
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 13 April 2023
Sec. Veterinary Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1133813
Objective: To report surgical site infections (SSI) after Tibial Plateau Leveling Osteotomy (TPLO), treatment course, associated risk factors, bacterial isolates and antimicrobial resistance.
Study design: Retrospective clinical cohort study.
Study population: Six hundred and twenty seven dogs and 769 TPLO procedures.
Methods: Data from electronic medical records of dogs undergoing TPLO between 2005 and 2015 at a single institution have been retrospectively reviewed. A generalized mixed logistic regression was used to determine possible risk factors. The Chi-Square test of independence was used to examine the relationship between the isolation of multidrug-resistant (MDR) bacteria and the development of major infections undergoing additional surgical treatment. To assess the correlation between number of SSI and number MDR isolate per year, Pearson's correlation coefficient was calculated.
Results: The overall complication rate was 19.3% (n = 149). SSI was most frequent with 8.5% (n = 65). Major SSI occurred in 6.8% (n = 52) TPLO (80.0% SSI). Staphylococcus (S.) pseudintermedius (n = 37) and S. aureus (n = 10) were most frequently isolated. Multidrug-resistant bacteria were identified in 2.7% (n = 21) TPLO (32.3% SSI) but were not associated with major SSI (p = 0.426). There was a strong positive correlation between number of MDR isolates per year and number of SSI per year [r(9) = 0.79, p = 0.004]. Factors associated with SSI were previous TPLO in the contralateral stifle (p = 0.02, OR = 2.01, 95% CI = 1.11–3.64) and German Shepherd dogs (p = 0.035, OR = 4.41, 95% CI = 1.11–17.54). The use of non-locking implants was found to be protective (p = 0.02, OR = 0.179, 95% CI = 0.18–0.77).
Clinical significance: Infection with multidrug-resistant bacteria is an emerging problem in veterinary practice and treatment is challenging. The incidence of major SSI was found to be high but was not associated with the isolation of MDR bacteria.
Tibial Plateau Leveling Osteotomy (TPLO) is one of the most commonly performed surgical procedures for the treatment of cranial cruciate ligament (CCL) disease in dogs (1, 2). Postoperative complication rates in the literature range from 9.7% to 27.8% (3–7). The reported incidence of surgical site infections (SSI) ranges from 2.9% to 17.3% (4, 5, 8–14) and is considered higher than expected in comparison to other sterile elective orthopedic procedures (15). The large variation in the literature can partially be explained by different definitions of key terms used for postoperative complication and infection. Due to the extensive usage of TPLO in veterinary surgery, SSI has a great economic impact on pet owners and influences patients' recovery from a surgery that is considered a standard procedure (16). Previous publications have suggested a variety of factors associated with SSI after TPLO including surgeon's experience, increased body weight, duration of anesthesia, breed predisposition for German Shepherd Dogs, and use of non-locking implants for dogs >50 kg (4, 9, 12, 14, 17).
The emergence of multidrug-resistant (MDR) bacteria is widely acknowledged and poses a major therapeutic challenge in both human and veterinary medicine (18, 19). Literature reports a prevalence of 20–40% multidrug-resistant Staphylococcus (S.) aureus and S. pseudintermedius isolates among dogs with SSI after TPLO (8, 10, 11, 16, 20, 21). For human patients suffering from implant-associated MRSA-infection, the Centers for Disease Control and Prevention (CDC) guidelines recommend long-term systemic antimicrobial therapy, surgical debridement, and, whenever possible, removal of the surgical implants (22). In veterinary medicine, the selection of antimicrobials is limited since many of the compounds remaining effective are considered of critical importance for treatment of infections with MDR bacteria in human medicine and should therefore be avoided (23). Consequently, effective systemic antimicrobial treatment is sometimes not possible. Furthermore, bacteria commonly involved in SSI in dogs, in particular Staphylococcus spp., are known to form biofilms that hinder hosts' immune response and effectively prevent appropriate concentrations of antimicrobials at the site of infection (24). For this reason, removal of orthopedic implants is often considered necessary but, in the case of TPLO, is only feasible when the degree of bone healing at the osteotomy site guarantees appropriate stability.
The aim of this retrospective study was firstly to analyze risk factors for SSI after TPLO and report bacterial isolates and their antimicrobial resistance pattern. Secondly, we sought to investigate the influence of occurrence of multidrug-resistant bacteria on treatment of SSI.
A retrospective cohort study was conducted. Medical records of dogs that underwent stifle arthroscopy and TPLO in a veterinary teaching hospital between January 2005 and December 2015 were reviewed. The minimum duration between data collection and surgical procedure was set to 6 months. Procedures were excluded, if medical records were incomplete or other orthopedic procedures were simultaneously performed on the same stifle apart from arthroscopic treatment of meniscal injury. Medical records were considered incomplete, if information on signalment, documentation of surgical procedure and anesthesia, or postoperative care was missing. Available follow up information was extracted from medical records in the form of documentation of in-house rechecks or communication logs. If no postoperative complications were recorded, the recovery was considered uneventful. Dogs undergoing bilateral staged TPLO were considered independent cases and the statistical model was designed to account for the dependence on multiple observations of a single animal by modeling a random intercept for each animal. Data collection included breed, age, sex, body weight, history of dermatitis, use of locking or non-locking implant, duration of anesthesia, pre-, intra-, and postoperative antimicrobial administration, and if previous contralateral TPLO had been performed (during or before the study period). In cases of postoperative complications, the following data was collected: Type and timepoint of complication, type of therapy and outcome. Results of bacteriological culture and antimicrobial susceptibility testing were reviewed.
Arthroscopy was used for inspection of the stifle joint before every TPLO. Remnants of ruptured CCL were debrided, and meniscal damage treated with a combination of meniscal knife, motorized shaver, and arthroscopic punch forceps. TPLO as described by Slocum and Slocum (25) was performed using a jig. Osteotomy was stabilized using adequately sized TPLO standard or broad plates by Slocum Enterprises (Eugen, Oregon, USA), New Generation Devices (Glen Rock, New Jersey, USA), Synthes (Synthes Vet, West Chester, Pennsylvania, USA) or Fixin (TraumaVet, Rivoli, Piedmont, Italy) depending on surgeon's preference and availability. Cefazolin (20 mg/kg iv.) was administered within 60 min before surgery and repeated every 90 min until the end of the procedure. Postoperative antimicrobials were not routinely prescribed.
Criteria for diagnosis of SSI were based on those published by the United States Centers for Disease Control and Prevention (CDC) (26), with minor modifications. One or more of the following criteria had to be met: (1) Purulent drainage from the incision site. (2) Spontaneous wound dehiscence or wound reopened by surgeon AND fever, localized pain, OR tenderness, unless the incision was cultured negative. (3) Abscess, fistula, or other evidence of infection on clinical examination, diagnostic imaging, surgery, cytology, or histopathology. (4) Signs of inflammation AND positive bacterial culture of an aseptically collected sample from the surgical site. Based on the categorization of Cook et al. (27), with minor adjustments, SSI were further defined as catastrophic, major and minor. A SSI was considered a catastrophic complication if it resulted in permanent unacceptable function or was directly related to death or euthanasia. A major SSI was defined as requiring additional surgical intervention. In contrast to Cook and colleagues, infections treated only medically were defined as minor. This modification is in consensus with previous studies on SSI after TPLO (4, 5, 9).
Bacterial cultures and antimicrobial susceptibility testing (AST) were performed at the Institute of Veterinary Bacteriology (IVB) at the Vetsuisse Faculty, University of Bern. Bacterial cultivation of swab/aspirate and implant samples followed standard diagnostic procedures. Identification of colonies were performed by Vitek™ 2 Compact technology (BioMérieux, Marcy l'Etoile, France) until 2010. Since 2011, isolates were identified by Matrix-Assisted-Laser-Desorption/Ionization-Time-of-Flight-Mass-Spectroscopy (MALDI-TOF MS) (Microflex LT, Bruker Daltonics GmbH, Bremen, Germany).
From 2005 until May 2010, AST was performed using ATP-Strips (BioMérieux) and interpretation of test results followed manufacturers' instructions. Since August 2010, AST was performed with the Vitek™ 2 Compact technology (BioMérieux). Isolates were classified as susceptible or resistant, according to clinical breakpoints published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. If no EUCAST breakpoints were available, veterinary clinical breakpoints of the Clinical and Laboratory Standards Institute (CLSI) were used. For Pasteurella spp. The above-mentioned methods were not applicable, therefore detection of beta-lactamase enzymatic activity using BBL™ DrySlide™ Nitrocefin (BD Becton Dickinson) was used. Bacteria were evaluated for multidrug resistance (MDR) according to criteria published by the European Center for Disease Control (ECDC) and Centers of Disease Control (CDC) (non-susceptible to ≥1 agent in ≥3 antimicrobial categories as described) (28).
Descriptive data were reported as mean ± standard deviation or median (range) as appropriate. Normality of data was assessed using Shapiro-Wilk Test. Statistical analyses were performed by use of statistical software (R Core Team [2015], R Foundation for Statistical Computing, Vienna, Austria). A generalized mixed logistic regression was fitted to the data with SSI as the response variable and sex, age, body weight, ASA (American Society of Anaesthesiologists Classification) grading, pre- and postoperative antimicrobial therapy, previous contralateral TPLO, duration of anesthesia, implant (locking or non-locking) and breed included as fixed predictors. Breed was defined as a categorical variable with a separate category for the most represented breeds (mixed breed, Labrador, Golden Retriever, Bernese Mountain Dog, Boxer, Rottweiler, Great Dane, German Shepherd). The remaining breeds were summarized in one category (other). The variance inflation index was investigated and was found to be between 1 and 2, hence no problematic correlations among independent variables were identified. The identifier of an animal (ID) was included as a random intercept to account for the dependence on multiple observations of a single animal (bilateral staged surgery). Significance was set as p < 0.05. Pearson's Chi-Square test was used to test for independence between the presence of MDR bacteria and the presence of major infection. To assess the correlation between number of SSI and number MDR isolate per year, pearson's correlation coefficient was calculated.
During the study period, 797 TPLO procedures were recorded. Twenty-eight (n = 28) procedures were excluded due to incomplete medical records. A total of 627 dogs were included in the study population, 142 (22.5%) of which underwent staged bilateral TPLO, resulting in total of 769 TPLO procedures. In addition to the bilateral staged procedures performed within the study population, four (n = 4, 0.5%) cases were found to have undergone TPLO before the study period, leading to a total of 146 (19.0%) cases with a history of previous TPLO surgery on the contralateral stifle. The study population consisted of 402 (52.3%) neutered and 83 (10.8%) intact females, and 187 (24.3%) neutered and 97 (12.6%) entire males. The mean age was 5.9 ± 2.7 years, and median body weight 35.8 kg (range 10–87 kg). The most common breeds were mixed breed (n = 170, 23.1%), Labrador Retriever (n = 108, 14.0%), Golden Retriever (n = 88, 11.5%), Bernese Mountain Dog (n = 54, 7.0%), Boxer (n = 38, 4.9%), Rottweiler (n = 20, 2.6%), Great Dane (n = 18, 2.3%) and German Shepherd (n = 17, 2.2%). Only seven (0.9%) dogs had ongoing antimicrobial therapy at the time of surgery for reasons unrelated to TPLO surgery. Follow up information was available after 494/769 (64.2%) procedures. Mean time to last follow up was 247 days (min. 10 days, max. 3,029 days). A summary of patient's demographics with and without SSI can be found in Table 1.
In 55.5% (n = 427) of procedures, TPLO was completed on the left stifle, and 44.5% (n = 342) on the right. All surgeries were performed by, or under the supervision of, an experienced board-certified surgeon. A complete rupture of the CCL was diagnosed during 472 (61.5%) procedures, a partial rupture during 297 (38.6%). Partial meniscectomy was necessary in 298 stifles (38.5%), of which 284 (95.0%) affected the medial, 12 (4.0%) the lateral and three (1.0%) both menisci. Total meniscectomy was performed on the medial meniscus in three cases and medial meniscal release procedure in seven. Mean duration of anesthesia was 255 ± 49 min. In 71 (9.2%) cases, antibiotic treatment was continued postoperatively. The rational of continued post-operative antibiotic treatment of these cases was found to be history of or suspected ongoing local dermatitis in 13/71 (18.3%) cases, continuation of pre-operative antibiotic treatment in 4/71 (5.6%), minor breach of sterility during the surgical procedure in 2/71 (2.8%) and unknown in 52/71 (73.2) cases. Non-locking implants were used in 124/769 (16.1%) cases and locking in 645/769 (83.9%). The distribution of usage of locking and non-locking implants over time is presented in Table 2.
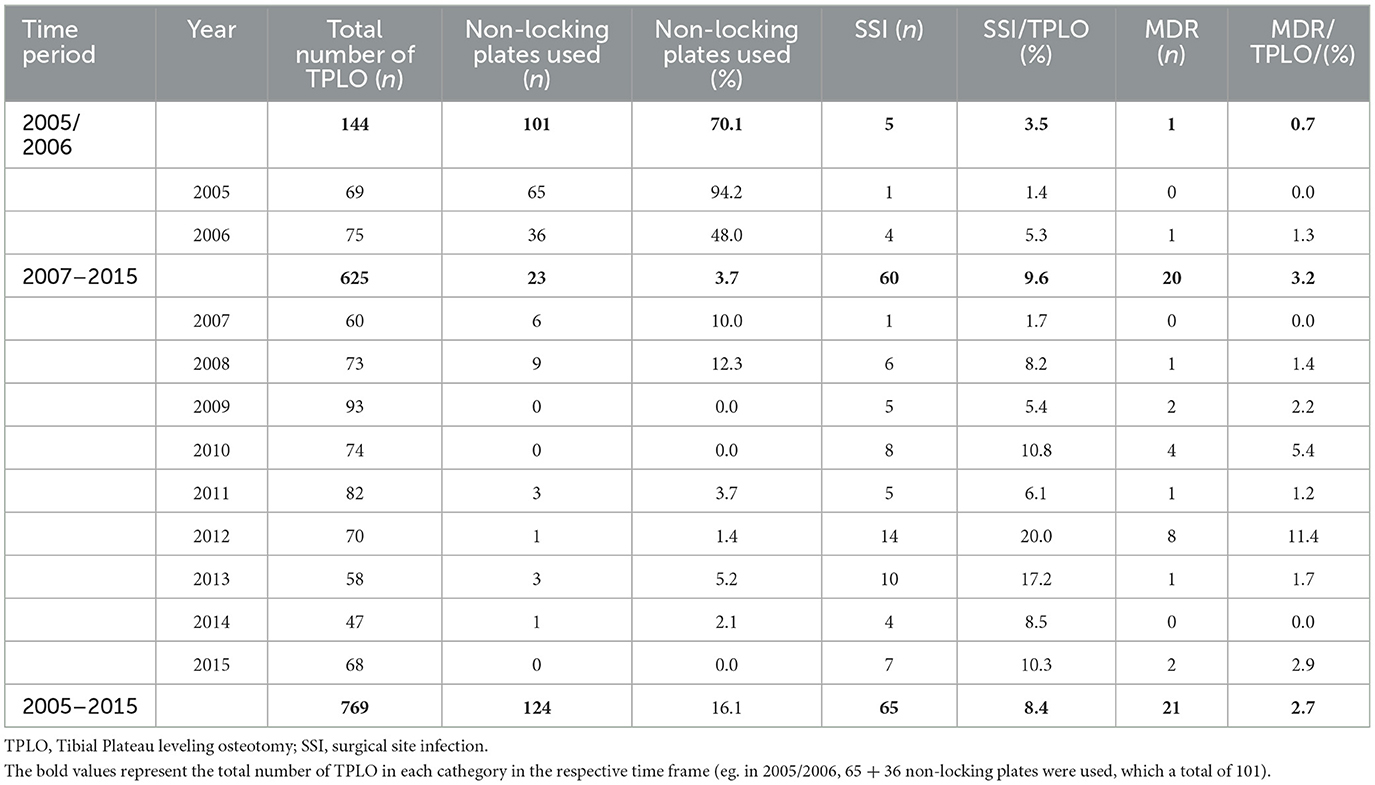
Table 2. Usage of non-locking implants, occurrence of SSI and isolation of MDR bacteria of 769 TPLO between 2005 and 2015.
An overview of encountered postoperative complications can be found in Table 3. Incidence of SSI was 8.5% (n = 65). Median duration between surgery and diagnosis of SSI was 14 days (range 4–855). Catastrophic SSI occurred after one TPLO (0.1% overall, 1.5% SSI). Major SSI was diagnosed in 52/65 (80.0%) SSI. Surgical implants were removed in 42/65 (64.6%). Median duration between surgery and implant removal was 132 days (range: 51–2,858 days). Antimicrobial therapy was initiated or continued after 15/42 explantation procedures. Overall, after 57/65 (87.7%) procedures with SSI antimicrobial therapy was prescribed, consisting of cefalexin (20 mg/kg BID po), amoxicillin/clavulanic acid (12.5 mg/kg BID po), clindamycin (5.5 mg/kg BID po), enrofloxacin (10 mg/kg SID po) or a combination of these. Median duration of antimicrobial treatment was 22 days (range: 5–52 days). According to our data, dogs that had previous TPLO on the contralateral stifle (p = 0.02, OR = 2.01, 95% CI = 1.11–3.64) and German Shepherd Dogs (p = 0.035, OR = 4.41, 95% CI = 1.11–17.54) were more likely to develop SSI. Furthermore, use of non-locking-implants was associated with lower odds of infection (p = 0.02, OR = 0.18, 95% CI = 0.18–0.77). Postoperative antibiotic treatment was not associated with development of SSI (p = 0.61).
Incidence of SSI involving MDR bacteria was found to be 2.7% (n = 21/769) of TPLO or 32.3% (n = 21/65) of SSI. One catastrophic complication occurred; A dog was euthanized upon owner's decision seven days post-surgery after being diagnosed with multiple complications including SSI with isolation of MDR bacteria and tuberositas tibia fracture. Major infection occurred after 18/21 (85.7%). Implants were removed in 14/21 (66.7%) cases, seven of which had been treated with adequate antimicrobials according to AST (Table 4). Comparing infection with MDR bacteria to infection with non-MDR bacteria, no association was found between isolation of MDR bacteria and development of major infection (p = 0.426). Absolute and relative number of SSI and SSI involving MDR bacteria is shown in Table 2. There was a strong positive correlation between number of MDR isolates per year and number of SSI per year [r(9) = 0.79, p = 0.004].
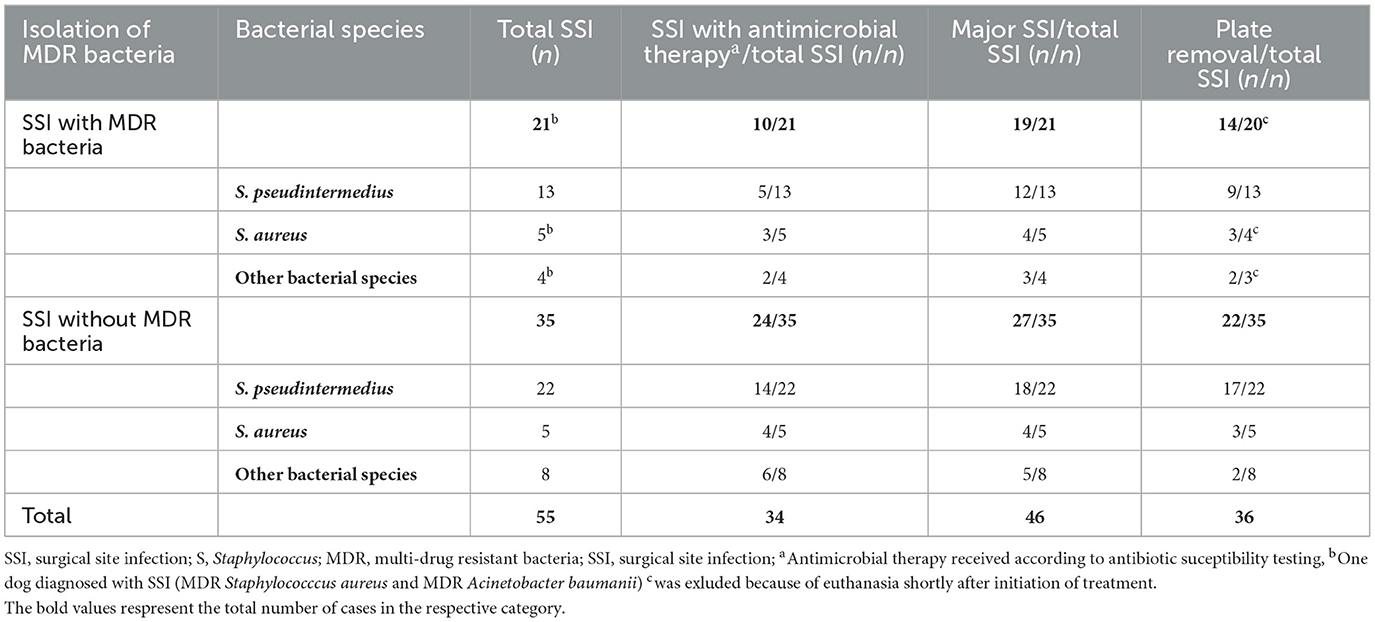
Table 4. Treatment and proportion of major SSI by isolated bacteria after 55 TPLO with positive bacterial culture results.
After 17 (2.2%) TPLO procedures, persistent lameness >50 days after TPLO without any evident cause was diagnosed. Six of seventeen dogs (35.3%) underwent conservative treatment consisting of non-steroidal anti-inflammatory drugs and restricted activity (n = 6, 35.3%), antimicrobial therapy with cefalexin 20 mg/kg for 7–10 days (n = 2, 11.8%), whilst 11 (64.7%) dogs underwent implant removal. Samples from removed implants for bacteriological culture were collected from 6/11 (54.5%), none of which yielded bacterial growth.
A total of 91 bacteriological culture results collected from 80 patients were available for analysis. In 69 (86.3%) dogs, a single culture was performed, and paired cultures in 11 (13.8%) dogs. Overall, in 69.2% (n = 63/91) of the samples bacterial growth was detected. Sixteen different bacterial species (Table 5) were isolated with S. pseudintermedius being by far most frequent (n = 37) followed by S. aureus (n = 10).
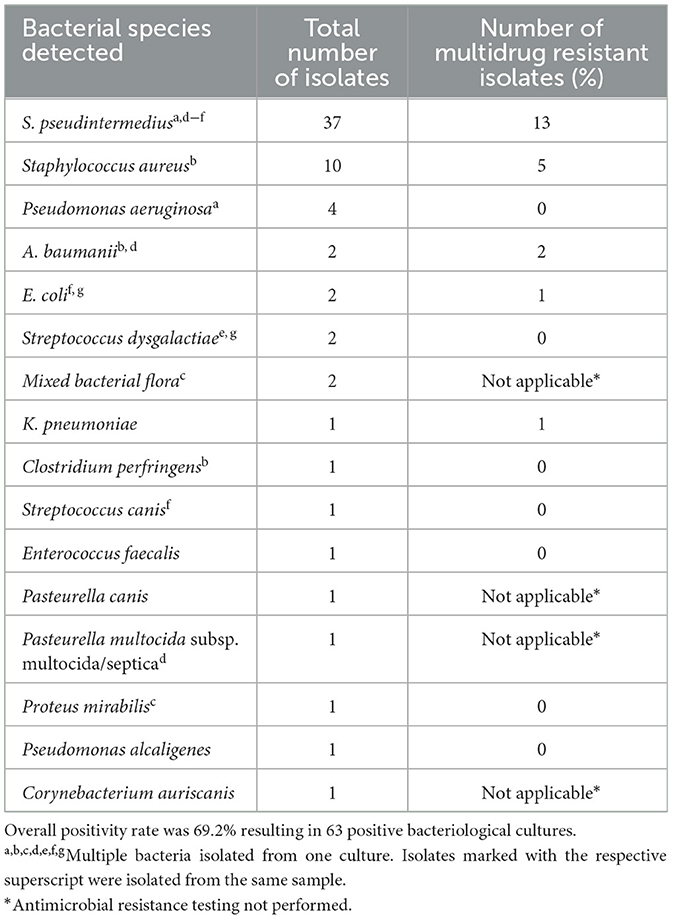
Table 5. Bacterial species and number of multidrug resistant bacteria isolated from 91 bacteriological culture collected from 80 dogs treated with TPLO.
In cases with a single bacterial culture, samples were submitted either when surgical site infection was first suspected (wound swabs/aspirates/tissue samples) or during removal of TPLO implants (screw, wound swab, tissue samples). Bacterial growth was detected in 72% (n = 50/69) of the samples. In 11 dogs, bacterial culture was performed when SSI was first suspected as well as during implant removal, which allowed pairing and comparison of results (Table 6). Paired samples were positive in six dogs. In all six cases, bacterial species isolated as well as the pattern of antimicrobial resistance were identical for both samples.
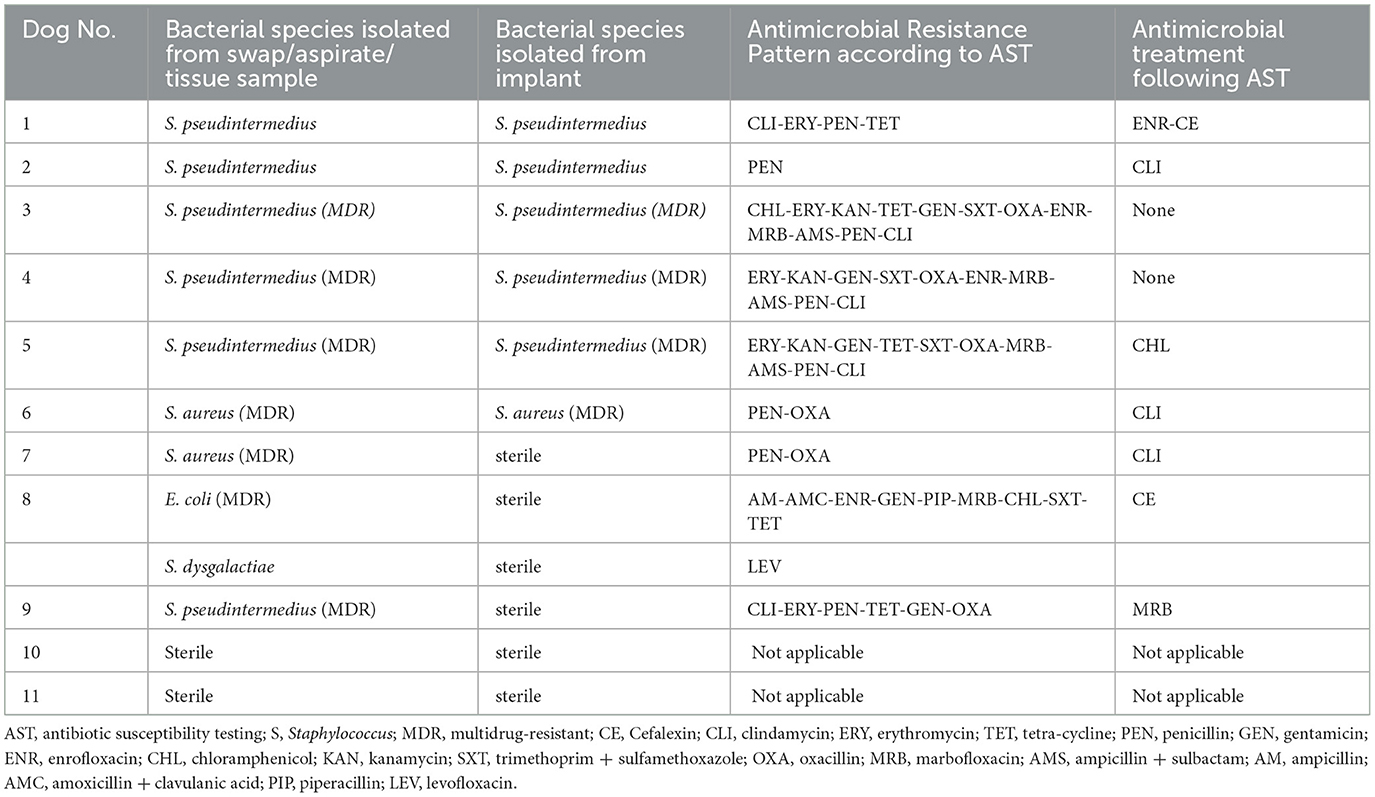
Table 6. Bacterial species and antimicrobial resistance pattern, antimicrobial treatment, and occurrence of complicated infection in 11 dogs with paired bacterial culture.
A total of 22 MDR bacteria were isolated after 21/769 TPLO (2.7% overall, 30.8% SSI). Thirteen of 37 (35.1%) S. pseudintermedius and five of ten (50.0%) S. aureus were classified as MDR. Other MDR isolates included Acinetobacter baumanii (n = 2), Klebsiella pneumoniae (n = 1) and Escherichia coli (n = 1) (Table 5). After one TPLO, two different MDR bacteria (S. aureus and A. baumanii) were isolated from the same surgical site.
The purpose of this study was to investigate SSI after TPLO, associated risk factors, isolated bacteria and respective antimicrobial resistance pattern, as well as the course of treatment. Postoperative SSI rate was found to be 8.5%, which is in line with previously published data (4, 8, 9, 29, 30). Similarly, the incidence of 2.7% MDR infections is comparable to the literature (8, 9, 11). We could confirm previous findings that German Shepherd Dogs have a higher risk to develop SSI after TPLO (13, 17, 30).
The incidence of major SSI of 80.0% in the subpopulation of patients diagnosed with a SSI is higher than previously reported (4, 5, 9, 31). The higher incidence of major SSI could be partially explained by different definitions for SSI and what is considered a major SSI. Since our implant removal rate (64.6%) were similar to other studies, the higher rate of major SSI could be attributed to cases that underwent a surgical intervention as part of the treatment of active infection. Therefore, this finding could indicate a more aggressive approach to treatment of SSI, prompting more frequent surgical intervention such as reopening and debridement of the wound and the resulting in the classification of a major SSI.
The rate of SSI subjectively shows a trend toward a higher incidence in the latter study period (Table 2). Similarly, MDR bacteria were more frequently isolated. This finding is conclusive, since we found a strong correlation between number of SSI and isolation of MDR bacteria per year. In both human and veterinary medicine, bacteria resistant to many available antibiotic compounds are considered one of the largest threats. Ironically, the use of antimicrobials itself is one of the main drivers behind this emerging problem, since exposure to antibiotics leads to selection and spread of resistant strains (18, 32). For TPLO surgery, perioperative antibiotic prophylaxis is considered a standard protocol but given the emergence of MDR bacteria, could be reconsidered in an effort to combat future infections. Prospective, long-term studies would be necessary to investigate the benefits and risks of this approach.
Our finding, that Staphylococcus spp. was dominant among isolates from SSI following TPLO, is in accordance with previously reported study results (4, 10, 12, 20). Analysis of AST of Staphylococcus spp. revealed high detection rates of MDR S. pseudintermedius and S. aureus (37.1% and 50.0%). In comparison, the prevalence of methicillin-resistance amongst isolated Staphylococci from infection sites after TPLO reported in literature varies between 20% and 40% (8, 10, 11, 16, 17, 20, 21). Other than beta-lactam antibiotics, clindamycin was frequently utilized for empiric therapy of suspected SSI. Resistance rates against these classes of antimicrobials amongst Staphylococcus spp. isolates in our population were shown to be rather high. For multiple reasons, these results should be interpreted with caution and proposing a general recommendation regarding the choice of perioperative antimicrobial prophylaxis and empiric antibiotic treatment should be avoided. Firstly, use of perioperative antimicrobials could have influenced these findings by selection of resistant isolates. Similarly, some bacterial culture samples and respective AST were obtained during implant removal, at which point previous antimicrobial treatment might have influenced culture results. Moreover, comparison of our results with previous studies suggests, that dominant bacterial species as well as patterns of antimicrobial resistance may vary greatly (4, 10).
When comparing bacterial isolates before and after implant removal in dogs with paired bacterial cultures, it was found that isolates, as well as antimicrobial resistance patterns, were identical within each dog, which contradicts a previous report (10). Furthermore, although antimicrobial therapy was appropriately adjusted with regards to AST, results after first positive cultivation in four cases demonstrated, that bacteria withstood treatment. From all four aforementioned cases, S. pseudintermedius (n = 3) or S. aureus (n = 1) spp. was isolated; a genus of bacteria known to form biofilm that impedes host response and curative effect of antimicrobials (24).
Our finding, that the use of non-locking vs. locking implants was protective against surgical site infection is in contrast with previous reports. Experimental and clinical studies have suggested a protective effect of implants using locking technology (14, 33–35). Locking implants are believed to lower infection rates by protecting the periosteal blood supply, provide more stable initial fixation and limit soft tissue dissection required for adequate implant placement (34, 36). The somewhat conflicting result of this study might be explained by the distribution of usage of non-locking implant and varying incidence of SSI during the study period. While 71% (n = 101/124) non-locking implants were used in 2005 and 2006, postoperative infection rates were found to only 3.5% vs. 9.6% during the later study period (Table 2). Considering that scientific evidence points in the opposite direction, it is unlikely that more frequent usage of locking implants caused the surge of SSI incidence. Instead, we hypothesize that other factors led to an increase. For example, the incidence of MDR bacteria in the later study period was higher, which might have overcome the beneficial effect of locking implants. Nevertheless, to our knowledge, a protective effect of locking implants for TPLO in a clinical setup has only been suggested for dogs weighting >50 kg (14, 35). Therefore, additional research in this field is needed to clarify this question.
We found that patients that had previous TPLO on the contralateral stifle had higher odds of postoperative infection. To our knowledge, this has not been reported before. In human medicine, patients that underwent previous surgery have been shown to be at greater risk for surgical site infection with methicillin-resistant S. aureus (37, 38). Similarly, in veterinary medicine, carriage of methicillin-resistant S. pseudintermedius has been associated with previous hospital stay and exposure to antimicrobials (39, 40). Nazarali et al. (11) have suggested, that carriage of methicillin-resistant S. pseudintermedius is a risk factor for development of SSI after TPLO. Nevertheless, since the number of dogs that meet these specific criteria in our study population is relatively low, investigation of associations between previous surgery/hospital stay and isolation of MDR-bacteria from an SSI site was not possible. Furthermore, we only included previous TPLO, but not previous surgery in general as a possible risk factor.
In total, 2.2% (n = 17) patients evaluated for ongoing lameness after TPLO were classified as having persistent lameness of unknown origin. Even though bacterial samples from implants of 6/11 (54.4%) were collected and did not yield bacterial growth, it is possible that the infection rate could have been underestimated due to a misclassification bias. On the other hand, lameness could have been caused be factors not directly related to TPLO surgery, such as progression of degenerative joint disease of the stifle. This applies mainly to cases, where the diagnosis was made several months after the surgery.
There are several limitations of this study. In respect to its retrospective nature, we wholly depended on completeness and accuracy of data collected from electronic medical records. Furthermore, subsequent patient information was not always available, due to owners electing to perform rechecks in a different practice. Additionally, the definition of SSI used was very narrow and would not include cases of early-stage SSI which only show signs of inflammation. For this reason, complication rates may have been underestimated.
We can conclude that incidence of major SSI was found to be high in the study population but was not associated to isolation of MDR bacteria. In contrast, isolation of MDR bacteria was correlated with SSI rates in general. Additional research is needed to investigate a possible link between previous hospital stay and use of non-locking vs. locking implants on risk for SSI after TPLO in general and SSI involving MDR bacteria in particular.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
BH, UR, GO, and FF designed the study. BH and GO collected the data. GO provided data on bacteriological examination of the SSI cases discussed. BH was responsible for the statistical analysis of the collected data. UR supervised data collection and analysis. BH, UR, and GO drafted the manuscript. All authors critically revised the manuscript and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. von Pfeil DJF, Kowaleski MP, Glassman M, Dejardin LM. Results of a survey of veterinary orthopedic society members on the preferred method for treating cranial cruciate ligament rupture in dogs weighing more than 15 kg (33 pounds). J Am Vet Med Assoc. (2018) 253:586–97. doi: 10.2460/javma.253.5.586
2. Leighton RL. Preferred method of repair of cranial cruciate ligament rupture in dogs: a survey of ACVS diplomates specializing in canine orthopedics. Vet Surg. (1999) 28:194–194.
3. Stauffer KD, Tuttle TA, Elkins AD, Wehrenberg AP, Character BJ. Complications associated with 696 Tibial Plateau Leveling Osteotomies (2001–2003). J Am Anim Hosp Assoc. (2006) 42:44–50. doi: 10.5326/0420044
4. Fitzpatrick N, Solano MA. Predictive variables for complications after TPLO with stifle inspection by arthrotomy in 1,000 consecutive dogs: predictive variables for TPLO complications. Vet Surg. (2010) 39:460–74. doi: 10.1111/j.1532-950X.2010.00663.x
5. Gatineau M, Dupuis J, Planté J, Moreau M. Retrospective study of 476 tibial plateau levelling osteotomy procedures: rate of subsequent “pivot shift,” meniscal tear and other complications. Vet Comp Orthop Traumatol. (2011) 24:333–41. doi: 10.3415/VCOT-10-07-0109
6. Hans EC, Barnhart MD, Kennedy SC, Naber SJ. Comparison of complications following tibial tuberosity advancement and Tibial Plateau Levelling Osteotomy in very large and giant dogs 50 kg or more in body weight. Vet Comp Orthop Traumatol. (2017) 7:1559. doi: 10.3415/VCOT-16-07-0106
7. Bergh MS, Rajala-Schultz P, Johnson KA. Risk factors for Tibial tuberosity fracture after Tibial Plateau Leveling Osteotomy in dogs. Vet Surg. (2008) 37:374–82. doi: 10.1111/j.1532-950X.2008.00391.x
8. Hagen CRM, Singh A, Weese JS, Marshall Q, Linden A, Gibson TWG. Contributing factors to surgical site infection after Tibial Plateau Leveling Osteotomy: a follow-up retrospective study. Vet Surg. (2020) 49:930–9. doi: 10.1111/vsu.13436
9. Spencer DD, Daye RM, A. prospective, randomized, double-blinded, placebo-controlled clinical study on postoperative antibiotherapy in 150 arthroscopy-assisted Tibial Plateau Leveling Osteotomies in dogs. Vet Surg. (2018) 47:E79–87. doi: 10.1111/vsu.12958
10. Gallagher AD, Mertens WD. Implant removal rate from infection after Tibial Plateau Leveling Osteotomy in dogs: implant removal after TPLO. Vet Surg. (2012) 41:705–11. doi: 10.1111/j.1532-950X.2012.00971.x
11. Nazarali A, Singh A, Moens NMM, Gatineau M, Sereda C, Fowler D, et al. Association between methicillin-resistant Staphylococcus pseudintermedius carriage and the development of surgical site infections following Tibial Plateau Leveling Osteotomy in dogs. J Am Vet Med Assoc. (2015) 247:909–16. doi: 10.2460/javma.247.8.909
12. Nazarali A, Singh A, Weese JS. Perioperative administration of antimicrobials during Tibial Plateau Leveling Osteotomy. Vet Surg. (2014) 43:966–71. doi: 10.1111/j.1532-950X.2014.12269.x
13. Coletti TJ, Anderson M, Gorse MJ, Madsen R. Complications associated with Tibial Plateau Leveling Osteotomy: a retrospective of 1519 procedures. Can Vet J. (2014) 55:6.
14. Solano MA, Danielski A, Kovach K, Fitzpatrick N, Farrell M. Locking plate and screw fixation after Tibial Plateau Leveling Osteotomy reduces postoperative infection rate in dogs over 50 kg: influence of TPLO locking plates on infection rate in dogs. Vet Surg. (2014) 44:59–64. doi: 10.1111/j.1532-950X.2014.12212.x
15. Eugster S, DrMedVet PS, Gaschen FDR, Ecvim-Ca D, Boerlin P, A. prospective study of postoperative surgical site infections in dogs and cats. Vet Surg. (2005) 33:542–50. doi: 10.1111/j.1532-950X.2004.04076.x
16. Nicoll C, Singh A, Weese JS. Economic impact of Tibial Plateau Leveling Osteotomy surgical site infection in dogs. Vet Surg. (2014) 43:899–902. doi: 10.1111/j.1532-950X.2014.12175.x
17. Lopez DJ, VanDeventer GM, Krotscheck U, Aryazand Y, McConkey MJ, Hayashi K, et al. Retrospective study of factors associated with surgical site infection in dogs following tibial plateau leveling osteotomy. J Am Vet Med Assoc. (2018) 253:315–21. doi: 10.2460/javma.253.3.315
18. Lloyd DH. Multi-resistant S. pseudintermedius: a wake-up call in our approach to bacterial infection. J Small Anim Pract. (2012) 53:145–6. doi: 10.1111/j.1748-5827.2011.01193.x
19. Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. (2014) 22:42–7. doi: 10.1016/j.tim.2013.11.003
20. Savicky R, Beale B, Murtaugh R, Swiderski-Hazlett J, Unis M. Outcome following removal of TPLO implants with surgical site infection. Vet Comp Orthop Traumatol. (2013) 26:260–5. doi: 10.3415/VCOT-11-12-0177
21. Weese JS, van Duijkeren E. Methicillin-resistant S. aureus and S. pseudintermedius in veterinary medicine. Vet Microbiol. (2010) 140:418–29. doi: 10.1016/j.vetmic.2009.01.039
22. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant S. aureus infections in adults and children: executive summary. Clin Infect Dis Off Publ Infect Dis Soc Am. (2011) 52:285–92. doi: 10.1093/cid/cir034
23. Perreten V, Kadlec K, Schwarz S, Gronlund Andersson U, Finn M, Greko C, et al. Clonal spread of methicillin-resistant S. pseudintermedius in Europe and North America: an international multicentre study. J Antimicrob Chemother. (2010) 65:1145–54. doi: 10.1093/jac/dkq078
24. Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. (2008) 322:207–28. doi: 10.1007/978-3-540-75418-3_10
25. Slocum B, Slocum TD. Tibial Plateau Leveling Osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet Clin North Am Small Anim Pract. (1993) 23:777–95.
26. Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, CDC. definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. (1992)13:606–8.
27. Cook JL, Evans R, Conzemius MG, Lascelles BDX, McIlwraith CW, Pozzi A, et al. Proposed definitions and criteria for reporting time frame, outcome, and complications for clinical orthopedic studies in veterinary medicine: proposed definitions and criteria for veterinary orthopedic studies. Vet Surg. (2010) 39:905–8. doi: 10.1111/j.1532-950X.2010.00763.x
28. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
29. Frey TN, Hoelzler MG, Scavelli TD, Fulcher RP, Bastian RP. Risk factors for surgical site infection-inflammation in dogs undergoing surgery for rupture of the cranial cruciate ligament: 902 cases (2005–2006). J Am Vet Med Assoc. (2010) 236:88–94. doi: 10.2460/javma.236.1.88
30. McDougall RA, Spector DI, Hart RC, Dycus DL, Erb HN. Timing of and risk factors for deep surgical site infection requiring implant removal following canine Tibial Plateau Leveling Osteotomy. Vet Surg. (2021) 50:999–1008. doi: 10.1111/vsu.13634
31. Pacchiana PD, Morris E, Gillings SL, Jessen CR, Lipowitz AJ. Surgical and postoperative complications associated with Tibial Plateau Leveling Osteotomy in dogs with cranial cruciate ligament rupture: 397 cases (1998–2001). J Am Vet Med Assoc. (2003) 222:184–93. doi: 10.2460/javma.2003.222.184
32. Tenover FC, McGowan JE. Reasons for the emergence of antibiotic resistance. Am J Med Sci. (1996) 311:9–16.
33. Moriarty TF, Schlegel U, Perren S, Richards RG. Infection in fracture fixation: Can we influence infection rates through implant design? J Mater Sci Mater Med. (2010) 21:1031–5. doi: 10.1007/s10856-009-3907-x
34. Eijer H, Hauke C, Arens S, Printzen G, Schlegel U, Perren SM. PC-Fix and local infection resistance: influence of implant design on postoperative infection development, clinical and experimental results. Injury. (2001) 32:B38–43. doi: 10.1016/S0020-1383(01)00124-3
35. Tuan J, Solano MA, Danielski A. Risk of infection after double locking plate and screw fixation of tibial plateau leveling osteotomies in dogs weighing greater than 50 kg. Vet Surg. (2019) 48:1211–7. doi: 10.1111/vsu.13308
36. Conkling AL, Fagin B, Daye RM. Comparison of tibial plateau angle changes after Tibial Plateau Leveling Osteotomy fixation with conventional or locking screw technology: screw type and TPA change. Vet Surg. (2010) 39:475–81. doi: 10.1111/j.1532-950X.2010.00656.x
37. Graffunder EM. Risk factors associated with nosocomial methicillin-resistant S. aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother. (2002) 49:999–1005. doi: 10.1093/jac/dkf009
38. Ayliffe GA. The progressive intercontinental spread of methicillin-resistant S. aureus. Clin Infect Dis Off Publ Infect Dis Soc Am. (1997) 24:S74–79.
39. Rynhoud H, Meler E, Gibson JS, Price R, Maguire T, Farry T, et al. Epidemiology of methicillin resistant Staphylococcus species carriage in companion animals in the Greater Brisbane Area, Australia. Res Vet Sci. (2021) 136:138–42. doi: 10.1016/j.rvsc.2021.02.012
Keywords: multidrug-resistant bacteria, MDR, SSI, surgical site infection, Tibial Plateau Leveling Osteotomy, TPLO
Citation: Husi B, Overesch G, Forterre F and Rytz U (2023) Surgical site infection after 769 Tibial Plateau Leveling Osteotomies. Front. Vet. Sci. 10:1133813. doi: 10.3389/fvets.2023.1133813
Received: 29 December 2022; Accepted: 20 March 2023;
Published: 13 April 2023.
Edited by:
Annika Bergström, Swedish University of Agricultural Sciences, SwedenReviewed by:
Hugo Schmökel, Evidensia Specialist Animal Hospital, SwedenCopyright © 2023 Husi, Overesch, Forterre and Rytz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Husi, YmVuamFtaW4uaHVzaUB1emguY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.