- 1Department of Small Animal Clinical Sciences, Texas A&M University School of Veterinary Medicine & Biomedical Sciences, College Station, TX, United States
- 2Department of Population Health Sciences, Virginia-Maryland College of Veterinary Medicine, Virginia Tech, Blacksburg, VA, United States
- 3Department of Clinical Sciences, NC State University College of Veterinary Medicine, Raleigh, NC, United States
- 4Department of Family Medicine, University of Washington, Seattle, WA, United States
Over the last few decades, frailty has become a pillar of research and clinical assessment in human gerontology. This complex syndrome, characterized by loss of physiologic reserves leading to decreased resilience to stressors, is of critical importance because it predicts higher risks of poor health outcomes, including mortality. Thus, identifying frailty among the elderly human population has become a key focus of gerontology. This narrative review presents current scientific literature on frailty in both humans and animals. The authors discuss the need for an accessible frailty instrument for companion dogs suitable for general use in veterinary medicine and the advances that would be facilitated by this instrument. A phenotypic frailty instrument for companion dogs, utilizing components that are easily collected by owners, or in the general practice setting, is proposed. The authors elaborate on the domains (physical condition, physical activity, mobility, strength, cognitive task performance, and social behavior), factors that will be included, and the data from the Dog Aging Project that inform each domain.
1. Introduction
1.1. Definition of frailty
Age carries a widely known association with increased risk for disease and adverse health outcomes. However, chronological age alone is neither a sensitive nor specific predictor of morbidity (1) when assessing health at the level of the individual. In lieu of chronological age, the phenomenon of frailty is now recognized in human gerontology as a superior way to assess the manifestations of aging and associated risks of disease and death within individuals and communities (1–5). Recognition of frailty adds a valuable dimension to the assessment of health and prognosis by documenting states of decline or loss of function which can emerge without any corresponding diagnosis of disease (6, 7). While frailty is associated with age, that association is complex in that individuals manifest frailty to varying degrees and at varying ages (8–11). Furthermore, frailty, unlike chronological age, can sometimes be reversed (12–14).
Frailty, an evolving concept in human gerontology, is a complex, multidimensional syndrome characterized by a loss of resilience to stressors associated with aging (2, 5, 15, 16). Resilience itself is also a multifaceted characteristic which is affected by patient-specific and environmental factors, and which confers both physiological and emotional reserves (4, 17). The underlying etiology of frailty is thought to be the dysregulation of multiple physiologic systems resulting in loss of these reserves (2, 3, 18). Underlying mechanisms of frailty are not yet fully elucidated, and possibilities include sarcopenia (19, 20), low-grade inflammation (2), immune dysfunction (20), endocrinopathies (20, 21), and genetic risk factors (2). Increased resilience has been shown to predict recovery from illness and/or injury and improved quality of life in individuals living with chronic conditions (17). By contrast, frailty is associated with an increased risk of health problems including falls/fractures (15, 20, 22), hospitalization (15, 22, 23), functional decline/disability (15, 20, 22), the requirement for long-term care or institutionalization (3, 24, 25), and death (15, 16, 22, 24). Because frailty leads to adverse health outcomes, including mortality, identifying frailty among elderly human individuals and populations has become an important focus of gerontology.
1.2. Human frailty applications
In 2001, Fried et al. proposed a phenotype of frailty to be applied in community-dwelling populations to identify at-risk individuals. It included components of unintentional weight loss, weakness, exhaustion, slowness, and low physical activity (15). Frailty, defined as the presence of three or more of these five characteristics, was predictive of adverse outcomes in relatively functional geriatric adults (15). Individuals identified as frail were six times more likely to die in the subsequent 3 years compared to their non-frail counterparts (15). The field of frailty has expanded dramatically over the last 20 years, with a multitude of instruments currently available to measure frailty in humans. These instruments typically can be categorized as Frailty Phenotype (FP) or Frailty Index (FI) instruments (25–27) (Table 1). Broadly, the FP model is based on the presence or absence of components that can be physically evaluated, while the FI model includes medical and laboratory findings and assigns numeric scores to those components. FP and FI instruments have been found to be comparable in the prediction of mortality (25, 27) and can be considered complementary in certain situations (26).
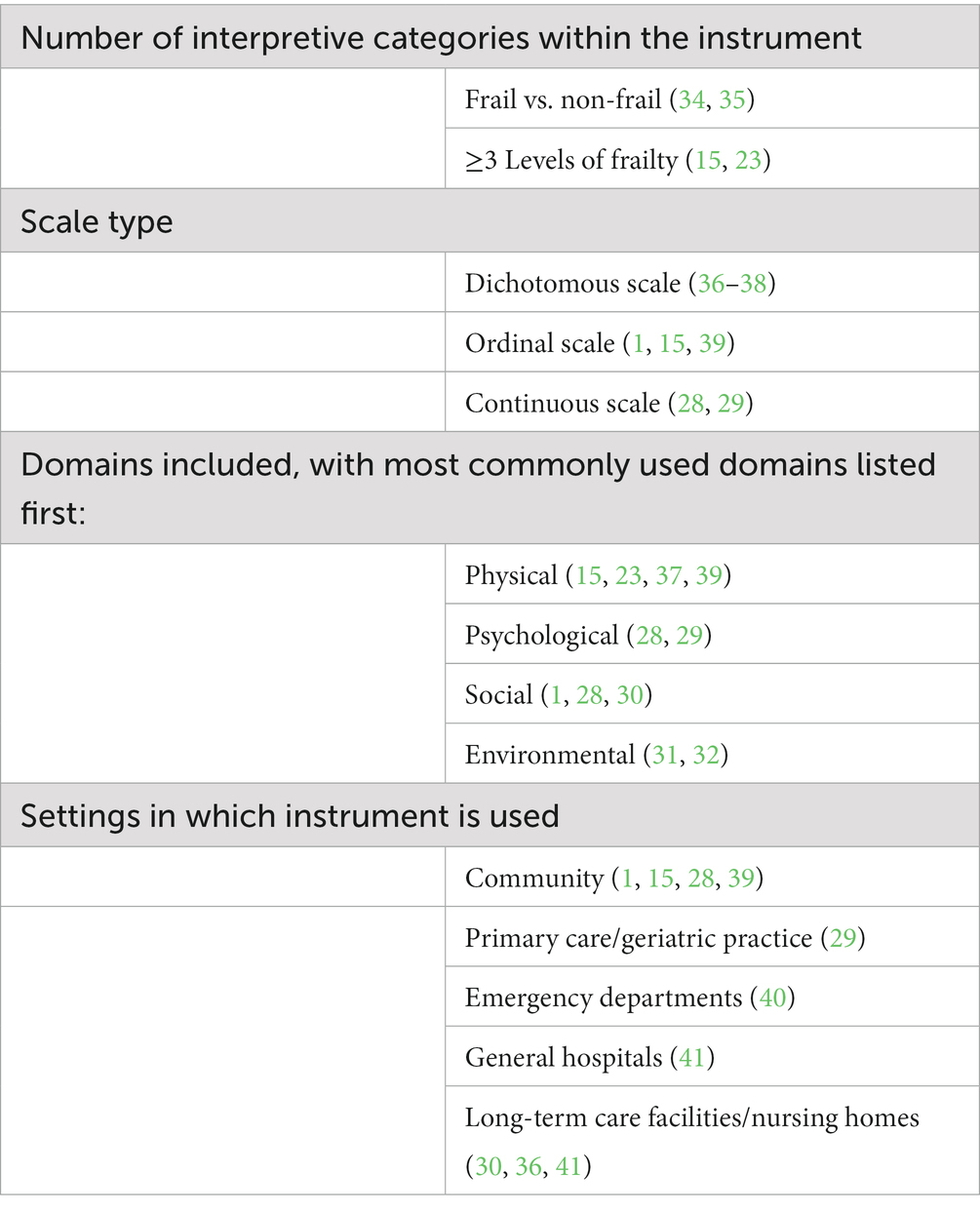
Frailty models have evolved over the years from physical-only models to ones that include frailty factors from psychological (28, 29), social (1, 29, 30), and environmental domains (31, 32). Currently, there are a plethora of different instruments to detect and measure frailty. A 2019 systematic review of studies evaluating frailty instruments used in human populations found a total of 51 diverse instruments, including numerous instruments using the FP model and many using the FI model (33). These instruments varied greatly in length/duration, use, interpretive categories, information collected, collection method, need for additional diagnostics, areas of investigation, etc. (Table 2). This diversity of frailty instruments is the product of different needs depending on the setting, administrator, available time, and aim of the measurements (42). However, these different instruments classify different subsets of the population as frail (43) which means that investigators must carefully select instruments for target applications. Across studies, the highest prevalence of frailty in human populations is observed in studies using multidimensional instruments (44), suggesting they may be more sensitive. There is also evidence that an increased number of included variables tends to result in a higher agreement between instruments and narrow prediction intervals, and that multidimensional instruments result in less error at the median point of frailty (43).
Along with the number of instruments and the domains they cover, the possible settings for the use of frailty assessment are also an expanding area of investigation. The identification of frail individuals informs prognosis and medical options and allows for several types of intervention directed at overall health and frailty itself. In human medicine, frailty can be used to stratify risk profiles and inform decision-making (16, 45). In a study of geriatric people who were treated for pelvic fractures, those identified as frail were at greater risk of mortality at the time of discharge and one-year post-discharge, and were also at risk of having reduced functionality and needing greater assistance at discharge (46). Frailty was associated with an increased risk of serious complications in geriatric trauma patients (47), and frailty status increased the risk of long-term mortality in COPD patients by 80% (48). This prognostic information is valuable for both patients and their families to understand prior to surgery or other medical treatment.
The identification of frail patients is also necessary to take any protective or preventive action, such as adjustments in the typical geriatric medical or surgical protocols to improve outcomes for a specific frail individual, or enrollment in programs targeted at altering the frailty state itself. In human geriatric patients, screening instruments can be utilized to identify frail patients simply to inform their healthcare team so that they can make treatment alterations as needed. One study looking at postoperative survival for human patients undergoing major elective surgical procedures found that simply implementing a frailty screening initiative resulted in improved survival (49). When a patient was identified as frail, anesthesia, surgery, critical care, and palliative care clinicians were informed and, if needed, a modified perioperative plan was developed (49). Decreased mortality using this approach was attributed to multiple factors, including increased vigilance for complications leading to earlier treatment and improved family involvement, leading to improved post-discharge care and social support (49).
As frailty is a dynamic process, influenced by both individual and environmental factors, it is susceptible to intervention. Frailty studies in human medicine have shown that early identification and targeted intervention can delay (50, 51), prevent (52), or reverse the progression of frailty (12–14). Early diagnosis is often noted to be important for a more positive outcome (12, 14, 51). Interventions are wide-ranging, including nutritional supplementation, medications, and exercise programs, among others (2). In a study of frailty scores, life-space (a measure of the geographical space in which a person’s life takes place) and quality of life, Chitalu and colleagues found that among people with high frailty scores, those with high life-space had better quality of life than similarly frail people with lower life-space; by contrast, life-space was a less relevant factor to quality of life among non-frail individuals (53). This finding suggests that specific interventions such as increasing life-space, may preferentially benefit frail individuals compared to their potential benefit on the greater geriatric population overall. An exploratory analysis of the MoveStrong exercises suggested improvement in frailty indicators (gait speed, balance, sit-to-stand functioning) and health-related quality of life in both frail and pre-frail individuals who used these exercises (54).
It is clear that instruments deployed in the assessment of human frailty fulfill a variety of roles. Some are used for screening large community populations for both pre-frailty and frailty (15), some are used within long-term care facilities (30, 36, 41) and others are used in healthcare facilities (29, 40, 41) and may include components of diagnostic data (34, 55, 56). The diverse instruments range from simple to complex and include assessment of numerous and varying domains. There are challenges inherent to the design of some instruments, and the mere fact that so many instruments exist creates challenges. The ability of a clinician or investigator to select the ideal human frailty instrument for the setting and goals is valuable, however, the existence of so many instruments and lack of consistency in use precludes large-scale assessment of their performance or comparison between groups of individuals or intervention techniques.
1.3. Frailty in other species
The importance and relevance of defining positive and negative trajectories of aging have been recognized widely. Outside of human gerontology, frailty scales developed in the laboratory setting for mice and rats have been shown to predict adverse outcomes (57–60), confirming that frailty is relevant and applicable to other species. By contrast, companion animal assessments rely heavily on owner-reported information. In this vein, veterinary specialties have developed quality of life (QoL) tools that are specific to a condition or body system [e.g., Canine Owner Reported QoL Questionnaire (61), Canine Symptom Assessment Scale (62), and questionnaires for QoL in patients with spinal cord injuries (63), patients with pain secondary to neoplasia (64), and patients undergoing chemotherapy (65)]. QoL questionnaires frequently capture a range of domains that are closely aligned with frailty and demonstrate the feasibility of companion animal frailty assessment by owner-reported metrics. Taking it a step further, McKenzie et al. proposed a Canine Geriatric Syndrome as a framework to evaluate physical, functional, behavioral, and metabolic changes in aging dogs to better understand and investigate the biological aging process; frailty is one of the proposed components of the syndrome (66). Despite this, the study of frailty in veterinary medicine, and specifically in dogs, is still in its infancy.
Hua et al. developed a FP instrument, evaluating five phenotype components derived from those used in human gerontology (67). They extrapolated findings relevant to these five components from the records of physical examinations performed on geriatric service dogs, and found that the presence of more than two of these components was significantly associated with decreased time until death, independent of age, health status, or subclinical and clinical diseases (67). Banzato et al. developed a questionnaire-based FI instrument including 33 health deficits, meant to reflect data collected in a routine health exam (45). Banzato et al.’s FI was shown to have moderate accuracy in predicting short-term mortality (45). In both Hua et al. and Banzato et al.’s work, the prevalence of frailty in dogs was noted to be similar to that of humans (15, 43–45, 67), and the overall mean FI calculated by Banzato et al. was similar to the overall FI for community-dwelling individuals (45, 57). In human gerontology, it has been reported that a FI of 0.7 is considered to be the “threshold when the accumulation of deficits becomes incompatible with life” (45, 68); Banzato et al. noted a similar cut-off point of 0.7 in their FI for dogs (45). Additionally, a new frailty phenotype based on owner responses to simple questions has shown strong predictive power for short term (6 months) mortality in a cohort of healthy senior dogs (69). Collectively, these studies demonstrate that a frailty syndrome similar to that described in human gerontology is also present, and can be assessed, in dogs. A standardized frailty assessment could become as important a tool in geriatric canine health as it is in human healthcare.
2. The need for frailty assessment in veterinary medicine
The importance of frailty assessment in human geriatric medicine has expanded as the elderly proportion of the human population has grown (22, 70, 71). Similarly, as the geriatric veterinary patient population expands, due to progress in diagnostic capability and therapeutic options that improve the overall management of pet health (72), the need for a clinically applicable frailty score in veterinary medicine is becoming clear. Age is the greatest risk factor for the development of frailty and, perhaps because of this strong association, the development of frailty and other age-related diseases within an aging individual is often viewed as inevitable (66). The belief that age alone is the cause of poor health outcomes may lead owners to forego opportunities to investigate actual underlying causes and provide potential interventions. This concept of a ‘normal’ state of poor health inevitably associated with aging is a barrier to the progress of geriatric veterinary medicine. The development and wide use of validated, objective frailty instruments in veterinary medicine are needed to advance the study of companion animal gerontology. Specifically, care of the geriatric companion dog is becoming a more important facet of veterinary general and specialty practice (73–75).
The ability to stratify risk profiles within the geriatric companion dog population, as was described above for human gerontology, could significantly impact prognostication and medical care for dogs. Humane euthanasia of companion dogs is a common manner of death, elected by owners due to a variety of reasons including old age, devastating illness or injury, the perception that recovery is unlikely, lack of access to or affordability of needed care, and poor quality of life, among others (76–78). Thus, veterinary medicine is uniquely impacted by the need to provide accurate prognostic information and to combat assumptions of poor prognosis based on age alone, because perceived poor prognosis can lead to elective euthanasia. Owners’ assessments of their dogs’ health-related quality of life (HRQL) have been shown to be heavily influenced by age itself, rather than survivability (79), and perception of poor HRQL could also promote the owner’s choice to euthanize (17, 18, 42, 80). By contrast, the deployment of a validated companion dog frailty instrument would enable studies to determine the actual stratified assessment of the risk of negative outcomes for geriatric dogs as a function of their frailty. Such a tool could then better support owner decision-making, avoiding the assumption of a poor prognosis simply based on the age of the dog. For example, a retrospective study of the outcomes of over 6,000 dogs entered into the Veterinary Committee on Trauma registry found that among moderately injured dogs, geriatric dogs had a significantly higher risk of death despite intervention, or of experiencing euthanasia due to grave prognosis, than their nongeriatric counterparts (81). The higher rate of deaths despite intervention among geriatric dogs suggests that they may have been frailer; by contrast, the higher rate of euthanasia among these dogs suggests that their owners or attending clinicians may have believed their prognosis was worse than the prognosis of younger dogs with similar injuries.
A frailty screening tool for veterinarians to use to easily identify frail dogs would also allow for adjustments in management protocols, from anesthesia to treatment, similar to the previously mentioned study in human geriatric elective surgical patients (49) – and ideally in the future allow veterinarians to more accurately predict recovery from specific events or illnesses. The first step in the ability to make frailty-specific protocol adjustments to improve frail patients’ outcomes is the ability to identify those patients. The identification of frail dogs would also allow for direct intervention in the frailty state as is seen in human medicine (2, 13, 14, 51, 52, 54). For instance, programs to target strength, balance, or maintenance of muscle mass could be implemented in veterinary medicine to directly improve the lives of geriatric companion dogs. Furthermore, there is a growing body of research into strategies to extend lifespan and healthspan in companion dogs (82–85). Lifespan is a challenging target to deploy in such clinical trials because it can be modified by owner-elected euthanasia in response to owner perception of the dog’s status, access to care, and other factors. Widespread adoption of a validated frailty instrument would enable frailty – physiologically meaningful, aging-associated phenotype – to replace lifespan as a valuable endpoint for such clinical trials. The ability to evaluate the effect of individual frailty on outcomes in specific situations and disease conditions as well as to appraise the utility and impact of an interventional program hinges on the ability to recognize and measure frailty. A widely used frailty assessment for dogs would also allow for comparison between medical institutions and different studies, longitudinal monitoring for individual patients, and an endpoint in clinical trials that assess interventions into aging and age-related decline.
3. Dog Aging Project proposed frailty instrument for dogs
The ideal companion dog frailty instrument would maximize the benefits while minimizing or eliminating the weaknesses associated with the diverse frailty instruments used in human gerontology (Table 3). As described above, diverse instruments available in human gerontology may satisfy the needs of different users but also make the collection of comparable data among sites or populations challenging. If frailty is to become a common component of the assessment of companion dogs, it would be beneficial to avoid this fragmentation of data by ensuring that a single tool is widely accessible and used in all dimensions of veterinary practice. The Dog Aging Project (DAP) is an open-data, long-term, longitudinal study of the genetic and environmental determinants of healthy aging in companion dogs (100, 101) and the ability to assess frailty in this population is paramount to fulfill the goals of the study. Enrolled dogs represent diverse household environments in all 50 US States and receive medical management from their primary care veterinarians throughout the study. A companion dog frailty instrument that can be used in all the practices in which these dogs receive routine medical attention will create an opportunity for the collection and clinical application of comparable frailty information not just from DAP-enrolled dogs, but from all companion dogs. The DAP has been previously described (100). Briefly, the DAP collects comprehensive information about dietary and exercise management, home environments, and health histories of enrolled dogs and it is likely that some of these experiences could influence, or be influenced by, frailty. The utility of a proposed frailty instrument would be assessed among these diverse groups of dogs, to better describe the manner in which varying attributes and experiences impact the trajectory of frailty in companion dogs.
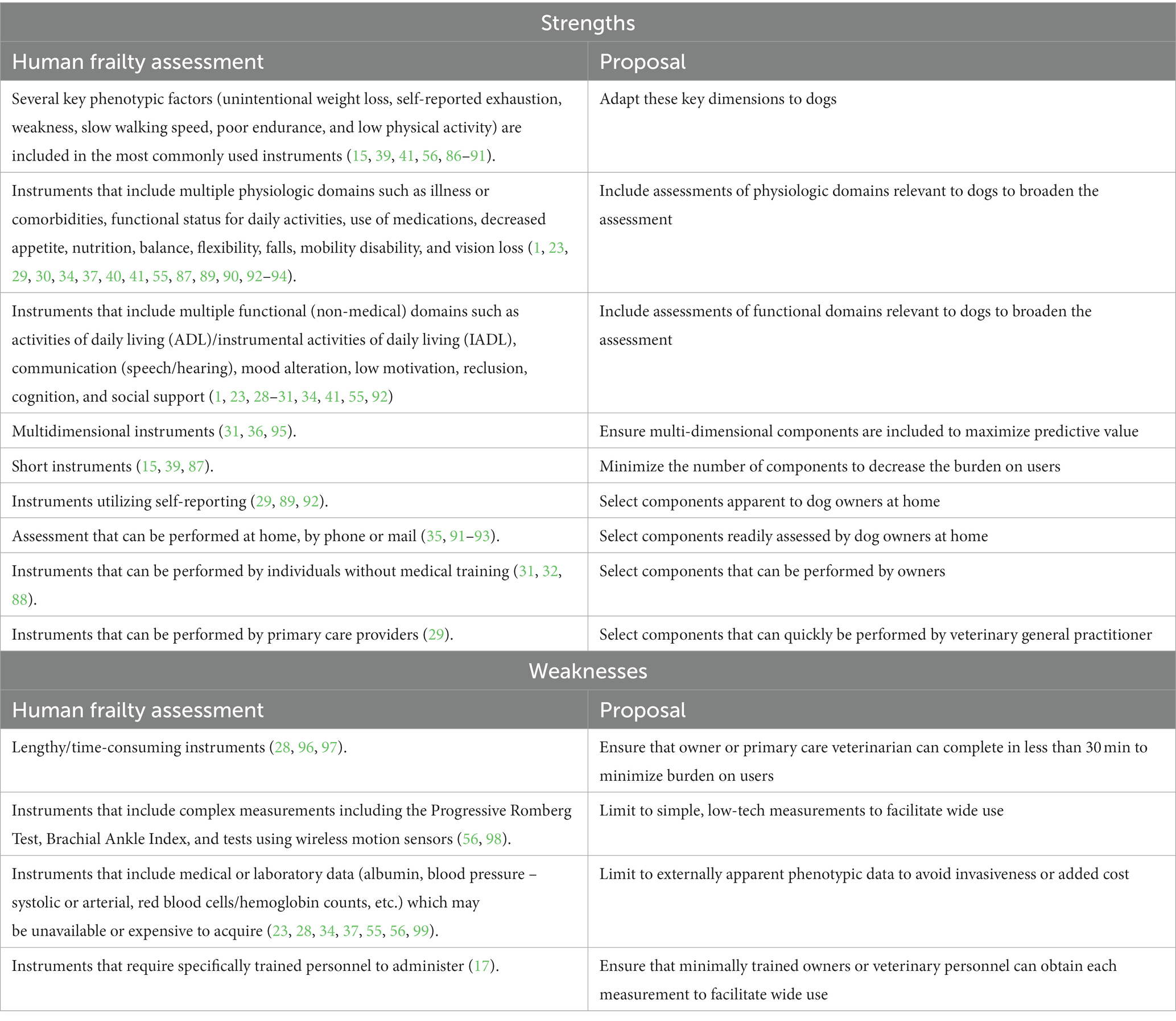
Table 3. Maximizing strengths and avoiding weaknesses of human gerontology frailty assessment in a proposed companion dog frailty instrument.
As discussed above, frailty instruments in human gerontology may be optimized for different settings, and ultimately a three-tiered structure (Screening, Assessment, Triage) for frailty evaluation in dogs seems valuable. The Screening step would enable owners to identify dogs likely to benefit most from frailty assessment, whereas the Triage step could be deployed in urgent medical settings to enable appropriate frailty-based protocol modifications for dogs who had not been previously assessed. Here we propose the design and rationale for an Assessment instrument, the Frailty Instrument for Dogs (FIDo).
Development of the FIDo is guided by several overarching goals, derived from observations about the strengths and weaknesses of various human frailty instruments (Table 3). To ensure that this instrument will be accessible to most veterinarians and owners, it must be easy to use, low-tech, low-cost, and of reasonable length (ideally less than 15 min for completion). To capture the complexity and multifactorial essence of frailty, the instrument must be a multi-dimensional tool, including physical, social, and psychological components. As previously discussed, there are two major models for frailty instruments utilized in human gerontology (FP and FI), however, there is no consensus on the better model. Both have been found to provide comparable predictions of mortality (27), and they may be complementary (26) in providing a more robust understanding of an individual. To achieve the goal that the FIDo is low-tech, low-cost, easily implemented, and widely available and utilized for the assessment of frailty, the DAP elected to build a phenotypic frailty model that includes externally apparent components. Use of a phenotypic instrument will allow the identification of frail patients using information that can be readily collected by owners and veterinarians without the need for medical or diagnostic interactions, that may hinder implementation due to financial or other barriers to care. A phenotypic approach also facilitates the identification of frail individuals that may be free of diagnosed disease (9, 26). Thus, in contrast to Banzato et al.’s FI instrument (45) diagnostic data and multimorbidity will be components of a separate assessment tool in the DAP framework. This multimorbidity tool would be available to use as a complementary assessment tool with FIDo for geriatric care in the future. In contrast to the FP instrument previously reported by Hua et al. (67), the components of the DAP FIDo will be prospectively collected by both owners and veterinarians.
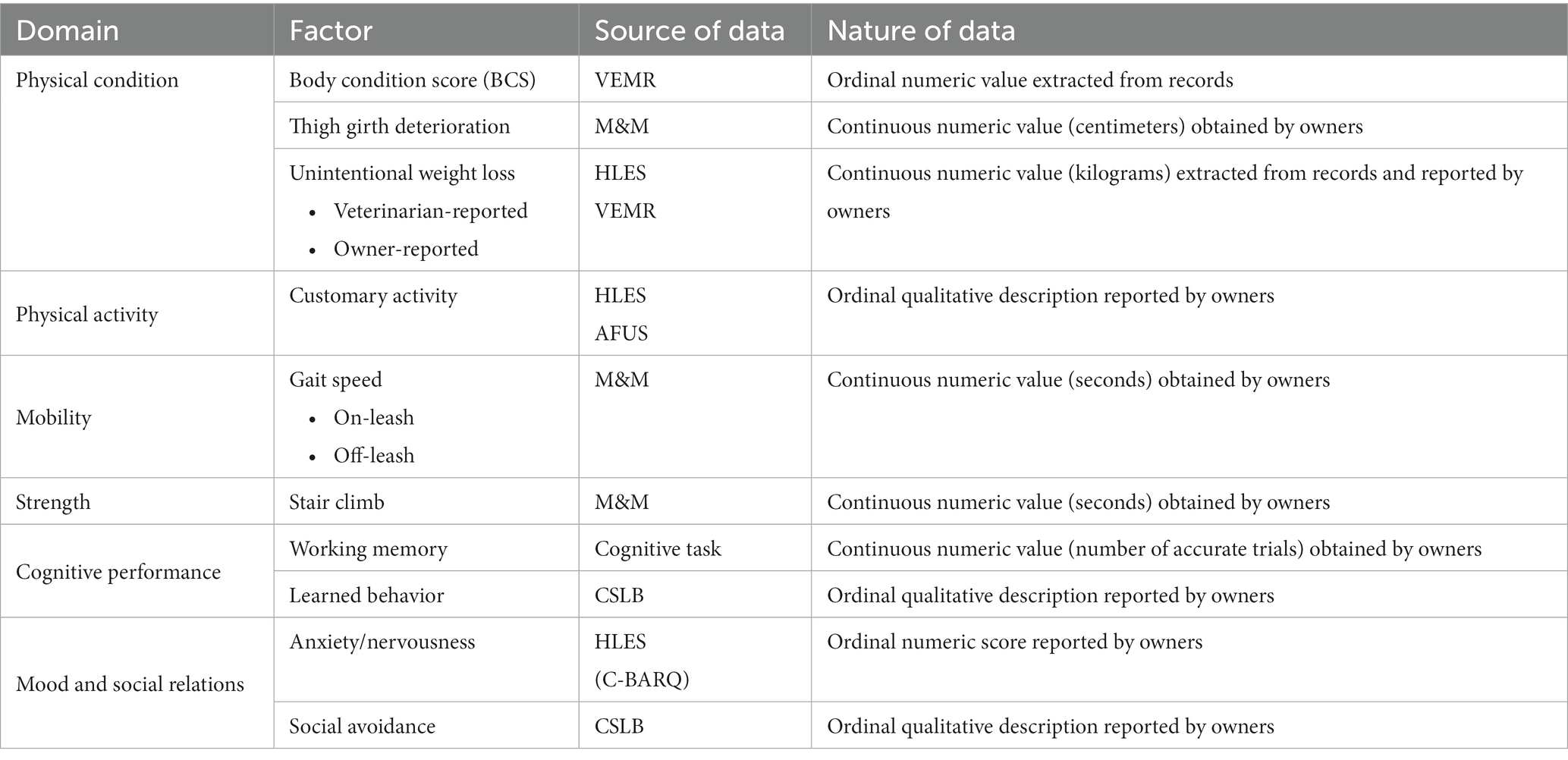
Domains for the DAP FIDo were carefully chosen to reflect those most often used in human gerontology which can be assessed in dogs. Multiple factors within each domain will be included in the initial analysis to determine which are the most relevant phenotypic markers of frailty. The domains that will be included in the DAP FIDo are physical condition, physical activity, mobility, strength, cognitive task performance, and mood and social relations (Table 4). In previous studies of congestive heart failure, diabetes mellitus, and osteoarthritis it was demonstrated that owners are sensitive and accurate reporters of their dog’s health status (102–104), thus owners will serve as primary data sources for most domains. Specific owner-provided information will be extracted from the DAP’s Health and Life Experience Survey (HLES), Annual Follow-Up Survey (AFUS), Canine Social and Learned Behavior Survey (CSLB) (105), Measurement and Mobility Activities (M&M), and Cognitive Tasks (100). Certain factors will also be extracted from veterinary electronic medical records (VEMRs) uploaded by participating owners. These components will initially be evaluated separately to determine which are predictive of mortality in dogs. As each component will be collected longitudinally, we will also assess whether the rate of change of each component is more strongly predictive of mortality than a discrete value at a single time point. All predictive components will be included in the first comprehensive model. Components that are not found to have a relationship with frailty will be removed. Stepwise analysis will allow removal of components that provide redundant information; remaining components will be weighted by a coefficient proportional to the association of each parameter with mortality.
3.1. Physical condition
Three different metrics of physical condition, body condition score (BCS), thigh girth deterioration, and unintentional weight loss, will be included in the initial DAP FIDo. Body condition score, a commonly used 1–9 scale used for the evaluation of an animal’s body fat from emaciated to obese, will be extracted from VEMRs. Increased BCS has been shown to be predictive of disease and mortality risk in canines (106), but a ‘protective effect’ associated with increased BCS has also been reported in the study of a canine frailty index model (45). Sarcopenia is an increasingly important factor in the assessment of human frailty and the mechanism by which it occurs is receiving increasing scrutiny (19, 107–109). As a component of frailty or an isolated finding, sarcopenia has been shown to be a marker for increased risk for disease and death (109). Sarcopenia has been described in the dog (110, 111) and a clinical assessment, the Muscle Condition Score (MCS) (111, 112), is available in veterinary practice. However, MCS is not yet a widely utilized tool in general practice and is found in only 5% of VEMRs submitted to the DAP. By contrast, it has been shown that minimally trained owners can obtain measurements of their dogs’ thigh circumference with strong agreement to those obtained by veterinary researchers (113). Thus, the DAP FIDo will use serial thigh circumference measurements to assess canine sarcopenia. Unintentional weight loss is a consistent factor in human frailty instruments. DAP participating owners provide their dogs’ weights annually (once in HLES, and subsequently in AFUS). We will also extract weights recorded at veterinary visits from VEMRs. As is the case for people, intentional weight loss in adult dogs is difficult for most owners to achieve (114, 115) the assumption will be that any detected weight loss was unintentional. However, the variable intervals at which weights are recorded at veterinary visits, and the difficulty of confirming whether any detected weight loss was truly unintentional, may preclude the value of this factor; validation analysis will determine whether it remains in the final instrument.
3.2. Physical activity
A variety of metrics of physical activity including objective kilocalorie expenditure, as well as self-reported activity, inactivity, exhaustion, and fatigue, are heavily utilized in human frailty instruments due to their predictive value. Importantly, these metrics are designed to detect or assess the individual’s customary amount and vigor of activity (15, 56, 86, 116), rather than the peak performance of which an individual is capable. Companion dogs often depend upon their owners for physical activity, such as being taken on leashed walks, or being given access to a park for play. For this reason, a simple assessment of the frequency of activity may reflect owner preferences more than dog ability. By contrast the dog’s interest in activity, vigor of activity, and change in activity patterns over time may be better suited to detect the onset of frailty. These topics of dog activity are addressed in HLES and AFUS, and we will derive a measurement of physical activity from owner responses to questions including, “Please choose the best description of your dog’s lifestyle over the past year. [not active/moderately active/very active],” “Over the past year, on average how much time per day is your dog physically active? Number of hours active: [0–8+], Number of minutes active: [0/10/20/30/40/50],” and “Over the past year, when your dog is active, what is the average intensity level of that activity? [low(walking)]/moderate [jogging]/[vigorous (sprinting)].” Other follow-up items identify the nature of the activity, including environmental conditions, on vs. off-leash, type of activity, etc. The use of multiple questions surrounding owner-reported activity allows for us to find the components that most accurately reflect the change in a dog’s activity as well as its relation to frailty.
3.3. Mobility
Despite significant variability between instruments used in human medicine, there is an overarching consensus that a measure of physical function is needed (117–119). A common measurement of mobility in human frailty instruments is walking speed, where an individual’s time to walk, a specified distance is compared to the lowest 20% of the population (15). Slower walking speeds in people have been shown to be predictive of worse postoperative outcomes (120–122), morbidity, and mortality (122–124); similar results were seen in rodents (125–127). Several small studies of mobility have shown a general trend of decline in functional capacity and spontaneous activity with but results also varied by location, breed and sex (128–130). Mobility scales for use in companion dog populations have previously been proposed, but they have not been validated or implemented in large populations (131, 132).
When developing a canine mobility scale Gonçalves et al. found a statistically significant difference in mobility scale scores between age quartiles (131), however, the presence of orthopedic and neurologic diseases was also noted to produce a statistically significant difference in scores (131). Banzato et al.’s frailty index study also reported poor mobility and low physical activity were significantly associated with time to death independently (45).
Morgan et al. morphologic and mobility trials found age was a weak but significant predictor of a dog’s speed for a given height (113), suggesting speed could be a useful frailty factor. They also showed that owners were able to perform low-tech assessments of their dogs’ speed with minimal training and were able to obtain measurements that strongly agreed with those obtained by investigators (113). Consequently, the DAP FIDo will include times to traverse a measured distance on-leash and off-leash over a flat-surface from our Measurement and Mobility instrument. A greater magnitude of change in off-leash speed with increasing age was reported (109), but this metric may not be a significant indicator of frailty among dogs of the same age. Thus, both on- and off-leash flat-surface speed will be evaluated separately in the initial model to determine whether either or both add predictive value to the frailty model independently of age alone.
3.4. Strength
Strength is also frequently included in human frailty instruments. In humans, strength can be assessed utilizing a variety of measurements including the ability to rise from a chair (39) and stair climbing (133); grip strength (15, 87) is the most popular measure. Grip strength is not a feasible metric in dogs, but stair climbing is a routine and accessible activity for many dogs. Elderly people often become reluctant to climb stairs as they age (127–129) and the authors’ clinical experience suggests that the same is true among companion dogs. Morgan et al.’s morphologic and mobility trials also assessed timed stair ascent and found that age was a weak but significant predictor of speed in this task as well (113). Other options that were considered as strength assessments, such as pressure-sensing chew toys (134), or systems that measure the strength with which a dog can pull against a measurement device (135, 136), are expensive, have limited availability, depend upon the dog’s training and interest, or some combination of those flaws. The DAP FIDo will use timed stair ascent as our measurement of canine strength.
3.5. Cognitive task performance
Cognitive decline is an important factor in many human frailty instruments (33, 137–139). There is an incompletely understood relationship among frailty, mild cognitive impairment, and the ability to perform the Instrumental Activities of Daily Living (IADL) in humans (55, 92, 140–142). While IADLs cannot readily be extrapolated to dogs, simple cognitive tasks that mimic daily activities have been developed and validated in dogs. Dogs, like humans, display evidence of mild cognitive decline with age, and their performance on these tasks also declines with age (143, 144). We will use performance on a purpose-built at-home cognitive task designed to assess working memory within our DAP FIDo. The purpose-built cognitive task is easily administered at home and does not require specialized equipment or dog training. Dogs are tested for their ability to (1) recall the location of a food item after varying delays [delayed search], or to recall which location still contains a food item after consuming food items from all other locations [radial array]. Instructions are provided through online video tutorials and owners respond to simple questions about their dog’s behavior through an interactive online survey.
Like humans, some dogs also develop dementia, called Canine Cognitive Dysfunction Syndrome (CCDS) (145, 146). The Canine Cognitive Dysfunction Rating Scale (CCDRS) is a validated survey used to diagnose CCDS among dogs with a cumulative score of 50 or greater (104, 105, 147). We have deployed this instrument within our DAP population as an annual survey, rebranded the Canine Social and Learned Behavior (CSLB) instrument, to avoid potential negative connotations of the diagnostic term, “cognitive dysfunction.” However, the purpose of a frailty instrument is to detect early, mild change in function, before an overt diagnosis, such as CCDS, is made. Thus, we will use individual items from the CSLB, rather than the total score, within our DAP FIDo. Specifically, we will use responses to the items “How often does your dog stare blankly at the walls or floor?” and “Compared with 6 months ago, does your dog have difficulty finding food dropped on the floor?” to detect changes in at-home cognitive performance over time. The total CSLB score, as well as owner-reported diagnoses in the Health Section of HLES, will be evaluated to ensure that the selected CSLB items do not reflect a diagnosis of CCD or another neurologic disease that may have its own impact on mortality.
3.6. Social behavior
Recent work in human frailty has promoted the importance of including social components in frailty assessments (1, 148). While questions about happiness and social support networks cannot be readily extrapolated to dogs, there is a large body of research describing normal companion dog behavior, including socially interactive behavior (149–152). The Canine Behavioral Assessment and Research Questionnaire (C-BARQ) is a validated survey used to objectively document companion dog behavior in a variety of settings (153–155). We have deployed the C-BARQ as the Behavior component of HLES and AFUS, so that it is completed annually by DAP participants. We will use responses to specific items from both C-BARQ and CSLB to represent mood and social relations in the DAP FIDo. Specifically, we will use the cumulative score from the Fear and Anxiety section of C-BARQ and the item on social avoidance, “How often does your dog walk away while, or avoid, being petted?” from CSLB.
These items addressing physical condition, physical activity, mobility, strength, cognitive task performance, and mood and social relations will be included in the first version of the DAP FIDo to identify those domains and specific factors that most strongly predict mortality among companion dogs. The model will be revised to include the fewest, but most informative elements, to facilitate wide deployment among companion dog owners and veterinary practices.
4. Discussion and conclusion
The assessment of frailty plays a central role in human gerontology as a superior means to understand, describe, and mitigate certain manifestations of aging and the associated risks of poor health outcomes, including death within individuals and communities. The numerous frailty instruments for humans that have been developed include diverse domains and factors within each domain, leading to challenges in comparing the efficacy and utility of these instruments across populations and settings. As companion dogs increasingly survive into geriatric ages, the ability to document their frailty, and to understand its impact on health outcomes, becomes increasingly valuable. The Dog Aging Project is collecting targeted data to enable the construction and validation of a companion dog frailty instrument designed to be uncomplicated, quick to complete, low-tech, low-cost, and accessible to dog owners and veterinary general practitioners. Use of this tool, once finalized, will enhance both research opportunities, and the ability to provide excellent veterinary care, to aging companion dogs.
Author contributions
AF, AR, KC, NO, and RM worked together to construct an outline of the manuscript. RM wrote the first draft of the manuscript. AR, NO, AF, and KC revised and edited sequential drafts of the manuscript. EP contributed to the visualization and revision. All authors have read and approved the final manuscript.
Funding
The Dog Aging Project is supported by U19 grant AG057377 from the National Institute on Aging, a part of the National Institutes of Health, and private donations. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
The Dog Aging Project thanks study participants, their dogs, and community veterinarians for their important contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer BM declared a past co-authorship with the author NO to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rolfson, DB, Majumdar, SR, Tsuyuki, RT, Tahir, A, and Rockwood, K. Validity and reliability of the Edmonton frail scale. Age Ageing. (2006) 35:526–9. doi: 10.1093/ageing/afl041
2. Walston, J, Hadley, EC, Ferrucci, L, Guralnik, JM, Newman, AB, Studenski, SA, et al. Research Agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging research conference on frailty in older adults: research agenda for frailty. J Am Geriatr Soc. (2006) 54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x
3. Markle-Reid, M, and Browne, G. Conceptualizations of frailty in relation to older adults: conceptualizations of frailty in relation to older adults. J Adv Nurs. (2003) 44:58–68. doi: 10.1046/j.1365-2648.2003.02767.x
4. Raphael, D, Cava, M, Brown, I, Renwick, R, Heathcote, K, Weir, N, et al. Frailty: a public health perspective. Can J Public Health. (1995) 86:224–7.
5. Buchner, DM, and Wagner, EH. Preventing frail health. Clin Geriatr Med. (1992) 8:1–17. doi: 10.1016/S0749-0690(18)30494-4
6. on behalf of the VIP1 study groupFlaatten, H, De Lange, DW, Morandi, A, Andersen, FH, Artigas, A, Bertolini, G, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years). Intensive Care Med. (2017) 43:1820–8. doi: 10.1007/s00134-017-4940-8
7. Franceschi, C, Garagnani, P, Morsiani, C, Conte, M, Santoro, A, Grignolio, A, et al. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med. (2018) 12:61. doi: 10.3389/fmed.2018.00061
8. Strawbridge, WJ, Shema, SJ, Balfour, JL, Higby, HR, and Kaplan, GA. Antecedents of frailty over three decades in an older cohort. J Gerontol Ser B Psychol Sci Soc Sci. (1998) 53B:S9–S16. doi: 10.1093/geronb/53B.1.S9
9. Fried, LP, Ferrucci, L, Darer, J, Williamson, JD, and Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol Ser A Biol Med Sci. (2004) 59:M255–63. doi: 10.1093/gerona/59.3.M255
10. Kim, MJ, Park, S, Jung, Y, Kim, SH, and Oh, IH. Exploring health-related quality of life and frailty in older adults based on the Korean frailty and aging cohort study. Qual Life Res. (2020) 29:2911–9. doi: 10.1007/s11136-020-02568-5
11. Bandeen-Roche, K, Seplaki, CL, Huang, J, Buta, B, Kalyani, RR, Varadhan, R, et al. Frailty in older adults: a nationally representative profile in the United States. GERONA. (2015) 70:1427–34. doi: 10.1093/gerona/glv133
12. Cesari, M, Prince, M, Thiyagarajan, JA, De Carvalho, IA, Bernabei, R, Chan, P, et al. Frailty: an emerging public health priority. J Am Med Dir Assoc. (2016) 17:188–92. doi: 10.1016/j.jamda.2015.12.016
13. Tarazona-Santabalbina, FJ, Gómez-Cabrera, MC, Pérez-Ros, P, Martínez-Arnau, FM, Cabo, H, Tsaparas, K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. (2016) 17:426–33. doi: 10.1016/j.jamda.2016.01.019
14. Hoogendijk, EO, Afilalo, J, Ensrud, KE, Kowal, P, Onder, G, and Fried, LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394:1365–75. doi: 10.1016/S0140-6736(19)31786-6
15. Fried, LP, Tangen, CM, Walston, J, Newman, AB, Hirsch, C, Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A Biol Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.M146
16. Morley, JE, Vellas, B, Abellan van Kan, G, Anker, SD, Bauer, JM, Bernabei, R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) 14:392–7. doi: 10.1016/j.jamda.2013.03.022
17. Windle, G. What is resilience? A review and concept analysis. Rev Clin Gerontol. (2011) 21:152–69. doi: 10.1017/S0959259810000420
18. Kuchel, GA. Frailty, allostatic load, and the future of predictive gerontology: Editorial. J Am Geriatr Soc. (2009) 57:1704–6. doi: 10.1111/j.1532-5415.2009.02406.x
19. Roubenoff, R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. (2000) 4:140–2.
20. Walston, J, and Fried, LP. Frailty and the older man. Med Clin N Am. (1999) 83:1173–94. doi: 10.1016/S0025-7125(05)70157-7
21. Clegg, A, and Hassan-Smith, Z. Frailty and the endocrine system. Lancet Diabetes Endocrinol. (2018) 6:743–52. doi: 10.1016/S2213-8587(18)30110-4
22. Clegg, A, Young, J, Iliffe, S, Rikkert, MO, and Rockwood, K. Frailty in elderly people. Lancet. (2013) 381:752–62. doi: 10.1016/S0140-6736(12)62167-9
23. Clegg, A, Bates, C, Young, J, Ryan, R, Nichols, L, Ann Teale, E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. (2016) 45:353–60. doi: 10.1093/ageing/afw039
24. Rockwood, K, Stadnyk, K, MacKnight, C, McDowell, I, Hébert, R, and Hogan, DB. A brief clinical instrument to classify frailty in elderly people. Lancet. (1999) 353:205–6. doi: 10.1016/S0140-6736(98)04402-X
25. Rockwood, K. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. (2005) 173:489–95. doi: 10.1503/cmaj.050051
26. Cesari, M, Gambassi, G, Abellan van Kan, G, and Vellas, B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. (2014) 43:10–2. doi: 10.1093/ageing/aft160
27. Kim, DJ, Massa, MS, Potter, CM, Clarke, R, and Bennett, DA. Systematic review of the utility of the frailty index and frailty phenotype to predict all-cause mortality in older people. Syst Rev. (2022) 11:187. doi: 10.1186/s13643-022-02052-w
28. Mitnitski, AB, Mogilner, AJ, and Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci World J. (2001) 1:323–36. doi: 10.1100/tsw.2001.58
29. Ravaglia, G, Forti, P, Lucicesare, A, Pisacane, N, Rietti, E, and Patterson, C. Development of an easy prognostic score for frailty outcomes in the aged. Age Ageing. (2008) 37:161–6. doi: 10.1093/ageing/afm195
30. Jones, DM, Song, X, and Rockwood, K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment: a frailty index from a comprehensive geriatric assessment. J Am Geriatr Soc. (2004) 52:1929–33. doi: 10.1111/j.1532-5415.2004.52521.x
31. Vernerey, D, Anota, A, Vandel, P, Paget-Bailly, S, Dion, M, Bailly, V, et al. Development and validation of the FRAGIRE tool for assessment an older person’s risk for frailty. BMC Geriatr. (2016) 16:187. doi: 10.1186/s12877-016-0360-9
32. De Witte, N, Gobbens, R, De Donder, L, Dury, S, Buffel, T, Schols, J, et al. The comprehensive frailty assessment instrument: development, validity and reliability. Geriatr Nurs. (2013) 34:274–81. doi: 10.1016/j.gerinurse.2013.03.002
33. Faller, JW, Pereira, D, Do, N, De Souza, S, Nampo, FK, Orlandi, F, et al. Instruments for the detection of frailty syndrome in older adults: a systematic review. PLoS One. (2019) 14:e0216166
34. Orouji Jokar, T, Ibraheem, K, Rhee, P, Kulavatunyou, N, Haider, A, Phelan, HA, et al. Emergency general surgery specific frailty index: a validation study. J Trauma Acute Care Surg. (2016) 81:254–60. doi: 10.1097/TA.0000000000001120
35. Derrick Chan, DC, Tsou, HH, Chen, CY, and Chen, CY. Validation of the Chinese-Canadian study of health and aging clinical frailty scale (CSHA-CFS) telephone version. Arch Gerontol Geriatr. (2010) 50:e74–80. doi: 10.1016/j.archger.2009.06.004
36. Peters, LL, Boter, H, Buskens, E, and Slaets, JPJ. Measurement properties of the Groningen frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. (2012) 13:546–51. doi: 10.1016/j.jamda.2012.04.007
37. Velanovich, V, Antoine, H, Swartz, A, Peters, D, and Rubinfeld, I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. (2013) 183:104–10. doi: 10.1016/j.jss.2013.01.021
38. Studenski, S, Hayes, RP, Leibowitz, RQ, Bode, R, Lavery, L, Walston, J, et al. Clinical global impression of change in physical frailty: development of a measure based on clinical judgment: change in frailty. J Am Geriatr Soc. (2004) 52:1560–6. doi: 10.1111/j.1532-5415.2004.52423.x
39. Ensrud, KE. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. (2008) 168:382–9. doi: 10.1001/archinternmed.2007.113
40. Goldstein, J, Hubbard, RE, Moorhouse, P, Andrew, MK, Mitnitski, A, and Rockwood, K. The validation of a care partner-derived frailty index based upon comprehensive geriatric assessment (CP-FI-CGA) in emergency medical services and geriatric ambulatory care. Age Ageing. (2015) 44:327–30. doi: 10.1093/ageing/afu161
41. Metzelthin, SF, Daniëls, R, van Rossum, E, de Witte, L, van den Heuvel, WJ, and Kempen, GI. The psychometric properties of three self-report screening instruments for identifying frail older people in the community. BMC Public Health. (2010) 10:176. doi: 10.1186/1471-2458-10-176
42. de Vries, NM, Staal, JB, van Ravensberg, CD, Hobbelen, JSM, Olde Rikkert, MGM, and Nijhuis-van der Sanden, MWG. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. (2011) 10:104–14. doi: 10.1016/j.arr.2010.09.001
43. Aguayo, GA, Donneau, AF, Vaillant, MT, Schritz, A, Franco, OH, Stranges, S, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. (2017) 186:420–34. doi: 10.1093/aje/kwx061
44. Collard, RM, Boter, H, Schoevers, RA, and Oude Voshaar, RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. (2012) 60:1487–92. doi: 10.1111/j.1532-5415.2012.04054.x
45. Banzato, T, Franzo, G, Di Maggio, R, Nicoletto, E, Burti, S, Cesari, M, et al. A frailty index based on clinical data to quantify mortality risk in dogs. Sci Rep. (2019) 9:16749. doi: 10.1038/s41598-019-52585-9
46. Perea, LL, Fort, LS, Morgan, ME, Brown, CT, Wang, S, and Bradburn, E. Frailty is associated with worse outcomes in geriatric pelvic fractures. Am Surg. (2022) 88:1573–5. doi: 10.1177/00031348221084943
47. Pecheva, M, Phillips, M, Hull, P, Carrothers, AOR, and Queally, J. The impact of frailty in major trauma in older patients. Injury. (2020) 51:1536–42. doi: 10.1016/j.injury.2020.04.045
48. Galizia, G, Cacciatore, F, Testa, G, Della-Morte, D, Mazzella, F, Langellotto, A, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res. (2011) 23:118–25. doi: 10.1007/BF03351076
49. Hall, DE, Arya, S, Schmid, KK, Carlson, MA, Lavedan, P, Bailey, TL, et al. Association of a Frailty Screening Initiative with Postoperative Survival at 30, 180, and 365 days. JAMA Surg. (2017) 152:233. doi: 10.1001/jamasurg.2016.4219
50. Wang, CH, Chang, WP, Chen, SR, Cheng, WJ, Chou, KR, and Pien, LC. Health literacy and exercise to treat frailty in community-dwelling older adults: a National Survey Study. IJERPH. (2022) 19:8711. doi: 10.3390/ijerph19148711
51. Gill, TM, Baker, DI, Gottschalk, M, Peduzzi, PN, Allore, H, and Byers, A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. (2002) 347:1068–74. doi: 10.1056/NEJMoa020423
52. Lang, PO, Michel, JP, and Zekry, D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. (2009) 55:539–49. doi: 10.1159/000211949
53. Chitalu, P, Tsui, A, Searle, SD, and Davis, D. Life-space, frailty, and health-related quality of life. BMC Geriatr. (2022) 22:646. doi: 10.1186/s12877-022-03355-2
54. Rodrigues, IB, Wagler, JB, Keller, H, Thabane, L, Weston, ZJ, Straus, SE, et al. Encouraging older adults with pre-frailty and frailty to “MoveStrong”: an analysis of secondary outcomes for a pilot randomized controlled trial. Health Promot Chronic Dis Prev Can. (2022) 42:238–51. doi: 10.24095/hpcdp.42.6.02
55. Joseph, B, Pandit, V, Zangbar, B, Kulvatunyou, N, Tang, A, O’Keeffe, T, et al. Validating trauma-specific frailty index for geriatric trauma patients: a prospective analysis. J Am Coll Surg. (2014) 219:10–17e1. doi: 10.1016/j.jamcollsurg.2014.03.020
56. García-García, FJ, Carcaillon, L, Fernandez-Tresguerres, J, Alfaro, A, Larrion, JL, Castillo, C, et al. A new operational definition of frailty: the frailty trait scale. J Am Med Dir Assoc. (2014) 15:371.e7–371.e13. doi: 10.1016/j.jamda.2014.01.004
57. Rockwood, K, Blodgett, JM, Theou, O, Sun, MH, Feridooni, HA, Mitnitski, A, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep. (2017) 7:43068. doi: 10.1038/srep43068
58. Kane, AE, Hilmer, SN, Boyer, D, Gavin, K, Nines, D, Howlett, SE, et al. Impact of longevity interventions on a validated mouse clinical frailty index. GERONA. (2016) 71:333–9. doi: 10.1093/gerona/glu315
59. Whitehead, JC, Hildebrand, BA, Sun, M, Rockwood, MR, Rose, RA, Rockwood, K, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol Series A. (2014) 69:621–32. doi: 10.1093/gerona/glt136
60. Liu, H, Graber, TG, Ferguson-Stegall, L, and Thompson, LV. Clinically relevant frailty index for mice. J Gerontol Ser A Biol Med Sci. (2014) 69:1485–91. doi: 10.1093/gerona/glt188
61. Giuffrida, MA, Brown, DC, Ellenberg, SS, and Farrar, JT. Development and psychometric testing of the canine owner-reported quality of life questionnaire, an instrument designed to measure quality of life in dogs with cancer. JAVMA. (2018) 252:1073–83. doi: 10.2460/javma.252.9.1073
62. Giuffrida, MA, Farrar, JT, and Brown, DC. Psychometric properties of the canine symptom assessment scale, a multidimensional owner-reported questionnaire instrument for assessment of physical symptoms in dogs with solid tumors. JAVMA. (2017) 251:1405–14. doi: 10.2460/javma.251.12.1405
63. Budke, CM, Levine, JM, Kerwin, SC, Levine, GJ, Hettlich, BF, and Slater, MR. Evaluation of a questionnaire for obtaining owner-perceived, weighted quality-of-life assessments for dogs with spinal cord injuries. JAVMA. (2008) 233:925–30. doi: 10.2460/javma.233.6.925
64. Yazbek, KVB. Fantoni DT, Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. JAVMA. (2005) 226:1354–8. doi: 10.2460/javma.2005.226.1354
65. Iliopoulou, MA, Kitchell, BE, and Yuzbasiyan-Gurkan, V. Development of a survey instrument to assess health-related quality of life in small animal cancer patients treated with chemotherapy. JAVMA. (2013) 242:1679–87. doi: 10.2460/javma.242.12.1679
66. McKenzie, BA, Chen, FL, Gruen, ME, and Olby, NJ. Canine geriatric syndrome: a framework for advancing research in veterinary Geroscience. Front Vet Sci. (2022) 9:853743. doi: 10.3389/fvets.2022.853743
67. Hua, J, Hoummady, S, Muller, C, Pouchelon, JL, Blondot, M, Gilbert, C, et al. Assessment of frailty in aged dogs. AJVR. (2016) 77:1357–65. doi: 10.2460/ajvr.77.12.1357
68. Searle, SD, Mitnitski, A, Gahbauer, EA, Gill, TM, and Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. (2008) 8:24. doi: 10.1186/1471-2318-8-24
69. Russell, K, Fefer, G, Mondino, A, and Olby, N. Establishing a frailty phenotype for aging dogs. Research Abstract JVIM. (2022) 36:2382–022.
70. Nan, J, Duan, Y, Wu, S, Liao, L, Li, X, Zhao, Y, et al. Perspectives of older adults, caregivers, healthcare providers on frailty screening in primary care: a systematic review and qualitative meta-synthesis. BMC Geriatr. (2022) 22:482. doi: 10.1186/s12877-022-03173-6
71. Sutton, JL, Gould, RL, Daley, S, Coulson, MC, Ward, EV, Butler, AM, et al. Psychometric properties of multicomponent tools designed to assess frailty in older adults: a systematic review. BMC Geriatr. (2016) 16:55. doi: 10.1186/s12877-016-0225-2
72. Cozzi, B, Ballarin, C, Mantovani, R, and Rota, A. Aging and veterinary Care of Cats, dogs, and horses through the Records of Three University Veterinary Hospitals. Front Vet Sci. (2017). 4. doi: 10.3389/fvets.2017.00014/full
73. Bartges, J, Boynton, B, Vogt, AH, Krauter, E, Lambrecht, K, Svec, R, et al. AAHA canine life stage guidelines*. J Am Anim Hosp Assoc. (2012) 48:1–11. doi: 10.5326/JAAHA-MS-4009
74. Metzger, FL. Senior and geriatric care programs for veterinarians. Vet Clin N Am Small Anim Pract. (2005) 35:743–53. doi: 10.1016/j.cvsm.2004.12.005
75. Fortney, WD. Implementing a successful senior/geriatric health care program for veterinarians, veterinary technicians, and office managers. Vet Clin N Am Small Anim Pract. (2012) 42:823–34. doi: 10.1016/j.cvsm.2012.04.011
76. Pegram, C, Gray, C, Packer, RMA, Richards, Y, Church, DB, Brodbelt, DC, et al. Proportion and risk factors for death by euthanasia in dogs in the UK. Sci Rep. (2021) 11:9145. doi: 10.1038/s41598-021-88342-0
77. Gorodetsky, E. Epidemiology of dog and cat euthanasia across Canadian prairie provinces. Can Vet J. (1997) 38:649–52.
78. Huang, WH, Liao, AT, Chu, PY, Zhai, SH, Yen, IF, and Liu, CH. A 3-year surveillance on causes of death or reasons for euthanasia of domesticated dogs in Taiwan. Prev Vet Med. (2017) 147:1–10. doi: 10.1016/j.prevetmed.2017.08.015
79. Rodger, S, Scott, EM, Nolan, A, Wright, AK, and Reid, J. Effect of age, breed, and sex on the health-related quality of life of owner assessed healthy dogs. Front Vet Sci. (2021): 603139. doi: 10.3389/fvets.2021.603139
80. Theou, O, Cann, L, Blodgett, J, Wallace, LMK, Brothers, TD, and Rockwood, K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res Rev. (2015) 21:78–94. doi: 10.1016/j.arr.2015.04.001
81. Young, AA, Cooper, E, Yaxley, P, and Habing, G. Evaluation of geriatric trauma in dogs with moderate to severe injury (6169 cases): a VetCOT registry study. J Vet Emergen Crit Care. (2022) 32:386–96. doi: 10.1111/vec.13165
82. © 2023 Cellular Longevity, Inc. More, healthier years with your pet. More, healthier years with your pet. Available at: https://loyalfordogs.com/products
83. Urfer, SR, Kaeberlein, TL, Mailheau, S, Bergman, PJ, Creevy, KE, Promislow, DEL, et al. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. GeroScience. (2017) 39:117–27. doi: 10.1007/s11357-017-9972-z
84. Adams, VJ, Watson, P, Carmichael, S, Gerry, S, Penell, J, and Morgan, DM. Exceptional longevity and potential determinants of successful ageing in a cohort of 39 Labrador retrievers: results of a prospective longitudinal study. Acta Vet Scand. (2015) 58:29. doi: 10.1186/s13028-016-0206-7
85. Waters, DJ. Aging research 2011: exploring the pet dog paradigm. ILAR J. (2011) 52:97–105. doi: 10.1093/ilar.52.1.97
86. Wu, C, Geldhof, GJ, Xue, QL, Kim, DH, Newman, AB, and Odden, MC. Development, construct validity, and predictive validity of a continuous frailty scale: results from 2 large US cohorts. Am J Epidemiol. (2018) 187:1752–62. doi: 10.1093/aje/kwy041
87. Romero-Ortuno, R, Walsh, CD, Lawlor, BA, and Kenny, RA. A frailty instrument for primary care: findings from the survey of health, ageing and retirement in Europe (SHARE). BMC Geriatr. (2010) 10:57. doi: 10.1186/1471-2318-10-57
88. Woo, J, Yu, R, Wong, M, Yeung, F, Wong, M, and Lum, C. Frailty screening in the community using the FRAIL scale. J Am Med Dir Assoc. (2015) 16:412–9. doi: 10.1016/j.jamda.2015.01.087
89. Cesari, M, Demougeot, L, Boccalon, H, Guyonnet, S, Abellan Van Kan, G, Vellas, B, et al. Self-reported screening tool for detecting community-dwelling older persons with frailty syndrome in the absence of mobility disability: the find questionnaire. PLoS One. (2014) 9:e101745. doi: 10.1371/journal.pone.0101745
90. Dapp, U, Minder, CE, Anders, J, Golgert, S, and von Renteln-Kruse, W. Long-term prediction of changes in health status, frailty, nursing care and mortality in community-dwelling senior citizens - results from the longitudinal urban cohort ageing study (LUCAS). BMC Geriatr. (2014) 14:141. doi: 10.1186/1471-2318-14-141
91. de Souto Barreto, P, Greig, C, and Ferrandez, AM. Detecting and categorizing frailty status in older adults using a self-report screening instrument. Arch Gerontol Geriatr. (2012) 54:e249–54. doi: 10.1016/j.archger.2011.08.003
92. Raîche, M, Hébert, R, and Dubois, MF. PRISMA-7: a case-finding tool to identify older adults with moderate to severe disabilities. Arch Gerontol Geriatr. (2008) 47:9–18. doi: 10.1016/j.archger.2007.06.004
93. Di Bari, M, Profili, F, Bandinelli, S, Salvioni, A, Mossello, E, Corridori, C, et al. Screening for frailty in older adults using a postal questionnaire: rationale, methods, and instruments validation of the INTER-FRAIL study. J Am Geriatr Soc. (2014) 62:1933–7. doi: 10.1111/jgs.13029
94. Chimukangara, M, Helm, MC, Frelich, MJ, Bosler, ME, Rein, LE, Szabo, A, et al. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc. (2017) 31:2509–19. doi: 10.1007/s00464-016-5253-7
95. Daniels, R, van Rossum, E, Beurskens, A, van den Heuvel, W, and de Witte, L. The predictive validity of three self-report screening instruments for identifying frail older people in the community. BMC Public Health. (2012) 12:69. doi: 10.1186/1471-2458-12-69
96. Rockwood, K, Theou, O, and Mitnitski, A. What are frailty instruments for? Age Ageing. (2015) 44:545–7. doi: 10.1093/ageing/afv043
97. Ma, L, Zhang, L, Tang, Z, Sun, F, Diao, L, Wang, J, et al. Use of the frailty index in evaluating the prognosis of older people in Beijing: a cohort study with an 8-year follow-up. Arch Gerontol Geriatr. (2016) 64:172–7. doi: 10.1016/j.archger.2015.11.002
98. Toosizadeh, N, Mohler, J, and Najafi, B. Assessing upper extremity motion: an innovative method to identify frailty. J Am Geriatr Soc. (2015) 63:1181–6. doi: 10.1111/jgs.13451
99. Afilalo, J, Lauck, S, Kim, DH, Lefèvre, T, Piazza, N, Lachapelle, K, et al. Frailty in older adults undergoing aortic valve replacement. J Am Coll Cardiol. (2017) 70:689–700. doi: 10.1016/j.jacc.2017.06.024
100. Creevy, KE, Akey, JM, Kaeberlein, M, and Promislow, DEL. The dog aging project consortium, Barnett BG, et al. an open science study of ageing in companion dogs. Nature. (2022 Feb 3) 602:51–7. doi: 10.1038/s41586-021-04282-9
101. Open data access. Dog Aging Project. Available at: https://dogagingproject.org/open_data_access/
102. Briggs, CE, Nelson, RW, Feldman, EC, Elliott, DA, and Neal, LA. Reliability of history and physical examination findings for assessing control of glycemia in dogs with diabetes mellitus: 53 cases (1995–1998). javma. (2000) 217:48–53. doi: 10.2460/javma.2000.217.48
103. Brown, DC, Boston, RC, Coyne, JC, and Farrar, JT. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. JAVMA. (2008) 233:1278–83. doi: 10.2460/javma.233.8.1278
104. Porciello, F, Rishniw, M, Ljungvall, I, Ferasin, L, Haggstrom, J, and Ohad, DG. Sleeping and resting respiratory rates in dogs and cats with medically-controlled left-sided congestive heart failure. Vet J. (2016) 207:164–8. doi: 10.1016/j.tvjl.2015.08.017
105. Salvin, HE, McGreevy, PD, Sachdev, PS, and Valenzuela, MJ. The canine cognitive dysfunction rating scale (CCDR): a data-driven and ecologically relevant assessment tool. Vet J. (2011) 188:331–6. doi: 10.1016/j.tvjl.2010.05.014
106. Salt, C, Morris, PJ, Wilson, D, Lund, EM, and German, AJ. Association between life span and body condition in neutered client-owned dogs. J Vet Intern Med. (2018) 33:jvim.15367. doi: 10.1111/jvim.15367
107. Wilson, D, Jackson, T, Sapey, E, and Lord, JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. (2017) 36:1–10. doi: 10.1016/j.arr.2017.01.006
108. Mijnarends, DM, Schols, JMGA, Meijers, JMM, Tan, FES, Verlaan, S, Luiking, YC, et al. Instruments to assess sarcopenia and physical frailty in older people living in a community (care) setting: similarities and discrepancies. J Am Med Dir Assoc. (2015) 16:301–8. doi: 10.1016/j.jamda.2014.11.011
109. Xu, J, Wan, CS, Ktoris, K, Reijnierse, EM, and Maier, AB. Sarcopenia is associated with mortality in adults: a systematic review and Meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
110. Freeman, LM. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med. (2012) 26:3–17. doi: 10.1111/j.1939-1676.2011.00838.x
111. Freeman, LM, Michel, KE, Zanghi, BM, Vester Boler, BM, and Fages, J. Evaluation of the use of muscle condition score and ultrasonographic measurements for assessment of muscle mass in dogs. Am J Vet Res. (2019) 80:595–600. doi: 10.2460/ajvr.80.6.595
112. Tufts University. Muscle condition score chart for dogs [internet]. WSAVA Global Nutrition Committee. (2013). Available at: https://wsava.org/wp-content/uploads/2020/01/Muscle-Condition-Score-Chart-for-Dogs.pdf
113. Morgan, EM, Heseltine, JC, Levine, GJ, Promislow, DEL, and Creevy, KE. Evaluation of a low-technology system to obtain morphological and mobility trial measurements in dogs and investigation of potential predictors of canine mobility. Am J Vet Res. (2019) 80:670–9. doi: 10.2460/ajvr.80.7.670
114. German, AJ, Titcomb, JM, Holden, SL, Queau, Y, Morris, PJ, and Biourge, V. Cohort study of the success of controlled weight loss programs for obese dogs. J Vet Intern Med. (2015) 29:1547–55. doi: 10.1111/jvim.13629
115. Porsani, MYH, Teixeira, FA, Amaral, AR, Pedrinelli, V, Vasques, V, Oliveira, AG, et al. Factors associated with failure of dog’s weight loss programmes. Vet Med Sci. (2020) 6:299–305. doi: 10.1002/vms3.229
116. Gobbens, RJJ, van Assen, MALM, Luijkx, KG, Wijnen-Sponselee, MT, and Schols, JMGA. The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc. (2010) 11:344–55. doi: 10.1016/j.jamda.2009.11.003
117. Howlett, SE, Rutenberg, AD, and Rockwood, K. The degree of frailty as a translational measure of health in aging. Nat Aging. (2021) 1:651–65. doi: 10.1038/s43587-021-00099-3
118. Fried, LP, Cohen, AA, Xue, QL, Walston, J, Bandeen-Roche, K, and Varadhan, R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. (2021) 1:36–46. doi: 10.1038/s43587-020-00017-z
119. Guralnik, JM, Simonsick, EM, Ferrucci, L, Glynn, RJ, Berkman, LF, Blazer, DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.M85
120. Afilalo, J, Kim, S, O’Brien, S, Brennan, JM, Edwards, FH, Mack, MJ, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. (2016) 1:314. doi: 10.1001/jamacardio.2016.0316
121. Lee, L, Patel, T, Costa, A, Bryce, E, Hillier, L, Slonim, K, et al. Screening for frailty in primary care: accuracy of gait speed and hand-grip strength. Can Fam Physician. (2017) 63:e51–7.
122. Pamoukdjian, F, Lévy, V, Sebbane, G, Boubaya, M, Landre, T, Bloch-Queyrat, C, et al. Slow gait speed is an independent predictor of early death in older cancer outpatients: results from a prospective cohort study. J Nutr Health Aging. (2017) 21:202–6. doi: 10.1007/s12603-016-0734-x
123. Liu, B, Hu, X, Zhang, Q, Fan, Y, Li, J, Zou, R, et al. Usual walking speed and all-cause mortality risk in older people: a systematic review and meta-analysis. Gait Posture. (2016) 44:172–7. doi: 10.1016/j.gaitpost.2015.12.008
124. Ferrante, LE, Pisani, MA, Murphy, TE, Gahbauer, EA, Leo-Summers, LS, and Gill, TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. (2015) 175:523. doi: 10.1001/jamainternmed.2014.7889
125. Oakland, K, Nadler, R, Cresswell, L, Jackson, D, and Coughlin, P. Systematic review and meta-analysis of the association between frailty and outcome in surgical patients. Ann R Coll Surg Engl. (2016) 98:80–5. doi: 10.1308/rcsann.2016.0048
126. Yorke, A, Kane, AE, Hancock Friesen, CL, Howlett, SE, and O’Blenes, S. Development of a rat clinical frailty index. J Gerontol Series A. (2017) 72:897–903. doi: 10.1093/gerona/glw339
127. Gomez-Cabrera, MC, Garcia-Valles, R, Rodriguez-Mañas, L, Garcia-Garcia, FJ, Olaso-Gonzalez, G, Salvador-Pascual, A, et al. A new frailty score for experimental animals based on the clinical phenotype: inactivity as a model of frailty. J Gerontol Series A. (2017) 72:885–91. doi: 10.1093/gerona/glw337
128. Siwak, CT, Murphey, HL, Muggenburg, BA, and Milgram, NW. Age-dependent decline in locomotor activity in dogs is environment specific. Physiol Behav. (2002) 75:65–70. doi: 10.1016/S0031-9384(01)00632-1
129. Siwak, CT, Tapp, PD, Zicker, SC, Murphey, HL, Muggenburg, BA, Head, E, et al. Locomotor activity rhythms in dogs vary with age and cognitive status. Behav Neurosci. (2003) 117:813–24. doi: 10.1037/0735-7044.117.4.813
130. Morrison, R, Penpraze, V, Greening, R, Underwood, T, Reilly, JJ, and Yam, PS. Correlates of objectively measured physical activity in dogs. Vet J. (2014) 199:263–7. doi: 10.1016/j.tvjl.2013.11.023
131. Gonçalves, LCVB, Simões, ADGA, Millis, DL, and De, MAJF. Development of a scale to evaluate mobility in dogs. Cienc Rural. (2016) 46:2210–5. doi: 10.1590/0103-8478cr20160123
132. Hesbach, A. A proposed canine movement performance test: the canine timed up and go test (CTUG). Orthoped Phys Ther Pract. (2003) 15:26.
133. Malmstrom, TK, Miller, DK, and Morley, JE. A comparison of four frailty models. J Am Geriatr Soc. (2014) 62:721–6. doi: 10.1111/jgs.12735
134. Lindner, DL, Marretta, SM, Pijanowski, GJ, Johnson, AL, and Smith, CW. Measurement of bite force in dogs: a pilot study. J Vet Dent. (1995) 12:49–52. doi: 10.1177/089875649501200202
135. Hamel, L, Brech, CL, Besnier, NJ, and Daculsi, LG. Measurement of biting-pulling strength developed on canine teeth of military dogs. J Vet Dent. (1997) 14:57–60. doi: 10.1177/089875649701400202
136. Fleyshman, DI, Wakshlag, JJ, Huson, HJ, Loftus, JP, Olby, NJ, Brodsky, L, et al. Development of infrastructure for a systemic multidisciplinary approach to study aging in retired sled dogs. Aging. (2021) 13:21814–37. doi: 10.18632/aging.203600
137. Tam, ACY, Chan, AWY, Cheung, DSK, Ho, LYW, Tang, ASK, Christensen, M, et al. The effects of interventions to enhance cognitive and physical functions in older people with cognitive frailty: a systematic review and meta-analysis. Eur Rev Aging Phys Act. (2022) 19:19. doi: 10.1186/s11556-022-00299-9
138. Auyeung, TW, Kwok, T, Lee, J, Leung, PC, Leung, J, and Woo, J. Functional decline in cognitive impairment – the relationship between physical and cognitive function. Neuroepidemiology. (2008) 31:167–73. doi: 10.1159/000154929
139. Graham, JE, Mitnitski, AB, Mogilner, AJ, and Rockwood, K. Dynamics of cognitive aging: distinguishing functional age and disease from chronologic age in a population. Am J Epidemiol. (1999) 150:1045–54. doi: 10.1093/oxfordjournals.aje.a009928
140. Mitnitski, AB, Graham, JE, Mogilner, AJ, and Rockwood, K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. (2002) 2:1. doi: 10.1186/1471-2318-2-1
141. Robertson, DA, Savva, GM, and Kenny, RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. (2013) 12:840–51. doi: 10.1016/j.arr.2013.06.004
142. Ellwood, A, Quinn, C, and Mountain, G. Psychological and social factors associated with coexisting frailty and cognitive impairment: a systematic review. Res Aging. (2022) 44:448–64. doi: 10.1177/01640275211045603
143. Stewart, L, MacLean, EL, Ivy, D, Woods, V, Cohen, E, Rodriguez, K, et al. Citizen science as a new tool in dog cognition research. PF Ferrari, PLoS One. (2015), 10: e0135176.
144. Watowich, MM, MacLean, EL, Hare, B, Call, J, Kaminski, J, Miklósi, Á, et al. Age influences domestic dog cognitive performance independent of average breed lifespan. Anim Cogn. (2020) 23:795–805. doi: 10.1007/s10071-020-01385-0
145. Salvin, HE, McGreevy, PD, Sachdev, PS, and Valenzuela, MJ. Growing old gracefully—behavioral changes associated with “successful aging” in the dog. J Veter. Behav. (2011) 6:313–20. doi: 10.1016/j.jveb.2011.04.004
146. Madari, A, Farbakova, J, Katina, S, Smolek, T, Novak, P, Weissova, T, et al. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia scale (CADES). Appl Anim Behav Sci. (2015) 171:138–45. doi: 10.1016/j.applanim.2015.08.034
147. Salvin, HE, McGreevy, PD, Sachdev, PS, and Valenzuela, MJ. Under diagnosis of canine cognitive dysfunction: a cross-sectional survey of older companion dogs. Vet J. (2010) 184:277–81. doi: 10.1016/j.tvjl.2009.11.007
148. Steptoe, A, Shankar, A, Demakakos, P, and Wardle, J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. (2013) 110:5797–801. doi: 10.1073/pnas.1219686110
149. Wanser, SH, MacDonald, M, and Udell, MAR. Dog–human behavioral synchronization: family dogs synchronize their behavior with child family members. Anim Cogn. (2021) 24:747–52. doi: 10.1007/s10071-020-01454-4
150. González-Martínez, Á, Martínez, MF, Rosado, B, Luño, I, Santamarina, G, Suárez, ML, et al. Association between puppy classes and adulthood behavior of the dog. J Vet Behav. (2019) 32:36–41. doi: 10.1016/j.jveb.2019.04.011
151. Duranton, C, and Gaunet, F. Behavioral synchronization and affiliation: dogs exhibit human-like skills. Learn Behav. (2018) 46:364–73. doi: 10.3758/s13420-018-0323-4
152. Kang, OD. Effects of environment enrichment on behavioral problems in dogs with separation anxiety. J Environ Sci Int. (2022) 31:131–9. doi: 10.5322/JESI.2022.31.2.131
153. McGreevy, PD, Georgevsky, D, Carrasco, J, Valenzuela, M, Duffy, DL, and Serpell, JA. Dog behavior co-varies with height, bodyweight and skull shape, PLoS One (2013) 8:e80529
154. Hare, E, Kelsey, KM, Serpell, JA, and Otto, CM. Behavior differences between search-and-rescue and pet dogs. Front Vet Sci. (2018) 5:118. doi: 10.3389/fvets.2018.00118
155. Serpell, JA, University of Pennsylvania. C-BARQ. Available at: https://vetapps.vet.upenn.edu/cbarq/
Keywords: aging, age-related disease, geriatric, phenotype, resilience
Citation: Melvin RL, Ruple A, Pearson EB, Olby NJ, Fitzpatrick AL and Creevy KE (2023) A review of frailty instruments in human medicine and proposal of a frailty instrument for dogs. Front. Vet. Sci. 10:1139308. doi: 10.3389/fvets.2023.1139308
Edited by:
Alasdair James Charles Cook, University of Surrey, United KingdomReviewed by:
Brennen McKenzie, Loyal, United StatesHolger Andreas Volk, University of Veterinary Medicine Hannover, Germany
Copyright © 2023 Melvin, Ruple, Pearson, Olby, Fitzpatrick and Creevy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate E. Creevy, a2NyZWV2eUB0YW11LmVkdQ==
 Rachel L. Melvin
Rachel L. Melvin Audrey Ruple
Audrey Ruple Elizabeth B. Pearson1
Elizabeth B. Pearson1 Natasha J. Olby
Natasha J. Olby Kate E. Creevy
Kate E. Creevy