- Department of Animal Science, College of Animal Science and Technology, Anhui Agricultural University, Hefei, China
This study investigated the effects of Clostridium butyricum on the growth performance, meat quality and intestinal health of broilers. A total of 800 one-day-old male Arbor Acres broilers were randomly assigned to two groups with 16 replicates of 25 broilers per group and fed with a basal diet (CON) or a basal diet supplemented with 1.5 × 109 cfu/kg C. butyricum and 5 × 108 cfu/kg C. butyricum at 1–21 d and 22–42 d, respectively (CB). The results indicated that C. butyricum significantly increased the final body weight, average daily gain at 1–42 d in the growth performance of broilers (P < 0.05). Moreover, C. butyricum significantly increased value and pH24h value of breast meat but reduced the drip loss and shear force (P < 0.05). Regarding serum antioxidant indices, C. butyricum significantly increased the total superoxide dismutase (T-SOD) and total antioxidative capacity activities and reduced the malondialdehyde content (P < 0.05). Furthermore, the broilers in the CB demonstrated an increase in jejunal lipase and trypsin activities, villus height (VH) and VH-to-crypt depth ratio at 42 d compared with those in the CON (P < 0.05). C. butyricum also upregulated the intestinal mRNA levels of zonula occludens-1, nuclear factor erythroid 2-related factor 2 (Nrf2), SOD1 and interleukin-10 in the jejunal mucosa (P < 0.05), but it downregulated the mRNA levels of nuclear factor kappa B (NF-κB) and tumor necrosis factor-α (P < 0.05). These results indicate that C. butyricum can improve the growth performance and meat quality of broilers. In particular, C. butyricum can improve the intestinal health of broilers, which is likely to be related to the activation of the Nrf2 signaling pathway and inhibition of the NF-κB signaling pathway.
1. Introduction
Generally, the growth performance of broilers is highly dependent on their intestinal health, which is influenced by a variety of factors, such as diet, stress and infection. These factors may increase the intestinal permeability of broilers and compromise the structural integrity of their intestinal epithelium, resulting in an increase in inflammatory responses and ultimately the restriction of normal growth (1). In recent years, because of the ban on antibiotics in poultry feed, unspecified intestinal diseases have emerged in poultry production (2). Therefore, new alternatives are urgently required to reduce the incidence of intestinal disease and increase the production efficiency of broilers. Among these potential alternatives, probiotics, owing to their ecofriendly and pollution-free characteristics, are typically fed directly to inhibit the growth of pathogens and regulate their intestinal function of broilers (2, 3). Studies have demonstrated that probiotics could prevent pathogenic bacteria from adhering to the intestinal mucosa and hence improve the microecological environment of the gastrointestinal tract in order to optimize the intestinal function of broilers, thereby enhancing their growth performance (4, 5). Hence, dietary supplementation with probiotics is regarded as an effective method for enhancing the growth performance and enteric diseases resistance of broilers.
Clostridium butyricum CBM 588 is a typical probiotic strain that produces spores that are resistant to high temperature, humidity, stomach acid and bile salts, indicating its high adaptability to the intestinal environment (6). C. butyricum metabolites contain digestive enzymes that can break down large molecules of nutrients into small molecules that can be easily absorbed, thereby enhancing the utilization efficiency of these nutrient substances and ultimately promoting the growth performance of the host (6). As a source of energy, short-chain fatty acids (SCFA), another metabolite of C. butyricum, not only retard the growth of pathogenic enteric bacteria, but also promote intestinal development. Studies have indicated that the addition of C. butyricum to the diet of broilers could increase their intestinal digestive capacity and improve their intestinal morphology, which in turn enhances their growth performance (7, 8). Liao et al. (9) reported that C. butyricum treatment increased the concentrations of polyunsaturated fatty acids in the meat and improved the meat quality of broilers. In addition, C. butyricum was found to positively affect the immune response of broilers, which could promote the secretion of anti-inflammatory factors and immunoglobulins (10, 11). A recent study on Pekin ducks revealed that dietary C. butyricum supplementation increased the SCFA content in the gut and optimized the intestinal flora (12). Although the effects of C. butyricum treatment on the growth performance and intestinal health have been extensively documented in the literature on poultry production, the results remain inconsistent. Therefore, the further research on more targeted indicators is necessary to accurately evaluate the benefits of C. butyricum and explore the mechanism of through which C. butyricum regulates intestinal health. In addition, because of C. butyricum and its metabolites showing certain immuno–antioxidant properties in poultry (9, 13), we hypothesize that C. butyricum could regulate the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway (a key signaling pathway for regulating antioxidant capacity) and nuclear factor-κB (NF-κB) signaling pathway (a key signaling pathway for regulating inflammatory response). Accordingly, the present study investigated the effects of C. butyricum on the growth performance, meat quality and intestinal health of broilers to elucidate the potential mechanism though which C. butyricum regulates intestinal health.
2. Materials and methods
2.1. Experimental design and diets
The experimental procedures performed on the animals included in this study were approved by the Animal Ethics Committee of Anhui Agricultural University. A total of 800 one-day-old male Arbor Acres (AA) broilers from the same batch were randomly assigned to two groups with 16 replicates of 25 broilers per group and fed with a basal diet (CON) or a basal diet supplemented with 1.5 × 109 cfu/kg C. butyricum and 5 × 108 cfu/kg C. butyricum at 1–21 d and 22–42 d, respectively (CB). The C. butyricum additive contained at least 5 × 109 cfu/g powder. The addition level of C. butyricum used in this study was based on an unpublished gradient addition trial which found that C. butyricum supplementation at 1.5 × 109 cfu/kg from 1 to 21 d and 5 × 108 cfu/kg from 22 to 42 d could maximize the growth performance of broilers. The broilers were provided with mash feed and fresh water ad libitum from 1 d until 42 d. The temperature was gradually reduced from 34°C ± 1°C to 23°C ± 1°C at a rate of 2°C or 3°C per week and then maintained at 23°C ± 1°C until the end of the experiment, and the lighting program was set to produce 1 h of darkness and 23 h of light. The coop was longitudinally ventilated using an exhaust fan, and the humidity maintained between 55 and 65%. The basal diets are formulated according to the requirements for broilers by NRC (1994), and their composition and nutritional level are displayed in Supplementary Table 1. Body weight (BW) at 21 d and 42 d and daily feed consumption were documented on a replicate basis to calculate the average daily BW gain (ADG), average daily feed intake (ADFI) and feed to gain ratio (F/G). These indexes of growth performance were calculated using the following formulas.
2.2. Sample collection
At 21 and 42 d, one broiler of the average pen BW was selected from each replicate and euthanized by electrical stunning, and the remaining broilers meeting the market requirement were sold after the end of the experiment. Blood samples were then collected from the wing vein after 12 h of fasting, placed in polypropylene tubes, and centrifuged at 1500 × g for 15 min at 4°C to obtain the serum, which was then stored at −20°C until the analysis of antioxidant-related indicators. After the slaughter and dissection processes, the right breast muscle was removed for the analysis of cooking loss and shear force, and the left breast muscle was removed and kept at 4°C for the analysis of pH, meat color and drip loss. To evaluate the digestive enzyme activity, duodenal, jejunal, and ileal digesta were collected in sterile centrifuge tubes, speedily removed in liquid nitrogen and kept at −80°C. Subsequently, segments measuring 2 cm were cut from the middle of the duodenum, jejunum and ileum and fixed in 10% buffered formalin for morphological evaluation. Mucosal samples were then obtained by gently scraping the jejunal wall using sterile slides, immediately frozen in liquid nitrogen, and stored at −80°C until gene expression analysis.
2.3. Meat quality
The color (L*, lightness; a*, redness; b*, yellowness) and pH of breast muscle were measured in triplicate using a chroma meter (CR-300, Minolta Camera, Osaka, Japan) and a pH meter (pH-STAR, SFK-Technology, Copenhagen, Denmark) at 45 min and 24 h postmortem, respectively. Subsequently, drip loss, cooking loss, and shear force were measured using our previously described methods (14).
2.4. Metabolite contents and enzyme activities
The intestinal contents were first homogenized in precooled phosphate-buffered saline and centrifuged at 1000 × g for 10 min at 4°C, and then the supernatants were obtained. Subsequently, malondialdehyde (MDA) level and the activities of catalase (CAT), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px) in serum, as well as intestinal amylase, lipase, and trypsin activities were determined by commercially available kits (Nanjing Jiancheng Biochemistry Institute, Nanjing, China) according to the manufacturer's instructions.
2.5. Intestinal morphology
Intestinal samples were dehydrated, embedded in paraffin, and stained with hematoxylin and eosin. Subsequently, under an inverted fluorescence microscope, villus height (VH) and crypt depth (CD) were measured and recorded using an image processing and analysis system (Leica Imaging Systems, Cambridge, UK) to evaluate the ratio of VH to CD (VH/CD).
2.6. Quantitative reverse transcription PCR
Total RNA was separated from jejunal mucosa using TRIzol (Yeasen, Shanghai, China). The RNA samples were then reverse-transcribed into cDNA for next analysis using a Hifair® II 1st Strand cDNA Synthesis Kit (Yeasen, Shanghai, China), and gene expression levels were quantified using real-time PCR with a Hieff® qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) and a real-time PCR system (Thermo Fisher Scientific, MA, USA). All primers (Supplementary Table 2) were synthesized by Hefei Qingke Biotechnology Co., Ltd. The reaction conditions and parameter settings followed our previous research (15). The relative expression of target genes was determined using the 2−ΔΔCt method based on the expression of β-actin.
2.7. Statistical analysis
Data were analyzed using SPSS 18.0 (SPSS, Chicago, IL, USA) and are expressed as mean ± standard error. Student's t-test was performed to analyze and compare the CON and CB groups. Statistical significance was set at P < 0.05.
3. Results
3.1. Growth performance
As shown in Table 1, the addition of C. butyricum significantly increased the BW and ADG of broilers at 42 d and 1–42 d, respectively (P < 0.05), and tended to improve their ADG at 22–42 d (P = 0.057). However, C. butyricum did not have significant effects on the ADFI or F/G at each stage in either group (P > 0.05).
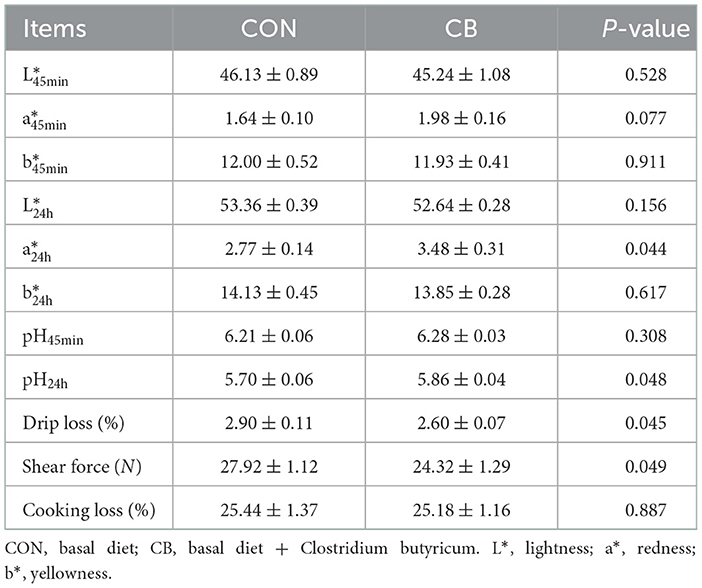
3.2. Meat quality
As listed in Table 2, the broilers in the CB group had higher a and pH24h values and a lower drip loss and shear force than did those in the CON group (P < 0.05). In addition, treatment with C. butyricum tended to increase their a value (P = 0.077).
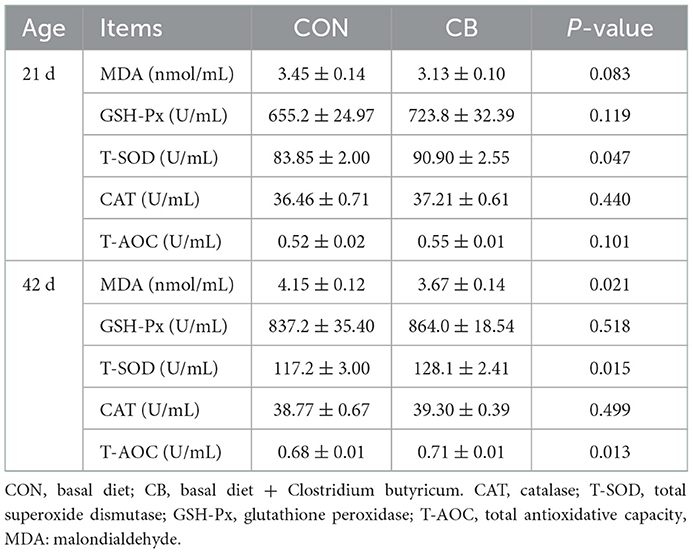
3.3. Serum antioxidant statues
As presented in Table 3, the broilers in the CB group recorded the lower (P < 0.05) content of MDA at 42 d and higher (P < 0.05) activities of T-AOC at 42 d and T-SOD at d 21 and d 42 than did those in the CON group. Furthermore, C. butyricum treatment tended to decrease the MDA content at 21 d (P = 0.083). However, no marked difference was observed in the activities of GSH-Px and CAT (P > 0.05).
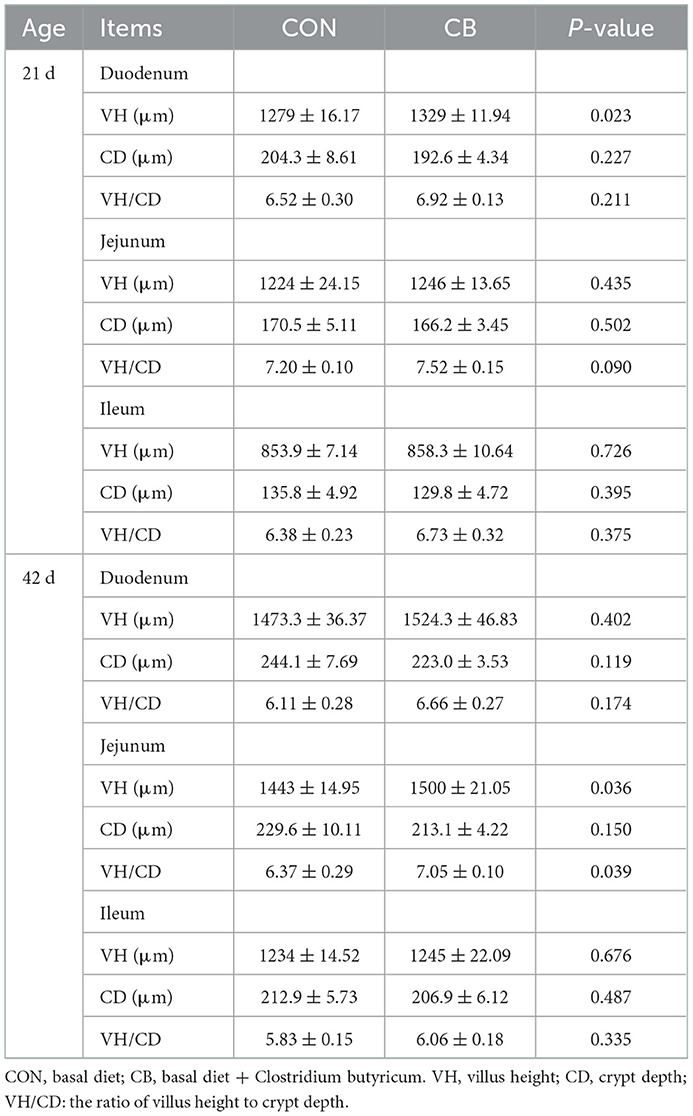
3.4. Intestinal morphology
As listed in Table 4, no significant difference was observed in CD between the CON and CB groups (P > 0.05). However, the addition of C. butyricum significantly increased the jejunal VH and VH/CD at 42 d (P < 0.05). Similarly, C. butyricum supplementation significantly improved the duodenal VH at 21 d (P < 0.05). However, no remarkable influence in ileal morphology was observed between the CON and CB groups (P > 0.05).
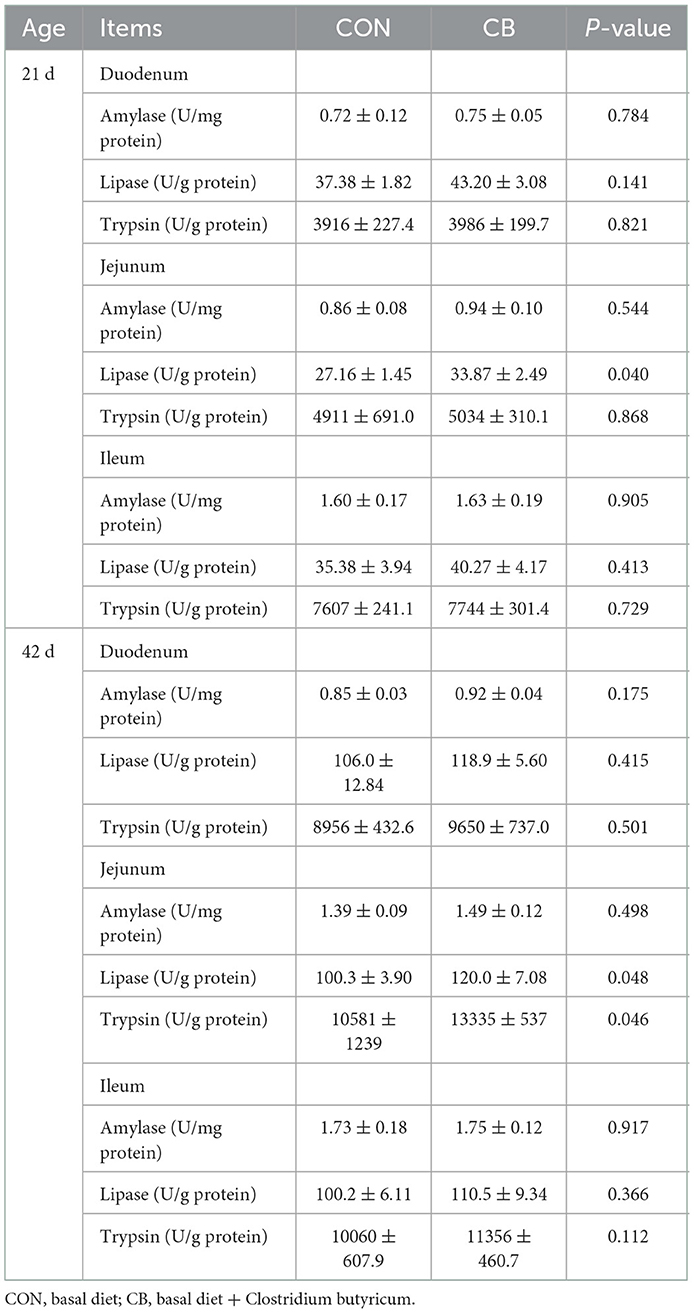
3.5. Digestive enzyme activity
As displayed in Table 5, no remarkable difference was observed in the digestive enzyme activity in the duodenum or ileum between the CON and CB groups (P > 0.05). Compared with the CON group, the CB group demonstrated higher lipase activity in the jejunum at 21 and 42 d (P < 0.05). Similarly, C. butyricum dramatically increased jejunal trypsin activity at 42 d (P < 0.05).
3.6. mRNA expression levels of intestinal barrier genes
As illustrated in Figure 1, the CB group had a higher expression level of the tight-junction-protein-related gene ZO-1 than did the CON group (P < 0.05). However, occludin level was similar in both groups (P > 0.05).
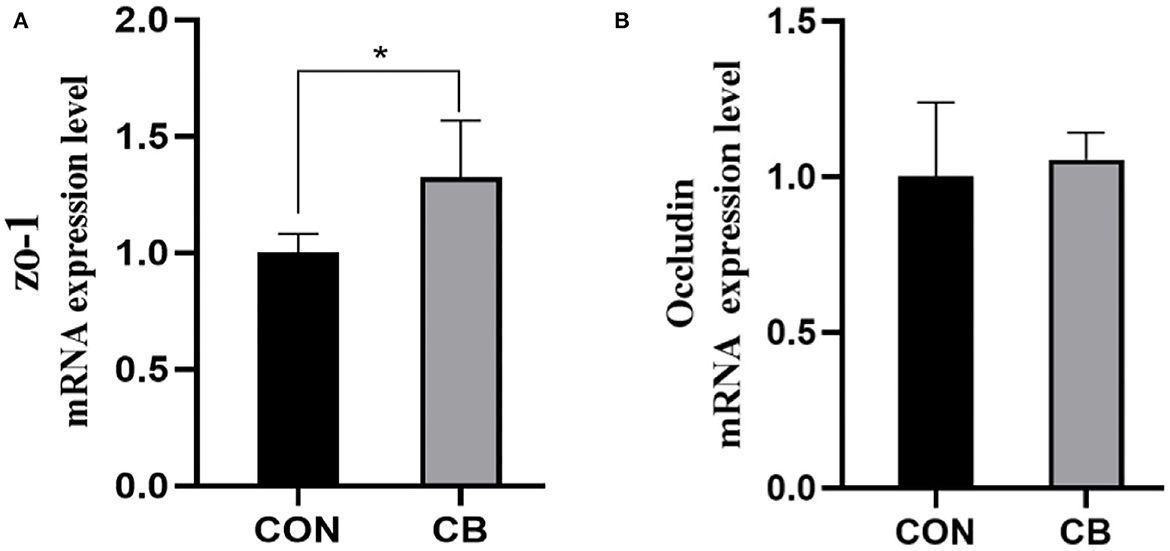
Figure 1. (A, B) Effect of C. butyricum on the mRNA expression levels of jejunal barrier genes of broilers. CON, basal diet; CB, basal diet + Clostridium butyricum. *Significant difference between group (P < 0.05). ZO-1, zonula occludin protein 1.
3.7. mRNA expression levels of antioxidant-related genes
As presented in Table 6, C. butyricum addition effectively increased the jejunal mRNA levels of Nrf2 and SOD1 in the broilers (P < 0.05). However, no significant differences were observed in the mRNA levels of CAT or GSH-Px (P > 0.05).
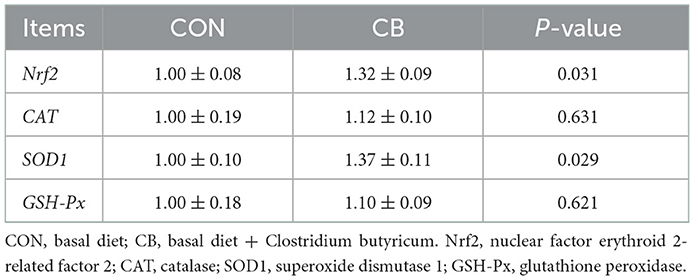
Table 6. Effect of C. butyricum on the mRNA expression levels of jejunal antioxidant-related genes of broilers.
3.8. mRNA expression levels of inflammation-related genes
As listed in Table 7, C. butyricum effectively downregulated jejunal mRNA expression of NF-κB and tumor necrosis factor-α (TNF-α) (P < 0.05), but it upregulated interleukin (IL)-10 mRNA expression (P < 0.05). However, no significant difference was observed in the mRNA expression levels of IL-1β or IL-6 between the CON and CB groups (P > 0.05).
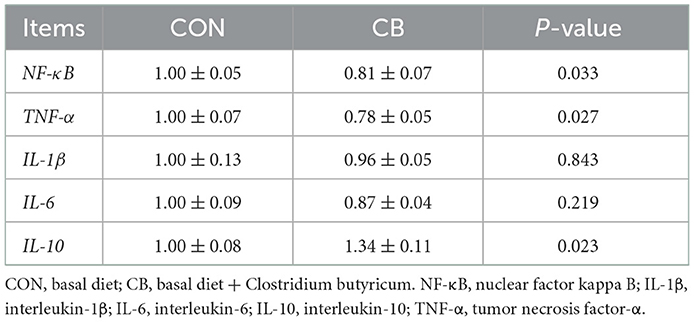
Table 7. Effect of C. butyricum on the mRNA expression levels of jejunal inflammation-related genes of broilers.
4. Discussion
The development of intensive production has predisposed poultry to diseases, which in turn inhibits their growth and development. Including probiotics such as C. butyricum in their diets is therefore regarded as an effective solution to this problem (16, 17). As the most reliable indicator of broiler growth and development, growth performance is the key to increase economic efficiency. According to a previous study, AA broilers fed with 1 × 109 cfu/kg C. butyricum exhibited an increase in ADG and a decrease in F/G at 1–21 and 1–42 days, respectively (18). Nevertheless, Yang et al. (11) and Liao et al. (16) discovered that different doses of C. butyricum addition significantly increased the ADG of broilers at 22–42 d but did not considerably affect their feed conversion ratio. In the present study, we investigated the effect of C. butyricum on the growth performance of AA broilers. Our results indicate that although C. butyricum did not considerably affect the growth performance of the broilers at 1–21 days, it significantly improved their final BW and ADG at 42 and 1–42 days, respectively, which is consistent with the results of Yang et al. (11). These results suggest that C. butyricum treatment improves the growth performance of broilers, which may be due to the production of butyric acid, vitamin B, and amylase by C. butyricum to enhance the utilization of feed nutrients (19). In addition, different effects of C. butyricum supplementation have been observed in various studies, which could be attributed to some essential factors such as additive dosage, broiler breed and physiological stage, diet composition, and feeding management.
With the rise in living standards, consumers have become increasingly concerned with meat quality, especially in China. Meat quality is an accurate indicator of the physical and chemical properties of meat. In general, L*, a*, and b* are used to evaluate the color of meat, which is a crucial indicator of the meat's freshness and quality (20). After broilers are slaughtered, the lactic acid is produced by glycolysis accumulates in the muscle tissue, resulting in a decrease in the pH value, protein denaturation, and a decline in the ability of muscle protein to bind water (21). Thus, the final pH and rate of pH decline in meat have a direct effect on the drip loss and ultimately on the water retention capacity and tenderness of the meat. Liu et al. (20) reported that C. butyricum treatment increased the pH45min and a* values but reduced the L* value, drip loss and shear force of in Peking duck breast meat. In another study, Li et al. (22) dicovered that Partridge shank chickens fed with a synbiotic diet including C. butyricum exhibited lower breast muscle drip loss and cooking loss and higher a and pH24h values. In the present study, we discovered that C. butyricum increased the a and pH24h values but reduced the drip loss and shear force of breast meat, indicating an improvement of the freshness, tenderness and water retention capacity of the meat. These favorable results indicate that C. butyricum can enhance the meat quality of broilers.
Antioxidant enzyme activity and oxidation product concentration are reliable biomarkers for assessing the antioxidant status of animals. T-SOD is a crucial component of the enzyme system, which effectively scavenges free radicals and inhibits peroxidation. MDA is one of the final peroxidation products derived from membrane lipids. Its level is regarded as a crucial index for quantifying the degree of peroxidation (14). According to previous studies, C. butyricum could mitigate peroxidation and improve the antioxidant enzyme activity in vivo (13, 16, 23). Li et al. (18) reported that the addition of C. butyricum to the diets of broilers enhanced the activities of their serum T-AOC, GSH-Px and SOD. Zhan et al. (13) discovered that C. butyricum supplementation resulted in the improvement of T-SOD and GSH?Px activities in the serum of laying hens. Similarly, Han et al. (23) found that C. butyricum addition enhanced serum GSH-Px activity in weaned piglets. In the present study, we discovered that C. butyricum supplementation significantly decreased the serum MDA content in broilers at 42 d, indicating the lower level of lipid peroxidation. In addition, C. butyricum addition significantly increased the activities of T-SOD and T-AOC at 42 d, indicating an improvement of antioxidant capacity. Generally, an improvement in antioxidant capacity may reduce the degree of protein and lipid oxidative damage in muscles and prolong the shelf life of meat (14), further confirming that C. butyricum improves the quality of meat.
Digestive enzymes are important for the digestion of nutrient materials and their activities have a substantial effect on the feed conversion and growth performance of broilers. Previous studies have recorded that probiotics can produce digestive enzymes and enhance their activities in the intestine. According to a previous study, Cobb broilers fed with C. butyricum exhibited elevated jejunal amylase, lipase, and protease activity (10). Wang and Gu (24) discovered that the addition of Bacillus coagulans NJ0516 to the diets of broilers resulted in a significant increase in their duodenal protease and amylase activities. However, Rodjan et al. (25) reported that the supplementation of Bacillus spores did not significantly affect the digestive enzyme activity of Ross 308 broilers. Similar to the findings of Zhang et al. (10), we found that C. butyricum increased the jejunal lipase and trypsin activities in the broilers. These favorable results suggest that C. butyricum significantly improves the digestive function of broilers; this is attributable to their metabolism of SCFA, which may stimulate the secretion of digestive enzymes by digestive glands. However, the currently available information is inconsistent and limited, necessitating further evaluation of the underlying mechanism.
The intestine is an essential conduit for the exchange of materials between the external environment and the host environment. Intestinal morphology is a major factor in the evaluation of the intestinal status and is closely linked with the development of the intestinal epithelial structure, which is typically evaluated though the VH, CD and VH/CD. A decrease in CD and an increase in VH and VH/CD indicate a natural increase in epithelial cell turnover and a well-differentiated intestinal mucosa, suggesting an enhanced digestive and absorptive capacity (26). A previous study reported that C. butyricum treatment significantly improved the duodenal VH and VH/CD and reduced the duodenal CD in broilers (27). Cao et al. (28) found that broilers fed with C. butyricum exhibited an increased VH and a decreased CD in the ileum. Furthermore, a recent study revealed that the addition of C. butyricum to the diet of broilers infected with Clostridium perfringens increased their intestinal VH/CD and ameliorated the degree of their intestinal damage (29). In the present study, we discovered that C. butyricum considerably improved the duodenal VH and jejunal VH and VH/CD of the broilers, indicating an improvement in intestinal morphology. These positive results suggest that C. butyricum has a promotive effect on the development of the intestinal epithelial structure and thereby improves the intestinal morphology.
The intestinal barrier is crucial for protecting the host against infection and other diseases and maintaining normal physiological function. In addition to preventing the entry of luminal bacteria and dietary allergens into the mucosa, an intact intestinal barrier selectively regulates the entry of nutrients, ions, and water into the body. ZO-1 and occludin, the main tight junction proteins of the intestinal barrier, play a major role in regulating the integrity and permeability of the gut barrier by binding to the actin cytoskeleton and contributing to the formation of a tight junction structure. According to Li et al. (18), the addition of C. butyricum to the diets of broilers increased the relative expression levels of claudin-1 and ZO-1 in the gut. Liu et al. (30) reported that treatment with C. butyricum significantly increased the expression of occludin and ZO-1 in the jejunum of broilers at 42 d. A similar result was observed in our study; that is, after 42 d of feeding broilers with a diet containing C. butyricum, we discovered that the jejunal mRNA levels of ZO-1 increased, indicating an improvement in the broilers' intestinal barrier integrity.
With the exception of the intestinal barrier, intestinal health is inseparable from intestinal antioxidant status and immune function. As a major regulator, Nrf2 is a basic leucine zipper protein that regulates the expression of genes related to antioxidants or detoxifying enzymes and protects the body against oxidative stress (31, 32). In general, Nrf2 exists in an inactive form in the cytoplasm binding to its inhibitor: Kelch-like epichlorohydrin-related protein 1 (Keap1). In response to oxidative stress, the Keap1–Nrf2 complex dissociates, allowing Nrf2 to translocate into the nucleus and bind to the antioxidant response element. This results in the transcription of antioxidant genes such as heme oxygenase-1, SOD and CAT. Hence, activating the Nrf2 signaling pathway may increase the expression levels of antioxidant genes (33). In our work, we first found that C. butyricum supplementation in broilers resulted in a significant increase in the expression levels of Nrf2 and SOD1 in their jejunal mucosa, indicating an improvement in their intestinal antioxidant capacity. This positive finding may be attributable to the activation of the Nrf2 signaling pathway, which effectively protects the intestinal mucosa against oxidative stress damage (33). Moreover, NF-κB, a key transcription factor involved in immunity, can recognize, rapidly respond to, and transcribe a variety of proinflammatory cytokines and inflammatory mediators, such as TNF-α and ILs, resulting in an inflammatory crisis (34). Reduced expression of genes encoding proinflammatory cytokines, such as interferon-γ, IL-1β and TNF-α, may retard the activation of the NF-κB signaling pathway. Li et al. (18) demonstrated that C. butyricum increased the expression levels of IL-1β and TNF-α in the ileum of broilers. Besides, a study on weaned piglets challenged with lipopolysaccharides revealed that C. butyricum downregulated the expression of IL-1β, TNF-α and NF-κB, thereby mitigating inflammatory damage (35). Similarly, in the present study, we discovered that C. butyricum treatment significantly downregulated the mRNA expression of NF-κB and TNF-α in the jejunal mucosa of the broilers. Moreover, we first observed that C. butyricum significantly increased the mRNA expression of IL-10 in their mucosa. These positive results indicate the lower inflammation and the improved immune status, which is by likely inhibiting NF-κB signaling pathway. Indeed, a potential cross-link exists between the Nrf2 and NF-κB signaling pathways (36), and inhibiting the Nrf2 signaling pathway reduces the antioxidant capacity and increases the likelihood of oxidative stress damage to the body, ultimately leading to inflammation through NF-κB signaling pathway activation (37, 38). Therefore, improvements in intestinal health, including the intestinal morphology, intestinal barrier, antioxidant capacity and immune status, induced by C. butyricum may be closely related to the activation of the Nrf2 signaling pathway and inhibition of the NF-κB signaling pathway.
5. Conclusions
The addition of C. butyricum to the diets of broilers can improve their growth performance and meat quality. C. butyricum can also improve intestinal health, which is likely to be linked with the activation of the Nrf2 signaling pathway and inhibition of the NF-κB signaling pathway. However, additional research is necessary to determine the exact mechanism of action of C. butyricum.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Ethics Committee of Anhui Agricultural University.
Author contributions
Conceptualization: ZL and XJ. Methodology: LL and YL. Investigation and writing—original draft preparation: ZL and LL. Data curation: QW and YL. Formal analysis: XJ and QW. Writing—review and editing: CZ and XC. Supervision: ZG and CZ. Funding acquisition: CZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Major Science and Technology Project of Huaibei City (Z2020013), the Technical Service Project of Poultry Breed Industry and Efficient Production Technology Innovation Center in Xuanzhou District of Anhui Province (21339032), and the Innovation and Entrepreneurship Cultivation Project for Graduate Students of Anhui Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1107798/full#supplementary-material
References
1. Gadde U, Oh ST, Lee YS, Davis E, Zimmerman N, Rehberger T, et al. The effects of direct-fed microbial supplementation, as an alternative to antibiotics, on growth performance, intestinal immune status, and epithelial barrier gene expression in broiler chickens. Probiotics Antimicrob Proteins. (2017) 9:397–405. doi: 10.1007/s12602-017-9275-9
2. Teirlynck E, Gussem MDE, Dewulf J, Haesebrouck F, Ducatelle R, Van Immerseel F. Morphometric evaluation of “dysbacteriosis” in broilers. Avian Pathol. (2011) 40:139–44. doi: 10.1080/03079457.2010.543414
3. Bhogoju S, Nahashon S. Recent advances in probiotic application in animal health and nutrition: a review. Agriculture. (2022) 12:304. doi: 10.3390/agriculture12020304
4. Ghasemi-Sadabadi M, Ebrahimnezhad Y, Shaddel-Tili A, Bannapour-Ghaffari V, Kozehgari H, Didehvar M. The effects of fermented milk products (kefir and yogurt) and probiotic on performance, carcass characteristics, blood parameters, and gut microbial population in broiler chickens. Arch Anim Breed. (2019) 62:361–74. doi: 10.5194/aab-62-361-2019
5. Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. (2017) 61:1600240. doi: 10.1002/mnfr.201600240
6. Guo PT, Zhang K, Ma X, He PL. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. (2020) 11:24. doi: 10.1186/s40104-019-0402-1
7. Han J, Wang Y, Song D, Lu Z, Dong Z, Miao H, et al. Effects of Clostridium butyricum and Lactobacillus plantarum on growth performance, immune function and volatile fatty acid level of caecal digesta in broilers. Food Agric Immunol. (2018) 29:797–807. doi: 10.1080/09540105.2018.1457013
8. Eeckhaut V, Van Immerseel F, Croubels S, De Baere S, Haesebrouck F, Ducatelle R, et al. Butyrate production in phylogenetically diverse Firmicutes isolated from the chicken caecum. Microb Biotechnol. (2011) 4:503–12. doi: 10.1111/j.1751-7915.2010.00244.x
9. Liao XD, Wu RJ, Ma G, Zhao LM, Zheng ZJ, Zhang RJ. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids Health Dis. (2015) 14:36. doi: 10.1186/s12944-015-0035-0
10. Zhang L, Zhang L., Zhan X, Zeng X, Zhou L, Cao G, et al. Effects of dietary supplementation of probiotic, Clostridium butyricum, on growth performance, immune response, intestinal barrier function, and digestive enzyme activity in broiler chickens challenged with Escherichia coli K88. J Anim Sci Biotechnol. (2016) 7:3. doi: 10.1186/s40104-016-0061-4
11. Yang CM, Cao GT, Ferket PR, Liu TT, Zhou L, Zhang L, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poult Sci. (2012) 91:2121–9. doi: 10.3382/ps.2011-02131
12. Liu Y, Liu C, An K, Gong X, Xia Z. Effect of Dietary Clostridium butyricum supplementation on growth performance, intestinal barrier function, immune function, and microbiota diversity of Pekin ducks. Animals. (2021) 11:2514. doi: 10.3390/ani11092514
13. Zhan HQ, Dong XY, Li LL, Zheng YX, Gong YJ, Zou XT. Effects of dietary supplementation with Clostridium butyricum on laying performance, egg quality, serum parameters, and cecal microflora of laying hens in the late phase of production. Poult Sci. (2019) 98:896–903. doi: 10.3382/ps/pey436
14. Zhang C, Luo J, Yu B, Zheng P, Huang Z, Mao X, et al. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. (2015) 102:15–21. doi: 10.1016/j.meatsci.2014.11.014
15. Zhao F, Wang X, Li Y, Chen X, Geng Z, Zhang C. Effects of dietary supplementation with epigallocatechin gallate on meat quality and muscle antioxidant capacity of broilers subjected to acute heat stress. Animals. (2021) 11:3296. doi: 10.3390/ani11113296
16. Liao XD, Ma G, Cai J, Fu Y, Yan XY, Wei XB, et al. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult Sci. (2015) 94:662–7. doi: 10.3382/ps/pev038
17. Lokapirnasari WP, Al Arif A, Soeharsono S, Fathinah A, Najwan R, Wardhani HCP, et al. Improves in external and internal egg quality of Japanese quail (Coturnix coturnix japonica) by giving lactic acid bacteria as alternative antibiotic growth promoter. Iran J Microbiol. (2019) 11:406–11. doi: 10.18502/ijm.v11i5.1959
18. Li W, Xu B, Wang L, Sun Q, Deng W, Wei F, et al. Effects of Clostridium butyricum on growth performance, gut microbiota and intestinal barrier function of broilers. Front Microbiol. (2021) 12:777456. doi: 10.3389/fmicb.2021.777456
19. Araki Y, Andoh A, Fujiyama Y, Takizawa J, Takizawa W, Bamba T. Short-term oral administration of a product derived from a probiotic, Clostridium butyrictim induced no pathological effects in rats. Int J Mol Med. (2002) 9:173–7. doi: 10.3892/ijmm.9.2.173
20. Liu YH, Li YY, Feng XC, Wang Z, Xia ZF. Dietary supplementation with Clostridium butyricum modulates serum lipid metabolism, meat quality, and the amino acid and fatty acid composition of Peking ducks. Poult Sci. (2018) 97:3218–29. doi: 10.3382/ps/pey162
21. Meng Q, Sun S, Bai Y, Luo Z, Li Z, Shi B, et al. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. (2020) 167:108176. doi: 10.1016/j.meatsci.2020.108176
22. Li J, Cheng YF, Chen YP, Qu HM, Zhao YR, Wen C, et al. Effects of dietary synbiotic supplementation on growth performance, lipid metabolism, antioxidant status, and meat quality in Partridge shank chickens. J Appl Anim Res. (2019) 47:586–90. doi: 10.1080/09712119.2019.1693382
23. Han Y, Tang C, Li Y, Yu Y, Zhan T, Zhao Q, et al. Effects of dietary supplementation with Clostridium butyricum on growth performance, serum immunity, intestinal morphology, and microbiota as an antibiotic alternative in weaned piglets. Animals. (2020) 10:2287. doi: 10.3390/ani10122287
24. Wang Y, Gu Q. Effect of probiotic on growth performance and digestive enzyme activity of arbor acres broilers. Res Vet Sci. (2010) 89:163–7. doi: 10.1016/j.rvsc.2010.03.009
25. Rodjan P, Soisuwan K, Thongprajukaew K, Theapparat Y, Khongthong S, Jeenkeawpieam J, et al. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens. J Anim Physiol Anim Nutr. (2018) 102:931–40. doi: 10.1111/jpn.12858
26. Shamoto K, Yamauchi K. Recovery responses of chick intestinal villus morphology to different refeeding procedures. Poult Sci. (2000) 79:718–23. doi: 10.1093/ps/79.5.718
27. Abdel-Latif MA, Abd El-Hack ME, Swelum AA, Saadeldin IM, Elbestawy AR, Shewita RS, et al. Single and combined effects of Clostridium butyricum and saccharomyces cerevisiae on growth indices, intestinal health, and immunity of broilers. Animals. (2018) 8:184. doi: 10.3390/ani8100184
28. Cao GT, Xiao YP, Yang CM, Chen AG, Liu TT, Zhou L, et al. Effects of Clostridium butyricum on growth performance, nitrogen metabolism intestinal morphology and cecal microflora in broiler chickens. J Anim Vet Adv. (2012) 11:2665–71. doi: 10.3923/javaa.2012.2665.2671
29. Xu X, Yang S, Olajide JS, Qu Z, Gong Z, Wang J, et al. Clostridium butyricum supplement can ameliorate the intestinal barrier roles in broiler chickens experimentally infected with Clostridium perfringens. Front Physiol. (2021) 12:737481. doi: 10.3389/fphys.2021.737481
30. Liu L, Ling HY, Zhang W, Zhou Y, Li YG, Peng N, et al. Functional comparison of Clostridium butyricum and sodium butyrate supplementation on growth, intestinal health, and the anti-inflammatory response of broilers. Front Microbiol. (2022) 13:914212. doi: 10.3389/fmicb.2022.914212
31. Li W, Kong A-N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog. (2009) 48:91–104. doi: 10.1002/mc.20465
32. Zhu Y, Zhang Y-J, Liu W-W, Shi A-W, Gu N. Salidroside suppresses HUVECs Cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. (2016) 21:1033. doi: 10.3390/molecules21081033
33. Song J, Kang SM, Lee WT, Park KA, Lee KM, Lee JE. Glutathione protects brain endothelial cells from hydrogen peroxide-induced oxidative stress by increasing nrf2 expression. Exp Neurobiol. (2014) 23:93–103. doi: 10.5607/en.2014.23.1.93
34. Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. (2018) 4:126–36. doi: 10.1016/j.aninu.2017.09.001
35. Chen L, Li S, Zheng J, Li W, Jiang X, Zhao X, et al. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechnol. (2018) 9:62. doi: 10.1186/s40104-018-0275-8
36. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-kappa B response pathways. Biochem Soc Trans. (2015) 43:621–6. doi: 10.1042/BST20150014
37. Gao W, Guo L, Yang Y, Wang Y, Xia S, Gong H, et al. Dissecting the crosstalk between Nrf2 and NF-kappa B response pathways in drug-induced toxicity. Front Cell Dev Biol. (2022) 9:809952. doi: 10.3389/fcell.2021.809952
Keywords: broilers, Clostridium butyricum, growth performance, meat quality, intestinal health
Citation: Li Z, Long L, Jin X, Li Y, Wu Q, Chen X, Geng Z and Zhang C (2023) Effects of Clostridium butyricum on growth performance, meat quality, and intestinal health of broilers. Front. Vet. Sci. 10:1107798. doi: 10.3389/fvets.2023.1107798
Received: 25 November 2022; Accepted: 09 January 2023;
Published: 24 January 2023.
Edited by:
Abdelrazeq M. Shehata, Al-Azhar University, EgyptReviewed by:
Mahmoud M. Alagawany, Zagazig University, EgyptSabreen Ezzat Fadl, Matrouh University, Egypt
Penka Mladenova Petrova, Bulgarian Academy of Sciences, Bulgaria
Subrat Kumar Bhanja, Central Avian Research Institute (ICAR), India
Copyright © 2023 Li, Long, Jin, Li, Wu, Chen, Geng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Zhang,  Y2hlbmcyMDA1MDUwMkAxMjYuY29t
Y2hlbmcyMDA1MDUwMkAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Zhen Li
Zhen Li Lingbo Long†
Lingbo Long† Xingyong Chen
Xingyong Chen Zhaoyu Geng
Zhaoyu Geng Cheng Zhang
Cheng Zhang