
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 17 January 2023
Sec. Veterinary Dentistry and Oromaxillofacial Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1114445
Introduction: Pituitary pars intermedia dysfunction (PPID) and dental disorders are of major concern in horses older than 15 years. Although PPID in geriatric horses and dental disorders in all age groups are well described, a connection between this endocrine disease and pathological changes in equine dental structures has not yet been investigated. In humans, periodontitis is considered to be a complication of systemic diseases like diabetes mellitus type 2, obesity and various conditions leading to an impaired immune response. In PPID, cross links to insulin and immune dysregulations are proven. The aim of this study was to compare histological findings of the gingiva and the sub gingival periodontal ligament of PPID affected horses with control horses.
Methods: In a case-control morphometric descriptive study, 145 dental locations of 10 PPID affected horses (27.3 ± 2.06 years) were compared with 147 dental locations of 10 controls (21.4 ± 4.12 years). Histological parameters were leukocyte infiltration, keratinization of gingival epithelium, blood vessel supply of the periodontium and structure of cementum.
Results: The distribution and localization of gingival leukocyte infiltrations (LI) in PPID affected horses was more often multifocal to coalescing (p = 0.002) and reached into deeper parts of the periodontium, sometimes down to the sub gingival periodontal ligament (PDL). Aged animals of both groups showed higher prevalence (PPID: OR 1.66; controls: OR 1.15) for severe leukocyte infiltration in the PDL. PPID was not significantly associated with increased LI. The cementum bordering the soft tissue in interdental locations showed four times more irregularities in PPID affected horses than in controls which predisposes for interdental food impaction and periodontal diseases.
Discussion: In summary, multifocal to coalescing leukocytes and irregular cementum are seen more often in PPID than in controls - however our findings mainly reflect an association of older age with periodontal disease.
In human and canine dentistry, precise descriptions of morphological and histological features of the gingiva exist (1–7). In contrast, the gingiva of the horse is addressed only in a few studies (8–10). The gingiva, along with the cementum, periodontal ligament and alveolar bone, forms the structural and functional group of tissue called the periodontium. The epithelium of the healthy gingiva is connected with the alveolar mucosa at the mucogingival junction (MGJ). The gingiva itself can be divided into an attached and a free part. The attached gingiva is firmly bound to the periosteum of the alveolus by connective tissue. The free gingiva lies unattached next to the tooth, forming a collar (5). The space between tooth and free gingiva is named the gingival sulcus (Sulcus gingivalis) and the epithelium forming the inner wall of the gingival sulcus is called the sulcular epithelium (11). At the bottom of the gingival sulcus, the so-called junctional epithelium attaches to the hard substance of the tooth. In contrast to brachydont species, where the junctional epithelium attaches to enamel, in hypsodont species like horses it attaches to the dental cementum (11). An intact junctional epithelium forms a permeable barrier with important immunological tasks, protecting the deeper tissues from the oral microbiotic flora and preventing periodontal diseases. In horses, the primary reasons for the onset of periodontal diseases are physical disorders of tooth growth, eruption and/or wear (9, 12, 13), causing gaps (diastemata) between neighboring cheek teeth and allowing food to become entrapped. Once food is impacted in widened interdental spaces, the integrity of the interdental junctional epithelium is lost and progressive periodontal disease results.
The most likely etiopathogenesis of periodontal disease in human and canine species is primarily bacterial, initiated by the development of plaque (3, 9), leading to gingival inflammation as the key factor for the onset of periodontitis (14). In human dentistry, hormonal changes due to puberty or pregnancy lead to changes in the immune system, which predispose for gingivitis (15–17). Hyperglycemia, like in diabetes mellitus type 1 and 2, hypercortisolism in Cushing's syndrome and immunodeficiencies (e.g. in leukemia, neutropenia, acquired immune deficiency syndrome) are also known to modify the course and severity of periodontitis. This proves the multifactorial nature of this seemingly localized inflammation (15, 17–19). Furthermore, obesity is recognized as a risk factor for the development of periodontitis (20). Impaired immune function due to increased production of proinflammatory cytokines from adipose tissue (adipokines) is proposed as the underlining pathophysiological mechanism (21).
PPID is the most common equine endocrine disease, affecting more than 20% of horses older than 15 years. The loss of dopaminergic neurons leads to loss of control of the melanotropic cells in the pars intermedia of the equine pituitary gland (22, 23). The result is an overproduction of proopiomelanocortin (POMC) (24). POMC is further processed into smaller peptides, e.g., adrenocorticotropin (ACTH), α-melanocyte-stimulating hormone (α-MSH), β-endorphin and corticotropin-like intermediate lobe peptide (CLIP). The different amounts and the variety of POMC-derived peptides are thought to cause the different clinical signs of PPID (23, 25). Although PPID is a specific equine endocrine disease, it is often associated with insulin dysregulation, delayed wound-healing, altered collagen metabolism and increased susceptibility to infections (16, 25, 26), which are known to predispose for periodontal disease in humans and dogs. The question arises, if – besides old age – a systemic disease like PPID may weaken the periodontal apparatus, leading to interdental widening, food entrapment and finally to periodontitis. We hypothesized, that periodontal disease in older equids with PPID is more common than in aged controls. Therefore, the main objective of this study was to describe histomorphological characteristics, including inflammatory signs of the equine gingiva and subgingival periodontium by comparing healthy horses older than 15 years with PPID affected horses.
PPID affected horses (n = 10) and non-affected controls (n = 10) needed to be 15 years or older. The inclusion criteria for the PPID group were the presence of micro- or macroadenoma in the pars intermedia of the pituitary gland and at least one clinical sign of PPID (e.g., hypertrichosis, muscle atrophy leading to a “sway back” or “pot-belly” appearance, abnormal fat distribution, hyperhidrosis, laminitis) or a history of PPID with at least one of the above mentioned clinical signs. The control group consisted of horses without PPID-associated alterations in the pituitary gland, and without a history or clinical signs of PPID (Table 1). The horses were euthanized due to medical reasons unrelated to this study.
The jaws were dissected with a band saw (type K440H, Kolbe Foodtec, Elchingen, Germany) to obtain the upper and lower incisor arcades as well as the four cheek teeth quadrants. The specimens were cleaned of loose food and debris with water and were subsequently fixed in 10% neutral buffered formalin. Macroscopic findings such as periodontal pocketing, diastema formation, food impaction and gingival texture changes are published elsewhere (28). The specimens were further sectioned to obtain histological samples from preassigned locations (Table 2, Figures 1, 2) with a diamond bladed band saw (Proxxon Type MBS 240/E No 27 172, Föhren, Germany). The samples were processed as described by Steinfort et al. (8). Specimens were decalcified in buffered ethylene diamine tetra-acetate (EDTA, pH 8.0) for 6–8 weeks at room temperature. Afterwards, the specimens were embedded in para?n wax, sectioned and stained with hematoxylin-eosin (HE). Samples were assessed via light microscopy (Leica DM750, Leica DM2500 and Leica ICC50 HD, Wetzlar, Germany). With slight modifications, the system of Steinfort et al. (8) was used to evaluate the following parameters in the dental and interdental gingiva and the subgingival periodontal tissue:
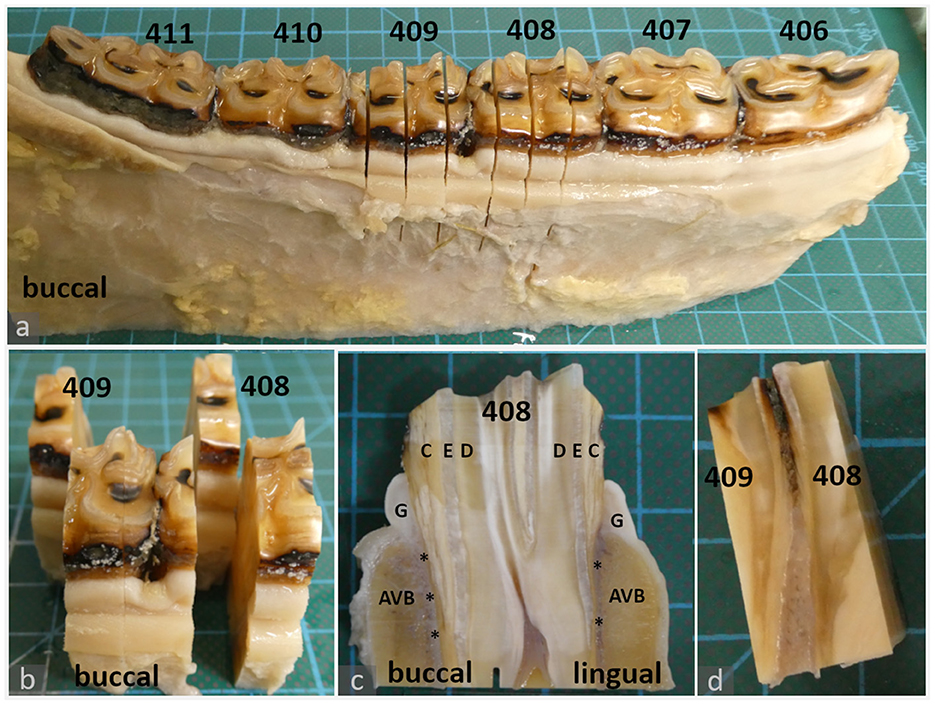
Figure 1. Histologic sampling, examples shown for teeth 408 and 409 (a–c): 20-year-old Icelandic mare (control) (d): 29-year-old Warmblood mare (PPID). (a, b): Dental gingiva samples were collected by transverse section of teeth 408 and 409 in mesial and distal positions of the tooth. The mesial position was processed and evaluated as described in material and methods. The distal position (background) was archived. (c): Sections were further divided to obtain separate specimens from the lingual and buccal aspect. Each specimen contained the gingiva (G), subgingival parts of the alveolar bone (AVB), subgingival periodontal ligament (asterisks) and dental substances: cement (C), enamel (E), dentin (D). (d): Interdental gingiva samples were collected by sagittal section through teeth 408 and 409.
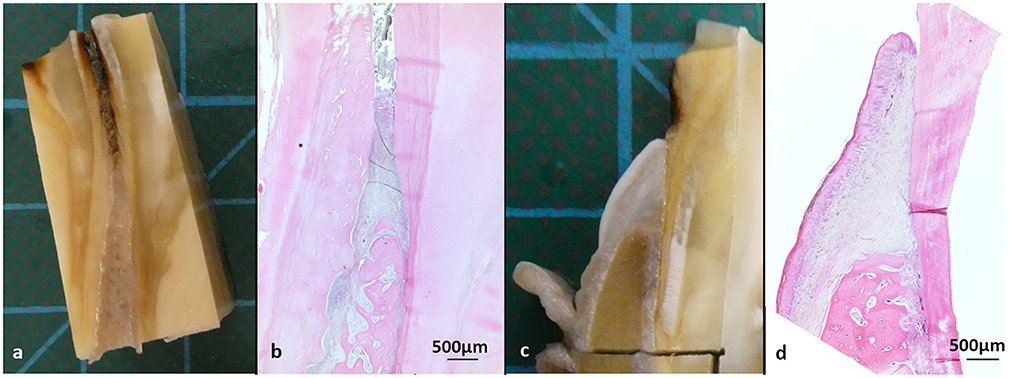
Figure 2. Macroscopic and histological overview, stained with hematoxylin-eosin (HE). (a, b) Interdental ginigva sample between tooth 408 and 409 of a 29-year-old Icelandic mare (PPID). (c, d) Dental gingiva sample, cheek teeth 409 lingual aspect of a 19-year-old Icelandic mare (control).
Epithelia were evaluated for the type of keratinization: non-keratinized, orthokeratinized (no nuclei in stratum corneum) and parakeratinized (cells with pyknotic nuclei in stratum corneum). In addition, the presence/absence of rete pegs was recorded.
SE and JE were each evaluated for the presence of locally or diffusely distributed leukocytic infiltration (LI) and leukocyte cell type, using 400x total-magnification.
The degree of LI was scored using 200x total-magnification within the complete SE and JE. For statistical reasons, the scores were summarized in the binominal categories of either “low” or “high”:
Low LI = 0: no leukocytic infiltration; 1: 1–2 leukocytes (mild);High LI = 2: 3–10 leukocytes (moderate); 3: > 10 leukocytes (severe).
The attached area of the JE on the cementum surface was scored as 0: no attachment; 1: attachment present with 1-5 cell layers; 2: attachment present with > 5 cell layers. For statistical reasons the scores 1 and 2 were combined to create the binominal categories of either “no attachment” or “attachment present”.
The distribution of leukocytes in the LP and PDL was recorded concerning localization (e.g., close to the gingival sulcus, the cementum, the oral gingival epithelium or the alveolar bone) and categorized as not present, multifocal or multifocal to coalescing.
The leukocyte cell types were evaluated in 400x total magnification.
The degree of LI was scored using 100x total-magnification within the complete LP. The following score was applied:
Low LI = 1: 0–50 leukocytes (mild);
High LI = 2: 51–100 leukocytes (moderate); 3: > 100 leukocytes (severe).
Eosinophilic infiltration was recorded separately as present or not present.
The distribution and localization of blood vessels in the lamina propria and the PDL was recorded and grouped either as homogenous (physiologic) or inhomogeneous, their quantity was scored using one optic field in 100x total-magnification:
Low numbers of blood vessels = 0–5 blood vessels;
High numbers of blood vessels = ≥ 6 blood vessels.
The collagen fiber arrangement was evaluated in the LP and PDL as regular or irregular (fibers running in criss-cross manner with irregular distances between connective tissue cells and matrix) in HE stain.
Higher mineralized cementum from “rest periods” in cementum formation was visible as parallel incremental lines as described by Fiorellini et al. (29). Evaluation of the alignment of incremental lines was recorded as regular or irregular.
The presence and localization of plaque (bluish deposition on cemental surface) and/or caries [brownish discoloration of the bluish plaque matrix and loss of calcified tissue forming flake or flask-like lesions, were defined as peripheral caries (30)] was recorded as present or not present, and classified as supragingival (above the sulcus gingivalis) or gingival (within the gingival sulcus).
The dataset was analyzed with the statistical software SAS® 9.4 (SAS Institute Inc., 2008). A t-test was calculated to detect the age difference between the groups. In all epithelia, keratinization was evaluated as present or not present. The degrees of leukocytic infiltration of the sulcular epithelium and the lamina propria were combined for statistical analysis (= degree of leukocytic infiltration of the gingiva: LIG). LIG was differentiated in “low” (0–2 leukocytes in SE, 0–50 leukocytes in LP) or “high” (≥ 3 leukocytes in SE, ≥ 51 leukocytes in LP). The junctional epithelium was evaluated separately because of its specialized tasks in the periodontium. Plaque was almost ubiquitous. Therefore, just localization of plaque was compared statistically between groups. Caries was categorized as present or absent.
All dependent variables, except the distribution of leukocytes in the PDL in dental and interdental locations, were analyzed using the GLIMMIX procedure with a binomial distribution (link-function = logit). For the distribution of leukocytes in the PDL in dental and interdental locations a multinomial model was created (link-function = cumlogit). The models included age as a covariable and group as a fixed effect. The interaction between age and group was also tested. If there was no significant interaction between age and group, the interaction was removed from the model. All model results were calculated for a mean age of 24.4 years. Due to rare occurence in some of the tested parameters (dental gingiva locations: oral gingival and junctional epithelium keratinization type, oral gingival and sulcular epithelium presence of rete pegs, PDL distribution of blood vessels; interdental gingiva locations: oral gingival epithelium presence of rete pegs, LP and PDL collagen fiber arrangement, PDL distribution of blood vessels and eosinophil infiltration) fitting of the model was not possible. In these cases, a Chi-Square test or Fisher exact tests were used to test the dependency of the group and the respective parameter. Age could not be considered in these parameters.
All statistical analyses were carried out separately for dental gingiva and interdental gingiva samples. Data is presented as mean ± SD if not mentioned otherwise. In all cases, a significance level of p ≤ 0.05 was used.
The PPID group (n = 10) consisted of four mares and six geldings, aged from 24 to 30 years (27.3 ± 2.06), including six Warmbloods, two Ponies, one Icelandic Horse and one Haflinger. Treatment with pergolid was reported in five horses of the PPID group. The control group (n = 10) consisted of six mares and four geldings, aged from 16 to 29 years (21.4 ± 4.12), including three Icelandic Horses, two Warmbloods, two Haflingers, one Pony, one Friesian and one Draft Horse. Horses in the PPID-affected group were significantly older than in the control group (p = 0.0007).
Table 3 summarizes the histological findings in the dental and interdental gingiva.
A parakeratinized stratified squamous epithelium was found in 99.1% (115/116) of the PPID and in 79.1% (91/115) of the control specimens. Only one PPID sample (0.9%) featured a non-keratinized stratified squamous epithelium (108 palatinal), whereas in controls, 20.9% of the specimens showed an orthokeratinized stratified squamous epithelium. Rete pegs were visible in all specimens and no blood vessels or leukocyte infiltrates were found in the oral gingival epithelium in either group. No significant differences between the groups were found.
In the PPID group, 50.9% of the specimens showed a keratinized stratified squamous epithelium, while in the control group 44.1% were keratinized (p = 0.3). None of the specimens showed orthokeratinization. In both groups, the coronal aspect of the SE was usually keratinized, and the apical aspect showed a non-keratinized stratified squamous epithelium. Rete pegs were visible in all PPID samples and in 97.4% (112/115) of the control specimens.
In both groups, approximately 15% of specimens showed no leukocytes within their SE. For statistical analysis, the degrees of LI in SE and LP were combined to leukocytic infiltration of the gingiva (LIG) and are displayed in the LP section. In both groups, the cell type of the LI in the SE consisted predominantly of lymphocytes, followed by neutrophils. Plasma cells were present only occasionally.
In both groups, the JE was classified as a non-keratinized stratified squamous epithelium. After controlling for age, rete pegs were present in 5.2% (5/97) of PPID specimens and 12.4% (14/113) of controls (Figure 3). Neither group nor age showed a significant influence on the presence of rete pegs. LI were present in 81.8% (81/99) of PPID samples and in 84.2% (96/114) of controls. Neither group (p = 0.35) nor age (p = 0.054) showed an influence on the degree of leukocytic infiltration in the JE. Like in the SE, the predominant cell types were lymphocytes, followed by neutrophils and plasma cells. An intact attachment of the JE to the tooth was not more often seen in the control group (96.5%) than in the PPID group (92.8%; p = 0.64).

Figure 3. Gingival sulcus and junctional epithelium with and without rete pegs, staining hematoxylin-eosin (HE) (a, b) oE, Oral epithelium; SE, Sulcular epithelium; JE, Junctional epithelium; LP, Lamina propria; C, Cementum; *Sulcus gingivalis (a) 22-year-old Friesian mare, control, 108 buccal aspect: Rete pegs (arrows) in JE present (b) 25-year-old Warmblood gelding, PPID, 109 buccal aspect: Rete pegs in JE not visible, notice leukocyte infiltration beneath and inside the JE.
The LP contained homogenously distributed blood vessels and a subepithelial capillary plexus beneath the JE and SE in every specimen. The number of blood vessels in the LP did not differ between the groups. Perivascular leukocyte infiltration was seen in all specimens of both groups in the gingiva. The PPID group showed more often (87.7%) a high degree of leukocytic infiltration of the gingiva (LIG) than the controls (79.3%), but there were no statistically significant differences, even if the adjustment for a mean age of 24.4 years was applied. However, the distribution of LI in the lamina propria showed a significant difference between both groups (p = 0.002). In the PPID group, 65.4% of the specimens showed multifocal LI and 34.6% multifocal to coalescing LI. Controls showed multifocal LI in 92.8% and multifocal to coalescing LI in only 7.2% (Figure 4). When comparing the groups at the mean age of 24.4 years, PPID affected animals showed a higher chance (OR: 6.83) for a multifocal to coalescing LI distribution in the LP than controls. The leukocyte cell populations consisted predominantly of lymphocytes and plasma cells. The third most common cell population in both groups in the LP were eosinophils. Eosinophils were observed in both groups as focal or multifocal infiltrations, lying close to the gingival sulcus or the cementum. Neither the group nor age influenced significantly the presence of eosinophils in the LP. The PPID group showed slightly fewer eosinophilic infiltrations (45.8%), than the controls (50.7%). Macrophages and neutrophils were never seen as the dominant cell population but were present, especially if there were macroscopically noted periodontal pockets close to the histological sampling location.
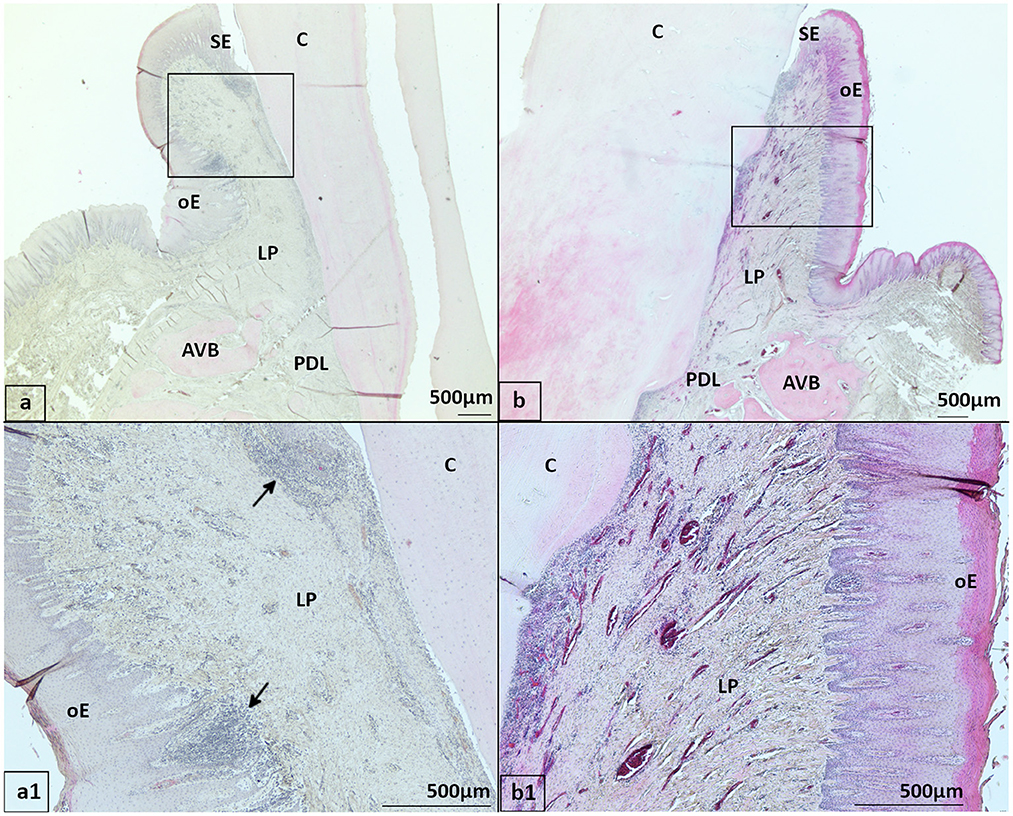
Figure 4. Distribution of leukocyte infiltrations (LI) in gingiva, HE stained (a, a1) 20-year-old Icelandic control mare, 409 buccal aspect with multifocal LI (arrows) in gingiva close to the gingival sulcus and oral epithelium. (b, b1) 29-year-old Warmblood gelding with PPID, 408 buccal aspect with multifocal to coalescing LI in the gingiva. oE, Oral epithelium; SE, Sulcular epithelium; LP, Lamina propria; PDL, Periodontal ligament; AVB, Alveolar bone; C, Cementum.
Blood vessels in the PDL of both groups were observed in every specimen, lying either close to the alveolar bone or close to the cementum in a homogenous distribution. Most of the specimens (65.8% in PPID and 75.8% in controls) showed high blood vessel numbers with no significant difference. Age but not group, had a major effect (p < 0.0001) on the amount of LI in the PDL. Animals older than 24.4 years had a higher chance (OR: 1.24) for high amounts of LI in the PDL (Figure 5). In the PPID group, 13.3% (15/113) showed no LI in the PDL, 65.5% showed multifocal LI and in 21.2%, a multifocal to coalescing distribution of LI in the PDL was noted. In the control group, 33.6% (38/113) showed no LI, 54% showed a multifocal distribution of LI and 12.4% a multifocal to coalescing LI in the PDL. Even after mathematically controlling for age, no significant effect on the distribution of LI in the PDL between groups was calculated. However, if the age decreases by one year, the chance to show none or multifocal LI in the PDL increases (OR: 1.1; p = 0.05). Leukocyte cell populations consisted predominantly of lymphocytes and plasma cells in both groups with some apoptotic lymphocytes. Eosinophils were seen more frequently in the PPID group (10.6%) than in the controls (2.7%), but neither group nor age showed a significant effect on the presence of eosinophils in the PDL (Figure 6). Neutrophils and macrophages were never the dominant cell population, but single such cells were observed in individual samples from both groups. There was no significant difference between the two groups concerning the presence of an irregular collagen fiber network (PPID 5.6%; controls 2.6%).
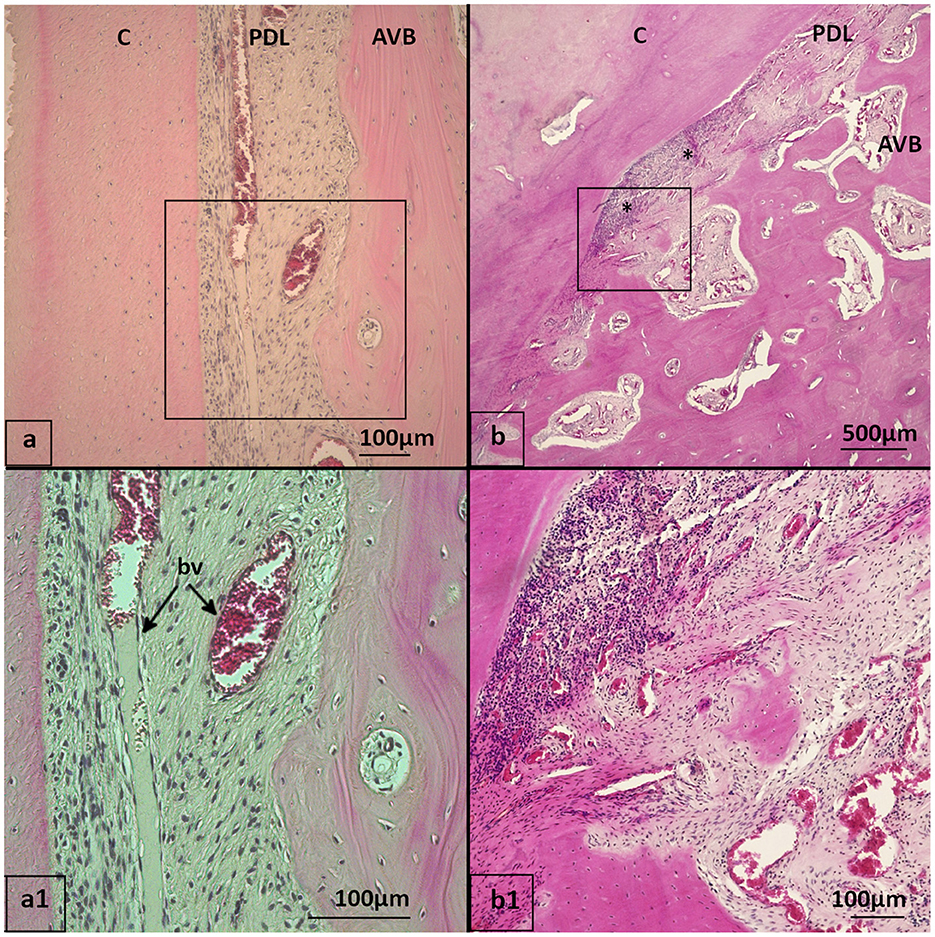
Figure 5. Leukocyte infiltrations (LI) in PDL, HE stained (a, a1) 21-year-old Haflinger gelding of the control group, 108 buccal aspect with no LI in PDL. The tooth position showed a gingival sulcus depth of < 1 mm and macroscopic gingiva alterations. No leukocyte infiltration within the PDL. Bv, blood vessels in longitudinal direction in the PDL. (b, b1) 28-year-old Warmblood mare with PPID, 409 buccal aspect with focal LI (*) in the PDL without periodontal pocket close to the observed area. The tooth position showed a gingival sulcus depth of 3 mm and macroscopical alterations of the gingiva. PDL, Periodontal ligament; AVB, Alveolar bone; C, Cementum.
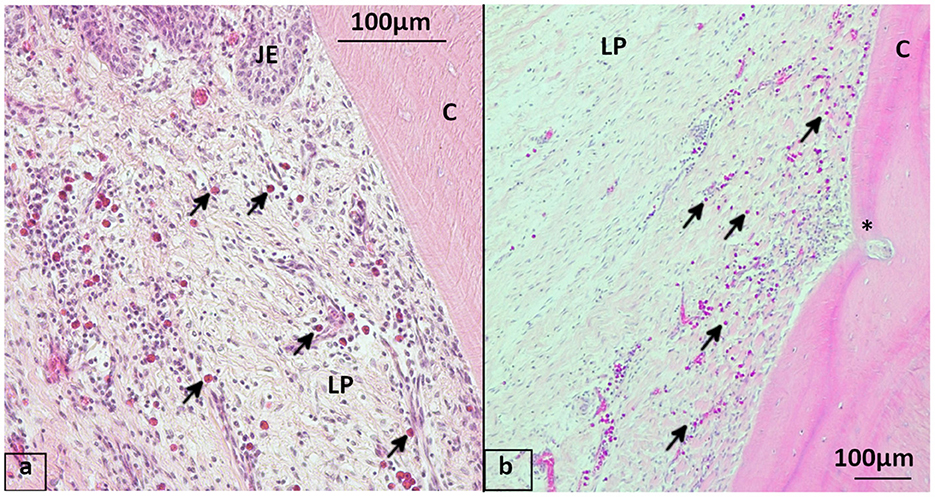
Figure 6. Eosinophils in the gingiva, staining hematoxylin-eosin (HE) (a) 19-year-old Icelandic mare from the control group, 409 lingual aspect with mulitfocal perivascular eosinophil infiltration (arrows) of the lamina propria beneath the junctional epithelium. (b) 29-year-old Icelandic PPID mare, 408 lingual aspect showing multiple eosinophils infiltrating the lamina propria, blood vessel running into cementum (*). JE, Junctional epithelium; LP, Lamina propria; C, Cementum.
Irregular cemental structures (Figure 7) were found in 15.5% of the PPID group and in 21.3% of the control samples. Within each group, an increase of 1 year in age results in a higher chance for irregular cemental structures (PPID OR: 1.66; control OR: 1.15). The interaction of age and group was significant (p = 0.005) in this parameter. Plaque was present within the gingival sulcus in 41.2% of the PPID and in 31.9% of the control samples, but there were no significant differences between the groups or age. Caries was found almost exclusively above the gingival sulcus and was recorded in 48.9% of PPID and 42.6% of control specimens. The interaction between age and group was significant with regard to the presence of caries (p = 0.007). In the (older) PPID group, an increase in age by 1 year even yields to a lower chance for caries (OR: 0.75).
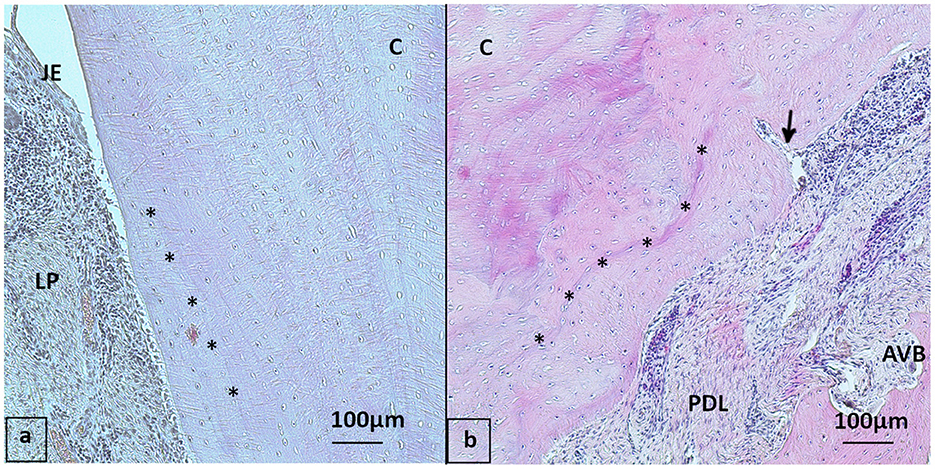
Figure 7. Cemental structure, HE stained (a) 20-year-old Icelanic mare from the control group, buccal aspect of 409 with regular cemental structure (asterisk: incremental lines of Salter almost parallel to each other and to the cemental surface). (b) 30-year-old Pony gelding from the PPID group, 109 buccal aspect with “irregular” cementum: Multiple signs of reparative cementum and incremental lines of Salter not parallel to each other (asterisk), note the blood vessel (arrow) running into the cementum. JE, Junctional epithelium; LP, Lamina propria; PDL, Periodontal ligament; AVB, Alveolar bone; C, Cementum.
Table 3 shows the detailed results of histological findings in interdental gingiva samples. Age significantly increased (p = 0.03) the degree of LI in the PDL. There was no difference between the groups (p = 0.91). An increase in age by 1 year yields to a higher chance (OR: 1.38) for high LI in the PDL. Eosinophilic infiltrations in the PDL were increased (p = 0.03) in the PPID group with 22.2% (4/18) compared to none in the control group. The interdental cementum of the interdental gingiva in the PPID group showed significantly more irregularities (15/29) than controls (4/31; p = 0.02). Comparing the groups mathematically at the same age of 24.4 years, horses affected with PPID had a much higher chance (OR: 8.5) to show irregular cemental structure in interdental positions than controls.
Equine periodontal disease is recognized as a clinically relevant entity, but is often seen as a secondary, mostly reversible process, if macroscopic lesions are corrected. Since the continuous eruption requires ongoing remodeling processes in the equine dentition anyway, healing of periodontal lesions in hypsodont species is assumed to occur with time (9). However, such lesions may lead to quidding, weight loss and early tooth loss when not treated (31, 32). To the knowledge of the authors, this is the first study that describes detailed periodontal, histological findings in PPID affected horses in comparison to unaffected controls. PPID, as an equine specific systemic endocrine disease, impairs multiple metabolic and immunological pathways. However, our hypothesis, that signs of periodontal structural weakening and inflammation in PPID affected horses are more severe than in controls, was supported in only two of our parameters: multifocal to coalescing leukocyte infiltrations in the LP of dental gingiva were found more often in PPID (p = 0.002), as well as an irregular cementum in interdental spaces (p = 0.02) and at non-interdental sites (age*group p = 0.005). This might point to a disproportionate inflammation in PPID affected horses and a structural weakening of the periodontal tissue predisposing for the development of diastemata.
Five of the ten horses of the PPID group were treated with pergolide. All still expressed classical clinical signs of PPID. A current study from Miller et al. (33) concluded that pergolide does not influence the immune function in horses with PPID. Therefore, we did not expect an influence of pergolide on the parameters we assessed and no separated evaluation of treated and non-treated horses was performed. A limitation of this study might be seen in the significantly older (27.3 ± 2.06 years) PPID than control group (21.4 ± 4.12 years, p < 0.001). The inclusion of horses aged at least 15 years could not prevent this effect. In human and canine dentistry, age was shown to be an associated factor for the development of periodontitis (34–37). The term immunosenescence as well as inflammaging (38–40) describes the influence of age on the immune system and getting older not only means a longer exposure time for destructive forces to develop (1, 35, 36). However, our results were statistically controlled for a mean age of 24.4 years and therefore the documented effects of age and PPID are regarded as valid results.
The predominant presence of a parakeratinized stratified epithelium in the oral gingiva of horses is in line with findings documented for brachydont species like dogs, cats and humans (41–43). The higher amount of orthokeratinized epithelia in controls might reflect a higher mechanical irritation in these sites (8, 44, 45). The types of epithelia within the oral gingiva, sulcular epithelium and junctional epithelium were not significantly different between PPID and controls. Diffusely distributed LI in the gingiva are already documented in a wide range of species and they are claimed to be a normal finding in the gingiva (8, 9, 46, 47). The JE shows unique features compared with other oral epithelia. On one hand, it forms an epithelial barrier against the oral cavity, but permits the passage of extracellular fluid, inflammatory cells and other components of the immunologic host defense system. On the other hand, the function of the JE is to provide a stable connection with the teeth, which is essential for a healthy periodontium (3). Macroscopically horses affected by PPID showed a higher number of gingival sulci of ≥ 1 mm than healthy controls (p = 0.004) (28). Our findings here show a higher but not significant number of PPID affected horses (7/97) with a smaller attachment zone or missing contact than controls (4/113). The remodeling and eruption of teeth in hypsodont species requires ongoing cellular renewal and activity in order to keep the JE attached and the gingival sulcus < 1 mm. A gingival sulcus deeper than 1 mm or a loss of attachment could be an expression of a poorer dental status, predisposing for food and bacterial entrapment in the gingival sulcus. In this study we could not show a difference between PPID affected and healthy controls concerning the gingival epithelia.
The collagen fiber arrangement in the LP showed no difference between groups and an irregular fiber network was seen in 7% (8/114) of PPID and 5.2% (6/116) of controls. However, collagen fiber arrangement was evaluated exclusively in HE stained specimens and not in collagen fiber specialized staining methods. Histological evaluations of tendons from PPID affected horses (n = 4) showed a reduced longitudinal arrangement of collagen fibers in the suspensory ligament and an accumulation of proteoglycans between suspensory ligament fibers in comparison to two groups of healthy control horses (48). Likewise, in humans with adrenal hypercortisolism, alterations in collagen and epidermal thinning were observed in histological sections from skin with no evidence of leukocyte infiltration (49). In human dentistry, the normal lamina propria is described as collagenous tissue that usually shows no inflammatory infiltrates except in the area of the gingival sulcus (3, 5, 50). The main finding of our study is that PPID affected horses showed significantly (p = 0.002) more multifocal to coalescing LI than controls in the LP. Cox et al. (9) described that leukocyte infiltration is a physiological aspect of the equine gingiva mainly in the superficial lamina propria of the free gingiva, while the deep lamina propria contained mild perivascular infiltrations of lymphocytes and plasma cells. In humans, risk factors for severe forms of periodontal disease with LI in LP and even PDL are diseases that influence immune function (e.g., diabetes mellitus, HIV, cushing's syndrome) or lead to exaggerated immune responses (21, 34, 51, 52). In our study, the higher presence of multifocal to coalescing LI in the PPID group could show the onset of immune dysregulation within the LP. The infiltrations consisted mostly of lymphocytes and plasma cells, consistent with a chronic inflammatory/immunological reaction. Interestingly, eosinophils represented the third most common leukocyte cell population within the infiltrates of the lamina propria and in the PDL. The role and function of eosinophils in the periodontium is currently not fully understood. The presence of eosinophils might play a role in gingival protection and might be a transient expression of remodeling rather than a pathological finding (10). Eosinophils beneath the gingival epithelium were reported as a result of parasitic infestation of the gingiva by larvae of botflies (53). In rats with experimentally induced periodontal disease, eosinophils were identified in the lamina propria and are thought to play a role in cell and collagen destruction (9, 54). Our findings of eosinophils are in line with the findings of Cox et al. (9) who found eosinophils “irrespective of the presence or degree of periodontal disease” in the lamina propria and submucosa of their specimens. In contrast, Steinfort et al. (8) found no eosinophils in specimens of horses free from dental and periodontal disorders. Therefore, we assume that the often clustered eosinophils found in this study are a sign of enhanced immunological activity in the gingiva. However, there were no statistical differences detected between groups or related to age in our older probands.
There are few studies in equine dentistry that evaluated the subgingival periodontal ligament and their results are consistent with those of our control group (9, 55). We could not detect a significant difference in the distribution of LI between the groups but 21.2% (24/113) of PPID affected horses showed a multifocal to coalescing distribution of LI in the PDL, while in the control group only 12.4% (14/113) showed this distribution of LI. The reason why only a part of the PPID group in our study showed a higher amount of multifocally to coalescing distributed LI could be the varying amounts of POMC-derived peptides and their multiple systemic effects. PPID is a slowly developing and progressing neurodegenerative disease. Therefore, effects from this disease might be balanced by physiological regulations of the body and its immune system. Recent papers on PPID affected horses showed that they suffer from an impaired immune function (38, 56). Their neutrophil function is decreased resulting in a low oxidative burst capacity and decreased adhesion. Interestingly, an unexpected finding in the study of McFarlane et al. (56) was the increased chemotaxis in healthy aged horses, which is contrary to humans showing an age-related loss of chemotactic ability. On the other hand, age-related inflammation leads to an up-regulation of chemotaxis. In PPID, the age-induced higher LI might be counteracted by immunosuppressant POMC-peptides (e.g., α-MSH and CLIP). This could lead to a multifocal to coalescing high leukocyte infiltration without obvious signs of tissue demarcation, like we have shown in the periodontium.
On interdental gingiva sites, the PPID group showed more than four times more irregularities in cemental structures than controls (p = 0.02). This might still be a normal reaction due to remodeling capacities of the cellular tertiary cementum in hypsodont species (57), possibly caused by trauma or infections, which lead to cemental deposition in resorptive and reparative lesions (2, 58). Irregular cemental structure could also be an age related occurrence (35) as geriatric horses have shorter teeth, which is accompanied by higher tooth mobility. This is counter-regulated by a feature of senile equine dentition: the formation of new cementum replacing and compensating the missing tooth substance (35, 57, 58). However, in our probands, age had no significant influence (p = 0.79) on the presence of cemental irregularities. Therefore, we speculate, that PPID might interfere with the building of new cementum.
Nearly all samples in both groups showed plaque within the gingival sulcus or below the gingival epithelium level in interdental locations. In dental locations, caries was found almost exclusively above the gingival sulcus. The nutrition of geriatric horses often involves feed with simpler carbohydrate content (e.g., senior mash with higher starch vs. cellulose content), therefore the high amount of soluble sugars with their cariogenic potential may explain these findings. However, information about diet was not available for the probands. There was no higher prevalence of peripheral caries or plaque in PPID horses. This contradicts the proposal of Simon and Herold (59), who hypothesized that older, but especially PPID affected horses will develop plaque and caries due to immunosuppressive mechanisms.
Histomorphological characteristics of periodontal tissues of PPID affected horses are described and compared with healthy controls for the first time. This study has some limitations, as the histological evaluations were not made blindly and only hematoxylin-eosin (HE) staining was used. Other stains and techniques for collagen, polarized light and immunohistochemistry for collagen, vascularisation, leukocytes could be further approaches for future studies. PPID affected horses showed a significantly higher prevalence of multifocal to coalescing leukocyte infiltrations within the lamina propria of the gingiva. Further characterization of lymphocytes would be needed in order to evaluate their possible role in destructive periodontal processes in PPID affected horses. However, in the periodontal ligament for the distribution and amount of leukocytic infiltration, age was more important than PPID status.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the animal study because according to German legislation, the post mortal collection of specimens does not need any permission of the animal welfare authority. Written informed consent was obtained from the owners for the participation of their animals in this study.
AZ wrote the manuscript and performed the histological evaluations of the gingiva. MG performed the histopathological scoring of the pituitary glands. CS and KF contributed to the concept and design of the study. KB performed the statistical analysis. All authors read the manuscript, contributed to manuscript revision, and approved the submitted version.
This study received funding from Boehringer Ingelheim Vetmedica. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. The APC was funded by the Open-Access-Fonds for junior scientists, Justus-Liebig-University GieSSen.
The authors would like to thank Jörg Vogelsberg for his excellent technical support and Andreas Schaubmar for the excellent support on statistics.
KF has a consultancy agreement with Boehringer Ingelheim Vetmedica.
The remaining authors declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Berglundh T, Lindhe J, Sterrett JD. Clinical and structural characteristics of periodontal tissues in young and old dogs. J Clin Periodontol. (1991) 18:616–23. doi: 10.1111/j.1600-051X.1991.tb00099.x
2. Berkovitz BK, Holland GR, Moxham BJ. Oral Anatomy, Histology and Embryology. Edinburgh: Elsevier (2018) p. 462.
3. Nanci A, Bosshardt DD. Structure of periodontal tissues in health and disease. Periodontol. (2006) 40:11–28. doi: 10.1111/j.1600-0757.2005.00141.x
4. Schroeder HE. Strukturbiologie. In:HF Wolf, EM Rateitschak, K-H Rateitschak, HE Schroeder, , editors. Parodontologie: Farbatlanten der Zahnmedizin 1. Stuttgart: Georg Thieme Verlag (2012). p. 7–21.
5. Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol. (1997) 13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x
6. Attström R, Graf-de Beer M, Schroeder HE. Clinical and histologic characteristics of normal gingiva in dogs. J Periodont Res. (1975) 10:115–27. doi: 10.1111/j.1600-0765.1975.tb00016.x
7. Steiniger B, Schwarzbach H, Stachniss V. Mikroskopische Anatomie der Zähne und des Parodonts. Stuttgart: Thieme (2010) p. 64. doi: 10.1055/b-002-41840
8. Steinfort S, Röcken M, Vogelsberg J, Failing K, Staszyk C. The equine gingiva: a histological evaluation. Front Vet Sci. (2019) 6:435. doi: 10.3389/fvets.2019.00435
9. Cox A, Dixon PM, Smith S. Histopathological lesions associated with equine periodontal disease. Vet J. (2012) 194:386–91. doi: 10.1016/j.tvjl.2012.04.026
10. Dixon PM, Du Toit N, Staszyk C. A fresh look at the anatomy and physiology of equine mastication. Vet Clin North Am Equine Pract. (2013) 29:257–72. doi: 10.1016/j.cveq.2013.04.006
11. Klugh DO. Equine periodontal disease. Clin Tech Equine Practice. (2005) 4:135–47. doi: 10.1053/j.ctep.2005.04.005
12. Staszyk C, Gasse H. Oxytalan fibres in the periodontal ligament of equine molar cheek teeth. Anat Histol Embryol. (2004) 33:17–22. doi: 10.1111/j.1439-0264.2004.00503.x
13. Masset A, Staszyk C, Gasse H. The blood vessel system in the periodontal ligament of the equine cheek teeth–part I: the spatial arrangement in layers: part I: the spatial arrangement in layers. Ann Anat. (2006) 188:529–33. doi: 10.1016/j.aanat.2006.06.010
14. Murakami S, Mealey BL, Mariotti A, Chapple IL. Dental plaque-induced gingival conditions. J Clin Periodontol. (2018) 45:17–27. doi: 10.1111/jcpe.12937
15. Novak JM. Chapter 7: Classification of Diseases and Conditions Affecting the Periodontium. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier Saunders (2018). p. 232–51.
16. Klokkevold PR, Mealey BL. Chapter 17: Influence of Systemic Disorders and Stress on the Periodontium. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier; Saunders (2018). p. 575–628.
17. Nirola A, Batra P, Kaur J. Ascendancy of sex hormones on periodontium during reproductive life cycle of women. J Int Clin Dent Res Organ. (2018) 10:3. doi: 10.4103/jicdro.jicdro_29_17
18. Caton JG, Armitage G, Berglundh T, Chapple IL, Jepsen S, Kornman KS, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol. (2018) 45:1–8. doi: 10.1111/jcpe.12935
19. Johnson NW, Griffiths GS, Wilton JM, Maiden MF, Curtis MA, Gillett IR, et al. Detection of high-risk groups and individuals for periodontal diseases. Evidence for the existence of high-risk groups and individuals and approaches to their detection. J Clin Periodontol. (1988) 15:276–82. doi: 10.1111/j.1600-051X.1988.tb01584.x
20. Beck JD, Arbes SJ. Chapter 8: Epidemiology of Gingival and Periodontal Diseases. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier; Saunders (2018) p. 251–90.
21. Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Periodontol. (2018) 89:S183–203. doi: 10.1002/JPER.16-0480
22. Horn R, Bamford NJ, Afonso T, Sutherland M, Buckerfield J, Tan RH, et al. Factors associated with survival, laminitis and insulin dysregulation in horses diagnosed with equine pituitary pars intermedia dysfunction. Equine Vet J. (2019) 51:440–5. doi: 10.1111/evj.13041
23. McFarlane D. Equine pituitary pars intermedia dysfunction. Vet Clin North Am Equine Pract. (2011) 27:93–113. doi: 10.1016/j.cveq.2010.12.007
24. McGowan TW, Pinchbeck GP, McGowan CM. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet J. (2013) 45:74–9. doi: 10.1111/j.2042-3306.2012.00578.x
25. Fey K. Störungen im hypothalamisch-hypophysären System. In:Brehm W, Gehlen H, Ohnesorge B, Wehrend A, Dietz O, Huskamp B, , editors. Handbuch Pferdepraxis. Stuttgart: Enke Verlag (2016). p. 831–9.
27. Miller MA, Pardo ID, Jackson LP, Moore GE, Sojka JE. Correlation of pituitary histomorphometry with adrenocorticotrophic hormone response to domperidone administration in the diagnosis of equine pituitary pars intermedia dysfunction. Vet Pathol. (2008) 45:26–38. doi: 10.1354/vp.45-1-26
28. Nitzsche AM, Fey K, Büttner K, Gröf M, Staszyk C. The gingiva of horses with pituitary pars intermedia dysfunction: a macroscopic anatomical evaluation. Front. Vet. Sci. (2022) 8:786971. doi: 10.3389/fvets.2021.786971
29. Fiorellini JP, Kim DM, Ishikawa SO. Chapter 5: Tooth-Supporting Structures. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier; Saunders (2018). p. 171–225.
30. Borkent D, Smith S, Dixon PM, A. histological and ultrastructural study of equine peripheral caries. Equine Vet J. (2020) 52:104–11. doi: 10.1111/evj.13134
32. Kennedy RS, Lappin DF, Dixon PM, Bennett D, Riggio MP. Gingival Toll-like receptor and cytokine messenger RNA levels in equine periodontitis and oral health. Equine Vet J. (2017) 49:294–9. doi: 10.1111/evj.12597
33. Miller AB, Loynachan AT, Bush HM, Hart KA, Barker VD, Campana-Emard AG, et al. Effects of pituitary pars intermedia dysfunction and Prascend (pergolide tablets) treatment on endocrine and immune function in horses. Domest Anim Endocrinol. (2021) 74:106531. doi: 10.1016/j.domaniend.2020.106531
34. Tonetti MS, Bottenberg P, Conrads G, Eickholz P, Heasman P, Huysmans M-C, et al. Dental caries and periodontal diseases in the ageing population: call to action to protect and enhance oral health and well-being as an essential component of healthy ageing - Consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. (2017) 44:S135–44. doi: 10.1111/jcpe.12681
35. Van der Velden U. Effect of age on the periodontium. J Clin Periodontol. (1984) 11:281–94. doi: 10.1111/j.1600-051X.1984.tb01325.x
36. Needleman I. Chapter 6: Aging and the Periodontium. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier Saunders (2018) p. 225–32.
37. Kyllar M, Witter K, Tichy F. Gingival stippling in dogs: clinical and structural characteristics. Res Vet Sci. (2010) 88:195–202. doi: 10.1016/j.rvsc.2009.07.013
38. McFarlane D. Immune dysfunction in aged horses. Vet Clin North Am Equine Pract. (2016) 32:333–41. doi: 10.1016/j.cveq.2016.04.009
39. Zak A, Siwinska N, Elzinga S, Barker VD, Stefaniak T, Schanbacher BJ, et al. Effects of advanced age and pituitary pars intermedia dysfunction on components of the acute phase reaction in horses. Domest Anim Endocrinol. (2020) 72:106476. doi: 10.1016/j.domaniend.2020.106476
40. Siard-Altman MH, Harris PA, Moffett-Krotky AD, Ireland JL, Betancourt A, Barker VD, et al. Relationships of inflamm-aging with circulating nutrient levels, body composition, age, and pituitary pars intermedia dysfunction in a senior horse population. Vet Immunol Immunopathol. (2020) 221:110013. doi: 10.1016/j.vetimm.2020.110013
41. Stepaniuk K, Hinrichs JE. The structure and function of the periodontium. In:BA Niemiec, , editor. Veterinary Periodontology. Ames, IW: John Wiley & Sons (2013). p. 3–18.
42. Winning TA, Townsend GC. Oral mucosal embryology and histology. Clin Dermatol. (2000) 18:499–511. doi: 10.1016/S0738-081X(00)00140-1
43. Fiorellini JP, Kim DM, Ishikawa SO. Chapter 4: The Gingiva. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier Saunders (2018). p. 126–71.
44. Caffesse RG, Nasjleti CJ, Kowalski CJ, Castelli WA. The effect of mechanical stimulation on the keratinization of sulcular epithelium. J Periodontol. (1982) 53:89–92. doi: 10.1902/jop.1982.53.2.89
45. Listgarten MA. Similarity of epithelial relationships in the gingiva of rat and man. J Periodontol. (1975) 46:677–80. doi: 10.1902/jop.1975.46.11.677
46. Garant PR, Mulvihill JE. The ultrastructure of clinically normal sulcular tissues in the beagle dog. J Periodont Res. (1971) 6:252–65. doi: 10.1111/j.1600-0765.1971.tb00616.x
47. Bulkacz J, Carranza FA. Chapter 20: Defense Mechanisms of the Gingiva. In:MG Newman, H Takei, PR Klokkevold, FA Carranza, , editors. Newman and Carranza's Clinical Periodontology. Philadelphia: Elsevier Saunders (2018). p. 684–706.
48. Hofberger S, Gauff F, Licka T. Suspensory ligament degeneration associated with pituitary pars intermedia dysfunction in horses. Vet J. (2015) 203:348–50. doi: 10.1016/j.tvjl.2014.12.037
49. Groves RW, MacDonald LM, MacDonald DM. Profound digital collagen atrophy: a new cutaneous presentation of adrenal-dependent Cushing's syndrome. Br J Dermatol. (1990) 123:667–71. doi: 10.1111/j.1365-2133.1990.tb01486.x
50. Zachinsky L. Range of histologic variation in clinically normal gingiva. J Dent Res. (1954) 33:580–9. doi: 10.1177/00220345540330042001
51. Jepsen S, Caton JG, Albandar JM, Bissada NF, Bouchard P, Cortellini P, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. (2018) 45:S219–29. doi: 10.1111/jcpe.12951
53. Cogley TP. Effects of migrating gasterophilus intestinalis larvae (diptera: gasterophilidae) on the mouth of the horse. Vet Parasitol. (1989) 31:317–31. doi: 10.1016/0304-4017(89)90081-2
54. Bertão JM, Almeida OP de, Do Nascimento A, Novaes PD, Bozzo L. Eosinophils during developing periodontal disease of rats. J Periodont Res. (1985) 20:467–74. doi: 10.1111/j.1600-0765.1985.tb00829.x
55. Wulff W,. Histologische Untersuchungen am Ligamentum periodontale des Pferdebackenzahns. (2005). Available online at: https://elib.tiho-hannover.de/receive/etd_mods_00002296
56. McFarlane D, Hill K, Anton J. Neutrophil function in healthy aged horses and horses with pituitary dysfunction. Vet Immunol Immunopathol. (2015) 165:99–106. doi: 10.1016/j.vetimm.2015.04.006
57. Mitchell SR, Kempson SA, Dixon PM. Structure of peripheral cementum of normal equine cheek teeth. J Vet Dent. (2003) 20:199–208. doi: 10.1177/089875640302000401
58. Dixon PM. The Gross, Histological, and Ultrastructural The Gross, Histological, and Ultrastructural Anatomy of Equine Teeth and Their Relationship to Disease. Proceedings of the 49th Annual Convention of the American Association of Equine Practitioners, Vol 48, p 421–37 Orlando, Florida. (2002).
Keywords: pituitary pars intermedia dysfunction (PPID), periodontal disease (PD), diastema, leukocytic infiltration, histology, equine teeth
Citation: Zapf AM, Fey K, Büttner K, Gröf M and Staszyk C (2023) Periodontal structures in horses with pituitary pars intermedia dysfunction: A histological evaluation. Front. Vet. Sci. 10:1114445. doi: 10.3389/fvets.2023.1114445
Received: 02 December 2022; Accepted: 02 January 2023;
Published: 17 January 2023.
Edited by:
Padraic Martin Dixon, University of Edinburgh, United KingdomReviewed by:
F. Capela e Silva, University of Evora, PortugalCopyright © 2023 Zapf, Fey, Büttner, Gröf and Staszyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne Maria Zapf,  emFwZi5hbm5lQGdteC5kZQ==
emFwZi5hbm5lQGdteC5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.