- 1Department for Horses, Faculty of Veterinary Medicine, Leipzig University, Leipzig, Germany
- 2Center for Clinical Veterinary Medicine, Faculty of Veterinary Medicine, Ludwig-Maximilians-University, Munich, Germany
Equine veterinarians face challenges in treating horses with osteoarthritic joint pain in routine veterinary practice. All common treatment options aim to reduce the clinical consequences of osteoarthritis (OA) characterized by persistent synovitis and progressive degradation of articular cartilage. A range of joint-associated cell types and extracellular matrices are involved in the not yet entirely understood chronic inflammatory process. Regeneration of articular tissues to re-establish joint hemostasis is the future perspective when fundamental healing of OA is the long-term goal. The use of intra-articular applied biologic therapeutics derived from blood or mesenchymal stroma cell (MSC) sources is nowadays a well-accepted treatment option. Although this group of therapeutics is not totally consistent due to the lack of clear definitions and compositions, they all share a potential regenerative effect on articular tissues as described in in vivo and in vitro studies. However, the current stage of science in regenerative medicine needs to be supported by clinical reports as in fact, in vitro studies as well as studies using induced OA models still represent a fragment of the complex pathomechanism of naturally occurring OA. This systemic review aims to determine the long-term effect of orthobiologic therapeutics in horses suffering naturally occurring OA. Thereby, a meta-analysis of randomized controlled trials (RCTs) is conducted to describe the efficiency and safety of intra-articular applied orthobiologics in terms of lameness reduction in the long-term. Using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) guidelines, thirteen studies met the inclusion criteria for the systemic review. Four of those studies have further been evaluated by the meta-analysis comparing the long-term effect in lameness reduction. Each study was examined for risk of bias. For data evaluation, a random-effects model was used, describing the overall outcome in a forest plot. The I2 statistic was used to assess heterogeneity. Results indicate, that orthobiologic therapies represent an effective long-term and safe OA treatment option. Due to the inhomogeneity of included studies, no statements are provided addressing specific orthobiologic therapies, affected joints, OA stage and horse's intended use. Future clinical trials should follow standardized study designs to provide comparable data.
1. Introduction
Osteoarthritis (OA) is an intensively researched condition in human and equine patients characterized by persistent articular inflammation leading to chronic synovitis, progressive destruction of articular cartilage, and consequently to a permanent loss of function and joint pain (1–3). Causative and stimulating factors of OA are still not fully investigated. In horses, the etiology of OA is assumed to be mainly post-traumatic. Therefore, OA in horses can be understood as the result of a failed repair of damaged articular and periarticular tissues. However, not only the nature of the initial structural tissue damage (repetitive microtrauma vs. single severe trauma), but also the degree and course of imbalance of the joint homeostasis seem to determine OA manifestation and progression (4).
In equine patients, joint related diseases including OA are considered the most common cause of lameness, as being involved in approximately 60% of all lameness cases (5–7). More than 70% of racehorses population suffer from lameness due to articular inflammation during their career (8, 9). However, the occurrence of OA is not only linked to high-speed and high-performance sport horse disciplines, such as horse racing (10–12) and show jumping (13, 14), but also to the increasing age of the patients (15–17). In OA-affected horses, the prognosis for long-term return to exercise and work on intended use varies between 30 and 50% and depends on the disease stage, the affected joints, and the horse's work level (17, 18).
In daily clinical practice, equine veterinarians face the challenge of treating OA as a persistent and chronic disease potentially affecting all joint associated tissues (10, 19, 20). Often the subsequent treatment choice is based on the veterinarian's personal experience, the owner's economic feasibility and the intended use for the horse in relation to disease stage. Although a broad spectrum of varying therapeutic concepts is stated (21, 22), conventional treatment options are limited in terms of modifying or reversing disease progression, thereby potentially being inferior in the long-term treatment success. However, the development of successful long-term treatment options is difficult, due to the intricate pathomechanisms of OA initiation as well as progression and the involvement of various cell types and extra-cellular matrices.
Recent studies have shown that biologic therapeutics derived from blood and mesenchymal stromal cell (MSC) sources hold a potentially regenerative potential for articular tissues in vitro (23–26) and in vivo (27–29). Beneficial clinical effects described after an intra-articular administration of biological therapeutics include reduction of lameness and joint effusion (30–32). It is assumed that clinically relevant effects of intra-articular administered blood products and MSCs in OA-affected joints in part are attributed to locally effective growth factors, cytokines, as well as secretomes and exosomes from delivered cells, which further innate on-site cell regeneration (33–35). Although the group of these so named orthobiologics or orthobiologic therapeutic agents is not totally consistent due to differences in manufacturing, processing and application, they all share potential regenerative effects on the described articular tissues proven in vitro (26, 36, 37) and in vivo (38–40) studies.
After more than 20 years of clinical experience in equine medicine, the use of intra-articularly applied orthobiologic therapeutics is considered as a safe and recognized treatment option for osteoarthritic joints today (41, 42). Yet, existing studies, which form the basis of our knowledge about the efficacy of orthobiologic therapeutics in equine medicine, differ in fundamental study design parameters like the availability of placebo groups or the type of researched OA (naturally occurring vs. experimentally induced OA). Consequences drawn from these studies are at best implemented in the treatment of clinical cases and provide evidence-based treatment concepts for equine OA. However, due to the heterogenicity of therapeutic products (blood-derived, tissue-derived), processing methods and components used (cell-free, blood-derived cells, tissue-derived cells), and treatment regimens (single injection, multiple injections), an unacceptably high number of subjects would be required to draw definitive conclusions. Therefore, the application of quantitative statistical methods summarizing primary data from clinical and experimental trials via meta-analysis is a useful tool to draw conclusions from a cohort of studies. The aim of the present study is to conduct a systematic review of current literature in the field of the intra-articular application of orthobiologic therapeutics in naturally occurring equine OA. Furthermore, a meta-analysis of in vivo and controlled studies has been carried out to assess the long-term effect of orthobiologic therapeutics on naturally OA-affected joints in horses.
2. Materials and methods
2.1. Definition of orthobiologic therapies
The present systematic review focuses on the following two intra-articularly applicable orthobiologic therapeutic concepts for equine OA.
2.1.1. Mesenchymal stromal cells
Caplan described the first approaches of stem cell therapy in 1991, proposing potential differentiation into desired tissues (43). The characteristic differentiation potential of these cells has laid the foundation to prove therapeutic concepts in various fields of medicine where tissue regeneration and restoration are the aimed effects (44–49). In the process of clinical stem cell application, orthopedic diseases such as OA were becoming an inherent part of scientific interest (35, 50). The common term “stem cell” is nowadays used in popular science and increasingly replaced by the more scientific expression of a “multipotent mesenchymal stromal cell (MSC)” because specific stem cell characteristics (51) [long in vivo survivability, ability for self-replication and multipotent differentiation into certain tissue types (43)] are insufficiently accurate to prove in therapeutic purposes. However, the term “MSC” is not used uniformly and is not subject to a clear definition. Due to increasing impact of MSCs via paracrine effects, the term “medicinal signal cell” has been proposed in recent publications (52, 53).
MSCs can be derived from mesenchymal tissues such as blood, bone marrow and adipose tissue, but do not represent a homogeneous stem cell population (41). In horses, commonly used MSC sources are fat, harvested from subcutaneous adipose tissue at the tail base (lipectomy) (54, 55), bone marrow obtained by puncturing the sternum (56) or venous blood (40, 57). Following tissue harvesting, the process of MSC isolation and cultivation under laboratory conditions requires several weeks to obtain cell numbers usually used for intra-articular applications (41). Besides these autologous cultivated MSCs, commercially available MSC therapeutics are approved by the European Medicines Agency (EMA). Currently, two off the shelf MSC therapeutics are available, one of which uses chondrogenic induced MSCs dissolved in allogeneic plasma (40, 57–59), whereas the other product uses MSCs derived from the umbilical cord (60, 61). These therapeutics contain a defined number of allogeneic MSCs from donor equids. A further alternative to commercially available ready-to-use products is the in-house production of therapeutics from tissue sources like blood, bone marrow or adipose tissue, usually received from the equine patient (autologous) (42). These so-called point-of-care products are readily available through a fast, standardized process of cell separation and MSC enrichment by medical devices (27, 62). Depending on the tissue sources and processing, the final solution contains a variety of different cell types in a mixed population of blood and adipose progenitor cells as well as differentiated cells (41, 42). The proportion of MSC-like cells within the final product is regarded low and not defined (63). With regard to obtain a high number of defined MSCs from the stated tissue sources, MSC isolation and cultivation has to be performed under laboratory conditions (autologous cultivated MSCs) (64, 65). As a result, several millions MSCs are available for application (66). The time between tissue sampling to MSC harvesting calculates several weeks, which must be considered for autologous treatment regimes.
2.1.2. Autologous blood products
Autologous blood products represent a wide range of therapeutics due to the variety of blood processing methods and individual blood components (33, 67). Basically, two groups of blood derived applicable therapeutics can be stated: (1) cell-based and (2) cell-free autologous blood products. For blood processing, commercially available medical devices are provided to equine practitioners.
Cell-based autologous blood products aim to increase the concentration of certain blood cells, mainly platelets, within the applicable therapeutic agent to transmit the regenerative potential of platelet containing growth factors into the joint (68, 69). Depending on the respective blood platelet number and the processing method, the increase in platelet concentration varies widely among products (70). The amount of transmitted growth factors and cytokines depends on the total number of applied platelets, on the injected solution and whether the therapeutic cells are solved in plasma or in a non-blood based injectable solution (71, 72). Platelet rich plasma (PRP) is one of the best-known representatives of this therapeutic group, with a defined 3- to 5-fold increase in platelet concentration in autologous plasma (73, 74). PRP is produced using a double-centrifugation method (41). Alternative processing methods such as single-centrifugation techniques and filtration provide therapeutics with deviating values of platelets and leucocytes from PRP (33, 75). In horses, cellular autologous blood products were commonly used in cases with tendon and ligament injuries (76). However, their use in joint-related diseases is described, and positive outcomes are documented, particularly in combination with MSC-treatments (40, 77). The therapeutic effects have not yet been clarified in detail, since not only growth factors play a pivotal role in tissue regeneration.
Cell-free, serum-based therapeutics represent another group of autologous blood products. After extended coagulation of the patient's blood at 37°C and subsequent centrifugation, the final orthobiologic therapeutic substance provides the full blood cell secretome (26, 78). In addition to the already serum-diluted cytokines, growth factors and proteins, the extended coagulation phase also stimulates de novo synthesis of proteins, which enrich the final product to a so far not totally defined extended secretome (79, 80). The mode of action of the acellular autologous blood products is in many aspects not fully defined (81, 82). The often referred increase of anti-inflammatory interleukin-1 receptor antagonist (IL-1Ra) concentration is only partially responsible for the described positive clinical effects (30, 83). The mechanism of action of enriched IL-1Ra as therapeutic agent is to block the receptors and therefore prevent the proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) released by the intra-articular inflammatory process from binding (33).
2.2. Inclusion criteria
A distinction was made between systematic analysis and meta-analysis. To obtain a general overview, all experimental studies with a follow-up time of more than 6 months were examined in the systematic review. In the meta-analysis, only randomized and controlled trials (RCTs) with a follow-up period of more than 6 months were examined according to the following inclusion and exclusion criteria. To present the results as clearly as possible, the PICO method was used. (a) Population: horses with naturally occurred OA; (b) Interventions: intra-articular therapy by MSCs alone or by MSCs in combination with autologous blood products, or autologous blood products alone; (c) Comparison: degree of lameness before and after intra-articular treatment (comparison of success rate, horses working on competition, horses working at trainings level, lame free horses); (d) Outcome: degree of lameness and adverse effects; (e) Study designs: for the systematic review all experimental studies were included, for the meta-analysis randomized controlled trials were included.
2.3. Exclusion criteria
The following studies were excluded: (a) treated animals other than horses and diseases other than OA; (b) use of treatment method other than intra-articular; (c) no clear lameness diagnostics used; (d) not published in English or German; German language was included as this is the authors mother language and articles could be assessed in detail (e) no complete replication of quantitative data of the treated animals (for example individual degree of lameness).
2.4. Search strategy
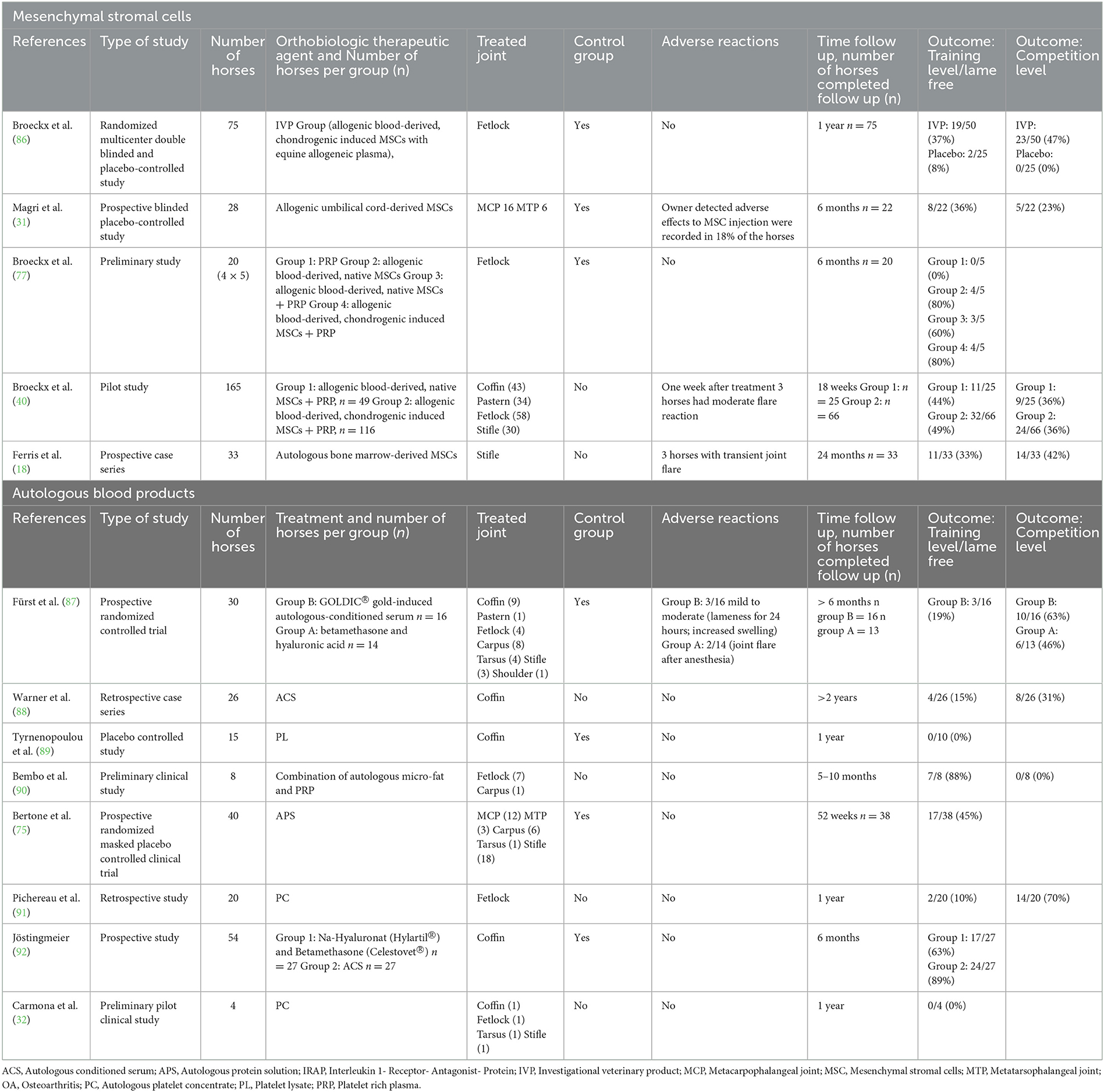
The following research platforms were used (listed according to weighting): PubMed, Google Scholar and CAB direct. Literature searches were carried out using the following keywords: “horse/equine,” “joint/osteoarthritis,” “intra-articular,” “regenerative therapy,” “return/performance.” The search terms could be summarized with the Boolean operators “AND” or “OR” (84). The research was conducted between January 2021 and March 2022. A comprehensive literature search on orthobiologic based joint therapies in horses was undertaken, including all studies published in English and German. This initial investigation summarized 271 findings, of which all studies were examined according to inclusion and exclusion criteria. Subsequently, this initial investigation delivered 86 results. In addition, the reference list of all 86 papers were manually checked for research-relevant studies. To ensure that no meta-analyses relevant to this topic were available, a hit query was performed on PubMed using the two keywords “horse” and “meta-analysis.” The response resulted in 79 meta-analyses. This compares to 18 matches with three real meta-analyses in 2017 (85). These results prove clearly that meta-analysis is becoming more and more relevant in evidence-based medicine. None of these 79 meta-analyses deals in a similar or identical way with the issue investigated in this research. The subsequent table lists the most important studies, sorted by intra-articular administered products, in horses with naturally occurring OA compared to induced OA. In addition, the study duration is indicated >6 months (Table 1).
2.5. Data extraction
To meet the aim of the topic, only in vivo studies were analyzed. For the systematic review, all experimental studies were included regardless their level of evidence or design, with and without a control group. The control group was defined as another horse, another leg (contralateral limb) or another treatment method. The following data were examined and listed according to the following aspects: author, year of publication, type of study (RCTs/ No-RCTs), sample size, treatment protocol, treated joint/joints, placebo-controlled, adverse reactions, follow-up time, lameness evaluation (horses working at trainings level/lame free horses/horses working on competition level/success rate).
For the intra-articular treatment regimen with orthobiologic therapeutics, there were no specifications regarding diagnostic methods, treatment frequency, dosage, and preparation of the appropriate therapeutics (intra-articular therapeutics with MSC and/or autologous blood products are allowed). In addition, studies with any joint with naturally occurring OA were included in the systematic review; there were no specifications on a specific localization. Finally, all studies were evaluated based on the lameness examination and classified into either a positive or a negative outcome. The positive outcomes were divided into two groups: horses working at training level and horses returning to competition. Horses with a negative outcome did not respond to treatment or had a relapse during the observation period. For the meta-analysis, the two positive outcome groups (horses working at training level/lame free and horses returning to competition) were combined due to a lack of study numbers.
2.6. Quality assessment
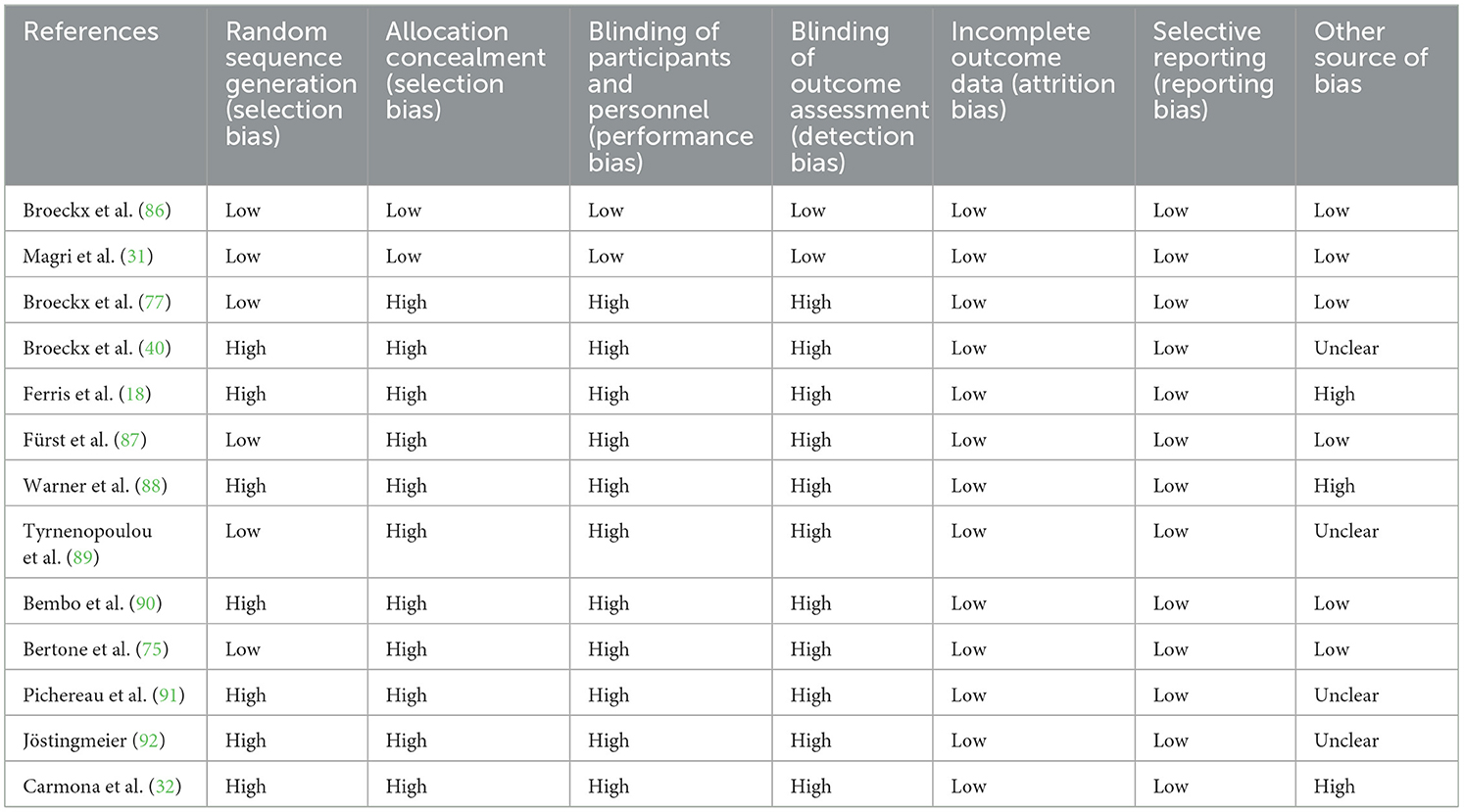
Each study in the systematic review was examined for the following 7 bias characteristics: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), other source of bias. Regarding each aspect, the studies were classified as high risk, low risk or unclear risk according to the PRISMA guidelines (93). For a better visualization, a traffic light table with “high risk” in red, “low risk” in green, and “unclear risk” in yellow was created. The classification into the category “unclear risk” occurs when relevant details for the classification into bias are not sufficiently substantiated in the respective study (94).
2.7. Statistical analysis
Using the PRISMA guidelines, 13 studies met the inclusion criteria for the systematic review (93). To compare dichotomous outcomes via meta-analysis, an odds ratio (OR) with 95% confidence interval (CI) was calculated using the R program (95). For data evaluation a random-effects model was used describing the overall outcome. Each study with its estimated effect size and corresponding confidence interval is graphically represented in the forest plot. Furthermore, the forest plot illustrates the extent to which the result from the individual study varies (96, 97). This variability is referred to as heterogeneity and is assessed by I2 in the following meta-analysis. Heterogeneity was determined to be significant at I2 > 50% or p < 0.1. A result was considered significant with p < 0.05.
2.8. Meta-analysis
In the meta-analysis, the results of lameness evaluation at different time periods of the studies were presented in the individual sections. In the short-term follow-up periods, one additional placebo-controlled and randomized trial was examined for better comparability (57). These will be discussed separately. All long-term studies are listed in the last row of the forest plot.
3. Results
3.1. Risk of bias
With all instruments that measure the risk of bias in clinical trials, it must be considered that they do not present an exact measurement method. Instead, it is an estimation in which the result always contains a subjective component. The purpose is to compare similar and homogenous treatment groups affected only by random variabilities (75).
All studies in the systemic review were assessed against the listed seven criteria and classified as high, low, or unclear risk (Table 2). The traffic light system (Figure 1) was used to illustrate the overall risk achieved by each study. Six studies avoided selection bias by randomly assigning participants (31, 75, 77, 86, 87, 89). Secrecy of the randomization scheme and blinding of veterinarians and patient owners was met by only two studies, both demonstrate a low risk of bias (31, 86). Blinding of treatment was achieved by generating two groups of examining and dispensing veterinarians at both study sites and owner's absence at administering the agent (86). For comparison, in the other study, the syringe was blinded so that owners and veterinarians did not know which treatment regime was selected. Blinding was maintained throughout the entire duration of the study (31).
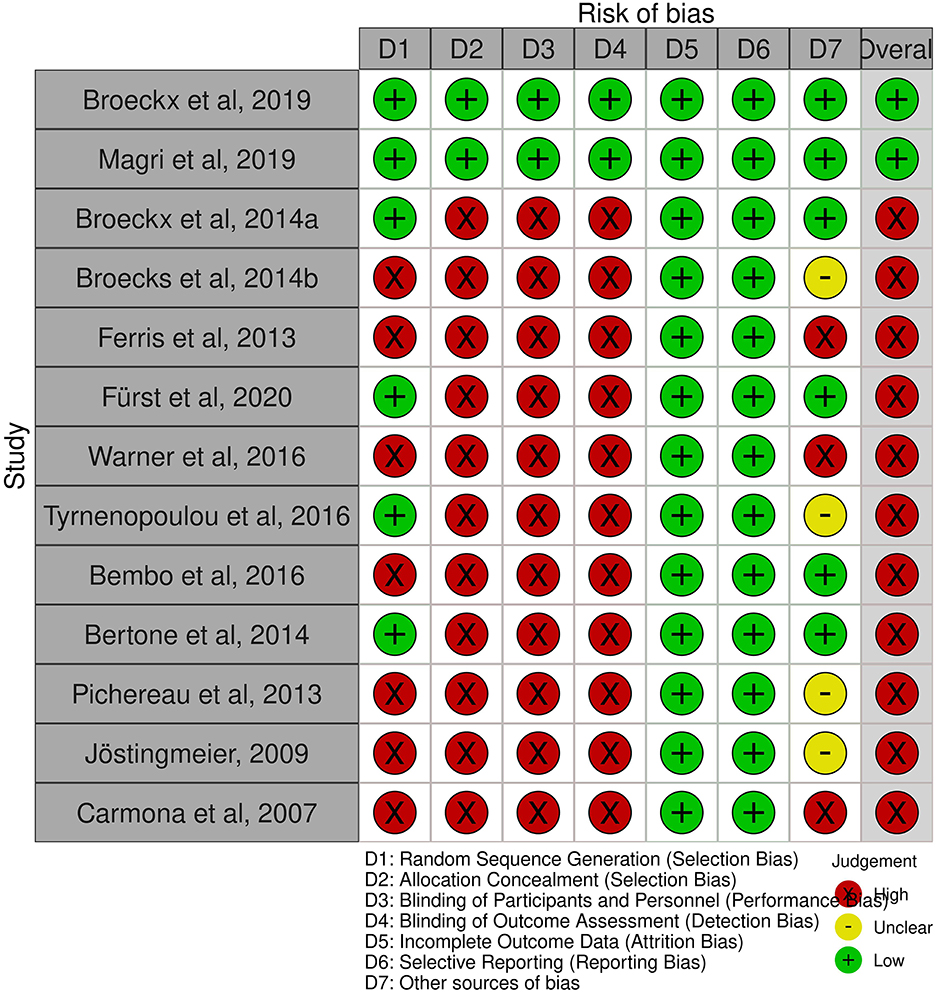
Figure 1. Summary of risk of bias (98).
All studies in the systematic review reported study discontinuations and missing outcome data. Therefore, almost all studies were considered to have a low risk of incomplete results and selective reporting. In addition, most studies used an owner questionnaire for long-term follow-up. In summary, eleven studies are at a high risk of bias (18, 32, 40, 75, 77, 87–92), due to the lack of blinding. The risk of bias graph shows the authors' assessment of each item in percentage (Figure 2). Overall, <25% of the studies included in the systematic review were found to be at a low risk of bias.

Figure 2. Risk of bias (98).
3.2. Systematic review
The flowchart (Figure 3) shows the detailed systematic analysis after initial electronic and manual research, with a total of 271 studies. This resulted in 28 studies being assessed for the qualitative synthesis after an initial review. These studies were further assigned to the defined orthobiologic therapies when treatment of equine OA was the scientific focus (Table 3). Following this, the biologic cell source of the selected 28 studies was assessed and listed (Table 4). This states, that based on their sources of orthobiologic therapeutics, 4 studies were included using blood-derived MSCs either non-induced (native) or chondrogenic-induced (40, 57, 77, 86), 5 studies focused on bone marrow-derived MSCs (18, 29, 99–101) from which 2 studies also included MSCs derived from adipose tissue for comparison (100, 101). Five studies included adipose tissue-derived MSCs only (27, 55, 100–102). The effect of umbilical cord-derived MSCs were studied in 2 publications (31, 61). Eight studies describe the use of cell-based autologous blood products as orthobiologic therapeutics (19, 32, 75, 89–91, 103, 104) and 6 studies a cell-free final therapeutic product (30, 81, 87, 88, 92, 105). The following results were obtained: 8 studies used MSCs as a therapeutic agent for naturally occurring OA (18, 31, 40, 55, 61, 77, 86, 102); 6 studies examined the effect of MSCs after inducing OA (27, 29, 57, 99–101); 14 studies treated with autologous blood products, with 1 study inducing OA (30) while the remaining studies examined naturally occurring OA (19, 32, 75, 81, 87–92, 103–105). Of the 14 MSC-related studies, 12 were placebo controlled (27, 29, 31, 55, 57, 61, 77, 86, 99–102) and 7 studies had an outcome with patient follow-up at least 6 months after treatment initiation (18, 27, 29, 31, 77, 86, 99). Comparatively, of the 14 groups treated with autologous blood products, 7 were placebo controlled (19, 30, 75, 87, 89, 92, 103) and 8 studies had a long-term follow-up (32, 75, 87–92). After screening the studies with the specified inclusion and exclusion criteria, 13 studies were examined for systematic analysis, and listed in Table 1 (18, 31, 32, 40, 75, 77, 86–92).
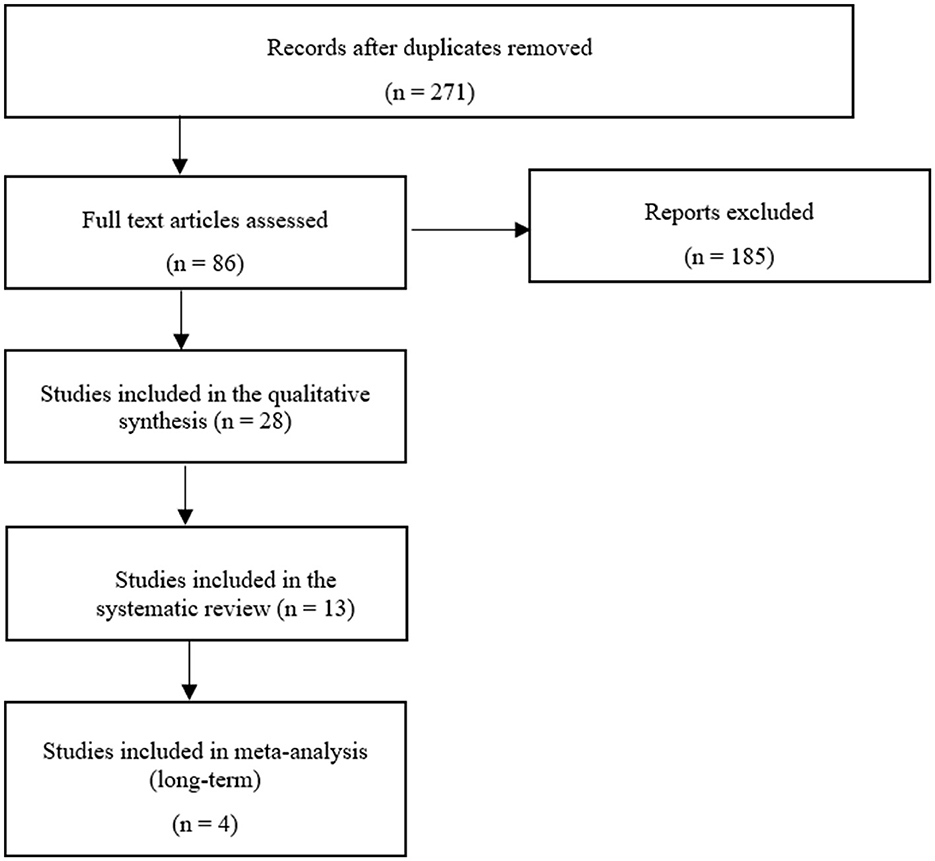
Figure 3. Flow chart showing the methods used for the systemic search (93).
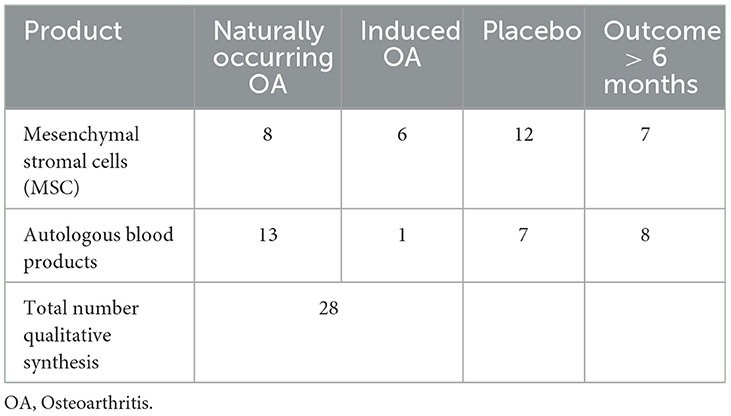
Table 3. Numbers of studies from qualitative synthesis including naturally occurring OA compared to induced OA, trials with a placebo group, and studies with a follow-up time over 6 months.
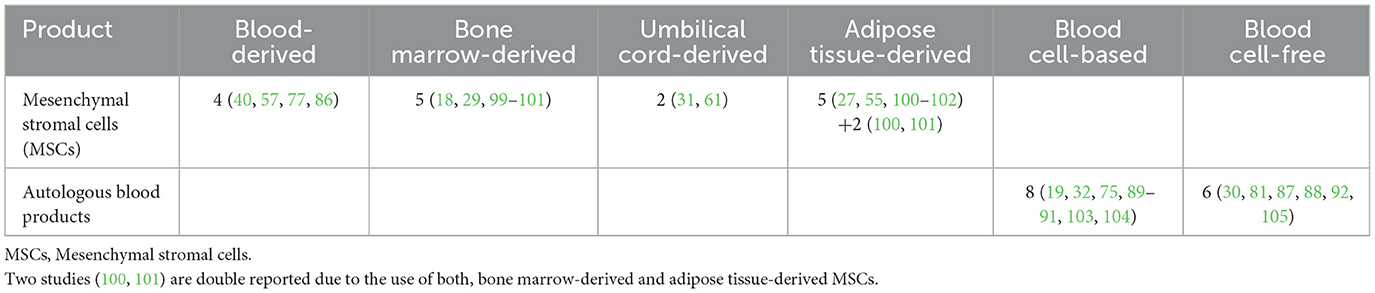
Table 4. Numbers of studies from qualitative synthesis demonstrating the source and composition of orthobiologic therapeutic agents.
Table 5 lists all 15 studies from the quantitative synthesis that were not included in the systematic review due to the lack of information on the individual degree of lameness. Therefore, an average value was given for the whole group. Other reasons for exclusion were short observation periods, induced OA, or an overall too short observation time.
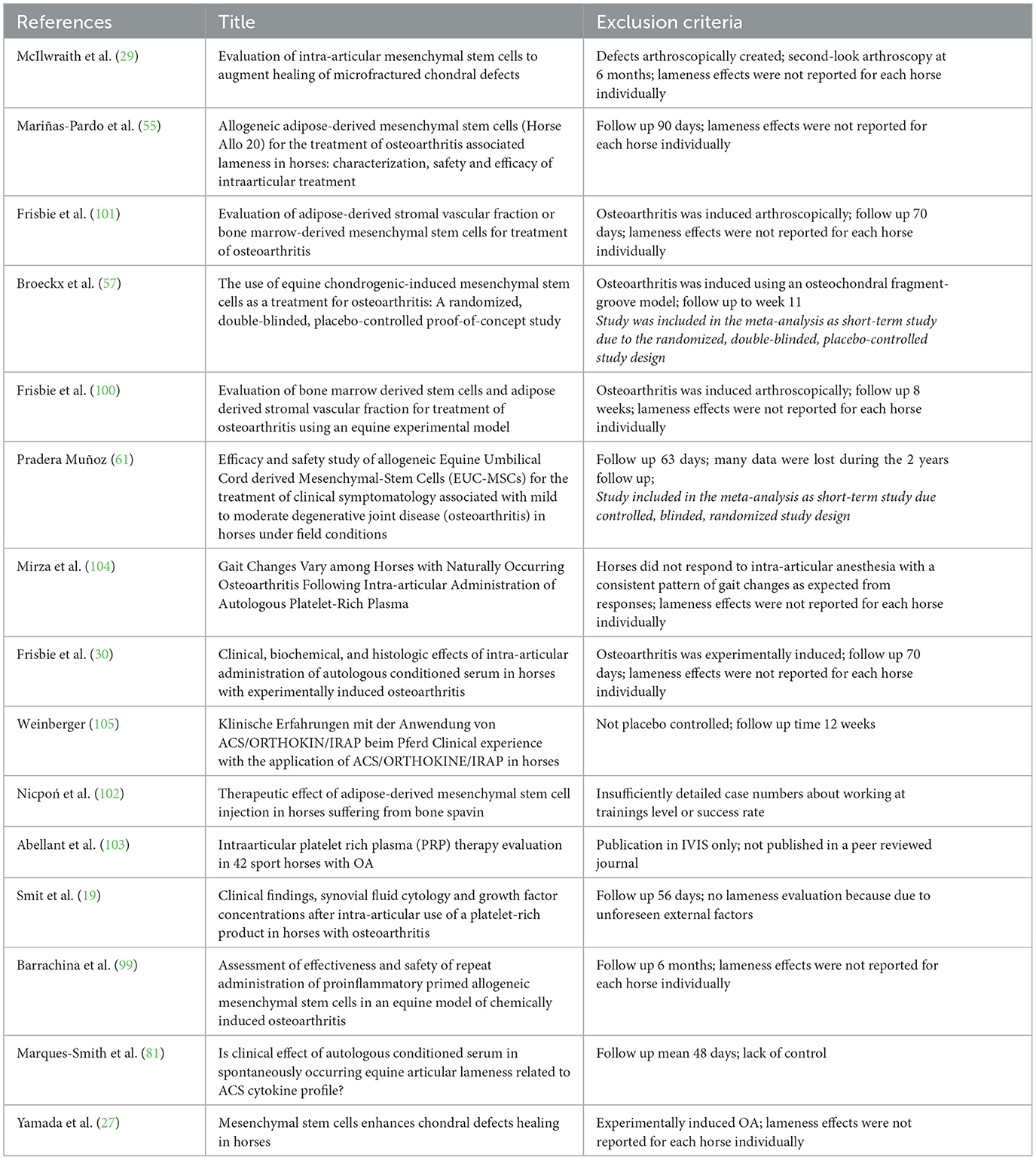
Table 5. Studies from literature review that are not included in the systematic review because of incomplete data referring to the set inclusion criteria.
The age, sex, breed, and disposition of the horses selected for the investigations varied among the studies. Most of the trials in the systematic review examined the effect of orthobiologic therapeutics for the coffin or fetlock joint (Table 1). Five of the 13 studies treated naturally occurring OA with MSCs (18, 31, 40, 77, 86), and 1 study subdivided the treatment groups into f4 subgroups (PRP; native MSCs, native MSCs with PRP; chondrogenic-induced MSCs with PRP) (77). This study used allogenic peripheral blood as MSC source and labeled isolated, non-induced MSCs as “native.” Due to lack of placebo-controlled studies, the PRP-subgroup was compared with the MSC-subgroups of different sources in combination with PRP in the following meta-analysis. The remaining studies treated horses with non-induced (“native”) MSCs, chondrogenic-induced MSCs, umbilical cord-derived MSCs and bone marrow-derived MSCs. None of the 5 reviewed studies using adipose-derived MSCs as orthobiologic agent met the inclusion criteria for meta-analysis (27, 55, 100–102). In general, all MSC-studies demonstrated a heterogenous group regarding manufacturing and processing methods of the particular cell source. Considering possible side effects, 3 studies observed a mild to moderate inflammatory response after intra-articular treatment with MSCs (18, 31, 40). At the final examination, all patients felt well. Therefore, no general side effects were concluded.
Eight of the 13 studies examined treatment outcomes with autologous blood products. Of these, 3 studies used autologous conditioned serum (ACS) products (87, 88, 92). The remaining studies used cellular autologous blood products with a high platelet-rich content (32, 75, 89–91). Mild side effects such as self-limiting local swelling and lameness were noted in 1 ACS study (87).
Concerning the post-treatment, every study designed a particular rehabilitation program. All horses received a 1- (86, 89) to 8-week (31) hand-walking program at the end of treatment, followed by individual retraining. Most studies graded the severity of lameness according to the AAEP (American Association of Equine Practitioners) scoring system (18, 32, 75, 77, 86–90).
Although several placebo-controlled studies were included, most of them lack long-term follow-up or control was not maintained throughout the entire duration of the study. For example, in one RCT, horse owners in the placebo group were offered treatment with autologous protein solution (APS) 14 days after the placebo treatment. The randomized controlled study was well-structured, but the observation time of the control group was too short to be included in our meta-analysis. The APS group improved significantly after the treatment compared with baseline or control group scores (75). In total, 5 studies were placebo controlled over the entire observation period, 4 of which were randomized (77, 86, 87, 89). These studies have also been included in the meta-analysis.
In summary, an average of 65% improvement in lameness grade was achieved after the treatment with intra-articular applied orthobiologic therapeutics, regardless of which therapeutic agent was used (Figure 4). Eleven studies showed a general positive effect after treatment, with horses working at trainings level or horses returning to competition (Table 1). Two outliers could be detected, that showed initial improvement in the first 7–8 months after treatment but then returned to their initial degree of lameness (32, 89). In both studies, the majority of horses responded positively at the beginning and maintained their high level of performance over a period of at least 6 months. Furthermore, horses showed no adverse reactions. This outcome suggests that platelet lysate (PL) and autologous platelet concentrate (PC) can be an efficient short-term therapy for horses suffering from OA (32, 89) (Figure 5). Looking at the average proportions without outliers, 80% of the horses involved in the studies showed lameness reduction after treatment with orthobiologic therapies (Figure 5).
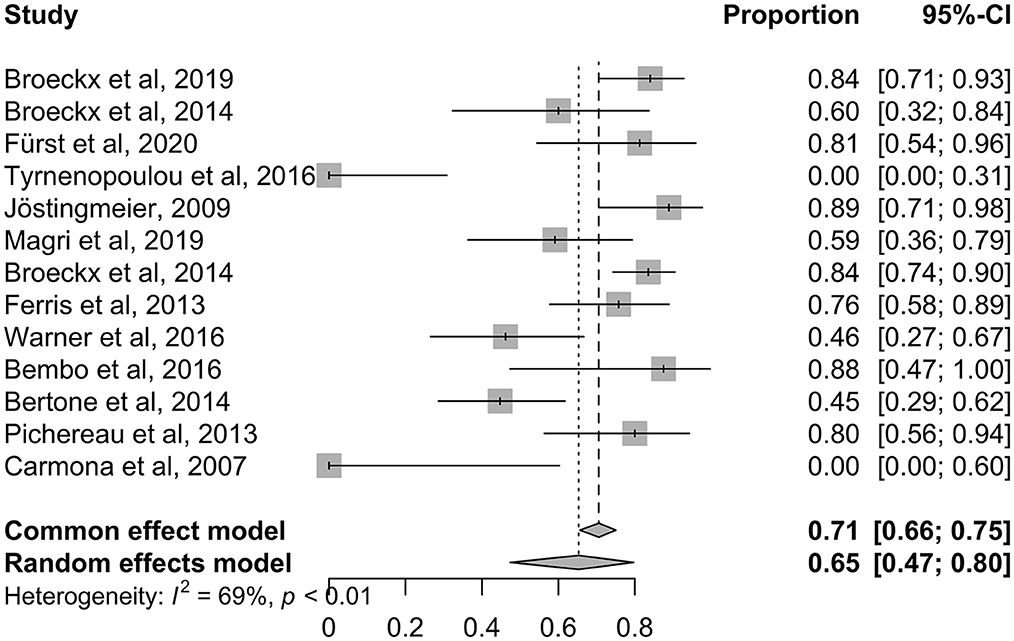
Figure 4. Shows the ratios of the individual studies in the systematic analysis graphically with outliers.
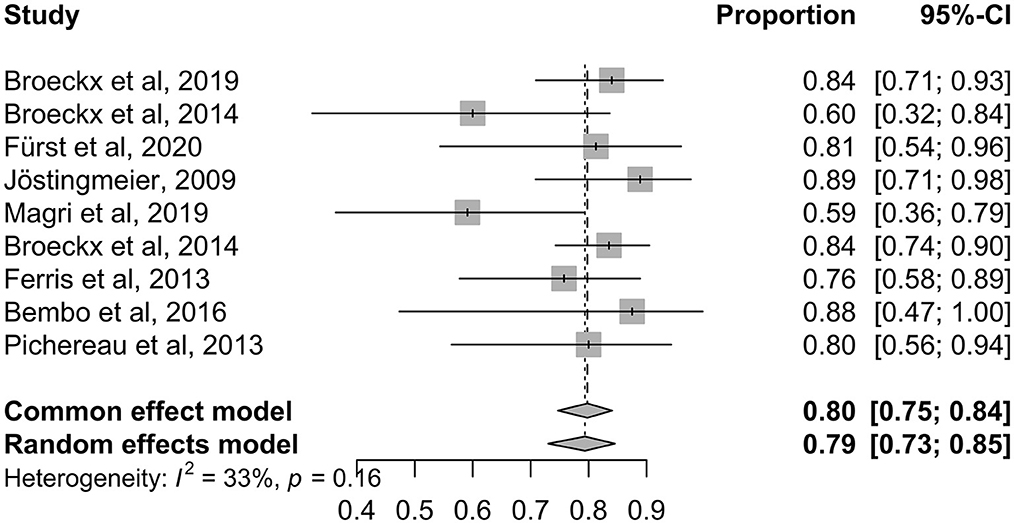
Figure 5. Shows the ratios of the individual studies in the systematic analysis graphically without outliers.
As previously stated, in 1 study treatment groups were divided into 4 subgroups, and interestingly, the group treated with chondrogenic induced MSCs had the most successful result, with 80% lameness-free horses and horses working at trainings level (77). Another pilot study compared non-induced (native) MSCs with chondrogenic induced MSCs, both in combination with PRP. This resulted in a higher average score for the beneficial effects using chondrogenic induced MSCs. However, the result was statistically non-significant (40).
Promising results with MSCs and ACS were justified over a 24-months follow-up period (18, 88). After intra-articular administration of MSCs postoperatively after arthroscopy of the stifle, 42% of horses returned to their previous level of work, and 33% returned to work after a mean follow-up period of 24 months (18). In a retrospective study from Warner et al. 31% of the horses returned to their previous level of work and 15% performed at exercise level after a period of at least 2 years following the ACS treatment of the coffin joint (88). Both studies were not blinded and without a control group, which significantly limits their validity. However, both studies provide indication of a long-term effect of MSC and ACS treatment in OA.
3.3. Meta-analysis
Four RCTs (77, 86, 87, 89) out of the 13 trials were included in the long-term meta-analysis with a follow-up time >6 months. The control groups were treated with saline (86, 89), other orthobiologic therapeutic agents (PRP) (77) or corticosteroids and hyaluronic acid (87). All studies included horses of different breed, sex, age, and level of performance. Moreover, the diagnosed and treated OA occurred in different joints, ranging from low to high motion joints (Table 1). Due to the scarcity of studies, no restrictions were made.
Figure 6 demonstrates a forest plot with outcomes at different time points. The focus was the set inclusion criteria of a follow-up period >6 months. Three studies (77, 86, 87) showed a positive impact of orthobiologic therapeutics compared to their control groups.

Figure 6. Forest plot showing results of selected studies using a meta-analysis to compare lameness reduction of experimental and control. The common effect model and the random effects model are shown. Depending on heterogeneity (I2 > 50%) the random effects model was used for studies with long-term follow-up. The greater the squares, the more participants included the study. The size of the squares is proportional to the weight of the study. The whiskers correspond to the 95% confidence interval (Cl).
One study reported a regression to its initial lameness level after an observation period of 1 year (89). In this study, no side effects were noted in the first 6 months after treatment and 9 out of 10 horses treated with PL returned to their normal activity. Lameness recurred from the 7th month, and all horses relapsed to their initial degree of lameness at the end of the study period. This study illustrates the correlation between duration of follow-up and recurrence of lameness. Within 6 months, horses returned to their previous level of performance. However, all horses relapsed to their initial degree of lameness, therefore only a temporary positive effect could be observed.
The forest plot illustrates the common effect model and the random effects model and whether heterogeneity could be stated as significant. Data demonstrating I2 > 50% were assigned to the random effects model. As demonstrated in the last row of the plot, all long-term follow-up studies showed moderate heterogeneity with I2 = 55% and p = 0.11 (Figure 6). A random effects model was used due to the assumption of moderate differences among study design and implementation in the clinical studies.
An odds ratio (OR) of 1 indicates no difference between the treatment and control group, whereas an OR > 1 indicates that lameness is more likely to be reduced in the experimental group. All studies with an OR values higher than 1 favor the experimental group (Figure 6: OR 17.02; 95% CI: 8.5474 to 33.8849 p < 0.0001). None of the studies crossed the line into ineffectiveness, suggesting that the treatment effect was estimated to be similar across studies.
The diamond square represents the average of all individual studies. If the limit of ineffectiveness is not exceeded, a significant difference in lameness reduction between the experimental and control groups is stated. It can be summarized, that the included orthobiologic therapeutics are safe and showed significant improvement in lameness reduction compared to their control groups. Three studies (77, 86, 87) showed a long and constant improvement over 6 months.
One short-term RCT was included in the forest plot for comparative reasons (57). We assessed at what time point the trial showed significance for treatment with an orthobiologic therapy and how effective the short-term trial was. Treatment success with chondrogenic induced MSCs in an induced OA model was demonstrated to be a time dependent factor, with decreasing lameness levels from 2 weeks after treatment throughout the observation period of 11 weeks (57).
In summary, the use of intra-articular administered orthobiologic therapeutic agents show an incidence of lameness reduction by 73% compared to the control in the long-term follow-up, whereas in the control group lameness was reduced by 17% (77, 86, 87, 89). According to the included studies, horses with naturally occurring OA demonstrated a significantly reduced degree of lameness after intra-articular treatment with orthobiologic therapeutics compared with the control in the long-term follow-up.
3.4. Publication bias
Publication bias occurs when the probability of a study being published depends significantly on its outcome. This means that it is more likely, that a study will be published if the results are consistent with the hypothesis or if the study results are significant (106).
The occurrence of publication bias can be tested by creating a funnel plot. Ideally, the individual data points form a symmetrical, inverted funnel. On the x-axis, the treatment effect is plotted against the study size on the y-axis. The largest studies are located at the top of the graph and plotted near the average. The smaller studies are distributed on both sides of the average and lie close to the x-axis.
The funnel plot showed almost the desired symmetrical shape, with the studies close to the midline. It is important to note, that studies that conducted lameness examinations at different time points are considered as individual studies. For instance, a study by Broeckx et al. (57) is plotted 4 times in the funnel plot, at each study time point. The hypothesis that studies with a smaller number of participants are more likely to be in the bottom range is correct. Overall, the publication bias can be classified as low (Figure 7).
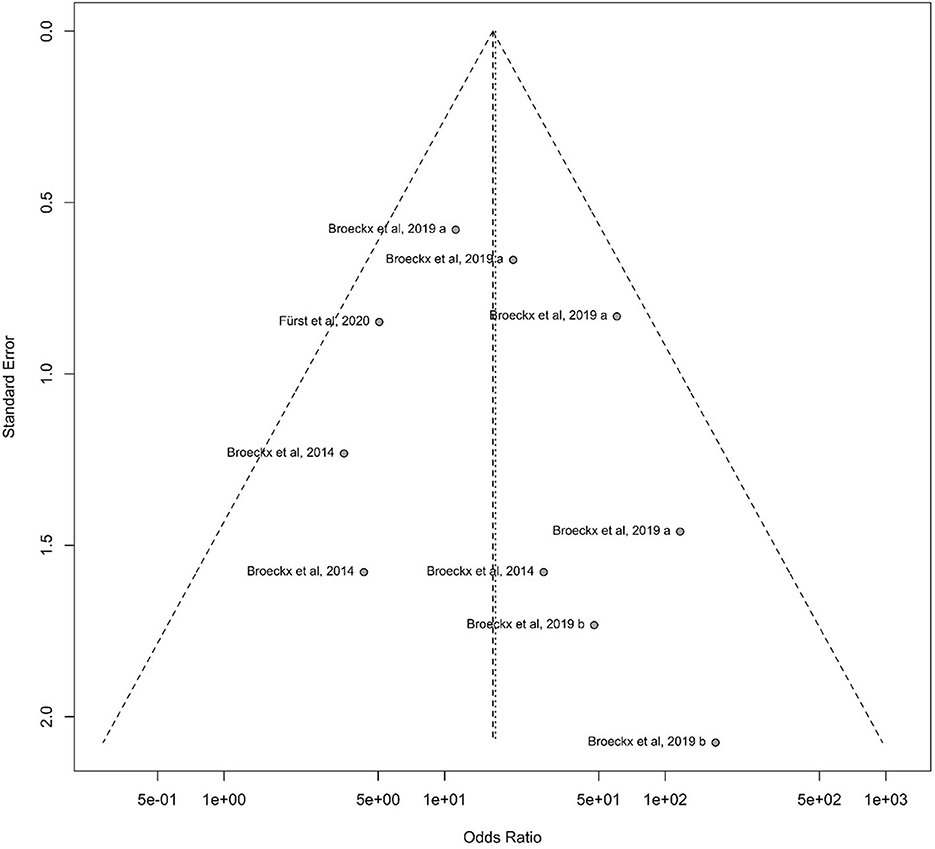
Figure 7. Funnel diagram demonstrating the standard error to the odds ratio according to the study design of participating horses. On the x-axis, the treatment effect is plotted against the study size on the y-axis. Largest studies are located at the top of the graph and plotted near the average. Smaller studies will spread on both sides of the average and lie close to the x-axis.
4. Discussion
OA is a leading cause of pain, disability and economic impact on the health system worldwide (3, 107). The demand for regenerative medicine to treat OA is steadily increasing in human and veterinary medicine. Therefore, it is important to obtain an up-to-date state of knowledge and to compare previous studies using meta-analysis (108, 109). There are two main reasons why the equine model is a suitable model for human medicine. First, horses spontaneously develop chondral defects and age-/trauma-induced OA that are very similar to humans (15). Second, there are numerous in vitro and in vivo studies, some even with experimentally induced OA, in which the therapeutic index of orthobiologic therapeutics can be assessed (109).
To our knowledge, this is the first meta-analysis comparing orthobiologic therapies with its control group in long-term in vivo studies for the treatment of OA. Overall, many topic-related articles were recorded, but of the 86 articles fully screened, only 13 (15%) were useful for the systematic review after passing the inclusion and exclusion criteria. Finally, only 4 (5%) of these studies could meet the criteria for the meta-analysis (Figure 3). This shows that although there is a great research interest in this topic area only a few studies examine long-term success compared with a control group. The result of this meta-analysis showed comparable studies with a moderate heterogeneity, which overall demonstrate a positive result in terms of orthobiologic therapy (Figure 6). By demonstrating the therapeutic efficiency of the mentioned therapies in the long-term in clinical cases of OA, the application of such therapeutics in equine veterinary practice is justifiable.
Major limitations were, that the number of comparable studies that met the inclusion criteria were low. Most studies suitable for systematic review lacked a control group. Another shortcoming was the absence of a uniform treatment pattern in the controlled trials. All controlled studies treated with different placebos [saline (86, 89), other potentially regenerative agents (77), cortisone and hyaluronic acid (87)]. Compared to other meta-analysis and systematic reviews, the lack of an adequate placebo group was also the main point of criticism (70, 110). From an animal welfare perspective, it is unethical to not treat animals suffering from joint-related pain. Moreover, it is difficult to find a homogenous control group, in which all horses are treated with the same agent. However, it is almost impossible to convince horse owners to participate in a long-term study without them knowing whether they will be receiving a placebo or a treatment. Especially, since there is a real chance that their horse will miss out on a potential therapy. In general, all privately owned horse owners wanted to be assured that everything was being done to get the horse well and back to work.
The lack of blinded study designs in RCTs is noticeable. Overall, only 2 long and 2 short-term studies were fully blinded (31, 57, 61, 86). Reasons for this include the high effort of blinding all medical staff and owners. In addition, it is often difficult to obtain permission from horse owners for placebo-controlled and blinded study participation for the entire study duration. The absence of blinding is often associated with excessive reasoning, especially when assessing subjective outcomes (111, 112). The lack of blinding is the main reason for the high risk of bias.
Another serious point of criticism is the difference of the joint localization. Due to the lack of studies, no restriction was made here, and all long-term studies could participate, regardless of the joint in which the OA occurred. The emphasis was placed on lameness reduction in a long-term follow-up. Of course, from a medical point of view, there is criticism on the comparability of the individual joints. No distinction was made between chronic or acute OA, mild or advanced OA. The absence of a homogenous concept shows the need for further studies. To avoid heterogenicity, a meta-analysis with naturally occurring chronic OA in the same joints would be useful.
The systematic analysis showed a positive result of 80% in all studies, except for the two outliers. In other words, over 80% of the horses treated with orthobiologic therapies showed a reduction in their degree of lameness. Lameness evaluation was uniformly investigated in 9 studies using the AAEP score (18, 32, 75, 77, 86–90); the other studies used their own clinical scores. In most studies, the endpoint survey was conducted using an owner survey. Although the owners' assessment is subjective, comparability can be established because health status and degree of lameness are collected in relation to the performance level before and after treatment.
Two short-term studies were double-blind, randomized, and placebo-controlled with a low potential for bias. This showed that a very safe study design is possible in studies with a shorter control period, as blinding can be maintained (57, 61). In summary, significant lameness improvement with orthobiologic therapy was observed in both groups from the 2nd (57) and 5th (61) week after treatment. In these models, accurate experimental design and maintenance of blinding is facilitated. However, most animal models are limited to a period of 8 to 12 weeks (Table 5). In addition, many studies reported only an average or mean values for lameness evaluation. Individual results are usually missing here (30, 57, 100, 101). Due to a missing randomization scheme and blinding, many studies show a high potential for bias.
Overall, moderate heterogeneity among the studies in the meta-analysis has been described. All product- and treatment-specific factors mentioned above have an unknown impact on treatment success. The aim of this study is to draw attention to the importance of a correct study design. The results indicate a significant improvement with orthobiologic therapies compared to their control for at least several months. However, due to the paucity of studies with long-term and placebo-controlled follow-up, no concrete statement can be made regarding effectiveness of specific orthobiologics, exemplary the preference of MSCs to autologous blood products and vice versa. However, equine practitioners can rely on a safe and effective treatment option when using orthobiologics but thereof no recommendation regarding specific products can be derived. In the future, more randomized, controlled, blinded studies and long-term studies are needed to make further informed conclusions. It is crucial to determine the exact composition and effect of all orthobiologic therapeutics in further studies to develop effective and standardized treatment protocols.
5. Conclusion
Apart from the limited and sometimes controversial findings, the systematic review and meta-analysis showed an overall support toward the orthobiologic therapeutic application. After treatment with orthobiologics, a beneficial effect on OA was demonstrated without significant adverse effects. Satisfactory effects were examined over a period of 6–12 months, with a high success rate. Limitations lie within the lack of homogeneous standardization protocols and outcome measurements. Future studies should focus on standardized study designs regarding patient details, treated joints and type of orthobiologic substances in RCTs to allow comparable conclusions about the long-term effect of intra-articular administered orthobiologic therapeutics.
Author contributions
AM, AT, YZ, and SR constructed the manuscript. AT, SR, and WB edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Open Access Publishing Fund of Leipzig University and supported by the German Research Foundation within the Program Open Access Publication Funding and the Junior Scientist Support Program financed by the Freundeskreis Tiermedizin, the Faculty of Veterinary Medicine, and by Ceva Santé Animale.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuyinu EL, Narayanan G, Nair LS, Laurencin CT. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. (2016) 11:19. doi: 10.1186/s13018-016-0346-5
2. McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. (2015) 52:803–18. doi: 10.1177/0300985815588611
3. Roseti L, Desando G, Cavallo C, Petretta M, Grigolo B. Articular cartilage regeneration in osteoarthritis. Cells. (2019) 8:1305. doi: 10.3390/cells8111305
4. Estrada Mcdermott J, Pezzanite L, Goodrich L, Santangelo K, Chow L, Dow S, et al. Role of Innate immunity in initiation and progression of osteoarthritis, with emphasis on horses. Animals. (2021) 11:3247. doi: 10.3390/ani11113247
5. Caron JP, Genovese RL. Principles and Practices of Joint Disease Treatment. In:M. Ross, S. Dyson, editors. Diagnosis and Management of Lameness in the Horse. Philadelphia: W.B. Saunders (2003) 746–64. doi: 10.1016/B978-0-7216-8342-3.50092-9
6. Mahmoud EE, Hassaneen ASA, Noby MA, Mawas AS, Abdel-Hady ANA. Equine osteoarthritis: an overview of different treatment strategies. SVU Int J Vet Sci. (2021) 4:85–96. doi: 10.21608/svu.2021.57242.1099
7. van Weeren PR, Back W. Musculoskeletal disease in aged horses and its management. Vet Clin North Am Equine Pract. (2016) 32:229–47. doi: 10.1016/j.cveq.2016.04.003
8. Morris EA, Seeherman HJ. Clinical evaluation of poor performance in the racehorse: the results of 275 evaluations. Equine Vet J. (1991) 23:169–74. doi: 10.1111/j.2042-3306.1991.tb02749.x
9. Niemelä TM, Tulamo RM, Hielm-Björkman AK. A randomised, double-blinded, placebo-controlled clinical study on intra-articular hyaluronan treatment in equine lameness originating from the metacarpophalangeal joint. BMC Vet Res. (2016) 12:60. doi: 10.1186/s12917-016-0687-7
10. Neundorf RH, Lowerison MB, Cruz AM, Thomason JJ, McEwen BJ, Hurtig MB. Determination of the prevalence and severity of metacarpophalangeal joint osteoarthritis in Thoroughbred racehorses via quantitative macroscopic evaluation. Am J Vet Res. (2010) 71:1284–93. doi: 10.2460/ajvr.71.11.1284
11. Bertuglia A, Pagliara E, Grego E, Ricci A, Brkljaca-Bottegaro N. Pro-inflammatory cytokines and structural biomarkers are effective to categorize osteoarthritis phenotype and progression in Standardbred racehorses over five years of racing career. BMC Vet Res. (2016) 12:246. doi: 10.1186/s12917-016-0873-7
12. Auer JA, Fackelmann GE. Treatment of degenerative joint disease of the horse: a review and commentary. Vet Surg. (1981) 10:80–9. doi: 10.1111/j.1532-950X.1981.tb00635.x
13. Yamada ALM, Pinheiro M, Marsiglia MF, Hagen SCF, Baccarin RYA, da Silva LCLC. Ultrasound and clinical findings in the metacarpophalangeal joint assessment of show jumping horses in training. J Vet Sci. (2020) 21:e21. doi: 10.4142/jvs.2020.21.e21
14. Baccarin RYA, Seidel SRT, Michelacci YM, Tokawa PKA, Oliveira TM. Osteoarthritis: a common disease that should be avoided in the athletic horse's life. Anim Front Rev Mag Anim Agric. (2022) 12:25. doi: 10.1093/af/vfac026
15. McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. (2012) 1:297–309. doi: 10.1302/2046-3758.111.2000132
16. Cantley CEL, Firth EC, Delahunt JW, Pfeiffer DU, Thompson KG. Naturally occurring osteoarthritis in the metacarpophalangeal joints of wild horses. Equine Vet J. (1999) 31:73–81. doi: 10.1111/j.2042-3306.1999.tb03794.x
17. Panizzi L, Barber SM, Lang HM, Carmalt JL. Carpometacarpal osteoarthritis in thirty-three horses. Vet Surg. (2009) 38:998–1005. doi: 10.1111/j.1532-950X.2009.00589.x
18. Ferris DJ, Frisbie DD, Kisiday JD, Mcilwraith CW, Hague BA, Major MD, et al. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet Surg. (2014) 43:255–65. doi: 10.1111/j.1532-950X.2014.12100.x
19. Smit Y, Marais HJ, Thompson PN, Mahne AT, Goddard A. Clinical findings, synovial fluid cytology and growth factor concentrations after intra-articular use of a platelet-rich product in horses with osteoarthritis. J S Afr Vet Assoc. (2019) 90:1019–9128. doi: 10.4102/jsava.v90i0.1721
20. McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr Cartil. (2010) 18 (Suppl 3):S93–105. doi: 10.1016/j.joca.2010.05.031
21. Goodrich LR, Nixon AJ. Medical treatment of osteoarthritis in the horse - a review. Vet J. (2006) 171:51–69. doi: 10.1016/j.tvjl.2004.07.008
22. Contino EK. Management and rehabilitation of joint disease in sport horses. Vet Clin North Am Equine Pract. (2018) 34:345–58. doi: 10.1016/j.cveq.2018.04.007
23. Carmona JU, Ríos DL, López C, Álvarez ME, Pérez JE, Bohórquez ME. In vitro effects of platelet-rich gel supernatants on histology and chondrocyte apoptosis scores, hyaluronan release and gene expression of equine cartilage explants challenged with lipopolysaccharide. BMC Vet Res. (2016) 12:135. doi: 10.1186/s12917-016-0759-8
24. Colbath AC, Dow SW, Phillips JN, McIlwraith CW, Goodrich LR. Autologous and allogeneic equine mesenchymal stem cells exhibit equivalent immunomodulatory properties in vitro. Stem Cells Dev. (2017) 26:503–11. doi: 10.1089/scd.2016.0266
25. Blázquez R, Sánchez-Margallo FM, Reinecke J, Álvarez V, López E, Marinaro F, et al. Conditioned serum enhances the chondrogenic and immunomodulatory behavior of mesenchymal stem cells. Front Pharmacol. (2019) 10:699. doi: 10.3389/fphar.2019.00699
26. Hraha TH, Doremus KM, Mcilwraith CW, Frisbie DD. Autologous conditioned serum: the comparative cytokine profiles of two commercial methods (IRAP and IRAP II) using equine blood. Equine Vet J. (2011) 43:516–21. doi: 10.1111/j.2042-3306.2010.00321.x
27. Yamada ALM, Carvalho A de M, Moroz A, Deffune E, Watanabe MJ, Hussni CA, et al. Mesenchymal stem cell enhances chondral defects healing in horses. Stem Cell Discov. (2013) 03:218–25. doi: 10.4236/scd.2013.34027
28. Frisbie DD, Kawcak CE, McIlwraith CW. 520 evaluation of autologous conditioned serum using an experimental model of equine osteoarthritis. Osteoarthr Cartil. (2008) 16:S222–3. doi: 10.1016/S1063-4584(08)60559-2
29. McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. (2011) 27:1552–61. doi: 10.1016/j.arthro.2011.06.002
30. Frisbie DD, Kawcak CE, Werpy NM, Park RD, Mcllwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. (2007) 68:290–6. doi: 10.2460/ajvr.68.3.290
31. Magri C, Schramme M, Febre M, Cauvin E, Labadie F, Saulnier N, et al. Comparison of efficacy and safety of single versus repeated intra-articular injection of allogeneic neonatal mesenchymal stem cells for treatment of osteoarthritis of the metacarpophalangeal/metatarsophalangeal joint in horses: a clinical pilot study. PLoS ONE. (2019) 14:e0221317. doi: 10.1371/journal.pone.0221317
32. Carmona JU, Argüelles D, Climent F, Prades M. Autologous platelet concentrates as a treatment of horses with osteoarthritis: a preliminary pilot clinical study. J Equine Vet Sci. (2007) 27:167–70. doi: 10.1016/j.jevs.2007.02.007
33. Camargo Garbin L, Morris MJ. A comparative review of autologous conditioned serum and autologous protein solution for treatment of osteoarthritis in horses. Front Vet Sci. (2021) 8:82. doi: 10.3389/fvets.2021.602978
34. Nakagami H, Morishita R, Maeda K, Kikuchi Y, Ogihara T, Kaneda Y. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. (2006) 13:77–81. doi: 10.5551/jat.13.77
35. Roth SP, Burk J, Brehm W, Troillet A, MSC. in tendon and joint disease: the context-sensitive link between targets and therapeutic mechanisms. Front Bioeng Biotechnol. (2022) 10:440. doi: 10.3389/fbioe.2022.855095
36. Aldrich ED, Cui X, Murphy CA, Lim KS, Hooper GJ, McIlwraith CW, et al. Allogeneic mesenchymal stromal cells for cartilage regeneration: a review of in vitro evaluation, clinical experience, and translational opportunities. Stem Cells Transl Med. (2021) 10:1500–15. doi: 10.1002/sctm.20-0552
37. Yin Z, Yang X, Jiang Y, Xing L, Xu Y, Lu Y, et al. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: an in vitro study. J Biomater Appl. (2014) 28:1039–50. doi: 10.1177/0885328213492573
38. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. (2003) 48:3464–74. doi: 10.1002/art.11365
39. Kriston-Pál É, Haracska L, Cooper P, Kiss-Tóth E, Szukacsov V, Monostori É, et al. Regenerative approach to canine osteoarthritis using allogeneic, adipose-derived mesenchymal stem cells. Safety results of a long-term follow-up. Front Vet Sci. (2020) 7:510. doi: 10.3389/fvets.2020.00510
40. Broeckx S, Suls M, Beerts C, Vandenberghe A, Seys B, Wuertz-Kozak K, et al. Allogenic mesenchymal stem cells as a treatment for equine degenerative joint disease: a pilot study. Curr Stem Cell Res Ther. (2014) 9:497–503. doi: 10.2174/1574888X09666140826110601
41. Roth SP, Brehm W, Troillet A. Cell-based therapeutic strategies for osteoarthritis in equine patients: Basic knowledge for clinical practitioners. Tierarztl Prax Ausgabe G Grosstiere Nutztiere. (2021) 49:189–202. doi: 10.1055/a-1482-7752
42. Bogers SH. Cell-based therapies for joint disease in veterinary medicine: what we have learned and what we need to know. Front Vet Sci. (2018) 5:1. doi: 10.3389/fvets.2018.00070
43. Voga M, Adamic N, Vengust M, Majdic G. stem cells in veterinary medicine—current state and treatment options. Front Vet Sci. (2020) 7:278. doi: 10.3389/fvets.2020.00278
44. Maniar HH, Tawari AA, Suk M, Horwitz DS. The current role of stem cells in orthopaedic surgery. Malaysian Orthop J. (2015) 9:1. doi: 10.5704/MOJ.1511.016
45. Capparè P, Tetè G, Sberna MT, Panina-Bordignon P. The emerging role of stem cells in regenerative dentistry. Curr Gene Ther. (2020) 20:259–68. doi: 10.2174/1566523220999200818115803
46. Mokbel AN, El Tookhy OS, Shamaa AA, Rashed LA, Sabry D, El Sayed AM. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. (2011) 12:259. doi: 10.1186/1471-2474-12-259
47. Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane database Syst Rev. (2014) 2014:CD007888. doi: 10.1002/14651858.CD007888.pub2
48. Aligholi H, Safahani M, Asadi-Pooya AA. Stem cell therapy in patients with epilepsy: a systematic review. Clin Neurol Neurosurg. (2021) 200:106416. doi: 10.1016/j.clineuro.2020.106416
49. MacDonald ES, Barrett JG. The potential of mesenchymal stem cells to treat systemic inflammation in horses. Front Vet Sci. (2020) 6:507. doi: 10.3389/fvets.2019.00507
50. Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. (2021) 13:448.
51. Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. (2005) 7:393–5. doi: 10.1080/14653240500319234
52. Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. (2011) 9:11–5. doi: 10.1016/j.stem.2011.06.008
53. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. (2017) 6:1445–51. doi: 10.1002/sctm.17-0051
54. Lawver J, Thaler R. Ultrasound-guided lipoaspiration for mesenchymal stromal cell harvest in the horse. Equine Vet Educ. (2016) 28:23–9. doi: 10.1111/eve.12398
55. Mariñas-Pardo L, García-Castro J, Rodríguez-Hurtado I, Rodríguez-García MI, Núñez-Naveira L, Hermida-Prieto M. Allogeneic adipose-derived mesenchymal stem cells (Horse Allo 20) for the treatment of osteoarthritis-associated lameness in horses: Characterization, safety, and efficacy of intra-articular treatment. Stem Cells Dev. (2018) 27:1147–60. doi: 10.1089/scd.2018.0074
56. Brehm W, Burk J, Delling U, Gittel C, Ribitsch I. Stem cell-based tissue engineering in veterinary orthopaedics. Cell Tissue Res. (2012) 347:677–88. doi: 10.1007/s00441-011-1316-1
57. Broeckx SY, Martens AM, Bertone AL, Van Brantegem L, Duchateau L, Van Hecke L, et al. The use of equine chondrogenic-induced mesenchymal stem cells as a treatment for osteoarthritis: a randomised, double-blinded, placebo-controlled proof-of-concept study. Equine Vet J. (2019) 51:787–94. doi: 10.1111/evj.13089
58. Broeckx SY, Spaas JH, Chiers K, Duchateau L, Van Hecke L, Van Brantegem L, et al. Equine allogeneic chondrogenic induced mesenchymal stem cells: a GCP target animal safety and biodistribution study. Res Vet Sci. (2018) 117:246–54. doi: 10.1016/j.rvsc.2017.12.018
59. CVMP. Arti-Cell Forte (Chondrogenic Induced Equine Allogeneic Peripheral Blood-Derived Mesenchymal Stem Cells). (2018). Available online at: https://www.ema.europa.eu/en/documents/overview/arti-cell-forte-epar-medicine-overview_en.pdf (accessed November 26, 2022).
60. CVMP. HorStem (Equine Umbilical Cord Mesenchymal Stem Cells) What is HorStem and what is it used for? How is HorStem used? How does HorStem work? (2019). Available online at: https://www.ema.europa.eu/en/documents/smop-initial/cvmp-summary-positive-opinion-horstem_en.pdf (accessed November 26, 2022).
61. Pradera Muñoz A. Efficacy and safety study of allogeneic Equine Umbilical Cord derived Mesenchymal Stem Cells (EUC-MSCs) for the treatment of clinical symptomatology associated with mild to moderate degenerative joint disease (osteoarthritis) in horses under field conditions. (Dissertation). Madrid, Spain, Universidad Autónoma de Madrid. (2019).
62. Schnabel L V, Fortier LA, Wayne McIlwraith C, Nobert KM. Therapeutic use of stem cells in horses: which type, how, and when? Vet J. (2013) 197:570–7. doi: 10.1016/j.tvjl.2013.04.018
63. Bruno I, Martinez R, Sanchez A, Friddle C, McClure SR. Characterization of nucleated cells from equine adipose tissue and bone marrow aspirate processed for point-of-care use. J Equine Vet Sci. (2014) 34:1118–27. doi: 10.1016/j.jevs.2014.06.023
64. Taylor SE, Clegg PD. Collection and propagation methods for mesenchymal stromal cells. Vet Clin North Am Equine Pract. (2011) 27:263–74. doi: 10.1016/j.cveq.2011.05.003
65. Koch TG, Thomsen PD, Betts DH. Improved isolation protocol for equine cord blood-derived mesenchymal stromal cells. Cytotherapy. (2009) 11:443–7. doi: 10.1080/14653240902887259
66. Bourzac C, Smith LC, Vincent P, Beauchamp G, Lavoie JP, Laverty S. Isolation of equine bone marrow-derived mesenchymal stem cells: a comparison between three protocols. Equine Vet J. (2010) 42:519–27. doi: 10.1111/j.2042-3306.2010.00098.x
67. Ionita CR, Troillet AR, Vahlenkamp TW, Winter K, Brehm W, Ionita JC. Comparison of humoral insulin-like growth factor-1, platelet-derived growth factor-BB, transforming growth factor-β1, and interleukin-1 receptor antagonist concentrations among equine autologous blood-derived preparations. Am J Vet Res. (2016) 77:898–905. doi: 10.2460/ajvr.77.8.898
68. Hessel LN, Bosch G, van Weeren PR, Ionita JC. Equine autologous platelet concentrates: a comparative study between different available systems. Equine Vet J. (2015) 47:319–25. doi: 10.1111/evj.12288
69. Textor J. Autologous biologic treatment for equine musculoskeletal injuries: platelet-rich plasma and IL-1 receptor antagonist protein. Vet Clin North Am Equine Pract. (2011) 27:275–98. doi: 10.1016/j.cveq.2011.05.001
70. Brossi PM, Moreira JJ, Machado TSL, Baccarin RYA. Platelet-rich plasma in orthopedic therapy: a comparative systematic review of clinical and experimental data in equine and human musculoskeletal lesions. BMC Vet Res. (2015) 11:98. doi: 10.1186/s12917-015-0403-z
71. Lee EB, Kim JW, Seo JP. Comparison of the methods for platelet rich plasma preparation in horses. J Anim Sci Technol. (2018) 60:20. doi: 10.1186/s40781-018-0178-4
72. Textor JA, Willits NH, Tablin F. Synovial fluid growth factor and cytokine concentrations after intra-articular injection of a platelet-rich product in horses. Vet J. (2013) 198:217–23. doi: 10.1016/j.tvjl.2013.07.020
73. Agrawal AA. Evolution, current status and advances in application of platelet concentrate in periodontics and implantology. World J Clin Cases. (2017) 5:159. doi: 10.12998/wjcc.v5.i5.159
74. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. (2001) 10:225–8. doi: 10.1097/00008505-200110000-00002
75. Bertone AL, Ishihara A, Zekas LJ, Wellman ML, Lewis KB, Schwarze RA, et al. Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res. (2014) 75:141–51. doi: 10.2460/ajvr.75.2.141
76. Velloso Alvarez A, Boone LH, Braim AP, Taintor JS, Caldwell F, Wright JC, et al. A Survey of Clinical Usage of Non-steroidal Intra-Articular Therapeutics by Equine Practitioners. Front Vet Sci. (2020) 7:579967. doi: 10.3389/fvets.2020.579967
77. Broeckx S, Zimmerman M, Crocetti S, Suls M, Mariën T, Ferguson SJ, et al. Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS ONE. (2014) 9:e85917. doi: 10.1371/journal.pone.0085917
78. Geburek F, Lietzau M, Beineke A, Rohn K, Stadler PM. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res Ther. (2015) 6:126. doi: 10.1186/s13287-015-0115-0
79. Meijer H, Reinecke J, Becker C, Tholen G, Wehling P. The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res. (2003) 52:404–7. doi: 10.1007/s00011-003-1197-1
80. Wehling P, Moser C, Frisbied D, McIlwraith CW, Kawcak CE, Krauspe R, et al. Autologous Conditioned Serum in the Treatment of Orthopedic Diseases. BioDrugs. (2012) 21:323–32. doi: 10.2165/00063030-200721050-00004
81. Marques-Smith P, Kallerud AS, Johansen GM, Boysen P, Jacobsen AM, Reitan KM, et al. Is clinical effect of autologous conditioned serum in spontaneously occurring equine articular lameness related to ACS cytokine profile? BMC Vet Res. (2020) 16:1–9. doi: 10.1186/s12917-020-02391-7
82. Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. (2002) 9:12–20. doi: 10.1038/sj.gt.3301608
83. Lasarzik J, Bondzio A, Rettig M, Estrada R, Klaus C, Ehrle A, et al. Evaluation of two protocols using autologous conditioned serum for intra-articular therapy of equine osteoarthritis—A pilot study monitoring cytokines and cartilage-specific biomarkers. J Equine Vet Sci. (2018) 60:35–42.e2. doi: 10.1016/j.jevs.2016.09.014
84. Ran J, Yang X, Ren Z, Wang J, Dong H. Comparison of intra-articular hyaluronic acid and methylprednisolone for pain management in knee osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. (2018) 53:103–10. doi: 10.1016/j.ijsu.2018.02.065
85. Doll S. Metaanalyse klinischer Studien 1983-2016 zur langfristigen Gebrauchsfähigkeit von Sportpferden nach Behandlung von natürlich entstandenen Erkrankungen der oberflächlichen und der tiefen Beugesehne und des Fesselträgers entweder allein mit kontrollierter Bewegung oder kombiniert mit einem potenziell regenerativen Therapeutikum (Dissertation). Leipzig, Germany, Veterinärmedizinische Fakultät der Universität Leipzig. (2019).
86. Broeckx SY, Seys B, Suls M, Vandenberghe A, Mariën T, Adriaensen E, et al. Equine allogeneic chondrogenic induced mesenchymal stem cells are an effective treatment for degenerative joint disease in horses. Stem Cells Dev. (2019) 28:410–22. doi: 10.1089/scd.2018.0061
87. Fürst A, Veith G, Eisenreich J. A prospective comparison of the GOLDIC ® technique and corticosteroid plus hyaluronic acid injections for arthrogenic lameness in horses. Medicine (Baltimore). (2020) 36:196–204. doi: 10.21836/PEM20200301
88. Warner K, Schulze T, Lischer CJ. Behandlung von osteoarthritis mit ACS (IRAP®) bei 26 pferden-retrospektive studie. Pferdeheilkunde. (2016) 32:241–8. doi: 10.21836/PEM20160307
89. Tyrnenopoulou P, Diakakis N, Karayannopoulou M, Savvas I, Koliakos G. Evaluation of intra-articular injection of autologous platelet lysate (PL) in horses with osteoarthritis of the distal interphalangeal joint. Vet Q. (2016) 36:56–62. doi: 10.1080/01652176.2016.1141257
90. Bembo F, Eraud J, Philandrianos C, Bertrand B, Silvestre A, Veran J, et al. Combined use of platelet rich plasma & micro-fat in sport and race horses with degenerative joint disease: Preliminary clinical study in eight horses. Muscles Ligaments Tendons J. (2016) 6:198–204. doi: 10.11138/mltj/2016.6.2.198
91. Pichereau F, Décory M, Cuevas Ramos G. Autologous platelet concentrate as a treatment for horses with refractory fetlock osteoarthritis. J Equine Vet Sci. (2014) 34:489–93. doi: 10.1016/j.jevs.2013.10.004
92. Jöstingmeier U. Vergleichende Betrachtung des Behandlungserfolges der intraartikulären kombinierten Behandlung mit Natriumhyaluronat und Betamethason mit der intraartikulären Behandlung mit autologem konditionierten Serum (IL-1 Ra) bei Pferden mit positiver Hufgelenkanästhesie- Eine Anwendungsbeobachtung (Dissertation). Berlin, Germany, Freie Universität Berlin. (2009).
93. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
94. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
95. R Core Team. R: A Language Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. (2022). Available online at: https://www.r-project.org (accessed November 8, 2022).
96. Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ Br Med J. (2001) 322:1479. doi: 10.1136/bmj.322.7300.1479
97. Dettori JR, Norvell DC, Chapman JR. Seeing the forest by looking at the trees: how to interpret a meta-analysisforest plot. Glob Spine J. (2021) 11:614. doi: 10.1177/21925682211003889
98. McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12:55–61. doi: 10.1002/jrsm.1411
99. Barrachina L, Remacha AR, Romero A, Vitoria A, Albareda J, Prades M, et al. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet Res. (2018) 14:1–17. doi: 10.1186/s12917-018-1556-3
100. Frisbie DD, Kawcak CE, Werpy NM, McIlwraith CW. 519 evaluation of bone marrow derived stem cells and adipose derived stromal vascular fraction for treatment of osteoarthritis using an equine experimental model. Osteoarthr Cartil. (2008) 16:S222. doi: 10.1016/S1063-4584(08)60558-0
101. Frisbie DD, Kisiday JD, Kawcak CE, Werpy NM, McIlwraith CW. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. (2009) 27:1675–80. doi: 10.1002/jor.20933
102. Nicpoń J, Marycz K, Grzesiak J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol J Vet Sci. (2013) 16:753–4. doi: 10.2478/pjvs-2013-0107
103. Abellanet I, Padres M. Intraarticular platelet rich plasma (PRP) therapy: Evaluation in 42 sport horse with OA. [Conference presentation]. In: Proceedings of the 11th International Congress of the World Equine Veterinary Association. Brazil. (2009). Available online at: https://www.ivis.org/library/weva/weva-internal-congress-brazil-2009/intraarticular-platelet-rich-plasma-prp-therapy (accessed September 28, 2022).
104. Mirza MH, Bommala P, Richbourg HA, Rademacher N, Kearney MT, Lopez MJ. Gait changes vary among horses with naturally occurring osteoarthritis following intra-articular administration of autologous platelet-rich plasma. Front Vet Sci. (2016) 3:1. doi: 10.3389/fvets.2016.00029
105. Weinberger T. Klinische Erfahrungen mit der Anwendung von ACS/ORTHOKIN/IRAP beim Pferd. Pferde Spiegel. (2008) 11:111–4. doi: 10.1055/s-0029-1225792
106. Rothstein HR, Bushman BJ. Publication bias in psychological science: comment on Ferguson and Brannick (2012). Psychol Methods. (2012) 17:129–36. doi: 10.1037/a0027128
107. Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. (2011) 29:802. doi: 10.1002/jor.21359
108. Vitale ND, Vandenbulcke F, Chisari E, Iacono F, Lovato L, Di Matteo B, et al. Innovative regenerative medicine in the management of knee OA: the role of autologous protein solution. J Clin Orthop Trauma. (2019) 10:49–52. doi: 10.1016/j.jcot.2018.08.019
109. Liu TP, Ha P, Xiao CY, Kim SY, Jensen AR, Easley J, et al. Updates on mesenchymal stem cell therapies for articular cartilage regeneration in large animal models. Front cell Dev Biol. (2022) 10:982199. doi: 10.3389/fcell.2022.982199
110. Montano C, Auletta L, Greco A, Costanza D, Coluccia P, Del Prete C, et al. The use of platelet-rich plasma for treatment of tenodesmic lesions in horses: a systematic review and meta-analysis of clinical and experimental data. Animals. (2021) 11:1–18. doi: 10.3390/ani11030793
111. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. (2002) 359:696–700. doi: 10.1016/S0140-6736(02)07816-9
Keywords: horse, degenerative joint disease, regenerative medicine, orthobiologics, autologous blood products, mesenchymal stromal cells (MSC), review–systematic, meta-analysis
Citation: Mayet A, Zablotski Y, Roth SP, Brehm W and Troillet A (2023) Systematic review and meta-analysis of positive long-term effects after intra-articular administration of orthobiologic therapeutics in horses with naturally occurring osteoarthritis. Front. Vet. Sci. 10:1125695. doi: 10.3389/fvets.2023.1125695
Received: 16 December 2022; Accepted: 31 January 2023;
Published: 23 February 2023.
Edited by:
Laura Barrachina, University of Galway, IrelandReviewed by:
Lauren Virginia Schnabel, North Carolina State University, United StatesStefano Grolli, University of Parma, Italy
Copyright © 2023 Mayet, Zablotski, Roth, Brehm and Troillet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonia Troillet,  dHJvaWxsZXRAdmV0bWVkLnVuaS1sZWlwemlnLmRl
dHJvaWxsZXRAdmV0bWVkLnVuaS1sZWlwemlnLmRl
 Anna Mayet1
Anna Mayet1 Yury Zablotski
Yury Zablotski Walter Brehm
Walter Brehm Antonia Troillet
Antonia Troillet