
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 06 February 2023
Sec. Parasitology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1109986
This article is part of the Research Topic Pathobiology, Epidemiology and Control of Protozoan Diseases of Veterinary Importance View all 8 articles
Introduction: Neospora caninum is one of the main causes of abortion in cattle. In wildlife, the occurrence and relevance of vertical infection have not been yet clearly evaluated. The aim of this study was to verify the possibility of vertical transmission of N. caninum in three wild species extensively distributed in Europe, namely roe deer Capreolus capreolus, wild boar Sus scrofa and red fox Vulpes vulpes.
Methods: A total of 190 fetuses (72 wild boars, 67 foxes and 51 roe deer) from 61 females were included in the study. All animals, which were either found dead or culled within selective control plans in North-western Italy, were tested, in parallel by PCR on central nervous system, skeletal muscle and kidney.
Results and discussion: The efficiency of vertical transmission in the three target species was of 87.5% (95% CI 69.00–95.66).
Neospora caninum is a protozoan parasite, first described in 1988 (1). The main hosts involved in the domestic cycle are dogs (2, 3) and cattle (4) as definitive and intermediate hosts, respectively. Neospora caninum is known to have a broad range of wild intermediate hosts. The existence of a sylvatic cycle was first demonstrated in North America, being maintained by coyotes Canis latrans (5) and white-tailed deers Odocoileus virginianus (6, 7). To date, wild carnivores recognized as competent definitive hosts for N. caninum include coyotes (5), dingoes Canis lupus dingo (8) and wolves Canis lupus (9). N. caninum has been detected in a wide spectrum of herbivores, carnivores, rodents and birds which might serve as intermediate hosts (10, 11). Several of the numerically most relevant ungulate species in Europe were found infected with N. caninum, including red deer Cervus elaphus, roe deer Capreolus capreolus, chamois Rupicapra rupicapra, alpine ibex Capra ibex (12–14), wild boar Sus scrofa, fallow deer Dama dama, mouflon Ovis ammon (15), moose Alces alces (16) and bison Bison bonasus bonasus (17). The parasite has been demonstrated to infect lagomorphs as brown hare Lepus europaenus, wild rabbit Oryctolagus cuniculus and eastern cottontail Sylvilagus floridanus (18–20). Rodents like the house mouse Mus musculus, field mouse Apodemus sylvaticus and brown rat Rattus norvegicus, are suspected to be an important link between domestic and sylvatic cycles, because of their cosmopolitan diffusion and their role as prey for both domestic and wild canids (10, 21).
N. caninum infection is known to produce neuromuscular clinical signs in dogs (22). The parasite is the primary infective cause of abortion in cattle herds worldwide (23, 24), resulting in serious economic losses in farm management (25), which globally amounts to $ 1,298 million (26). Clinical presentation of N. caninum infection in cattle includes embryonic resorption, fetal death, abortion, stillbirth or birth of persistently infected animals without clinical signs (22). Vertical transmission is a highly effective route of infection, which in cattle has been reported to occur with a frequency between 81 and 95% (25). Congenital transmission occurs through the passage of tachyzoites across the placenta after ingestion of environmental oocysts during pregnancy or by reactivation of quiescent bradyzoites in chronically infected animals. This is the main route for the maintenance of N. caninum in cattle herds (27). The existence of congenital infection has been demonstrated in other species like dog, cat (28), sheep (29), goat (30), non-human primates (31) and mouse (32).
Few case reports dealt with the hypothesis of a vertical transmission of N. caninum in captive wild animals. N. caninum was diagnosed in a stillborn Eld's deer Cervus eldi siamensis (33), in a fallow deer with neurological clinical signs compatible with N. caninum infection (34), in two full-termed stillborn twins of lesser kudu Tragelaphus imberbis (35) and an Axis deer Axis axis died shortly after birth (36). A recent study assessed seroconversion following vertical transmission of N. caninum in captive South American deer species (37) while to our knowledge, only one study assessing the possibility of congenital infection in free ranging wild animals, was conducted in North America on white tailed deer (38). Two experimental studies carried out on breeding foxes (Vulpes vulpes and Alopex lagopus) identified N. caninum in cub's tissues, suggesting the occurrence of vertical transmission in this species (39, 40). No data are available about congenital transmission in the target species. In the study area (Piedmont Region, North-western Italy), N. caninum is known to circulate within wildlife in ruminants (12, 13), rodents (21), lagomorphs (20) and in domestic dogs (41), cats (42) and cattle (43). In this context, we reported data from the first study conducted with the specific aim of assessing vertical transmission of N. caninum in roe deer, wild boar and fox. These three species were chosen due to their high abundance, wide geographical range, and different feeding strategies that make them a good model of the parasite's life cycle in wild animals.
All the animals included in the study came from five different provinces of North Western Italy (NUTS3–Nomenclature of Territorial Units for Statistics, level 3), namely Alessandria (area A), Biella (area B), Cuneo (area C), Novara (area D) and Torino (area E) (Figure 1), and were taken for necropsy, to the Dept. of Veterinary Sciences (University of Turin) over a period of three consecutive years, if found dead or killed for nationally-authorized selective plans of demographic control. A total of 190 fetuses (n = 72 wild boar Sus scrofa, n = 67 fox Vulpes vulpes and n = 51 roe deer Capreolus capreolus) were sampled from 61 pregnant females (n = 16 wild boar S. scrofa, n = 17 red fox V. vulpes, n = 28 roe deer C. capreolus) (Table 1). Adult females and fetuses were dissected under clean laboratory conditions with sterile scalpels, in order to minimize the risk of cross contamination. After full necroscopic examination, a portion of central nervous system (CNS–medulla oblongata), skeletal muscle (quadriceps femoris) and kidney of both pregnant females and fetuses were collected and stored at −20°C until further processing. These three tissues were chosen because they were reported to be the most frequently infected in bovine fetuses (43). Samples were immediately frozen and stored in single vials at −20°C until DNA extraction and PCR amplification. Data about origin, weight and sex of the fetuses (in case the stage development allowed sexing procedure) were taken for each animal, and an identification number was assigned to allow an univocal matching between each mother and her fetuses.
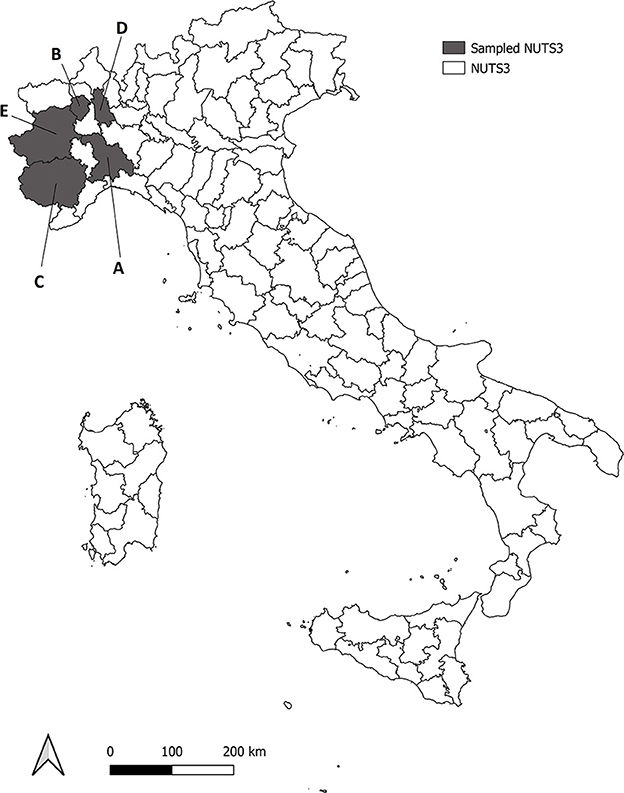
Figure 1. Roe deer, wild boar and red fox analyzed were sampled from five Provinces (NUTS3) in North western Italy, in gray in the map (NUTS3–Nomenclature of Territorial Units for Statistics, level 3). Sampled NUTS3 are Alessandria (area A), Biella (area B), Cuneo (area C), Novara (area D) and Torino (area E).
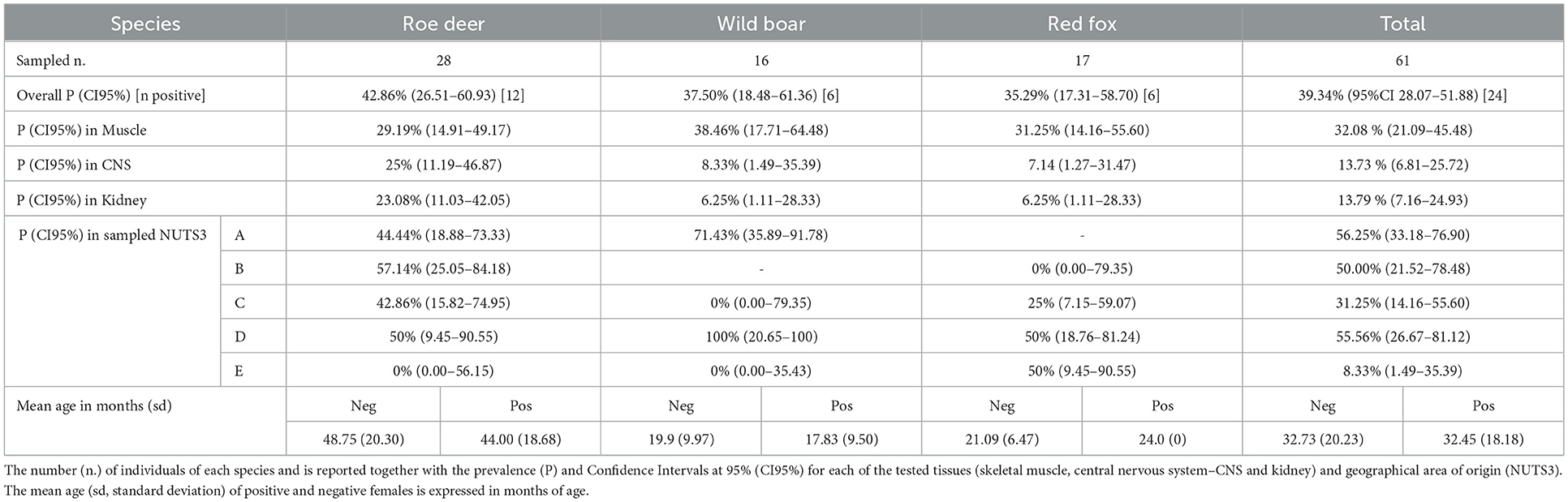
Table 1. Prevalence of Neospora caninum infection in adult females of roe deer, wild boar and red fox.
Total genomic DNA was extracted from 25 mg of tissue using GenElute Mammalian genomic MiniPrep Kit (Sigma–Aldrich, St. Louis, MO, USA) according to manufacturer's instructions. A specific 337 bp fragment of the NC5 region of N. caninum was amplified using primers Np6-plus (5′-CTCGCCAGTCAACCTACGTCTTCT-3′) and Np21-plus (5′-CCCAGTGCGTCCAATCCTGTAAC-3′), modified from (44), according to (20). Negative controls (Toxoplasma gondii DNA and distilled water) were included in each step (extraction, amplification and electrophoresis run), while positive controls (DNA extracted from cultured N. caninum NC1 tachyzoites) were included in amplification and electrophoresis run. Positive samples (one amplicon for each mother and litter) were purified using the commercial kit Nucleospin Extract II kit (Macherey-Nagel, Düren, Germany) and sequenced on both strands (MacrogenEurope, The Netherlands) to confirm PCR results. The resulting sequences were compared to those already available in GenBank.
Data were analyzed with R-3.4.4 (45). The vertical transmission parameter was calculated as the proportion of PCR positive females that produced PCR positive offspring (at least 1 positive fetus per litter). Uncorrected Chi-square test and Odds ratio were used to determine the association between maternal and fetal infection status. Potential risk factors were analyzed by Generalized Linear Model (GLM), in order to evaluate their effect on the outcome of PCR analysis. Risk factors evaluated were: year of sampling, geographical origin, species, weight (as proxy of gestational stage normalized for each species), tissue tested, sex, PCR positivity of the mother and age of the mother at death. The model with the lower AIC (Akaike Information Criterion) was selected (46). Tests were considered significant when p-value (p) was equal or lower than 0.05. In statistical analysis, animals were considered positives if N. caninum DNA was found at least in one of the three tissues tested. Cochran Q Test and post-hoc McNemar's test were used to evaluate which of the tissue tested (CNS, kidney or skeletal muscle) allowed for higher sensitivity in N. caninum identification. Assessment of inter-rater reliability (IRR) to assess PCR results consistency among the three tested tissues was calculated using Light's Kappa (k) for multiple raters (47).
The overall prevalence of infection in the adult pregnant females was P=39.34% (95%CI 28.07–51.88). N. caninum was detected with comparable prevalence among the three species (Table 1). Geographical origin and age (age in months of positive females mean = 32.45, sd = 18.18 vs. age of negative females mean = 32.73, sd = 20.23) were not significantly associated with PCR results (p>0.05). In the three tested species, skeletal muscle provided the highest sensitivity compared to CNS (χ2 = 4.05; p < 0.05) and kidney (χ2 = 5.79; p < 0.05).
The vertical transmission rate of N. caninum from PCR positive females was 87.5% (95% CI 69.00–95.66) ranging from 83.33% in roe deer and wild boar, to 100% in red fox (Table 2). In multi-fetus litters (number of fetuses min = 2, max = 8, sd = 1.68) of infected females, N. caninum DNA was detected on average, in 80.00% of the fetuses of the litter (CI95% 0.00–100%). The best performing GLM with N. caninum infection in fetuses as independent variable, included two dependent factors, namely fetus weight and PCR status of the mother. Normalized fetus weight resulted as a significant risk factor and likelihood of congenital infection increased with fetus body weight and thus with the gestational stage. In roe deer and wild boar positive fetuses had a significantly higher normalized weight (p = 0.0242 and p = 0.0002 respectively) compared to negative ones. Congenital infection was strongly associated with positivity of the mother to N. caninum (χ2 = 47.88, p = 0.000; OR = 11.98; CI95% 5.39–28.41%). The association between fetal and maternal infection in the three species is reported in Table 2. No significant correlations were found with the other variables considered (species, year of sampling, origin, sex, mother age) (Table 3). Prevalence of infection in the different tissues ranged from 10.58% (CI95% 6.96–15.78%) in kidneys, to 11.58% (CI95% 7.77–16.91%) in CNS, and 18.09% in skeletal muscle (CI95% 13.24–24.21%). Mc Nemar's test evidenced a significant difference in diagnostic sensitivity between muscle and the other tissues (skeletal muscle-kidney: χ2=7.53, p = 0.0061; skeletal muscle-CNS χ2 = 4.00, p = 0.0455) and Light's K underlined a minimal IRR between the three tissues analyzed (k = 0.26).
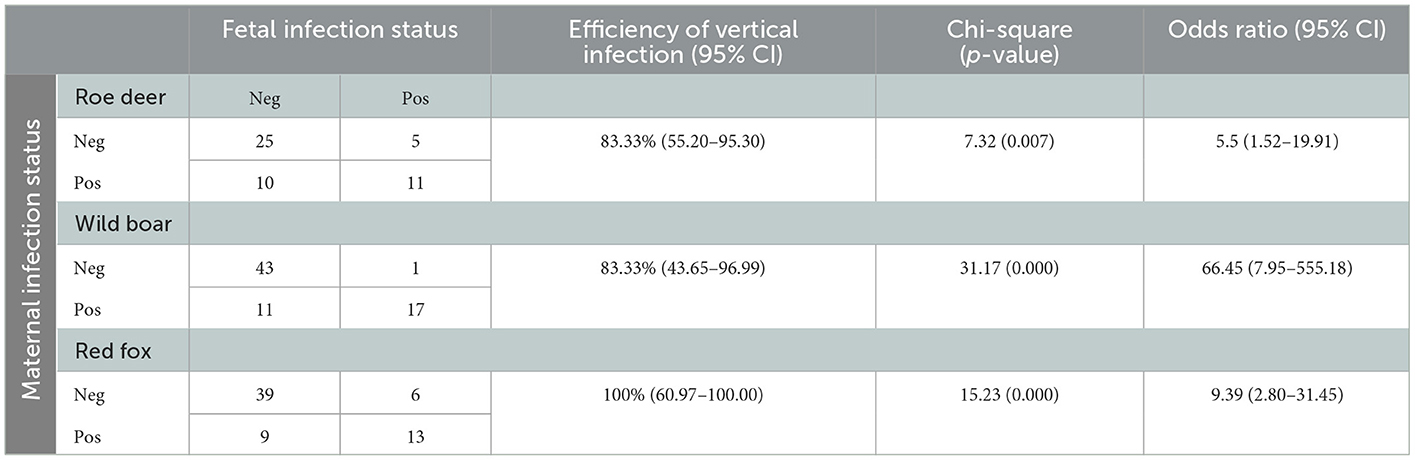
Table 2. Association between fetal and maternal positivity to N. caninum by PCR in the three species roe deer, wild boar, and red fox.
A total of 45 positive samples (each of 24 positive pregnant females and one isolate for each of the 21 positive litters) were sequenced and deposited in GenBank under accession numbers MT346029 to MT346031. Minor differences (max 3 bp) were detected among the isolates, and in all cases isolates from fetuses of each litter were 100% identical to the isolate detected in their respective mothers.
Analysis of the NC5 DNA sequences showed a maximum identity ranging from 98 to 100% (query coverage of 100%), with N. caninum isolates from rodents (GenBank accession numbers: EF202079–EF202082) and cattle (GenBank accession numbers: KP715561, KP715562) from the same study area.
To the best of our knowledge, this study evidenced for the first time the possibility of congenital transmission of N. caninum in roe deer, wild boar and fox, also assessing vertical transmission on a large number of free-ranging individuals. Indeed, previous evidence of congenital infection in free-ranging wildlife was given only in the white-tailed deer (O. virginianus) (38). Transplacental transmission in captive animals was hypothesized by serological evidence (37) or when stillbirth or perinatal neurological signs were indicative of N. caninum infection (33–36). N caninum DNA was detected in fetal tissues of all the three species considered (wild boar, roe deer, and red fox). At necropsy, none of the fetuses showed any alteration possibly attributable to N. caninum infection. PCR positivity for N. caninum of the mother was strongly associated with fetus positivity in all three species (OR = 11.98). In wild boar the recorded OR is 66.45 (95%CI 7.95–555.18), while in Roe deer and Fox the OR values are 5.5 (95% CI 1.52–19.91) and 9.39 (95%CI 2.80–31.45) respectively. The recorded efficiency of vertical infection is high in all three target species and falls within the range previously reported for cattle (25). DNA sequencing showed 100% identity between fetal and maternal isolates of N. caninum. Even if skeletal muscle showed a significant higher sensitivity than the other tissues, considered the minimal IRR among tissues tested, multiple tissues should always be analyzed in parallel in order to avoid underestimation of parasite's prevalence in fetuses. Fetal weight was considered indicative of gestational age and significantly related to N. caninum infection in roe deer and wild boar as previously reported in cattle (22, 48). Pregnancy-induced immunomodulation with reduced cell-mediated response and INFγ production, is a major responsible of parasite's replication control, and allows transplacental transmission of tachyzoites in chronically as well as in newly infected subjects (49–51). Vertical transmission had already been hypothesized in roe deer because of the lack of correlation between seropositivity and age of the tested subjects (12). The authors suggested that the high seroprevalences in animals of all ages, including yearlings, is the result of a concomitant occurrence of both vertical and horizontal transmission. Horizontal transmission of N. caninum occurs trough the ingestion of oocyst spread with feces of definitive hosts or trough consumption of preys infected with tissue cysts. Remarkably, horizontal transmission connects the domestic and sylvatic cycles of N. caninum. In the study area the main hosts involved in the domestic cycle are cattle and rural shepherd dogs (41). These latter are considered the primary connection with the sylvatic cycle, providing both contamination of pastures with oocysts and by occasionally feeding on wild ungulates. Moreover, the role of wolves in N. caninum transmission in the study area is possibly evolving since presence and density of C. lupus has been increasing steadily in the past decade and roe deer and wild boars are two of its main preys (52). N. caninum isolates, sequenced in this study, are identical to N. caninum isolates of rodents and cattle from the same area (21). This finding further corroborates the hypothesis that the sylvatic cycle is maintained by vertical transmission and amplified by the presence of wild predators, acting as definitive hosts. Moreover, this sylvatic cycle seems correlated with the domestic one, not just by domestic dogs, but also by rodents (21). This finding further supports the hypothesis that these species could act as a link between domestic and wild cycle, due to their wide territorial distribution and their role as prey for various domestic and wild species.
The results obtained in this study assessed, for the first time, the possibility of transplacental transmission in roe deer, wild boar and red fox. The role of vertical transmission in establishing and maintaining N. caninum's sylvatic cycle must be confirmed by demonstrating that transplacental infection can lead to the onset of persistent infection in offspring. The high prevalence, found in the wild species here analyzed, underlines their role as intermediate host in the parasite life cycle in an extensive geographical area, characterized by a variety of wild-domestic interfaces. The assessment of the vertical transmission and of an existing connection between wild and domestic parasitic cycles, leads to the need of reconsidering management measures and epidemiological surveillance, with the aim of controlling transmission between wild and domestic animals. Further research is also needed to quantify the relevance of wild carnivores as definitive hosts especially in light of current increasing population trends and geographical expansion of competent hosts (Canis lupus) (9).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on animals in accordance with the local legislation and institutional requirements.
SZ: investigation, data curation, formal analysis, and writing final version of manuscript. MP: investigation, writing—original draft, and review and editing. EF: conceptualization, funding acquisition, and writing—review and editing. All authors contributed to the article and approved the submitted version.
This study was partially funded by the Piemonte Regional Administration (Convenzione tra la Regione Piemonte e il Dip. di Scienze Veterinarie dell'Università degli Studi di Torino per la razionalizzazione ed integrazione delle attività di raccolta e smaltimento degli animali selvatici morti o oggetto di interventi di contenimento).
The authors gratefully acknowledge the precious help of Drs. Davide Grande, Simona Bruno, and Carpignano Maria Grazia for their support during necropsies and sample collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dubey JP, Barr BC, Barta JR, Bjerkås I, Björkman C, Blagburn BL, et al. Redescription of Neospora caninum and its differentiation from related coccidia. Int J Parasitol. (2002) 32:929–46. doi: 10.1016/s0020-7519(02)00094-2
2. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol. (1998) 28:1473–9. doi: 10.1016/S0020-7519(98)00138-6
3. Lindsay DS, Dubey JP, Duncan RB. Confirmation that the dog is a definitive host for Neospora caninum. Vet Parasitol. (1999) 82:327–33. doi: 10.1016/S0304-4017(99)00054-0
4. Thilsted JP, Dubey JP. Neosporosis-like abortions in a herd of dairy cattle. J Vet Diagn Invest. (1989) 1:205–9. doi: 10.1177/104063878900100301
5. Gondim LFP, McAllister MM, Pitt WC, Zemlicka DE. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int J Parasitol. (2004) 34:159–61. doi: 10.1016/j.ijpara.2004.01.001
6. Dubey JP, Hollis K, Romand S, Thulliez P, Kwok OCH, Hungerford L, et al. High prevalence of antibodies to Neospora caninum in white-tailed deer (Odocoileus virginianus). Int J Parasitol. (1999) 29:1709–11. doi: 10.1016/S0020-7519(99)00142-3
7. Dubey JP, Jenkins MC, Kwok OCH, Zink RL, Michalski ML, Ulrich V, Gill J, Carstensen M, Thulliez P. Seroprevalence of Neospora caninum and Toxoplasma gondii antibodies in white-tailed deer (Odocoileus virginianus) from Iowa and Minnesota using four serologic tests. Vet Parasitol. (2009) 161:330–4. doi: 10.1016/j.vetpar.2009.01.002
8. King JS, Šlapeta J, Jenkins DJ, Al-Qassab SE, Ellis JT, Windsor PA. Australian dingoes are definitive hosts of Neospora caninum. Int J Parasitol. (2010) 40:945–50. doi: 10.1016/j.ijpara.2010.01.008
9. Dubey JP, Jenkins MC, Rajendran C, Miska K, Ferreira LR, Martins J, et al. Gray wolf (Canis lupus) is a natural definitive host for Neospora caninum. Vet Parasitol. (2011) 181:382–7. doi: 10.1016/j.vetpar.2011.05.018
10. Gondim LFP. Neospora caninum in wildlife. Trends Parasitol. (2006) 22:247–52. doi: 10.1016/j.pt.2006.03.008
11. Dubey JP, Hemphill A, Calero-Bernal R, Schares GN. Neosporosis in Animals. New York: CRC Press. (2017) p. 530. doi: 10.1201/9781315152561
12. Ferroglio E, Rossi L. Prevalence of Neospora caninum antibodies in wild ruminants from the Italian Alps. Vet Rec. (2001) 11:79–91. doi: 10.1136/vr.148.24.754
13. Ferroglio E, Bassano B, Trisciuoglio A, Rossi L. Antibodies to Neospora caninum in Alpine ibex from the Italian Alps. Z Jagdwiss. (2001) 47:226–8. doi: 10.1007/BF02241552
14. Bregoli M, Gioia C, Stefano N, Mariapia C, Claudio P. Serological survey of Neospora caninum in free-ranging wild ruminants. Vet Arh. (2006) 76:
15. Almeria S, Vidal D, Ferrer D, Pabón M., Fernández-de-Mera MIG, Ruiz-Fons F, Alzaga V, Marco I, Calvete C, Lavin S, et al. Seroprevalence of Neospora caninum in non-carnivorous wildlife from Spain. Vet Parasitol. (2007) 143:21–8. doi: 10.1016/j.vetpar.2006.07.027
16. Moskwa B, Gozdzik K, Bień J, Kornacka A, Cybulska A, Reiterová K, et al. Detection of antibodies to Neospora caninum in moose (Alces alces): the first report in Europe. Folia Parasitol (Praha). (2014) 61:34–6. doi: 10.14411/fp.2014.014
17. Bień J, Moskwa B., Cabaj W. In vitro isolation and identification of the first Neospora caninum isolate from European bison (Bison bonasus bonasus L). Vet Parasitol. (2010) 173:200–5. doi: 10.1016/j.vetpar.2010.06.038
18. Ferroglio E, Trisciuoglio A. Antibodies to Neospora caninum in European brown hare (Lepus europaeus). Vet Parasitol. (2003) 115:75–8. doi: 10.1016/S0304-4017(03)00201-2
19. Hughes JM, Thomasson D, Craig PS, Georgin S, Pickles A, Hide G. Neospora caninum: detection in wild rabbits and investigation of co-infection with Toxoplasma gondii by PCR analysis. Exp Parasitol. (2008) 120:255–60. doi: 10.1016/j.exppara.2008.07.011
20. Zanet S, Palese V, Trisciuoglio A, Cantón Alonso C, Ferroglio E. Encephalitozoon cuniculi, Toxoplasma gondii and Neospora caninum infection in invasive Eastern Cottontail Rabbits Sylvilagus floridanus in Northwestern Italy. Vet Parasitol. (2013) 197:682–4. doi: 10.1016/j.vetpar.2013.05.014
21. Ferroglio E, Pasino M, Romano A, Grande D, Pregel P, Trisciuoglio A. Evidence of Neospora caninum DNA in wild rodents. Vet Parasitol. (2007) 148:346–9. doi: 10.1016/j.vetpar.2007.06.031
22. Dubey JP, Schares G. Neosporosis in animals-the last five years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
23. Almería S, Serrano-Pérez B, López-Gatius F. Immune response in bovine neosporosis: Protection or contribution to the pathogenesis of abortion. Microb Pathog. (2017) 109:177–82. doi: 10.1016/j.micpath.2017.05.042
24. Khan A, Fujita AW, Randle N, Regidor-Cerrillo J, Shaik JS, Shen K, et al. Global selective sweep of a highly inbred genome of the cattle parasite Neospora caninum. Proc Natl Acad Sci U S A. (2019) 116:22764–73. doi: 10.1073/pnas.1913531116
25. Liu Y, Reichel MP, Lo WC. Combined control evaluation for Neospora caninum infection in dairy: Economic point of view coupled with population dynamics. Vet Parasitol. (2020) 277:108967. doi: 10.1016/j.vetpar.2019.108967
26. Reichel MP, Alejandra Ayanegui-Alcérreca M, Gondim LFP, Ellis JT. What is the global economic impact of Neospora caninum in cattle–the billion dollar question. Int J Parasitol. (2013) 43:133–42. doi: 10.1016/j.ijpara.2012.10.022
27. Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev. (2007) 20:323–67. doi: 10.1128/CMR.00031-06
28. Dubey JP, Lindsay DS. Transplacental Neospora caninum infection in cats. J Parasitol. (1989) 75:765–71. doi: 10.2307/3283062
29. Dubey JP, Lindsay DS. Neospora caninum induced abortion in sheep. J Vet Diagn Invest. (1990) 2:230–3. doi: 10.1177/104063879000200316
30. Lindsay DS, Rippey NS, Powe TA, Sartin EA, Dubey JP, Blagburn BL. Abortions, fetal death, and stillbirths in pregnant pygmy goats inoculated with tachyzoites of Neospora caninum. Am J Vet Res. (1995) 56:1176–80.
31. Barr BC, Conrad PA, Sverlow KW, Tarantal AF, Hendrickx AG. Experimental fetal and transplacental Neospora infection in the nonhuman primate. Lab Investigat. (1994) 71:236–42.
32. Cole RA, Lindsay DS, Blagburn BL, Dubey JP. Vertical transmission of Neospora caninum in mice. J Parasitol. (1995) 81:730–2. doi: 10.2307/3283962
33. Dubey JP, Rigoulet J, Lagourette P, George C, Longeart L, LeNet JL. Fatal transplacental neosporosis in a deer (Cervus eldi siamensis). J Parasitol. (1996) 82:338-−9. doi: 10.2307/3284175
34. Soldati S, Kiupel M, Wise A, Maes R, Botteron C, Robert N. Meningoencephalomyelitis caused by Neospora caninum in a juvenile fallow deer (Dama dama). J Vet Med A Physiol Pathol Clin Med. (2004) 51:280–3. doi: 10.1111/j.1439-0442.2004.00646.x
35. Peters M, Wohlsein P, Knieriem A, Schares G. Neospora caninum infection associated with stillbirths in captive antelopes (Tragelaphus imberbis). Vet Parasitol. (2001) 97:153–7. doi: 10.1016/S0304-4017(01)00401-0
36. Basso W, Moré G, Quiroga MA, Balducchi D, Schares G, Venturini MC. Neospora caninum is a cause of perinatal mortality in axis deer (Axis axis). Vet Parasitol. (2014) 199:255–8. doi: 10.1016/j.vetpar.2013.10.020
37. Baldini MHM, Sandoval EDP, Duarte JMB. Assessment of transplacental transmission of Neospora caninum and Toxoplasma gondii in Neotropical deer: an estimative based on serology. Vet Parasitol. (2022) 303:109677. doi: 10.1016/j.vetpar.2022.109677
38. Dubey JP, Jenkins MC, Kwok OCH, Ferreira LR, Choudhary S, Verma SK, et al. Congenital transmission of Neospora caninum in white-tailed deer (Odocoileus virginianus). Vet Parasitol. (2013) 196:519–22. doi: 10.1016/j.vetpar.2013.03.004
39. Schares G, Wenzel U, Müller T, Conraths FJ. Serological evidence for naturally occurring transmission of Neospora caninum among foxes (Vulpes vulpes). Int J Parasitol. (2001) 31:418–23. doi: 10.1016/S0020-7519(01)00118-7
40. Yu X, Chen N, Hu D, Zhang W, Li X, Wang B, et al. Detection of Neospora caninum from farm-bred young blue foxes (Alopex lagopus) in China. J Vet Med Sci. (2009) 71:113–5. doi: 10.1292/jvms.71.113
41. Ferroglio E, Pasino M, Ronco F, Benà A, Trisciuoglio A. Seroprevalence of antibodies to Neospora caninum in urban and rural dogs in north-west Italy. Zoonoses Public Health. (2007) 54:135–9. doi: 10.1111/j.1863-2378.2007.01033.x
42. Ferroglio E, Guiso P, Pasino M, Accossato A, Trisciuoglio A. Antibodies to Neospora caninum in stray cats from north Italy. Vet Parasitol. (2005) 131:31–4. doi: 10.1016/j.vetpar.2005.04.012
43. Gennero MS, Bergagna S, Pasino M, Romano A, Grande D, Trisciuoglio A, et al. Detection of Neospora caninum DNA by PCR analysis in bovine aborted foetuses. Epidemiologie et Sante Animale. (2008) 53:51–6. doi: 10.1128/JCM.40.4.1194-1198.2002
44. Muller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol. (1996) 34:2850–2. doi: 10.1128/jcm.34.11.2850-2852.1996
45. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2019)
46. Johnson JB, Omland KS. Model selection in ecology and evolution. Trends Ecol Evol. (2004) 19:101–8. doi: 10.1016/j.tree.2003.10.013
47. Hallgren KA. Computing Inter-Rater reliability for observational data: an overview and tutorial. Quant Meth Psych. (2012) 8:23–34. doi: 10.20982/tqmp.08.1.p023
48. Innes EA, Andrianarivo AG, Björkman C, Williams DJL, Conrad PA. Immune responses to Neospora caninum and prospects for vaccination. Trends Parasitol. (2002) 18:497–504. doi: 10.1016/S1471-4922(02)02372-3
49. Quinn HE, Ellis JT, Smith NC. Neospora caninum: A cause of immunemediated failure of pregnancy? Trends Parasitol. (2002) 18:391–4. doi: 10.1016/S1471-4922(02)02324-3
50. Hemphill A, Vonlaufen N, Naguleswaran A. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology. (2006) 133:261–78. doi: 10.1017/S0031182006000485
51. Williams DJL, Hartley CS, Björkman C, Trees AJ. Endogenous and exogenous transplacental transmission of Neospora caninum–how the route of transmission impacts on epidemiology and control of disease. Parasitology. (2009) 136:1895–900. doi: 10.1017/S0031182009990588
52. Marucco F, Avanzinelli E, Bassano B, Bionda R, Bisi F, Calderola S, et al. La popolazione di lupo sulle Alpi Italiane 2014–2018. (2018). Available online at: https://www.lifewolfalps.eu/wp-content/uploads/2020/10/Report_monitoraggio_Alpi_completo.pdf (accessed January 24, 2023).
Keywords: vertical transmission, Neospora caninum, roe deer, wild boar, red fox
Citation: Zanet S, Poncina M and Ferroglio E (2023) Congenital transmission of Neospora caninum in wild ungulates and foxes. Front. Vet. Sci. 10:1109986. doi: 10.3389/fvets.2023.1109986
Received: 28 November 2022; Accepted: 18 January 2023;
Published: 06 February 2023.
Edited by:
Charoonluk Jirapattharasate, Mahidol University, ThailandReviewed by:
Dadin Prando Moore, National Scientific and Technical Research Council (CONICET), ArgentinaCopyright © 2023 Zanet, Poncina and Ferroglio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Zanet,  c3RlZmFuaWEuemFuZXRAdW5pdG8uaXQ=
c3RlZmFuaWEuemFuZXRAdW5pdG8uaXQ=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.