- 1College of Animal Science and Technology, Hebei Agricultural University, Baoding, China
- 2Baoding Xingrui Agriculture and Animal Husbandry Development Co., Ltd., Baoding, China
- 3School of Basic Medical Sciences, Hebei University, Baoding, China
- 4National Engineering Laboratory for Animal Breeding and MOA Key Laboratory of Animal Genetics and Breeding, College of Animal Science and Technology, China Agricultural University, Beijing, China
Eggshell translucency severely affects external egg quality, and variations in the eggshell or eggshell membrane are considered the structural basis of the trait. Research has shown that 1.85% additional mixed fatty acids in the diet would greatly decrease the occurrence of eggshell translucency. Only a few studies have examined the phenotypic regularity of eggshell translucency with the increasing age of hens. Therefore, two strains, 1139 Rhode Island Red-White (RIR-White) and 836 Dwarf Layer-White (DWL-White), were used, and from each strain, 30 hens each that consecutively laid translucent or opaque eggs at 67 wks of age were selected. Subsequently, eggshell translucency, internal quality and external quality of eggs, and total cholesterol, albumin, calcium binding protein and other physiological indicators related to lipid, lipoprotein, and calcium metabolisms at the 75th, 79th, and 83rd wks of age in the late phase of the laying cycle were determined. Results: (1) In terms of flocks, for both strains, the translucency scores of the translucent groups were significantly higher than those of the opaque groups (P < 0.05); in terms of individuals, 81.1% RIR-White and 82.8% DWL-White hens consecutively laid eggs of the same or similar translucency, indicating the stability of the trait with increasing hen age; (2) In RIR-White, the eggshell strength of the translucent group at 75 weeks was significantly higher than that of the opaque group (P < 0.05); in DWL-White, the eggshell membrane thickness of the translucent group at the 75th and 83rd weeks was significantly lower than that of the opaque group (P < 0.05); (3) Compared to the opaque groups, the translucent groups had lower total cholesterol content in both RIR-White and DWL-White, lower albumin content in DWL-White at the 79th weeks (P < 0.05), and higher calcium-binding protein (CALB1) in RIR-White at the 83rd weeks (P < 0.05). In summary, this study illustrates the stability of eggshell translucency in late-phase laying hens and provides a reference of physiological indicators for exploring the formation of translucent eggs.
Introduction
Eggshell translucency in hens was first reported in 1932 and is directly caused by penetration and accumulation of moisture in the eggshell (1, 2). Studies have shown that eggshell translucency is widely present in pure lines, commercial hens, and Chinese local strains, such as Rhode Island Red, White Leghorn, Brown-Egg Dwarf Layers, Hy-Line Brown hens, No.1 of Jing Hong laying hens, and Taihang chickens (2–4). In addition, compared with opaque eggs, the eggshell of translucent eggs is more easily penetrated by the Bifidobacterium and Escherichia coli, leaving a hidden danger to food safety (5). Traits of shell translucency are partly regulated by genetic factors, manifesting in differences in the severity of shell translucency among strains and between individuals. Furthermore, hens have laid eggs with the same level of translucency on consecutive days (3, 6). In previous studies, the heritabilities of eggshell translucency were estimated to be 0.18–0.22 in 63-wk-old Brown-Egg Dwarf Layers and a 52-wk-old F2 resource population generated from a cross between Dongxiang Blue-shelled and White Leghorn hens (2). Nevertheless, eggshell translucency is also partly affected by other factors, such as the temperature and humidity of the environment and the level of phosphorus in the diet (7). Additionally, eggshell translucency in older hens (60 weeks) appears to be more severe than that in younger hens (24 and 42 weeks) (8). However, the stability of the eggshell translucency trait in flocks and the continuity of the traits in individuals during the late phase of laying cycles has not been systematically investigated.
The structural basis of the translucent eggshell phenotype are variations of the eggshell's mineralized layer or eggshell membrane (2, 5, 8), of which the primary reason for eggshell translucency is yet to be confirmed. It has been reported that the eggshell thickness of translucent eggs was significantly higher than that of opaque eggs (9); however, certain studies have reported no difference between translucent and opaque eggs (7, 10). Furthermore, prominent, continuous grooves in the mammillary layer of eggshells and variations in mammillary caps were observed (5, 8); however, the results were not statistically analyzed. The eggshell is composed of 96% calcium, 2% organic matter, phosphorus, magnesium and other trace elements (11). Compared to hens laying opaque eggs, lower expression of Ca2+-ATPase mRNA was detected in the uterus tissues of hens laying translucent eggs, implying a low concentration of calcium ions in uterine fluids during eggshell formation (12). Scanning of normal eggshell ultrastructure using X-ray micro-computed tomography demonstrated that pore space accounts for almost one-third of the total eggshell volume (13). Bubble pores with 200–400 nm diameter are the main pore system in eggshells and play a paramount role in regulating gas exchange (14). However, bubble pores may also provide adequate space for moisture accumulation, resulting in eggshell translucency. In different studies, differences in eggshell thickness and strength were not consistent between translucent and opaque eggs (2, 7, 10). Furthermore, there was no difference in the quantity and size of bubble pores between translucent and opaque eggs (15), indicating that variations in the eggshell structure were important but may not be an essential factor affecting eggshell translucency.
The eggshell membrane is a fibrous network structure between the egg white and the mineralized eggshell layer. It not only provides a substrate for the initial mineralization during eggshell formation but also surrounds the egg contents and acts as a barrier, preventing egg contents from penetrating and accumulating in eggshells (11, 16). Therefore, the eggshell membrane may be an essential factor affecting eggshell translucency (2). Liu et al. (17) first discovered that the eggshell membrane of translucent eggs was thinner than that of opaque eggs. Further studies on the physical structure and chemical composition of the eggshell membrane demonstrated that the maximum longitudinal tensile break force and the total amino acid, proline, and lysine contents of the membrane in translucent eggs was significantly lower than those of opaque eggs (2), indicating that the contents of translucent eggs may penetrate through the eggshell membrane and accumulate in the eggshell more easily. Regarding nutrition, adding 1.85% mixed fatty acids, such as stearic acid, oleic acid, and linoleic acid, in the hens' diets for 4 weeks significantly reduced the percentage of translucent eggs from 15.9 to 4.4%, with no effect on the eggshell thickness and strength (18). Fatty acids may improve the quality of eggshell membrane by regulating the activity or expression of proteins and other substances involved in eggshell membrane formation through the lipid metabolism (19–21), thereby decreasing the percentage of translucent eggs.
The current study aimed to systematically investigate the characteristics of eggshell translucency in the late-phase laying cycle of hens, the stability of the translucency trait in the hen flock, and the continuity of shell translucency with the increasing age of the hens. We also compared the differences in the egg quality, blood lipid metabolism indicators, calcium metabolism related to the eggshell membrane, and the eggshell's mineralized layer between translucent and opaque eggs. These factors were chosen to provide a reference for determining the causes of eggshell translucency to help design strategies for reducing its occurrence.
Materials and methods
Experimental population and eggshell translucency identification
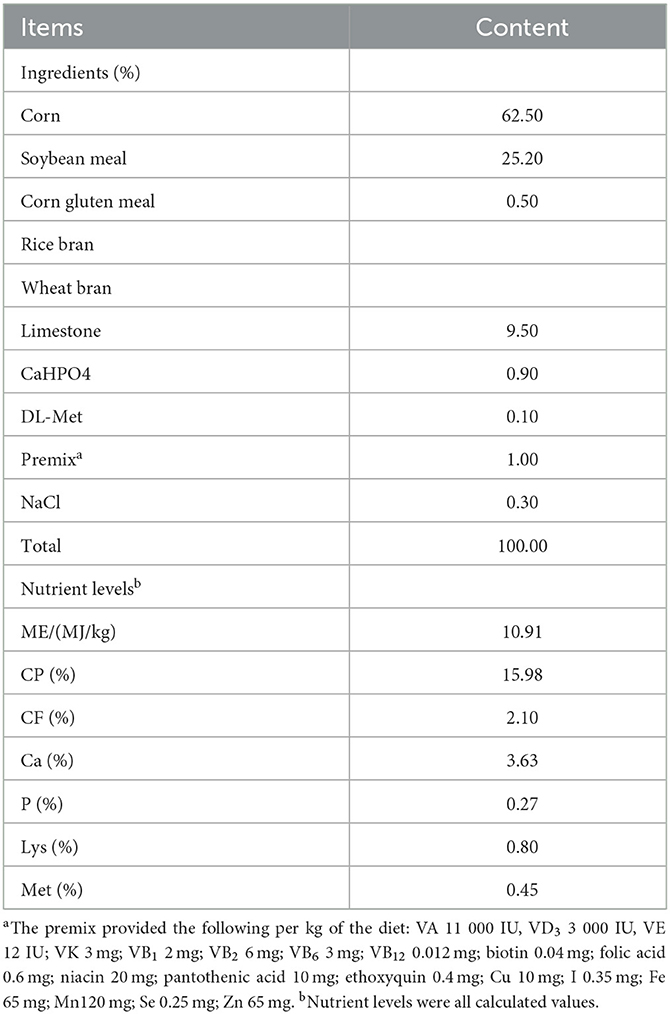
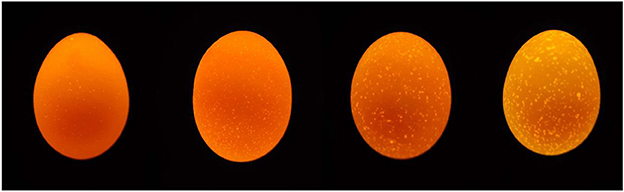
Sixty-seven-week-old hens of 1,139 Rhode Island Red-White (RIR-White) and 836 Dwarf Layer-White (DWL-White) strains were used in the experiment; all hens were raised in Hebei Rongde Poultry Breeding Co., Ltd. (Hengshui, China). Both RIR-White and DWL-White were bred for seven generations. RIR-White hens are white, lay brown-shelled eggs, have evolved from Rhode Island Red, and their body weight while laying the first egg is approximately 1.82 kg; DWL-White hens are white, lay pink-shelled eggs, and have evolved from Brown-Egg Dwarf Layers, which were developed by backcrosses of male meat-type dwarf ISA-Vedette to the female CAU brown egg layers (22). The two strains were reared in a fully enclosed underground house in individual cages (length 30 cm, width 40 cm, height 40 cm in front, and 33 cm in behind), with free access to food and water, a 16:8 h light-dark cycle, and automatic control of mechanical ventilation. Composition and nutrient levels of the basal diet are shown in Table 1. From each individual, we collected all eggs over five consecutive days and recorded the corresponding individual number. Meanwhile, abnormal eggs, such as broken, soft-shelled, or deformed eggs, were excluded. For each hen, if three qualified eggs were selected, we stopped collecting eggs from that hen in the next few days, and if less than three eggs were selected on the 5th day, we also stopped collecting eggs. The collected eggs were stored in a stable environment (temperature: 20–25°C humidity: 40–50%) for 5 days and then classified based on their translucency levels (quantity and size of translucent spots on the eggshell) using a 1–4 scoring system (4). The reference sample used in the scoring method is shown in Figure 1. The higher the score, the more severe the eggshell translucency. The eggshell translucency level of each hen was determined by the average score value of 3 eggs. Finally, for each strain, 30 individuals with a higher eggshell translucency score and 30 individuals with a lower eggshell translucency score were selected and classified as translucent and opaque groups, respectively. Subsequently, the eggshell translucency levels of each hen were recorded at 75, 79, and 83 weeks of age; the method and process of eggshell translucency identification were the same as above.
Egg quality measurement
After eggs were stored for 7 d during the 75th, 79th, and 83rd weeks, we randomly selected 30 eggs each from the translucent and opaque groups of both RIR-White and DWL-White strains and measured their egg quality. The indicators studied were as follows: egg weight, 7-d water loss, egg shape index, eggshell thickness, eggshell membrane thickness, eggshell strength, Haugh unit, yolk color, eggshell weight, eggshell proportion, egg white weight, and yolk weight. Egg weight, yolk color, and Haugh unit were measured using an egg quality tester (EA-01; Israel Aoke Co., Ltd., Israel). The 7-d water loss, eggshell weight, egg white weight, and yolk weight were measured using an electronic analytical balance (PL1502-S; Mettler Toledo Instruments Co., LTD., Switzerland). The 7-d water loss rate is the ratio of 7-d water loss to initial egg weight; eggshell proportion is the ratio of eggshell weight to egg weight; the eggshell was first washed using deionized water to remove albumen and left for 30 min to dry naturally. Then the eggshells were weighed using an electronic analytical balance as above. Egg shape index is the ratio of the long diameter of an egg to its short diameter and was measured using an NFN384 egg quality analyzer (FHK, Tokyo, Japan). Eggshell thickness and eggshell membrane thickness were measured using digital vernier calipers (MNT-LG11-121-1; Guangzhou Lige Technology Co., Ltd., Guangzhou); eggshell membrane thickness is the value of the total eggshell thickness minus the thickness of the mineralized eggshell layer. Eggshell strength was determined using an eggshell strength tester (ESTG-01; Israel Aoke Co., Ltd., Israel).
Plasma biochemistry
On the 67th of the 75th, 79th, and 83rd wks, after completing the egg collection procedure, we collected 3 mL of inferior pterygoid venous blood from each hen and put it into an anticoagulant tube with heparin sodium as an anticoagulant (Shandong Junnuo Medical Devices Co., Ltd., Shandong, China). Plasma samples were prepared by centrifugation at 3,000 rpm for 15 min (LG10-2.4A; Beijing Rebel centrifuge Co., Ltd., China). The glycerine phosphate oxidase peroxidase (GPO-PAP) method (23) and the cholesterol oxidase phenol 4-aminoantipyrine peroxidase (CHOD-PAP) assay (24) were used to measure triglyceride and total cholesterol contents, respectively. The contents of total protein were detected by bicinchoninic acid (BCA) assay (25) and albumin was detected by bromocresol green (BCG) assay (26), while the methylthymol blue (MTB) method (27), and the molybdenum blue method (28) were used to determine the calcium and phosphorus levels, respectively (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The globulin content is the difference between the total protein and albumin contents. The contents of estrogen, calcium-binding protein (CALB1), and calcium pump were measured using double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) (29) kits (Shanghai Jianglai Biotechnology Co., Ltd., Shanghai, China).
Statistical analysis
Using non-parametric Mann-Whitney tests (30), the ordinal shell translucency score data per hen strain were compared at different weeks of age between translucent and opaque groups, as well as across age within each group. All egg quality and blood indicators of the translucent and opaque groups at the same week were compared using a t-test (all data were normally distributed). The between groups variance of egg quality and blood indicators among the different weeks of hen age was analyzed using SPSS software (version 23.0, SPSS Inc., Chicago, IL) in the general linear model (GLM), and the model used is shown in the following equation:
where Xij is the measurement value of the different indicators, μ is the overall average, αi is the effect of groups, and eij is the residual error. Differences in the average values among groups for each indicator were compared using Duncan's multiple range test. The significance level was set at 0.05.
Results
Eggshell translucency
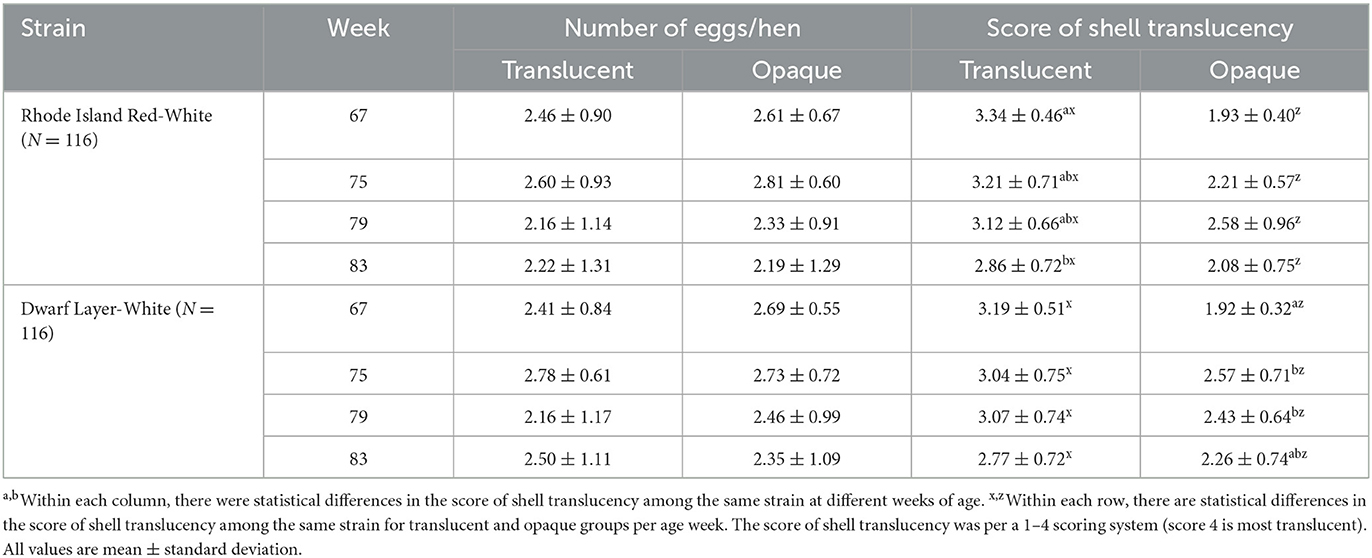
Variations in eggshell translucency with the increase in hen age of both RIR-White and DWL-White strains are shown in Table 2. For RIR-White, in the 67th wk, the mean eggshell translucency score of the translucent group (3.34) was significantly higher than that of the opaque group (1.93; P < 0.05). In the 75th, 79th, and 83rd weeks, the trend was consistent with that of the 67th wk; the mean translucency scores of the translucent group were still significantly higher than those of the opaque group (P < 0.05). For DWL-White, the mean translucency score of the translucent group at the 67th wk was 3.19, which was significantly higher than that of the opaque group (1.92; P < 0.05). In the 75th, 79th, and 83rd weeks, the results were consistent with those of RIR-White; mean translucency scores of the translucent group were still significantly higher than those of the opaque group (P < 0.05). For the translucent group, the translucency score of the 83rd wk was significantly lower than those of the 67th weeks in RIR-White (P < 0.05). For the opaque group, the translucency score of the 67th wk was significantly lower than those of 75th and 79th weeks in the DWL-White strain (P < 0.05). In RIR-White strain, the average translucency score of the translucent group decreased with hen age, and in DWL-White strain, the average translucency score of the opaque group increased with hen age.
Regarding individuals, variations of eggshell translucency with hen age are presented in Figure 2. In RIR-White, 81.1% of hens successively laid eggs of the same or adjacent translucency scores on the 67th, 75th, 79th, and 83rd weeks. Among these, 31% (18 individuals) of hens successively laid eggs of the same score (1.7, 8.6, and 12.1%, 8.6% of hens laid eggs of scores 1, 2, 3, and 4, respectively); 50% (29 individuals) of hens laid eggs of adjacent scores (5.2% of hens laid eggs of score 1 and 2, 22.4% of hens laid eggs of score 2 and 3, and 22.4% of hens laid eggs of scores 3 and 4); 18.9% (11 individuals) of hens laid eggs covering over three score levels. In DWL-White, similar to RIR-White, 82.8% of hens laid eggs of the same or adjacent translucency scores. Among these, 22.4% (13 individuals) of hens laid eggs of the same score, 60.3% (35 individuals) of hens laid eggs of adjacent translucency scores, (6.9% of hens laid eggs of scores 1 and 2, 31% of hens laid eggs of scores 2 and 3, and 22.4% of hens laid eggs of scores 3 and 4), and 17.2% (10 individuals) of hens laid eggs covering over three score levels.

Figure 2. The Venn diagram of average scores of eggshell translucency at 67th, 75th, 79th, and 83rd weeks in 2 strains. (A) Venn diagram of Rhode Island Red-White; (B) Venn diagram of Dwarf Layer-White.
Egg quality
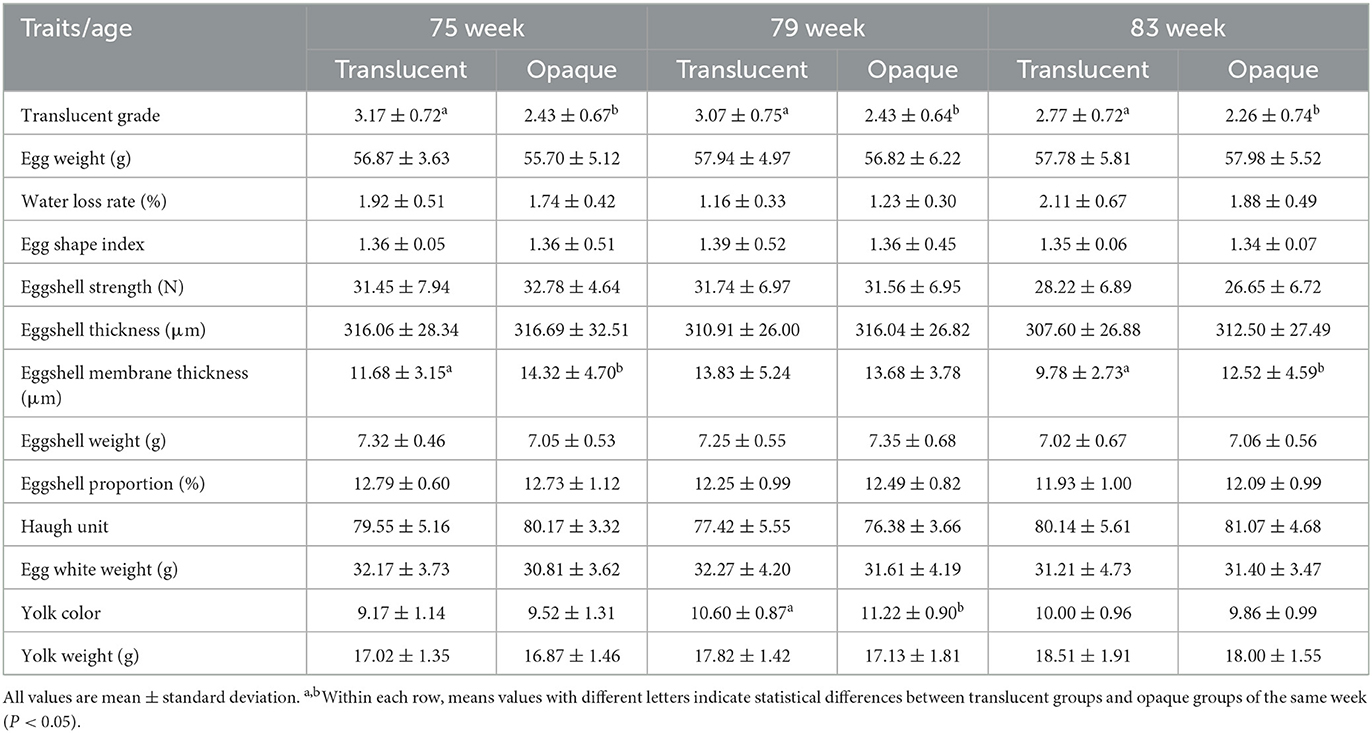
Detailed metrics of egg quality of the translucent and opaque groups of both RIR-White and DWL-White strains are shown in Tables 3, 4. In RIR-White, the egg weight, eggshell strength, and eggshell weight of the translucent group were significantly higher than those of the opaque group at 75 weeks of age (P < 0.05), whereas the eggshell thickness tended to be higher than that of the opaque group. In 83-wk-old hens, the egg weight, egg white weight, and yolk weight of the translucent group were significantly higher, and the 7-d water loss rate was significantly lower than those of the opaque group (P < 0.05), whereas the eggshell strength of the translucent group tended to be higher than that of the opaque group. In DWL-White, the eggshell membrane thickness of the translucent group was significantly thinner than that of the opaque group at 75 and 83 weeks of age (P < 0.05), and there were no significant differences in other indicators. In the 79th wk of age, the yolk color of the translucent group was significantly higher than that of the opaque group in RIR-White (P < 0.05), whereas the opposite was true in DWL-White.
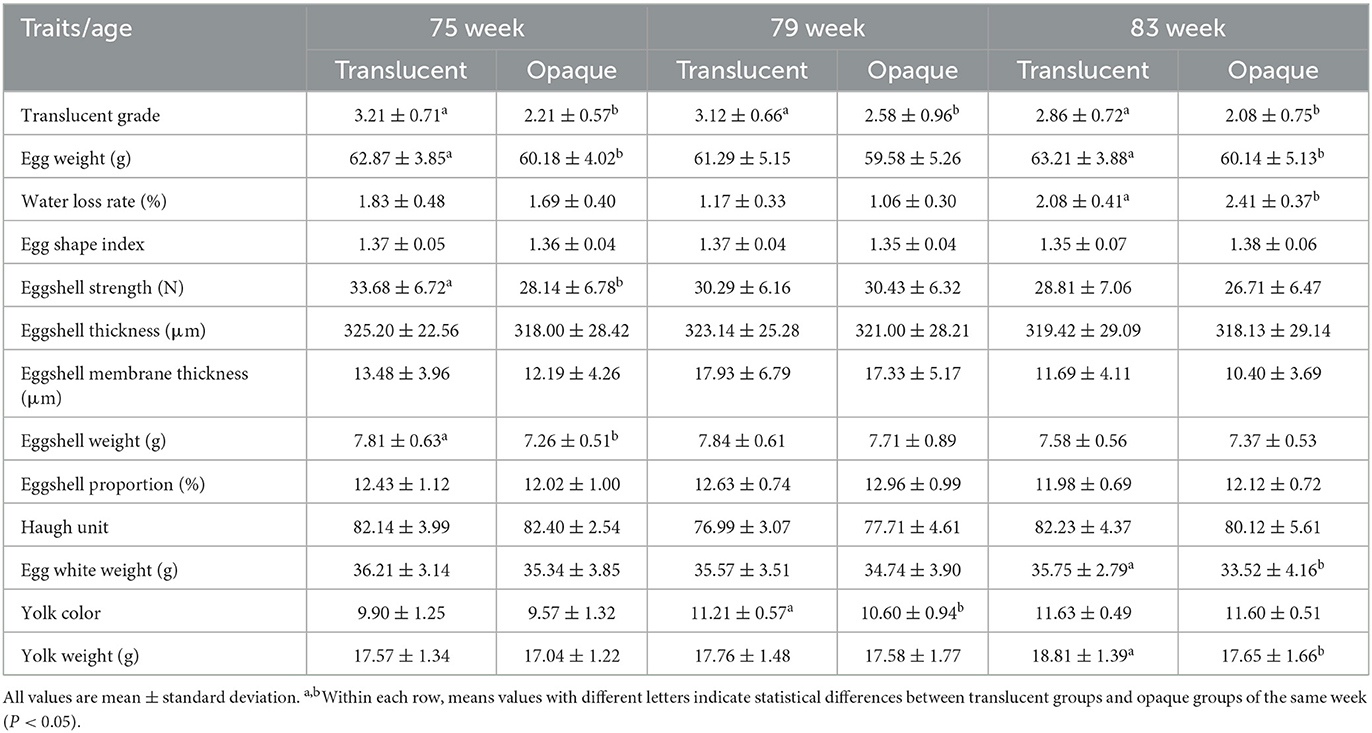
Table 3. Comparison of egg quality between translucent and opaque groups of Rhode Island Red-White across age.
Blood indicators
The results for all blood indicator analyses are presented in Tables 5, 6. In RIR-White, at the 75th wk, the total cholesterol of the translucent group was significantly lower than that of the opaque group (P < 0.05). In DWL-White, similar results were obtained at the 79th and 83rd weeks; the total cholesterol of the translucent group was significantly lower than those of the opaque group (P < 0.05), and the same trend was also observed at the 75th wk. At the 83rd wk in RIR-White, the CALB1 levels of the translucent group were significantly higher than those of the opaque group (P < 0.05) and the 79th wk in DWL-White found the same trend. Furthermore, in RIR-White, at the 83rd wk, the phosphorus content of the translucent group was significantly lower than that of the opaque group (P < 0.05). In DWL-White, the albumin content of the translucent group was significantly lower than that of the opaque group at the 79th week of age, whereas the estrogen content of the translucent group was significantly higher than that of the opaque group at the 75th week. There were no differences in other indicators.
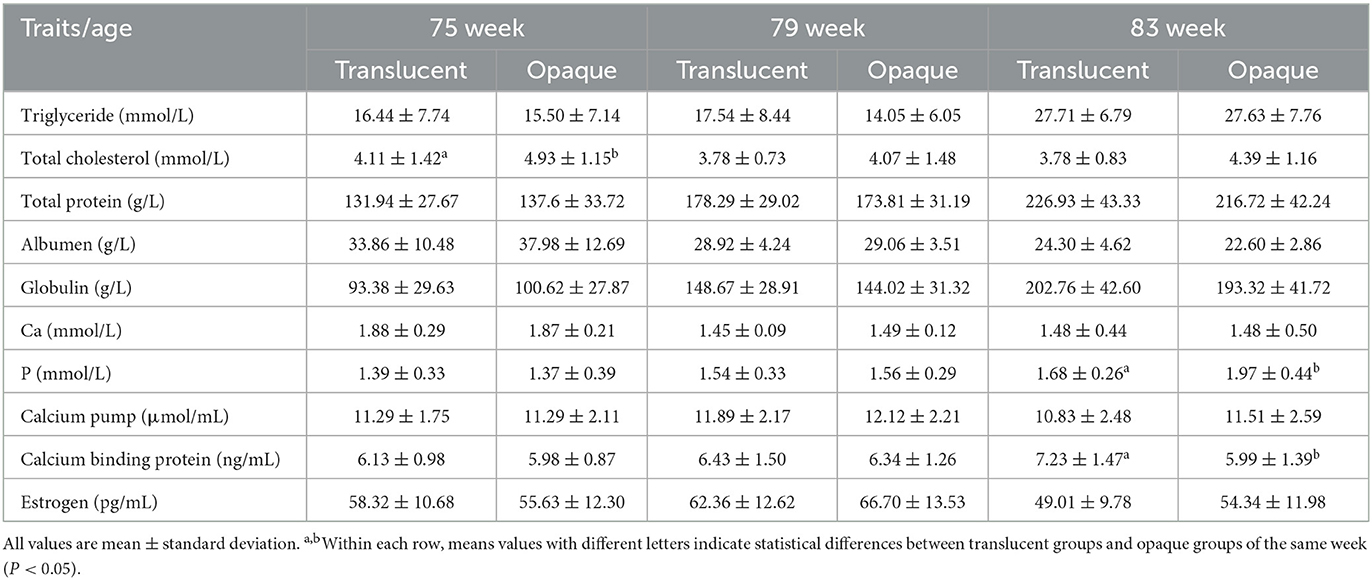
Table 5. Comparison of plasma biochemistry indexes between translucent and opaque groups of Rhode Island Red-White.
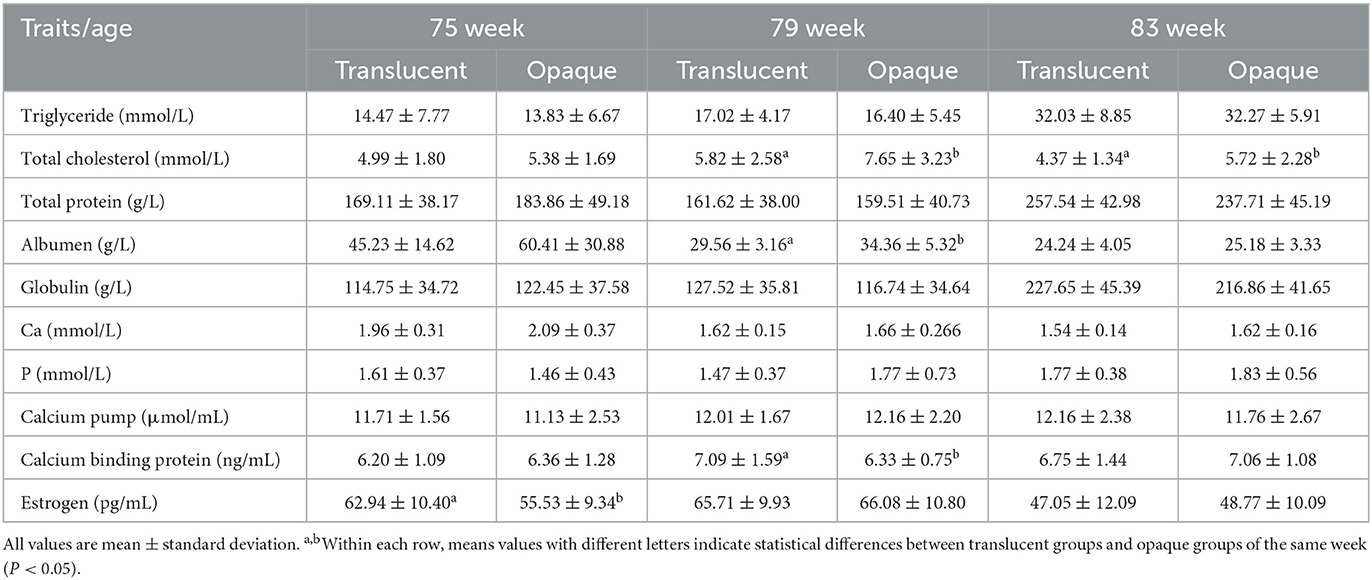
Table 6. Comparison of plasma biochemistry indexes between translucent and opaque groups of Dwarf Layer-White.
Discussion
Eggshell translucency
This experiment tracked the eggshell translucency of RIR-White and DWL-White strains at the 67th, 75th, 79th, and 83rd weeks of age. In terms of the hen flock, consistent differences were observed in the average eggshell translucency score between the translucent and opaque groups in both strains from the 67th to 83rd week, implying the trait stability of eggshell translucency. With the increase in the age of the hens, the difference in shell translucency between the translucent and opaque groups became smaller. Shell translucency of the opaque group tended to be more severe with the increasing age of hens, whereas shell translucency of the translucent group tended to be less with the increasing age of hens; these observations are consistent with those of Solomon (8). Tables 3, 4 show that for both strains, the eggshell strength and thickness of the translucent groups tended to be lower and thinner (31), respectively, with the increase in hens' age. It is speculated that variations in the eggshell may affect the conditions for water accumulation in the eggshell (13), resulting in the decrease of shell translucency. However, the translucent group maintained a high score for eggshell translucency, suggesting that variations in the eggshell may not be the essential cause for eggshell translucency.
Regarding individual hens, 81% RIR-White and 82.7% DWL-White hens laid eggs of the same or adjacent eggshell translucency score from the 67th to 83rd wk, indicating the stability of the eggshell translucency trait in late-phase laying hens. Measurement error may be an important reason for the occurrence of adjacent scores. When using the scoring method, an egg with a specific score of shell translucency has an approximately 50% probability of being misclassified into the adjacent score (4). Another reason may be that the shell translucency phenotype did change slightly (Table 2), and these changes may be caused by non-essential factors for the occurrence of shell translucency. Above all, the results correspond with previous studies suggesting that hens laid translucent eggs on consecutive days (2, 6) and prove the trait stability of eggshell translucency in the late-phase laying cycle of hens.
Egg quality
In DWL-White hens, the eggshell membrane thickness of the translucent group was significantly lower than that of the opaque group, and this was consistent with the thinner eggshell membrane and lower maximum tensile break strength of the translucent group reported in Brown-Egg Dwarf Layers (2). Translucent spots on the eggshell were the result of accumulation and uneven distribution of moisture from egg contents (1). The eggshell membrane is a complex network of fibers that wrap egg contents and act as a barrier for preventing egg contents from penetrating the eggshell (11, 16). However, the eggshell membrane is also porous, and the porosity was estimated to be 52.06% using an atomic force microscope (32). During storage, changes in the environmental temperature and the release of carbon dioxide in egg whites (33) may cause changes in the volume of egg contents, promoting the rupture of thinner or less tough eggshell membranes, allowing egg contents and moisture to penetrate the eggshell membrane and accumulate in the eggshell. However, because there is no difference in pore distribution between translucent eggs and opaque eggs, more water can gather in the eggshell, forming translucent eggs (34).
In RIR-White, at the 75th wk, the eggshell strength of the translucent group was significantly higher than that of the opaque group, and the eggshell thickness at the 75th wk and eggshell strength at the 83rd wk also showed the same trend. These results are consistent with previous research results in White Leghorn layer hens and Brown-Egg Dwarf Layers (9). Eggshell strength is directly related to the eggshell thickness (35), crystal ultrastructure of the eggshell (36), and the attachment strength between the eggshell membrane and mammillary layer (37). In 40-wk-old Isa Brown laying hens, it was reported that the cap structure of mammillary cores in the translucent group was more fully in contact with the eggshell membrane (5), contributing to the enhancement of eggshell strength and eggshell thickness (38). In the current study, the differences in eggshell and eggshell membrane between the translucent and opaque groups of the two strains were not entirely consistent. But the results do not conflict with the fact that the shell membrane of translucent eggs is thinner (7), the eggshell strength is higher (2), and the eggshell thickness is higher (39). One important reason for these results may be the thinner eggshell membrane in the late-phase laying cycle of the two strains, with average values of approximately 11–13 μm, which were lower than those in White Leghorn and Brown-Egg Dwarf Layers (18–21 μm) (9). We speculate that a thinner eggshell membrane may be a prerequisite for eggshell translucency.
Most studies have shown no difference in protein height, haugh unit, yolk weight and in other internal egg quality measurements between translucent and opaque groups (1, 40). However, in this study, in RIR-White, the egg weight, eggshell weight, egg yolk weight, and egg white weight of the translucent group at the 75th or 83rd week were higher than those of the opaque group, and the water loss rate was lower than that of the opaque group. Among these parameters, egg weight is mainly affected by body weight (41), indicating that the physiological status of layers were all affected by the eggshell translucency to a certain extent. Eggshell weight, yolk weight, and egg white weight was all affected by egg weight, which was positively correlated with egg weight (42). In addition, the smaller the egg weight, the larger the surface area/volume ratio (43), which may be responsible for the higher water loss rate in the opaque group.
Plasma biochemistry
Studies have shown that including 1.85% of mixed fatty acids in hens' diets decreases eggshell translucency but does not affect eggshell strength and thickness (18); however, it may improve the quality of the eggshell membrane. Total cholesterol is an important indicator of blood lipid metabolism (44). In this experiment, the total cholesterol of the translucent group was lower than that of the opaque group, and this corresponded with the fact that the candidate gene for the translucency trait, Acetyl-CoA carboxylase alpha (ACACA), was associated with abdominal fat through epistatic effects (2, 45). During egg formation, when eggs travel through the isthmus of the fallopian tube, the expression of proteins such as collagen X, fibrillin-1 (FBN1), lysyl oxidase (LOX), and sulfhydryl oxidase 1 (QSOX1) play important roles in the formation of eggshell membranes (46). FBN1 affects the elasticity of eggshell membranes (46), and the expression of FBN1 is significantly up-regulated in individuals with high levels of body fat (20). In addition, LOX activity was overactivated in lipopolysaccharide-treated rat lungs (47), and the expression of the LOX family genes in the oviduct affected the structure of the eggshell membrane by promoting the connection between fibrous protein and collagen in the eggshell membrane (21). In DWL-White, the albumin content of the opaque group was higher than that of the translucent group. It has been reported that elevated plasma albumin would promote wound repair of liver cells (48) and that lipids, lipoproteins, and proteins synthesized by the liver are important precursors involved in egg formation (49). Thus, it is hypothesized that lipid synthesis disorder in the liver may affect eggshell membrane formation, resulting in a thinner eggshell membrane and causing eggshell translucency.
In both RIR-White and DWL-White, higher levels of CALB1 were identified in the translucent group, and a higher estrogen level was also observed in the translucent group of DWL-White at the 75th week. Since both higher levels of CALB1 and estrogen promote calcium deposition during eggshell formation (12, 50, 51), the observations are consistent with the higher eggshell strength in the translucent group. The CALB1 in the intestine and uterus tissue of chickens is related to the absorption and secretion of calcium ions (50, 51). In the duodenum, the expression of the CALB1 decreased when hens laid soft-shelled eggs and increased when hens laid normal-shelled eggs (50, 51), whereas the concentration of CALB1 in the uterine tissue was regulated by estrogen levels (51). In addition, estrogen could also stimulate osteoblasts to produce the medullary bone (50), and the decomposition and reconstruction of medullary bone is closely related with eggshell formation (50, 51). However, in the current study, the expression of CALB1 in the translucent group contradicts with previous research (7, 10), which is probably because in the studies by Nie (7) and Jiang (10), the differences in eggshell and shell membrane were not identified. In RIR-White, the inorganic phosphorus content of the opaque group at the 83rd wk was significantly higher than that of the translucent group, and this observation is consistent with the observation by Nie (7).
Conclusion
In RIR-White and DWL-White, for hen individuals and flocks, the trait of eggshell translucency is stable during the late-phase laying cycle (from the 67th to 83rd week), indicating the feasibility to solve eggshell translucency from a genetic perspective. For eggshell and eggshell membrane, the eggshell strength and thickness of the translucent group tended to be higher than those of the opaque group, and the eggshell membrane thickness tended to be thinner than that of the opaque group. While both eggshell and eggshell membrane contributed to formation of eggshell translucency, the eggshell membrane may play a more important role. For physiological indicators, the total cholesterol and albumin contents of the translucent group tended to be lower than those of the opaque group. The CALB1 and estrogen levels of the translucent group tended to be higher than those of the opaque group. In the two strains of RIR-White and DWL-White, parts of egg quality and physiological indicators of translucent and opaque hen group are not completely consistent, which may caused by characteristics of hen strain or random errors. This study provides reference physiological indicators for exploring the formation of translucent eggs from a genetic perspective.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
All procedures used in this study were approved by the Animal Use and Ethical Committee of Hebei Agricultural University (University Identification No. HB/2019/03). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
Main experimental methods: D-HW, L-HL, and R-YZ. Writing: H-LR. Operation: X-YZ. Supervision: K-QD, HC, and C-SN. Manuscript modification: D-HW and E-YH. All authors read and approved the final manuscript.
Funding
This work was supported by China Agriculture Research System of MOF and MARA(CARS-40), National Youth Science Fund Project of National Natural Science Foundation of China (31902141), and Key R&D Program Project of Hebei Province-Target Project of Modern Breeding Science and Technology (20326631D).
Acknowledgments
The authors would like to thank Hebei Rongde Poultry Breeding Co., Ltd. for its support to this experiment.
Conflict of interest
X-YZ is an employee of Baoding Xingrui Agriculture and Animal Husbandry Development Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Holst W, Almquist H, Lorenz F. A study of shell texture of the hen's egg. Poult Sci. (1932) 11:144–9. doi: 10.3382/ps.0110144
2. Wang DH. Exploration and Research on the Formation Mechanism of Translucent Eggs. China: Agricultural University. (2017).
3. Zhang HD, Zhao XF, Ren ZZ, Tong MQ, Chen JN Li SY, et al. Comparison between different breeds of laying hens in terms of eggshell translucency and its distribution in various ends of the eggshell. Poult Sci. (2021) 100:101510. doi: 10.1016/j.psj.2021.101510
4. Wang DH, Chen H, Zhou RY, Huang CX, Gao HX, Fan BL, et al. Study of measurement methods on phenotype of translucent eggs. Poult Sci. (2019) 98:6677–83. doi: 10.3382/ps/pez539
5. Chousalkar KK, Flynn P, Sutherland M, Roberts JR, Cheetham BF. Recovery of salmonella and escherichia coli from commercial egg shells and effect of translucency on bacterial penetration in eggs. Int J Food Microbiol. (2010) 142:207–13. doi: 10.1016/j.ijfoodmicro.2010.06.029
6. Baker R, Curtiss R. Individual hen differences in egg shell mottling and the relationship of shell mottling to clutch size, internal quality and weight loss. Poult Sci. (1957) 36:904–8. doi: 10.3382/ps.0360904
7. Nie W. Effects of Dietary Phosphorus Levels on Egg Laying Performance, Eggshell Quality and Calcium and Phosphorus Absorption in Dwarf Laying Hens [PhD]. China Agricultural University (2013).
9. Wang DH Li YJ, Liu L, Liu JS, Bao M, Yang N, et al. Traits of eggshells and shell membranes of translucent eggs. Poult Sci. (2017) 96:351–8. doi: 10.3382/ps/pew328
10. Jiang MJ. Effects of Calcium Metabolism in Caged Laying Hens on Eggshell Quality and Its Mechanism [PhD]. Shandong Agricultural University (2015).
11. Nys Y, Gautron J, Garcia-Ruiz JM, Hincke MT. Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. Comptes Rendus Palevol. (2004) 3:549–62. doi: 10.1016/j.crpv.2004.08.002
12. Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Compar. Biochem. Physiol A. (2009) 152:447–69. doi: 10.1016/j.cbpa.2008.11.020
13. Riley A, Sturrock CJ, Mooney SJ, Luck MR. Quantification of eggshell microstructure using X-ray micro computed tomography. Br Poult Sci. (2014) 55:311–20. doi: 10.1080/00071668.2014.924093
14. Zhou J, Wang S, Nie F, Feng L, Zhu G, Jiang L. Elaborate architecture of the hierarchical hen's eggshell. Nano-research. (2011) 4:171–9. doi: 10.1007/s12274-010-0067-8
15. Talbot C, Tyler C. A Study of the fundamental cause of natural translucent areas in egg shells. Br Poult Sci. (1974) 15:197–204. doi: 10.1080/00071667408416096
16. Arias JL, Fink DJ, Xiao SQ, Heuer AH, Caplan AI. Biomineralization and eggshells: cell-mediated acellular compartments of mineralized extracellular matrix. Int Rev Cytol. (1993) 145:217–50. doi: 10.1016/S0074-7696(08)60428-3
17. Liu JC, Gao HJ, Wang L. Causes of thin translucent on eggshells. Chin Poultry. (2007) 29:49–51. doi: 10.16372/j.issn.1004-6364.2007.10.017
18. Zhang YL, Cheng X, Gao Q, Fan CD, Xia AS, Zeng D, et al. Effects of balanced oil on production performance and egg quality of laying hens. Chinese poultry. (2020) 42:36–9. doi: 10.16372/j.issn.1004-6364.2020.07.007
19. Summers L, Kielty C, Pinteaux E. Adhesion to fibronectin regulates interleukin-1 beta expression in microglial cells. Mol Cell Neurosci. (2009) 41:148–55. doi: 10.1016/j.mcn.2009.02.007
20. Davis MR, Arner E, Duffy CR, De Sousa PA, Dahlman I, Arner P, et al. Expression of Fbn1 during adipogenesis: relevance to the lipodystrophy phenotype in marfan syndrome and related conditions. Mol Genet Metab. (2016) 119:174–85. doi: 10.1016/j.ymgme.2016.06.009
21. Chen Z, Xie X, Jiang N, Li J, Shen L, Zhang Y. Ccr5 signaling promotes lipopolysaccharide-induced macrophage recruitment and alveolar developmental arrest. Cell Death Dis. (2021) 12:184. doi: 10.1038/s41419-021-03464-7
22. Yang N, Ning Z, Wang Q, Li J, Wu C. Application of the Sex-Linked Dwarf Gene to Improving Feed Efficiency of Layers. In: Proceedings of the 20th World's Poultry Congress, New Delhi, India. (1996).
23. Sullivan DR, Kruijswijk Z, West CE, Kohlmeier M, Katan MB. Determination of serum triglycerides by an accurate enzymatic method not affected by free glycerol. Clin Chem. (1985) 31:1227–8. doi: 10.1093/clinchem/31.7.1227
24. Siedel J, Schlumberger H, Klosc S, Zicgenhorn J, Wahlefeld AW. Improved reagent for the enzymatic determination of serum cholesterol. J Clin Chem Clin Biochem. (1981) 19:838–9.
25. Walker JM. The bicinchoninic acid (Bca) assay for protein quantitation. Protein Protoc Handb. (1994) 32:5–8. doi: 10.1385/0-89603-268-X:5
26. Webster D. The immediate reaction between bromcresol green and serum as a measure of albumin content. Clin Chem. (1977) 23:663–5. doi: 10.1093/clinchem/23.4.663
27. Yang C, Liu W, Zhao Z, Wu H. [Determination of the Content of Serum Calcium with Methylthymol Blue as Chromogenic Reagent]. Spectrosc Spectral Anal. (1998) 18:485.
28. Katrdna PT, Wallin HC. Determination of Total Phosphorus in Foods by Colorimetric Measurement of Phosphorus as Molybdenum Blue after Dry-Ashing: Nmkl Interlaboratory Study. J AOAC Int. (1994) 6:1557. doi: 10.1093/jaoac/77.6.1557
29. Tabatabaei MS, Ahmed M. Enzyme-Linked Immunosorbent Assay (ELISA). Methods Mol Biol. (2022) 2508:115–34. doi: 10.1007/978-1-0716-2376-3_10
30. Birnbaum ZW. On a use of the Mann-Whitney statistic. In: Proceedings of the third Berkeley symposium on mathematical statistics and probability, Berkeley, CA, USA. (1956). doi: 10.1525/9780520313880-005
31. Park JA, Sohn SH. The influence of hen aging on eggshell ultrastructure and shell mineral components. Korean J Food Sci Animal Resour. (2018) 38:1080–91. doi: 10.5851/kosfa.2018.e41
32. Torres FG, Troncoso OP, Piaggio F, Hijar A. Structure-property relationships of a biopolymer network: the eggshell membrane. Acta Biomater. (2010) 6:3687–93. doi: 10.1016/j.actbio.2010.03.014
33. Brake J, Walsh TJ, Benton CE, Petitte JN, Meijerhof R, Peñalva G. Egg Handling and Storage. Poultry Sci. (1997) 76:144–51. doi: 10.1093/ps/76.1.144
34. Ray A, Roberts J R. The structural basis of egg shell translucency and its role in food safety of table eggs. In: Proceedings of the Australian Poultry Science Symposium, Sydney, New south wales. (2013).
35. Li Q, Duan Z, Sun C, Zheng J, Xu G, Yang N. Genetic variations for the eggshell crystal structure revealed by genome-wide association study in chickens. BMC Genomics. (2021) 22:786. doi: 10.1186/s12864-021-08103-1
36. Ketta M, Tumová E. Relationship between eggshell thickness and other eggshell measurements in eggs from litter and cages. Ital J Anim Sci. (2018) 17:234–9. doi: 10.1080/1828051X.2017.1344935
37. Örberg J. Relationship between the shell membrane-shell bond and shell deformation in hens' eggs. Br Poult Sci. (1990) 31:249–54. doi: 10.1080/00071669008417254
38. Johansson K, Orberg J, Carlgren AB, Wilhelmson M. Selection for egg shell strength in laying hens using shell membrane characteristics. Br Poult Sci. (1996) 37:757–63. doi: 10.1080/00071669608417905
39. Caglayan T, Kirikci K, Aygun A. Comparison of hatchability and some egg quality characteristics in spotted and unspotted partridge (Alectoris Chukar) eggs. J Appl Poultry Res. (2014) 23:244–51. doi: 10.3382/japr.2013-00899
40. Baker R, Curtiss R. Strain differences in egg shell mottling, internal quality, shell thickness, specific gravity, and the interrelationships between these factors. Poult Sci. (1958) 37:1086–90. doi: 10.3382/ps.0371086
41. Huxley JS. On the relation between egg-weight and body-weight in birds. Zool J Linn Soc. (1927) 36:457–66. doi: 10.1111/j.1096-3642.1927.tb02180.x
42. Shi S, Wang K, Dou T, Yang H. Egg weight affects some quality traits of chicken eggs. J Food Agr Environ. (2009) 7:432–4. doi: 10.3168/jds.2009-92-4-1827
43. Zigler KS, Raff RA. A shift in germ layer allocation is correlated with large egg size and facultative planktotrophy in the echinoid clypeaster rosaceus. Biol Bull. (2013) 224:192–9. doi: 10.1086/BBLv224n3p192
44. Mohajeri D, Nazeri M. Inhibitory effect of crocin on hepatic steatosis in the rats fed with high fat diet. J Animal Veter Adv. (2012) 11:2373–9. doi: 10.3923/javaa.2012.2373.2379
45. Hu G, Wang S, Tian J, Chu L, Li H. Epistatic effect between acaca and fabp2 gene on abdominal fat traits in broilers. J Genet Genomics. (2010) 37:505–12. doi: 10.1016/S1673-8527(09)60070-9
46. Sah N, Mishra B. Regulation of egg formation in the oviduct of laying hen. World's Poultry Sci J. (2018) 74:509–22. doi: 10.1017/S0043933918000442
47. Harris ED, Blount JE, Leach RM. Localization of lysyl oxidase in hen oviduct: implications in egg shell membrane formation and composition. Science. (1980) 208:55–6. doi: 10.1126/science.6102412
48. Panda AK, Rama Rao SS, Raju MV, Sharma SS. Effect of Probiotic (Lactobacillus Sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of white leghorn layer breeders. J Sci Food Agric. (2008) 88:43–7. doi: 10.1002/jsfa.2921
49. Fujii M, Odawara K, Fukunaga T, Koga K, Tojo H. Changes of estradiol and lipid concentrations in serum and liver fatty acid of maturing hen and cockerel. Agric Biol Chem. (1984) 48:2197–203. doi: 10.1080/00021369.1984.10866484
50. Whitehead CC. Overview of bone biology in the egg-laying hen. Poult Sci. (2004) 83:193–9. doi: 10.1093/ps/83.2.193
Keywords: late-phase laying hen, eggshell translucency, egg quality, physiological indicator, calcium
Citation: Ren H-L, Zhao X-Y, Di K-Q, Li L-H, Hao E-Y, Chen H, Zhou R-Y, Nie C-S and Wang D-H (2023) Eggshell translucency in late-phase laying hens and its effect on egg quality and physiological indicators. Front. Vet. Sci. 10:1133752. doi: 10.3389/fvets.2023.1133752
Received: 29 December 2022; Accepted: 02 May 2023;
Published: 18 May 2023.
Edited by:
Dana L. M. Campbell, Commonwealth Scientific and Industrial Research Organisation (CSIRO), AustraliaReviewed by:
Alba Rodriguez Mengod, Catholic University of Valencia San Vicente Mártir, SpainKarim El-Sabrout, Alexandria University, Egypt
Sophie Réhault-Godbert, Institut National de recherche pour l'agriculture, l'alimentation et l'environnement (INRAE), France
Copyright © 2023 Ren, Zhao, Di, Li, Hao, Chen, Zhou, Nie and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-He Wang, dGhlY29uY2VydGV2ZW50QGNhdS5lZHUuY24=
 He-Ling Ren
He-Ling Ren Xiao-Yu Zhao2
Xiao-Yu Zhao2 Er-Ying Hao
Er-Ying Hao Rong-Yan Zhou
Rong-Yan Zhou