
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 03 February 2023
Sec. Livestock Genomics
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1085474
This article is part of the Research Topic Epigenomics Implication for Economic Traits in Domestic Animals View all 10 articles
Wei pig (WP) and Large White pig (LP) are fatty and lean breeds, respectively. Extrachromosomal circular DNA (eccDNA) plays an important role in regulating signaling pathway processes of cell. However, there are few reports regarding the eccDNA and ecDNA profiles in WP and LP. The present work aimed to investigate the eccDNA and ecDNA profiles between WP and LP. Three WPs and three LPs (100 ± 1.3 kg) were selected for analysis of eccDNA and ecDNA in the ear samples. Results showed that there were 39,686,953,656–58,411,217,258 and 53,824,168,657–58,311,810,737 clean data for WP and LP, respectively. Sequencing yielded 15,587–25,479 and 71,123–79,605 eccDNAs from the ear samples of WP and LP, respectively. There were 15,111 and 22,594 eccDNA-derived genes in the WP and LP, respectively, and 13,807 eccDNA-derived genes were common in the ear samples of both pigs. Sequencing yielded 13–19 and 27–43 ecDNAs in the ears of WP and LP, respectively. There were 1,005 and 1,777 ecDNA-derived genes in WP and LP, respectively, and 351 ecDNA-derived genes were common in the ear samples of both pigs. The most significant KEGG pathways of eccDNA-derived genes were axon guidance, focal adhesion, metabolic pathways, MAPK signaling pathway, Hedgehog signaling pathway, microRNAs in cancer, tight junction, phospholipase D signaling pathway, endocytosis, and sphingolipid signaling pathway. Furthermore, the most significant KEGG pathways of ecDNA-derived genes were olfactory transduction, B cell receptor signaling pathway, and chemical carcinogenesis. The eccDNA00044301 was lower abundance, while the ecDNA00000060 was higher abundance in WP compared with that in LP. Summary, we found that eccDNAs and ecDNAs are common in WP and LP and occur in sizes large enough to carry one or several partial or complete genes. These findings have expanded the knowledge repertoire of circular DNA in pig and will provide a reference for the use of pigs as a medical model and help discovery of new genetic markers to select high-quality breeds.
Extrachromosomal circular DNA (eccDNA), an unconventional presence of extrachromosomal DNA, is a circular DNA molecule derived from genomic DNA (1, 2). It can be categorized as microDNA, spcDNA, and ecDNA. The ecDNA which found in cancerous cell are double-stranded circular DNA molecule ranging from a few 100 kb to several Mbs in size (3, 4). It has recently been shown that eccDNA plays an important role in regulating biological processes including signal pathway, telomere trimming, and stress response (5, 6). The special distribution of eccDNA enhances the ability to use it as a biomarker for some diseases, especially cancer (7). With the development of high-throughput sequencing, eccDNA sequencing may be helpful for researchers to further explore the potential biological functions of eccDNA.
The pig is an ancient omnivorous mammal. As an economically important animal in agriculture, pigs are a main source of meat products and are in high demand worldwide (8, 9). The similarities between pig and human, in terms of genome, immunology, and, anatomical structure, enhance its usefulness as a biological model (10–12). Pigs have become an important biomedical research model for studying obesity, diabetes, hypertension, and other human diseases (13).
The Wei pig (WP) is a famous fatty breed in Anhui Province of China; it is resistant to stress and has a high reproductive rate and fat content (14). The characteristics of Large White pig (LP, an excellent lean breed) include a fast growth speed, high lean rate, and low feed gain ratio (14, 15). There are some differences between the two varieties owing to differences in their genetic backgrounds. However, few reports are available regarding the differences in eccDNA and ecDNA between WP and LP. The present work used high-throughput sequencing to determine eccDNA and ecDNA profiles from the ear samples of WP and LP. Bioinformatics was used to assess the similarities and differences of eccDNA and ecDNA between WP and LP. This information could provide a reference for the potential use of pigs as a medical model and help discover new genetic markers to select high-quality breeds.
This experiment was approved by the Animal Care and Use Committee of Anhui Science and Technology University. Three male WPs and three male LPs (weight 100 ± 1.3 kg) were selected from cooperative farm of Anhui Science and Technology University were dived into CW and CY groups. The ear samples were collected from these pigs and stored at −70°C until the DNA extraction procedure.
The DNA of ear sample was extracted use a commercial kit (Tiangen, China) and the quality was detected using Qubit (ThermoFisher, USA) and NanoDrop (ThermoFisher, USA). The library construction and sequencing were carried out according to the methods described by Møller et al. (16). The samples were processed by exonuclease V (NEB, USA) to degrade the linear genomic DNA For the restriction enzyme-based approach, circular DNA were digested using MspI (NEB, USA). Then, the library was sequenced on Novaseq 6000 (Illumina, USA) and 150 paired-end reads were generated (GeneDenovo, China). The high-quality clean reads were obtained from raw reads by Ffastp software. Q20 and Q30 were used to determine the quality of circular DNA. Large circular DNA in tumors is generally > 100 kb, while the other types of circular DNA are usually below 100 kb. Therefore, we used 100 kb as a threshold to distinguish eccDNA from ecDNA. The corresponding correlation analysis was performed independently.
EccDNA and ecDNA were annotated according to its source region. We divided the genome into exons, introns, gene_up2k, gene_down2k, and intergenic regions and annotated each eccDNA and ecDNA based on the region to which it belonged. If the eccDNA and ecDNA spanned multiple regions, it was classified according to the following priority: exon > intron > gene_ up2k > gene_ down2k > intergenic. The eccDNA and ecDNA related genes were derived from coding gene regions. The common and unique eccDNA and ecDNA related gene between CW and CY groups were analyzed by Venn analysis (https://www.omicshare.com).
The differential abundance of eccDNA and ecDNA were analyzed using EdgeR software. The eccDNA and ecDNA with a p-value < 0.05 and a |log2FC| > 1 was regard as the significant difference between CW and CY groups.
The enriched pathway of eccDNA and ecDNA related genes were performed using GO and KEGG database in www.omicshare.com.
Sequencing yielded 39,686,953,656–58,411,217,258 and 53,824,168,657–58,311,810,737 clean data for WP and LP, respectively (Table 1). The average Q30 values for WP and LP were 93.69 and 92.4%, respectively. An average of 80.46% (WP) and 81.44% (LP) of reads were mapped along the Sus scrofa reference genome (Table 1). The average GC content of WP and LP was 49.78 and 54%, respectively.
As shown in Figure 1A, sequencing yielded 15,587–25,479 and 71,123–79,605 eccDNAs in the ears of WP and LP, respectively. Most eccDNAs belonged to introns and intergenic regions (Figure 1B). There were 15,111 and 22,594 eccDNA-derived genes in the WP and LP, respectively, and 13,807 eccDNA-derived genes were common in the ears of both pig types (Figure 1C).
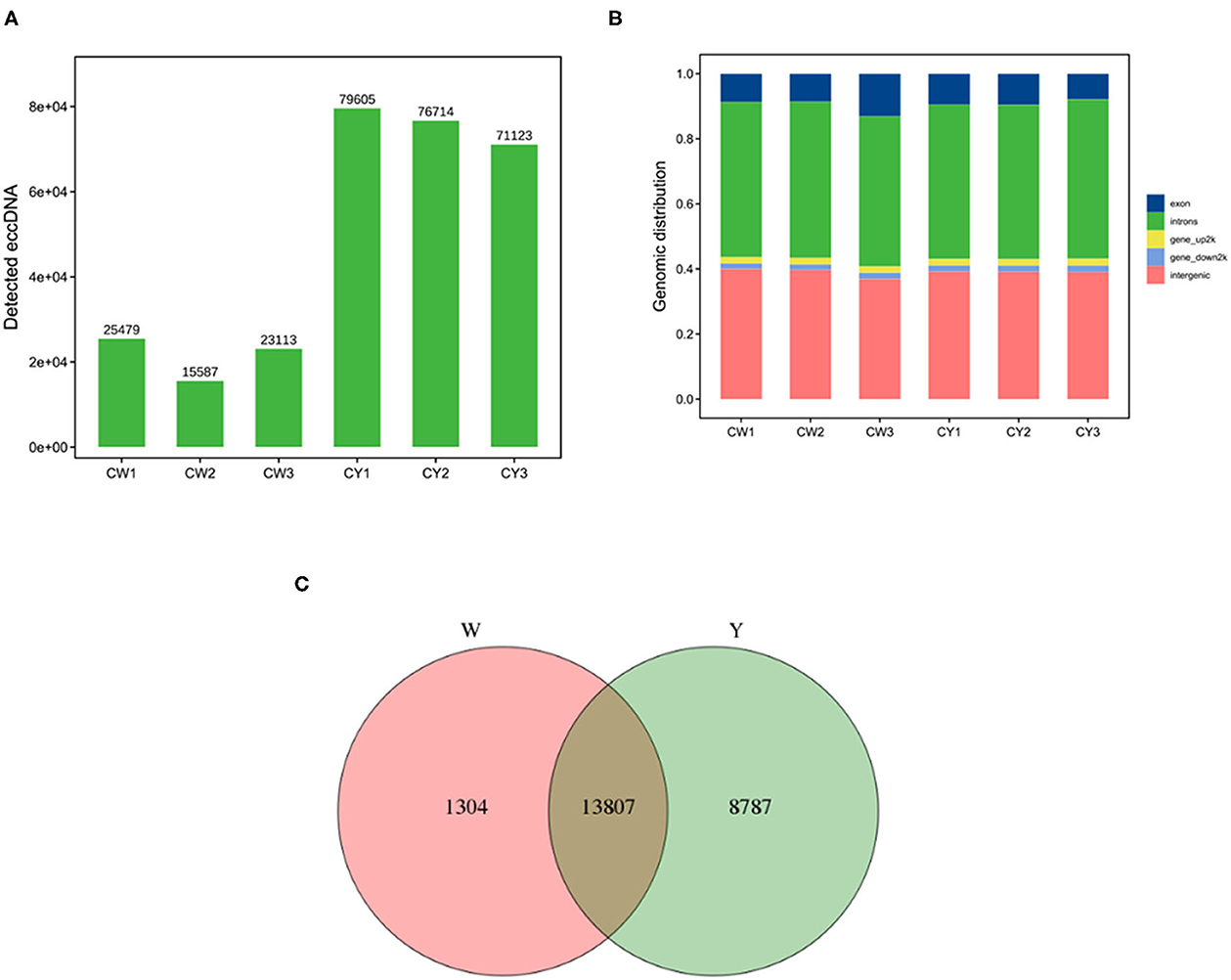
Figure 1. Identification of eccDNA and its derived genes. (A) Identification of eccDNA in Wei (CW) and Large White (CY) pigs. (B) Distribution of eccDNA. (C) Venn analysis of eccDNA derived genes in Wei and Large White pigs.
Figure 2A shows that sequencing yielded 13–19 and 27–43 ecDNAs in the ears of WP and LP, respectively. There were 1,005 and 1,777 ecDNA-derived genes in WP and LP, respectively, and 351 ecDNA-derived genes were common in the ears of both pig types (Figure 2B).
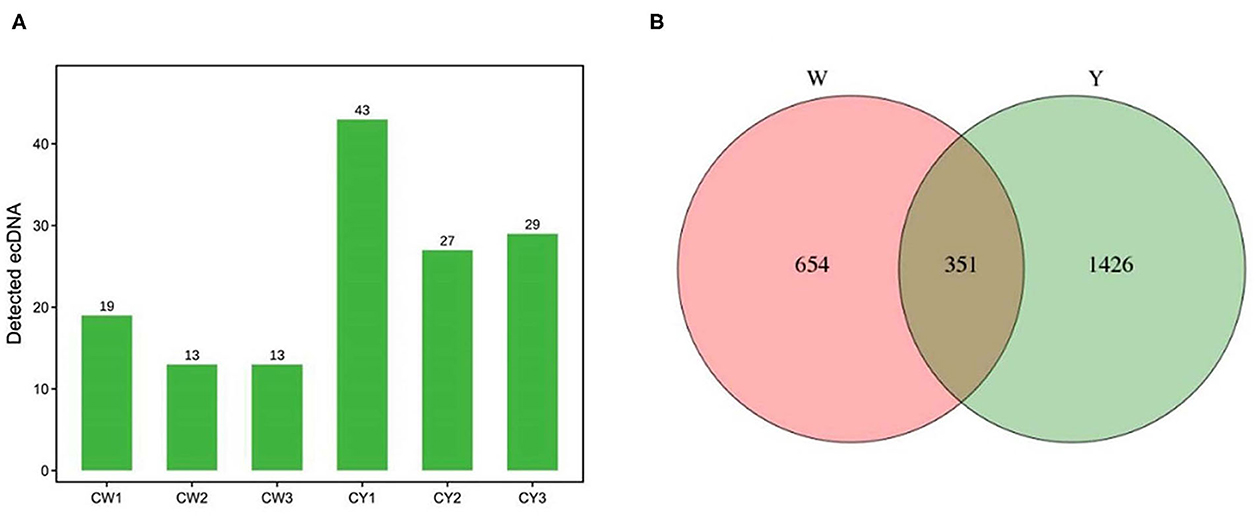
Figure 2. Identification of ecDNA and its derived genes. (A) Identification of ecDNA in Wei (CW) and Large White (CY) pigs. (B) Venn analysis of ecDNA derived genes in Wei and Large White pigs.
As shown in Table 2, the GO enriched terms of eccDNA-derived genes including biological processes (cellular component organization, cellular component organization or biogenesis, metabolic process, etc.), cellular components (cell, cell part, intracellular etc.), and molecular functions (protein binding, catalytic activity, ion binding etc.) in the WP and LP.
Figure 3 shows the top 20 most significant KEGG pathways, including axon guidance, focal adhesion, metabolic pathways, MAPK signaling pathway, Hedgehog signaling pathway, microRNAs in cancer, tight junction, phospholipase D signaling pathway, endocytosis, and sphingolipid signaling pathway in the WP and LP.
Table 3 shows the GO enriched terms of ecDNA-derived genes, including biological processes (G protein-coupled receptor signaling pathway), cellular components (membrane, integral component of membrane, intrinsic component of membrane, etc.), and molecular functions (olfactory receptor activity, N-acyltransferase activity, G protein-coupled receptor activity, etc.) in the WP and LP.
As shown in Table 4, the top 3 most significant KEGG pathways including olfactory transduction, B cell receptor signaling pathway, and chemical carcinogenesis in the WP and LP.
As shown in Table 5, eccDNA00044301 was lower abundance in WP compared with that in LP. Furthermore, the gene derived from eccDNA00044301 is a rhomboid 5 homolog 2 (RHBDF2). The ecDNA00000060 was higher abundance in WP compared with that in LP.
Generally, most DNA is linear in eukaryote. Nevertheless, some unconventional DNAs, existed in extrachromosomal region, are found to be circular (2, 17). The extrachromosomal cDNA is highly correlated with disease occurrence (7, 18). Pigs and humans are not only highly similar in disease occurrence and physiological characteristics, but also have a high homology in genome and chromosome structure; therefore, pigs have been used as an important mammalian model in human research (19). Many studies have used high-throughput sequencing to study the differences in mRNA, microRNA, and lncRNA in different breeds of pigs, but to the best of our knowledge, there are no previous reports on the high-throughput sequencing of circular DNA in pigs (20–22). In this study, high-throughput sequencing was used to investigate their differences in circular DNA between fatty and lean pig breeds to lay a foundation for their use as human research models and in livestock breeding.
DNA is mainly located in the nucleus of the cells of humans, animals, and plants. However, in special cases, excluding exogenous nucleic acid invasions such as exogenous virus infection, some DNA particles are present outside the chromosome (23, 24). These extrachromosomal DNA particles can be linear or circular. Initially, cDNA was identified in human tumor cells and was thought to be associated with tumor heterogeneity and drug resistance (25, 26). Further studies showed that eccDNA was present in yeast, tumor samples, and cancer cell lines (27, 28). Møller et al. (16) suggested that cDNAs are found in the somatic tissue of healthy humans (16). Similar results were obtained in this study. About > 80% clean reads were mapped to S. scrofa genome, and many eccDNAs and ecDNAs were isolated from the ear samples of WP and LP. Most eccDNAs belonged to the intron and intergenic regions. These results demonstrated that the eccDNA and ecDNA were not only existed in the tumor cells, but also existed in the somatic tissue.
EccDNA and ecDNA usually carry partial or complete genes and functional elements, and participate in aging, drug resistance, and tumors (27, 29, 30). In the GO enrichment analysis, the eccDNA- and ecDNA-derived genes were involved in some pathways, including metabolic process, cell, cell part, olfactory receptor activity, and catalytic activity. The KEGG pathway, detected in terms of the number of genes and q value, was analyzed to further discover the potential biological functions of eccDNA and ecDNA-derived genes in the present study. The most significant KEGG pathways of eccDNA-derived genes were annotated as lipid metabolism, folding, sorting and degradation, signal transduction, transport and catabolism, endocrine system, and infectious diseases. Furthermore, the most significant KEGG pathways of ecDNA-derived genes were annotated as organismal systems and human diseases. These results suggest that eccDNA and ecDNA may be important in regulating metabolism and disease occurrence (18, 31). In particular, KEGG pathway annotation of human diseases (e.g., cancers, infectious diseases, endocrine and metabolic diseases, and immune diseases) suggested that WP and LP have significant potential to construct biomedical disease models.
To further investigate the differences in cDNA, the differential abundance of eccDNA and ecDNA was analyzed in WP and LP. EccDNA00044301 was higher abundance in LP than that in WP. The gene derived from EccDNA00044301 is RHBDF2. This gene is important for iRhoms related to physiological targets, development, disease, and targeting therapeutic opportunities (32). The iRhoms have crucial functions in neurological disorders (Alzheimer's and Parkinson's), inflammation, wound healing, skin diseases, and cancer (33). In addition, RHBDF2 participates in cellular oxidative stress and inflammatory reactions (34).
EccDNAs and ecDNAs are common in WP and LP. These cDNAs are large enough to carry one or several partial or complete genes. Both WP and LP can potentially be used to construct human biomedical disease models. Besides, there were existed differential abundance of eccDNA (EccDNA00044301) and ecDNA (EcDNA00000060) between both breeds. Future studies should focus on the functional verification of EccDNA00044301 and EcDNA00000060.
The data presented in the study are deposited in the China National Center for Bioinformation (CNCB), Genome Sequence Archive (GAS) repository, accession number CRA009400.
The animal study was reviewed and approved by Animal Care and Use Committee of Anhui Science and Technology University.
HH and AW design the study and directed study implementation. WZ managed the fieldwork. HH and WZ performed laboratory work. HH managed data. AW and KW analyzed data. HH, AW, and WZ developed the first draft of the paper. All authors reviewed and edited drafts of the manuscript and approved the final version.
This research was supported by the Special Fund for Anhui Agriculture Research System (AGCYJSTX-05-14/15), Key Research and Development Projects of Anhui Province (202004f06020048), the University Synergy Innovation Program of Anhui Province (GXXT-2021-055), and Scientific Research Project of Anhui Science and Technology University (2021zrzd04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liao Z, Jiang W, Ye L, Li T, Yu X, Liu L. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA (ecDNA) in tumor heterogeneity and progression. BBA Rev Cancer. (2020) 1874:188392. doi: 10.1016/j.bbcan.2020.188392
2. Sin STK, Jiang P, Deng J, Lu J, Cheng SH, Dutta A, et al. Identification and characterization of extrachromosomal circular DNA in maternal plasma. Proc Natl Acad Sci USA. (2020) 117:1658–65. doi: 10.1073/pnas.1914949117
3. Hung KL, Mischel PS, Chang HY. Gene regulation on extrachromosomal DNA. Nat Struct Mol Biol. (2022) 29:736–44. doi: 10.1038/s41594-022-00806-7
4. Hung KL, Yost KE, Xie L, Shi Q, Helmsauer K, Luebeck J, et al. ecDNA hubs drive cooperative intermolecular oncogene expression. Nature. (2021) 600:731–6. doi: 10.1038/s41586-021-04116-8
5. Ling X, Han Y, Meng J, Zhong B, Chen J, Zhang H, et al. Small extrachromosomal circular DNA (eccDNA): Major functions in evolution and cancer. Mol Cancer. (2021) 20:1–15. doi: 10.1186/s12943-021-01413-8
6. Li R, Wang Y, Li J, Zhou X. Extrachromosomal circular DNA (eccDNA): An emerging star in cancer. Biomark Res. (2022) 10:1–13. doi: 10.1186/s40364-022-00399-9
7. Yang L, Jia R, Ge T, Ge S, Zhuang A, Chai P, et al. Extrachromosomal circular DNA: Biogenesis, structure, functions and diseases. Sig Transduct Target Ther. (2022) 7:1–21. doi: 10.1038/s41392-022-01176-8
8. Monger XC, Gilbert AA, Saucier L, Vincent AT. Antibiotic resistance: From pig to meat. Antibiotics. (2021) 10:1209. doi: 10.3390/antibiotics10101209
9. Natonek-Wiśniewska M, Piestrzynska-Kajtoch A, Koseniuk A, Koseniuk A, Krzyścin P. Current analytical methods and research trends are used to identify domestic pig and wild boar DNA in Meat and Meat Products. Genes. (2022) 13:1825. doi: 10.3390/genes13101825
10. Bassols A, Costa, C, Eckersall PD, Osada J, Sabrià J, Tibau J. The pig as an animal model for human pathologies: A proteomics perspective. Prot Clin Appl. (2014) 8:715–31. doi: 10.1002/prca.201300099
11. Heinritz S, Mosenthin R, Weiss E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr Res Rev. (2013) 26:191–209. doi: 10.1017/S0954422413000152
12. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol. (2012) 20:50–7. doi: 10.1016/j.tim.2011.11.002
13. Lunney JK, Van Goor A, Walker KE, Hailstock T, Franklin J, Dai C. Importance of the pig as a human biomedical model. Sci Transl Med. (2021) 13:eabd5758. doi: 10.1126/scitranslmed.abd5758
14. Xu J, Wang C, Jin E, Gu Y, Li S, Li Q. Identification of differentially expressed genes in longissimus dorsi muscle between Wei and Yorkshire pigs using RNA sequencing. Genes Genom. (2018) 40:413–21. doi: 10.1007/s13258-017-0643-3
15. Zhang D, Wu W, Huang X, Xu K, Zheng C, Zhang J. Comparative analysis of gene expression profiles in differentiated subcutaneous adipocytes between Jiaxing Black and Large White pigs. BMC Genomics. (2021) 22:61. doi: 10.1186/s12864-020-07361-9
16. Møller HD, Mohiyuddin M, Prada-Luengo I, Sailani MR, Halling JF, Plomgaard P. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat Commun. (2018) 9:1–12. doi: 10.1038/s41467-018-03369-8
17. Wu S, Turner KM, Nguyen N, Raviram R, Erb M, Santini J, et al. Circular ecDNA promotes accessible chromatin and high oncogene expression. Nature. (2019) 575:699–703. doi: 10.1038/s41586-019-1763-5
18. Qiu GH, Zheng X, Fu M, Huang C, Yang X. The decreased exclusion of nuclear eccDNA: From molecular and subcellular levels to human aging and age-related diseases. Ageing Res Rev. (2021) 67:101306. doi: 10.1016/j.arr.2021.101306
19. Schoenly KG, Tarone AM, Villet MH. Pigs vs. people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int J Legal Med. (2020) 134:793–810. doi: 10.1007/s00414-019-02074-5
20. Liu X, Liu K, Shan B, Wei S, Li D, Han H, et al. A genome-wide landscape of mRNAs, lncRNAs, and circRNAs during subcutaneous adipogenesis in pigs. J Animal Sci Biotechnol. (2018) 9:1–13. doi: 10.1186/s40104-018-0292-7
21. Zhang X, Cai S, Chen L, Yuan R, Nie Y, Ding S, et al. Integrated miRNA–mRNA transcriptomic analysis reveals epigenetic-mediated embryonic muscle growth differences between Wuzhishan and Landrace pigs. J Anim Sci. (2019) 97:1967–78. doi: 10.1093/jas/skz091
22. Miao Z, Wang S, Wang Y, Wei P, Khan MA, Zhang J, et al. Comparison of microRNAs in the intramuscular adipose tissue from Jinhua and Landrace pigs. J Cell Biochem. (2019) 120:192–200. doi: 10.1002/jcb.27298
23. Gu X, Yu A, Chai P, Ge S, Fan X. Novel insights into extrachromosomal DNA: Redefining the onco-drivers of tumor progression. J Exp Clin Cancer Res. (2020) 39:1–10. doi: 10.1186/s13046-020-01726-4
24. Wu P, Liu Y, Zhou R, Liu L, Zeng H, Xiong F, et al. Extrachromosomal circular DNA: A new target in cancer. Front Oncol. (2022) 12:814504. doi: 10.3389/fonc.2022.814504
25. Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. (2017) 543:122–5. doi: 10.1038/nature21356
26. Verhaak RGW, Bafna V, Mischel PS. Extrachromosomal oncogene amplification in tumour pathogenesis and evolution. Nat Rev Cancer. (2019) 19:283–8. doi: 10.1038/s41568-019-0128-6
27. Zhou T, Ma S, Zhao Y, Guo D, Wang H, Kuang M, et al. Identification and characterization of extrachromosomal circular DNA in alcohol induced osteonecrosis of femoral head. Front Genet. (2022) 13:918379. doi: 10.3389/fgene.2022.918379
28. Arrey G, Keating ST, Regenberg B. A unifying model for extrachromosomal circular DNA load in eukaryotic cells. Semin Cell Dev Biol. (2022) 128:40–50. doi: 10.1016/j.semcdb.2022.03.002
29. Møller H, Parsons L, Jørgensen TS, Botstein D, Regenberg B. Extrachromosomal circular DNA is common in yeast. Proc Natl Acad Sci USA. (2015) 112:3114–22. doi: 10.1073/pnas.1508825112
30. Lin C, Chen Y, Zhang F, Liu B, Xie C, Song Y. Encoding gene RAB3B exists in linear chromosomal and circular extrachromosomal DNA and contributes to cisplatin resistance of hypopharyngeal squamous cell carcinoma via inducing autophagy. Cell Death Dis. (2022) 13:171. doi: 10.1038/s41419-022-04627-w
31. Zhao Y, Yu L, Zhang S, Su X, Zhou X. Extrachromosomal circular DNA: Current status and future prospects. Elife. (2022) 11:e81412. doi: 10.7554/eLife.81412
32. Burzenski LM, Low BE, Kohar V, Shultz LD, Wiles MV, Hosur V. Inactive Rhomboid proteins RHBDF1 and RHBDF2 (iRhoms): A decade of research in murine models. Mamm Genome. (2021) 32:415–26. doi: 10.1007/s00335-021-09910-3
33. Lichtenthaler SF, O'Hara BF, Blobel CP. iRhoms in the brain–a new frontier? Cell Cycle. (2015) 14:3003–4. doi: 10.1080/15384101.2015.1084187
34. Xu MX, Dai XL, Kuang Q, Zhu L, Hu LF, Lou DS. Dysfunctional Rhbdf2 of proopiomelanocortin mitigates ambient particulate matter exposure-induced neurological injury and neuron loss by antagonizing oxidative stress and inflammatory reaction. J Hazard Mater. (2020) 400:123158. doi: 10.1016/j.jhazmat.2020.123158
Keywords: eccDNA, ecDNA, Wei pig, Large White pig, high-throughput sequencing
Citation: Wen A, Zhang W, Xu J, Wang K and Hu H (2023) Identification and characterization of extrachromosomal circular DNA in Wei and Large White pigs by high-throughput sequencing. Front. Vet. Sci. 10:1085474. doi: 10.3389/fvets.2023.1085474
Received: 31 October 2022; Accepted: 03 January 2023;
Published: 03 February 2023.
Edited by:
Sihua Jin, Anhui Agricultural University, ChinaReviewed by:
Hui Diao, Sichuan Animal Science Academy, ChinaCopyright © 2023 Wen, Zhang, Xu, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Hu,  aGFpeWFuZ2hoQDE2My5jb20=
aGFpeWFuZ2hoQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.