
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 23 September 2022
Sec. Veterinary Pharmacology and Toxicology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.979794
This article is part of the Research TopicRising Stars in Veterinary Pharmacology and Toxicology, 2021: Pharmacokinetics and PKPD ModelingView all 7 articles
The pharmacokinetics of butorphanol after intravenous (IVB) and intramuscular (IMB) administration in donkeys were determined in this preliminary study. Healthy male gelded donkeys (n = 5), aged 6–12 years old, were administered 0.1 mg/kg butorphanol IV or IM in a randomized, crossover design. Blood samples were obtained at predetermined intervals for 24 h (IVB) and 48 h (IMB) after administration. Plasma butorphanol concentrations were determined by high performance liquid chromatography and pharmacokinetic parameters were calculated. Following IVB administration, mean (± SE) apparent volume of distribution, elimination half-life, total body clearance, and area under the plasma concentration time curve from time 0 to infinity (AUC0−∞) were 322 ± 50 mL/kg, 0.83 ± 0.318 h, 400 ± 114 mL/h/kg, 370 ± 131 h·ng/mL, respectively. After IMB administration, a maximum plasma drug concentration of 369 ± 190 ng/mL was reached at 0.48 ± 0.09 h. The IMB AUC0−∞ was 410 ± 60 h·ng/mL. Bioavailability of IMB was 133 ± 45%. The pharmacokinetics of butorphanol in healthy donkeys was characterized by faster elimination half-life compared to values from the equine literature.
As of 2018, the worldwide donkey population was estimated to be over 50 million animals, with most serving in the capacity of working animals and essential to the livelihood of people in developing countries (1, 2). However, there is also a growing trend of donkeys kept as pets or used for physical or emotional therapy in children with special needs or to visit the elderly (3). Typically, veterinarians treat donkeys as small horses, even though the majority of drug pharmacokinetic data collected in donkeys shows a difference in metabolism between the species (4–11). Suggested drug dose protocols for horses must be cautiously extrapolated to the donkey and mule patient, along with careful monitoring for any unintended effects (10).
Butorphanol is a synthetic κ-opioid receptor agonist and a μ-opioid receptor antagonist drug that was originally labeled for antitussive purposes in dogs, but is also approved as an analgesic in cats and horses (12). The effects of butorphanol in horses have been studied, specifically to determine the optimal dose (13), the pharmacokinetic effects (13–16), age considerations (17), and effects of exercise (18). The sedative and analgesic effects of detomidine-butorphanol was determined to be an effective combination for standing procedures in donkeys (19), as was the combination of xylazine and butorphanol in donkeys (20). Butorphanol combined with xylazine and ketamine has also been compared to other injectable anesthetic agents in Mammoth asses (21) and mules (22). It is known that the cardiopulmonary effects after administration of butorphanol in horses causes no significant changes in heart rate, mean and diastolic arterial pressure, or cardiac output (23). Butorphanol has been associated with adverse behavioral and gastrointestinal tract effects in horses including ataxia, decreased borborygmi, and decreased defecation (14). To our knowledge, there are no studies determining the pharmacokinetics of butorphanol in donkeys. The specific aim of the study was a preliminary description of the pharmacokinetics of butorphanol in donkeys after intravenous and intramuscular administration.
Eight adult, male-castrated, university-owned healthy donkeys (Equus asinus) weighing 146.7 ± 12.9 kg, aged 8.2 ± 1.6 years, with body condition score 3.8 ± 0.5 (out of 5), respectively were enrolled in this study, but only five donkeys (n = 5) were able to be used for cross-over comparison.
They were determined to be healthy by means of physical examination, complete blood count and serum biochemistry. The donkeys were kept as a herd within a grass pasture and were also provided local Guinea grass twice a day and water ad libitum. Food, but not water, was withheld for 6 h prior to and for 2 h following drug administration. Water was available ad libitum throughout the study. The donkeys did not receive any medications for at least 2 weeks prior to commencement of the study and were acclimatized to the study environment during this period. At the completion of data collection, all donkeys were monitored for 1 week to ensure their health prior to returning to the entire herd.
The study was approved by the Institutional Animal Care and Use Committee at Ross University School of Veterinary Medicine (IACUC #: 14-5-024 approved on 5/29/14). The donkeys were randomly assigned (http://www.random.org/lists) to receive each of the two treatments, intravenous butorphanol (IVB) and intramuscular butorphanol (IMB), in a crossover design, with at least 7 days of washout between treatments. Prior to drug administration and under aseptic conditions, a 14 gauge, 5.5” catheter (Abbocath, Hospira Inc., Lake Forest IL, USA) was percutaneously placed in each external jugular vein. Local anesthesia (up to 3 mL of 2% lidocaine hydrochloride) was placed subcutaneously in the area where the jugular catheter was placed. The right jugular vein catheter was used for drug administration, while the left jugular vein catheter was used for blood sample collection. Each donkey was weighed on an electronic scale immediately prior to drug administration for both treatment groups. The donkeys received an intravenous (IV) dose of butorphanol at 0.1 mg/kg (Torbugesic Injection; Fort Dodge Animal Health, Fort Dodge, IO 50501 USA), administered slowly over 1 min and flushed afterwards with 10 mL, 0.9% heparinized (2 units/mL) saline solution. For intramuscular (IM) administration, 0.1 mg/kg of butorphanol was given intramuscularly (18G × 1.5” needle) within the base of the right side of the neck. However, only one IV catheter in the left external jugular vein was placed when IMB treatments were given.
For the IVB treatment: blood samples (5 mL each) were collected at time 0 (before drug administration) and at 3, 5, 10, 15, 30, and 45 min, and at 1, 2, 4, 8, 12, and 24 h. For the IMB treatment: blood samples (5 mL each) were collected at time 0, 5, 10, 15, 30, and 45 min, and at 1, 2, 4, 8, 12, 24, 36, and 48 h. The samples were collected by using a 3- syringe technique to avoid sample dilution. A blood volume of 10 mL was pulled from the left jugular catheter by using a 12 mL syringe containing 2 mL of heparinized saline. Then the blood sample was collected using a second empty sterile syringe. The blood contained in the first syringe was given back to the subject. Finally, a third syringe with 10 mL heparinized (2 units/mL) 0.9% saline solutions was used for flushing the left jugular catheter after collection of each blood sample. Catheters were removed either following flushing in the butorphanol (right side) or following collection of the 12-h sample (left side) and the remaining samples were collected by direct venipuncture (18G × 1.5” needle attached to a 12 mL syringe). Blood samples were collected into lithium heparin blood tubes (Monoject/Kendall; Tyco Healthcare Group, Mansfield, MA 02048, USA) and were centrifuged at 3,000 × g for 10 min. Plasma was immediately transferred into cryogenic vials (Corning Inc., Corning, NY 14831, USA) and stored at −80°C until analysis.
Routine health monitoring of the subjects including heart rate, respiratory rate, borborygmi (1–2-min auscultation of four quadrants) and rectal temperature were recorded at −15 and 0 min pre-drug administration and at 15, and 30 min, and 1, 2, 4, 8, 12, and 24 h post-drug administration for the IVB treatment and additionally for 36 and 48 h post-drug administration for the IMB treatment. Any adverse behavior (e.g., agitation, compulsive chewing, increased locomotor activity, ataxia, head jerking, nystagmus) or gastrointestinal tract effects (e.g., decreased borborygmi, decreased defecation, colic) were recorded at each sampling time.
Analysis of butorphanol in plasma samples was conducted using the Yarbrough et al. (24) method. The system consisted of a 2,695 separations module and a 2,475 fluorescence detector (Waters, Milford, MA, USA). Separation was attained on a Waters Symmetry C18 (4.6 × 75 mm, 5 μm) column with a Symmetry guard column. The mobile phase was a mixture of A: ammonium acetate buffer (0.05 M; pH 4.1), and B: acetonitrile. All solutions were prepared fresh daily filtered through a 0.22 μm filter and degassed before their use. The mixture was pumped at a starting condition of 77% A and 23% B for 2.7 min, then changed to 65% A and 35% B over 9.3 min and finally returned to initial conditions for the final 3 min. The flow rate was 1.3 mL/min. Fluorescence was measured at an excitation of 200 nm and an emission of 325 nm with the gain at 1000X. The column was maintained at an ambient temperature of 25°C.
Butorphanol was extracted from plasma samples using a liquid-liquid extraction. Previously frozen plasma samples were thawed and vortexed. One hundred microliters of plasma was transferred to a screw top test tube, followed by 10 μl of propylparaben (100 μg/mL internal standard). Two milliliters of ethyl acetate: hexane (40:60) was added. The tubes were vortexed for 1 min, and then centrifuged for 20 min at 1,700 × g. The organic layer was removed to a clean glass tube and evaporated to dryness with nitrogen. Samples were reconstituted in 225 μl of mobile phase. The solution was transferred to chromatography vials, and 100 μl was injected.
Standard curves for plasma analysis were prepared by spiking untreated plasma, from multiple individuals in the same herd of donkeys, with butorphanol which produced a linear concentration range from 5 to 1,500 ng/mL. The quality control samples used were 7, 300, 700 and 1,300 ng/mL. The acceptance criterion was ± 15% deviation from the nominal value except the LLOQ which was 20%. Intra-assay variability ranged from 1.7 to 8.4% and inter-assay variability ranged from 6.0 to 9.7%. The average recovery was 92%. The recovery of the internal standard was 97%. The lower limit of limit of quantification (LLOQ) was 5 ng/mL which represents a peak approximately five times baseline noise.
Plasma butorphanol concentrations were analyzed for each individual animal by compartmental and noncompartmental approaches using Phoenix 64 WinNonlin (Pharsight Corp., Mountain View, CA). Biexponential equations for a two-compartment model Cp = Ae−αt + Be−βt (where A and B are y-intercept constants, α is the rate constant of the distribution phase, and β is the rate constant of the elimination phase) were fit to the data. Weighting of the data using the reciprocal weighting of residual errors, using re-iterative fitting (i.e., 1/Yhat of the concentration) was used to improve the line fit and residual plots. The goodness of fit of the data with the model was determined by visual examination of the line fits, residual plots, and Akaike's information criteria (25). Values for elimination rate constant (λz), plasma half-life (t½), maximum plasma concentration (Cmax), time to maximum plasma concentration (Tmax), apparent volume of distribution (Vdarea), apparent volume of distribution at steady state (Vdss), total body clearance (Cl), and area under the plasma concentration time curve (AUC0−∞) from time 0 to infinity were calculated from non-compartmental analysis. While A, distribution intercept; B, elimination intercept; α, distribution constant; β, elimination constant; t½α, distribution half-life; t½ β, elimination half-life were calculated using a compartmental analysis. The AUC and AUMC were calculated using the log-linear trapezoidal rule. Mean residence time (MRT) was calculated as AUMC0−∞/AUC0−∞.
For butorphanol the extraction ratio (Ebody) was calculated as previously described (26, 27) with:
First, calculations for each individual, and then combined for a mean value, with cardiac output described by Toutain et al. (26), as follows:
Parameter values were reported as the arithmetic mean of individually estimated parameters.
Variability in pharmacokinetic parameters was expressed as the standard deviation. In the case of the half-life, the harmonic mean and pseudo standard deviation were used instead.
No adverse behaviors (defined as agitation, compulsive chewing, increased locomotor activity, ataxia, head jerking, nystagmus) were noted throughout the study or for 48 h afterwards.
Decreased borborygmi was noted in all subjects for both treatment groups for the first 1–2 h following administration of butorphanol but returned to baseline by the time the 4-h sample was obtained. The pharmacokinetic parameters after single IVB and IMB dosing are displayed in Table 1. Time vs. concentration curves are displayed in Figure 1 for IVB and IMB administration. Eight donkeys were originally enrolled in the study, but samples from 5 donkeys were used for the pharmacokinetic analysis due to loss of usable sample during international shipment.
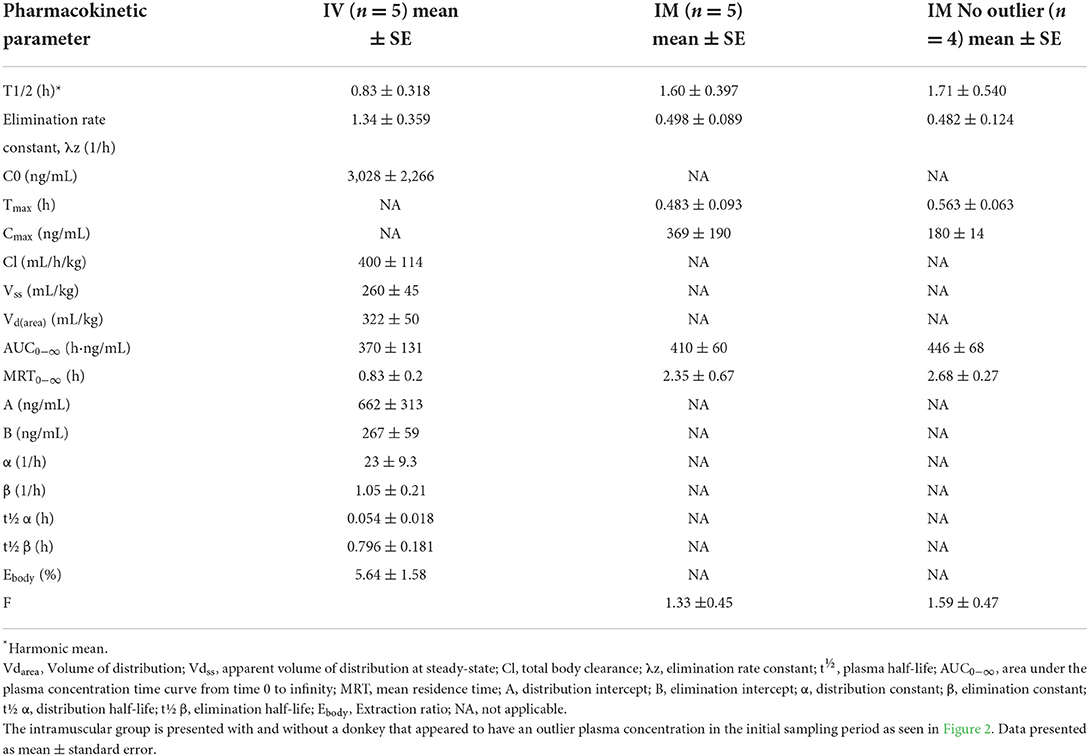
Table 1. Plasma pharmacokinetic parameters of intravenous and intramuscular administered butorphanol (0.1 mg/kg) in donkeys.
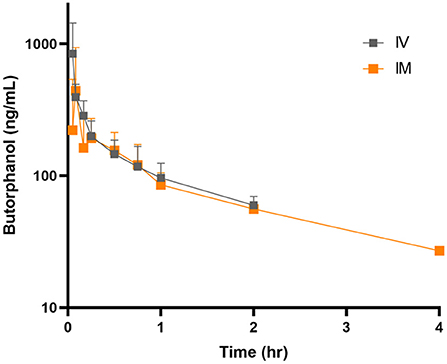
Figure 1. Mean ± SE plasma concentrations following intravenous (gray squares) and intramuscular (orange squares) administration of butorphanol (0.1 mg/kg) to donkeys.
To the best of the authors' knowledge, this is the first report on the pharmacokinetics of butorphanol in donkeys. The results of this investigation in donkeys demonstrates several differences from the pharmacokinetics of butorphanol previously reported in horses. Other studies have evaluated the pharmacokinetics of intravenously administered butorphanol in horses (13, 14, 16–18). The plasma clearance reported in this study of donkeys (400 mL/kg/h) is less than reported in a population of seven Thoroughbred geldings (646 mL/kg/h) (18), ten horses of different breeds (650 mL/kg/h) (16), a population of ten Thoroughbreds (690 mL/kg/h) (13), as well as a study of six Arabian-pony cross foals (1,882 ± 261 mL/kg/h) (17). The reported extraction ratio of 5.64% would be considered a low overall body extraction ratio (26). The Cmax in these donkeys, 369 ± 190 ng/mL, was higher than the reported Cmax of 99.2 ± 29.5 ng/mL in adult horses that received a lower dose of 0.08 mg/kg butorphanol IM (15). Interestingly, the horses that received a lower IM dose of butorphanol had a shorter Tmax of 0.32 h compared to the donkeys with a Tmax of 0.48 h after IM butorphanol administration. As seen in Figure 2, there is one donkey that had an unexpected concentration time curve making it most likely an outlier due to a potential analytical lab labeling error. Analysis of the remaining four donkeys resulted in a Cmax of 180 ±14 ng/mL which is lower than when all five donkeys are considered but still higher than reported Cmax in horses. The Tmax when the outlier is excluded from the IM butorphanol is 0.56 h and the AUC increases from 410 ± 60 to 446 ± 68 h·ng/mL. These changes in PK parameters when the outlier donkey in the IM butorphanol group is excluded should be taken into consideration for future studies.
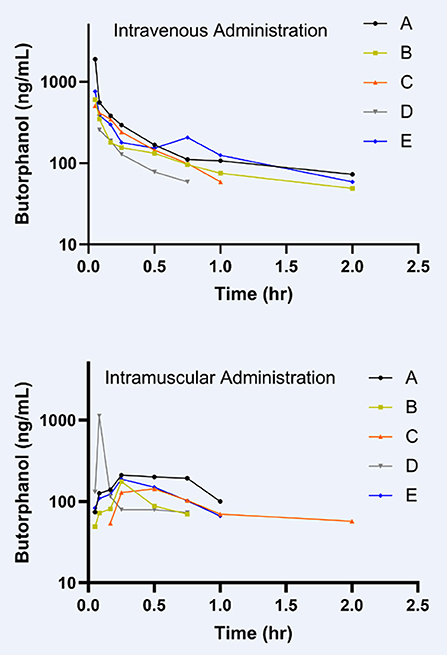
Figure 2. Individual plasma concentration time curves of each of the five animals (A–E) for the IV butorphanol and IM butorphanol treatment groups.
The plasma half-life (T1/2) reported in these donkeys after IVB administration of 0.83 h was shorter than the ranges of 2.31–7.77 h reported in adult horses (13, 14, 16). T1/2 after IMB administration of 1.60 h was longer than that noted in horses of 0.57 h (15). In horses, the longer elimination after IV vs. IM administration was attributed to a deep compartment after IV administration in horses that was not detected after IM administration (16). This deep compartment was not observed in the donkeys in our study. An explanation for the difference between half-lives after IV and IM administration in these donkeys could be flip-flop kinetics where elimination and absorption overlap or the rate of drug absorption is slower than the rate of elimination. The inter-individual variations in pharmacokinetic parameters (T1/2, MRT, AUC) between the two treatment groups as seen in Table 1 were not statistically different between groups. Pharmacokinetic parameters can be influenced by assay sensitivity, with lower limits of detection and quantification extending reported parameters such as elimination half-life (28). This could explain the differences in parameters reported in the donkeys in this study when compared to horses as this study's method had a limit of detection of 5 ng/mL compared to 0.1 ng/mL for several reported equine studies (13–16). However, analytical sensitivity would not impact parameters such as Cmax and Tmax.
The bioavailability of butorphanol after IM administration to horses has been described as 37.3% (15) and 87% after subcutaneous administration (16). The mean ± standard error IM bioavailability in the five donkeys in this study was approximately 133 ± 45%. While this could be suggestive of a species-specific difference in bioavailability between horses and donkeys, it could also be an artifact of the observed areas under the curve. Bioavailability can be affected by a number of parameters within individuals. Factors such as drug concentration at the site of administration, absorption site surface area, the pKa of a drug, the molecular size of a drug, and the pH of the fluid surrounding the site of drug administration could all affect the bioavailability (29).
The authors can only speculate that one of these factors might be the cause of the difference seen or there may be alterations in muscle blood flow in these individuals that could account for the changes seen. A more likely cause was that an insufficient number of early samples were obtained to adequately describe the absorption phase. Alternatively, it could be due to inter-individual variability in the rate of absorption as some individuals did not adhere to expected rise and fall from the IM administration. A bioavailability of >100% for morphine has been reported in goats (30), llamas (31), and dogs (32) and this could be attributed to a limited or minimal distribution phase.
Limitations of this study include the small sample size. However, for veterinary pharmacokinetic studies, a sample size of 4 to 6 animals typically is sufficient to describe the pharmacokinetics of a test drug (33). The authors recognize that most pharmacokinetic investigations in other species studied at least 6 animals. The data in this study is being presented as a preliminary study because of the small sample size that was able to be evaluated in a cross-over manner.
Future studies of the pharmacokinetics of butorphanol in donkeys should consider higher doses, pharmacodynamic effects, and increased number of sampling timepoints to more reliably determine bioavailability. Additional work could involve utilizing nonlinear mixed-effects modeling to determine factors for the variation of pharmacodynamics of butorphanol in the donkey population (34). Determining the effective dose of butorphanol in donkeys would benefit those working with this species in clinical practice.
In conclusion, in this preliminary study, administration of 0.1 mg/kg butorphanol, by both the IM and IV route, to healthy adult donkey geldings is characterized by a rapid elimination half-life.
Higher peak concentrations were achieved after IVB administration. However, butorphanol was detectable for longer after IM administration (4 vs. 2 h).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Ross University Institutional Animal Care and Use Committee.
LE, OO, BS, and IL contributed to study design and implementation. SC developed the analytical method and performed the pharmacokinetic analysis. JS contributed to analysis and results interpretation. All authors contributed to manuscript construction.
This project was funded by a Ross University School of Veterinary Medicine intramural grant. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors would like to thank the following people for their contribution in the collection of data: Crace Lewis, Kathy Alston, Andrew Ebner, Julia Carter, Jen Dupre, Dr. Michelle Cerullo, Michaela Witcher, Dr. Allison Greco, and Dr. Christelle Follett.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Upjohn MM, Pfeiffer DU, Verheyen KL. Helping working Equidae and their owners in developing countries: monitoring and evaluation of evidence-based interventions. Vet J. (2014) 199:210–6. doi: 10.1016/j.tvjl.2013.09.065
2. Norris SL, Little HA, Ryding J, Raw Z. Global donkey and mule populations: figures and trends. PLoS ONE. (2021) 16:e0247830. doi: 10.1371/journal.pone.0247830
3. The Donkey Sanctuary Knowledge Advice. (2022). Available online at: http://www.thedonkeysanctuary.org.uk/donkey-assisted-therapy (accessed May 30, 2022).
4. Lizarraga I, Janovyak E. Comparison of the mechanical hypoalgesic effects of five α2- adrenoceptor agonists in donkeys. Vet Rec. (2013) 173:294. doi: 10.1136/vr.101684
5. Senior JM. Not small horses: improving treatments for donkeys. Vet Rec. (2013) 173:292–3. doi: 10.1136/vr.f5646
6. Mealey KL, Matthews NS, Peck KE, Burchfield ML, Bennett BS, Taylor TS. Pharmacokinetics of R(-) and S(+) carprofen after administration of racemic carprofen in donkeys and horses. Am J Vet Res. (2004) 65:1479–82. doi: 10.2460/ajvr.2004.65.1479
7. Sinclair MD, Mealey KL, Matthews NS, Peck KE, Taylor TS, Bennett BS. Comparative pharmacokinetics of meloxicam in clinically normal horses and donkeys. Am J Vet Res. (2006) 67:1082–5. doi: 10.2460/ajvr.67.6.1082
8. Matthews NS, Peck KE, Mealey KL, Taylor TS, Ray AC. Pharmacokinetics and cardiopulmonary effects of guaifenesin in donkeys. J Vet Pharmacol Ther. (1997) 20:442–6. doi: 10.1046/j.1365-2885.1997.00081.x
9. Lizarraga I, Sumano H, Brumbaugh G. Pharmacological and pharmacokinetic differences between donkeys and horses. Equine Vet Educ. (2004) 16:102–12. doi: 10.1111/j.2042-3292.2004.tb00275.x
10. Mendoza FJ, Perez-Ecija A, Toribio RE. Clinical pharmacology in donkeys and mules. Vet Clin North Am Eq Pract. (2019) 35:589–606. doi: 10.1016/j.cveq.2019.08.011
11. Chapuis RJJ, Smith JS, French HM, Toka FN, Peterson EW, Little EL. Nonlinear mixed- effect pharmacokinetic modeling and distribution of doxycycline in healthy female donkeys after multiple intragastric dosing–preliminary investigation. Animals. (2021) 11:2047. doi: 10.3390/ani11072047
12. KuKanich B, Wiese A. Opioids. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA. Veterinary anesthesia and analgesia: The Fifth Edition of Lumb and Jones. Ames, IA: Wiley-Blackwell. (2015). p. 220–1.
13. Kalpravidh M, Lumb WV, Wright M, Heath RB. Analgesic effects of butorphanol in horses: dose-response studies. Am J Vet Res. (1984) 45:211–6. doi: 10.1111/j.1365-2885.2012.01385.x
14. Sellon DC, Monroe VL, Roberts MC, Papich MG. Pharmacokinetics and adverse effects of butorphanol administered by single intravenous injection or continuous intravenous infusion in horses. Am J Vet Res. (2001) 62:183–9. doi: 10.2460/ajvr.2001.62.183
15. Sellon DC, Papich MG, Palmer L, Remund B. Pharmacokinetics of butorphanol in horsesafter intramuscular injection. J Vet Pharmacol Ther. (2009) 32:62–5. doi: 10.1111/j.1365-2885.2008.01004.x
16. Chiavaccini L, Claude AK, Lee JH, Ross MK, Meyer RE, Langston VC. Pharmacokinetics and pharmacodynamics comparison between subcutaneous and intravenous butorphanol administration in horses. J Vet Pharmacol Ther. (2015) 38:365–74. doi: 10.1111/jvp.12191
17. Arguedas MG, Hines MT, Papich MG, Farnsworth KD, Sellon DC. Pharmacokinetics of butorphanol and evaluation of physiologic and behavioral effects after intravenous and intramuscular administration to neonatal foals. J Vet Intern Med. (2008) 22:1417–26. doi: 10.1111/j.1939-1676.2008.0200.x
18. Paine SW, Bright J, Scarth JP, Hincks PR, Pearce CM, Hannan C, et al. The intravenous pharmacokinetics of butorphanol and detomidine dosed in combination compared with individual dose administrations to exercised horses. J Vet Pharmacol Ther. (2020) 43:162–70. doi: 10.1111/jvp.12838
19. Joubert KE, Briggs P, Gerber D, Gottschalk RG. The sedative and analgesic effects of detomidine-butorphanol and detomidine alone in donkeys. J S Afr Vet Assoc. (1999) 70:112–8. doi: 10.4102/jsava.v70i3.769
20. Lizarraga I, Castillo-Alcala F. Sedative and mechanical hypoalgesic effects of butorphanol in xylazine-premedicated donkeys. Equine Vet J. (2015) 47:308–12. doi: 10.1111/evj.12274
21. Matthews NS, Taylor TS, Hartsfield SM, Williams JD. A comparison of injectable anaesthetic regimens in Mammoth asses. Equine Vet J Suppl. (1992) 11:37–40. doi: 10.1111/j.2042-3306.1992.tb04770.x
22. Matthews NS, Taylor TS, Skrobarcek CL, Williams JD. A comparison of injectable anaesthetic regimens in mules. Equine Vet J Suppl. (1992) 1:34–6. doi: 10.1111/j.2042-3306.1992.tb04769.x
23. Robertson JT, Muir WW, Sams R. Cardiopulmonary effects of butorphanol tartrate in horses. Am J Vet Res. (1981) 42:41–4.
24. Yarbrough J, Greenacre C, Cox S. Determination of butorphanol using high performance liquid chromatography in small volume plasma samples. J Liq Chromatogr Relat Technol. (2014) 37:1270–7. doi: 10.1080/10826076.2013.789798
25. Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. (1978) 6:165–75. doi: 10.1007/BF01117450
26. Toutain PL, Bousquet-Melou A. Plasma clearance. J Vet Pharmacol Ther. (2004) 27:415–25. doi: 10.1111/j.1365-2885.2004.00605.x
27. Smith JS, Marmulak TL, Angelos JA, Lin Z, Rowe JD, Carlson JL, et al. Pharmacokinetic parameters and estimated milk withdrawal intervals for domestic goats (Capra Aegagrus Hircus) after administration of single and multiple intravenous and subcutaneous doses of flunixin meglumine. Front Vet Sci. (2020) 7:213. doi: 10.3389/fvets.2020.00213
28. Smith JS, Coetzee JF, Fisher IWG, Borts DJ, Mochel JP. Pharmacokinetics of fentanyl citrate and norfentanyl in Holstein calves and effect of analytical performances on fentanyl parameter estimation. J Vet Pharmacol Ther. (2018) 41:555–61. doi: 10.1111/jvp.12501
29. Price G, Patel DA. Drug Bioavailability. StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
30. Gordon E, Dirikolu L, Liu CC, Ulanov AV, Li Z, Welborn M, et al. Pharmacokinetic profiles of three dose rates ofmorphine sulfate following single intravenous, intramuscular, and subcutaneous administration in the goat. J Vet Pharmacol Ther. (2022) 45:107–16. doi: 10.1111/jvp.13011
31. Uhrig SR, Papich MG, KuKanich B, Mama KR, Wagner AE, Chapman PL, et al. Pharmacokinetics and pharmacodynamics of morphine in llamas. Am J Vet Res. (2007) 68:25–34. doi: 10.2460/ajvr.68.1.25
32. Barnhart MD, Hubbell JA, Muir WW, Sams RA, Bednarski RM. Pharmacokinetics, pharmacodynamics, and analgesic effects of morphine after rectal, intramuscular, and intravenous administration in dogs. Am J Vet Res. (2000) 61:24–8. doi: 10.2460/ajvr.2000.61.24
33. Riviere JE, Chittenden J. Study design and data analysis. In: Riviere JE, editor. Comparative Pharmacokinetics. 2 ed. Ames, IA: Wiley Blackwell (2011). p. 311.
34. Bon C, Toutain PL, Concordet D, Gehring R, Martin-Jimenez T, Smith J, et al. Mathematical modeling and simulation in animal health. Part III: Using nonlinear mixed-effects to characterize and quantify variability in drug pharmacokinetics. J Vet Pharmacol Ther. (2018) 41:171–83. doi: 10.1111/jvp.12473
Keywords: butorphanol, opioid, Equus asinus L. (donkey), pharmacokinetics, donkey
Citation: Ebner L, O O, Simon B, Lizarraga I, Smith J and Cox S (2022) Pharmacokinetics of butorphanol following intravenous and intramuscular administration in donkeys: A preliminary study. Front. Vet. Sci. 9:979794. doi: 10.3389/fvets.2022.979794
Received: 27 June 2022; Accepted: 11 August 2022;
Published: 23 September 2022.
Edited by:
Benito Soto-Blanco, Federal University of Minas Gerais, BrazilReviewed by:
Cengiz Gokbulut, Balıkesir University, TurkeyCopyright © 2022 Ebner, O, Simon, Lizarraga, Smith and Cox. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa Ebner, bGlzYS5lYm5lckBsbXVuZXQuZWR1
†Present Address: Ignacio Lizarraga, AgResearch Limited, Grasslands Research Centre, Palmerston North, New Zealand
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.