
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 26 January 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1078259
Background: Autophagy was reported to play a crucial role in maintaining general and skin health.
Methods: The study used a synthesized autophagy inducer (AI) (Aquatide™ cospharm Inc.; Daejeon, Korea), for evaluating the effects of autophagy on skin and hair in dogs. Twenty-two dogs with poor skin and hair which were diagnosed with canine atopic dermatitis (CAD) or pituitary-dependent hyperadrenocorticism (PDH) were included. Clinical scores using Canine Atopic Dermatitis Extent and Severity Index-04 (CADESI-04), Pruritus Visual Analog Scale (PVAS) and skin barrier function using measurement of transepidermal water loss (TEWL) were evaluated and canine keratinocytes were also used in vitro investigation of pro-inflammatory cytokines after AI treatment.
Results: In the AI group, clinical scores and skin barrier function were improved at week 8 significantly compared to in the other groups. In particular, the AI significantly improved the hair surface damage at 8 weeks compared to the baseline. In vitro, the AI reduced pro-inflammatory cytokines by activating the 78-kDa glucose-regulated protein (GRP78).
Conclusion: AI improve skin barrier function and hair damage and reduce pro-inflammatory cytokines by inhibiting reactive oxygen species (ROS) production in dogs.
The canine skin and hair coat are important predictors of underlying systemic diseases and the general health status. Symptoms of poor skin and hair conditions in dogs are very broad, but commonly include localized or extensive itching, dryness, hair loss, and other unusual behaviors such as excessive licking and scratching (1, 2). Diseases with bad effects on the dogs' skin and hair representatively include many conditions like atopic dermatitis, hyperadrenocorticism, and malignant tumors (3–5). Therapeutic options for these diseases vary; canine atopic dermatitis (CAD) can be treated with anti-inflammatory drugs, antipruritic drugs, and allergen-specific immunotherapy (6), hyperadrenocorticism is treated with trilostane (7), and anti-cancer drugs like toceranib, lomustine, and vincristine are used in tumors (8, 9). However, the efficacy of current therapeutic options could be limited in some cases of CAD and additional treatments might be needed (10).
Autophagy is central to skin health and plays a critical role in controlling skin aging, inflammation, and immune responses (11–13). Under external (e.g., UV irradiation) and internal (e.g., stress) conditions, induced-autophagy removes aged subcellular organelles and proteins while it also regulates skin homeostasis, functions of keratinocytes, proliferation, and differentiation of epidermal stem cells to protect skin health (13–15). In addition, autophagy can influence hair development by improving the period of the hair cycle, shortening the period between the telogen phase to the anagen phase, and inducing hair regeneration (13, 16). Therefore, maintenance of appropriate autophagy is critical for host skin homeostasis, and modulation of the autophagy and function is a promising therapeutic strategy. However, the studies of mechanism and clinical effects of autophagy on the skin health are not enough.
Heptasodium hexacarboxymethyl dipeptide-12 is a recently synthesized autophagy inducer branded as Aquatide™ (Incospharm Incorporation, Dajeon, Korea) (17). Aquatide™ also contains Resveratrol, which activates sirtuin-1, and pyrrolidone carboxylic acid, which is a skin moisturizing ingredient (17–19). Recent evidence indicates that Aquatide™ can exert the anti-oxidant and anti-aging effects in humans by activating autophagy through direct binding onto sirtuin-1 (20).
In the present study, we firstly investigated the effects of autophagy induction on the skin and hair conditions topically in dogs and it may be a useful model for investigations of autophagy in human.
Synthesized Aquatide™ (Incospharm Inc., Daejeon, Korea) was used for evaluating the effects of autophagy inducer on dogs' skin and hair. In a prior study, safety assessments were completed in Beagles and the research also complied with the Institutional Animal Care and Use Committee guidelines at Chonnam National University (identification code No. CNU IACUC-YB-2019-26).
Two types of topical formulation were prepared for the study; the test products contained 2% Aquatide™, glycerin, propylene glycol, and 1,3-butylene glycol and the vehicle products contained the same components without Aquatide™. The topical formulation was applied twice a day to the whole body for 8 weeks.
A total of 31 client-owned dogs who visited the veterinary teaching hospital were included. All the owners approved the study and signed an informed consent. Twenty-two patient dogs with poor skin and hair conditions based on physical examination were included regardless of the underlying diseases. The average range of their age was 7.79 ± 3.62 years. They consisted of three females, 16 spayed females, one male, and 13 neutered males. The patients included six Poodles, six Pomeranian, six Malteses, four Mixed, two Spitzs, two Cocker Spaniels, one Chihuahua, one French Bulldog, one Bichon Frise, one Shih tzu, one Jindo, one Miniature Pinscher and one Siberian Huskey. Healthy control group consisted of nine dogs with the average range of age of 6.00 ± 3.40 years. They consisted of two females, three spayed females, and four neutered males. The healthy conrols included three Poodles, one Siberian Huskey, one Cocker Spaniel, one Spitz, one miniature Pinscher, and two mixed breeds.
The enrolled dogs were maintained for the treatment for their underlying diseases during the study 90 (for 8 weeks). For example, CAD patients were treated with anti-inflammatory drugs (e.g., cyclosporine (Cipol-N® oral solution, CKD Pharm, Seoul, Korea) and hypoallergenic diets (e.g., hypoallergenic, Royal Canine, Dissay, France) and PDH patients were prescribed trilostane (Vetoryl®, Dechra, Northwich, United Kingdom).
The dogs were divided into 3 groups in a randomized and double-blinded manner. Staff not involved in the treatment randomly allocated the dogs into three groups using a random number table procedure. Five dogs with CAD (age 6.60 ± 3.07 years) and two dogs with PDH (age 13 ± 1 years) were applied the Aquatide™ product. Three dogs with CAD (age 6.33 ± 4.11 years) and three dogs with PDH (age 10.33 ± 3.30 years) were applied the vehicle product. Other seven dogs with CAD (age 7.43 ± 2.13 years) and two dogs with PDH (age 7.50 ± 2.50 years) were not applied with any topical product. Nine client-owned dogs were recruited as the healthy control (age 6.00 ± 3.39 years). These dogs were determined to be healthy based upon medical history and physical examination. To assess the skin and hair conditions, skin examinations and hair damage in healthy controls at week 0 and in experimental groups at weeks 0, 4, and 8 were evaluated. As for methods used for diagnosis, Favrot's criteria was used in CAD. Adrenocorticotropic hormone stimulation test and high-dose dexamethasone suppression test was used in PDH.
Not only atopic dermatitis, but also all the patients were evaluated using Canine Atopic Dermatitis Extent and Severity Index-04 (CADESI-04) (3) for skin lesion scoring.
TEWL was measured through the closed-chamber method using GPSkin Barrier® (GPOWER Inc., Seoul, Korea) (21–23). The assessment regions, including the left and right sides of the concave surface of the pinna, axilla, and inguinal area were repeatedly measured. All sites were measured three times by placing the probe in the same location. All the TEWL values were measured after stabilizing the dogs for 30 min in a controlled room at room temperature, with temperature of 26 ± 1°C and relative humidity of 50 ± 5%.
For collective evaluation of skin condition, Pruritus Visual Analog Scale (PVAS) was graded for the assessment of itching (24). The scores ranged from 0 to 10.
The collected hair samples from flank including hair bulb were stored at room temperature until analysis. Grades of hair damage include hair surface damage, hair cuticle layers damage, and hair cortex damage (25). The hair surface damage was evaluated using scanning electron microscopy (EM-30, 30 kV; COXEM, Daejeon, Korea). Each hair was photographed three times and evaluated by two investigators. The score of hair surface damage is 0 to 4.
Canine epidermal keratinocytes were purchased from the CELLnTEC and were maintained at 37°C in a humidified incubator at 5% CO2 in CnT-09 culture medium. For the experiment, Canine keratinocytes (5 × 105) were seeded in a 60 mm cell culture dish, incubated for 24 h, and pre-treated with Aquatide™ (1, 10, 100 μg/mL) or rapamycin (5 μM) for 1 h. The dose of Aquatide™ was determined based on a prior study (17, 20). After removing the treatment media, cells were washed with PBS and cultured in media with lipopolysaccharide (LPS) (10 μg/mL) for 4 h. Then, the cells were washed twice with cold PBS and harvested. MTT test was performed to check cell viability.
Realtime PCR was performed with TaqMan Gene Expression Assays from Life Technologies (GRP78, HS00946086_g1; GapdH, Cf04419463_gH). The protein levels of Glucose regulated protein (GRP-78), Interleukin (IL) 4 and IL-13 in keratinocyte were measured using western blotting analysis. The relative expression of GRP-78 was measured using qPCR. Each signal was normalized against GAPDH signals in the same sample. Details are provided in the methods of the Supplementary material.
All the statistical analyses were performed using GraphPad Prism version 9 (GraphPad Software, La Jolla, CA, USA). The Shapiro-Wilk tests were performed to analyze the normality of all data. The unpaired T-test was used to compare skin condition between healthy control and patient group at the starting point of the trial. Other data was analyzed using ANOVA with subsequent Tukey's multiple comparison tests to establish the difference between groups. Mean ± standard deviation (SD) was used to describe the distribution of continuous variables. Statistical significance was set at P < 0.05.
The demographics of the total study population at the starting point of the trial are shown in Figure 1. Among these experimental groups, diseases of patients are presented in Table 1. CADESI-04, PVAS, and hair scoring was significantly higher in the treatment group (CAD + PDH) than the healthy controls (P < 0.01). TEWL was statistically higher in the treatment group than in the control group (P < 0.0001).
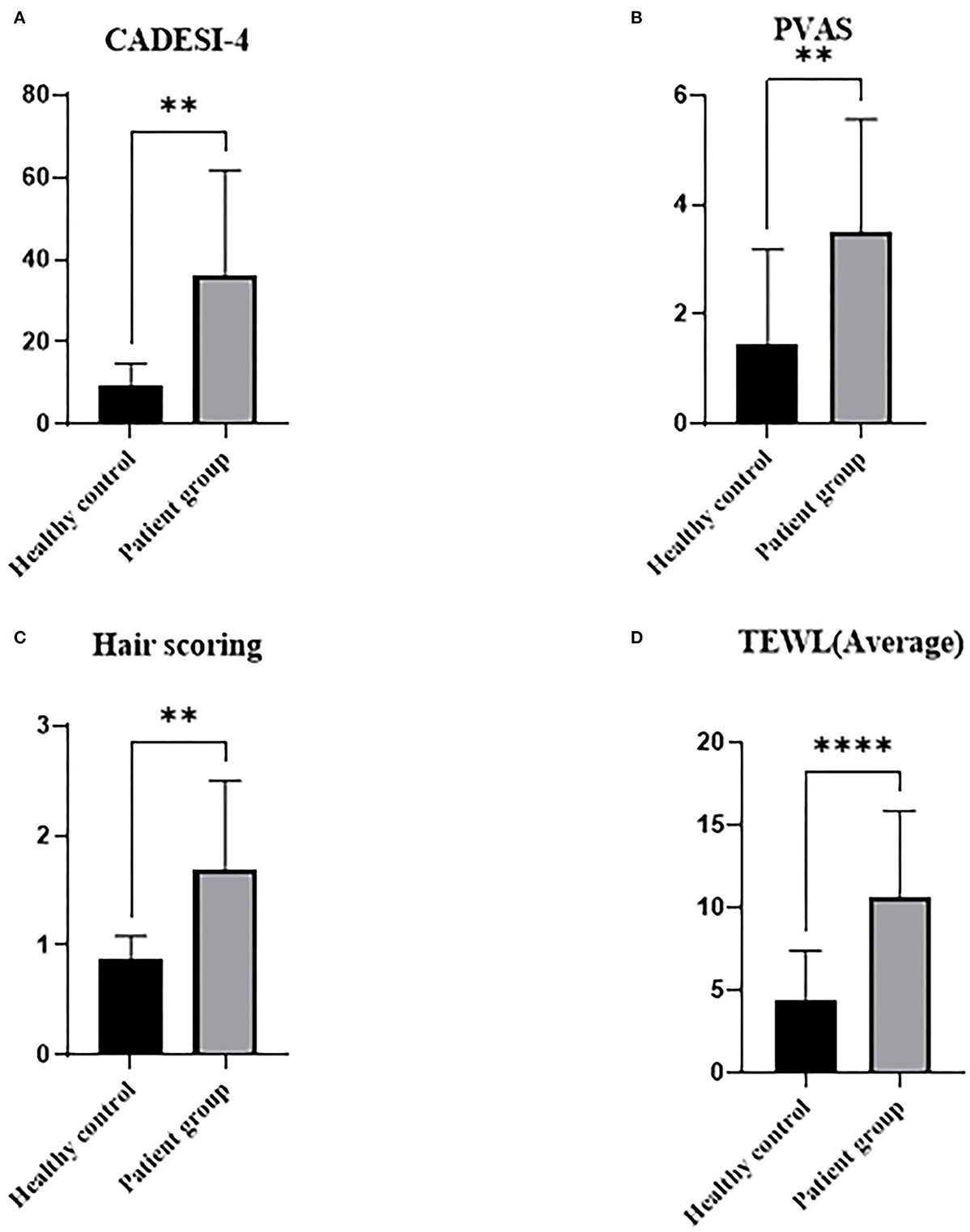
Figure 1. Graphs of skin and hair conditions by underlying diseases in dogs at starting point. Healthy controls were determined based upon medical history and physical examination. Treatment group includes CAD and PDH. CAD, canine atopic dermatitis; CADESI-04, canine atopic dermatitis extent and severity index-04; PDH, pituitary dependent hyperadrenocorticism; PVAS, pruritus visual analog scale; TEWL, transepidermal water loss. **p < 0.01, ****p < 0.0001.
To investigate whether autophagy induction has the potential to alleviate skin conditions, we applied the Aquatide™ topical formulation twice a day to the whole body for 8 weeks. In continuous visual evaluations, the dogs who were applied AquatideTM showed improved skin lesions and hair conditions after the 8 weeks compared to week 0 (Figure 2). In addition, the dogs who were applied Aquatide™ showed significantly decreased TEWL in the skin than the negative controls (axilla, P < 0.05; ear pinna, P < 0.05; inguinal regions, P < 0.0001; Figures 3C–E). The dogs in the Aquatide™ group also had a lower CADESI-4 and PVAS compared to the vehicle group, although there was no significant difference (Figures 3A, B). In assessments of the hair conditions in these dogs, Aquatide™ treatment reduced hair surface damage and hair cuticle layers compared to the negative and vehicle control groups (P < 0.05; Figure 4). These findings indicate that Aquatide™ treatment effectively alleviated skin conditions and hair quality in dogs with underlying diseases.
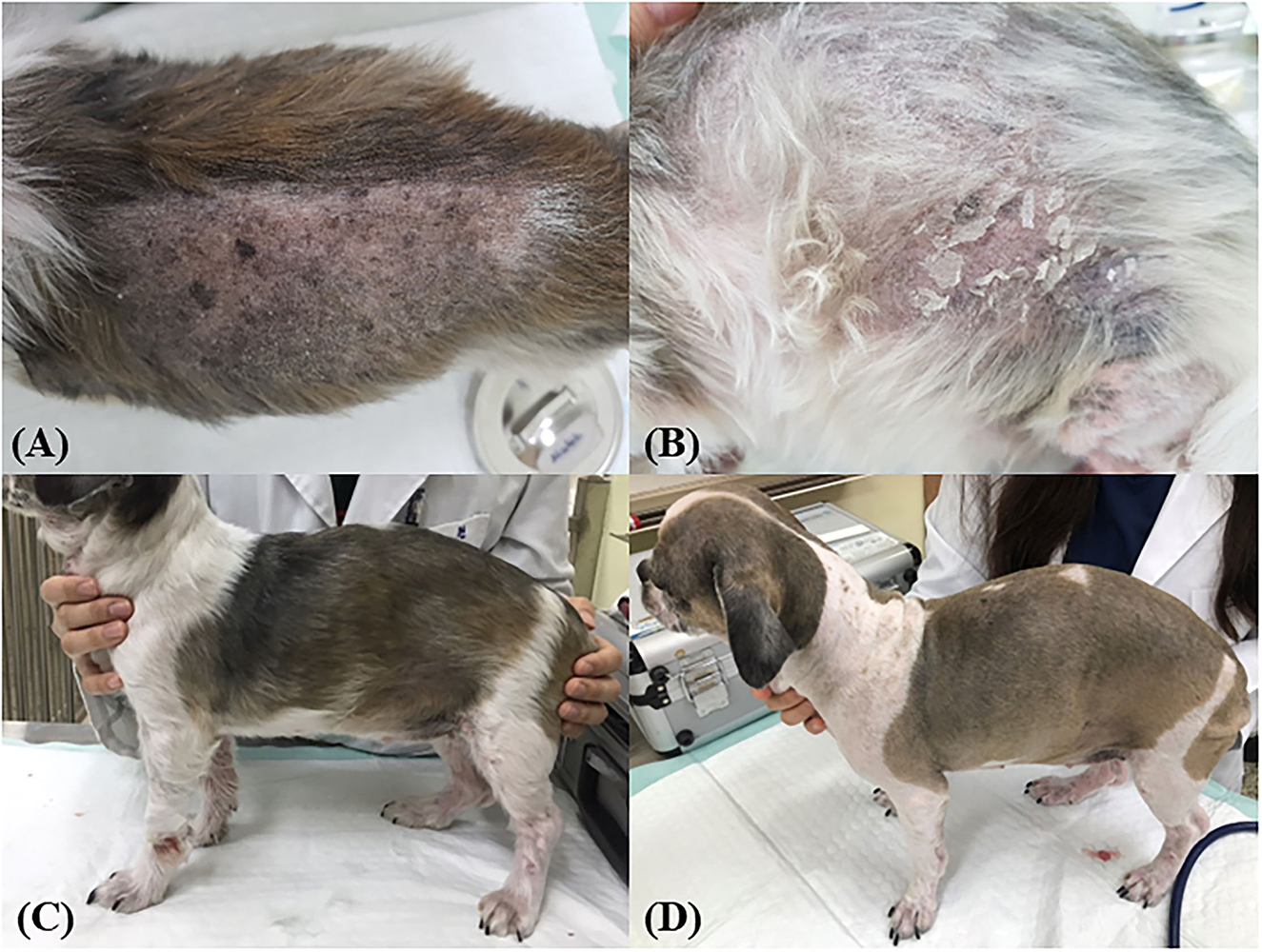
Figure 2. Induction of autophagy improves skin lesions and hair quality in dogs with CAD. The alopecia on the left flank and many keratins were identified on the back and left flank at week 0 (A, B). After 4 weeks, the lesions and the hair quality were improved (C). At week 8, improved lesions were maintained (D).
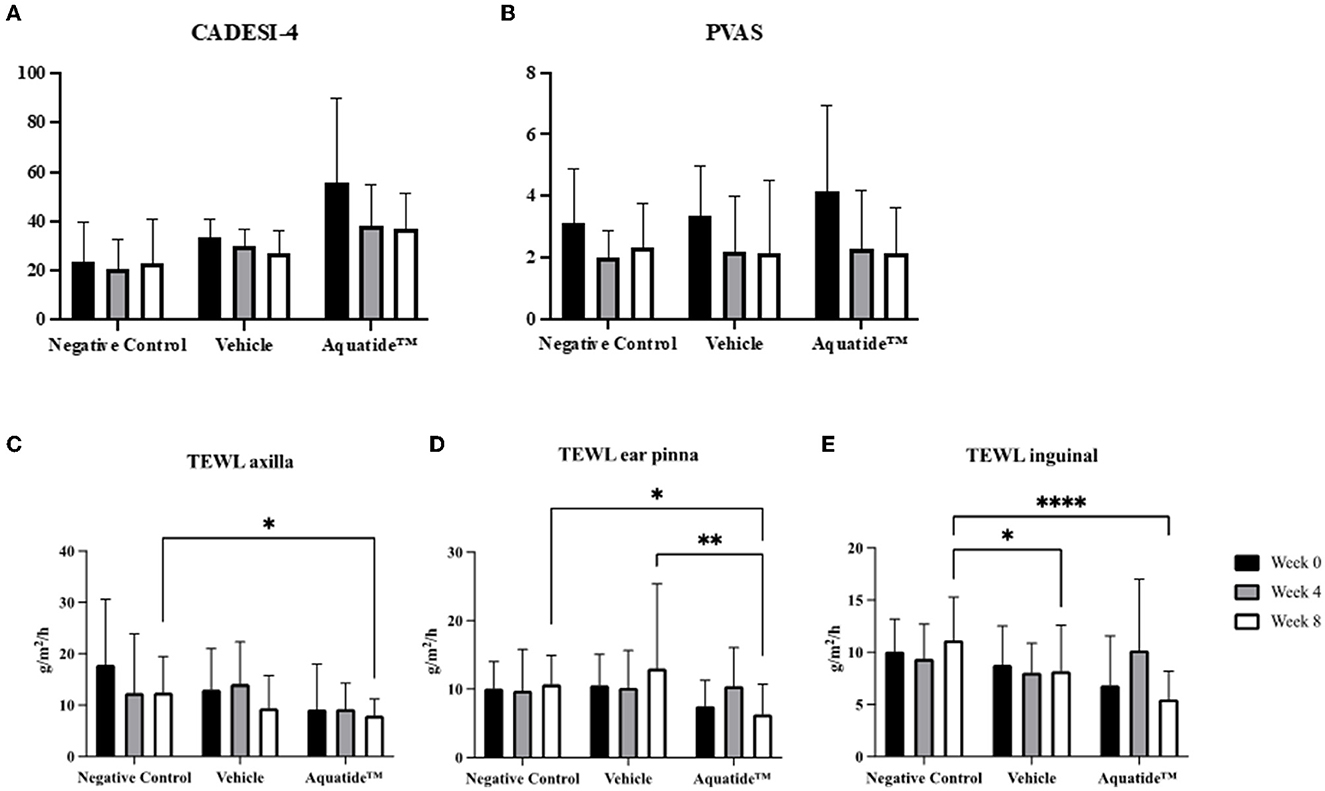
Figure 3. Graphs of CADESI-4, PVAS and TEWL at week 0, 4, and 8 in the treatment group. Induction of autophagy significantly reduces TEWL in dogs with underlying diseases. CADESI-04, canine atopic dermatitis extent and severity index-04; VAS, visual analog scale; TEWL, transepidermal water loss. *p < 0.05, **p < 0.01, and ****p < 0.0001.
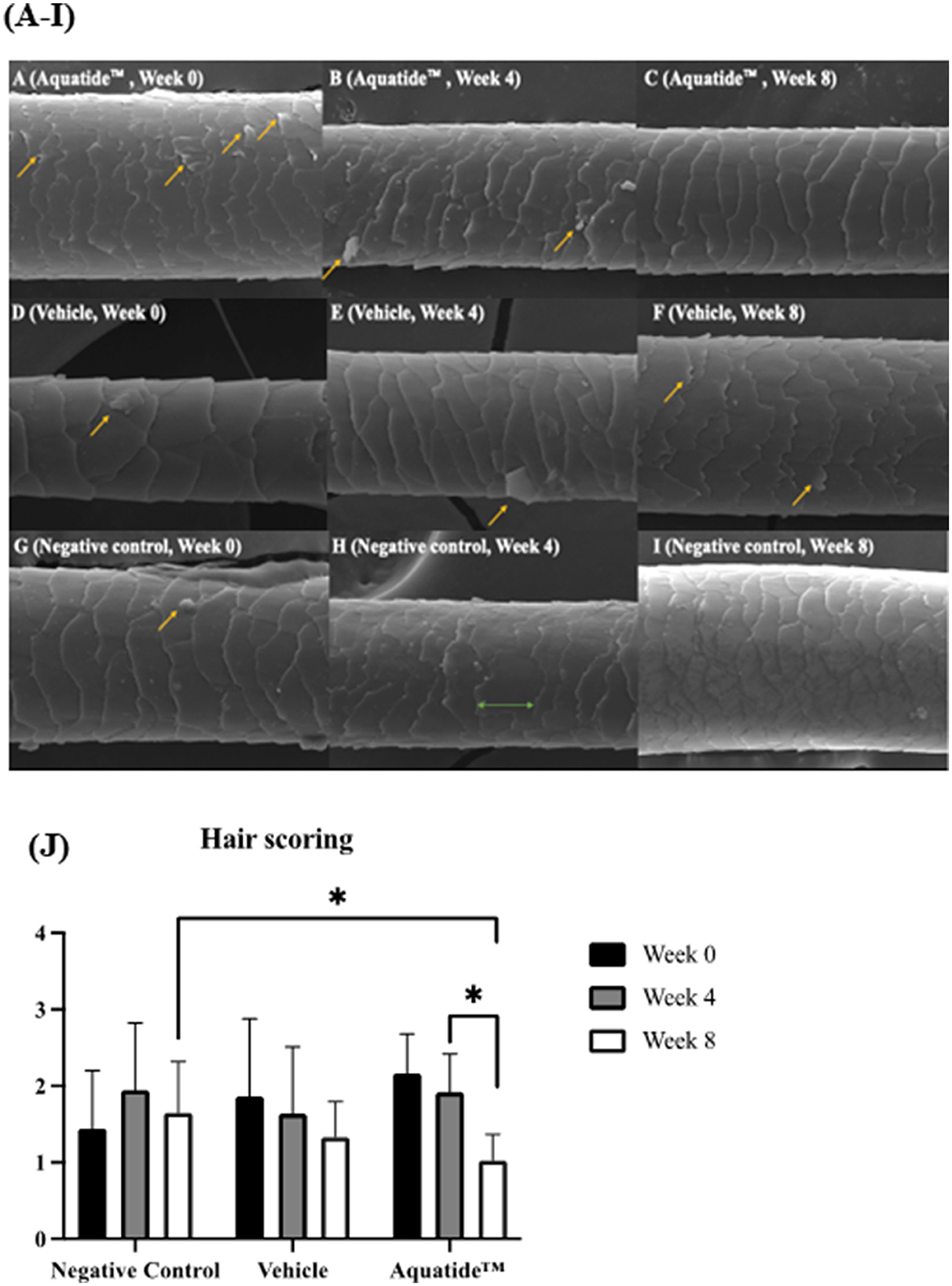
Figure 4. Induction of autophagy improves hair surface damage and hair cuticle layers in dogs with underlying diseases. (A–I) Scanning electron microscopic photographs for evaluating hair surface damage. The arrows points to the hair surface damage. It was confirmed that the hair surface was improved at week 8 after Aquatide™ was applied. (J) Hair scoring in the treatment group. When Aquatide™ was applied, hair scoring was significantly decreased at week 8 compared to week 0 and week 4, and was significantly lower than in the negative control. *p < 0.05.
To confirm if activation of autophagy regulates oxidative stress, we examined the levels of GRP-78 after Aquatide™ treatment in vitro. As shown in Figures 5A, B, the GRP-78 protein level in the Aquatide™ group gradually increased in a dose-dependent manner than those of the control and cells incubated with rapamycin (positive control group). Next, we examined whether Aquaitde-induced activation alters pro-inflammatory cytokine levels after LPS stimulation. As shown in Figure 5C, D, pretreatment of Aquatide™ significantly lowered pro-inflammatory cytokines (IL-4 and IL-13) in keratinocytes, compared with non-treated cells after LPS stimulation. In addition, pretreatment with Aquatide™ significantly increased GRP-78 gene expression after LPS stimulation. These findings indicate that pretreatment with Aquatide™ effectively decreases pro-inflammatory cytokines in the keratinocytes by activating GRP-78 in vitro.
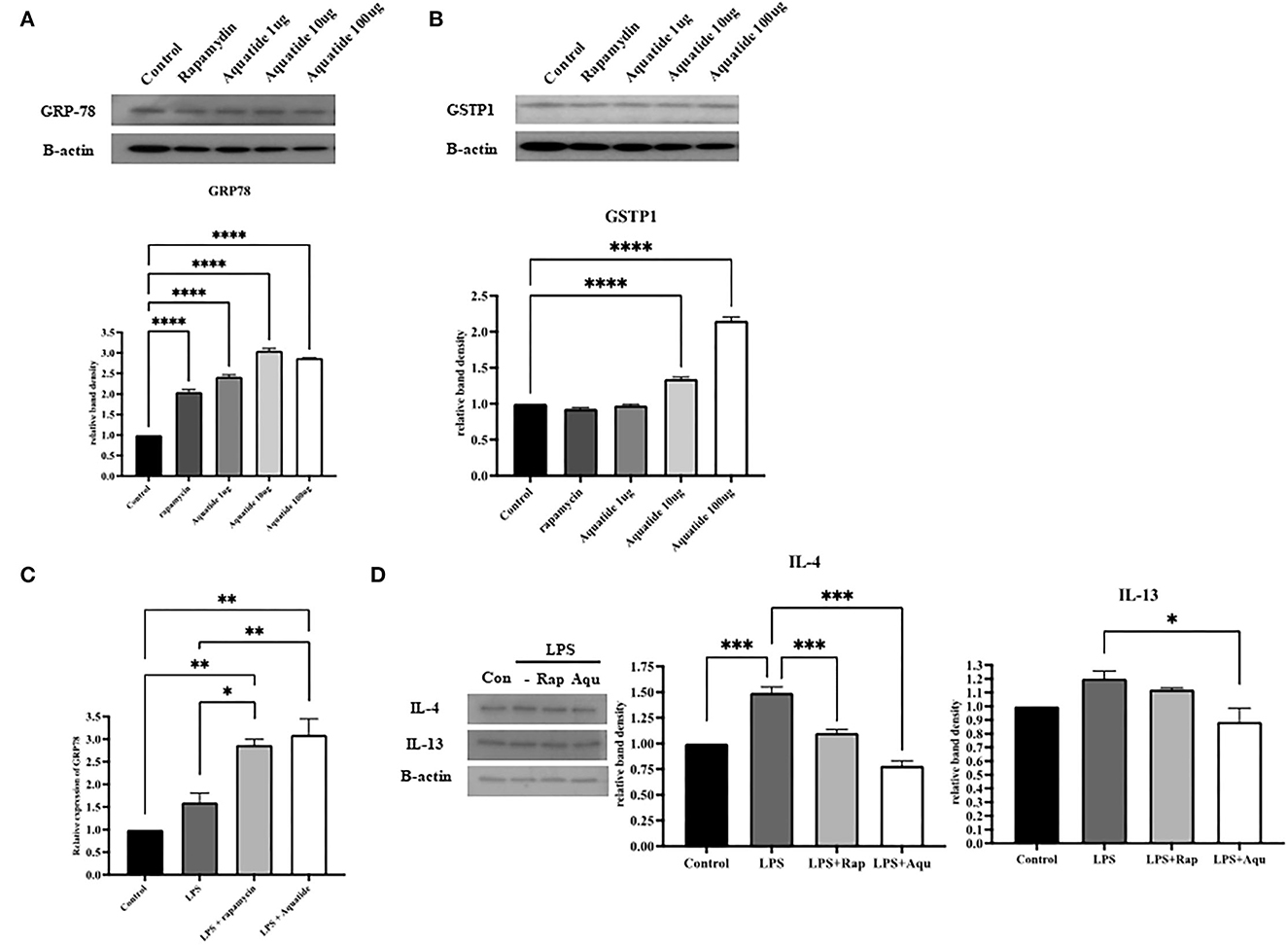
Figure 5. Preconditioning-induced autophagy reduces pro-inflammatory cytokines through GRP78 activation in canine keratinocyte cells. The antioxidant-related protein levels of (A) GRP-78 and (B) GSTP1 and mRNA levels of (C) GRP-78 were assessed by western blot and real-time PCR. (D) pro-inflammatory cytokines (IL-4 and IL-13) were assessed by western blot analysis. Statistical significance was determined using ANOVA and Tukey's multiple comparison tests. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Here, we demonstrated that topical Aquatide™ treatment in dogs with skin disease alleviated skin and hair conditions. In addition, preconditioning-induced autophagy decreased pro-inflammatory cytokines and increased GRP-78 protein levels after LPS stimulation in the keratinocyte cells. Our results indicated that increased autophagy activation ameliorates skin and hair conditions via the modulation of oxidative stress-mediated inflammation in the skin of canine skin disorder.
Previous studies have reported that an imbalance of skin homeostasis from underlying diseases contributes to the development of skin and hair disorders (26–28). In this study, the dogs enrolled had underlying diseases that affect skin health, such as CAD and PDH. Those canine primary diseases may show poor skin conditions, such as pruritic inflammation in CAD (3). Alopecia and seborrhea sicca can be seen in hyperadrenocorticism (4).
Application of Aquatide™ improved skin clinical features, which were considered typical abnormalities induced by underlying diseases. Previously, some studies reported that the induction of autophagy has important anti-oxidant effects with preventive properties in skin disorders by reducing inflammation (29, 30). In addition, autophagy activation is reported to provide an anti-inflammatory promoting activity in the mouse skin (31). Therefore, Aquatide™ application might have exerted improved effects against skin disorders by blocking inflammatory-related responses, especially with anti-oxidation through induction of autophagy.
In this study, it was found that the effect of improved hair quality was greater than that of clinical scores such as CADESI-04 and PVAS in dogs. In vitro, anti-inflammatory effects were observed, but no statistical significance was observed in CADESI-04 and PVAS, which are related to clinical symptoms. The discrepancy in the outcomes between the clinical and preclinical experiments may be due to not effective concentration of AI on the skin or some different environmental factor in skin in vivo and cells in vitro.
Chronic skin inflammation is associated with the overproduction of reactive oxygen species (ROS), defined as oxidative stress (32). Excessive ROS can directly cause damage to the cells, and eventually exceeds the defense capacity of the antioxidant system, leading to the pathogenesis of AD, as well as cutaneous diseases (33–35). Autophagy is an evolutionarily conserved homeostatic cellular process to promote cell survival and adaptive responses during oxidative and/or genotoxic stress conditions (36, 37).
Previous studies have reported that autophagy inducers such as rapamycin and Aquatide™ provide optimal protection against skin inflammation and cellular senescence via suppressing the production of ROS and the maintenance of cellular homeostasis (17, 20, 31, 38). In addition, as an autophagy inducer, rapamycin alleviated oxidative stress-related ER stress in cellular-protection via the promotion of GRP78/mTOR signaling pathway (39). ROS causes death of hair cells, but autophagy can decrease ROS levels (40, 41). Therefore, the hair quality improvement effects could be also attributed to the ROS-related mechanism.
In addition, the expression of IL-4 and IL-13, which are pro-inflammatory cytokines associated with AD, was decreased while the expression of GRP-78 was increased in the keratinocyte cells after Aquatide™ treatment. These results suggest that induction of autophagy promotes GRP78-related function directly or indirectly, and therefore may suppress inflammation in the skin.
Our present study has some limitations. Autophagy triggers hair regeneration (16), but we could not evaluate the effect of hair cycle improvement in dogs. It was not easy to evaluate the effect of hair regeneration in this study because the evaluation period was short, and many dogs had been hair clipping, instead, we evaluated the hair quality improvements effects through scanning electron microscopy. Importantly, to date, a direct relationship between Aquatide™ and anti-oxidant in canines has not been identified. Further studies are required to confirm this relationship and elucidate the underlying mechanism. Finally, in the Aquatide™ group, CADESI-04 and PVAS decreased, but there were significant differences because the number of dogs in this study was relatively small. Further studies should overcome this limitation. Nevertheless, this is the first study to evaluate the effects of a topical formulation containing an autophagy inducer on the dogs' skin and hair conditions.
We demonstrated that Aquatide™ application improves skin and hair conditions resulting from underlying diseases; the results indicated a possible antioxidant-related mechanism. The present study could be helpful to understand the effects of autophagy on the skin and hair conditions in humans.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee at Chonnam National University (CNU IACUC-YB-2019-26). Written informed consent was obtained from the owners for the participation of their animals in this study.
YK, S-HL, and YS conducted the experiments and data analysis and wrote the manuscript. SJ contributed to manuscript preparation. H-JK conceived the experiments and revised the entire manuscript. All authors reviewed the manuscript.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and the Republic of Korea (NRF-2016R1D1A3B04934798 and NRF-2020R1A2C2005364).
SJ is a current employee of Incospharm Corp.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1078259/full#supplementary-material
1. Hensel P, Santoro D, Favrot C, Hill P, Griffin C. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet Res. (2015) 11:196. doi: 10.1186/s12917-015-0515-5
2. Olivry T, DeBoer DJ, Favrot C, Jackson HA, Mueller RS, Nuttall T, et al. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the international task force on canine atopic dermatitis. Vet Dermatol. (2010) 21:233–48. doi: 10.1111/j.1365-3164.2010.00889.x
3. Olivry T, Saridomichelakis M, Nuttall T, Bensignor E, Griffin CE, Hill PB, et al. Validation of the canine atopic dermatitis extent and severity index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet Dermatol. (2014) 25:77–85. doi: 10.1111/vde.12107
4. Ettinger SJ, Feldman EC, Cote E. Textbook of Veterinary Internal Medicine. 8th ed. St. Louis, MO: Elsevier (2017).
5. Hänel KH, Cornelissen C, Lüscher B, Baron JM. Cytokines and the skin barrier. Int J Mol Sci. (2013) 14:6720–45. doi: 10.3390/ijms14046720
6. Nuttall TJ, Marsella R, Rosenbaum MR, Gonzales AJ, Fadok VA. Update on pathogenesis, diagnosis, and treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. (2019) 254:1291–300. doi: 10.2460/javma.254.11.1291
8. Yamazaki H, Tanaka T, Mie K, Nishida H, Miura N, Akiyoshi H. Assessment of postoperative adjuvant treatment using toceranib phosphate against adenocarcinoma in dogs. J Vet Intern Med. (2020) 34:1272–81. doi: 10.1111/jvim.15768
9. Brown PM, Tzannes S, Nguyen S, White J, Langova V. LOPP chemotherapy as a first-line treatment for dogs with T-cell lymphoma. Vet Comp Oncol. (2018) 16:108–13. doi: 10.1111/vco.12318
10. Villatoro AJ, Hermida-Prieto M, Fernández V, et al. Allogeneic adipose-derived mesenchymal stem cell therapy in dogs with refractory atopic dermatitis: clinical efficacy and safety. Vet Rec. (2018) 183:654. doi: 10.1136/vr.104867
11. Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. (2013) 15:713–20. doi: 10.1038/ncb2788
12. Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. (2009) 5:527–49. doi: 10.1016/j.chom.2009.05.016
13. Jeong D, Qomaladewi NP, Lee J, Park SH, Cho JY. The role of autophagy in skin fibroblasts, keratinocytes, melanocytes, and epidermal stem cells. J Invest Dermatol. (2020) 140:1691–7. doi: 10.1016/j.jid.2019.11.023
14. Wang M, Charareh P, Lei X, Zhong JL. Autophagy: multiple mechanisms to protect skin from ultraviolet radiation-driven photoaging. Oxid Med Cell Longev. (2019) 2019:8135985. doi: 10.1155/2019/8135985
15. Rajawat YS, Hilioti Z, Bossis I. Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev. (2009) 8:199–213. doi: 10.1016/j.arr.2009.05.001
16. Chai M, Jiang M, Vergnes L, Fu X, Barros SC, Doan NB, et al. Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. (2019) 27:3413–21. doi: 10.1016/j.celrep.2019.05.070
17. Lim CJ, Lee YM, Kang SG, Lim HW, Shin KO, Jeong SK, et al. Aquatide activation of SIRT1 reduces cellular senescence through a SIRT1-FOXO1-autophagy axis. Biomol Ther. (2017) 25:511–8. doi: 10.4062/biomolther.2017.119
18. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. (2010) 1:e10. doi: 10.1038/cddis.2009.8
19. Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, et al. Safety assessment of PCA (2-Pyrrolidone-5-carboxylic acid) and its salts as used in cosmetics. Int J Toxicol. (2019) 38:5S–11S. doi: 10.1177/1091581819856568
20. Lim J, Lim CJ, Kim S, Nam G, Chang M, Park K, et al. Antiaging and antioxidant effects of topical autophagy activator: a randomized, placebo-controlled, double-blinded study. J Cosmet Dermatol. (2019) 18:197–203. doi: 10.1111/jocd.12530
21. Ye L, Wang Z, Li Z, Lv C, Man M-Q. Validation of GPSkin Barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res Technol. (2019) 25:25–9. doi: 10.1111/srt.12590
22. Lau-Gillard PJ, Hill PB, Chesney CJ, Budleigh CB, Immonen A. Evaluation of a hand-held evaporimeter (VapoMeter) for the measurement of transepidermal water loss in healthy dogs. Vet Dermatol. (2010) 21:136–45. doi: 10.1111/j.1365-3164.2009.00738.x
23. Oh WS, Oh TH. Measurement of transepidermal water loss from clipped and unclipped anatomical sites on the dog. Aust Vet J. (2009) 87:409–12. doi: 10.1111/j.1751-0813.2009.00481.x
24. Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. (2012) 92:497–501. doi: 10.2340/00015555-1265
25. Kim YD, Jeon SY, Ji JH, Lee WS. Development of a classification system for extrinsic hair damage: standard grading of electron microscopic findings of damaged hairs. Am J Dermatopathol. (2010) 32:432–8. doi: 10.1097/DAD.0b013e3181c38549
26. Sotiropoulou PA, Blanpain C. Development and homeostasis of the skin epidermis. Cold Spring Harb Perspect Biol. (2012) 4:a008383. doi: 10.1101/cshperspect.a008383
27. Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. (2009) 10:209–17. doi: 10.1038/nrm2636
28. Olivry T. Is the skin barrier abnormal in dogs with atopic dermatitis?. Vet Immunol Immunopathol. (2011) 144:11–16. doi: 10.1016/j.vetimm.2011.07.014
29. Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. (2011) 146:682–95. doi: 10.1016/j.cell.2011.07.030
30. Nagar R. Autophagy: a brief overview in perspective of dermatology. Indian J Dermatol Venereol Leprol. (2017) 83:290–7. doi: 10.4103/0378-6323.196320
31. Yang F, Tanaka M, Wataya-Kaneda M, Yang L, Nakamura A, Matsumoto S, et al. Topical application of rapamycin ointment ameliorates Dermatophagoides farina body extract-induced atopic dermatitis in NC/Nga mice. Exp Dermatol. (2014) 23:568–72. doi: 10.1111/exd.12463
32. Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. (2020) 59:101036. doi: 10.1016/j.arr.2020.101036
33. Bertino L, Guarneri F, Cannavò SP, Casciaro M, Pioggia G, Gangemi S. Oxidative stress and atopic dermatitis. Antioxidants. (2020) 9:196. doi: 10.3390/antiox9030196
34. Nakai K, Yoneda K, Kubota Y. Oxidative stress in allergic and irritant dermatitis: from basic research to clinical management. Recent Pat Inflamm Allergy Drug Discov. (2012) 6:202–9. doi: 10.2174/187221312802652839
35. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. (2015) 21:16–29. doi: 10.1016/j.arr.2015.01.001
36. Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. (2007) 6:1837–49. doi: 10.4161/cc.6.15.4511
37. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. (2011) 147:728–41. doi: 10.1016/j.cell.2011.10.026
38. Kim HR, Kim JC, Kang SY, Kim HO, Park CW, Chung BY. Rapamycin alleviates 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced aggravated dermatitis in mice with imiquimod-induced psoriasis-like dermatitis by inducing autophag. Int J Mol Sci. (2021) 22:3968. doi: 10.3390/ijms22083968
39. Wu J, Yang F, Zhang X, Chen G, Zou J, Yin L, et al. Hydrogen sulfide inhibits endoplasmic reticulum stress through the GRP78/mTOR pathway in rat chondrocytes subjected to oxidative stress. Int J Mol Med. (2021) 47:34. doi: 10.3892/ijmm.2021.4867
40. Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. (2015) 35:615–21. doi: 10.1007/s10571-015-0166-x
Keywords: autophagy, dog, skin, keratinocytes, hair, TEWL
Citation: Kim Y, Lee S-H, Song Y, Jeong S and Kim H-J (2023) Induction of autophagy improves skin and hair conditions in dogs with underlying diseases. Front. Vet. Sci. 10:1078259. doi: 10.3389/fvets.2023.1078259
Received: 24 October 2022; Accepted: 09 January 2023;
Published: 26 January 2023.
Edited by:
Muhammad Saqib, University of Agriculture, Faisalabad, PakistanReviewed by:
Marcin Szczepanik, University of Life Sciences in Lublin, PolandCopyright © 2023 Kim, Lee, Song, Jeong and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ha-Jung Kim,  a2ltaGo2MTRAam51LmFjLmty
a2ltaGo2MTRAam51LmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.