- 1Forestry College, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
- 2Forest Pest and Disease Control and Quarantine Station, Forestry Bureau of Fuzhou City, Fuzhou, Fujian, China
- 3Fujian Academy of Forestry, Fuzhou, Fujian, China
- 4Provincial Key Laboratory of Integrated Pest Management in Ecological Forests, Fujian Agriculture and Forestry University, Fuzhou, Fujian, China
Introduction: Beauveria spp. and Dastarcus helophoroides Fairmaire adults were simultaneously released to attack elder larvae or pupae of Monochamus alternatus in pine forests in China. However, little is known about the pathogenicity virulence and biosafety of Beauveria spp. on beneficial adults of D. helophoroides, and specific Beauveria bassiana (Bb) strains should be selected for synthetic release together with D. helophoroides.
Methods: A total of 17 strains of Beauveria spp. were collected, isolated, and purified, and then their mortality, cadaver rate, LT50, spore production, spore germination rate, and growth rate of D. helophoroide adults were calculated based on 0–20 days data after spore suspension and powder contact.
Results and discussion: The lethality rate of BbMQ, BbFD, and BbMH-03 strains to D. helophoroides exceeded 50%, and the cadaver rate reached 70.6%, among which the mortality rate (82.22%), cadaver rate (47.78%), spore production (1.32 × 109 spores/ml), spore germination rate (94.71%), colony dimension (49.15 mm2), and LT50 (10.62 d) of the BbMQ strain were significantly higher than those of other strains (P < 0.01), and the mortality of D. helophoroides adults increased significantly with increased spore suspension concentration, with the highest mortality reaching 92.22%. This strain was identified as Beauveria bassiana by morphological and molecular methods, while the BbWYS strain had a minimum lethality of only 5.56%, which was safer compared to other strains of adult D. helophoroide. Consequently, the biological characteristics and pathogenicity of different Beauveria bassiana strains varied significantly in their effects on D. helophoroide adults, and the safety of different strains should be assessed when they are released or sprayed to control multiple pests in the forest. The BbMQ strain should not be simultaneously sprayed with releasing D. helophoroide adults in the same forest, while the BbWYS strain can be used in concert with D. helophoroide to synergize their effect.
1. Introduction
Beauveria spp. contains a variety of insect pathogenic fungi comprising one of the most important natural enemies and components of integrated pest management (IPM) for agricultural pests, attracting much attention and widely used in application efforts worldwide (1, 2). Recently, they were commonly used to control some important defoliators and longhorn beetles in China such as Dendrolimus punctatus Walker (3, 4), D. houi Lajonquiere (5) and Monochamus alternatus Hope (6), achieving significant control effects (5, 7, 8) and becoming popular biological pesticides. Meanwhile, M. alternatus, as the main vector of pinewood nematode (Bursaphelenchus xylophilus) and the causal agent of pine wilt disease in China, directly or indirectly kills hundreds of millions of pine trees in 19 provinces (9, 10). Dastarcus helophoroides Fairmaire, a dominant endoparasitic beetle, which also has been released to kill M. alternatus in the forests achieved good levels of control (6, 11–15).
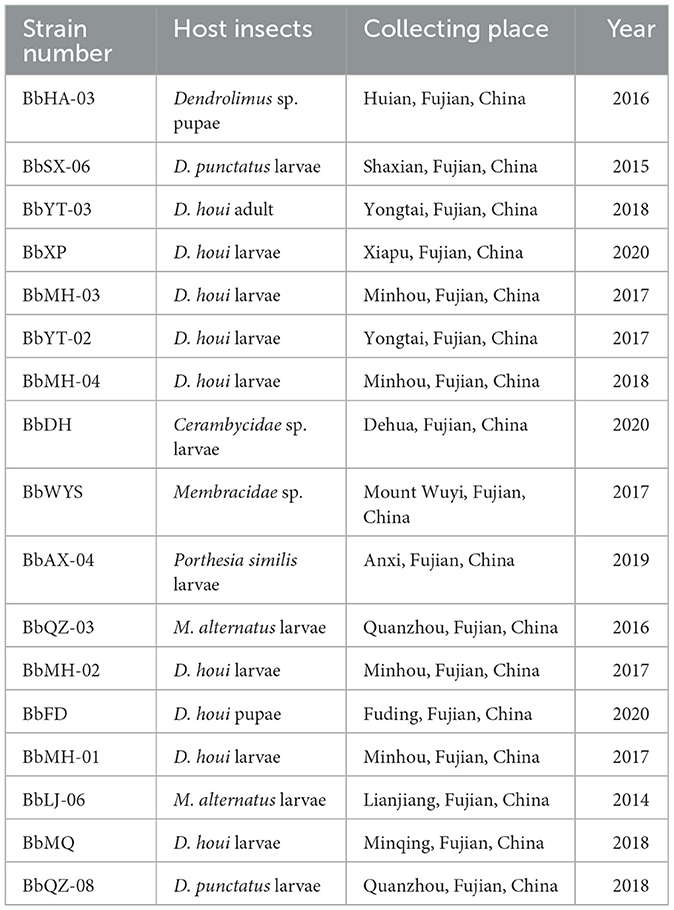
Earlier research revealed that D. punctatus and M. alternatus have overlapping life cycles, and they annually coexist from March to May in the same P. massoniana forests (16, 17). Coincidentally, Beauveria spp. was sprayed to suppress the larvae of D. punctatus during this period to optimize its effect due to optimal temperature and humidity in the forests, and the infected caterpillars of D. punctatus will probably provide the pathogen for their next generation. Meanwhile, it is also the best period for the mass release of D. helophoroides adults to kill the old larvae or pupae of M. alternatus (15). Obviously, these two natural enemies will inevitably coexist in the same forest. What will happen if D. helophoroides adults encounter Beauveria spp.? Could it infect D. helophoroides and thus affect its population stability and pest control effectiveness? To date, except for a few reports of Beauveria bassiana (Bb) strain having very low mortality in D. helophoroides adults (18), little is known about pathogenicity and biosafety of Beauveria spp. on D. helophoroides. Consequently, 17 strains of Beauveria spp. were collected, isolated, purified, and inoculated on D. helophoroides adults to screen and obtain the highest and lowest virulence strains for practice use and biosafety evaluation (19).
2. Methods and materials
2.1. Insect acquisition, rearing, and screening methods
The 20-day-old adults of D. helophoroides were purchased from the Southern Natural Enemy Breeding and Application Engineering Technology Research Center, the State Forestry Administration (Changsha, Hunan, China), and stored in the refrigerator under 4°C at Fujian Agriculture and Forestry University (Fuzhou, Fujian, China). Prior to experiments, 30 healthy D. helophoroides adults were taken out and put into a modified plastic box (16 × 10.5 × 5 cm, L × W × H) under room temperature (25 ± 1°C), allowed 5 min to recover, and then transferred to an artificial climate chamber (Shanghai Yiheng, MGC-300H, 20L) under 25 ± 1°C, L:D = 12:12 h, RH = 70 ± 10% for 2 d. All samples were screened by removing dead and dying individuals in the lab during the process of mass rearing, and three replicates were prepared.
The plastic box was modified to be a rearing box for D. helophoroides adults by making 15 ventilation holes (d = 1 mm) by using a soldering iron and placing two paper towels (15 × 5 cm, length × width) on the bottom of the rearing box. In total, five pieces of P. massoniana bark and one section of P. massoniana wood (d = 4 cm, h = 6 cm) were also placed on the paper towels to simulate habitat. Finally, a centrifuge tube (3 ml) containing an artificial diet for D. helophoroides and a moist cotton ball was placed to provide food and water, respectively.
Formulation of an artificial diet for D. helophoroides adults was as follows: 20 g larval powder of M. alternatus larvae, 60 g powder of Zophobas morio, 20 g powder of Tenebrio molitor, 10 g powder of Antheraea pernyi pupae, 10 g dried yeast, 10 g glucose, 10 g lyophilized yolk powder, 50 g pine powder (containing 15 g epidermal powder plus 35 g xylem powder), and 20 g sunflower oil, with all ingredients thoroughly mixed (20). The prepared artificial diet was put into centrifuge tubes (3 ml) and stored in the refrigerator at −4°C.
2.2. Collection, isolation, and purification of tested strains
Most strains of Beauveria spp. were naturally collected from infected insect cadavers in the forests in Fujian, China, and a few strains were obtained from the Fujian Academy of Forestry (FAF, Fujian, China). The PDA medium was used to obtain pure cultures after delineation, and by continuous isolation in a sterile ultra-clean bench in the lab. A total of 17 strains of Beauveria spp. were obtained and identified based on the fungal morphological characteristics (Table 1) and stored at 4°C in the refrigerator.
2.3. The virulence of Beauveria spp. spore suspension on D. helophoroides adults
The 17 strains were inoculated in line on sterile Petri dishes (d = 10 cm) containing the PDA medium and incubated purely in an artificial incubator (Shanghai Yiheng, LRH-150) under 26 ± 1°C, 80 ± 10% humidity for 14 d; then each strain after full sporulation was eluted with sterile water containing 0.01% Tween-80 in a 50 ml centrifuge tube and fixed to 10 ml, cultivated in the incubator shakers for 30 min under 25 °C (speed 200 rpm/min), then diluted to make suspension (108 spores/ml). A total of 30 healthy D. helophoroides adults were placed into the pathogenic fungus suspension for 10 s to allow sufficient contact at the point where they were transferred into clean rearing boxes with sterile forceps. Each strain had three replicates. Samples immersed in the sterile water (Tween-80) were used as control (CK). The artificial diet and moist cotton balls were renewed daily, and their mortality, number of dead individuals, and time of death were recorded. Dead D. helophoroides adults were routinely placed into sealed centrifuge tubes (5 ml) inside an artificial incubator (Panasonic, Japan, MIR-154-PC) for 14 d. The incubation conditions were 26 ± 1°C and 80 ± 10% humidity, and colony growth on the surface of D. helophoroides adult cadaver was observed daily.
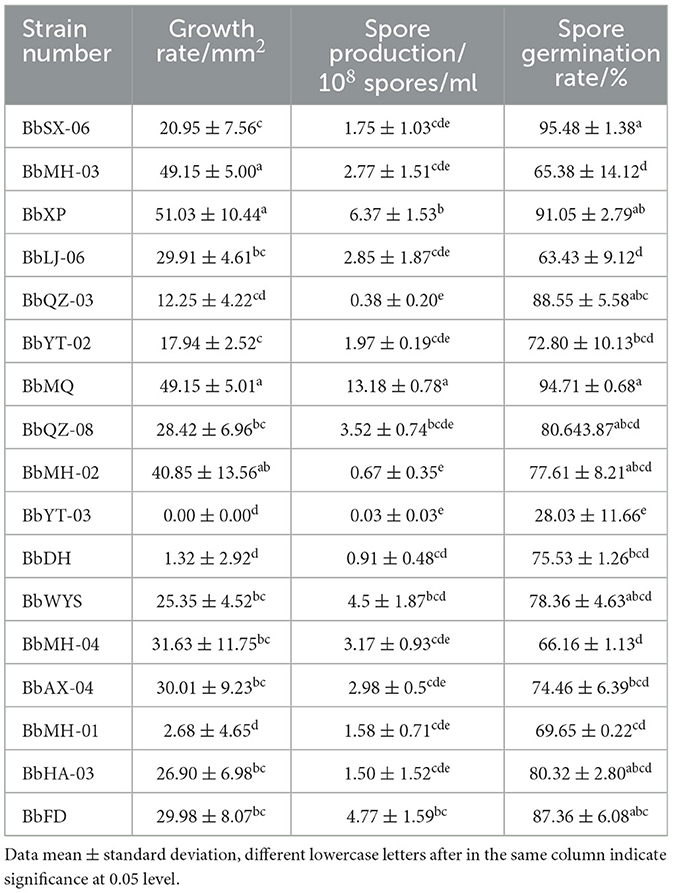
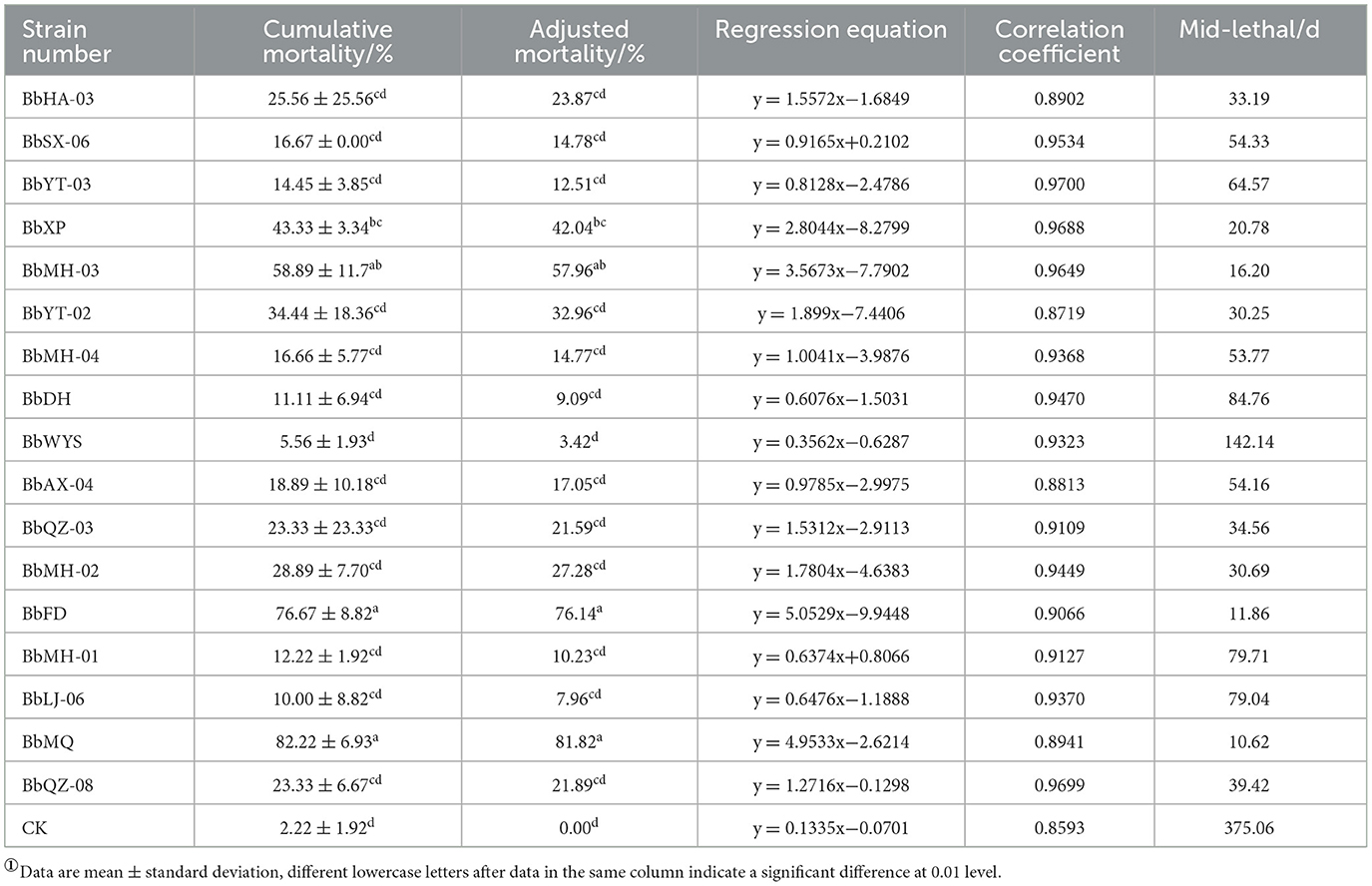
All 17 strains were screened based on the spore production, growth rate, spore germination rate, mortality, cadaver rate, and lethality duration, and the strains with the highest and lowest pathogenicity to D. helophoroides adults were determined.
2.4. The virulence of the highest pathogenic strains against D. helophoroides adults
The strain with the highest pathogenicity was fully eluted with sterile water containing 0.01% Tween-80 in a 50 ml centrifuge tube and fixed to 10 ml, cultivated in the incubator shakers for 30 min under 25°C (speed 200 rpm/min) and then diluted to make 1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 spores/ml suspensions. For each suspension, 30 healthy D. helophoroides adults were submerged for 5 s before they were placed back into rearing boxes and reared on an artificial diet for 20 d of observation. Sterile water containing 0.01% Tween-80 was used as the control, and each treatment had three replicates.
2.5. Biological traits of different strains and identification of highly pathogenic strains
The pure culture of 17 strains was inoculated on the PDA medium, and their spore germination rates were measured. The colony traits were morphologically observed at 24 h, and then their growth rates were measured by the crossover method. Finally, spore production was counted by using a hemocytometer plate after full sporulation at 14 d, and their spore morphology, size, and spore peduncle were also observed and recorded.
DNA from different strains was extracted by using the Ezup column-based fungal genomic DNA extraction kit (Sangon Biotech Co., Ltd., Shanghai, China) and amplified by using the fungal universal primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC). The 25 ul PCR amplification system contained Taq PCE Master Mix 12.5 ul, template DNA 1uL, forward primers, and reverse primers (10 uM) per 1 ul, ddH2O 9.5 ul, respectively. The PCR reaction procedure was as follows: pre-denaturation at 95°C for 5 min, denaturation at 94°C for 30 s, annealing at 57°C for 30 s, and extension at 72°C for 90 s for 30 cycles, and finally, all reactions were extended for 10 min at 72°C and detected by electrophoresis using 1% agarose gel. Some PCR samples were specifically selected based on ITS region amplification and sequenced to confirm them as Beauveria species. The PCR amplified samples with the ITS region confirmed as Beauveria bassiana by electrophoresis were sent to Biotech Bioengineering (Shanghai) Co., for sequencing, then the ITS sequences were submitted to the NCBI database for BLASTN comparison, and 23 fungal sequences were downloaded to construct a phylogenetic evolutionary tree by using the maximum likelihood method (ML) and MEGA10.0 software to determine their species.
2.6. Data processing
The cumulative mortality rate [mortality rate (%) = (number of deaths / total number of insects tested) × 100], corrected mortality rate [corrected mortality rate (%) = [(mortality rate in the treatment group–mortality rate in CK) / (1–CK mortality rate)] × 100], and cadaver rate, [cadaver rate, (%) = (number of cadaver / total number of insects tested) × 100] were calculated for each strain. All data were analyzed by using IBM SPSS Statistics 26 software. Figures of cadaver rate and cumulative mortality curve were drawn using Origin 2021b 64 bits. A DSLR camera (Canon EOS 77D) with a macro lens (LAOWA 100 mm Macro) was used to take pictures of the cadavers of D. helophoroide.
3. Results and analysis
3.1. Spore production and virulence of different strains against D. helophoroides adults
The spore production of 17 strains varied after 14 d cultivation, of which the BbMQ strain was 1.32 × 109 spores/ml, the spore germination rate was 94.71%, and the colony area was 49.15 mm2, which were significantly higher than those of other strains (Table 2, F18,50 = 16.931, p < 0.05).
All tested Beauveria spp. were pathogenic to all D. helophoroides adults, of which the corrected mortality of the BbMQ strain was 81.82%, indicating that it was the most pathogenic and virulent strain, followed by BbFD strains (76.14%). In addition, the cumulative mortality of the BbMQ strain reached 82.22% at 20 d, which was significantly higher than the other 15 strains (p < 0.05). We also found that the mortalities of BbHD, BbWYS, BbMH-01, BbLJ-06, and BbYT-03 strains were <15%, of which the BbWYS strain was only 5.56% (Table 3, F17,36 = 11.896, p < 0.01). All 17 strains could fit the univariate linear regression equation (F18,38 = 12.181, p < 0.01, R2 > 0.85), while the mid-lethal time of the BbMQ strain was theoretically 10.62 d, which was remarkably shorter than the BbFD, BbMH-03 strains (Figure 1, Table 3, F18,38 = 12.181, p < 0.01).
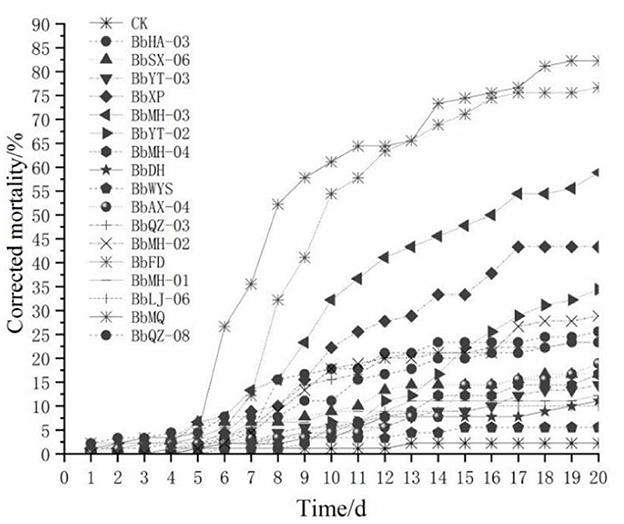
Figure 1. Mortality change of D. helophoroides adults infected by different strains of Beauveria bassiana.
3.2. Infection symptoms and process of D. helophoroides adults by Beauveria spp.
The infected D. helophoroides adults tended to move slowly and showed slight anorexia during the initial stage of infection. At the beginning of infection (4 ~ 6 d), the number of D. helophoroides adults feeding on the diet gradually decreased, and approximately two adults showed daily foraging behavior, and no adults were seen to have fed on the diet during the final period (10 ~ 15 d), while five adults fed daily on the diet in the control groups.
Dead beetles were characterized by having three pairs of legs bent and unfolded after infection, with tarsus clutching the wood, or three pairs of legs stretched out with the abdomen upwards (Figures 2A, E). During 14 d, moisturized culture and the growth of the BbMQ strains was observed as a model: mycelium started from the head mouthparts, thoracic and abdominal ends, and intersegmental areas between each foot of the adult insect after 1 ~ 2 d infection/death, then the whole adult body was covered by the mycelium (Figure 2F), the mycelium grew rapidly on the day 3 (Figure 2J), and a large number of spores started to cover the body surface on day 6 to day 11 (Figure 2H). In contrast, the dead adults from control groups with the moisturizing cultivation did not produce any mycelia and spores during the entire process (0–11 d) (Figures 2B–D).

Figure 2. Symptom and process of D. helophoroides adults infected by the BbMQ strain. (A) 1 d CK; (B) 2 d Ck; (C) 3 d Ck; (D) 11 d CK; (E) 1 d BbMQ; (F) 2 d BbMQ; (J) 3 d BbMQ; (H) 11 d BbMQ.
3.3. Cadaver rate of infected D. helophoroides adults
Out of a total of 17 strains, only 12 strains were found to produce cadavers by moisturizing and culturing the infected carcasses, accounting for 70.6% of all tested strains, and the cadaver rates of BbFD and BbMQ strains were 54.45 and 47.78%, respectively (Figure 3, F18,38 = 23.781 p < 0.05). The virulence and infestation of Beauveria spp. on the D. helophoroides adult was relatively stable, and the survival of D. helophoroides adults might be seriously threatened by the natural Beauveria spp. in the forests.
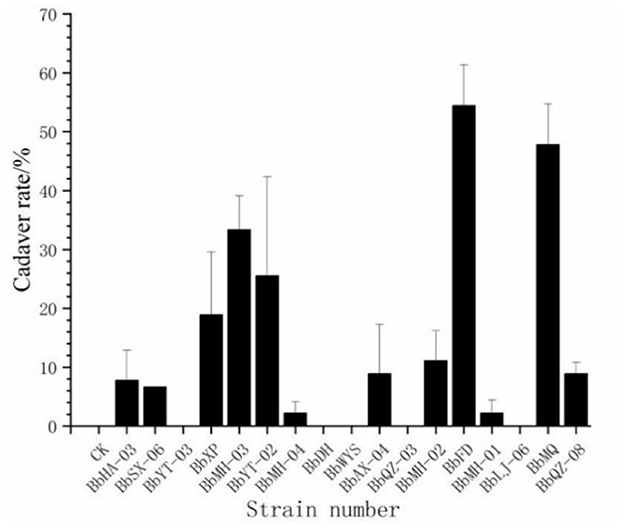
Figure 3. Cadaver rate percentage change of D. helophoroides adults infected by different strains of Beauveria bassiana.
3.4. Virulence of BbMQ strain suspensions
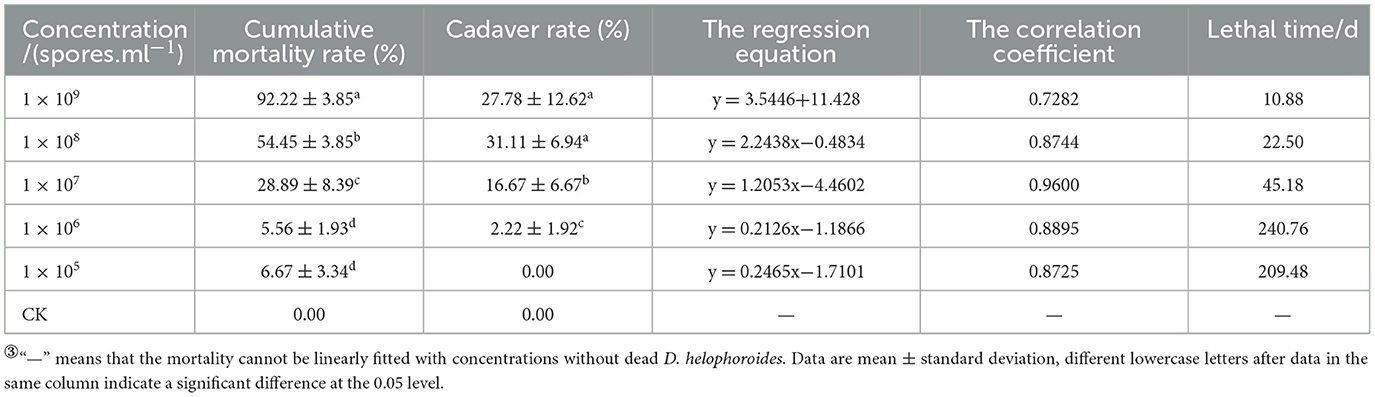
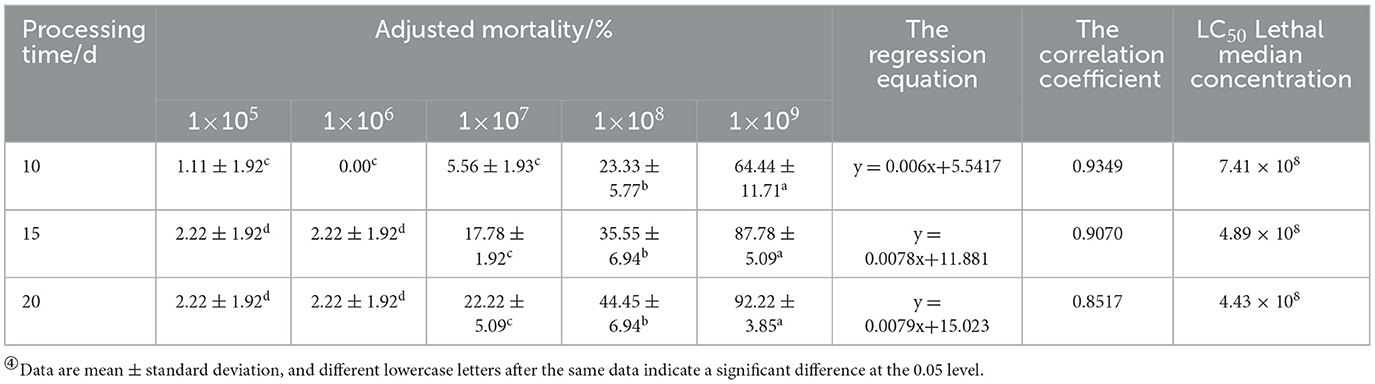
Based on the mortality rate, lethality time, cadaver rate, spore production, spore germination rate, and colony growth area of 17 strains, the BbMQ strain was thought to be the most dangerous to D. helophoroides adults. Further results showed that both the cumulative mortality rate and cadaver rate of D. helophoroides adults decreased significantly with decreased concentrations of the spore suspension, the lethal time increased significantly with decreasing concentrations of the suspension, and the lethal median time was the shortest (10.88 d) under 1 × 109 spores/ml suspension, indicating that the lethality of the BbMQ strain on the D. helophoroides adults was determined by the concentration of the spore suspensions (Table 4, F5,12 = 203.738, p < 0.05). The semi-lethal concentrations were 7.41 × 108, 4.89 × 108, and 4.43 × 108 spores/ml, respectively, when D. helophoroides adults were infected by the BbMQ strain at days 10, 15, and 20, and all fit the univariate linear regression equation (F4,10 = 220.038, p < 0.05, R2 > 0.85) (Table 5).
3.5. BbMQ strain identification
3.5.1. Morphological identification
The colonies of the BbMQ strain were white flocculent at the early stage (0–5 d) and then the mycelium began to produce spores on the day 6 after inoculation. The middle of the colony was white powder-like, the edge was fluffy and neat, and the whole colony was white powder-like on day 8, with a small amount of oil-like droplets on the surface, and the back of the colony was yellowish (Figure 4a). There were some transparent round conidia (Figure 4b) in the sporulation axis extended from the sporulation cell under an optical microscope (NIKON E100) preliminarily indicating Beauveria bassiana.
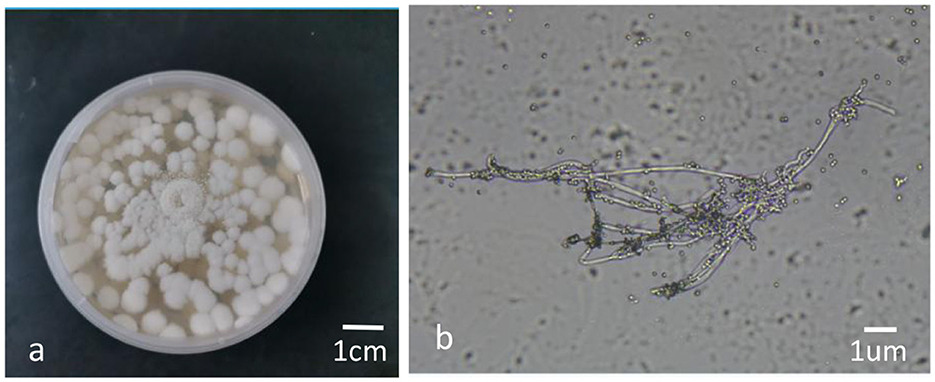
Figure 4. Colony morphology and conidia of BbMQ. (a) Colony morphology of the BbMQ strain, (b) hypha and conidia of the BbMQ strain.
3.5.2. Molecular biology identification
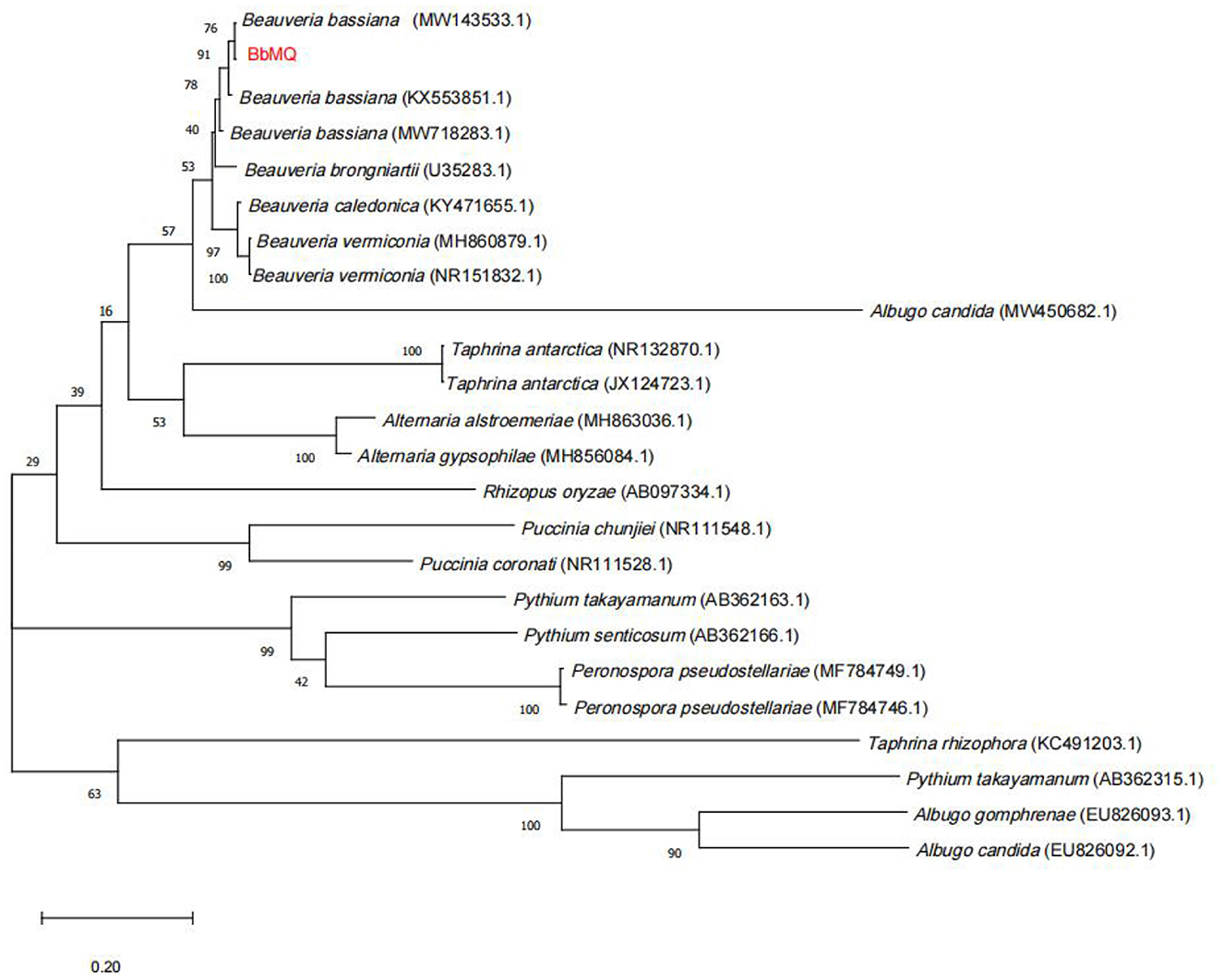
The 533bp amplified sequence was obtained after the BbMQ genomic DNA was used as the template, and the common primers ITS1 and ITS4 were used for amplification, recovery, cloning, and sequencing. The obtained ITS sequences were compared in the NCBI database by BLASTN homology. The sequence homology between BbMQ and Beauveria bassiana in the NCBI library was above 99%. In total, 23 sequences with high similarity to Beauveria bassiana were selected from NCBI, then a phylogenetic evolutionary tree was constructed using MEGA 10.0 software with a Bootstrap value of 1,000, indicating that BbMQ was in the same sister group as Beauveria bassiana (MW143533) strain with high homology (Figure 5). Therefore, the morphology, NCBI blast, and evolutionary tree comparison indicated that the BbMQ strain was Beauveria bassiana. The Gen Bank registry of the BbMQ strain was ON386272.
4. Conclusion and discussion
Beauvaria spp. is the most popular pathogen for some agricultural pests worldwide, especially for defoliators and branch-shoot pests in the forests, and their mass production and technology are mature with promising application prospects (21, 22), easy operation, and bio-control advantages (23). For example, Beauveria bassiana was used to control D. punctatus (8) and D. houi (5) in Fujian, China, achieving maximum mortality rates of 80%, and 92%, respectively. Beauveria bassiana from wild strains had a significantly higher lethality than those from commercial strains to control Scatella tenuicosta in Texas, USA (24), and mortality of coffee berry borer (CBB, Hypothenemus hampei) was more than 90% when Beauveria bassiana was applied in the coffee orchard in Hawaii, USA (21).
However, as broad-host insect pathogenic fungus Beauveria spp. was widely distributed and persists in various fields worldwide, but it was rarely applied to control M. alternatus, (6, 13, 25–27), and its pathogenicity against natural enemies of M. alternatus has not been clarified. In this study, we found that 17 strains of Beauveria bassiana had significant differences in pathogenicity to D. helophoroides adults, with the highly pathogenic strain being BbMQ based on the mortality rate, lethality time, cadaver rate, spore production, spore germination rate, and colony growth dimension. The lethal strain was morphologically and molecular biologically identified as a Beauveria bassiana and potentially threatens the safety of D. helophoroides adults, while the BbWYS strain had a minimum lethality of only 5.56% but higher lethality (more than 95%) to adults of Monochamus alternatus and larvae of Dendrolimus punctatus, the most important pests of masson pine forests, which was safer than all other strains to the adult D. helophoroide and can be simultaneously released in the pine forests. It should be used cautiously in pine forests to control other pests, such as targeting D. punctatus to avoid harming the D. helophoroides adults and other natural enemies; meanwhile, the low pathogenic BbWYS strain did not endanger the D. helophoroides and would be an ideal alternative natural enemy of controlling other important pests in the pine forests. Additionally, previous studies have shown that Beauveria bassiana was highly pathogenic to both adults and larvae of M. alternatus, resulting in survival rates of <10%, but mortality was <15% if a higher concentration (1 × 108 spores/ml) suspension was provided to D. helophoroides adult and larvae without any cadaver (18), which was significantly different from our findings and presumably related to the pathogenicity differences of various strains.
The combined application of multiple natural enemies can significantly improve the control effect of agricultural and forestry pests. For example, the infected Bactericera cockerelli by Beauveria bassiana did not have a significant impact on the parasitism of wasps Tamarixia triozae (28), indicating that synergistic effects might occur in the pest population when pathogenic fungi and parasitoids were simultaneously released in the forest. The use of Trichogramma dendrolimi carrying Beauveria bassiana in the field resulted in a 28.1 and 22.8% reduction in the rate of infested Zea mays by Ostrinia furnacalis and the infesting holes, respectively, and a significant increase in the cadaver rate of O. furnacalis (29), while the emergence and survival rate of T. dendrolimi was not influenced by Beauveria bassiana. An obvious synergistic effect occurred between Scleroderma guani and Beauveria bassiana when they were released to control the fourth instar overwintering larvae of M. alternatus in Lishui County, Jiangsu, China, and the control effect order was S. guani plus Beauveria bassiana > S. guani > Beauveria bassiana (30). The combined use of Scleroderma sichuanensis and Beauveria bassiana was found to cause some damage to S. sichuanensis, but it also generally improved the control effect on M. alternatus (31).
Physiologically, the spores of different Beauveria bassiana strains have different abilities to secrete extracellular enzymes such as chitinase, Pri, and protease to degrade the body wall during the process of breaking through the insect body wall, as well as the amount of secretion, thus showing some infection variability among their hosts (32, 33). We also assumed that differences in their pathogenicity were caused by their different ability to secrete extracellular enzymes.
Overall, the strategy of using natural enemies carrying Beauveria bassiana to control agroforestry pests is feasible; however, several concerns need to be addressed. The biosafety assessment of different Beauveria spp. strains is necessary prior to their widespread application, which will be beneficial to improve the control effect of forest pests and can also effectively protect some important natural enemies, such as D. helophoroides.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: NCBI-ON386272.1.
Author contributions
GL and YZ: conceptualization. GL, YZ, and SY: methodology and writing—original draft preparation. CL, ZC, and XF: software and data analysis. YZ and ZC: validation. YZ, ZZ, and SC: samples collection. YZ, XF, and ZC: investigation. FZ and GL: resources. GL: writing—review and editing and funding acquisition. All authors reviewed and approved the final manuscript.
Funding
This study was financially supported by the Forestry Peak Discipline Construction Project of Fujian Agriculture and Forestry University (No.72202200205), the National Key Research and Development Program of China (2018YFC1200400), and the National Natural Science Fund of China (No. 31870641).
Acknowledgments
We are grateful to the Fujian Academy of Forestry, Fuzhou, Fujian, China, for partially providing lab strains of Beauveria bassiana, and Fujian Natural Enemy Biological Control Technology Co., LTD., (Quanzhou, Fujian, China) for providing D. helophoroides adults as well. We also thank Dr. Jacob Wickham, a native English speaker and editor of Integrative Zoology, for professional editing the English in this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Loko YLE, Toffa J, Bada B, Dassou GA, Zanzana K, Gavoedo D, et al. Potential of Metarhizium anisopliae and Beauveria bassiana to control Dinoderus porcellus (Coleoptera: Bostrychidae) infesting yam chips. J Asia Pac Entomol. (2022) 25:101885. doi: 10.1016/j.aspen.2022.101885
2. Steven JR, Dalton KB, David GM, Diana ML. Mycoinsecticide formulations of Beauveria bassiana and Metarhizium anisopliae reduce populations of lesser mealworm, Alphitobius diaperinus, in chicken-broiler houses. Biol Control. (2020) 144:104234–104234. doi: 10.1016/j.biocontrol.2020.104234
3. Li ZZ, Han BY, Fan MZ, Tang J. Strategies of applying Beauveria Bassiana against Mason pine caterpillar and their biodiversity basis. Chinese J Appl Ecol. (1998) 9:503–10. doi: 10.13287/j.1001-9332.1998.0108
4. Li ZZ. History, progress and current status of the application of against pest insects in China. Chinese J Biol Cont. (2015) 31:699–711. doi: 10.16409/j.cnki.2095-039x.2015.05.010
5. Cai GG. Screening and application study of Beauveria bassiana Bbd3 strain with high virulence to Dendrolimus houi Lajionquiere. Journal of Nanjing Forestry University. (2006) 30:105–8.
6. Zhang YN, Yang ZQ. Studies on the natural enemies and biocontrol of Monochamus alternatus Hope (Coleoptera: Cerambycidae). Plant Prot. (2006) 32:9–14. doi: 10.11707/j.1001-7488.20190901
7. Kovač M, Lacković N, Pernek M. Effect of Beauveria bassiana fungal infection on survival and feeding behavior of pine-tree lappet moth (Dendrolimus pini L.). Forests. (2020) 11:974. doi: 10.3390/f11090974
8. Huang JS, Tang CS, He XY, Xu YC, Wu SD. Efficacy of oil formulation of Beauveria bassiana to Dendrolimus punctatus by ULV spraying. Scientia silvae sinicae. (2003) 39:194–7.
9. Zheng YN, Liu PX, Shi Y, Wu H, Yu HY, Jiang SW, et al. Difference analysis on pine wilt disease between Liaoning province of northeastern China and other epidemic areas in China. J Beijing For Univ. (2021) 43:155–60. doi: 10.12171/j.1000-1522.20200108
10. Ye JR. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Scientia silvae sinicae. (2019) 55:1–10. doi: 10.11707/j.1001-7488.20190901
11. Lee B-H, Yang J-O, Beckett S, Ren Y. Preliminary trials of the ethanedinitrile fumigation of logs for eradication of Bursaphelenchus xylophilus and its vector insect Monochamus alternatus. Pest Manag Sci. (2017) 73:1446–52. doi: 10.1002/ps.4476
12. Guo Y, Wang Y, O'Donoghue AJ, Jiang Z, Carballar-Lejarazú R, Liang G, et al. Engineering of multiple trypsin/chymotrypsin sites in Cry3A to enhance its activity against Monochamus alternatus Hope larvae. Pest Manag Sci. (2020) 76:3177–26. doi: 10.1002/ps.5866
13. Chen JX, Cheng J, Lin T. Analysis of potential molecular targets in Monochamus alternatus (Coleoptera: Cerambycidae) inoculated with Beauveria bassiana (Deuteromycotina: Hyphomycetes). J Entomol Sci. (2018) 53:533–42. doi: 10.18474/JES17-139.1
14. Li X, Dong G, Fang J, Liu H, Yang L, Guo W, et al. Host foraging behavior of Dastarcus helophoroides (Coleoptera: Bothrideridae) Adults, a Coleopteran Parasitoid of Monochamus alternatus (Coleoptera: Cerambycidae). J Insect Behav. (2016) 29:108–16. doi: 10.1007/s10905-016-9547-3
15. Yang ZQ, Wang XY, Zhang YN. Recent advances in biological control of important native and invasive forest pests in China. Biol Control. (2014) 68:117–28. doi: 10.1016/j.biocontrol.2013.06.010
16. Zhang S, Kong X, Zhang Z. Research Progress on the Dendrolimus spp. pheromone: from identification to molecular recognition. Front Cell Dev Biol. (2022) 10:829826. doi: 10.3389/fcell.2022.829826
17. Zhang ZY, Zha YP, Cai SS, Hong CH, Liang P, Chen JY, et al. Application of harmonic radar to analyze dispersal behavior of the Japanese pine sawyer beetle, Monochamus alternatus (Coleoptera: Cerambycidae). Entomol Res. (2020) 50:50–8. doi: 10.1111/1748-5967.12411
18. Deng JD, Zhuang WX, Liu YJ, Song LW, Zhang LW. Pathogenicity of white muscardine fungus Beauveria bassiana against Japanese pine sawyer beetle Monochamus alternatus and its compatibility with ectoparasitic beetle Dastarcus helophoroides. Plant Prot. (2021) 48:602–9.
19. Thomas SR, Elkinton JS. Pathogenicity and virulence. J Invertebr Pathol. (2004) 85:146–51. doi: 10.1016/j.jip.2004.01.006
20. Yan XW, Ji BZ, Zhou G, He Z, Yu JX, Lu M, et al. The Dastarcus helophoroides to a feed and breeding technology for artificial and large-scale raising of herbivore. Jiangsu: CN102960559A. (2013).
21. Wraight SP, Galaini WS, Howes RL, Castrillo LA, Griggs MH, Carruthers RI, et al. Efficacy of Beauveria bassiana strain GHA spray applications against coffee berry borer Hypothenemus hampei on Hawai‘i Island. Biol Control. (2021) 161:104587. doi: 10.1016/j.biocontrol.2021.104587
22. Agboyi LK, Ketoh GK, Kpindou OK, Martin T, Glitho IA, Tamó M. Improving the efficiency of Beauveria bassiana applications for sustainable management of Plutella xylostella (Lepidoptera: Plutellidae) in West Africa[J]. Biological Control. (2020) 144:104233. doi: 10.1016/j.biocontrol.2020.104233
23. Xu WJ, Sui L, Gao P. Study and application of wettable powder of beauveria bassiana to pyrausta nubilalis. Chinese J Biol Cont. (2020) 36:862–5. doi: 10.16409/j.cnki.2095-039x.2020.06.018
24. Ugine TA, Wraight SP, Sanderson JP. Microbial biological control potential of three strains of Beauveria bassiana s. l. against greenhouse shore fly Scatella tenuicosta : Assessment of virulence, mass production capacity, and effects on shore fly reproduction. Biol Cont. (2013) 65:348–56. doi: 10.1016/j.biocontrol.2013.03.014
25. Li XJ, Dong GP, Fang JM, Liu HJ, Guo WL. Identification of differentially expressed immunity-related genes in Monochamus alternatus Hope (Coleoptera: Cerambycidae) larvae parasitized by Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Entomol Res. (2018) 48:248–61. doi: 10.1111/1748-5967.12275
26. Shi H, Zhou J, Chen Y, Wang Q, Pan Y, Zhang J, et al. A comparison of fitness-related traits in the coleopteran parasitoid Dastarcus helophoroides (Coleoptera: Bothrideridae) reared on two factitious hosts. J Econ Entomol. (2020) 113:2634–40. doi: 10.1093/jee/toaa212
27. Awan UA, Xia S, Meng L, Raza MF, Zhang Z, Zhang H, et al. Isolation, characterization, culturing, and formulation of a new Beauveria bassiana fungus against Diaphorina citri. Biological Control. (2021) 158:104586. doi: 10.1016/j.biocontrol.2021.104586
28. Fernando TM, Patricia TG, Ariel WGF, Ricardo GF. Can Beauveria bassiana Bals. (Vuill) (Ascomycetes: Hypocreales) and Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) be used together for improved biological control of Bactericera cockerelli (Hemiptera: Triozidae). Biol Cont. (2015) 90:42–48. doi: 10.1016/j.biocontrol.2015.05.014
29. Yang Z, Lu Y, Mao G, Zhao Y, Zhang QH, Sui L, et al. Use of Trichogramma dendrolimi carry Beauveria bassiana in control of Ostrinia furnacalis in field. Chinese Journal of Biological Control. (2020) 36:52–7. doi: 10.16409/j.cnki.2095-039x.2019.05.011
30. Liu HJ, Pu CG, Wang LF, Shen XC, Zheng RZ, Shu QL, et al. Biocontrol of Monochamus alternatus by Beauveria bassiana and Scleroderma guani. Scientia silvae sinicae. (2007) 43:64–8.
31. Yang CP, Tao YY, Xiang MR, Yang W, Yang H. The potential for using Scleroderma sichuanensis as a vector for fungal pathogens of Monochamus alternatus. Chin J Entomol. (2021) 58:425–36. doi: 10.7679/j.issn.2095-1353.2021.0444
32. Elawati NE, Pujiyanto S, Kusdiyantini E. Production of extracellular chitinase Beauveria bassiana under submerged fermentation conditions. J Phys Conf Ser. (2018) 1025:012074. doi: 10.1088/1742-6596/1025/1/012074
Keywords: Dastarcus helophoroides Fairmaire, Beauveria bassiana, Monochamus alternatus Hope, pathogenicity, biological control, virulence
Citation: Zhou Y, Lu C, Chen Z, Ye S, Fang X, Zhang Z, Cai S, Zhang F and Liang G (2023) Pathogenicity virulence of Beauveria spp. and biosafety of the BbMQ strain on adult ectoparasitic beetles, Dastarcus helophoroides Fairmaire (Coleoptera: Colydiidae). Front. Vet. Sci. 10:1077473. doi: 10.3389/fvets.2023.1077473
Received: 25 October 2022; Accepted: 31 March 2023;
Published: 16 May 2023.
Edited by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomReviewed by:
Javier A. Garza-Hernandez, Universidad Autónoma de Ciudad Juárez, MexicoRoberto Senas Cuesta, University of Arkansas, United States
Copyright © 2023 Zhou, Lu, Chen, Ye, Fang, Zhang, Cai, Zhang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanghong Liang, ZmpsaGdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Youjun Zhou1†
Youjun Zhou1† Ciding Lu
Ciding Lu Xinyuan Fang
Xinyuan Fang Guanghong Liang
Guanghong Liang