- 1College of Veterinary Medicine, Washington State University, Pullman, WA, United States
- 2Faculty of Veterinary Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria
- 3College of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Nigeria
- 4College of Veterinary Medicine, Cornell University, Ithaca, NY, United States
- 5College of Veterinary Medicine, Mississippi State University, Starkville, MS, United States
- 6Department of Veterinary Parasitology, Universidade Federal de Parana, Curitiba, Brazil
Usutu virus (USUV) is an arthropod-borne virus (arbovirus) of the flaviviridae family (genus Flavivirus) which belong to the Japanese encephalitis virus complex. Culex mosquitoes have been implicated in the transmission of this pathogen. The major susceptible hosts of USUV are migratory birds, thereby potentiating its ability to spread from one region to another globally. Nigeria has the largest economy in Africa with a significant percentage of the gross domestic product relying on the agricultural and animal production industry. This review explores the zoonotic potentials of the virus in Africa, especially Nigeria, with special focus on the devastating sequelae this might lead to in the future if necessary precautionary policies are not enacted and adopted to bolster the surveillance system for mosquito-borne viruses.
Introduction
Usutu virus (USUV) previously restricted to sub-Saharan Africa, is now considered an emerging arboviral pathogen attracting the attention of the scientific and public health community due to its potential to spread to USUV-free areas, cause substantial mortalities in several avian species worldwide and consequential zoonotic impact on humans (1). USUV is an arbovirus of the flaviviridae family (genus Flavivirus) in the Japanese encephalitis virus complex. Other related viruses in this complex includes the Japanese encephalitis virus (JEV), West Nile virus (WNV), Yellow fever virus, Dengue and Murray Valley encephalitis virus (MVEV) which are some of the most pathogenic arboviruses of health impact to humans and animals (1). USUV was first discovered from mosquitoes in South Africa in 1959 and has since been reported in animal (rats, horses and dogs) and human hosts (Figure 1) in African regions like South Africa (4), West Africa (2), Central African Republic (1), Northern Africa (5), and East Africa (6) (Figure 2). Also, USUV has spread from Africa to Europe (emerged in the continent in 2001) and been documented in wide range of animals (birds, boars, squirrels, chimpanzees, reptiles, or horses) and humans in member countries such as Germany (7), Czech Republic (8), Hungary (9), Italy (10), Portugal (11), Spain (12), Austria (13), and the United Kingdom (14). The natural transmission cycle of USUV involves mosquitoes (Culex and to a lesser extent Aedes vectors) and birds that serve as amplifying hosts. Humans and other mammals are considered incidental (“dead-end”) hosts to this virus (15). The emergence and increase of USUV in wild birds, mosquitoes and humans shows the virus may now be endemic in several European countries as in African nations and may become a potential global health threat in the future. It is interesting to know that despite being a virus originating from Africa, little information still exists about the epidemiology, ecology, transmission dynamics and cycle of the virus, and the zoonotic potential and burden in humans in Africa.
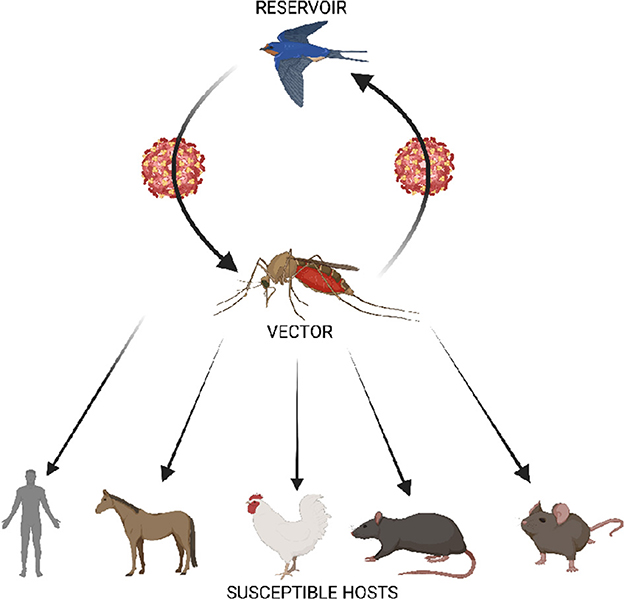
Figure 1. Schematic representation of the transmission cycle of USUV showing the enzootic cycle between Culex mosquito vector and birds, and the biological transmission of the virus to susceptible hosts. Humans have been identified as dead end host. Serological evidence of USUV infection has been reported in horse and avian population in Slovakia and Poland, and in African countries such as Central African Republic, Cote d'lvoire, Senegal Nigeria, and Uganda (2). In addition, USUV has been found in other animals including dogs, bats, red deer, rodents (shrews) (3).
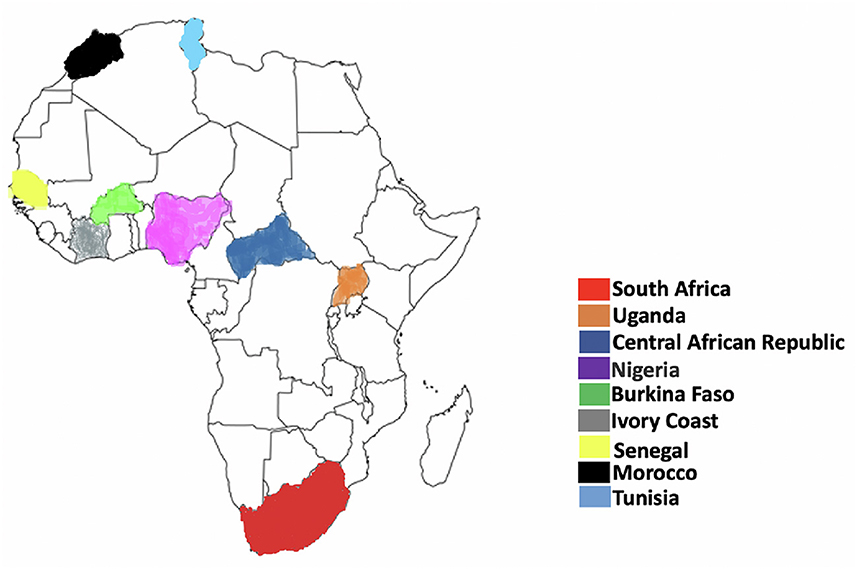
Figure 2. Geographical representation of Usutu virus distribution in Africa. The map represents countries where there have been reported presence of USUV. As depicted here, USUV has been detected at least once in Burkina Faso, Central African Republic, Ivory Coast, Morocco, Nigeria, Senegal, Tunisia, and Uganda. There have been reported presence of USUV also in Kenya, Madagascar, Mali, and Swaziland. Adapted from Clé et al. (1).
Genetic diversity of Usutu virus in Africa
As related to other flaviviruses, USUV has a (+)-strand RNA genome of 11,064 nucleotides that encodes a single polyprotein of 3,434 amino acids that is consequently sliced into structural (C, E, and prM) and non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) proteins (16). USUV is categorized into three (2) lineages which are diverse in African countries, based on the geographic origin of isolation (Figure 3). A phylogenetic study of the Nonstructural protein 5 (NS5) gene confirmed that USUV strains from Africa belong to 3 discrete lineages (Africa I-III) (17). The Africa lineage I comprise only one strain (CAR-1969 strain) isolated in 1969 in the Central African Republic. Also, the Africa II lineage investigated in South Africa in the mid-1940's includes USUV strains isolated in Senegal (16). Furthermore, the Africa III lineage comprises strains isolated in Senegal and a human isolate from the Central African Republic isolated in 1981. Currently, there are few whole genome sequences of USUV strains available from Africa. Future research on USUV involving the phylogenetic analysis of USUV strains isolated from African birds would bolster the understanding of the virus dispersal between Africa and Europe (4). According to Cadar et al. (18), there is sequence identity across 94% of USUV complete genome sequences considered, with the exclusion of the CAR-1969 isolate which showed a genome identity of 78.3% related to other USUV strains. A comparison of untranslated regions (UTR) revealed that 5′ UTR conserves a comparable secondary structure and size among diverse lineages, with the exclusion of Africa I lineage. At the same time, specific nucleotide mutations are seen in 5′ UTR in lineages in African countries (A3T, T4C, C10T, and T14C) (19). In 3′ UTR, highly adjustable size heterogeneity is seen among different lineages. Relative genomic analysis between USUV proteins showed specific amino acid mutations associated with the geographical origin of hosts and isolation. Geographic-specific mutations observed in all African lineages are based on changes in the Non-structural protein 4B (M16I) and Capsid protein (A120V). In similar fashion, host-specific mutations have been seen in birds (Y120N, prM). Also, in humans, unique amino acid mutations were discovered in the CAR-1981 strain (NS2A, S154L; NS3 Y474H; NS5, H173Q), and the USUV strain isolated from patients in Africa with skin rashes.
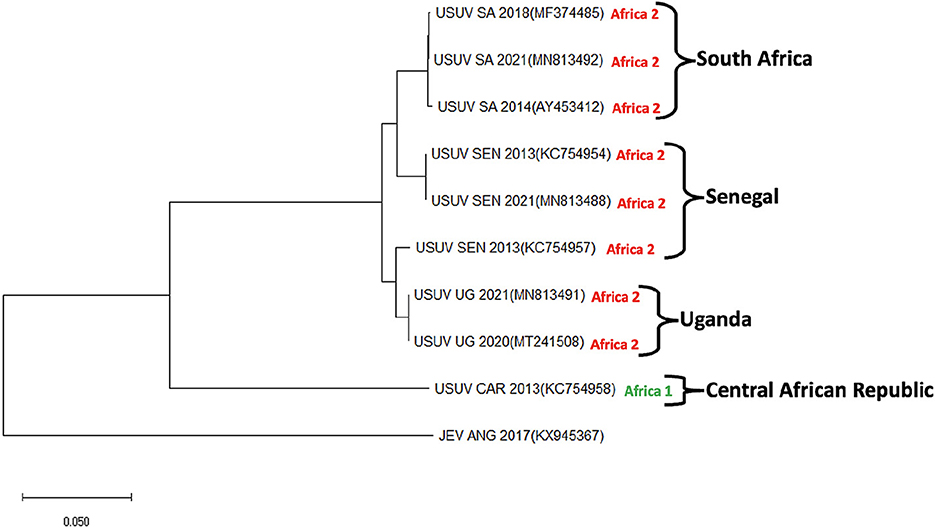
Figure 3. Phylogenetic tree showing Usutu virus strains isolated in 4 African countries (South Africa, Senegal, Uganda, and Central African Republic). USUV strains isolated from Africa can be classified into three clades (I–III). Sequences for Clade III strains specifically isolated from Africa are not currently available and thus not included in this tree. The evolutionary history was deduced using the maximum Likelihood method and Tamura-Nei model. Evolutionary analyses were conducted in the MEGA-X. This analysis involved 10 nucleotide sequences. The sequences were derived from Genbank repository with accession numbers: Usutu virus strains (USUV); MF374485, MN813492, AY453412, KC754954, MN813488, KC754957, MN813491, MT241508, and KC754958 and Japanese encephalitis virus (JEV); KX945367. JEV serves as an outgroup.
Vectors and co-circulation dynamics of Usutu virus
Mosquito
The environment is a critical factor in the successful circulation of viral infections. USUV has been associated with multiple birds' deaths and has been linked to neurotropic symptoms in humans (20). Culex mosquitoes which are the main mosquitoes involved in transmitting USUV, Japanese Encephalitis virus, and West Nile virus, are ornithophilic, greatly contributing to birds' involvement in this virus transmission. In Africa, USUV has been isolated from Culex neavei, and Culex univittatus (21). Importantly, common Culex mosquitoes which have been identified in Nigeria such as Culex perfuscu, and Culex quinquefasciatus, a member of Culex pipiens complex, are also involved in the circulation of USUV to migratory birds (4). However, Culex quinquefasciatus is the most abundant in a domestic environment and the most anthropophilic among the mosquito vectors for USUV (4). The possible involvement of Aedes albopictus, which has also been identified predominantly in southeastern parts of Nigeria (22), in the transmission cycle of USUV was confirmed in a study conducted in Italy (10). This study showed the simultaneous circulation of both West Nile and USUV by Aedes albopictus mosquito. Overall, Culex pipiens is the most competent vector for USUV because Aedes albopictus shows a lower competence even though the virus can replicate in the Mosquito's gut (23). Currently, more investigations are ongoing to identify more competent vectors involved in the zoonotic transmission cycle of USUV and other flaviviruses in Nigeria and Africa at large. In North America, Culex pipiens, and Culex quinquefasciatus were identified as competent vectors in the transmission of USUV following the infection of these mosquito vectors (23) USUV detection was also confirmed in mosquito species such as: Aedes japonicus, Aedes vexans, Anopheles maculipennis, Anopheles plumbeus, Coquillettidia richiardii, Culiseta annulata, and some Ochlerotatus species (24). To date, no studies have confirmed the presence of USUV in ticks, even in regions with significant USUV circulation (24). Routine surveillance is important in monitoring the adaptation of USUV in new mosquito vectors. A single mutation was necessary to adapt Chikungunya virus in Aedes albopictus (25). This surveillance will aid the rapid detection of USUV circulation into new geographical settings (3).
Birds
In Africa and Europe, birds act as the main natural and amplifying hosts in the transmission cycle of many pathogens including USUV. So far, the infection has been identified in over 93 different species of birds in thirty-five families (26). There is a correlation between birds' abundance and the circulation of USUV in mosquito vectors. In a survey carried out in Spain to screen mosquitoes for the presence of USUV genome, there was a link between passerine bird abundance and the presence of USUV in Culex vectors (9). In Africa and Europe, more than 60 avian species and migratory birds contribute to the circulation of USUV. Specifically in Nigeria, USUV was isolated from Kurrichane thrush (Turdus libonyanus), piping hornbill (Bycanistes sharpei), and little greenbull (Andropadus virens) in 1972 (27). Generally, the main clinical signs of USUV in infected birds include ataxia, anorexia, loss of weight, disorientation, and recumbency. The predominant gross pathologies include enlargement of the liver and spleen. Histologically, there is usually inflammatory infiltrates and necrotic foci in the spleen, heart, brain, and kidney of infected birds (28). In certain areas in Europe, such as Germany, France, and the Netherlands, USUV infection resulted in high mortality in the bird population. More studies aimed at investigating the mortality of birds and the basis for the variation in pathogenicity among different bird species in Nigeria and Africa is required.
Humans
So far, USUV infection has a very low incidence in humans in Africa and Europe, where the virus is circulating in the Culex mosquito vectors. To date, only few human cases have been identified. In Africa, the first detection of USUV infection occurred in 1981 in the Central African Republic. The patient had a fever and body rash. The second case was identified in a patient from Burkina Faso in 2004 who had fever and jaundice (4). In humans, USUV infection is usually characterized by fever, headache, tremors in the hand and hyperreflexia. In Europe, the first human report of USUV infection was made in Italy in 2009. The patient had a preexisting B cell lymphoma tumor and had a fever and neurologic symptoms. Also, in 2009, a woman in Italy was diagnosed with a sickness related to USUV infection following a liver organ transplant because of thrombotic thrombocytopenic purpura that developed during an acute episode of USUV infection. The first two human cases of USUV associated neuroinvasive infection were confirmed in immunocompromised patients in Italy in 2009 (29). The case report by Cavrini et al. (29) confirmed USUV by heminested RT-PCR assay targeting the NS5 gene of flaviviruses. Also, a retrospective analysis of human samples (serum and CSF) collected between 2008 and 2011 with or without neurological impairments in Modena, Italy documented a seroprevalence of about 6.6% (30). Similarly, USUV was detected in birds and mosquitoes, and partial sequencing of the virus genome revealed high nucleotide sequence similarity within hosts and strains from Central Europe. In 2014 and 2016–2018, much higher prevalence of the virus (18.1 and 46.3%, respectively) was detected in forest workers and healthy blood donors by 29, suggesting a rise and threat to human health. In 2013 another three cases of USUV neuro-invasive infection (Europe lineage 2) was reported in Croatia during a WNV outbreak (31). Like the Italian report, two of the cases were immunocompromised with arterial hypertension, hyperlipidemia, and diabetes mellitus, thus indicating comorbidities may have a role in the pathogenicity of USUV. Patients presented meningitis and meningoencephalitis with clinical signs such as headache, fever, nuchal rigidity, hand tremor, hyperreflexia, and memory loss and speech fluency difficulty. Another study in Croatia in 2018 further detected three cases, one of the patients was immunocompromised with chronic lymphocytic leukemia and fatal meningoencephalitis (15). USUV has also been reported in animals such as bats in 2013 (32), wild and captive birds in 2011 (28), Owls (33) and humans (18) in Germany. A survey of wild birds in 2011 detected USUV in 38.6% in dead birds using real-time RT-PCR (28). These data showed that the virus spread and caused epizootics among wild and captive birds in south-west Germany in 2011. The incidence of the virus has then increased and over 1300 cases were detected since 2013. Furthermore, in January 2012, a total of 4,200 serum samples from healthy blood donors from south-west Germany were analyzed for USUV-specific antibodies. Although all serum samples from the patients tested negative, one person was confirmed with IgG- and IgM antibodies and USUV infection, after eliminating the possibility of serological cross-reactivity to other flaviviruses (34). Likewise, during the 2016 epizootics, a surveillance of WNV and USUV from 13, 032 blood donors confirmed the presence of USUV Europe 3 lineage in a blood donor, which later revealed an acute USUV infection by immunofluorescence, PCR and genome sequencing assays (18). USUV from the donor plasma showed 99% homology sequence with those found in the birds during the epidemic in 2016. Hungary is another European country that has documented the presence of USUV in animals and humans. The country reported the first incidence during the country's dead bird surveillance program in a black bird (Turdus merula) in August 2005 and further six birds in 2006 by reverse transcription-PCR, immunohistochemistry, in situ hybridization, viral isolation and whole genome sequencing. A total of 332 dead birds belonging to various species were tested for USUV infection between 2003 and 2006. The Hungary USUV strain shared 99.9% identity with the circulating Austrian strain indicating that the USUV strain responsible for the epidemic in blackbird in spread from Austria to Hungary (9). Moreover, results of passive surveillance of USUV in Austria and Hungary (between 2010 and 2016) revealed 12 birds tested positive for USUV infection in 2016 in Hungary (35). Similar pathological conditions as previously described by Bakonyi et al. (35) were reported. The pathological examinations showed moderately autolytic condition, emaciation, moderate to severe splenomegaly, mild hepatomegaly and general congestion of internal organs (Table 1). The nucleotide sequences of USUVs detected cluster together with sequences identified in a human plasma sample and in blackbirds between 2009 and 2010 in Italy. The first human case of USUV was reported by Nagy et al. (44) during a surveillance programme for WNV in 2018. Patients with clinical suspicion of acute WNV infection were tested in parallel for WNV, tick-borne encephalitis virus and Usutu virus (USUV) by serological methods. The Europe lineage 2 USUV infection was confirmed in 2018 in a patient with aseptic meningitis by serology and molecular assays. Furthermore, in 2019, a year after the largest WNV epidemic in Europe, a comprehensive sero survey for WNV and USUV was conducted among blood in Hungary to assess the exposure of the Hungarian population (44). A total of five blood donors were USUV seropositive out of 3,005 donors tested (0.17%). This shows an increase in human donors with possible clinical manifestation expected in Hungary (44). The first emergence of USUV European lineage 2 was reported in Austria in 2001 where it received wide public attention due to the epidemics and high mortality among Eurasian blackbirds (Turdus merula) and great gray owls (Strix nebulosa) in the Vienna (38). A 3-year nationwide surveillance of the USUV in dead wild birds demonstrated 92 birds (in 2003), 11 (in 2004) birds, and 4 (in 2005) birds positive to the infection. However, USUV associated avian deaths reduced during the course of the years due to the establishment of herd immunity in wild birds in Austria (52). Between 2010 and 2015, a few wild birds tested were all tested negative to the USUV infection (15). In 2017, USUV nucleic acid was detected in six of out of 12,047 blood donations from eastern Austria between July and August 2017. These detections were from healthy individuals (35). However, the first case of human USUV was recently reported by Graninger et al. (47). The patient, an 81-year-old man was presented to a hospital in Vienna, Austria in early September 2021. The patient was febrile and displayed confusion and difficulty in responding to questions. The patient later showed the intermittent clonic jerks of his right upper extremity. Subsequently, Serum and CSF samples were tested for USUV, and the CSF came out positive via USUV-specific RT-PCR (47). A partial sequence of the virus genome was 100% identical to sequences from humans, birds, and mosquitoes in the Czech Republic, Hungary, and Austria and confirmed as the Europe 2 lineage (47). Similarly, in France and the Netherlands, the first outbreak of USUV were obtained in 2015 (48) and 2016 (50), respectively, which involved number of case reports of disease-associated and unusual mortality in blackbirds. In the subsequent year (2016) in France, the first human case was presented and associated with atypical neurologic signs (49). In the Netherlands, from April –September 2018, plasma from blood donors in three Dutch provinces with significant USUV activity and blackbird population were collected and analyzed for USUV infection using molecular assays (51). Six of the 12,040 Dutch blood donations were positive.
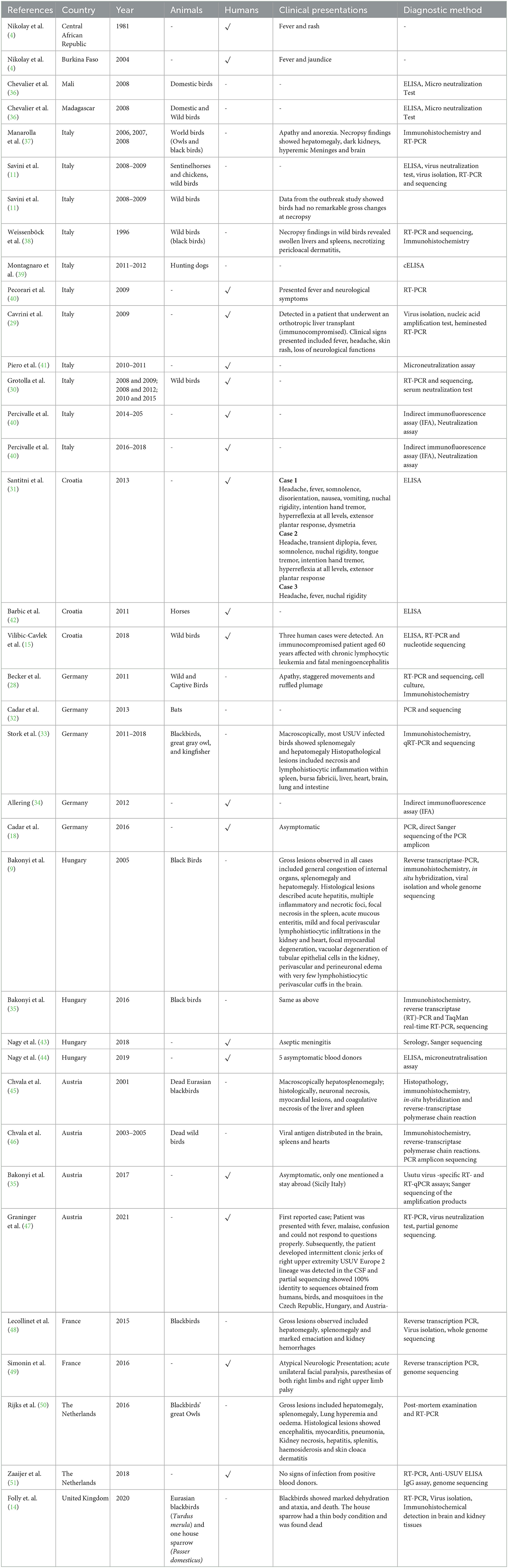
Table 1. A summary of countries that have reported both animal and human cases (incidental hosts) of USUV with confirmed vectors being Culex and Aedes mosquitoes.
Currently, in Africa, particularly Nigeria, there are limited studies investigating the seroprevalence of USUV infection in humans. More work needs to be done to assess the risk of the virus transmission between migratory birds and competent mosquito vectors, and eventually to humans.
USUV in other mammals
Some studies have identified USUV in other mammals such as dogs, horses, rodents, and bats (50). These findings oppose the current enzootic cycle of USUV among birds, mosquitoes. In Senegal, USUV was isolated and sequenced from samples obtained from rodents and shrews, which is the first evidence of USUV in rodents (2). Also, in Germany, USUV has been isolated from dead bats (Pipistrellus) mainly in the brain tissues. It is important to investigate further the contribution of bats to the transmission cycle of USUV (32). Apart from rodents, shrews, and bats, USUV infection has been detected in horses, dogs, wild boars, and wild ruminants. Antibodies specific for USUV has been identified in some horses raised in Poland, Croatia, Italy, Serbia, and Spain. (53). In addition, in 2012, USUV exposure was identified in military dogs and horses in Morocco (53). This was confirmed by virus neutralization tests. In 2014, a study also detected the presence of antibodies specific to USUV in horses in the southwestern region of Tunisia (54). In Spain, USUV-specific antibodies were also detected in wild ruminants (55). The co-infection of USUV with WNV indicates that more work needs to be done to identify which animals are truly acting as USUV reservoir hosts and which ones act as spill-over and dead-end hosts. This can help in preventing the transboundary circulation of this virus.
The role of migratory birds in mosquito-borne virus transmission
Migratory birds are important sentinels for the introduction of some flaviviruses like WNV into multiple regions largely because the outbreak of these viruses within temperate regions normally happens in the summer or beginning of fall, which incidentally also heralds a massive number of migratory birds and vectors like mosquitoes (56). Specifically, this WNV outbreak occurred among humans living in or near wetlands where high concentrations of birds come into contact with large numbers of ornithophilic mosquitoes (57); the principal vectors from which the virus has been isolated are mainly ornithophilic mosquitoes (Culex univittatus in the Middle East and C. pipiens in Europe) (58); antibodies to the virus have been found in the blood of many migratory bird species in Eurasia (58); migratory birds have been linked with transporting related viruses in the Western Hemisphere (59); WNV has been isolated from some species of actively migrating birds e.g., the Barred Warbler [Sylvia risoria] in Cyprus and the Turtle Dove [Streptopelia turtur] in Slovakia (60); viremia sufficiently long-term to infect vector mosquitoes has been documented in several bird species (58), and migration places substantial physiologic stress on birds. For example, stress has been shown to promote immunosuppression and enhanced replication of WNV in rodents (61). Further support for the possibility that migratory birds play a major role in virus transport comes from study of related viruses. For instance, both Eastern (EEE) and Western equine encephalomyelitis alphaviruses, ecologic relatives of WNV, have been isolated from actively migrating birds in the United States (59). Evidence also indicates that the 1962 epidemic of EEE in Jamaica resulted from transport of the virus by birds from the continental United States (61). Unlike the 1999 New York City epidemic, during which large numbers of dead and dying birds, especially crows, were observed concurrently with clinical reports of human infection with the virus (62), the Old-World epidemics of WNV had few concurrent reports of deaths of infected birds (58). This difference could indicate lack of both exposure and adaptation to the virus among New World avian populations compared with Old World species. Old World data indicate that susceptibility to fatal infection with the virus varies markedly for adult and young birds, with high death rates in juveniles and high incidence of circulating antibodies in adult birds (63). Susceptibility to infection also varies considerably among species. Hooded Crows (Corvus corone) had both a high death rate in young birds in laboratory experiments and high levels of circulating antibodies in adults, while Rock Doves (Columba livia) appeared to be much less susceptible to both infection and death from the virus (63).
Migratory birds as sentinels for USUV disease outbreak in Nigeria
Nigeria is a West African country located within the East-Africa-Asia, the Atlantic-America, and the Black Sea/Mediterranean migratory bird flyways, some of which overlap. The dry and dusty harmattan wind flows through the Sahara in a north-easterly direction with high daytime temperatures/low humidity and cool nighttime temperatures during winter in Europe (equivalent to harmattan cold in Nigeria) (64). Innate circadian clock genes frequently control the migration of these birds throughout the winter to climatically favorable regions in the tropics. These genes may also control photoperiodic responses and the timing of life cycle events like mating and eating. These directly impact how ecosystems adapt to warmer environments and places (65). In Nigeria, birds that migrate through West Africa's sub-region and stay there are influenced by the Nigeria biotype phenology. Seasonal solid patterns are seen throughout the sizeable biological zone that runs from Siberia's Pacific coast to Western Europe's Atlantic shoreline (Figure 4). As a result, many bird species nest in this area and migrate south to spend the winter in Africa and South or Southeast Asia. These birds, originating in Europe, travel over or around the Mediterranean as they migrate to Africa (67). Nearly 2 billion songbirds, waders, birds of prey, and waterfowl each year migrate from Europe to sub-Saharan Africa, with Nigeria inclusive (68). Many of these birds during the harmattan in Nigeria undergo a north-south transatlantic migration because of the country's abundance of wetlands, rivers, natural lakes, floodplains, and dug-out dams (64).
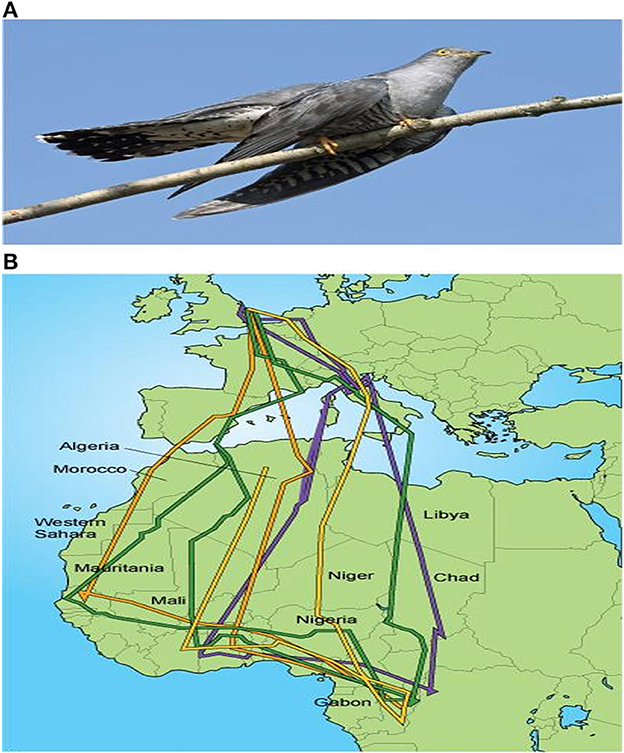
Figure 4. (A) Common cuckoo (Cuculus canorus), a long distance migrant that travels between the UK and Africa. (B) Migration paths of several cuckoos tracked from the UK to Africa and back. The multi-colored lines indicate migration paths of different individual bird species (66).
Zoonotic potentials oF USUV
The interaction between humans, animals, the environment, and significant health-related issues, especially the emergence and re-emergence of infectious pathogens, is not new and has been gaining global attention through active integrated, multisectoral, and multidisciplinary efforts. Likewise, the emergence and potential risk of USUV to human health are gaining scientific community recognition and entomological surveillance due to the possible impact of globalization and climate change on the genetic evolution of the virus propagation, amplification, persistence, and transmission among Culex mosquitoes; the adaptability in new hosts including humans, geographical locations and ecological niches; and capacity to be endemic in mosquito–bird life cycle and to co-circulate with WNV (19). As shown in Table 1, USUV is in countries that have reported the zoonotic possibility of the virus. Humans may get infected with USUV through Culex mosquito bites, and the zoonotic potential of USUV has been reported in a growing number of human cases worldwide. The zoonotic impact of USUV presents another picture in humans in Europe. Over 80 cases of sub-clinical cases of USUV infection have been reported during the surveillance of WNV, of which moderate flu-like signs were reported—rash, generalized headache, and weakness (69). The associated risk of USUV to humans was first described in Africa, where two human cases were documented in Central Africa and Burkina Faso in the 1980's and 2000's, respectively. For these two symptomatic infections, mild clinical signs involving fever, skin rashes, and jaundice, but no neurological symptoms were documented. Subsequently, newer cases have yet to be reported across Africa, which may be associated with little or no targeted surveillance programs to investigate, detect and monitor the virus and transmission dynamics among vectors, wild birds, humans, and the environment. Considering the distribution of the Culex mosquito vector in Nigeria (70) and the sub-optimal hygiene practices in most municipal slaughterhouses across the country (71), there is a possibility for the transmission of USUV from the mosquitoes and migratory birds to humans and vice versa. Although there is no substantial information from the literature if infected humans with viremia can infect the mosquito vectors in Nigeria, this could be a good project for investigation in the future. In the one health context, limited information still exists on USUV epidemiology and factors for emergence, ecology, phylogeny, and pathogenicity to human and animal health despite the intense circulation of the virus among mosquitoes and wild birds in Nigeria and many African countries (36).
Current surveillance program for mosquito-borne viruses in Nigeria
The entomological surveillance system is a vital component of vector control since it gives valuable information on mosquito vector species, their spatio-temporal distribution, density, bionomics, and, more critically, the susceptibility and resistance of those vectors to typical insecticides used (72). The major entomological surveillance projects in Nigeria are carried out in six designated sentinel sites across five ecological zones. However, this is primarily focused on Malaria since it is the predominant mosquito-borne disease in the country (73). Currently, no standard surveillance system is enacted to prevent an outbreak of most Flaviruses, including WNV and USUV. Taking a cue from the previous outbreak and occurrences of Highly Pathogenic Avian Influenza (HPAI) in Nigeria, its association with migratory birds (74), and the devastating socio-economic impact on the poultry industry in Nigeria (75), there must be an improvement in policy formation and development of surveillance programs in Nigeria and Africa. Also, routine serological evaluation of humans living near regions where migratory birds are known to be in Nigeria would help understand the effect of the virus on humans. Significantly, proper documentation and efficient disease reporting as valuable tools in the epidemiological assessment of mosquito-borne viruses should be emphasized. It could also be helpful to incorporate screening for Flaviviruses during blood transfusions.
Vaccines and therapeutics
Currently, there are no specific licensed vaccines and drugs for managing USUV in humans and animals. The prevention of USUV infection in humans and animals can be achieved through controlling mosquito vectors involved in the circulation of the virus. In addition, an interesting critical control point for the prevention of USUV would be the mosquito-human interface. However, there is a very low incidence of USUV infection in the human population; thus, there is little urgency to develop potent vaccines and therapeutic solutions. Many challenges are associated with developing vaccines needed to elicit protective immunity against some Flaviviral infections. There are limited models available for use in vaccine production. Adult mice serve as good models for the development of vaccines because they serve as a good platform to access immune response to immunization and complications linked to vaccination, but only suckling mice are susceptible to USUV infection, and this is a major constraint (76). To further identify more models for USUV vaccine development, a research study evaluated the effect of USUV infection in Gallus gallus domesticus embryonated chicken eggs. Following infection, the USUV isolate could replicate well within the chorioallantoic membrane and the allantoic fluid. Also, there was dose-dependent death of the chicken embryos after infection. This study sheds more light on the pathogenesis of USUV infection and contributes to vaccine development (69). The envelope (E), and the membrane (M) proteins are good antigenic targets for flaviviral vaccines. In the quest to develop a vaccine for USUV, a single administration of recombinant plasmid encoding the envelope (E), and the pre-membrane (M) protein was performed intramuscularly in mice. This immunization elicited a good immune response and protection against USUV challenge (77). West Nile and Usutu virus share antigenic similarity. A study checked if attenuated West Nile vaccine can induce neutralizing antibodies against USUV infection using ifnar−/− mouse model. Mice vaccinated with the attenuated West Nile virus vaccine were challenged with African and European strains of USUV. The vaccinated mice exhibited lower viremia compared to unvaccinated group. This study shows that attenuated West Nile virus vaccine is protective against USUV (78). Some studies have revealed the ability of acetyl-CoA carboxylase inhibitors to block the replication of USUV in cell culture. Also, the use of drugs that prevent autophagy shows some potency against the replication of USUV in vitro. (12). Favipiravir, a broad-spectrum viral RNA polymerase inhibitor, was shown to reduce USUV load in a mice model. IFN-/ and IFN-receptor knockdown AG129 mice susceptible to USUV infection were used to evaluate the effect of Favipiravir on viremia caused by Usutu virus. AG129 mice were inoculated with the virus and treated with Favipiravir. The viral RNA was significantly reduced compared to the control animals. In addition, there was a reduction in the viral RNA level in body tissues. (79). Currently, more studies channeled toward vector control, discovery of vaccines, and antivirals specific for USUV infection are needed in Africa, especially Nigeria.
Recommendations and conclusion
The genetic variability of the USUV strains discovered in Europe, highlights the numerous importations from Africa as well as the adaptability of the strains circulating there. The complete prevalence, regional range, and seasonality of USUV infection are yet to be identified (though one could predict more cases to occur in warmer months when other mosquito-borne arboviral infections are more common) (80). Therefore, there is an urgent need for an integrated surveillance systems (“One Health”) and intelligence for emerging and re-emerging pathogens at animal, human and vectors interface to be developed and implemented in Africa and other continents to elude underreporting and underestimation of arboviral infections. A robust and responsive animal and human surveillance system should be developed and improved upon, with focus on Veterinarians and animal handlers who are constantly having interactions with wild birds and other animals. For example, sentinel chickens have been extensively used in the United States and Europe to survey for WNV. Figure 5 shows the distribution of the poultry industry in Nigeria and a cluster of this are located near the Atlantic, where some migratory birds are found in the country. The use of sentinel chickens could be an excellent tool for USUV surveillance across the six geo-political zones in Nigeria. In addition, the Nigeria government, and by extension other Africa countries, needs to build and establish facilities for confirmatory diagnosis of arboviral infections for the enhanced integrated and targeted surveillance and mitigation. For future seroprevalence studies, positive serology assays must be confirmed by several methods most especially molecular techniques (currently underutilized in Africa), and virus neutralization methods to distinguish USUV from other Flaviviruses especially WNV in order to prevent false positive or false negative conclusions, leading to underestimation of USUV infections and future negative impact on the poultry industry, animal and human populations in that clime.
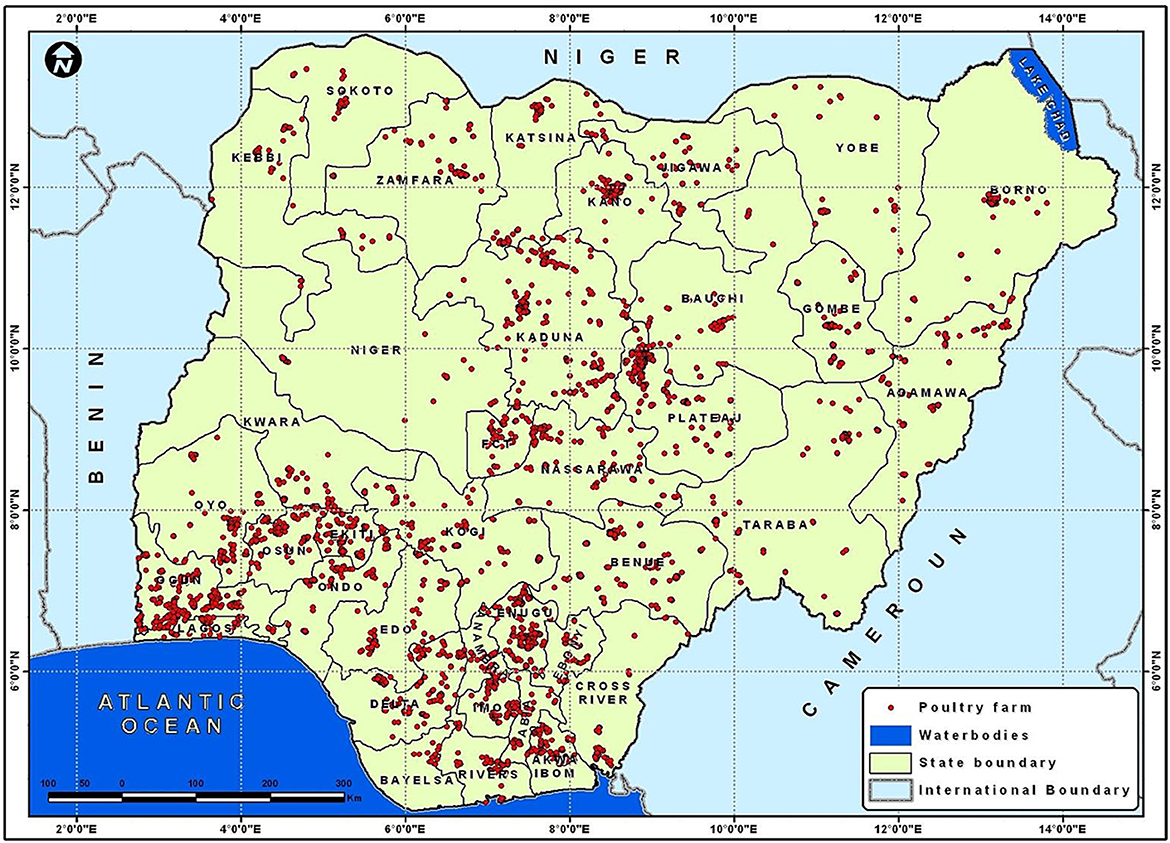
Figure 5. A map of Nigeria showing the distribution of poultry industry Omodele T and Okere I A (81).
Author contributions
OCA did the conceptualization. OCA, ROA, AB, and OOA designed the table and figures. OCA, OOA, AB, SCO, IPO, ROA, and AB wrote and reviewed the draft and final versions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Clé M, Beck C, Salinas S, Lecollinet S, Gutierrez S, Van de Perre P, et al. Usutu virus: a new threat?. Epidemiol Infect. (2019) 3:1–11. doi: 10.1017/S0950268819001213
2. Diagne MM, Ndione MHD, Di Paola N, Fall G, Bedekelabou A. P, Sembène P. M, et al. Usutu virus isolated from rodents in Senegal. Viruses. (2019) 11:81. doi: 10.3390/v11020181
3. Benzarti E, Linden A, Desmecht D, Garigliany M. Mosquito-borne epornitic flaviviruses: an update and review. J Gen Virol Microbiol Soc. (2019) 119–32. doi: 10.1099/jgv.0.001203
4. Nikolay B, Diallo M, Boye CSB, Sall AA. Usutu virus in Africa. Vector-Borne Zoonotic Dis. (2011) 5:1417–23. doi: 10.1089/vbz.2011.0631
5. Medrouh B, Lafri I, Beck C, Leulmi H, Akkou M, Abbad L, et al. First serological evidence of West Nile virus infection in wild birds in Northern Algeria. Comp Immuno Microbiol Infect Dis. (2020) 69:415. doi: 10.1016/j.cimid.2020.101415
6. Moseley PL. Exercise, stress, and the immune conversation. Exc Sci Rev. (2000) 28:128–32. Available online at: https://pubmed.ncbi.nlm.nih.gov/10916705/
7. Jöst H, Bialonski A, Maus D, Sambri V, Eiden M, Groschup MH, et al. Isolation of Usutu virus in Germany. Am J Trop Med Hyg. (2011) 85:551–3. doi: 10.4269/ajtmh.2011.11-0248
8. Hubálek Z, Rudolf I, Capek M, Bakonyi T, Betášová L, Nowotny N. Usutu virus in blackbirds (Turdus merula) Czech Republic 2011–2012. Transbound Emerg Dis. (2014) 61:273–6. doi: 10.1111/tbed.12025
9. Bakonyi T, Erdélyi K, Ursu K, Ferenczi E, Csörgo T, Lussy H, et al. Emergence of usutu virus in Hungary'. J Clin Microbiol. (2007) 45:3870–4. doi: 10.1128/JCM.01390-07
10. Calzolari M, Bonilauri P, Bellini R, Albieri A, Defilippo F, Maioli G, et al. Evidence of simultaneous circulation of west Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS ONE. (2010) 5:4234. doi: 10.1371/journal.pone.0014324
11. Savini G. Usutu virus in ITALY: an emergence or a silent infection?. Vet Microbiol. (2011) 151:264–74. doi: 10.1016/j.vetmic.2011.03.036
12. Vázquez González A., Ángel Jiménez-Clavero M. USUTU virus-potential risk of human disease in Europe Pathogenesis of arbovirus infections. View Proj. (2011) 3:9935. doi: 10.2807/ese.16.31.19935-en
13. Weidinger P., Kolodziejek J., Bakonyi T., Brunthaler R., Erdélyi K., Weissenböck H., et al. Different dynamics of Usutu virus infections in Austria and Hungary 2017–2018. Transbound Emerg Dis. (2020) 67:298–307. doi: 10.1111/tbed.13351
14. Folly AJ. Detection of Usutu virus infection in wild birds in the United Kingdom 2020. Eurosurveillance. (2020) 25:1–5. doi: 10.2807/1560-7917.ES.2020.25.41.2001732
15. Vilibic-Cavlek T, Petrovic T, Savic V, Barbic L, Tabain I, Stevanovic V, et al. Epidemiology of usutu virus: the European scenario. Pathogens. (2020) 9:1–19. doi: 10.3390/pathogens9090699
16. Bakonyi T. Complete genome analysis and molecular characterization of Usutu virus that emerged in Austria in 2001: comparison with the South African Strain SAAR-1776 and other flaviviruses. Virology. (2004) 328:301–10. doi: 10.1016/S0042-6822(04)00525-2
17. Engel D, Jost H, Wink M, Börstler J, Bosch S, Garigliany MM. Reconstruction of the evolutionary history and dispersal of Usutu virus, a neglected emerging arbovirus in Europe and Africa. (2016) 7:e01938e15. doi: 10.1128/mBio.01938-15
18. Cadar D. Widespread activity of multiple lineages of Usutu virus Western Europe 2016. Eurosurveillance. (2017) 22: 30452. doi: 10.2807/1560-7917.ES.2017.22.4.30452
19. Nikolay B. A review of West Nile and Usutu virus co-circulation in Europe: how much do transmission cycles overlap?'. Trans R Soc Trop Med Hyg. (2015) 109:609–18. doi: 10.1093/trstmh/trv066
21. Mcintosh BM, Jupp PG, De Sousa J. Mosquitoes feeding at two horizontal levels in gallery forest in Natal. South Africa. With reference to possible vectors ofchikungunya virus. J Ent Soc Afr. (1972) 45:12–52.
22. Adeleke MA. Twenty-three years after the first record of aedes albopictus in Nigeria: its current distribution and potential epidemiological implications. Af Entomol. (2015) 23:348–355. doi: 10.4001/003.023.0203
23. Cook CL, Huang YJ, Lyons AC, Alto BW, Unlu I, Higgs S, et al. North American Culex pipiens and Culex quinquefasciatus are competent vectors for Usutu virus. PLoS Neg Trop Dis. (2018) 12:8. doi: 10.1371/journal.pntd.0006732
24. Rahbari M, Rahlfs S, Jortzik E, Bogeski I, Becker K. H2O2 dynamics in the malaria parasite Plasmodium falciparum'. PLoS ONE. (2017) 12:e0174837. doi: 10.1371/journal.pone.0174837
25. Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. (2007) 3:1895–906. doi: 10.1371/journal.ppat.0030201
26. Roiz D, Vázquez A, Ruiz S, Tenorio A, Soriguer R, Figuerola J. Evidence that passerine birds act as amplifying hosts for usutu virus circulation. Ecohealth. (2019) 16:734–42. doi: 10.1007/s10393-019-01441-3
27. Arbovirus CDC catalog (1985). Available online at: www.ncid.cdc.gov/arbocat (accessed April 21 2011).
28. Becker N, Jöst H, Ziegler U, Eiden M, Höper D, Emmerich P, et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE. (2012) 7:604. doi: 10.1371/journal.pone.0032604
29. Cavrini F, Gaibani P, Longo G, Pierro AM, Rossini G, Bonilauri P, et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation Italy August-September 2009. Eurosurveillance. (2009) 14:9448. doi: 10.2807/ese.14.50.19448-en
30. Grottola A. Usutu virus infections in humans: a retrospective analysis in the municipality of Modena Italy. Clin Microbiol Infect. (2017) 23:33–7. doi: 10.1016/j.cmi.2016.09.019
31. Santini M, Vilibic-Cavlek T, Barsic B, Barbic L, Savic V, Stevanovic V, et al. First cases of human Usutu virus neuroinvasive infection in Croatia August–September 2013: clinical and laboratory features. J NeuroVirol. (2015) 21:92–7. doi: 10.1007/s13365-014-0300-4
32. Cadar D, Becker N, Campos Rd, Börstler J, Jöst H, Schmidt-Chanasit J. Usutu virus in bats Germany 2013. Emerg Infect Dis. (2014) 5:1771–3. doi: 10.3201/eid2010.140909
33. Störk T, Roi Md, Haverkamp AK, Jesse ST, Peters M, Fast C, et al. Analysis of avian Usutu virus infections in Germany from 2011 to 2018 with focus on dsRNA detection to demonstrate viral infections. Scient Rep. (2021) 11:5. doi: 10.1038/s41598-021-03638-5
34. Allering L. Detection of Usutu virus infection in a healthy blood donor from south-west Germany 2012. Euro Surveill. (2012) 17:50. doi: 10.2807/ese.17.50.20341-en
35. Bakonyi T, Erdélyi K, Brunthaler R, Dán Á, Weissenböck H, Nowotny N. Usutu virus Austria and Hungary 2010–2016. Emerg Microb Infect. (2017) 6:72. doi: 10.1038/emi.2017.72
36. Chevalier V, Marsot M, Molia S, Rasamoelina H, Rakotondravao R, Pedrono M, et al. Serological evidence of west nile and usutu viruses circulation in domestic and wild birds in wetlands of mali and madagascar in 2008. Int J Environ Res and Public Health. (2020) 17:1998. doi: 10.3390/ijerph17061998
37. Manarolla G, Bakonyi T, Gallazzi D, Crosta L, Weissenböck H, Dorrestein GM, et al. Usutu virus in wild birds in northern Italy. Vet Microbiol. (2010) 141:159–63. doi: 10.1016/j.vetmic.2009.07.036
38. Weissenböck H, Kolodziejek J, Url A, Lussy H, Rebel-Bauder B, Nowotny N. Emergence of Usutu virus an African mosquito-borne Flavivirus of the Japanese encephalitis virus group central Europe. Emerg Infect Dis. (2002) 8:652–6. doi: 10.3201/eid0807.020094
39. Montagnaro S, Piantedosi D, Ciarcia R, Loponte R, Veneziano V, Fusco G, et al. Serological evidence of mosquito-borne flaviviruses circulation in hunting dogs in Campania Region, Italy. Vector Borne Zoonotic Dis. (2019) 19:142–7. doi: 10.1089/vbz.2018.2337
40. Percivalle E, Sassera D, Rovida F, Isernia P, Fabbi M, Baldanti F, et al. usutu virus antibodies in blood donors and healthy forestry workers in the lombardy region. Northern Italy. Vector-Borne Zoonotic Dis. (2017) 17:658–61. doi: 10.1089/vbz.2017.2126
41. Pierro A, Gaibani P, Spadafora C, Ruggeri D, Randi V, Parenti S, et al. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010–2011. Clin Microbiol Infect. (2013) 19:E451–3. doi: 10.1111/1469-0691.12241
42. Barbic L, Vilibic-Cavlek T, Listes E, Stevanovic V, Gjenero-Margan I, Ljubin-Sternak S, et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. (2013) 13:772–4. doi: 10.1089/vbz.2012.1236
43. Nagy A, Mezei E, Nagy O, Bakonyi T, Csonka N, Kaposi M, et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Eurosurveillance. (2019) 2:1900038. doi: 10.2807/1560-7917.ES.2019.24.28.1900038
44. Nagy A, Csonka N, Takács M, Mezei E, Barabás É. West Nile and Usutu virus seroprevalence in Hungary: A nationwide serosurvey among blood donors in 2019. PLoS ONE. (2022) 17:6840. doi: 10.1371/journal.pone.0266840
45. Chvala S, Kolodziejek J, Nowotny N, Weissenböck H. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J Comp Pathol. (2004) 131:176–85. doi: 10.1016/j.jcpa.2004.03.004
46. Chvala S, Bakonyi T, Bukovsky C, Meister T, Brugger K, Rubel F, et al. Monitoring of Usutu virus activity and spread by using dead bird surveillance in Austria, 2003–2005. Vet Microbiol. (2007) 122:237–45. doi: 10.1016/j.vetmic.2007.01.029
47. Graninger M, Hubmer S, Riederer F, Kettner S, Hauk M, Auf T, et al. The first case of usutu virus neuroinvasive disease in Austria 2021. Open Forum Infect Dis. (2022) 9:255. doi: 10.1093/ofid/ofac255
48. Lecollinet S, Blanchard Y, Manson C, Lowenski S, Laloy E, Quenault H, et al. Dual emergence of usutu virus in common blackbirds, Eastern France, 2015. Emerg Infect Dis. (2016) 22:2225. doi: 10.3201/eid2212.161272
49. Simonin Y, Sillam O, Carles MJ, Gutierrez S, Gil P, Constant O, et al. Human Usutu virus infection with atypical neurologic presentation Montpellier France 2016. Emerg Infect Dis. (2018) 24:875–8. doi: 10.3201/eid2405.171122
50. Rijks JM, Kik M, Slaterus R, Foppen R, Stroo A, IJzer J, et al. Widespread Usutu virus outbreak in birds in The Netherlands 2016. Eurosurveillance. (2016) 21:30391. doi: 10.2807/1560-7917.ES.2016.21.45.30391
51. Zaaijer HL, Slot E, Molier M, Reusken CB, Koppelman MHGM. Usutu virus infection in Dutch blood donors. Transfusion. (2019) 59:2931–7. doi: 10.1111/trf.15444
52. Meister T. Serological evidence of continuing high Usutu virus (Flaviviridae) activity and establishment of herd immunity in wild birds in Austria. Vet Microbiol. (2007) 127:237–48. doi: 10.1016/j.vetmic.2007.08.023
53. Bazanów B. A survey on West Nile and Usutu viruses in horses and birds in Poland. Viruses. (2018) 10:87. doi: 10.3390/v10020087
54. Hassine TB, De Massis F, Calistri P, Savini G, Mohamed BB, Ranen A, et al. First detection of co-circulation of West Nile and Usutu viruses in equids in the south-west of Tunisia. Transbound Emerg Dis. (2014) 61:385–9. doi: 10.1111/tbed.12259
55. García-Bocanegra I Paniagua J, Gutiérrez-Guzmán A. V, Lecollinet S, Boadella M, Arenas-Montes A, et al. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet Res. (2016) 12:4. doi: 10.1186/s12917-016-0876-4
56. Nir Y, Goldwasser R, Lasowski Y, Avivi A. Isolation of arboviruses from wild birds in Israel. Am J Epidemiol. (1967) 86:372–8. Available online at: https://www.cabdirect.org/cabdirect/abstract/19682703969
57. Reeves WC. Overwintering of arboviruses. Progress in medical virology Fortschritte der medizinischen. Virusforschung Progres en virologie medicale. (1974) 17:193–220.
58. Hubálek Z, Halouzka J. Arthropod-borne viruses of vertebrates in Europe. Acta Scientiarum Naturalium Brno. (1996) 30:1–95. Available online at: https://www.cabdirect.org/cabdirect/abstract/20053130573
59. Calisher CH, Maness KS, Lord RD, Coleman PH. Identification of two South American strains of eastern equine encephalomyelitis virus from migrant birds captured on the Mississippi Delta 1. Am J Epidemiol. (1971) 4:172–8. doi: 10.1093/oxfordjournals.aje.a121309
60. Ernek E. Arboviruses in birds captured in Slovakia. Journal of hygiene epidemiology microbiology and immunology. (1977) 21:353–9.
62. Anderson JF, Andreadis TG, Vossbrinck CR, Tirrell S, Wakem EM, French RA, et al. Isolation of West nile virus from mosquitoes crows and a cooper's hawk in connecticut John. Trends Endocrinol Metab. (1999) 5:2331–3. doi: 10.1126/science.286.5448.2331
63. Work TH, Hurlbut HS, Taylor RM. Indigenous wild birds of the Nile Delta as potential West Nile virus circulating reservoirs. Am J Trop Med Hygiene. (1955) 4:872–88. doi: 10.4269/ajtmh.1955.4.872
64. Lotem A. Sex differences in begging vocalizations of nestling barn swallows. Hirundo rustica. Animal Behav. (2003) 66:1003–10. doi: 10.1006/anbe.2003.2295
65. Bazzi G, Cecere JG, Caprioli M, Gatti E, Gianfranceschi L, Podofillini S, et al. Clock gene polymorphism. Migratory behaviour and geographic distribution: a comparative study of trans-Saharan migratory birds. Mol Ecol. (2016) 25:6077–91. doi: 10.1111/mec.13913
66. Vega ML, Willemoes M, Thomson RL, Tolvanen J, Rutila J, Samaš P, et al. First-time migration in juvenile common cuckoos documented by satellite tracking. PLoS One. (2016) 11:e0168940. doi: 10.1371/journal.pone.0168940
67. Cambridge CI. Migrant Birds in the West African Sahel. Cambridge Conservation Initiative. (2010) p 1–2. Available online at: https://www.cambridgeconservation.org (accessed December 4, 2022).
68. Hahn S, Bauer S, Liechti F. The natural link between Europe and Africa - 2.1 billion birds on migration. Oikos. (2009)118:624–6. doi: 10.1111/j.1600-0706.2008.17309.x
69. Benzarti E, Sarlet M, Franssen M, Cadar D, Schmidt-Chanasit, J Rivas JF, et al. Usutu Virus Epizootic in Belgium in 2017 and 2018: evidence of Virus Endemization and Ongoing Introduction Events. Vector-Borne Zoonotic Dis. (2020) 20:43–50. doi: 10.1089/vbz.2019.2469
70. Uttah E. The taxa structure and composition of zooplankton communities of bonny estuary : a bio-indication of anthropogenic activities. Des Study Area. (2013) 14:635–41. Available online at: https://core.ac.uk/reader/32226860
71. Akinyera B. Microbial loads of beef and hygienic practice of butchers in jos municipal abattoir. Asian J Res Ani Vet Sci. (2018) 5:1–9. doi: 10.9734/AJRAVS/2018/42570
72. Balthazar T. Managing patients with short-term mechanical circulatory support: JACC review topic of the week. J Am Coll Cardiol. (2020) 77:1243–56. doi: 10.1016/j.jacc.2020.12.054
73. Visa TI, Ajumobi O, Bamgboye E, Ajayi I, Nguku P. Evaluation of malaria surveillance system in Kano State Nigeria 2013–2016. Infect Dis Pov. (2020) 9:1–9. doi: 10.1186/s40249-020-0629-2
74. Meseko C, Globig A, Ijomanta J, Joannis T, Nwosuh C, Shamaki D, et al. Evidence of exposure of domestic pigs to Highly Pathogenic Avian Influenza H5N1 in Nigeria. Sci Rep. (2018) 8:1–9. doi: 10.1038/s41598-018-24371-6
75. Obayelu AE. Socio-economic analysis of the impacts of avian influenza epidemic on households poultry consumption and poultry industry in Nigeria: empirical investigation of Kwara State. Livestock Res Rural Develop. (2007) 19:4. Available online at: https://lrrd.cipav.org.co/lrrd19/1/obay19004.htm
76. Blázquez AB. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology. (2015) 482:67–71. doi: 10.1016/j.virol.2015.03.020
77. Martín-Acebes MA. A recombinant DNA vaccine protects mice deficient in the alpha/beta interferon receptor against lethal challenge with Usutu virus. Vaccine. (2016) 34:2066–2073. doi: 10.1016/j.vaccine.2016.03.015
78. Salgado R, Hawks SA, Frere F, Vázquez A, Huang CYH, Duggal NK. West nile virus vaccination protects against usutu virus disease in mice. Viruses. (2021) 13:2352. doi: 10.3390/v13122352
79. Segura, Guerrero NA. Favipiravir inhibits in vitro Usutu virus replication and delays disease progression in an infection model in mice. Antiviral Res. (2018) 160:137–42. doi: 10.1016/j.antiviral.2018.10.026
80. Gill CM, Kapadia RK, Beckham JD, Piquet AL, Tyler KL, Pastula DM. Usutu virus disease: a potential problem for North America?. J Neurovirol. (2020) 26:149–54. doi: 10.1007/s13365-019-00818-y
81. Omodele T, Okere IA. GIS application in poultry production: identification of layers as the major commercial product of the poultry sector in Nigeria. Livestock Res Rural Develop. (2014) 26. Available online at: http://www.lrrd.org/lrrd26/5/omod26097.html
Keywords: Usutu virus, Culex mosquitoes, migratory birds, flavivirus, public health
Citation: Akinsulie OC, Adesola RO, Bakre A, Adebowale OO, Adeleke R, Ogunleye SC and Oladapo IP (2023) Usutu virus: An emerging flavivirus with potential threat to public health in Africa: Nigeria as a case study. Front. Vet. Sci. 10:1115501. doi: 10.3389/fvets.2023.1115501
Received: 04 December 2022; Accepted: 27 January 2023;
Published: 16 February 2023.
Edited by:
Mahmoud Darweesh, Uppsala University, SwedenReviewed by:
Helen Roberts, Food and Rural Affairs, United KingdomBenjamin Cull, University of Minnesota Twin Cities, United States
Copyright © 2023 Akinsulie, Adesola, Bakre, Adebowale, Adeleke, Ogunleye and Oladapo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olalekan Chris Akinsulie,  b2xhbGVrYW4uYWtpbnN1bGllQHdzdS5lZHU=
b2xhbGVrYW4uYWtpbnN1bGllQHdzdS5lZHU=
†Present address: Olalekan Chris Akinsulie, Department of Veterinary Microbiology and Pathology, College of Veterinary Medicine, Washington State University, Pullman, WA, United States
 Olalekan Chris Akinsulie
Olalekan Chris Akinsulie Ridwan Olamilekan Adesola
Ridwan Olamilekan Adesola Adetolase Bakre
Adetolase Bakre Oluwawemimo Oluseun Adebowale
Oluwawemimo Oluseun Adebowale Richard Adeleke
Richard Adeleke Seto Charles Ogunleye
Seto Charles Ogunleye Ifeoluwa Peace Oladapo
Ifeoluwa Peace Oladapo