
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 13 February 2023
Sec. Parasitology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1122092
This article is part of the Research Topic Pathobiology, Epidemiology and Control of Protozoan Diseases of Veterinary Importance View all 8 articles
Introduction: Neospora caninum and Toxoplasma gondii are closely related obligate intracellular protozoan parasites. They are considered to be the major causes of infectious abortions and congenital abnormalities in livestock worldwide resulting in huge economic losses. Currently, there are no reports on the prevalence of neosporosis or toxoplasmosis in cattle in Beheira, Egypt's most important region for cattle industry.
Methods: The current study investigated the presence of anti-N. caninum and anti-T. gondii antibodies in apparent healthy cattle from eight localities representing the whole area of Beheira. A total of 358 plasma samples were randomly collected from 6 dairy and 10 beef farms and analyzed by commercially available ELISAs. Production type (dairy versus beef), sex (female vs male), age (< 3 years, 3–5, and > 5 years old), breed (mixed vs Holstein vs Colombian Zebu), and location (various locations) were assessed as risk factors for N. caninum and T. gondii infections.
Results and discussion: Of the samples, 88 (24.6%) and 19 (5.3%) were positive for anti-N. caninum and anti-T. gondii antibodies, respectively, and mixed infection was detected in 7. Of the 16 herds, 6 dairy and 7 beef herds were positive for antibodies to N. caninum. Antibodies to T. gondii were detected in 4, and 5 of dairy and beef herds, respectively. Production type (dairy) and, therewith, sex (female), age (aged over 5 years), and location were considered as risk factors for N. caninum infection. No factors statistically associated with T. gondii infection were identified. Overall, this study provided the first serological detection of N. caninum and T. gondii infections in cattle from Beheira, demonstrating the endemicity of both parasites in the main cattle rearing region of Egypt. This study also confirmed earlier reports of N. caninum being more present in dairy cattle than in beef cattle. Routine monitoring of N. caninum and T. gondii infections and the implementation of control strategies are urgently needed.
Neospora caninum and Toxoplasma gondii are single-celled, obligate intracellular protozoan parasites causing neosporosis and toxoplasmosis, respectively (1). They affect a variety of warm-blooded animals, including humans for T. gondii, and inflict serious clinical and economic losses, especially in the global cattle industry (2, 3). The reproductive stages of T. gondii are exclusively observed in members of the Felidae family (4), while canids serve as final hosts of N. caninum (5, 6). Neospora caninum is recognized as the most common infectious cause of abortion in cows globally (7). Early embryonic death and resorption, abortion, stillbirth, delivery of a calf with deformities, and the birth of healthy carrier offspring are all possible outcomes of N. caninum infection in pregnant cows (8). Early culling of seropositive cattle has been recorded (9) due to increased veterinary medical treatment expenditures and a drop in growth rates in these animals (10). Primary infection with T. gondii in cattle during gestation can cause abortion or congenital anomalies, including severe generalized toxoplasmosis with a possible fatal outcome (11). Infection in immunocompetent non-pregnant hosts, on the other hand, is frequently silent, resulting in mild or no clinical signs (12). The presence and spread of bovine neosporosis have been reported in more than 34 countries from Europe, Asia, America and Australia (13). However, few studies have investigated the prevalence of N. caninum infection in African countries (14). Toxoplasma gondii has been isolated from almost all geographical regions worldwide except Antarctica (15).
Egypt has a cattle population of about 5.1 million cattle (16) with the highest density in Beheira province. Beheira province is located in the Nile Delta, the northern (lower) part of Egypt. It embraces the whole of the delta west of the Rosetta branch with a considerable desert region to the south. Economically, agriculture is the most important sector in Beheira province and produces wheat, rice, and corn as the major crops. Other cereals, potatoes, sugar beets, tomatoes, and sesame are also harvested (17). The unique location of Beheira province made it an attractive area for investment in cattle production, and currently, it has the highest number of cattle farms in Egypt with approximately 20% of the total cattle population, i.e., about 1 Mio heads (16). The majority of cattle herds are either mixed native breeds, raised in small to medium-sized herds by local farmers, or imported cattle breeds from Europe, Africa, and South America with improved meat and milk production characteristics, including Holstein Friesian, Zebu, Brown Swiss, and Simmental (16, 18). Cattle farming systems are either intensive (more than 200 cattle), semi-intensive (20–200 cattle per farm), or smallholder (5–20 cattle per farm) systems (19).
Several investigations have been conducted in Egypt to determine the prevalence of neosporosis and toxoplasmosis in various animal species and people from different geographic locations (southern and northern) (3, 19–30). However, there has never been a thorough investigation of the prevalence of these parasites in cattle of the Beheira area. As reported in a literature review, only 15 blood samples from an abattoir in Beheira were analyzed in 1977 (31), and 7 (46.6%) of those samples proved positive for T. gondii antibodies. Therefore, this study aimed to investigate the presence of anti-N. caninum and anti-T. gondii antibodies in apparently healthy cattle from different localities and farms in Beheira province, and to provide insight into the risk factors related to such infections. We opted for commercial ELISAs in this study to obtain objective results that will allow for comparisons between different laboratories and countries.
This research has obtained the approval of the Ethics of the Institutional Committee of the Faculty of Veterinary Medicine at Damanhour University, Egypt (DMU/VetINF-2019-/0145). The project and procedures were explained to the animal owners, and verbal consent was obtained, as requested by the Ethics Committee.
A total number of 358 apparently healthy cattle of different ages: < 3 years (n = 142), 3–5 years (n = 143), and >5 years (n = 73); sexes: males (n = 142), and females (n = 216); production types: beef (n = 142), and dairy (n = 216); breeds: mixed (n = 205), Holstein (n = 109), and Colombian zebu (n = 44) were randomly selected and stratified according to cattle population size from 16 herds (6 dairy and 10 beef) comprising a total of 4,795 heads in 8 different localities (Table 1) namely: Damanhour (n = 20), Edku (n = 22), Abu Hommus (n = 78), Nubariyah (n = 56), Abu Almatamer (n = 27), Dilinjat (n = 60), Kafr El-Dawar (n = 72), and Housh Eissa (n = 23) in Beheira province, northern Egypt (Figure 1), during the first half of 2022. The number of the tested herds per locality varied between one and three (Table 3). About 10% of the animals of each farm were sampled (Table 1).
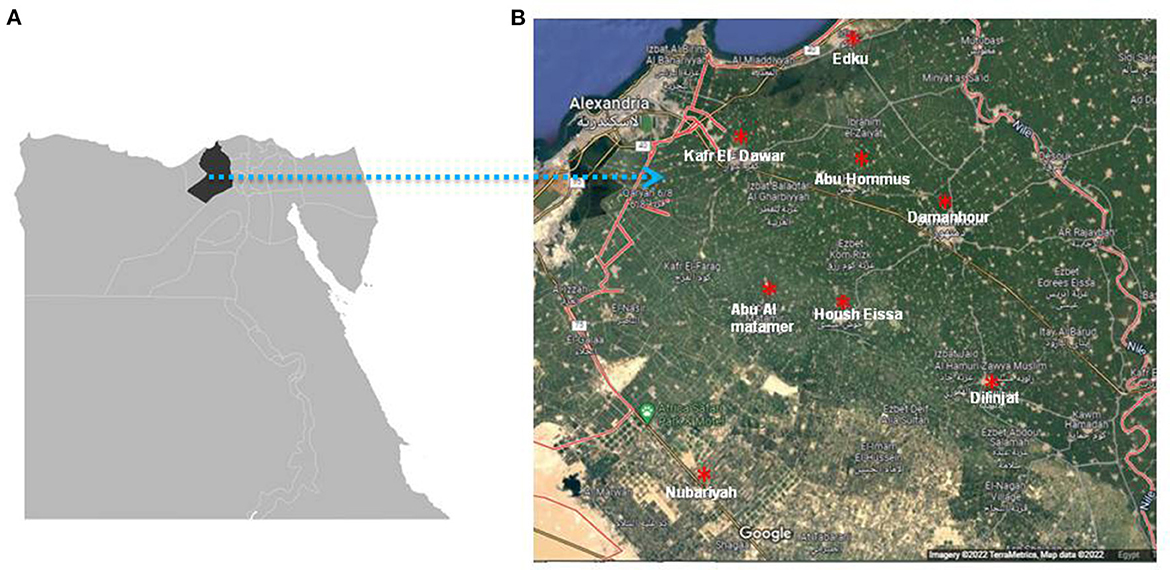
Figure 1. The geographical map of Egypt (Google imagery terraMetrics, Map data 2022) shows the location of Beheira province indicated by black color (A) and landscape showing the geographical distribution of eight localities in Beheira which indicated by red asterisks (B).
Whole blood samples were collected from cattle using the tail vein puncture procedure and were stored in a glass tube with K2 EDTA anticoagulant. Blood samples were transported immediately to the laboratory and kept at 4°C. For serological investigation, plasma samples were separated by centrifugation at 3,000 rpm for 15 min at room temperature and kept at −20°C prior to analysis.
All 358 collected cattle plasma samples were serologically investigated for infection by N. caninum or T. gondii or mixed infection via indirect ELISA assay, using commercial ELISA kits. For N. caninum, samples were analyzed with the competitive multi-species ELISA for neosporosis (ID.vet, Grabels, France). Such test has the potential to detect IgG and IgM through the use of purified extract of N. caninum as coated antigen and anti-N. caninum-HRP, with recorded sensitivity (100%; CI 95%: 98.8–100%) and specificity (100%; CI 95%: 99.63–100%) as provided by the manufacturer. Plasma samples and controls were diluted 1:2. The ODs obtained were used to calculate the percentage of sample (S) to negative (N) ratio (S/N%) for each of the test samples according to the following formula S/N (%) = OD sample/OD negative control × 100. Samples with an S/N% greater than 60% were considered negative; if the S/N% was between 50 and 60%, the result was considered doubtful, and considered positive if the S/N% was less than 50%.
Regarding T. gondii, the samples were analyzed with the indirect multi-species ELISA for toxoplasmosis (ID.vet, Grabels, France) according to the manufacturers' instructions. This tool was specified to detect IgG through the use of P30 as coated antigen and anti-multispecies IgG-HRP, with recorded sensitivity (98.36%; CI 95%: 95.29–99.44%) and specificity (99.42%; CI 95%: 98.8–100%). Plasma samples and controls were diluted 1:10. The optical density (OD) obtained was used to calculate the percentage of sample (S) to positive (P) ratio (S/P%) for each of the test samples according to the following formula: S/P (%) = (OD sample–OD negative control)/(OD positive control–OD negative control) × 100. Samples with an S/P% less than 40% were considered negative; if the S/P% was between 40 and 50%, the result was considered doubtful, and considered positive if the S/P% was greater than 50%.
The ODs of all ELISA results were read at 450 nm and measured with an Infinite® F50/Robotic ELISA reader (Tecan Group Ltd., Männedorf, Switzerland).
The significance of the differences in the infection rates of the different diseases and risk factors was determined by the Fisher Exact Probability Test (two-tailed) using online statistics software http://vassarstats.net/ and GraphPad Prism version 5. A P value of < 0.05 was considered statistically significant. Odds ratios and the 95% confidence intervals were calculated using www.vassarstats.net and GraphPad Prism version 5.
Out of the 358 individual samples, 88 (24.6%) were positive for anti-N. caninum antibodies, 17 (4.7%) doubtful, and 253 (70.7%) were negative. A lower prevalence rate of anti-T. gondii antibodies was shown: 19 (5.3%) were positive, 22 (6.1%) doubtful, and 317 (88.5%) negative. Furthermore, 7 were positive and 3 were doubtful for both anti-N. caninum and anti-T. gondii antibodies (Table 2).
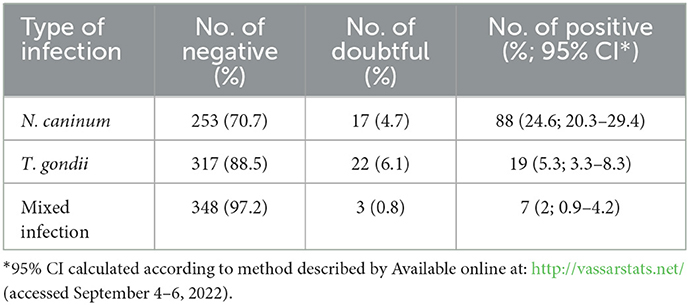
Table 2. Seroprevalence of N. caninum, T. gondii, and mixed infection in individual samples (n = 358).
On the herd level, 6/6 (100%) dairy and 7/10 (70%) beef herds tested positive for N. caninum antibodies, while T. gondii antibodies were detected in 4/6 (66.6%) dairy and 5/10 (50%) beef herds, respectively (Table 3). A higher seroprevalence of N. caninum was reported in dairy herds of mixed breeds with percentages of 54.6, 50, 34.8, and 11.1% in herds # 3, 12, 9, and 8, respectively, than in herds of Holstein cows (27.5, and 10.7% in herds # 4, and 7, respectively). Among beef herds, Colombian zebu herds # 11, and 2 showed the highest seroprevalence of N. caninum, with percentages of 60 and 40%, respectively, however zebu herds # 14 and 16 were totally negative. Herds with both, mixed and Holstein beef cattle, showed seroprevalences of 5, 16.7, 29.6, 20, and 7.1% in herds # 5, 6, 10, 13, and 15, respectively, however herd # 1 tested negative (Table 3).
Regarding T. gondii seroprevalence in dairy herds, positive cases were reported only in mixed breed herds with percentages of 9, 11.1, 4.3, and 14.6% in herds # 3, 8, 9, and 12, respectively. Also for seropositive beef herds, 4 herds were mixed cattle herds with infection rates of 13.3, 5, 6.7, and 7.1% in herds # 1, 5, 13, and 15, respectively, and one was a Colombian zebu herd (#14) with 11.1% infected animals. Co-infection of the same animal with N. caninum and T. gondii was detected only in dairy herds # 8, 9, and 12, all with mixed cattle breed, with co-infection rates of 3.7, 4.3, and 10.4%, respectively (Table 3).
The impact of several risk factors such as age, sex and production type, breed, and location of the tested cattle on the seroprevalence of N. caninum and T. gondii was investigated. For N. caninum infection, prevalence in cattle < 3 years old was 24/142 (16.9%), and a non-significant increase [p = 0.23; odds ratio (OR) = 1.5] in seropositivity was observed in cattle aged 3–5 years (33/143; 23.1%). A significant increase in N. caninum seroprevalence (p < 0.0001; OR = 3.6) was demonstrated in older cattle aged >5 years (31/73, 42.5%; Table 4). Female cattle (dairy cows) showed a significantly higher seroprevalence for N. caninum (64/216, 29.6%; p = 0.008, OR = 2.1) than males that were used for beef production (24/142, 16.9%) (Table 4). Only non-significant differences between different breeds were found. The lowest prevalence of N. caninum infection was detected in Colombian zebu (18.2%). Holstein cattle showed a higher seroprevalence (21.1%), and mixed cattle had the highest infection rate (27.8%) (Table 4). Seroprevalence for N. caninum varied between the localities. The prevalence in Damanhour (2/20; 10%) was taken as a reference value, and thus cattle in Edku (12/22; 54.5%), Kafr El-Dawar (27/72; 37.5%), and Dilinjat (22/60; 36.7%) had significantly higher values in comparison (p = 0.003, 0.027; 0.026; ORs = 10.8, 5.4, 5.2, respectively) (Table 4). No significant differences compared to Damanhour were found in Abu Hommus (15/78; 19.2%), Abu Almatamer (3/27; 11.1%), Nubariyah (6/56; 10.7%), and Housh Eissa (1/23; 4.3%) (Table 4).
For T. gondii, none of the tested factors (age, sex and production type, breed, and locality) had a significant effect on the seroprevalence. The lowest infection rate was found in cattle 3–5 years old (3.5%) followed by the youngest group (< 3 years; 4.2%). A slight, non-significant increase was found in the oldest group (>5 years; 11%) (p = 0.15, OR = 2.8) (Table 5). The infection rate with T. gondii in males was lower than in females, but also non-significantly (4.2 vs. 6%) (Table 5). Seroprevalence rates in the differed breeds varied from 0% in Holstein cattle, 2.3% in Colombian zebu cattle, up to 8.8% in mixed cattle breed, but there were no statistical differences (Table 5). Regarding location, cattle samples from Kafr El-Dawar showed the highest seroprevalence for T. gondii (12.5%) followed by Abu Almatamer (11.1%), Damanhour (10%), Edku (9.1%), Hosh Eissa (4.3%), Dilinjat (1.7%), and Abu Hommus (1.3%), but no significant differences between these regions were found (Table 5). Noticeably, a complete absence of T. gondii infection was reported in tested cattle from Nubariyah but there was no significant difference to Damanhour (Table 5).
This study provided the first report on the seroprevalence of N. caninum and T. gondii in cattle from Beheira province as well as a respective risk factor analysis (age, sex, and therewith production type, breed, and location). Our findings indicated a greater individual infection rate with N. caninum (24.6%) than with T. gondii (5.3%), which was also reflected on the herd level with 13 herds positive for N. caninum compared to 9 herds for T. gondii. Reportedly, cattle are naturally more susceptible to N. caninum than T. gondii infection and N. caninum can persist in a herd due to the highly efficient vertical transmission from cow to calf (32, 33). Indeed, in endemically infected dairy cattle herds up to 95% of calves of seropositive cows are born infected (13, 34). In our study, the seroprevalence in the youngest age group (< 3 years) was almost 17%, very close to the one reported before for this age group (17.5%) by Fereig et al. (24). Furthermore, owners frequently use domestic dogs as guardian dogs in cattle farms, thus the final host for N. caninum is often in close contact with livestock. In addition, total numbers of dogs have increased significantly in recent years (35), and a large number of stray canines and wolves are present in Egypt (22, 36). Thus, environmental contamination with N. caninum oocysts is potentially high in Egypt, especially on cattle farms. The observed increase of seroprevalence for N. caninum with age in our study suggests horizontal infection with oocysts as important route of infection, in addition to vertical transmission.
In comparison to the previous studies on N. canimum in cattle in Egypt, this work reported a similar prevalence rate (24.6%) to the 20.43% reported in cattle of the Delta region (23), a neighboring region of Beheira governorate, and also to the 18.9% in cattle from southern Egypt (24). Recently, three of ten bulk milks from different farms and different regions in Egypt were shown to contain antibodies to N. caninum, and one of these bulk milks was additionally positive for N. caninum DNA (26). For other livestock like camels, variable seroprevalences of 3.6% (37), 11% (21), and 25.7% (25) have been reported. A seroprevalence of even 68% was found in water buffalo (1). Our findings for T. gondii showed a lower infection rate (5.3%) compared to earlier research that estimated the seroprevalence of anti-T. gondii antibodies in cattle in Egypt. For instance, using a TgSAG2t-ELISA, 10.8% of the cattle sera from the Delta region (23), and 23.6% of the cattle from Qena, Kafr Elsheikh, and Minoufia tested positive (19). In Shebein El-Kom, molecular detection in cattle milk revealed 19.35% (6/31) positive for T. gondii DNA (30), while one in ten bulk milks tested positive for T. gondii DNA in a recent study (26). Cattle meat samples from Sohag city showed 31.3% positive by PCR (20). Despite the fact that the area of investigation differ between the studies, no earlier studies have revealed a lower prevalence of T. gondii infection in cattle than ours. As cats are the primary source of infection with this protozoan, this could be a sign of improved cat control on cattle farms in Beheira. Indeed, the majority of cats in Beheira are now domesticated, and the number of stray cats has dropped considerably in past years. As an outcome, a progressive reduction in the number of new cattle infections was anticipated.
The positive rate in female dairy cattle (29.6%) was significantly higher than in male beef cattle (16.9%). This supports earlier observations by Quintanilla-Gozalo et al. (38), who recorded higher seroprevalences to N. caninum in dairy cattle (83.2 and 36.8%) than those in beef cattle (55.1 and 17.9%) at the herd and individual level, respectively. Age might be an important factor in this equation, as in our study most of the males were younger than 3 years old and females were >3 years. This might also explain the contrast with an earlier study that did not identify sex as a risk factor for infection with N. caninum (24). Therefore, further investigation into the relationship between age, sex, and production type in cattle is necessary to determine the exact contribution of each variable to the infection risk. In accordance with an earlier study (19), the breed of cattle had no impact on the prevalence of N. caninum infection. However, a trend toward higher infection rates for both, N. caninum and T. gondii, was seen in mixed breed herds when compared to Holstein and Colombian zebu cattle in our study. Moreover, co-infection was reported only in 3 dairy mixed herds. These data could be linked to the management of these herds. Most of mixed cattle herds were not completely fenced against stray animals, suffered from shortage in veterinary services and regular screening of diseases, and were restocking with their own calves. The importance of the management system on the prevalence rate of N. caninum was also reflected by the significantly higher seroprevalence in Edku (only mixed breed analyzed), Dilinjat (all three breeds), and Kafr El-Dawar (mixed breed and zebu), respectively. This could be attributed to the nature of farming systems and the density of farms in these localities where most of farms are semi-intensive in comparison to the well-controlled intensive farms in other localities of the sampling area. These intensive herds mainly kept foreign breeds and the management was more professional. Furthermore, most of these cattle were imported as certified-free from diseases prior to stocking. Biosecurity, good veterinary practices, and use of artificial insemination in breeding system were also advantageous (16, 18). Neither age, sex and production type, nor breed were identified as risk factors for T. gondii infection in the examined cattle. This result was consistent with an investigation of the risk variables linked to T. gondii infection in cattle in southern Egypt (19). It's important to note that no Holstein cattle tested positive for anti-T. gondii antibodies, most probably for the management reasons discussed above.
In conclusion, this study provided the first serological survey on subclinical infection with N. caninum and T. gondii in cattle in Beheira province. The findings indicated a higher prevalence of N. caninum compared to T. gondii among the cattle tested, as well as in comparison to earlier investigations carried out in Egypt. The most important risk factors for N. caninum infection in the cattle studied were age, sex, and therewith production type, and locality. However, the current investigation found no risk factors for T. gondii infection. Future research is needed to provide more detailed information about the prevalence of N. caninum and T. gondii in Egypt. The elaboration of effective control strategies and biosecurity measures in cattle farms has become an urgent need, because no vaccines or effective drugs against N. caninum or T. gondii are currently available.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This research has obtained the approval of the Ethics of the Institutional Committee of the Faculty of Veterinary Medicine at Damanhour University, Egypt (DMU/VetINF-2019-/0145). Written informed consent for participation was not obtained from the owners because oral consent was obtained from each owner, as was agreed upon by the Ethics Committee (see respective letter). In Egypt, written informed consent is very hard to obtain because verbal explanations and consequent consent is the usual way of reaching agreements.
Conceptualization, design, resources, and shared materials: SM, RF, and CF. Experiments, formal analysis, and investigation: SM, RF, RH, KS, and CF. Writing—original draft: SM, RF, and RH. Writing—review and editing, project administration, and funding acquisition: RF and CF. All authors have read and agreed to the published version of the manuscript.
We acknowledge Prof. Nabil Bkear and Dr. Yassien Badr at the Department of Animal Medicine, Faculty of Veterinary Medicine, Damanhour University, Egypt for helping in the collection of samples as well as the owners of animals for providing the animal's data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dubey JP, Romand S, Hilali M, Kwok OCH, Thulliez P. Seroprevalence of antibodies to Neospora caniuum and Toxoplasma gondii in water buffaloes (Bubalus bubalis) from Egypt. Int J Parasitol. (1998) 28:527–9. doi: 10.1016/S0020-7519(97)00190-2
2. Rojas-Pirela M, Medina L, Rojas MV, Liempi AI, Castillo C, Pérez-Pérez E, et al. Congenital transmission of apicomplexan parasites: a review. Front Microbiol. (2021) 12:751648. doi: 10.3389/fmicb.2021.751648
3. Bishr NM, Abdel-Rahman AAH, Ashour AM, Ibrahim HM. Biochemical effects of Toxoplasma gondii and Neospora caninium infection on diary bovine models in Menoufia Province, Egypt. Adv Anim Vet Sci. (2020) 9:379–86. doi: 10.17582/journal.aavs/2021.379.386
4. Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. (2009) 39:877–82. doi: 10.1016/j.ijpara.2009.01.005
5. Ortega-Mora L, Bruno G, Franz JC, David B. Protozoal Abortion in Farm Ruminants: Guidelines for Diagnosis and Control. Cambridge, MA, CABI (2007) 1–309. doi: 10.1079/9781845932114.0000
6. Dubey JP, Hemphill A, Calero-Bernal R, Schares G. Neosporosis in Animals. Boca Raton: CRC Press. (2017). doi: 10.1201/9781315152561
7. Reichel MP, Ayanegui-alcérreca MA, Gondim LFP, Ellis JT. What is the global economic impact of Neospora caninum in cattle – The billion dollar question. Int J Parasitol. (2013) 43:133–42. doi: 10.1016/j.ijpara.2012.10.022
8. Dubey JP, Buxton D, Wouda W. Pathogenesis of bovine neosporosis. J Comp Pathol. (2006) 134:267–89. doi: 10.1016/j.jcpa.2005.11.004
9. Pare J, Thurmond MC, Hietala SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res. (1996) 60:133.
10. Barling KS, McNeill JW, Paschal JC, Mccollum Iii FT, Craig TM, Adams LG, et al. Ranch-management factors associated with antibody seropositivity for Neospora caninum in consignments of beef calves in Texas, USA. Prev Vet Med. (2001) 52:53–61. doi: 10.1016/S0167-5877(01)00233-1
11. Montoya JG, Huffman HB, Remington JS. Evaluation of the immunoglobulin G avidity test for diagnosis of toxoplasmic lymphadenopathy. J Clin Microbiol. (2004) 42:4627–31. doi: 10.1128/JCM.42.10.4627-4631.2004
12. Milne G, Webster JP, Walker M. Toxoplasma gondii: an underestimated threat? Trends Parasitol. (2020) 36:959–69. doi: 10.1016/j.pt.2020.08.005
13. Dubey JP, Schares G, Ortega-Mora L. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. (2007) 20:323–67. doi: 10.1128/CMR.00031-06
14. Nayeri T, Moosazadeh M, Sarvi S, Daryani A. Neospora caninum infection in aborting bovines and lost fetuses : A systematic review and meta-analysis. PLoS ONE. (2022) 17:e0268903. doi: 10.1371/journal.pone.0268903
15. Dubey JP, Rajendran C, Costa DGC, Ferreira LR, Kwok OCH, Qu D, et al. New Toxoplasma gondii genotypes isolated from free-range chickens from the Fernando de Noronha, Brazil: unexpected findings. J Parasitol. (2010) 96:709–12. doi: 10.1645/GE-2425.1
16. Food Agriculture Organaisation (FAO). Agency US, Development I. Livestock production systems spotlight cattle and buffaloes and poultry sectors Livestock production systems spotlight cattle and buffaloes, and poultry sectors in Egypt. (2018). Available online at: http://www.fao.org/ag/againfo/programmes/en/ASL2050.html (accessed November, 2022).
17. Wilson P, Grigoropoulos, D. The West Delta regional survey, Beheira Kafr el-Sheikh Provinces. (2009). Available online at: https://en.wikipedia.org/wiki/Beheira_Governorate#cite_note-sis.gov.eg-3 (accessed November, 2022).
18. Abdi A, Roushdy S, Beillard MJ. Egypt Livestock and Products Annual 2019 Egyptian Beef Prices Stable. U. S. Beef Imports Challenged. (2020) 1–12.
19. Fereig RM, Mahmoud HYAH, Mohamed SGA, Rezk M, Abdel-wahab A, Ahmed S, et al. Seroprevalence and epidemiology of Toxoplasma gondii in farm animals in different regions of Egypt. Vet Parasitol. (2016) 3:1–6. doi: 10.1016/j.vprsr.2016.05.002
20. Abdel-Aziz NM, Hassanien AA, Arafa MI. Detection of Toxoplasma gondii in aborted women and meat of slaughtered sheep and cattle in Sohag city, Upper Egypt. Adv Anim Vet Sci. (2020) 8:680–6. doi: 10.17582/journal.aavs/2020/8.6.680.686
21. Selim A, Abdelhady A. Neosporosis among Egyptian camels and its associated risk factors. Trop Animal Health Prod. (2020) 52:3381–5. doi: 10.1007/s11250-020-02370-y
22. Aboelwafa SS, Ali AO, Hamada R, Mahmoud H. Seroprevalence of Toxoplasma gondii and Neospora caninum in small ruminants in Luxor, Egypt. Adv Anim Vet Sci. (2022) 10:412–20. doi: 10.17582/journal.aavs/2022/10.2.412.420
23. Ibrahim HM, Huang P, Salem TA, Talaat RM, Nasr MI, Xuan X, et al. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am J Trop Med Hyg. (2009) 80:263–7. doi: 10.4269/ajtmh.2009.80.263
24. Fereig RM, Rezk M, Mohamed SGA, Mahmoud HYAH, Ali AO, Ali AF, et al. Serological detection and epidemiology of Neospora caninum and Cryptosporidium parvum antibodies in cattle in southern Egypt. Acta Trop. (2016) 162:206–11. doi: 10.1016/j.actatropica.2016.06.032
25. Fereig RM, Abdelbaky HH, El-Alfy E-S, El-Diasty M, Elsayed A, Mahmoud HYAH, et al. Seroprevalence of Toxoplasma gondii and Neospora caninum in camels recently imported to Egypt from Sudan and a global systematic review. Front Cell Infect Microbiol. (2022) 12:1721. doi: 10.3389/fcimb.2022.1042279
26. Fereig RM, Abdelbaky HH, Mazeed AM, El-Alfy E-S, Saleh S, Omar MA, et al. Prevalence of Neospora caninum and Toxoplasma gondii antibodies and DNA in raw milk of various ruminants in Egypt. Pathogens. (2022) 11:1305. doi: 10.3390/pathogens11111305
27. Fereig RM, Wareth G, Abdelbaky HH, Mazeed AM, El-Diasty M, Abdelkhalek A, et al. Seroprevalence of specific antibodies to Toxoplasma gondii, Neospora caninum, and Brucella spp in sheep and goats in Egypt. Animals. (2022) 12:3327. doi: 10.3390/ani12233327
28. Salama DB, Fereig RM, Abdelbaky HH, Shahat MS, Arafa WM, Aboelhadid SM, et al. Toxoplasma gondii and Neospora caninum antibodies in dogs and cats from Egypt and risk factor analysis. Pathogens. (2022) 11:1464. doi: 10.3390/pathogens11121464
29. Ahmed NE, Mohamed L, Akabway A, Ramadan MY, Mohamed S. Serological and PCR-sequencing assays for diagnosis of Toxoplasma gondii and Neospora caninum infecting camels in Egypt. Benha Vet Med J. (2017) 33:200–210. doi: 10.21608/bvmj.2017.30466
30. Byomi A, Zidan S, Salama S, Elsify A, Hadad G, EissaN. Public health implications of toxoplasmosis in animals and women in selected localities of menoufia governorate, Egypt. Assiut Vet Med J. (2018) 64:120–30. doi: 10.21608/avmj.2018.168920
31. Abbas IE, Villena I, Dubey JP. A review on toxoplasmosis in humans and animals from Egypt. Parasitology. (2020) 147:135–59. doi: 10.1017/S0031182019001367
32. Dubey JP, Schares G. Neosporosis in animals—the last five years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
33. De Barros LD, Garcia JL, Bresciani KDS, Cardim ST, Storte VS, Headley SA, et al. review of toxoplasmosis and neosporosis in water buffalo (Bubalus bubalis). Front Vet Sci. (2020) 7:455. doi: 10.3389/fvets.2020.00455
34. Villa L, Gazzonis AL, Fumagalli E, Zanzani SA, Manfredi MT. The Utility of serological analysis for Neospora caninum infection in dairy cattle farms management : serological investigation and evaluation of the effects on reproductive and productive performances in two study herds in Northern Italy. Animals. (2022) 12:786. doi: 10.3390/ani12060786
35. Egyptian kennel federation (2020). Available online at: https://www.fci.be/en/statistics/ByNco.aspx?iso=EG (accessed November, 2022).
36. Mahdy MAA, Mohamed WF. Comparative craniometric measurements of two Canid species in Egypt: the Egyptian red fox and the Egyptian Baladi dog. BMC Vet Res. (2022) 18:1–13. doi: 10.1186/s12917-022-03275-8
37. Hilali M, Romand S, Thulliez P, Kwok OCH, Dubey JP. Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Vet Parasitol. (1998) 75:269–71. doi: 10.1016/S0304-4017(97)00181-7
Keywords: neosporosis, toxoplasmosis, seroprevalence, risk factors, cattle, Beheira, Egypt
Citation: Metwally S, Hamada R, Sobhy K, Frey CF and Fereig RM (2023) Seroprevalence and risk factors analysis of Neospora caninum and Toxoplasma gondii in cattle of Beheira, Egypt. Front. Vet. Sci. 10:1122092. doi: 10.3389/fvets.2023.1122092
Received: 12 December 2022; Accepted: 26 January 2023;
Published: 13 February 2023.
Edited by:
Simona Gabrielli, Sapienza University of Rome, ItalyCopyright © 2023 Metwally, Hamada, Sobhy, Frey and Fereig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline F. Frey,  Y2Fyb2xpbmUuZnJleUB1bmliZS5jaA==; Ragab M. Fereig,
Y2Fyb2xpbmUuZnJleUB1bmliZS5jaA==; Ragab M. Fereig,  cmFnYWIuZmVyYWVnMkB2ZXQuc3Z1LmVkdS5lZw==
cmFnYWIuZmVyYWVnMkB2ZXQuc3Z1LmVkdS5lZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.