- 1Department of Zoology, Government Sadiq College Women University, Bahawalpur, Punjab, Pakistan
- 2Department of Pathology, Faculty of Veterinary and Animal Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 3Shandong Vocational Animal Science and Veterinary College, Weifang, China
- 4Department of Entomology, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 5Department of Physiology, Faculty of Veterinary and Animal Sciences, The Islamia University of Bahawalpur, Bahawalpur, Pakistan
- 6Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan
Despite being an essential trace element for numerous metabolic processes and micronutrients, copper (Cu) has induced adverse effects on the environment and public health due to its continuous and widespread use for the last several decades. The current study assessed the hematological and histopathological alterations in the freshwater fish (Labeo rohita) exposed to graded concentrations of copper sulfate. For this purpose, L. rohita fish (n = 72), weighing ~200–215 g, were randomly divided into four experimental groups and then exposed to acute doses of CuSO4, i.e., control, 0.28, 0.42, and 0.56 μgL−1. For comparative analysis of hematological and biochemical changes, blood/serum samples were obtained on 12, 24, and 36 days. Overall, the body weight of fish decreased with the time and dose of CuSO4; as the dose increases, body weight decreases. Dose and time-dependent results were observed in other parameters also. Results showed a significant increase in leukocytes, whereas red blood cells count, Hb, and Hct were significantly reduced in treated groups compared to the control. The mean corpuscular hemoglobin (MHC) and mean corpuscular hemoglobin concentration (MCHC) showed a non-significant decrease in treated groups compared to the control group. Serum biochemical parameters, including total proteins, albumin, and globulin, decreased significantly (p < 0.05). At the same time, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), glucose, and cholesterol were significantly (p < 0.05) increased in the treated groups compared to the control group. Significantly (p < 0.05) increased levels of lipid peroxidation while decreased values of antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (RGSH) in the blood of fish were recorded. Histopathological examination of fish gills, liver, and kidneys showed inflammation and degenerative changes due to CuSO4 exposure. In the brain tissue, degenerative changes like neuron necrosis, intracellular edema, cytoplasmic vacuolization, and congestion were observed. In conclusion, the study indicates that exposure to copper sulfate, even in smaller concentrations, can cause adverse hematological and histopathological changes in L. rohita fish.
Introduction
Increased agricultural activities and extensive industrialization ultimately enhance the levels of various metals in the aquatic environment. Copper (Cu), an essential micronutrient for humans and animals, is an important and vital trace element needed for the multiple normal metabolic functions in various living organisms (1, 2). Copper also has an essential role in the regular activity of connective tissue, iron metabolism, and several functions in the central nervous system (3, 4). Plants and animals need copper to perform everyday tasks in different organs, e.g., hemoglobin synthesis. It is also a cofactor for various enzymes (5–7).
Widespread application of copper sulfate (CuSO4) in various sectors like building materials, agricultural sector, electronic industry, water pipes, transportation sector, intra-uterine contraceptive devices, and as an antifungal agent and growth promoter in poultry has also induced toxicological impacts in the exposed species (8). Copper sulfate is used worldwide as a fungicide in agriculture and as an algaecide in aquaculture (9). Unlike other synthetic and organic pesticides, Cu cannot be degraded and can easily be deposited in various tissues of the treated animals (8, 10, 11). Due to frequent application, Cu can damage the cells due to its potential to catalyze the generation of reactive oxygen species (12). Copper sulfate (CuSO4) negatively affected male Nile tilapia's reproductive function (13). Studies have reported that persistent dietary and environmental Cu exposure may cause different neurological and reproductive disorders and hepatic diseases in various animals (8, 14, 15). Cu also causes toxic impacts in terms of poor feed intake, increased oxidative stress, reduced body mass, and biochemical and hematological changes (5, 8, 16).
Over the last years, aquatic contamination has become a great concern for marine life and public health (17–19). Anthropogenic activities and accidental industrial waste discharge have become major causes of aquatic pollution in freshwater (20). Pollution due to heavy metals occur in the aquatic ecosystem due to agricultural discharge, geological weathering, municipal, direct air deposition, and other industrial waste products (21–24). Due to anthropogenic activities, several toxicants, including CuSO4, enter the aquatic ecosystem and cause detrimental effects on the exposed aquatic organisms (13). Fishes are considered an important component of the aquatic food chain among different aquatic organisms and reflect almost all the adverse effects upon exposure to toxicants (25–28). High pollution due to various heavy metals in water has become a significant problem because of the threat to aquatic life and public health concerns related to fish consumption (23, 29, 30). In the natural environment and laboratory specimens, various metals have been linked with different fish abnormalities (31). Constant subjection to several metals, like Zn, Cu, Pb, Cd, Ni, and As, causes harmful effects in organisms (32).
Biochemical parameters are often considered indicators when a clinical diagnosis of aquatic organisms' physiology is applied to determine the effects of external stressors and toxic substances (33). For the contaminants, cellular enzymes (33) and biochemical and oxidative stress parameters (34–40) serve as a biomarker for assessing the risk of environmental hazards (41). The microscopic evaluation of target tissues using histopathological examination is the endpoint in determining the risks of contaminants in the environment (42). Histopathological alterations can be employed to analyze the effects of numerous environmental contaminants on organisms as well as the general health of the aquatic population (43, 44). Investigation of biochemical parameters is recommended to give early indicators regarding modifications in the organisms exposed to toxicants and is especially effective in identifying target organs with toxicity and animal health (26, 45). Thus, the objectives of this study were to ascertain the toxicological impact of CuSO4 in Labeo rohita along with dose and time-dependent manner so that a suitable safe, and eco-friendly dose of CuSO4 could be determined for the removal of various contaminants from fish pounds.
Materials and methods
Experimental species, management, and treatments
A total of 72 freshwater fish L. rohita, F. Hamilton, 1822, healthy and free from any clinical ailments, with approximately the same age and weight (200–215 g), were collected from a local fish hatchery and breeding center. All the fish were immediately transported to the laboratory and reared in glass aquaria having 100 L of water at 26 ± 1.0 °C and photo-regime (12/12 h) light/dark for 2 weeks under laboratory conditions. Before the start and during the trial, all the fish were offered Optimum Aquarium Fish Food having 28% crude proteins (Perfect Companion, Thailand). The feed was provided to all the fish at 2–3% of body mass twice daily. After 2 weeks, the fish were divided randomly into equal four groups (A-D) having 18 fish in each group. Copper sulfate (CuSO4) of analytical grade was procured from the local market. The concentrations of CuSO4 were selected following the previous studies (46, 47). Briefly, CuSO4 was dissolved in water @ 0.28, 0.42, and 0.56 μg.L−1, respectively and fish in groups B-D were treated with the prepared solution for 36 days.
Physical parameters
Clinical and behavioral signs include loss of equilibrium, mucus secretion from mouth and gills, air gulping, increased swimming, rapid operculum movement, bulging eyes, loss of coordination, erratic swimming, and swimming in isolation in fish treated with various concentrations of copper sulfate were estimated numerically roughly. Each sign was given score – to +++ (– means no sign while + to +++ denoted mild, moderate, and severe, respectively (48). Data thus collected were averaged and presented.
Blood sampling and estimation of hemato-biochemical parameters
On days 12th, 24th, and 36th post-treatment, ~2.0 mL of blood from the caudal vein of six randomly selected fish was collected using a 26-gauge hypodermic sterile needle after the fish was anesthetized with clove oil (4 mg.L−1) (27). A blood sample was placed in anticoagulant-coated glass test tubes on each collection day. Various hematological parameters, including hemoglobin (Hb) quantity, hematocrit (Hct), red blood cells (RBCs) counts, total and differential white blood cells (WBCs) counts, lymphocytes, and neutrophils were determined as described previously (28). Total proteins (Cat # 997180) were assessed according to the earlier method (27). After centrifugation, serum was separated and stored at −20°C. Serum biochemical parameters like aspartate aminotransferase (AST; Cat # 30243), alanine aminotransferase (ALT; Cat # 30254), creatinine (Cat # 99108), cholesterol (Cat # 30183), alkaline phosphatase (ALP; Cat # 30134) and triglycerides (Cat # 30364) were measured using commercial kits (Canovelles; Barcelona, Spain). The quantity of lactate dehydrogenase (LDH) was measured by using a commercial kit (Cat # 21213; Analytical, Germany), while the quantity of urea (Cat # 996060) and albumin (Cat # 997258) was measured with the help of commercially available kits (Quimica Clinica Aplicada, S.A. Spain) using chemistry analyzer (Model: Rx Monza # 328-15-1001; UK) at days 12th, 24th and 36th of the trial (27).
Body mass, absolute and relative organ weight, and histopathology
Randomly selected, six fish were weighed on the 12th, 24th, and 36th day of the trial, then an autopsy was conducted. Visceral organs like the kidneys, brain, gills, and liver were dissected and weighed immediately. The relative weight was measured as a percent of the body weight. Similarly, the liver, kidneys, brain, and gills were preserved in 10% paraformaldehyde for microscopic studies. After fixation, all the visceral tissues were processed for histopathological changes using eosin and hematoxylin staining procedures (27). Tissue slides were examined for the presence of lesions by two pathologists, and if discrepancy come across, then a third pathologist was asked for judgment. Thus, collected histopathological lesions were graded by earmarking numerics. All the slides in each group were studied; median of the numerics was calculated and an cumulative picture of each lesion in each group was drawn. The severity/degree of different histopathological lesions were indicated on the basis of scoring of lesions as mild (25%), moderate (50%), severe (75%) and very severe (100%).
Status of oxidative stress parameters and antioxidant enzymes in erythrocytes
TBARS values were determined according to the procedure already described (49), while reduced glutathione (RGSH), superoxide dismutase (SOD), and catalase (CAT) were determined according to modified methods of Beutler et al. (50), Marklund and Marklund (51) and Chance and Maehly (52), respectively.
Data analysis
The data thus collected from hemato-biochemical, oxidative stress, and antioxidant enzymic parameters were presented as means ± standard error (SE). The data were subjected to ANOVA (one-way analysis of variance) using statistical software (IBM SPSS Statistics, v.22.00) followed by post hoc Tukey's test to determine the significant variation at p < 0.05.
Results
Physical observations
The present investigation observed no mortality in the treatment groups (B, C, D) during the trial. Clinical and behavioral signs include loss of equilibrium, mucus secretion from mouth and gills, air gulping, increased swimming, rapid operculum movement, bulging eyes, loss of coordination, erratic swimming, and swimming in isolation detected in the treated fish. The severity of various clinical and behavioral signs increases parallelly with increased concentration and exposure time to CuSO4 (Table 1).
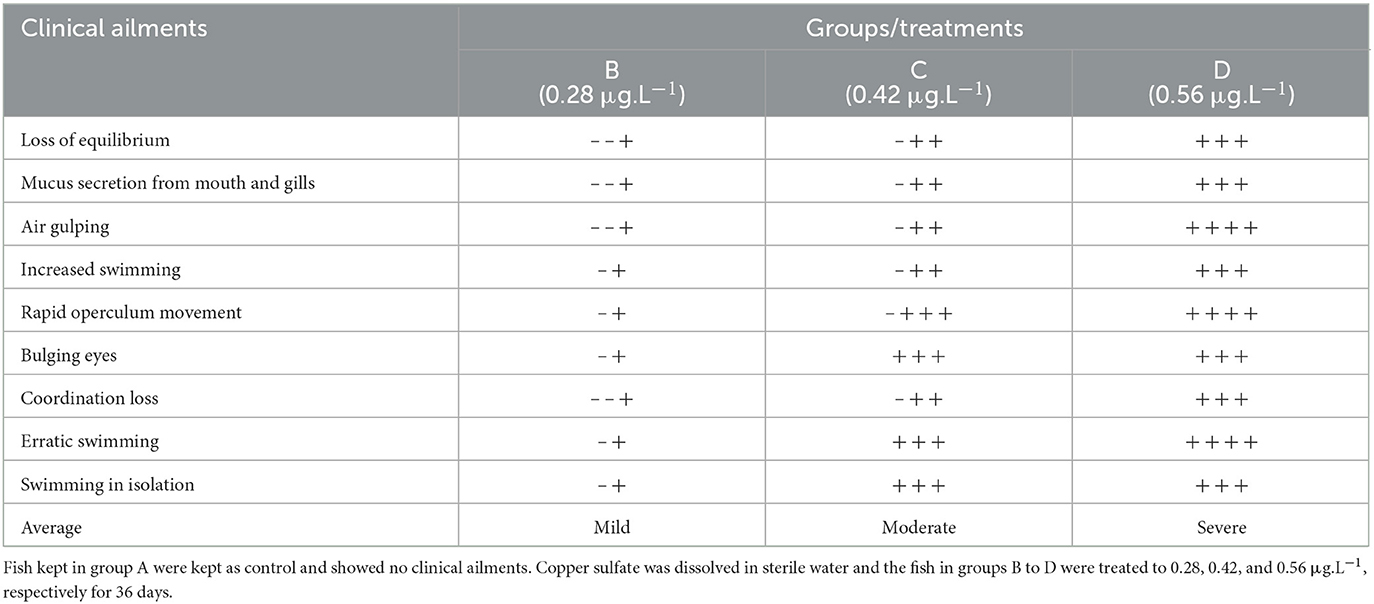
Table 1. Intensity of various clinical signs exhibited by the Labeo rohita treated with various concentrations of copper sulfate.
The body weight and absolute weight of various visceral tissues of Labeo rohita
Results showed that the body weight of fish decreased significantly (p < 0.05) in group D (CuSO4: 0.56 μgL−1) compared to the control group on the 36th experimental day (Table 2). The absolute weight of the brain, kidneys, and liver increased gradually at CuSO4 doses of 0.28 μgL−1 and 0.48 μgL−1 on experimental days 12 and 24. In contrast, at the CuSO4 dose of 0.56 μgL−1, the absolute weight of these organs increased significantly (p < 0.05) than the control group on experimental days 12, 24, and 36 (Table 2). Moreover, the absolute weight of the kidneys and liver also increased significantly (p < 0.05) in the control group at CuSO4 dose 0.48 μgL−1 on experimental day 36. The absolute weight of gills increased significantly (p < 0.05) at CuSO4 dose 0.56 μgL−1 than the control group on the 36th experimental day (Table 2).
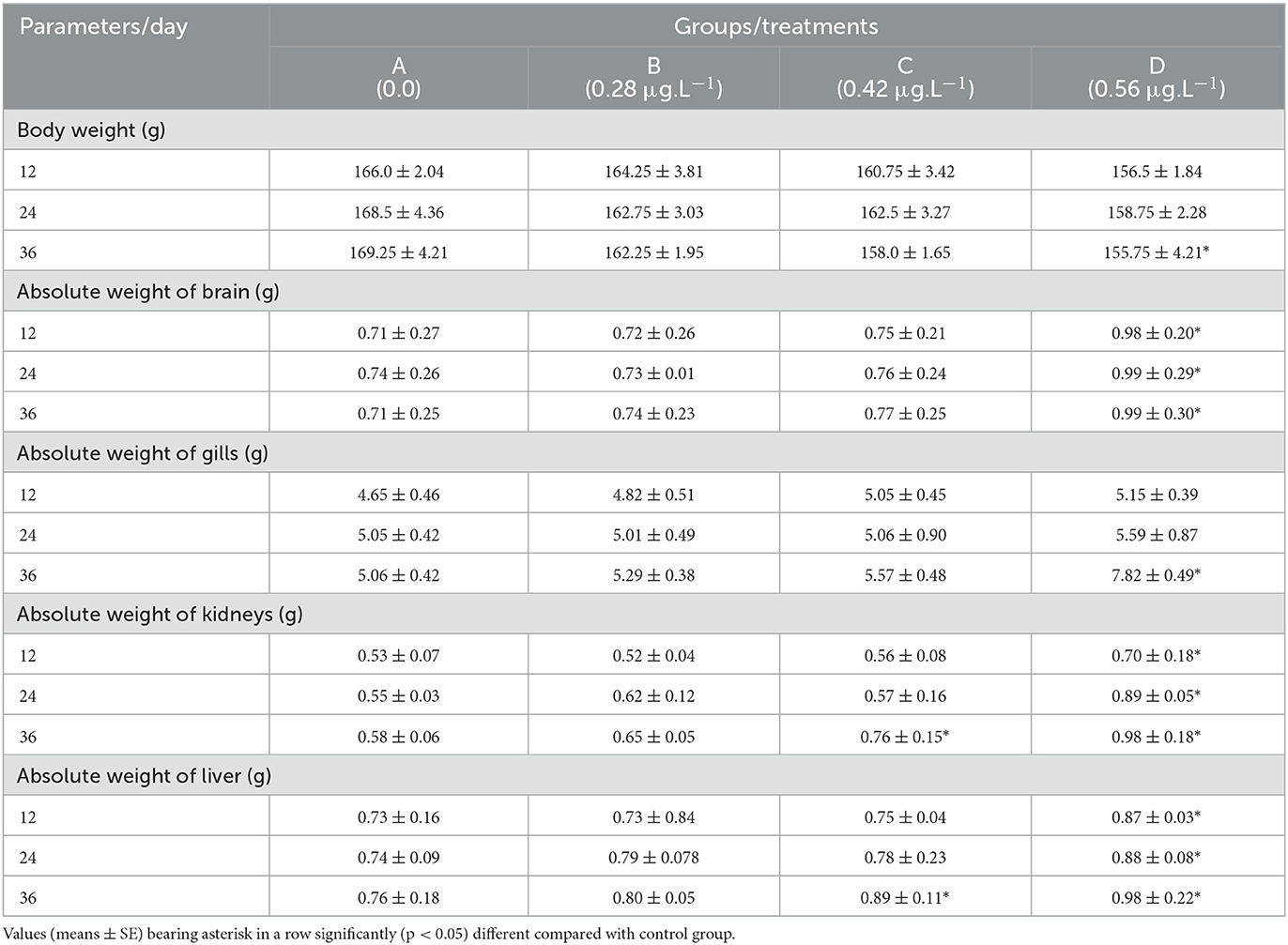
Table 2. Body weight and absolute weight of various visceral organs of Labeo rohita treated to copper sulfate with different doses.
The relative weight of visceral organs of Labeo rohita
Results showed that the relative liver weight of fish increased significantly (p < 0.05) at CuSO4 doses of 0.48 μgL−1 and 0.56 μgL−1 on experimental day 36th than the control group (Table 2). In contrast, the relative gills weight increased significantly (p < 0.05) on experimental days 24th and 36th in groups C (CuSO4: 0.48 μgL−1) and D (CuSO4: 0.56 μgL−1) compared to the control group. As far as the relative weight of the kidneys is concerned, that showed a significant (p < 0.05) increase on experimental days 12th, 24th, and 36th in group D (CuSO4: 0.56 μgL−1) and also in group C (CuSO4: 0.48 μgL−1) on experimental day 24 compared to control group (Table 3). Significantly (p < 0.05) relative weight of the brain increased on experimental days 12th, 24th, and 36th in groups C (CuSO4: 0.48 μgL−1) and D (CuSO4: 0.56 μgL−1) compared to the control group (Table 3).
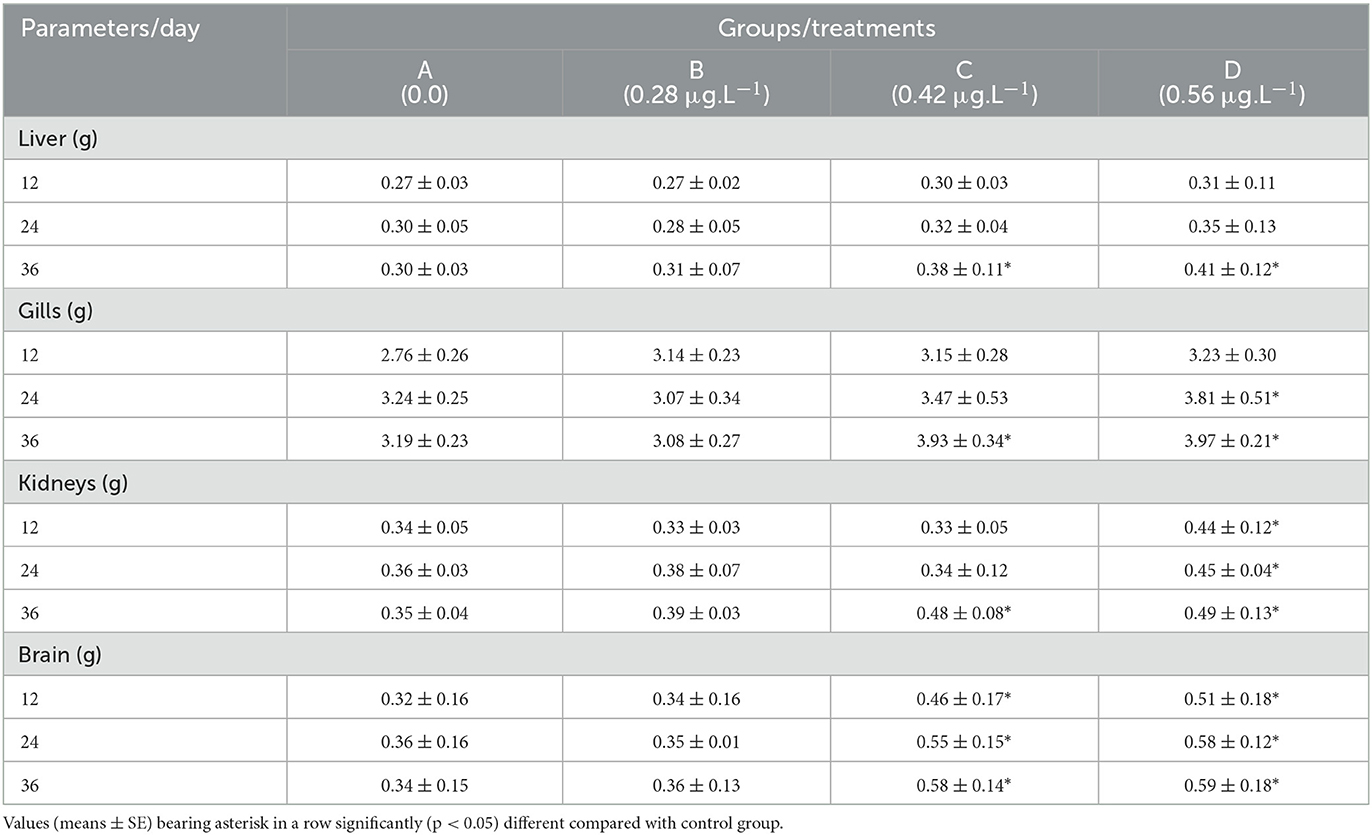
Table 3. Relative weight of visceral organs of Labeo rohita treated with various concentrations of copper sulfate.
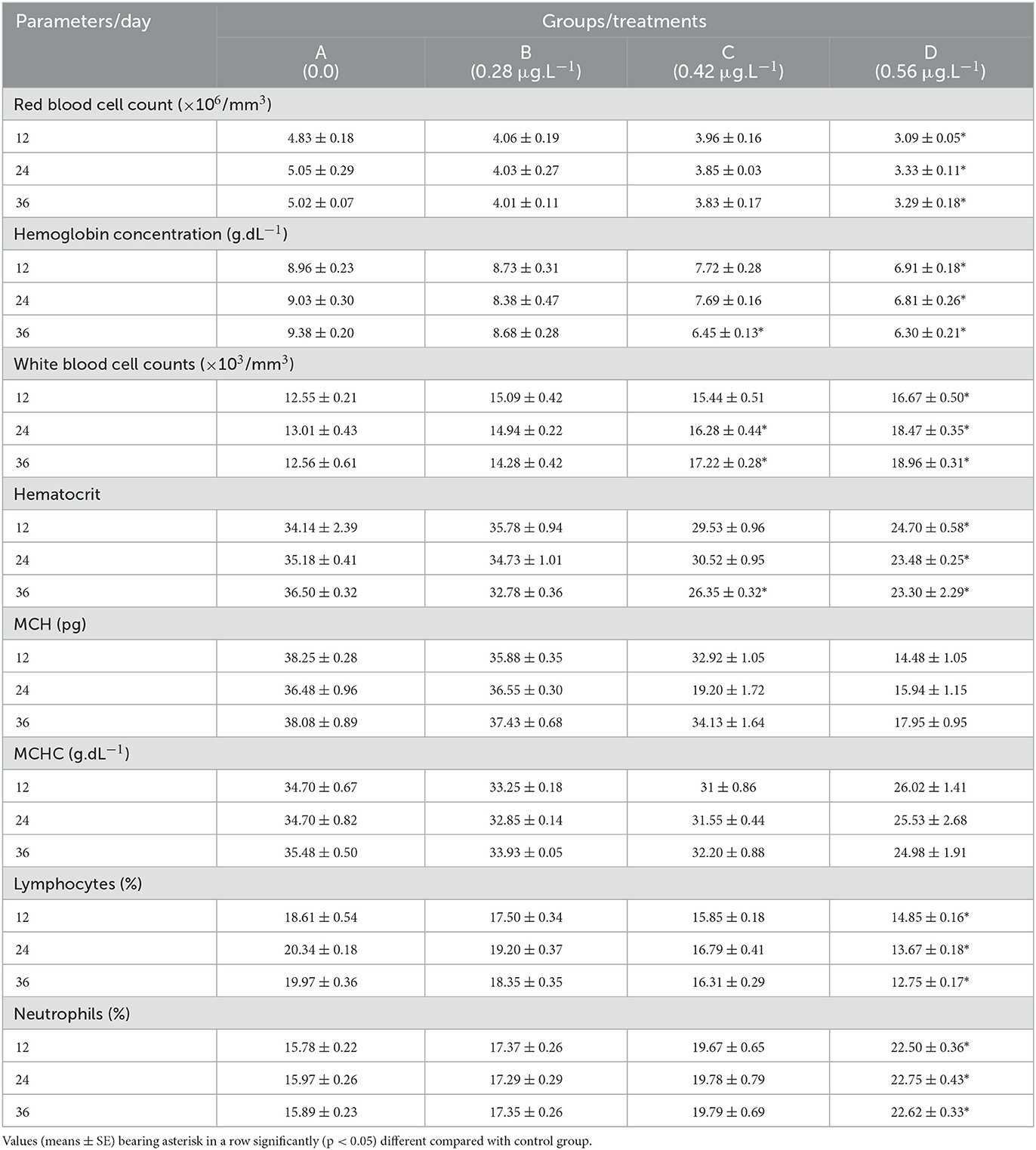
Hematological parameters
Different hematological parameters of fish exposed to several concentrations of CuSO4 are presented in Table 4. Results showed that RBC counts, hemoglobin, hematocrit, and lymphocytes decreased significantly (p < 0.05) while WBC counts and neutrophils increased significantly (p < 0.05) on experimental days 12, 24, and 36 in high dose group (CuSO4: 0.56 μg.L−1) compared to control group. In addition, hemoglobin and hematocrit decreased significantly (p < 0.05) at CuSO4 dose 0.42 μg.L−1 on experimental day 36 compared to the control group. WBCs counts also increased significantly (p < 0.05) on experimental day 24 and 36 at CuSO4 dose 0.42 μg.L−1 compared to control group (Table 4). MHC and MCHC in all treatment groups varied non-significant (p > 0.05) throughout the experiment as compared to the control group.
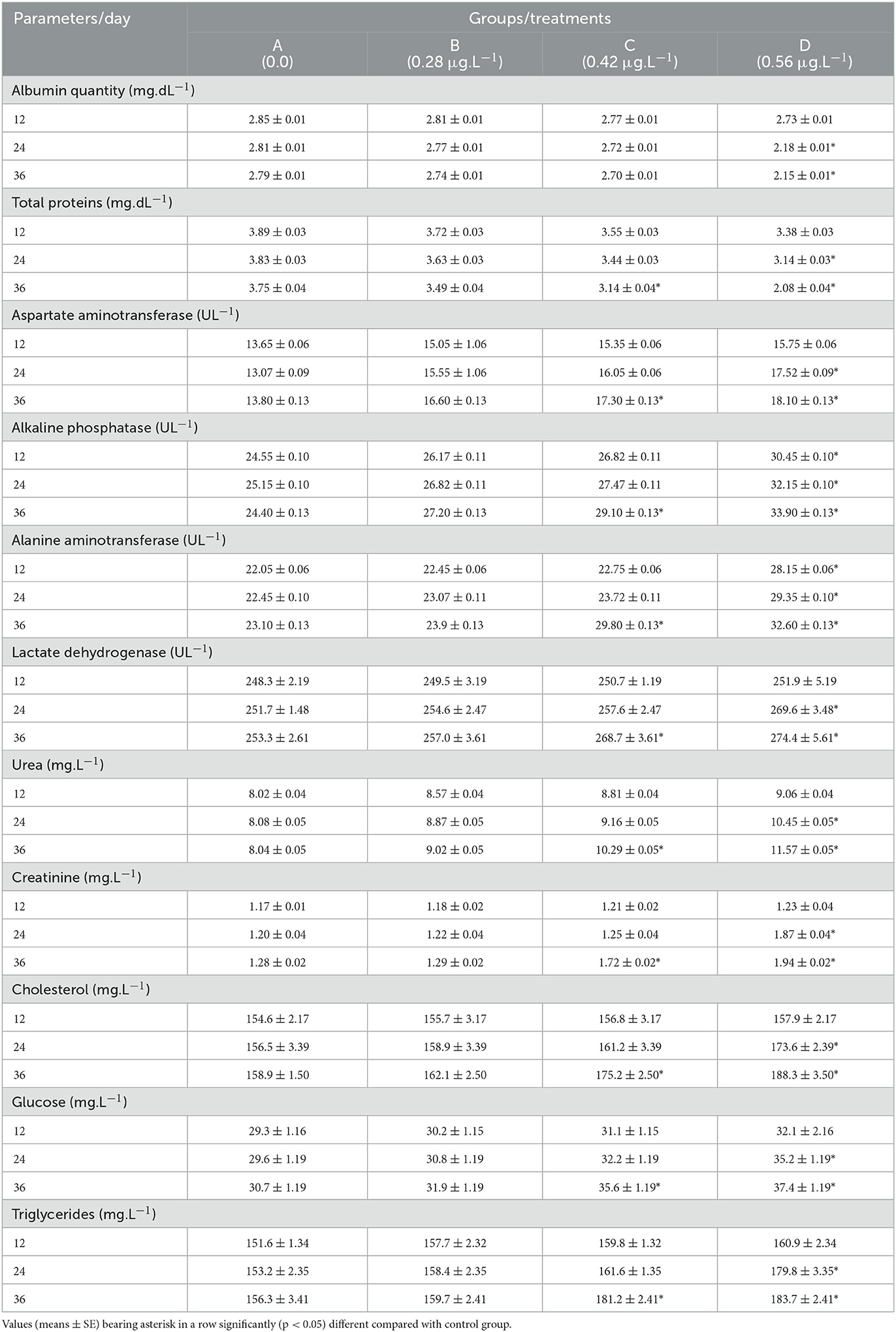
Serum biochemical parameters
Serum biochemical parameters of fish exposed to several concentrations of CuSO4 are presented in Table 5. The concentration of urea, creatinine, AST, LDH, cholesterol, glucose, and triglycerides increased significantly (p < 0.05) in the blood serum of fish treated with CuSO4 dose 0.56 μg.L−1 on experimental days 24 and 36 and also with CuSO4 dose 0.42 μg.L−1 on experimental day 36 compared to control group. In contrast, ALP and ALT increased significantly (p < 0.05) in fish treated with CuSO4 dose 0.56 μg.L−1 on experimental days 12, 24, and 36 and also with CuSO4 dose 0.42 μg.L−1 on experimental day 36 compared to control group. Total proteins and albumin concentration decreased significantly (p < 0.05) with the treatment of CuSO4 dose 0.56 μg.L−1 on experimental days 24 and 36, and total proteins also decreased significantly (p < 0.05) with the treatment of CuSO4 dose 0.42 μg.L−1 on experimental day 36 (Table 4).
Oxidative stress and antioxidant enzymes
The results of oxidative stress (Thio-barbituric acid reactive substances) and different antioxidant enzymes (reduced glutathione, superoxide dismutase, and catalase) are presented in Table 6. Results recorded a significantly (p < 0.05) increased quantity of TBARS in fish treated with CuSO4 dose 0.56 μg.L−1 on experimental days 24 and 36 and with CuSO4 dose 0.42 μg.L−1 on experimental day 36 compared to the control group. The TBARS also increased significantly (p < 0.05) with the treatment of CuSO4 dose 0.42 μg.L−1 on experimental day 36 (Table 5). There were significantly (p < 0.05) decreased concentrations of different antioxidant enzymes, including reduced glutathione, superoxide dismutase, and catalase in erythrocytes of fish treated with CuSO4 dose 0.56 μg.L−1 on experimental days 24 and 36 and also with CuSO4 dose 0.42 μg.L−1 on experimental day 36 compared to control group (Table 6).
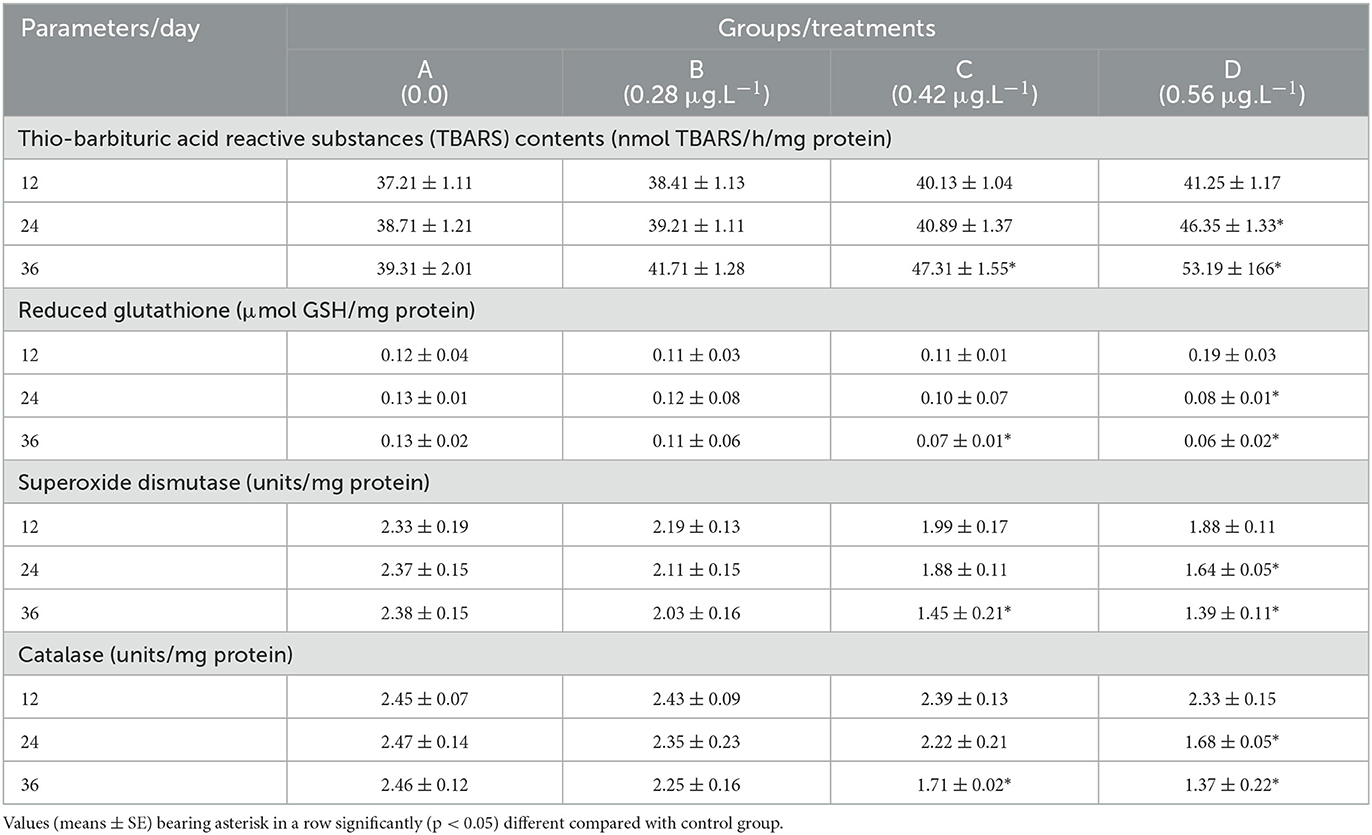
Table 6. Oxidative stress and antioxidant enzymes in erythrocytes of fish (Labeo rohita) treated to copper sulfate.
Histological alterations in various tissues
Histological investigation of the liver, gill, kidneys, and brain tissues on experimental days 12, 24, and 36 of the control and copper sulfate treated fish was carried out by the use of H and E staining, and various alterations in the tissue morphology were observed and presented below in Tables 7–9 and Figures 1, 2. Dose and time-dependent histopathological alterations in kidneys, gill, liver, and brain tissues occurred; those were fewer at CuSO4 dose 0.28 μg.L−1 and experimental day 12 and increased significantly (p < 0.05) with increasing the dose of CuSO4 from 0.42 μg.L−1 to 0.56 μg.L−1 and experimental day from 24 to 36. The degree of histopathological lesions was moderate to severe (Figure 1) at experimental day 36 with CuSO4 dose 0.42 μg.L−1 whereas these were severe to very severe (Figure 2) at CuSO4 dose 0.56 μg.L−1 at day 36. The Control group did not exhibit any histopathological alterations.
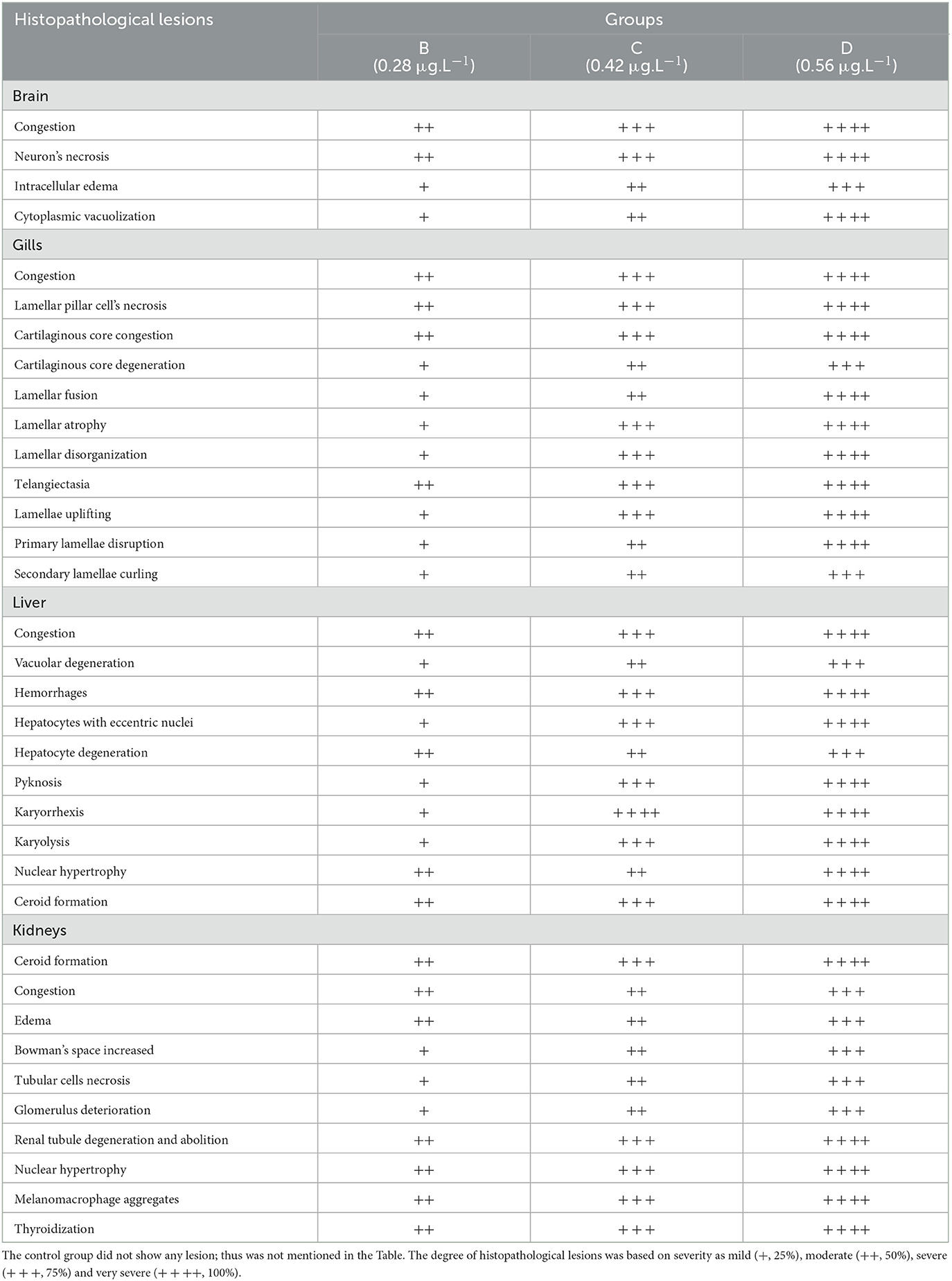
Table 7. Severity/degree of different histopathological lesions in various organs/tissues of Labeo rohita treated to various concentrations of copper sulfate at 12th day.
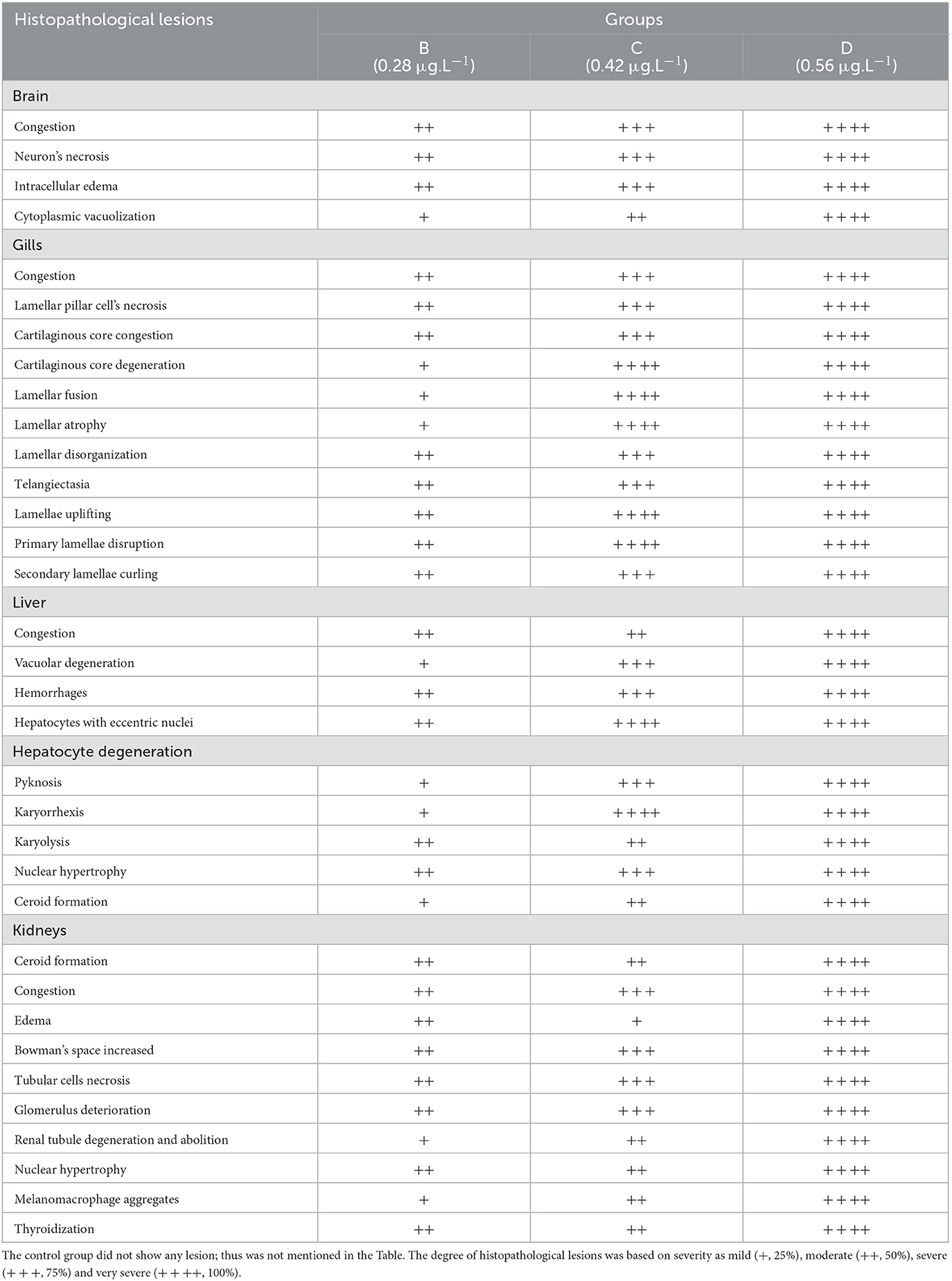
Table 8. Severity/degree of different histopathological changes in various tissues of Labeo rohita exposed to various concentrations of copper sulfate at day 24th.
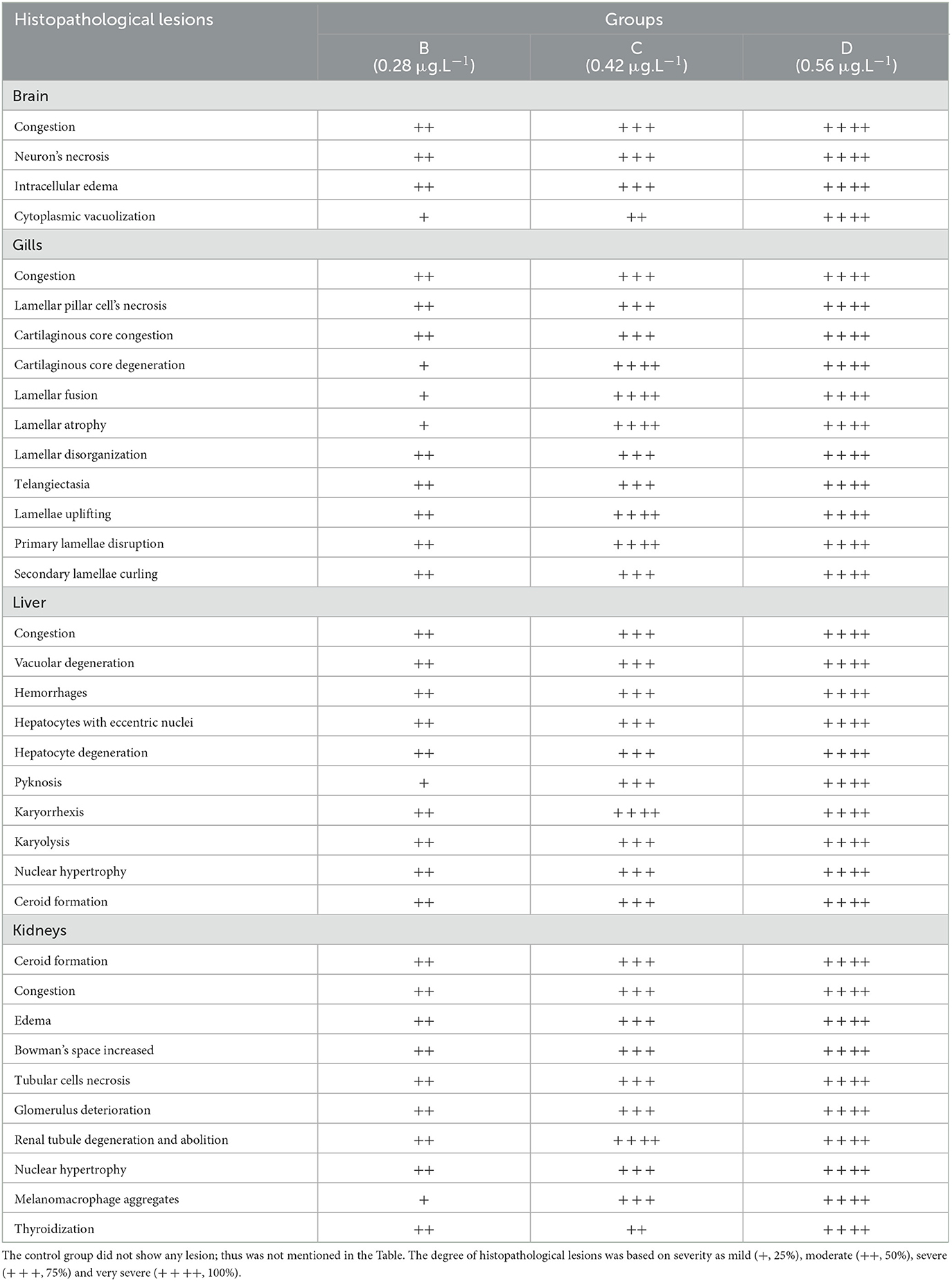
Table 9. Severity/degree of different histopathological changes in various tissues of Labeo rohita exposed to different concentrations of copper sulfate at day 36.
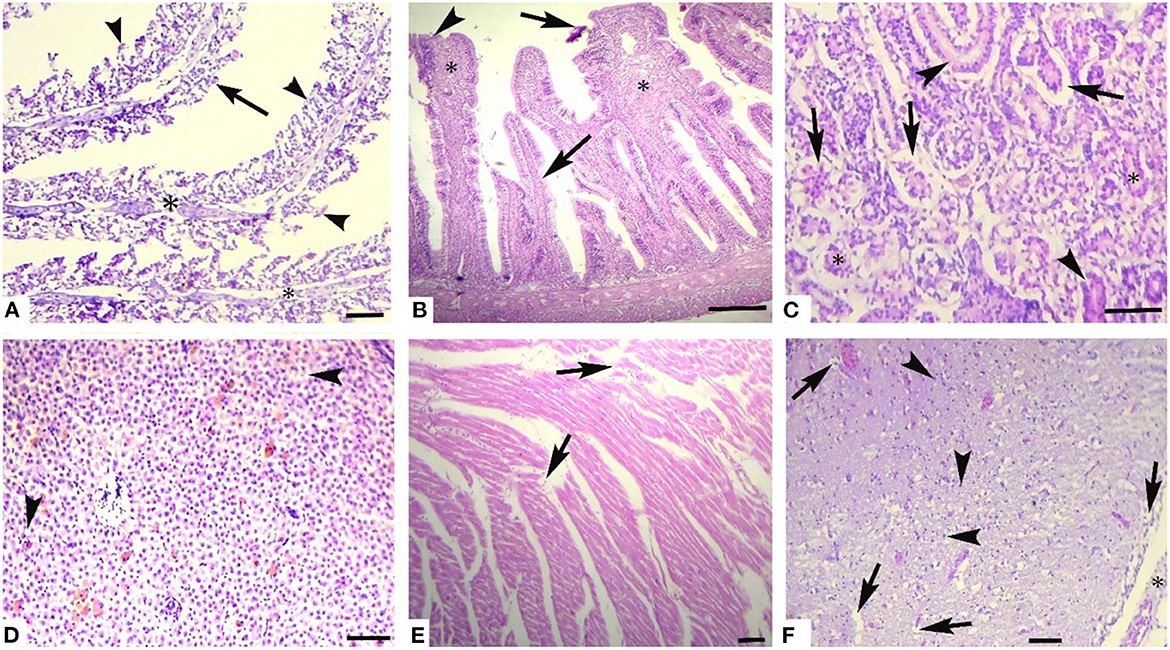
Figure 1. Photomicrograph of different tissues of freshwater fish treated with CuSO4 (0.42 μg.L−1) at experimental day 36: (A) Gills showing aneurysm (arrowheads), disorganization of primary lamellae (double asterisk) and necrosis of epithelium (arrows), (B) Intestine showing sloughing of epithelium from villi (arrows), necrosed epithelium (arrowheads) and necrosis of middle portio of villi (asterisk), (C) kidneys showing detachment of epithelium from the basement membrane (arrows), necrosis of renal tubules epithelial cells (arrowheads), protenecious material in tubulat lumen (asterisk) (D) Liver showing vacuolar degeneration (arrowheads) and necrosis of hepatocytes, (E) Heart showing disorganization of cardiac muscles (arrow), and (F) Brain showing necrosis of neurons (arrowheads), vacuolar degeneration of neurons (arrows) and microgliosis (asterisk). H and E. Bar is equal to (A) and (E) = 50 μm, and (B–D) and (F) = 100 μm.
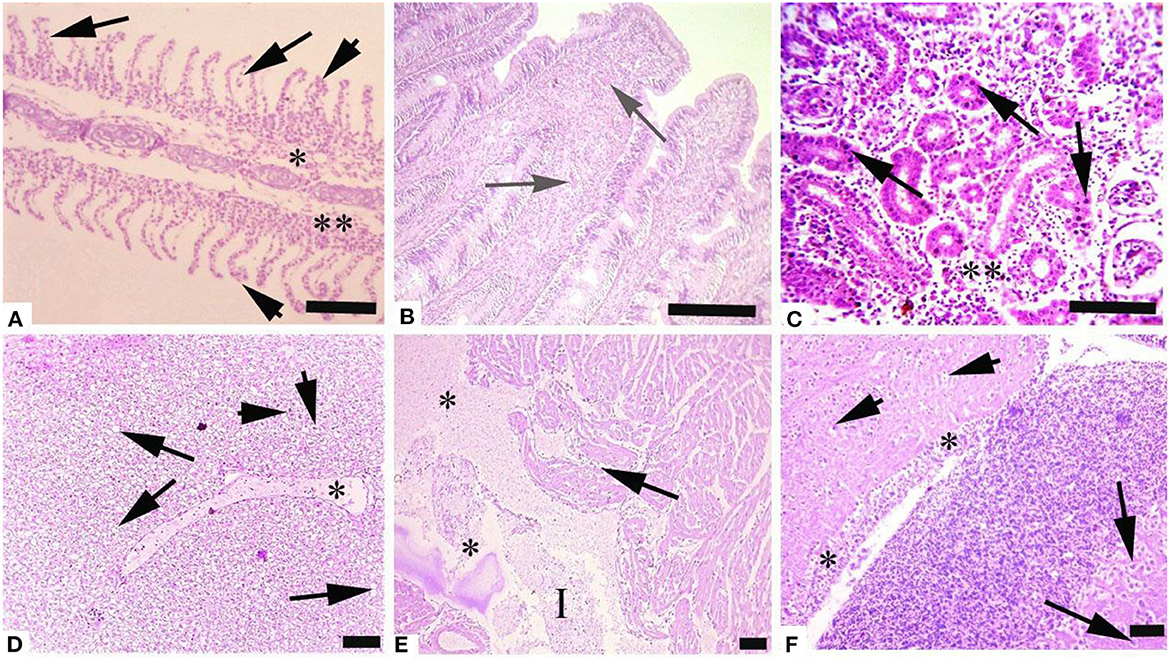
Figure 2. Photomicrograph of different tissues of freshwater fish treated with CuSO4 (0.56 μg.L−1) at experimental day 36: (A) Gills showing aneurysm (arrowheads), disorganization of primary lamellae (double asterisk) and necrosis of epithelium of secondary lamellae (arrows), (B) Intestine showing hemorrhages (arrows), (C) kidneys showing necrosis of renal tubules (asterisk) and necrosis of tubular epithelial cells (arrows), (D) Liver showing edema (asterisk), vacuolar degeneration (arrows) and necrosis (arrowheads), (E) Heart showing disorganization of cardiac muscles (arrow), edema (asterisks) and inflammatory exudate (I) and (F) Brain showing necrosis of neurons (arrowheads), degeneration of neurons (arrows) and microgliosis (asterisk). H and E. Bar is equal to (A) and (E) = 50 μm, and (B–D) and (F) = 100 μm.
Discussion
Copper sulfate has been reported to cause deleterious effects at higher levels (53–55). Sometimes the water quality, like pH, dissolved organic carbon, and hardness, also affect the toxicity of metal (e.g., Cu, Zn, Ni) in aquatic environments (22). Among all the environmental issues, metal pollution is the most serious and major hazard to the safety of humans and worldwide ecosystems (21, 44, 56–58).
The current study showed that various concentrations of CuSO4 affect fish health in several ways. In our trial, we observed no mortality in both control and CuSO4-treated experimental groups. However, the treated groups showed different clinical and behavioral signs like rapid operculum movement, tremors of fins, hypersecretion of mucus, erratic swimming, increased swimming area, loss of equilibrium, loss of coordination, increased surface breathing, and air gulping. Though no mortality was recorded in the present study, however high survival rate in the Japanese Medaka (Oryzias latipes) fish has been reported with exposure to low (10 BBP) compared with high (100 BBP) dose of CuSO4 (59). In the present study, the relative and absolute weight of fish exposed to CuSO4 showed a significant (p < 0.05) reduction in the treated groups compared to the control group. Similarly, it has been reported that Epinephelus coioides supplemented with CuSO4 and copper nanoparticles (Cu-NPs) rearing water exhibited lower weight gain than the control group (60). A decrease in growth performance could be due to two factors: in homeostasis and detoxification, metabolic expenditure increases, and higher exposure to Cu reduces feed intake, which ultimately leads to growth reduction (61).
In the present trial, various hematological and histopathological parameters were studied. Hematological signs are important for environmental monitoring, toxicological research, and indicators for many diseases. These parameters are critical for determining the physiological condition of fish under stress caused by metals or contaminants. In the current study, hematological parameters such as WBCs, and neutrophils were increased with the increase in concentration and duration. However, erythrocytes (4.83 ± 0.18 vs. 3.09 ± 0.05 × 106/mm3), hematocrit (34.14 ± 2.39 vs. 24.70 ± 0.58 %), and hemoglobin (8.96 ± 0.23 vs. 6.91 ± 0.18 g.dL−1), and lymphocytes (18.61 ± 0.54 vs. 14.85 ± 0.16 %) were decreased significantly (p < 0.05) after the exposure to CuSO4 (0.28 μg.L−1) on all experimental days. Similarly, CuSO4 (0.05 mg/L) exposure to fish Oreochromis niloticus had been reported to significantly (p < 0.05) decrease erythrocytes (1.78 ± 0.03 vs. 1.41 ± 0.02 106/μL), hematocrit (31.95 ± 0.74 vs. 24.83 ± 0.37 %), and hemoglobin (8.38 ± 0.09 vs. 5.94 ± 0.12 g/dL) values (17). It is also on record that CuSO4-NPs render more reduction in these parameters than CuSO4 itself (17). The lower hematological values in this study might be due to hemoglobin oxidation, erythrocyte destruction, and hemolysis (23, 47, 53, 56). Previous studies also described a decline in RBCs, Hb, and Hct contents in freshwater fishes exposed to Cd and Ni (58). In the current study, the hematological abnormalities might be due to the destruction or decreased production of RBCs in hematopoietic tissues, increased production of free radicals, and insufficient supply of oxygen to blood-forming tissues through gills. Moreover, several other studies have reported similar lower hematological values in different organisms like albino rats (62), yellow catfish (61), freshwater fish (47), and Catla Catla (23) exposed to various toxicants or pollutants. Additionally, the increased WBCs values and neutrophil percentage could be related to the stress conditions induced by the inflammatory response.
In the present study, except total proteins (3.75 ± 0.04 vs. 2.08 ± 0.04 mg.dL−1), all biochemical parameters including serum urea (8.04 ± 0.05 vs.11.57 ± 0.05 mg.L−1), creatinine (1.28 ± 0.02 vs. 1.94 ± 0.02 mg.L−1), cholesterol (158.9 ± 1.50 vs. 188.3 ± 3.50 mg.L−1), glucose (30.7 ± 1.19 vs. 37.4 ± 1.19 mg.L−1), triglycerides (156.3 ± 3.41 vs.183.7 ± 2.41 mg.L−1) along with serum enzymes i.e., ALT (23.10 ± 0.13 vs. 32.60 ± 0.13 UL−1), AST (13.80 ± 0.13 vs.18.10 ± 0.13 UL−1), ALP (24.40 ± 0.13 vs. 33.90 ± 0.13 UL−1) and LDH (253.3 ± 2.61 vs. 274.4 ± 5.61 UL−1) increased significantly (p < 0.05) in L. rohita with the exposure of CuSO4 (Table 5). However, along with other biochemical parameters such as glucose (105 ± 1.1 vs. 115 ± 0.5 mg/dL), AST (55.8 ± 0.3 vs. 57.4 ± 0.2 μg/L), ALT (28.6 ± 0.4 vs. 30.8 ± 0.5 μg/L) and uric acid (12.7 ± 0.04a 13 ± 0.1 mg/dL), total proteins (5.6 ± 0.1 vs. 6.4 ± 0.1 g/dL) also increased significantly (p < 0.05) in Nile tilapia (Oreochromis niloticus) with the treatment of both CuSO4 (15 mg/L) and CuO-NP (24). Increased total proteins in Nile tilapia with the treatment of CuSO4 (24) could be the reason that during exposure to Cu, fish may require higher protein concentration to meet the increased demand for repairing tissue and enhance the immunological response (20). Similarly, Atta et al. (63) reported that birds supplemented with excessive CuSO4 revealed an increase in ALP, ALT, and AST activities while reducing albumin, globulin levels, and total serum protein are consistent with our results. The hypoproteinemia in this study could be due to impairment of protein synthesis or the functional damage to the liver (31), and excessive loss of protein caused by degenerated glomerulus or could be explained due to the oxidative stress of copper on the liver and kidney tissue (64). There is also the possibility that the release of intracellular enzymes as a result of hepatic degeneration and necrosis caused by CuSO4 could lead to lipid peroxidation, which resultantly ends in hypoproteinemia (65). In the present research, the cholesterol levels increased significantly in L. rohita exposed to CuSO4 in a dose-dependent manner. Similarly, the findings of Almansour (66) are consistent with our results, who observed an increase in the cholesterol, total lipid, and LDL with no variation in HDL level in quail that were exposed to copper. Contrarily, no alteration was seen in total plasma cholesterol levels in broilers exposed to CuSO4 (67).
In this experimental trial, TBARS (39.31 ± 2.01 vs. 53.19 ± 166 nmol TBARS/h/mg protein) increased significantly (p < 0.05) whereas RGSH (0.13 ± 0.02 vs. 0.06 ± 0.02 μmol GSH/mg protein), SOD (2.38 ± 0.15 vs. 1.39 ± 0.11 units/mg protein) and CAT (2.46 ± 0.12 vs. 1.37 ± 0.22 units/mg protein) decreased significantly (p < 0.05) in red blood cells of L. rohita with the exposure of CuSO4 (Table 6). It has been reported that antioxidant enzymes such as SOD (11.6 ± 0.1 vs. 12.1 ± 0.13 IU/L), TAC (1.1 ± 0.01 vs. 0.9 ± 0.02 μM/L) and CAT (10.7 ± 0.1 vs. 11.5 ± 0.1 IU/L) increased significantly (p < 0.05) in Nile tilapia (Oreochromis niloticus) with the treatment of both CuSO4 (15 mg/L) and CuO-NP (24). The reduced quantity of different oxidant enzymes in erythrocytes of CuSO4 treated fish could be due to higher turnover of free radicals leading to depletion of antioxidants enzymes and induction of oxidative stress (40, 55, 68–70). Reduction in different antioxidant enzymes, including SOD, CAT, POD, and RGSH in erythrocytes of fish can possibly be due to different physiological disorders in erythrocytes and poor integrity of erythrocytic membranes in association with induction of low-grade inflammatory mechanisms in treated fish leading to inhibition of release of TNF-α and IL-1 (71). Studies have recorded that monitoring and evaluation of oxidative stress are of vital importance, which plays a vital role in the development of different physiological disorders in animals and also leads to lipid peroxidation, DNA damage, apoptosis, enzymic deactivation, and cell necrosis (71–75).
Histopathological changes have been extensively utilized as biomarkers while assessing the health of exposed fish to toxins/pollutants (76). Moreover, histopathology is the microscopic assessment of alteration in morphology that indicates disease in an organism (77, 78). We observed histopathological alterations in liver tissues, like ceroid formation, congestion, vacuolar degeneration, hemorrhages, pyknosis, karyorrhexis, degenerated hepatocytes, hepatocytes with eccentric nuclei, and karyolysis. While in kidney tissues, histopathological changes like widened Bowman's space, congestion, necrosis of tubular cells, edema, ceroid formation, melano-macrophage aggregates, glomerulus deterioration, thyroidization, and degeneration of kidney tubules were observed in L. rohita exposed to copper sulfate. These changes were dose-dependent.
The liver is considered as cutting edge immune organ (79). CuSO4 may possibly accrue in the liver. Liver is involved in the detoxification of metals at cellular level (80). Continual exposure to copper sulfate could have many detrimental effects on living beings (81). Copper have a tendency to bioaccumulate, disturbing human/animals food chain and eventually life menacing. Overexposure to copper generates inflammatory response, and oxidative stress, thus leading to liver injury (82–84).
Copper sulfate produces multiorgan (lungs, liver, gastrointestinal tract, and kidneys) injury/damage leading to disfunction via ROS production rendering oxidative stress (85). In hepatotoxicity, a vicious circle between oxidative stress and inflammatory response has been reported (86). Direct effect of copper usually leads to DNA and cell damage which then results in oxidative stress or enhanced discharge of ROS due to stimulation of phagocytic cells and increased tissue damage (6, 40, 70, 72, 87) as have been observed at high doses of CuSO4 in the present study. Moreover, endoplasmic reticulum and oxidative stress leads to nephrotoxicity as a result of CuSO4-exposure (5). Injury to various organs may result in fish mortality (88). Copper sulfate toxicity usually causes degeneration in renal tubules and glomerulus, and hepatocytes necrosis. These types of lesions hamper kidney and liver functions (89–91) which result in elevated enzymes such as LDH, ALT, and AST that has been seen in the present study.
Similarly, Abdel-Latif et al. (92) conducted a study on Nile tilapia. They found that exposure to sub-lethal concentration of CuO-NPs resulted in necrosis, hepatocytes degeneration, and severe congestion of blood sinusoids corresponding to the exposure dose, as well as increased Bowman's spaces, pyknotic nuclei, necrosis of kidney tubules, multi-focal inter-tubular hemorrhages and renal edema.
The gills are the first organ affected by exposure to CuSO4 in water (81). In the current study, histopathological alterations of gill tissues such as congestion of the cartilaginous core, congestion, telangiectasia, necrosis of lamellar pillar cells, lamellar fusion, lamellar atrophy, cartilaginous core degeneration, primary lamellae disruption, uplifting of lamellae, and disorganization of lamellar were observed, while in brain tissue degenerative changes like neurons necrosis, intracellular edema, cytoplasmic vacuolization, and congestion were observed in the studied fish at different exposure intervals during the experiment, which increases with an increase in concentration. Previously it has been indicated edematous changes in the gills could possibly due increased capillary permeability (88). Copper sulfate causes hyperplasia of gills epithelial cells, and it is dose dependent (93, 94) and it also leads to primary and secondary lamellar epithelial cells degeneration (76). Hyperplasia of gills epithelium along with degeneration and complete to partial lamellar fusion could be responses of the gills to pollutants (95, 96). Hyperplasia with thickening of gill filament epithelium may lead to the lamellar fusion (97). Augmented absorbency of the gill capillary walls subsequent vessel distension at the site of toxicant injury might be liable for the noted lamellar edema (98). There is another possibility that hyperplasia of gills could be a defensive aspect against irritants/heavy metals by reducing the mucosal membrane of respiratory tract and increasing the distance between toxicant–blood dissemination and its escalation which could result in the thickness of epithelial layers that could support by the intensifications of lamellar width and epithermus. Hence, lesions found in the current study could possibly impede the secretory, excretory, and respiratory functions of fish gills (81, 88, 99).
In the present study, CuSO4 dose and time-dependent histopathological alterations in brain tissues including congestion, cytoplasmic vacuolization, intracellular edema, and neuron necrosis were recorded. In the published literature, CuSO4 or CuO-NPs post disclosure, neuropil degeneration was obvious in Nile tilapia exposed to copper (24). These lesions could have developed as copper can cross blood-brain barrier (100–102). Several other studies (103–105) have reported similar lesions in brain of in response to various toxic chemicals and pollutants. Histopathological lesions in brain of L. rohita (106), O. punctatus (107), C. gariepinus (103), C. catla (108), C. Carpio (104), and O. mossambicus (97, 105) have been reported due to several toxicants exposure.
Conclusion
Many changes in the hematological and serum biochemical parameters and histopathological status in Labeo rohita were observed in the current study. These changes directly affect the health status of fish and all other aquatic organisms exposed to these pollutants/chemicals/compounds. This study observed that exposure to even a low concentration of CuSO4 (0.28 μg.L−1) might result in physiological and histopathological alterations in multiple fish tissues. Based on the results derived from this study, further studies are proposed to find out the more suitable dose of CuSO4 for regular use to decontaminate fish ponds. It is also suggested that due to possible effects on the ecosystem, aquatic organisms, and animal and human health, we should regularly monitor exposure to CuSO4 in waters and continuously observe the environment/ecosystem. Hence, we must prioritize studies about environmental monitoring of pollutants and their possible effects on different organisms.
Novelty of the study
While studying copper sulfate toxicity in fish in the past, people have reported mostly hematological or biochemical parameters singly or in combination. Whereas, in this study, we have reported comprehensively and simultaneously, physical, hematological, or biochemical, oxidative stress parameters along with gross and histopathology of fish exposed to various doses and at various time intervals.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Bioethics Committee, The Islamia University Bahawalpur, Pakistan.
Author contributions
SN and RH had the initial idea for the study. They were involved in the experiment, laboratory, data analysis, writing and development of the article, and the final proof stage. RH, SJ, and AC were involved in experimental studies, laboratory studies, and the article's writing. ML was involved in experimental studies, laboratory studies, and data analysis. ZG and AK performed and interpreted the statistical analysis. ZR arranged the facilities (enzymes, reagents). AK valuable suggestions regarding histopathological studies. All authors have read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; ANOVA, Analysis of variance; AST, Aspartate aminotransferase; Cd, Cadmium; CAT, Catalase; Cu-NPs, Copper nanoparticles; CuO-NPs, Copper oxide nanoparticles; CuSO4-NPs, Copper Sulfate nanoparticles; CuSO4, Copper Sulfate; Cu, Copper; Cr, Cromiuim; Hct, Hematocrit; Hb, Hemoglobin; HDL, High-density lipoprotein; IL-1, Interleukon-1; LDH, Lactate dehydrogenase; Pb, Lead; LDL, Low-density lipoprotein; MCHC, Mean corpuscular hemoglobin concentration; MHC, mean corpuscular hemoglobin; Ni, Nickle; RBCs, Red blood cells; RGSH, Reduced glutathione; SOD, Superoxide dismutase; TBARS, Thio-barbituric acid reactive substances; TNF-α, Tissue necrosis factor alpha; WBCs, White blood cells; Zn, Zinc.
References
1. Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I. Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol. (2016) 35:107–15. doi: 10.1016/j.jtemb.2016.02.006
2. Guo H, Wang Y, Cui H, Ouyang Y, Yang T, Liu C, et al. Copper induces spleen damage through modulation of oxidative stress, apoptosis, DNA damage, and inflammation. Biol Trace Elem Res. (2022) 200:669–77. doi: 10.1007/s12011-021-02672-8
3. Cimen ICC, Danabas D, Ates M. Comparative effects of Cu (60-80 nm) and CuO (40 nm) nanoparticles in Artemia salina: accumulation, elimination and oxidative stress. Sci Total Environ. (2020) 717:137230. doi: 10.1016/j.scitotenv.2020.137230
4. Tahir R, Ghaffar A, Abbas G, Turabi TH, Kausar S, Xiaoxia Du, et al. Pesticide induced hematological, biochemical and genotoxic changes in fish: a review. Agrobiol Rec. (2021) 3:41–57. doi: 10.47278/journal.abr/2021.005
5. Dai C, Liu Q, Li D, Sharma G, Xiong J, Xiao X. Molecular insights of copper sulfate exposure-induced nephrotoxicity: involvement of oxidative and endoplasmic reticulum stress pathways. Biomolecules. (2020) 10:1010. doi: 10.3390/biom10071010
6. Ijaz MU, Aziz S, Faheem M, Abbas K, Nasir S, Naz H, et al. Orientin attenuates cisplatin-induced renal toxicity by reducing oxidative stress and inflammation. Pak Vet J. (2021) 41:574–8. doi: 10.29261/pakvetj/2021.076
7. Akram R, Ghaffar A, Hussain R, Khan I, Santana VLDA, Mehmood K, et al. Hematological, serum biochemistry, histopathological and mutagenic impacts of triclosan on fish (bighead carp). Agrobiol Rec. (2022) 7:18–28. doi: 10.47278/journal.abr/2021.009
8. Wang Y, Li A, Mehmood K, Hussain R, Abbas RZ, Chang YF, et al. Long-term exposure to the fluoride blocks the development of chondrocytes in the ducks: The molecular mechanism of fluoride regulating autophagy and apoptosis. Ecotoxicol Environ Saf. (2021) 217:112225. doi: 10.1016/j.ecoenv.2021.112225
9. Firat Ö, Erol R, Firat Ö. Effects of individual and co-exposure of copper oxide nanoparticles and copper sulphate on nile tilapia Oreochromis niloticus: Nanoparticles enhance pesticide biochemical toxicity. Acta Chim Slov. (2022) 69:81–90. doi: 10.17344/acsi.2021.6995
10. Wiseman CL, Zereini F, Püttmann W. Metal translocation patterns in Solanum melongena grown in close proximity to traffic. Environ Sci Pollut Res Int. (2014) 21:1572–81. doi: 10.1007/s11356-013-2039-5
11. Ahmad L, Gul ST, Saleemi MK, Hussain R, Rehman AU, Naqvi SNH, et al. The effect of different repeated doses of cypermethrin on the behavioral and histological alterations in the brain of rabbits (Oryctolagus cuniculi). Int J Vet Sci. (2021) 10:347–54. doi: 10.47278/journal.ijvs/2021.092
12. Canaparo R, Foglietta F, Limongi T, Serpe L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials. (2020) 14:53. doi: 10.3390/ma14010053
13. Oda SS, El-Manakhly EM, Abou-Srag MA, Tohamy HG. Assessment of reproductive toxicity of carbofuran and copper sulfate in male Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res Int. (2022) 29:15896–904. doi: 10.1007/s11356-021-16965-x
14. Hsu HW, Bondy SC, Kitazawa M. Environmental and dietary exposure to copper and its cellular mechanisms linking to Alzheimer's disease. Toxicol Sci. (2018) 163:338–45. doi: 10.1093/toxsci/kfy025
15. De Jong WH, De Rijk E, Bonetto A, Wohlleben W, Stone V, Brunelli A, et al. Toxicity of copper oxide and basic copper carbonate nanoparticles after short-term oral exposure in rats. Nanotoxicology. (2019) 13:50–72. doi: 10.1080/17435390.2018.1530390
16. Shahzad MN, Javed MT, Shabir S, Irfan M, Hussain R. Effects of feeding urea and copper sulphate in different combinations on live body weight, carcass weight, percent weight to body weight of different organs and histopathological tissue changes in broilers. Exp Toxicol Pathol. (2012) 64:141–7. doi: 10.1016/j.etp.2010.07.009
17. Firat Ö, Erol R, Firat Ö. An investigation on freshwater fish Oreochromis niloticus (Linnaeus, 1758): assessing hemotoxic effects of different copper compounds used as nanomaterial or pesticide. Bull Environ Contam Toxicol. (2022) 108:549–54. doi: 10.1007/s00128-021-03320-6
18. Jabeen G, Manzoor F, Arshad M. Effect of cadmium exposure on hematological, nuclear and morphological alterations in erythrocyte of fresh water fish (Labeo rohita). Continental Vet J. (2021) 1:20–4.
19. Namratha ML, Lakshman M, Jeevanalatha M, Kumar BA. Hematological alterations induced by glyphosate and ameliorative effect of ascorbic acid in Wistar rats. Continental Vet J. (2021) 1:32–6. doi: 10.5455/ijlr.20191012074803
20. Al-Abdan MA, Bin-Jumah MN, Ali D. Investigation of biological accumulation and eco-genotoxicity of bismuth oxide nanoparticle in fresh water snail Lymnaea luteola. J King Saud Univ–Sci. (2021) 33:101355. doi: 10.1016/j.jksus.2021.101355
21. Authman MMN, Zaki MS, Khallaf EA, Abbas HH. Use of fish as bio-indicator of the effects of heavy metals pollution. J Aquacult Res Develop. (2015) 6:328. doi: 10.4172/2155-9546.1000328
22. Ali D, Ali H, Alarifi S. Genotoxicity in the freshwater gastropod Lymnaea luteola L: assessment of cell type sensitivities to lead nitrate. Chem Ecol. (2017) 33:171–9. doi: 10.1080/02757540.2016.1275587
23. Naz S, Hussain R, Ullah Q, Chatha AMM, Shaheen A, Khan RU. Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ Sci Pollut Res Int. (2021) 28:6533–39. doi: 10.1007/s11356-020-10980-0
24. Soliman HAM, Hamed M, Sayed AEH. Investigating the effects of copper sulfate and copper oxide nanoparticles in Nile tilapia (Oreochromis niloticus) using multiple biomarkers: the prophylactic role of Spirulina. Environ Sci Pollut Res Int. (2021) 28:30046–57. doi: 10.1007/s11356-021-12859-0
25. Canli EG, Canli M. Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ Toxicol Pharmacol. (2015) 39:606–13. doi: 10.1016/j.etap.2014.12.019
26. Akram R, Iqbal R, Hussain R, Ali M. Effects of bisphenol a on hematological, serum biochemical, and histopathological biomarkers in bighead carp (Aristichthys nobilis) under long-term exposure. Environ Sci Pollut Res Int. (2022) 29:21380–95. doi: 10.1007/s11356-021-17329-1
27. Yamagishi T, Kawano M, Watanabe H, Yagi A, Shintaku Y, Ohno K, et al. Severity classification of clinical signs and defining the moribund state as an experimental endpoint for acute toxicity testing using Japanese Medaka (Oryzias latipes). Environ Toxicol Chem. (2022) 41:1089–95. doi: 10.1002/etc.5294
28. Li A, Wang Y, Wang Y, Dong H, Wu Q, Mehmood K, et al. Microbiome analysis reveals soil microbial community alteration with the effect of animal excretion contamination and altitude in Tibetan Plateau of China. Int Soil and Water Conserv Res. (2021) 9:639–48. doi: 10.1016/j.iswcr.2021.04.011
29. Faheem M, Zahid Z, Ferreira NGC. Toxicity assessment of dibutyl phthalate in Grass carp: an integrated biomarker approach. Pak Vet J. (2021) 41:365–71. doi: 10.29261/pakvetj/2021.031
30. Choudhury R, Das P. Cytochrome b gene based population study of Tenualosailisha (Hamilton) in the Brahmaputra river system of India. Asian J Agri Biol. (2021) 2:201901033. doi: 10.35495/ajab.2019.01.033
31. Padrilah SN, Ahmad SA, Yasid NA, Sabullah MK, Daud HM, Khalid A, et al. Toxic effects of copper on liver and cholinesterase of Clarias gariepinus. Environ Sci Pollut Res Int. (2017) 24:22510–23. doi: 10.1007/s11356-017-9923-3
32. Aljarba NH, Ali H, Alkahtani S. Synergistic Dose Permutation of Isolated Alkaloid and Sterol for Anticancer Effect on Young Swiss Albino Mice. Drug Des Devel Ther. (2021) 15:4043–52. doi: 10.2147/DDDT.S322769
33. Yildirim NC, Benzer F, Danabas D. Evaluation of environmental pollution at Munzur River of Tunceli applying oxidative stress biomarkers in Capoeta trutta (Heckel, 1843). J Anim Plant Sci. (2011) 21:66–71.
34. Yildirim NC, Yildirim N, Danabas D, Danabas S. Use of acetylcholinesterase, glutathione S-transferase and cytochrome P450 1A1 in Capoeta umbla as biomarkers for monitoring of pollution in Uzuncayir Dam Lake (Tunceli, Turkey). Environ Toxicol Pharmacol. (2014) 37:1169–76. doi: 10.1016/j.etap.2014.04.001
35. Danabas D, Yildirim NC, Yildirim N, Onal AO, Uslu G, Unlu E, et al. Changes in antioxidant defense system in gills of Capoeta umbla caught from Uzuncayir Dam Lake, Turkey. Biochem Syst Ecol. (2015) 63:72–9. doi: 10.1016/j.bse.2015.09.029
36. Nwabueze AA, Nwabueze EO. Impact of environmental variables on abundance, growth and condition factor of Gymnarchus niloticus (Curvier, 1829) from Umueze-Ossissa lake system, Southern Nigeria. Asian J Agri Biol. (2021) 3:202011567. doi: 10.35495/ajab.2020.11.567
37. Aksu Ö, Yildirim NC, Danabas D, Yildirim N. Biochemical impacts of the textile dyes remazol brilliant blue R and congo red on the crayfish Astacus Leptodactylus (Decapoda, Astacidae). Crustaceana. (2017) 90:1563–74. doi: 10.1163/15685403-00003738
38. Li A, Wang Y, He Y, Liu B, Iqbal M, Mehmood K, et al. Environmental fluoride exposure disrupts the intestinal structure and gut microbial composition in ducks. Chemosphere. (2021) 277:130222. doi: 10.1016/j.chemosphere.2021.130222
39. Abdel-Saeed H, Salem NY. Evaluation of total antioxidant capacity, malondialdehyde, catalase, proteins, zinc, copper and IgE response in ovine verminous pneumonia. Int J Vet Sci. (2019) 8:255–8.
40. Aziz S, Abdullah S, Anwar H, Latif F, DNA. damage and oxidative stress in economically important fish, Bighead carp (Hypophthalmichthys nobilis) exposed to engineered copper oxide nanoparticles. Pak Vet J. (2022) 42:1–8. doi: 10.29261/pakvetj/2022.002
41. Widowati W, Prahastuti S, Hidayat M, Hasiana ST, Wahyudianingsih R, Afifah E, et al. Protective effect of ethanolic extract of jati belanda (Guazuma ulmifolia L.) by inhibiting oxidative stress and inflammatory processes in cisplatin-induced nephrotoxicity in rats. Pak Vet J. (2022) 42:376–82. doi: 10.29261/pakvetj/2022.050
42. Rehma T, Naz S, Hussain R, Chatha AMM, Ahmad F, Yamin A, et al. Exposure to heavy metals causes histopathological changes and alters antioxidant enzymes in fresh water fish (Oreochromis niloticus)”, Asian J Agri Biol. (2021) 1. doi: 10.35495/ajab.2020.03.143
43. Naseem S Ghaffar A Hussain R and Khan A 2022. Inquisition of toxic effects of pyriproxyfen on physical, hemato-biochemical and histopathological parameters in Labeo rohita fish. Pak Vet J. (2022) 42:308–15. doi: 10.1155/2022/5859266
44. Yacoub AM, Mahmoud SA, Abdel-Satar AM. Accumulation of heavy metals in tilapia fish species and related histopathological changes in muscles, gills and liver of Oreochromis niloticus occurring in the area of Qahr El-Bahr, Lake Al-Manzalah, Egypt. Oceanol Hydrobiol Stud. (2021) 50:1–15. doi: 10.2478/oandhs-2021-0001
45. Aziz S, Abdullah S, Anwar H, Latif F, Mustfa W. Effect of engineered nickel oxide nanoparticles on antioxidant enzymes in fresh water fish, Labeo rohita. Pak Vet J. (2021) 41:424–8. doi: 10.29261/pakvetj/2021.044
46. Gharedaashi E, Nekoubin H, Imanpoor MR, Taghizadeh V. Effect of copper sulfate on the survival and growth performance of Caspian Sea kutum, Rutilus frisii kutum. SpringerPlus. (2013) 2:498. doi: 10.1186/2193-1801-2-498
47. Sawsan HA, Amira HM. Mostafa MB, Nashaat AMM. Hematological and serum biochemical studies in fresh water fish exposed to acute and chronic copper and mercury toxicity. J Fish Pathol. (2017) 30:25–39. doi: 10.7847/jfp.2017.30.1.025
48. Ghaffar A, Hussain R, Ahmad N, Ghafoor R, Akram MW, Khan I, et al. Evaluation of hemato-biochemical, antioxidant enzymes as biochemical biomarkers and genotoxic potential of glyphosate in freshwater fish (Labeo rohita). Chem Ecol. (2021) 37:646–67. doi: 10.1080/02757540.2021.1937141
49. Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Analytic Biochem. (1978) 86:271–8. doi: 10.1016/0003-2697(78)90342-1
50. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. (1963) 61:882–8.
51. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. (1974) 47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x
52. Chance B, Maehly AC. Assay of catalase and peroxidase. Meth Enzymol. (1955) 2:764–75. doi: 10.1016/S0076-6879(55)02300-8
53. Ghaffar A, Hussain R, Aslam M, Abbas G, Khan A. Arsenic and urea in combination alters the hematology, biochemistry and protoplasm in exposed Rahu fish (Labeo rohita) (Hamilton, 1822). Turk J Fish Aquat Sci. (2016) 16:289–96. doi: 10.4194/1303-2712-v16_2_09
54. Boyle D, Clark NJ, Handy RD. Toxicities of copper oxide nanomaterial and copper sulphate in early life stage zebrafish: effects of pH and intermittent pulse exposure. Ecotoxicol Environ Saf. (2020) 190:109985. doi: 10.1016/j.ecoenv.2019.109985
55. Habotta OA, Elbahnaswy S, Ibrahim I. Neurotoxicity of singular and combined exposure of Oreochromis niloticus to methomyl and copper sulphate at environmentally relevant levels: assessment of neurotransmitters, neural stress, oxidative injury and histopathological changes. Environ Toxicol Pharmacol. (2022) 94:103935. doi: 10.1016/j.etap.2022.103935
56. Ismail HTH. Toxic impact of exposure to calcium hypochlorite and granular activated carbon on African catfish (Clarias gariepinus): a study of the alterations in hemato-biochemical profile and oxidative indices. Int J Vet Sci. (2022) 11:129–40. doi: 10.47278/10.47278/journal.ijvs/2021.082
57. Taha MG, SMA El-Hamamsy, NS Ahmed, MM Ali. Amelioration effect of Carica papaya fruit extracts on doxorubicin – induced cardiotoxicity in rats. Int J Vet Sci. (2020) 9:349–54.
58. Ali D, Yadav PG, Kumar S, Ali H, Alarifi S, Harrath AH. Sensitivity of freshwater pulmonate snail Lymnaea luteola L, to silver nanoparticles. Chemosphere. (2014) 104:134–40. doi: 10.1016/j.chemosphere.2013.10.081
59. Mason MW, Bertucci EM, Leri FM, Parrott BB. Transient copper exposure during embryogenesis and temperature affect developmental rate, survival, and fin regeneration in Japanese Medaka (Oryzias latipes). Environ Toxicol Chem. (2022) 41:748–57. doi: 10.1002/etc.5276
60. Wang T, Long X, Cheng Y, Liu Z, Yan S, A. Comparison effect of copper nanoparticles versus copper sulphate on Juvenile Epinephelus coioides: Growth parameters, digestive enzymes, body composition, and histology as biomarkers. Int J Genomics. (2015) 2015:783021. doi: 10.1155/2015/783021
61. Ikeogu CF, Okpala-Ezennia KP, Egwudike AC. Growth performance of African catfish (clarias gariepinus) juveniles cultured with fish feeds formulated with different nutritional components. Agrobiol Rec. (2022) 9:1–6. doi: 10.47278/journal.abr/2022.008
62. Lin S, Qiao N, Chen H, Tang Z, Han Q, Mehmood K, et al. Integration of transcriptomic and metabolomic data reveals metabolic pathway alteration in mouse spermatogonia with the effect of copper exposure. Chemosphere. (2020) 256:126974. doi: 10.1016/j.chemosphere.2020.126974
63. Atta AH, Fathy S, Gohar M. Prolonged administration of high doses of copper nicotinate to rats: Effect on biochemical and cellular constituents of blood and on copper level in serum, liver and muscle. Int J Med Sci. (2009) 1:178–83.
64. Hashem MA, Abd El Hamied SS, Ahmed EMA, Amer SA, El-Sharnouby ME. Mitigating the growth, biochemical changes, genotoxic and pathological effects of copper toxicity in broiler chickens by supplementing vitamins C and E. Animals. (2021) 11:1811. doi: 10.3390/ani11061811
65. Zhang SS, Noordin MM, Rahman SO, Haron J. Effects of copper overload on hepatic lipid peroxidation and antioxidant defense in rats. Vet Hum Toxicol. (2000) 42:261–4.
66. Almansour MI. Biochemical effects of copper sulfate, after chronic treatment in quail. J Biol Sci. (2006) 6:1077–82. doi: 10.3923/jbs.2006.1077.1082
67. Zhang H, Wang Y, Mehmood K, Chang YF, Tang Z, Li Y. Treatment of tibial dyschondroplasia with traditional Chinese medicines: “Lesson and future directions”. Poult Sci. (2020) 99:6422–33. doi: 10.1016/j.psj.2020.08.055
68. Bhutta ZA, Kulyar MFA, Jahanzaib, Sarwar I, Shabbir S, Boruh P, et al. Evaluation of hematological, antioxidant enzymes and oxidative stress parameters in buffaloes infected with babesiosis. Continental Vet J. (2022) 2:29–34.
69. Wang JQ, Hussain R, Ghaffar A, Afzal G, Saad AQ, Ahmad N, et al. Clinicohematological, Mutagenic, and Oxidative Stress Induced by Pendimethalin in Freshwater Fish Bighead Carp (Hypophthalmichthys nobilis). Oxid Med Cell Longev. (2022) 2022:2093822. doi: 10.1155/2022/2093822
70. Wang Y, Lu J, Qu H, Cai C, Liu H, Chu J. β-Carotene extracted from Blakeslea trispora attenuates oxidative stress, inflammatory, hepatic injury and immune damage induced by copper sulfate in zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol. (2022) 258:109366. doi: 10.1016/j.cbpc.2022.109366
71. Rossi E, Efendi R, Rahmayuni, BrSinulingga MS, Yoenissa R. Utilization of waste mixed pangasius fish fillet and pineapple core to produce peptone for lactic acid bacteria growth media. Int J Vet Sci. (2022) 11:272–9. doi: 10.47278/journal.ijvs/2021.126
72. Mahmood Y, Hussain R, Ghaffar A, Ali F, Nawaz S, Mehmood K, et al. Acetochlor affects bighead carp (Aristichthys Nobilis) by producing oxidative stress, lowering tissue proteins, and inducing genotoxicity. Biomed Res Int. (2022) 2022:9140060. doi: 10.1155/2022/9140060
73. Malhotra N, Ger TR, Uapipatanakul B, Huang JC, Chen KH, Hsiao CD. Review of copper and copper nanoparticle toxicity in fish. Nanomaterials. (2020) 10:1126. doi: 10.3390/nano10061126
74. Ouyang Z, Yang B, Yi J, Zhu S, Lu S, Liu Y, et al. Exposure to fluoride induces apoptosis in liver of ducks by regulating Cyt-C/Caspase 3/9 signaling pathway. Ecotoxicol Environ Saf. (2021) 224:112662. doi: 10.1016/j.ecoenv.2021.112662
75. Yang B, Liu Y, Li Y, Zhu S, Li Y, Yi J, et al. Exposure to the herbicide butachlor activates hepatic stress signals and disturbs lipid metabolism in mice. Chemosphere. (2021) 283:131226. doi: 10.1016/j.chemosphere.2021.131226
76. Gopinathan S, Binukumari S. Histopathological alterations in the gills of fresh water fish, Labeo rohita (hamilton-buchanan) exposed to heavy metal mixture (Cd, Cr 8#x38; Pb). J Adv Sci Res. (2021) 12:253–6. doi: 10.55218/JASR.202112134
77. Wavumbah NW, Janet M, Rose K. 2021. Canine testicular histopathology ensuing chemical castration. Agrobiol Rec. (2021) 5:28–31. doi: 10.47278/journal.abr/2021.002
78. Ahmed HH, El-Toukhey NES, Abd El-Rahman SS, Hendawy AKH. Efficacy of melatonin against oxidative stress, DNA damage and histopathological changes induced by nicotine in liver and kidneys of male rats. Int J Vet Sci. (2021) 10:31–6. doi: 10.47278/journal.ijvs/2020.022
79. Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. (2018) 36:247–77. doi: 10.1146/annurev-immunol-051116-052415
80. López-Alonso M, Prieto F, Miranda M, Castillo C, Hernández J, Benedito JL. The role of metallothionein and zinc in hepatic copper accumulation in cattle. Vet J. (2005) 169:262–7. doi: 10.1016/j.tvjl.2004.01.019
81. Tavares-Dias M. Toxic, physiological, histomorphological, growth performance and antiparasitic effects of copper sulphate in fish aquaculture. Aquaculture. (2021) 535:736350. doi: 10.1016/j.aquaculture.2021.736350
82. Wang Y, Tian J, Shi F, Li X, Hu Z, Chu J. Protective effect of surfactin on copper sulfate-induced inflammation, oxidative stress, and hepatic injury in zebrafish. Microbiol Immunol. (2021) 65:410–21. doi: 10.1111/1348-0421.12924
83. Hernandez PP, Undurraga C, Gallardo VE, Mackenzie N, Allende ML, Reyes AE. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol Res. (2011) 44:7–15. doi: 10.4067/S0716-97602011000100002
84. Leite CE, Maboni Lde O, Cruz FF, Rosemberg DB, Zimmermann FF, Pereira TC, et al. Involvement of purinergic system in inflammation and toxicity induced by copper in zebrafish larvae. Toxicol Appl Pharmacol. (2013) 272:681–9. doi: 10.1016/j.taap.2013.08.001
85. Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. (2016) 2016:5698931. doi: 10.1155/2016/5698931
86. Liu J, Zhao H, Wang Y, Shao Y, Li J, Xing M. Alterations of antioxidant indexes and inflammatory cytokine expression aggravated hepatocellular apoptosis through mitochondrial and death receptor-dependent pathways in Gallus gallus exposed to arsenic and copper. Environ Sci Pollut Res Int. (2018) 25:15462–73. doi: 10.1007/s11356-018-1757-0
87. Olivari FA, Hernández PP, Allende ML. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. (2008) 1244:1–12. doi: 10.1016/j.brainres.2008.09.050
88. Farhangi M, Jafaryan H. The comparison of acute toxicity (96h) of Copper (CuSO4) in Cyprinus Carpio and Rutilus Rutilus. Environ Pollut. (2019) 8:21–30. doi: 10.5539/ep.v8n2p21
89. Farhangi M, Kanani HG, Aliakbariyan A, Kashani M. Effect of copper sulphate on behavioral and histopathological changes in roach, Rutilus rutilus caspicus. Caspian J Env Sci. (2014) 12:73–9.
90. Peng X, Dai C, Zhang M, Das Gupta S. Molecular mechanisms underlying protective role of quercetin on copper sulfate-induced nephrotoxicity in mice. Front Vet Sci. (2021) 7:586033. doi: 10.3389/fvets.2020.586033
91. El Bialy BE, Hamouda RA, Abd Eldaim MA, El Ballal SS, Heikal HS, Khalifa HK, et al. Comparative toxicological effects of biologically and chemically synthesized copper oxide nanoparticles on mice. Int J Nanomedicine. (2020) 15:3827–42. doi: 10.2147/IJN.S241922
92. Abdel-Latif HMR, Dawood MAO, Mahmoud SF, Shukry M, Noreldin AE, Ghetas HA, et al. Copper oxide nanoparticles alter serum biochemical indices, induce histopathological alterations, and modulate transcription of cytokines, HSP70, and oxidative stress genes in Oreochromis niloticus. Animals. (2021) 11:652. doi: 10.3390/ani11030652
93. Atabati A, Keykhosravi A, Askari-Hesni M, Vatandoost J, Motamedi M. Effects of Copper Sulfate on gill histopathology of grass carp (Ctenopharyngodon idella). Iranian J Ichthyol. (2015) 2:35–42. doi: 10.22034/iji.v2i1.13
94. Abalaka SE, Enem SI, Idoko IS, Sani NA, Tenuche OZ, Ejeh SA, et al. Heavy metals Bioaccumulation and health risks with associated histopathological changes in Clarias gariepinus from the Kado Fish Market, Abuja, Nigeria. J Health Pollut. (2020) 10:200602. doi: 10.5696/2156-9614-10.26.200602
95. Mallat J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci. (1985) 42:630–48. doi: 10.1139/f85-083
96. Delahaut V, Raskovi'c B, Salvado MS, Bervoets L, Blust R, De Boeck G. Toxicity and bioaccumulation of cadmium, copper and zinc in a direct comparison at equitoxic concentrations in common carp (Cyprinus carpio) juveniles. PLoS ONE. (2020) 15:e0220485. doi: 10.1371/journal.pone.0220485
97. Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM, Carrola J, Matos P, et al. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesq Vet Bras. (2007) 27:103–9. doi: 10.1590/S0100-736X2007000300004
98. Olurin K, Olojo E, Mbaka G, Akindele A. Histopathological responses of the gill and liver tissue of Clarias gariepinus fingerlings to the herbicide, glyphosate. Afr J Biotechnol. (2006) 5:2480–7.
99. Khoshnood Z, Jamili Sh, Khodabandeh S, Mashinchian MA, Motallebi Moghanjoghi AA. Histopathological effects and toxicity of atrazine herbicide in Caspian Kutum, Rutilus frisii kutum, fry. Iranian J Fish Sci. (2014) 13:702–18.
100. Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. (2009) 1248:14–21. doi: 10.1016/j.brainres.2008.10.056
101. Fehse S, Nowag S, Quadir M, Kim KS, Haag R, Multhaup G. Copper transport mediated by nanocarrier systems in a blood-brain barrier in vitro model. Biomacromolecules. (2014) 15:1910–9. doi: 10.1021/bm500400k
102. Alnuaimi ShI and Al-Abdaly YZ. Neurobehavioral toxicity of copper sulfate accompanied by oxidative stress and histopathological alterations in chicks' brain. Iraqi J Vet Sci. (2023) 37:53–60. doi: 10.33899/ijvs.2022.133416.2224
103. Ayoola SO, Ajani EK. Histopathological effects of cypermethrin on juvenile African cdingatfish Clarias gariepinus. World J Biol Res. (2008) 1:1–14.
104. Lakshmaiah G. Brain histopathology of the fish Cyprinus carpio exposed to lethal concentrations of an organophosphate insecticide phorate. Int J Adv Res Dev. (2017) 2:668–72. doi: 10.7439/ijasr.v2i4.3233
105. Gobi N, Vaseeharan B, Rekha R, Vijayakumar S, Faggio C. Bioaccumulation, cytotoxicity and oxidative stress of the acute exposure selenium in Oreochromis mossambicus. Ecotoxicol Environ Saf. (2018) 162:147–59. doi: 10.1016/j.ecoenv.2018.06.070
106. Das KM, Mukherjee SC, A. histopathological study of carp (Labeo rohita) exposed to hexachlorocyclohexane. Vet Arhiv. (2000) 70:169–80.
107. Pugazhvendan SR, Narendiran JN, Kumaran RG, Kumaran S, Alagappan KM. Effect of malathion toxicity in the freshwater fish Ophiocephalus punctatus-A histological and histochemical study. World J Fish Marine Sci. (2009) 1:218–24.
Keywords: copper sulfate, Labeo rohita, blood-biochemistry, oxidative stress, histopathology
Citation: Naz S, Hussain R, Guangbin Z, Chatha AMM, Rehman ZU, Jahan S, Liaquat M and Khan A (2023) Copper sulfate induces clinico-hematological, oxidative stress, serum biochemical and histopathological changes in freshwater fish rohu (Labeo rohita). Front. Vet. Sci. 10:1142042. doi: 10.3389/fvets.2023.1142042
Received: 11 January 2023; Accepted: 15 February 2023;
Published: 09 March 2023.
Edited by:
Isa Ozaydin, Kafkas University, TürkiyeReviewed by:
Ola Habotta, Mansoura University, EgyptSamar Elblehi, Alexandria University, Egypt
Snur Hassan, University of Sulaimani, Iraq
Copyright © 2023 Naz, Hussain, Guangbin, Chatha, Rehman, Jahan, Liaquat and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riaz Hussain, ZHIucmlhei5odXNzYWluQGl1Yi5lZHUucGs=; Ahrar Khan, YWhyYXIxMTIyQHlhaG9vLmNvbQ==
†ORCID: Riaz Hussain orcid.org/0000-0003-3058-1371
Zia Ur Rehman orcid.org/0000-0003-1452-3745
Ahrar Khan orcid.org/0000-0001-5492-4266
 Saima Naz
Saima Naz Riaz Hussain
Riaz Hussain Zhang Guangbin
Zhang Guangbin Ahmad Manan Mustafa Chatha4
Ahmad Manan Mustafa Chatha4 Ahrar Khan
Ahrar Khan