toxoplasmosis in pregnant women by ELISA at Minia University Hospital, Egypt. Korean J Parasitol. (2015) 53:605. doi: 10.3347/kjp.2015.53.5.605
14. Abdelbaset AE, Hamed MI, Abushahba MF, Rawy MS, Sayed AS, Adamovicz JJ. Toxoplasma gondii seropositivity and the associated risk factors in sheep and pregnant women in El-Minya Governorate, Egypt. Vet World. (2020) 13:54. doi: 10.14202/vetworld.2020.54-60
15. Farooq R, Rashid I, Akbar H, Shehzad W, Islam S, Bajwa AA, et al. DNA amplification techniques for the detection of T. gondii tissue cysts in meat producing animals: a narrative review article. Iran J Parasitol. (2016) 11:431–40.
17. Remington JS, Klein JO. Infectious Diseases of the Fetus and Newborn Infant. London: WB Saunders (2001).
18. Uttah E, Ogban E, Okonofua C. Toxoplasmosis: a global infection, so widespread, so neglected. IJSRP. (2013) 3:1–6. Available online at: http://www.ijsrp.org/research-paper-0613.php?rp=P181351
19. Hill DE, Sreekumar C, Jones J, Dubey JP., Toxoplasma gondii. In: Simjee S, editor. Foodborne Diseases. Totowa, NJ: Humana Press (2007).
20. El Gamal R, Selim M, Mohamed S, Fathy G, Abdel Rahman S. Comparison of PCR with ELISA in diagnosis of recent toxoplasmosis in pregnant women. J Am Sci. (2013) 9:824–32. Available online at: http://www.jofamericanscience.org
21. Ibrahim HM, Mohamed AH, El-Sharaawy AA, El-Shqanqery HE. Molecular and serological prevalence of T. gondii in pregnant women and sheep in Egypt. Asian Pac J Trop Med. (2017) 10:996–1001. doi: 10.1016/j.apjtm.2017.09.012
22. Lin M-H, Chen T-C, Kuo T-T, Tseng C-C, Tseng C-P. Real-time PCR for quantitative detection of T. gondii. J Clin Microbiol. (2000) 38:4121–5. doi: 10.1128/JCM.38.11.4121-4125.2000
23. Nassef NE, Abd El-Ghaffar MM, El-Nahas NS, Hassanain ME-DA, El-Din SAS, Ammar AI. Seroprevalence and genotyping of T. gondii in Menoufia governorate. Menoufia Med J. (2015) 28:617–28. doi: 10.4103/1110-2098.165828
24. El-Nawawy A, Soliman AT, El Azzouni O, Amer E-S, Karim MA, Demian S, et al. Maternal and neonatal prevalence of toxoplasma and cytomegalovirus (CMV) antibodies and hepatitis-B antigens in an Egyptian rural area. J Trop Pediatr. (1996) 42:154–7. doi: 10.1093/tropej/42.3.154
25. Ibrahim HM, Huang P, Salem TA, Talaat RM, Nasr MI, Xuan X, et al. Short report: prevalence of Neospora caninum and T. gondii antibodies in northern Egypt. Am J Trop Med Hyg. (2009) 80:263–7. doi: 10.4269/ajtmh.2009.80.263
26. Amany M, Merwad A. Epidemiology and molecular detection of zoonotic T. gondii in cat feces and seroprevalence of anti-T. gondii antibodies in pregnant women and sheep. Life Sci J. (2012) 9:133–46. Available online at: http://www.lifesciencesite.com
27. Ahmed HA, Shafik SM, Ali ME, Elghamry ST, Ahmed AA. Molecular detection of T. gondii DNA in milk and risk factors analysis of seroprevalence in pregnant women at Sharkia, Egypt. Vet World. (2014) 7:594–600. doi: 10.14202/vetworld.2014.594-600
28. CAPMAS. Population Count in Egypt's Governates. Cairo: Central Agency for Public Mobilization and Statistics (2022).
29. Kapperud G, Jenum PA, Stray-Pedersen B, Melby KK, Eskild A, Eng J. Risk factors for T. gondii infection in pregnancy: results of a prospective case-control study in Norway. Am J Epidemiol. (1996) 144:405–12. doi: 10.1093/oxfordjournals.aje.a008942
30. Soulsby E. Helminthes, Arthropod and Protozoa of Domesticated Animals. 7th edn. London: Bailliere Tindal and Cassell Ltd (1982).
31. Tavassoli M, Esmaeilnejad B, Malekifard F, Soleimanzadeh A, Dilmaghani M. Detection of T. gondii DNA in Sheep and Goat Milk in Northwest of Iran by PCR-RFLP. Jundishapur J Microbiol. (2013) 6:e8201. doi: 10.5812/jjm.8201
32. Couronné R, Probst P, Boulesteix A-L. Random forest vs. logistic regression: a large-scale benchmark experiment. BMC Bioinform. (2018) 19:270. doi: 10.1186/s12859-018-2264-5
34. Grömping U. Variable importance assessment in regression: linear regression vs. random forest. Am Stat. (2009) 63:308–19. doi: 10.1198/tast.2009.08199
35. Hampton MM. Congenital toxoplasmosis: a review. Neonatal Netw. (2015) 34:274–8. doi: 10.1891/0730-0832.34.5.274
36. Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. (2010) 26:190–6. doi: 10.1016/j.pt.2010.01.009
37. Bittencourt LH, Lopes-Mori FM, Mitsuka-Breganó R, Valentim-Zabott M, Freire RL, Pinto SB, et al. Seroepidemiology of toxoplasmosis in pregnant women since the implementation of the surveillance program of toxoplasmosis acquired in pregnancy and congenital in the western region of Paraná, Brazil. Rev Bras Ginecol Obstet. (2012) 34:63–8.
38. Demiroglu T, Akin Polat Z, Çelik C. Investigation of the risk factors affecting T. gondii seropositivity in women of reproductive age applying to the maternity clinic of kilis state hospital Turkiye. Parazitolojii Dergisi. (2015) 39:299–304. doi: 10.5152/tpd.2015.4078
39. Agmas B, Tesfaye R, Koye DN. Seroprevalence of T. gondii infection and associated risk factors among pregnant women in Debre Tabor, Northwest Ethiopia. BMC Res Notes. (2015) 8:107. doi: 10.1186/s13104-015-1083-2
40. Alsammani MA. Sero-epidemiology and risk factors for T. gondii among pregnant women in Arab and African countries. J Parasit Dis. (2016) 40:569–79. doi: 10.1007/s12639-014-0558-8
41. Olariu TR, Ursoniu S, Hotea I, Dumitrascu V, Anastasiu D, Lupu MA. Seroprevalence and Risk Factors of T. gondii infection in pregnant women from western. Romania Vect Borne Zoonotic Dis. (2020) 20:763–7. doi: 10.1089/vbz.2019.2599
42. Antolova D, Janicko M, Halanova M, Jarcuska P, Geckova AM, Babinska I, et al. Exposure to T. gondii in the Roma and non-Roma inhabitants of Slovakia: a cross-sectional seroprevalence study. Int J Environ Res Public Health. (2018) 15:408. doi: 10.3390/ijerph15030408
43. Mwambe B, Mshana SE, Kidenya BR, Massinde AN, Mazigo HD, Michael D, et al. Sero-prevalence and factors associated with T. gondii infection among pregnant women attending antenatal care in Mwanza, Tanzania. Parasit Vect. (2013) 6:1–5. doi: 10.1186/1756-3305-6-222
44. van Enter BJD, Lau YL, Ling CL, Watthanaworawit W, Sukthana Y, Lee WC, et al. Seroprevalence of T. gondii infection in refugee and migrant pregnant women along the thailand-myanmar border. Am J Trop Med Hyg. (2017) 97:232–5. doi: 10.4269/ajtmh.16-0999
45. Abamecha F, Awel H. Seroprevalence and risk factors of T. gondii infection in pregnant women following antenatal care at Mizan Aman General Hospital, Bench Maji Zone (BMZ), Ethiopia. BMC Infect Dis. (2016) 16:460. doi: 10.1186/s12879-016-1806-6
46. Eroglu S, Asgin N. Awareness, knowledge and risk factors of T. gondii infection among pregnant women in the Western Black Sea region of Turkey. J Obstet Gynaecol. (2021) 41:714–20. doi: 10.1080/01443615.2020.1789954
47. Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, Jenum PA, et al. Sources of toxoplasma infection in pregnant women: European multicentre case-control study. Eur Res Netw Cong Toxoplasm BMJ. (2000) 321:142–7. doi: 10.1136/bmj.321.7254.142
48. Bahia-Oliveira LM, Jones JL, Azevedo-Silva J, Alves CC, Oréfice F, Addiss DG. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. (2003) 9:55–62. doi: 10.3201/eid0901.020160
49. Negero J, Yohannes M, Woldemichael K, Tegegne D. Seroprevalence and potential risk factors of T. gondii infection in pregnant women attending antenatal care at Bonga Hospital, Southwestern Ethiopia. Int J Infect Dis. (2017) 57:44–9. doi: 10.1016/j.ijid.2017.01.013
50. Bamba S, Cissé M, Sangaré I, Zida A, Ouattara S, Guiguemdé RT. Seroprevalence and risk factors of T. gondii infection in pregnant women from Bobo Dioulasso, Burkina Faso. BMC Infect Dis. (2017) 17:482. doi: 10.1186/s12879-017-2583-6
51. Iddawela D, Vithana SMP, Ratnayake C. Seroprevalence of toxoplasmosis and risk factors of T. gondii infection among pregnant women in Sri Lanka: a cross sectional study. BMC Public Health. (2017) 17:930. doi: 10.1186/s12889-017-4941-0
52. Dubey JP, Thayer DW. Killing of different strains of T. gondii tissue cysts by irradiation under defined conditions. J Parasitol. (1994) 80:764–7. doi: 10.2307/3283255
53. Castillo-Morales VJ, Acosta Viana KY, Guzmán-Marín Ed S, Jiménez-Coello M, Segura-Correa JC, Aguilar-Caballero A, et al. Prevalence and risk factors of T. gondii infection in domestic cats from the tropics of Mexico using serological and molecular tests. Interdiscip Perspect Infect Dis. (2012) 2012:29108. doi: 10.1155/2012/529108
54. Bizhga B, Selami F, LaÇi D, Shehdula D, Lika E. The evaluation of T. gondii infection in cats at tiranacity. Ann Univ Craiova-Agricult Montanol Cadastre Ser. (2016) 46:50–5.
Keywords: toxoplasma, PCR, cat, human, risk factor
Citation: Hassanen EAA, Makau DN, Afifi M, Al-Jabr OA, Abdulrahman Alshahrani M, Saif A, Anter RGA, El-Neshwy WM, Ibrahim D and Abou Elez RMM (2023) Interplay between cross sectional analysis of risk factors associated with Toxoplasma gondii infection in pregnant women and their domestic cats. Front. Vet. Sci. 10:1147614. doi: 10.3389/fvets.2023.1147614
Received: 18 January 2023; Accepted: 07 March 2023;
Published: 24 March 2023.
Edited by:
Vikrant Sudan, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Jay Prakash Yadav, Guru Angad Dev Veterinary and Animal Sciences University, IndiaMorteza Shams, Medical University of Ilam, Iran
Abdol Sattar Pagheh, Birjand University of Medical Sciences, Iran
Copyright © 2023 Hassanen, Makau, Afifi, Al-Jabr, Abdulrahman Alshahrani, Saif, Anter, El-Neshwy, Ibrahim and Abou Elez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doaa Ibrahim, ZG9pYnJhaGltQHZldC56dS5lZHUuZWc=
†ORCID: Doaa Ibrahim orcid.org/0000-0003-3402-1216
 Eman A. A. Hassanen
Eman A. A. Hassanen Dennis N. Makau2
Dennis N. Makau2 Omar A. Al-Jabr
Omar A. Al-Jabr Ahmed Saif
Ahmed Saif Doaa Ibrahim
Doaa Ibrahim Rasha M. M. Abou Elez
Rasha M. M. Abou Elez