- 1San Marco Veterinary Clinic and Laboratory, Padua, Italy
- 2Departament de Medicina i Cirurgia Animal, Facultat de Veterinària, Universitat Autònoma de Barcelona, Barcelona, Spain
- 3Department of Statistical Sciences, University of Padova, Padua, Italy
In Europe, Cytauxzoon spp. infection was documented in domestic and wild felids. Cats often develop a subclinical infection, while fatal disease is rare. Currently, information on the epidemiology, risk factors and clinicopathological findings of Cytauxzoon spp. infection remains limited and obtained by a single subject or small groups of cats. The objective of this case-control study was to evaluate clinicopathological findings and to describe risk factors associated with Cytauxzoon spp. infection in domestic cats. Infected cats (n = 39) and non-infected (n = 190) cats were selected from the database of the referral San Marco Veterinary Laboratory between 2008 and 2021. Demographic information, a preset questionnaire considering lifestyle, environment, and clinical status, and a CBC performed contextually with the PCR analysis were recorded for all cats. Data on the biochemical profile and serum protein electrophoresis were also evaluated when available. Compared to the control group, infection was more likely to occur in stray cats (24/39, 61.5%, P < 0.001), living totally/partially outdoors (36/39, 92.3%, P < 0.001), in an urban context (37/39, 94.9%, P = 0.002), taken or recently adopted from colonies (34/35, 97.1, P < 0.001), with irregular or absent parasite preventive treatments (39/39, 100%, p = 0.005), without fleas (28/35, 80%, P = 0.047) and without clinical signs (22/39, 56.4%, p = 0.026) at the time of medical evaluation. Anemia was not associated with infection, but in cats without clinical signs, the percentage of anemic-infected cats (7/22, 31.8%, P = 0.009) was higher compared to non-infected cats (5/65, 7.7%). Furthermore, a decrease in total iron serum concentration approximating the lowest reference interval [median values (IQR): 79 μg/dL (52.25) vs. 50.5 μg/dL (34), P = 0.007] was likely in infected cats. No other laboratory findings were associated with infection. Interestingly, a partial/total outdoor lifestyle was a risk factor for infection (OR: 8.58, 95% CI: 2.90–37.0, P < 0.001). In conclusion, the present study revealed that Cytauxzoon spp. infection manifests itself prevalently as a subclinical infection, based on physical examination and laboratory findings, in domestic European cats. However, subclinical infected cats were more likely to be anemic compared to non-infected.
Introduction
Cytauxzoonosis is a protozoan tick-transmitted disease that affects wild and domestic felids, traditionally caused by Cytauxzoon felis. It is particularly reported in central, south-eastern, and south-central USA (1, 2), although it was described in other parts of the world such as Brazil (3), Iran (4), and China (5). Domestic cats generally develop a severe disease that rapidly progresses to death (6) while wild felids are mostly affected by a subclinical infection that rarely has a fatal evolution (7). However, sick cats that survive C. felis infection (8, 9) and subclinical infected (10–13) have been increasingly documented.
In Europe, a piroplasm named Cytauxzoon spp. has been reported in both domestic felids (14–16) and wild felids (17–20). The protozoan was molecularly similar, but not superimposable, to C. felis (14). The first focus of infection was found in cats living in Trieste in northeastern Italy with a prevalence of 23% (14). Later, Cytauxzoon spp. infection has been reported in cats from Portugal (21), France (22), Spain (16), Switzerland (23, 24), Germany (25), and Russia (26). Although sometimes lethal (14, 21, 25), subclinical infection appeared to be more likely and frequent in domestic felines (14, 22, 23). Recently, three Cytauxzoon spp. genotypes indicated as Cytauxzoon europeus, Cytauxzoon otrantorum, and Cytauxzoon banethi were described in European wild cat (Felis silvestris) based on 18S rRNA, mitochondrial cytochrome b (CytB) and cytochrome c oxidase subunit I (COI) genes (27). In addition, domestic cats infected with C. europeus were documented in Switzerland (24). The available information on the epidemiology and clinicopathological findings of Cytauxzoon spp. infection in Europe is currently limited and often based on the evaluation of a single subject or of small groups (21–23, 28).
Given this context, the case-control study reported here was aimed at (1) evaluating clinicopathological findings and (2) describing risk factors associated with Cytauxzoon spp. infection in domestic cats in Italy.
Materials and methods
Study design and setting
This case-control study compared cats with and without Cytauxzoon spp. infection. Data were extracted from the database of PCR analysis performed at the San Marco Veterinary Laboratory (SMVL) referred from May 2008 to July 2021. The source database included the records of blood molecular analysis of Cytauxzoon spp. obtained from (1) cats published in previous works (14, 15) (n = 120) and (2) cats collected by clinicians distributed throughout Italy during routine veterinary procedures or illnesses, with or without suspected Cytauxzoon spp. infection (n = 526). The SMVL provides services to veterinary clinics spread throughout Italy. Approximately 1,400 molecular analyzes per year are performed in feline samples at SMVL.
A focus of Cytauxzoon spp. (14) was previously described in Trieste (45°39′01″ N, 13°46′13″ E), a seaport city in northeastern Italy. It is located in the northern part of the high Adriatic Sea near the border with Slovenia. It is characterized by a long coastline bordered by grasslands, forests, and karstic areas (https://en.wikipedia.org/wiki/Trieste). Ticks (29) and wild felids such as Eurasian Lynx (Lynx lynx) and the wild cat (Felis silvestris) (20) are reported in the region named Friuli Venezia Giulia, where Trieste is located.
Patient selection, inclusion criteria, sampling, questionnaire, and variable characteristics
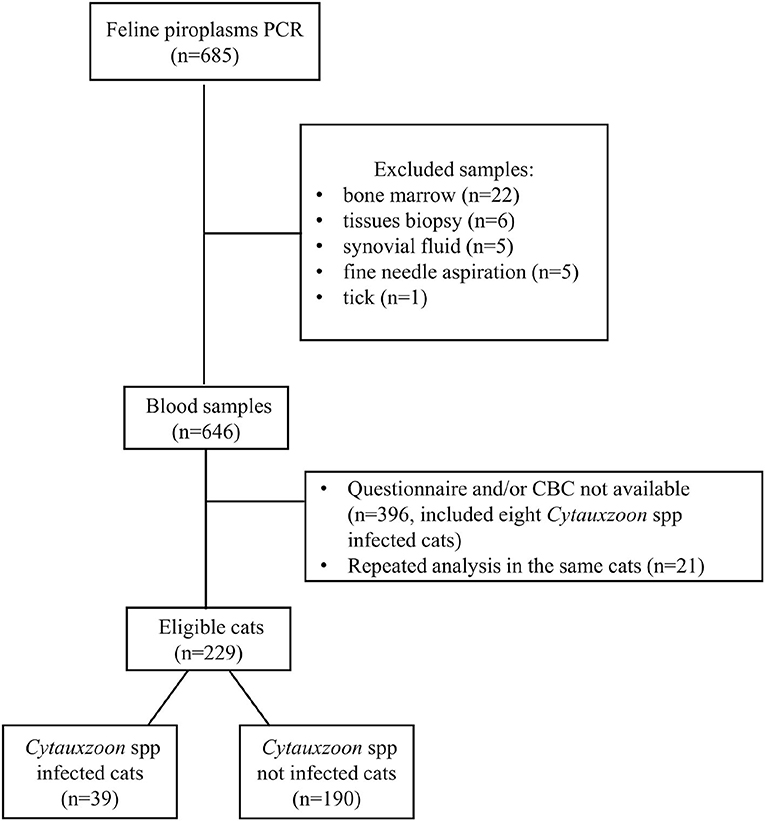
The selection of cats included in the present study is shown in Figure 1. The inclusion criteria were the following: (1) a blood piroplasm PCR analysis performed on the 18S rRNA gene, (2) a preset questionnaire, and (3) a CBC performed contextually to the PCR analysis.
Therefore, according to the inclusion criteria described above, the studied data set included random healthy/unhealthy, stray/owned (1) cats living in the Trieste focus (n = 90) (14, 28), and (2) cats (n = 139) living in the north (n = 112), in the center (n = 20), and in the south (n = 7) of Italy.
Based on the results of the PCR analysis, eligible cats were assigned to Cytauxzoon spp. infected (case) or not infected (control) groups. For the purposes of the case definition, 18S rRNA gene sequencing was performed randomly for the identification of piroplasm species in cats living in Trieste (14, 28), while it was considered mandatory for the others. The unmatched control group involved cats enrolled between 2008 and 2011, while cases were obtained throughout the study period.
Blood samples were taken from stray cats that lived in urban Trieste colonies as part of spay/neuter programs and from owned cats that underwent routine blood screening for various medical reasons.
The clinicians completed a multiple choice questionnaire at the same time the blood sample was collected. The questionnaire concerned patient characteristics (age, sex, breed), type of cat (owned or stray), lifestyle (indoor or partially/total outdoor), environment (rural/city), type of housing (taken/recently adopted from colony or cattery/household), parasite preventive treatments (regular or irregular/absent), ticks and/or flea presence and detection of clinical signs at the time of medical examination. The results of the questionnaire were already available on the cats observed between 2008 and 2011. The questionnaire of infected cats diagnosed subsequently was retrieved through a telephone call from one of the authors (EC). Only questionnaires based on clinical records collected by clinicians at the time of sampling were considered suitable.
When available, the biochemical profile and serum protein electrophoresis performed at the same time as the other assays were also included.
All samples were collected solely for the cat's health benefit.
When the age of the cats was not available, the clinicians provided an estimated age value. For this reason, this variable was not considered reliable for statistical analysis.
Cats were also stratified according to their geographical origin (north, central, or southern Italy) and the focus of Trieste. The dichotomic variable “anemia” was created using the red blood cell (RBC) concentration value of 6.35 × 106/μL (lower limit of the reference interval of SVML) rather than using hematocrit (Hct) as discriminator, to avoid possible incorrect classification due to storage artifacts (30).
The analytic methods
All clinicopathological tests were performed at the SMVL within 24–36 h after collection. The CBC was performed on K3EDTA whole blood samples using automatic cell counters (ADVIA® 120, ADVIA® 2120, and ADVIA® 2120i Hematology System, Siemens Healthineers GmbH, Erlangen, Germany) and was always accompanied by a microscopic evaluation of the blood smear. The biochemical profile was assessed on serum samples using an automated analyzer (Olympus AU2700, Olympus Diagnostic, Hamburg, Germany and Atellica CH930, Siemens Healthineers, GmbH, Erlangen, Germany). Serum protein electrophoresis was performed using Capillarys 1 and Capillarys 2 (Sebia, Florence, Italy).
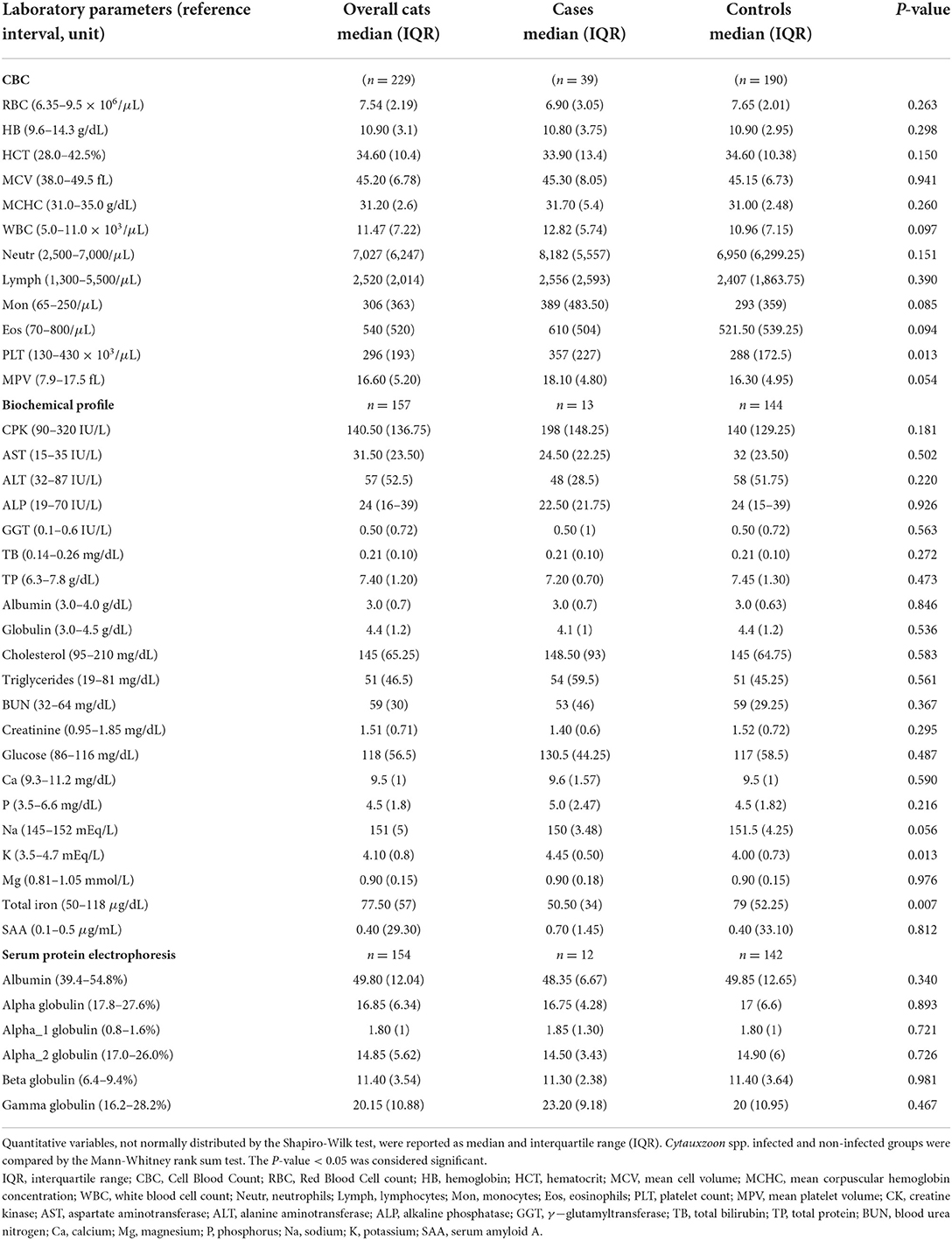
Laboratory parameters evaluated included the following: RBC, hemoglobin, Hct, mean cell volume, mean corpuscular hemoglobin concentration, white blood cell concentration, neutrophils, lymphocytes, monocytes, eosinophils, platelet count (PLT), mean platelet volume, creatine kinase, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, γ−glutamyltransferase, total bilirubin, total protein, albumin, globulin, cholesterol, triglycerides, blood urea nitrogen, creatinine, glucose, calcium, magnesium, phosphorus, sodium, potassium, total iron (Fe), serum amyloid A, albumin (by serum protein electrophoresis), α-globulin, α1-globulin, α2-globulin, β-globulin, and γ-globulin.
Molecular analysis, sequencing, and phylogenetic analysis
The molecular investigation was performed by (1) panpiroplasm end-point PCR, followed by sequencing of amplicons for species identification, carried out throughout the study period and used for the detection of Cytauxzoon spp. with a diagnostic purpose, (2) a phylogenetic analysis evaluated on the sequences of the nearly complete 18S rRNA of the stored samples still available at the time of the conceptualization of the study.
DNA was obtained from 200 μL of blood from each cat using the High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). Blood was incubated with 40 μL of proteinase K and 200 μL of binding buffer at 72°C for 1 h. Subsequent steps were performed according to the manufacturer's instructions. DNA was eluted in 50 μL of elution buffer. Molecular detection of piroplasms was carried out using a conventional PCR assay using the sense primer PIROA (5′-AATACCCAATCCTGACACAGGG-3′) and the antisense primer PIROB (5′-TTAAATACGAATGCCCCCAAC-3′) that amplify a fragment of ~412 bp of the 18S rRNA gene (31). PCR reactions were carried out in a final volume of 25 μL containing 2.5 μL of DNA template, 0.4 μM of each primer, 1.8 mM of MgCl2, 200 μM of each dNTP, 2.5 U of FastStart High Fidelity Enzyme, and 1X of FastStart High Fidelity Reaction Buffer (Roche Diagnostics GmbH, Mannheim, Germany). The thermal cycling conditions consisted of an initial denaturation at 95°C for 2 min, 35 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s and extension at 72°C for 30 s, followed by a final extension step at 72°C for 5 min. Positive controls and negative controls (water) of the PCR were included in each run. All reactions were performed using an automated thermal cycler (Applied Biosystems 2720, Carlsbad, CA, USA). The amplicons were visualized by UV transillumination after electrophoresis of 5 μL of the reaction solution in a 2.2% agarose FlashGel DNA cassette (Lonza, Rockland, ME, USA). All PCR products containing expected size amplicons were submitted to an external laboratory for direct sequencing on both strands (BMR Genomics srl, Padua, Italy). The nucleotide sequences obtained were compared with those available in the GenBank database using the BLAST program (32) for species identification.
To perform phylogenetic analysis, Cytauxzoon spp. positive DNA extracts from −20°C stored still available samples were subjected to amplification and sequencing of the nearly complete 18S rRNA gene as previously described (33). The contigs were assembled and edited using BioEdit 7.2.5 software, and the sequences obtained were aligned with a set of 34 relevant corresponding sequences of Cytauxzoon spp. longer than 1,000 bp retrieved from GenBank. Alignment was carried out using the MUSCLE algorithm (34) implemented in MEGA version 11 (35) and a maximum likelihood (ML) tree was inferred with the same software. Gaps or missing data in the alignment were deleted and the substitution model with the lowest Bayesian information criterion (BIC), calculated using the specific function of MEGA 11, was selected. The reliability of the tree was evaluated by performing 1,000 bootstrap replicates.
The nucleotide sequences obtained in this study were submitted to the GenBank database under accession numbers OM004051-OM004057.
Statistical analysis
Statistical analysis was performed using R for MacOS (version 4.1.0). After rejecting the normality assumption for quantitative variables with the Shapiro-Wilk test, quantitative data were expressed as medians and interquartile ranges (IQR). Categorical variables were expressed as counts and percentages in each category. The differences between the infected Cytauxzoon spp. and the control groups were analyzed in bivariate analysis with the Mann-Whitney rank sum for quantitative variables and with Pearson's chi-square test with Yates continuity correction or Fisher's exact test with categorical variables.
Two sub datasets that included subclinical and sick cats, respectively, were created to better assess the role of the anemic status during infection. Categoric variable anemia was analyzed referring to the presence/absence of Cytauxzoon spp. in each sub dataset.
The percentages of dichotomous variables such as sex, breed, type of cat, lifestyle, environment, ticks, fleas, clinical signs, and anemia that occur in the infected cat group were analyzed using the one sample proportion test considering a true proportion not equal to 0.5 as an alternative hypothesis. For each estimated proportion, the 95% confidence interval (95% CI) was calculated to provide a range of values that is likely to include the proportion of the real population of each variable.
To identify the risk factors for Cytauxzoon spp. infection, a multivariable logistic regression model was estimated, after backward variables selection. The presence of anemia was included in the model due to its clinical relevance during infection. The results of the fitted logistic regression were expressed as odds ratio (OR), 95% CI and p-value.
The statistical significance was declared for P < 0.05.
Results
Case and control cats
During the evaluation period, piroplasm PCR analysis was performed in 646 feline blood samples (Figure 1), with a diagnosis of Cytauxzoon spp. infection in 47 cats (7.3%).
Four hundred and seventeen cats were excluded for various reasons. Finally, 229 cats met the inclusion criteria and, according to the PCR results, were divided into case (infected, n = 39) and control (non-infected, n = 190) groups (Figure 1).
Cytauxzoon spp. infected cats were detected in the northeast (n = 32, 82.1%) and in the center (n = 7, 17.9%) of Italy (Figure 2).
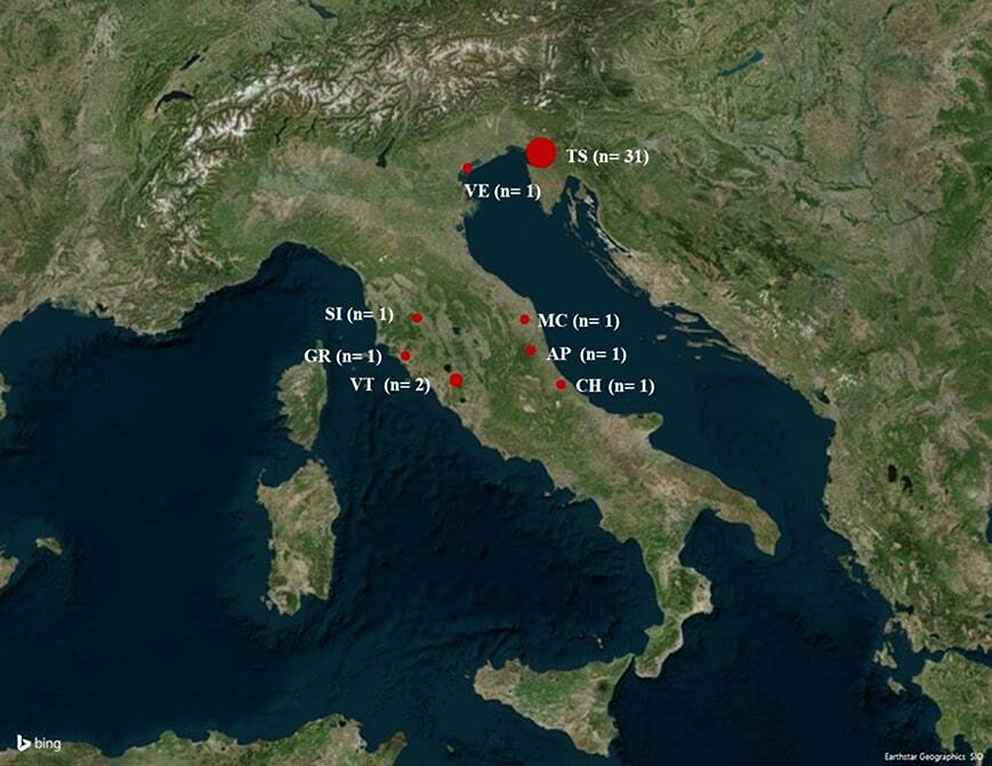
Figure 2. Map of Italy showing the cities where Cytauxzoon spp. infected cats were diagnosed. The map was obtained by Microsoft 3D Maps in Excel 2016 software. AP, Ascoli Piceno; CH, Chieti; GR, Grosseto; MC, Macerata; SI, Siena; TS, Trieste; VE, Venezia; VT, Viterbo.
The demographic information and clinical characteristics of the case and control groups are summarized in Table 1. The cats infected with Cytauxzoon spp. were predominantly female (25/39, 64.1%) and mixed breed (37/39, 94.9%) with a median age (IQR) of 24 months (60). Compared to the control group, infection was more likely to occur in stray cats, living partially/total outdoors, in an urban context, taken or recently adopted from colonies, with irregular or absent parasite preventive treatments, without fleas and without clinical signs at the time of the medical evaluation. By the one sample proportional test the Cytauxzoon spp. infected cat population was likely represented by high percentages of mixed breed cats (94.9%, 95% CI: 81.4–99.1%, P < 0.001) that live prevalently or totally outdoors (92.3%, 95% CI: 78–98%, P < 0.001) in urban areas (94.9%, 95% CI: 81.4–99.1%, P < 0.001), with no presence of flea (80%, 95% CI: 62.5–90.9%, P < 0.001) and ticks (82.9%, 95% CI: 65.7–92.8%, P < 0.001) at the time of clinical examination (Figure 3).
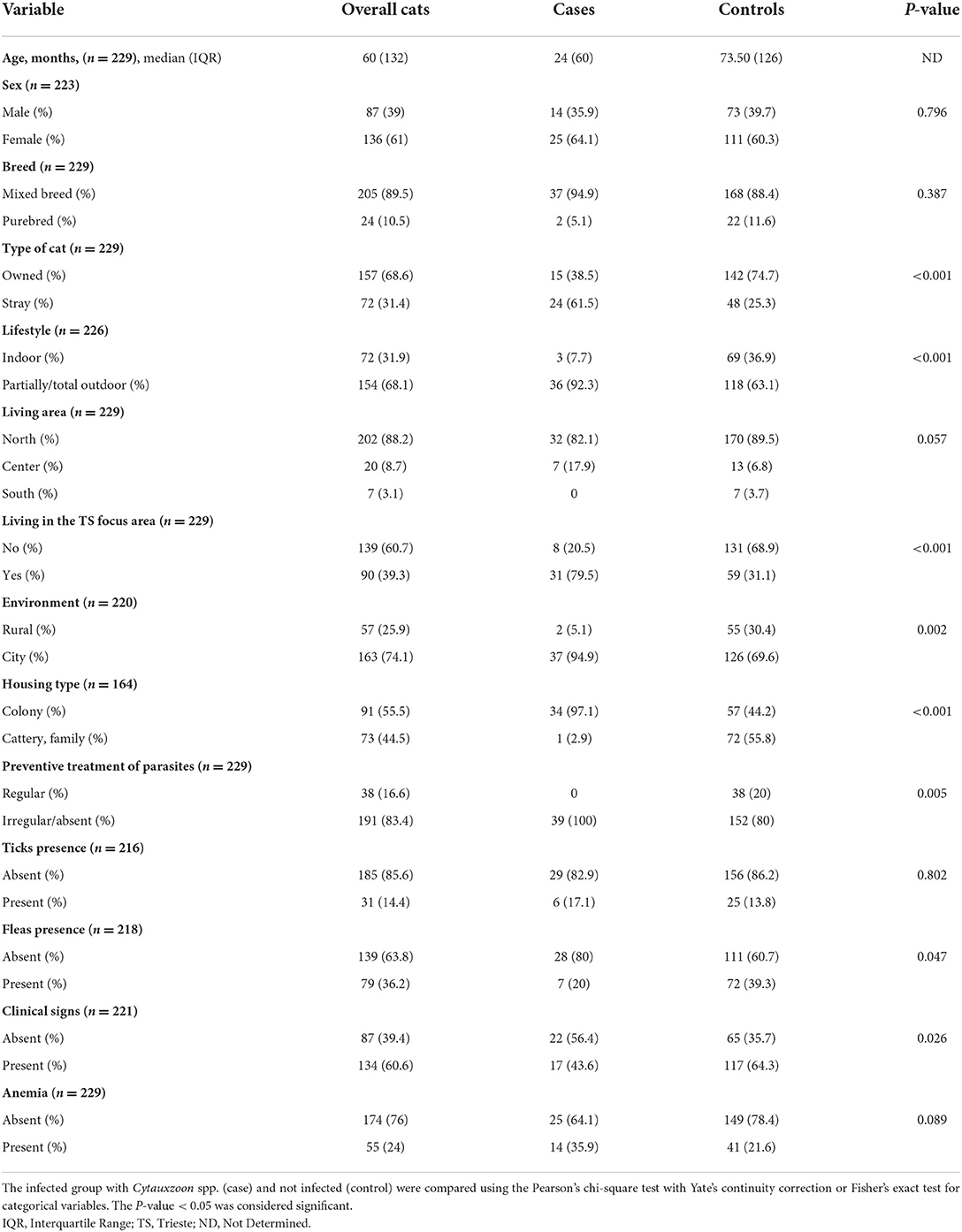
Table 1. Characteristics of patients at baseline in the total study population and in the case and control groups.
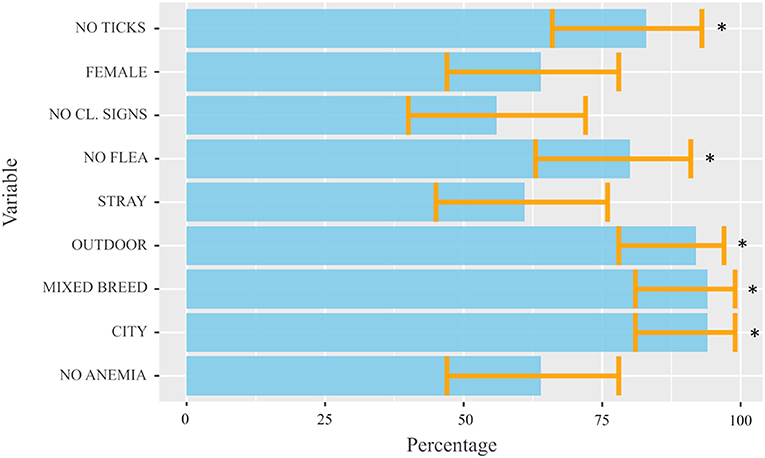
Figure 3. Estimated proportions for each dichotomous variable obtained by the one-sample proportional test from cats infected with Cytauxzoon spp. included in the study. The light blue bars represent the estimated proportions for each dichotomous variable obtained by the one-sample proportional test, considering a true proportion not equal to 0.5 as an alternative hypothesis. The orange lines (error bars) represent the width of the 95% IC calculated for each variable and provide interval estimations (and the uncertainty of the measurement) for the real population proportion of each variable. The P-value < 0.05 was considered significant and was indicated by *. CL, clinical.
Anemia was present in 14/39 (35.9%) of infected cats and was not associated with infection compared to the control group (41/149, 21.6%, P = 0.089). When the sub dataset of cats without clinical signs was analyzed at the time of diagnosis (n = 87), the percentage of anemia was likely higher in cats infected with Cytauxzoon spp. compared to those not infected (7/22, 31.8% infected vs. 5/65, 7.7% not infected, P = 0.009). In the subgroup of cats that presented clinical signs at the time of diagnosis (n = 134), anemia was not associated with infection (7/17, 41.2% infected vs. 36/117, 30.8% not infected, P = 0.401). The clinical signs observed by clinicians for cats infected with Cytauxzoon spp. were a non-specific (i.e., neurological signs, slimming, diarrhea, stomatitis, dermatitis, among others).
Laboratory findings are shown in Table 2. Compared to the control group, cats infected with Cytauxzoon spp. showed a higher platelet concentration [median values (IQR): 357 × 103/μL (227) vs. 288 × 103/μL (172.5), P = 0.013] and a lower total iron [median values (IQR): 50.5 μg/dL (34) vs. 79 μg/dL (52.25), P = 0.007]. No other statistical differences were observed with respect to laboratory findings.
In the multivariable analysis Cytauxzoon spp. infection was strongly associated with a partially/total outdoor lifestyle (OR: 8.58, 95% CI: 2.9–37, P < 0.001) and living in urban areas (OR: 11.9, 95% CI: 3.38–76.7, P = 0.001). The categorical variable anemia included in the logistic model has shown the following OR: 2.22, 95% IC: 0.97–5.05, P = 0.056. The AUC (area under the curve) of the model was 0.78 (95% IC: 0.71–0.85).
Sequencing and phylogenetic analysis
The DNA amplicon sequences obtained for diagnostic purposes based on pan-piroplasm end point PCR (31, 33) of cats living in the Trieste focus (n = 13) and in other parts of Italy (n = 8) showed a nucleotide identity >99% with reference sequences from Cytauxzoon spp. available in GenBank.
Sequencing of the nearly complete 18S rRNA gene was successful in seven cases.
The ML phylogenetic tree (Figure 4) was estimated at 979 sites, due to the complete deletion of gaps and missing data in the alignment; the Tamura 3-parameter (35) nucleotide substitution model with discrete gamma distribution showed the lowest BIC and was selected.
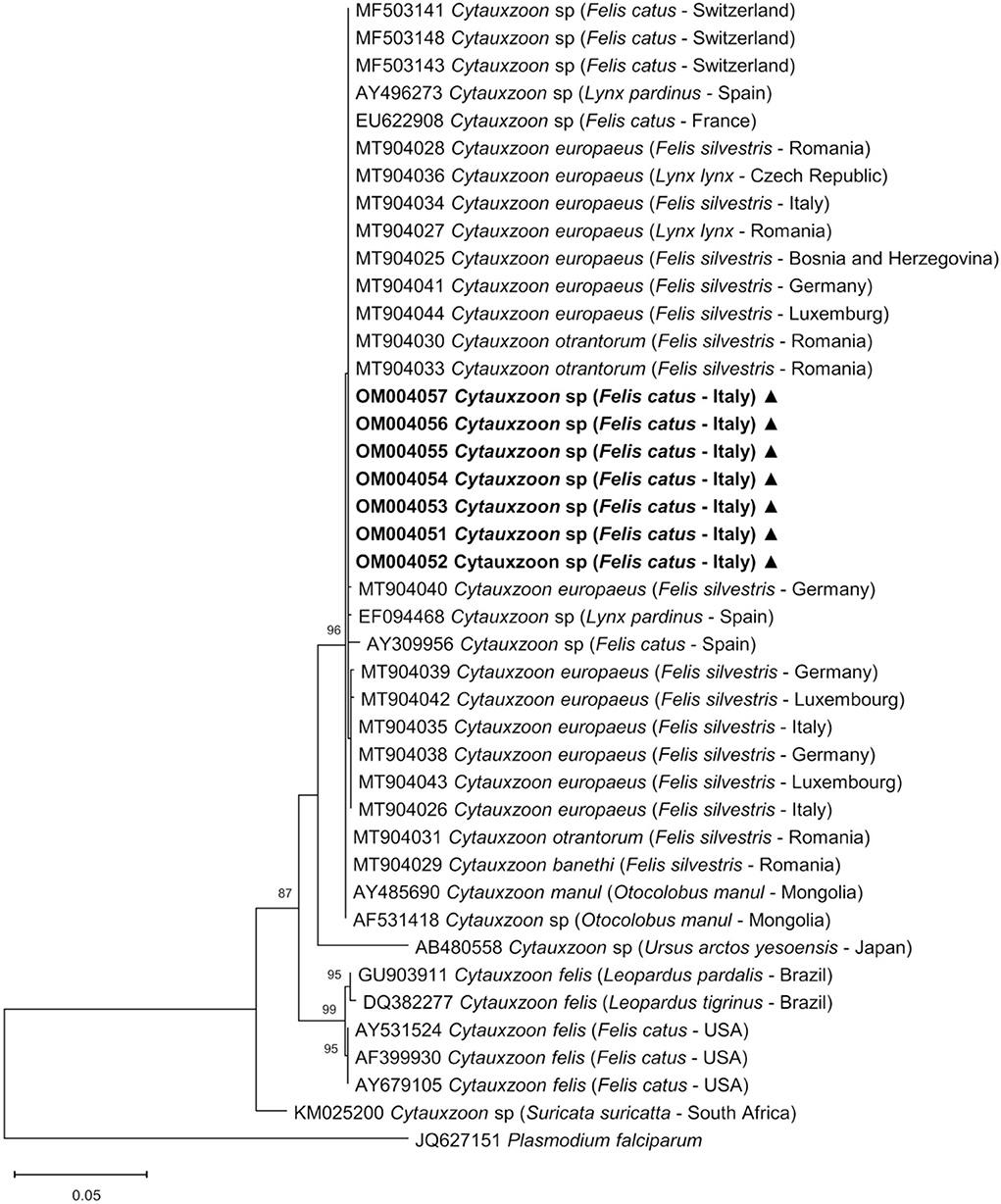
Figure 4. Phylogenetic relationship of Cytauxzoon spp. inferred from an alignment of 979 sites of the 18S rRNA gene. The maximum likelihood nucleotide tree inferred from an alignment of 979 sites of Cytauxzoon spp. 18S rRNA gene sequences using the Tamura 3-parameter substitution model with discrete gamma distribution. The GenBank accession number, species, host, and country of origin are included for each Cytauxzoon spp. sequence. The nucleotide sequences generated in this study are in bold and marked with a triangle. Plasmodium falciparum was included in the analysis as an out group. Bootstrap values <70% are indicated in the respective branches.
Phylogenetic analysis showed that the seven 18S rRNA piroplasmid gene sequences obtained in this study grouped with other European Cytauxzoon spp. from domestic and wild felids and with two sequences of C. manul detected in Pallas' cats from Mongolia, while C. felis sequences from North and South America grouped together in a clearly distinct cluster.
Discussion
To the best of the authors' knowledge, this is the first case-control study and, until now, the most substantial work on demographic and clinical data on Cytauxzoon spp. infection in domestic cats carried out in Europe. In fact, apart from the first description of the Trieste focus (14) and a recent paper mainly focused on the epidemiology and genetics of the parasite (24), most of the papers available in domestic cats are single case reports (21, 22, 25) or descriptions of a small group of infected subjects (16, 23, 28, 36).
Although in the present study a wide range of hematological and biochemical parameters were analyzed, few laboratory findings were statistically associated with Cytauxozoon spp. infection, with values not exceeding the reference interval. Infection appears more likely in cats without clinical signs and with alterations in few or absent laboratory parameters at the time of diagnosis. Therefore, this study supports, from a laboratory perspective, the idea that Cytauxzoon spp. infection in Italy is often subclinical (14, 28), as is occasionally the case for infection with C. felis in the USA (10, 11, 37, 38).
At a significance level of 0.05, anemia does not appear to be associated with Cytauxzoon spp. However, it should be noted that the P-values obtained for this categorical variable are borderline for significance by both the Pearson chi-square test and the multivariable logistic regression. Furthermore, while for the most part non-anemic, subclinical infected cats showed a significantly higher percentage of anemia compared to those not infected. It could be reasonable that in apparently healthy infected cats, parasitized erythrocytes are removed from the bloodstream or hemolyzed, giving rise to a variable rate of reduction of RBC that is not necessarily so marked that it evolves in an anemic state. Furthermore, considering the low concentration of iron associated with this infection, the mechanism of anemia of inflammatory disease (39) could be another explanation for our findings. It is important to note that anemia needs to be further investigated, by adding more subjects to the sample and evaluating the presence/absence of regeneration, to confirm and understand its real role and the mechanisms involved during Cytauxzoon spp. infection in both subclinical and sick cats.
Although many biochemical parameters were evaluated in the present study, even if just above the minimum value, only the lower iron concentration observed in the case group seems to be clinically relevant compared to the control group. Hypoferremia could be caused by a shift of iron to the storage site (e.g., inflammation disease) while other factors, such as the young age of the animals or Fe deficiency (39) appeared to be less likely. It is important to note that the biochemical and serum electrophoresis parameters were obtained from a lower number of cats compared to the CBC parameters. Data from more subjects might be needed to find mild differences between infected and uninfected cats.
In the present study, living partially or completely outdoors was strongly associated with Cytauxzoon spp. infection. Additionally, infection was more likely in stray cats. These results agree with previous works on both Cytauxzoon spp. (14, 16, 28) and C. felis (4, 40). They suggest that this lifestyle is a risk factor for Cytauxzoon spp. infection. Outdoor cats could be exposed to the sources of infection traditionally reported for C. felis as the tick vectors (6, 41) and wild felids as reservoirs (42). However, to our knowledge, the highly competent vectors Amblyomma Americanum (41, 43) and Dermacentor variabilis (44) that experimentally transmitted C. felis are not found in Europe. Furthermore, Cytauxzoon spp. has not yet been isolated from ticks collected in European countries (36, 45). However, Cytauxzoon spp. was isolated from European wild cats (Felis silvestris) in Italy (20), Romania (19), Bosnia and Herzegovina (46), and France (24) as well as in the Eurasian lynx (Lynx lynx) in Romania (19) and in the Iberian lynx (Lynx pardinus) in Spain (17, 18). Domestic cats and wild cats or European lynx are considered to live in sympatry, inbreed, and share pathogens (20, 47). Consistent with previous findings, the infected cats reported in the present work lived interestingly in the north-east and central Italy in areas almost overlapping those in which Cytauxzoon spp. was isolated from wild cats (20). These findings reinforce the possible role of the wild felid as a reservoir for this infection in Italy.
However, the frequent subclinical Cytauxzoon spp. infection described in the present work is consistent with previous studies (6, 9, 12, 14, 40) and contributes to assigning to apparently healthy infected cats the role of “long-life domestic reservoir” for this parasite. This role appears to be more important than the one played by wild felids, due to the greater possibility of contact between infected and non-infected domestic cats. Thus, Cytauxzoon spp. could be introduced more easily in a geographic area where it was not endemic, for example, during owner relocation (12) or after the adoption of an infected subject from a colony or a cattery. More studies are needed to determine the dynamics of the spillover of Cytauxzoon spp. from felines in sylvatic ecosystems to domestic cats in urban locations, the role of domestic infected cats as reservoirs, and if there is another potential competent vector for this infection.
Cats living in an urban area appeared to be strongly associated with Cytauxzoon spp. compared to other settings (rural, hilly, home). This result disagrees with previous papers reporting that C. felis and Cytauxzoon spp. are more likely in outdoor cats, especially in low-density residential or rural areas where contact with ticks and wild felids might be more frequent (16, 48). It is interesting to note that, in the present study, cats living in the colony, at the time of evaluation or recently adopted from it, were more likely to be infected by Cytauxzoon spp. than cats living in the cattery or family. As most of the colony cats were from the Trieste urban area focus (14, 28), a bias referring to the result of the urban environment could be suspected. Consequently, it is likely that our observations might mainly reflect the characteristics of a particular habitat as a colony rather than the context of the urban environment. In colonies, the interaction of cats with each other or with other animals (as wild felids) or a vector appears possibly more like a “natural” condition. This could lead to some reflections on the pathogenesis of Cytauxzoon spp. and could remark on the possible key role domestic cats play over wild cats as reservoirs of infection (9, 12, 14). Furthermore, based on feline behavior lifestyle in environments such as colonies (49), other means of transmission, rather than traditional ones involving vectors and wild felids, could be hypothesized to contribute to the spread of Cytauxzoon spp. infection in domestic cats. We suspected that the close co-existence between cats in colonies is characterized by friendly and agonistic behaviors and could create a favorable environment for horizontal transmission of the parasite by blood. In fact, infection and parasitemia, without clinical signs, were experimentally obtained by inoculating blood infected with C. manul from Pallas' cats (Otocolobus manul) to cats (50). Furthermore, since blood transfusions could represent a route of transmission, as a precaution, only cats negative for PCR were recommended as donors in endemic areas (23, 51, 52). Vertical transmission is another possible way for Cytauxoon spp. to spread in a colony setting where, despite spay/neuter control programs, mating continues to occur. However, vertical transmission has not been fully confirmed even if young cats infected with Cytauxzoon spp. (23) and C. felis (53) were reported. Future studies are needed to better understand the main ways of transmission of Cytauxzoon spp. in cats, especially when they live in a socially complex society as a colony.
A limitation of the present work is that we have not performed a more in-depth molecular study than the routine PCR analysis and sequencing used to make the diagnosis. A molecular analysis of the 18S rRNA gene was completely evaluated only in the most recent samples, and it was not possible to evaluate other genes such as CytB and COI, although it would be interesting to know if the C. europeus, C. otrantorum, and C. banethi subspecies (27) were present in Italy in domestic cats. Different genotypes could vary in virulence and geographical prevalence and could cause diseases with different pathogenicity and clinical course and different responses to treatment (6, 12, 24, 54). Future studies are necessary to deepen our understanding of the genotypes present in Italy, their biological behavior, and their role in the evolution of the disease. In addition, results of biochemical and serum electrophoresis were obtained a smaller number of cats than CBC. Even if our observations appear plausible and not anecdotal, data from more subjects must be considered to find mild differences between infected and not infected cats.
Conclusions
This study revealed that Cytauxzoon spp. commonly cause subclinical infections in domestic cats in Italy. In fact, no difference in clinical signs or laboratory findings was found in infected cats vs. non infected cats. Interestingly, although most of the cats were not anemic, the subclinical infected cats showed a significantly higher percentage of anemia compared to those not infected.
The infection was more likely to occur in stray cats living partially/completely outdoors, taken or recently adopted, from colonies, with irregular or absent parasite preventive treatments, without fleas and without clinical signs at the time of the medical evaluation.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author. The accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OM004051; https://www.ncbi.nlm.nih.gov/genbank/, OM004052; https://www.ncbi.nlm.nih.gov/genbank/, OM004053; https://www.ncbi.nlm.nih.gov/genbank/, OM004054; https://www.ncbi.nlm.nih.gov/genbank/, OM004055; https://www.ncbi.nlm.nih.gov/genbank/, OM004056; https://www.ncbi.nlm.nih.gov/genbank/, OM004057.
Ethics statement
Ethical review and approval and written informed consent from the owners of the animals was not required for the study on animals in accordance with the local legislation and institutional requirements.
Author contributions
EC: study design, sample collection, data analysis and interpretation, statistical analysis, and writing manuscript. LS-G: study design, data analysis and interpretation, and revised manuscript. SD: molecular biology and phylogenetic analysis and interpretation. LV: statistical analysis. EL: molecular biology sample processing. TF: data analysis and interpretation and revised manuscript. All authors contributed to the article and approved the submitted version.
Dedication
This paper is dedicated to the memory of Dr. Marco Caldin (1961–2022), a mentor and a great teacher to all of us.
Acknowledgments
The authors acknowledge the staff and the molecular biology workgroup of the San Marco Laboratory for technical support, and special mention goes to Dr. Michele Trotta. The authors are also grateful to Dr. Stefania Uccheddu for the feline behavioral medicine input and to all clinicians who kindly responded to the questionnaire.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Birkenheuer AJ, Le JA, Valenzisi AM, Tucker MD, Levy MG, Breitschwerdt EB. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998–2004). J Am Vet Med Assoc. (2006) 228:568–71. doi: 10.2460/javma.228.4.568
2. Meinkoth JH, Kocan AA. Feline cytauxzoonosis. Vet Clin North Am Small Anim Pract. (2005) 35:89–101. doi: 10.1016/j.cvsm.2004.08.003
3. Peixoto PV, Soares CO, Scofield A, Santiago CD, Fraņa TN, Barros SS. Fatal cytauxzoonosis in captive-reared lions in Brazil. Vet Parasitol. (2007) 145:383–7. doi: 10.1016/j.vetpar.2006.12.023
4. Moghaddam MR, Zaeemi M, Razmil GR. Preliminary study of Cytauxzoon felis infection in outdoor cats in Mashhad, Iran. Parasitol Res. (2020) 119:4177–83. doi: 10.1007/s00436-020-06780-7
5. Zou FC, Li Z, Yang JF, Chang JY, Liu GH, Lv Y, et al. Cytauxzoon felis infection in domestic cats, Yunnan province, China, 2016. Emerg Infect Dis. (2019) 25:353–4. doi: 10.3201/eid2502.181182
6. Reichard MV, Sanders TL, Weerarathne P, Meinkoth JH, Miller CA, Scimeca RC, et al. Cytauxzoonosis in North America. Pathogens. (2021) 10:1170. doi: 10.3390/pathogens10091170
7. Blouin EF, Kocan AA, Kocan KM, Hair J. Evidence of a limited schizogonous cycle for Cytauxzoon felis in bobcats following exposure to infected ticks. J Wildl Dis. (1987) 23:499–501. doi: 10.7589/0090-3558-23.3.499
8. Walker DB, Cowell RL. Survival of a domestic cat with naturally acquired cytauxzoonosis. J Am Vet Med Assoc. (1995) 206:1363–5.
9. Meinkoth JH, Kocan AA, Whitworth L, Murphy G, Fox JC, Woods JP. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997–1998). J Vet Intern Med. (2000) 14:521–5. doi: 10.1111/j.1939-1676.2000.tb02270.x
10. Haber MD, Tucker MD, Marr HS, Levy JK, Burgess J, Lappin MR, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol. (2007) 146:316–20. doi: 10.1016/j.vetpar.2007.02.029
11. Brown HM, Latimer KS, Erikson LE, Cashwell ME, Britt JO, Peterson DS. Detection of persistent Cytauxzoon felis infection by polymerase chain reaction in three asymptomatic domestic cats. J Vet Diagn Invest. (2008) 20:485–8. doi: 10.1177/104063870802000411
12. Rizzi TE, Reichard MV, Cohn LA, Birkenheuer AJ, Taylor JD, Meinkoth JH. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasit Vectors. (2015) 8:13. doi: 10.1186/s13071-014-0618-z
13. Nagamori Y, Slovak JE, Reichard MV. Prevalence of Cytauxzoon felis infection in healthy free-roaming cats in north-central Oklahoma and central Iowa. JFMS Open Rep. (2016) 2:2055116916655174. doi: 10.1177/2055116916655174
14. Carli E, Trotta M, Chinelli R, Drigo M, Sinigoi L, Tosolini P, et al. Cytauxzoon spp. infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol. (2012) 183:343–52. doi: 10.1016/j.vetpar.2011.07.025
15. Carli E, Trotta M, Bianchi E, Furlanello T, Caldin M, Pietrobelli M, et al. Cytauxzoon spp. infection in two free ranging young cats: clinicopathological findings, therapy and follow up. Turkiye Parazitol Derg. (2014) 38:185–9. doi: 10.5152/tpd.2014.3540
16. Díaz-Regañón D, Villaescusa A, Ayllón T, Rodríguez-Franco F, Baneth G, Calleja-Bueno L, et al. Molecular detection of Hepatozoon spp. and Cytauxzoon spp. in domestic and stray cats from Madrid, Spain. Parasit Vectors. (2017) 13:112. doi: 10.1186/s13071-017-2056-1
17. Luaces I, Aguirre E, García-Montijano M, Velarde J, Tesouro MA, Sánchez C et al. First report of an intraerythrocytic small piroplasm in wild Iberian lynx (Lynx pardinus). J Wildl Dis. (2005) 41:810–5. doi: 10.7589/0090-3558-41.4.810
18. Millán J, Naranjo V, Rodríguez A, Perez de la Lastra JM, Mangold AJ, de la Fuente J. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitology. (2007) 34:995–1001. doi: 10.1017/S003118200700248X
19. Gallusová M, Jirsová D, Mihalca AD, Gherman CM, D'Amico G, Qablan MA, et al. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic space: further evidence for a different Cytauxzoon species in European felids. J Parasitol. (2016) 102:377–80. doi: 10.1645/15-881
20. Veronesi F, Ravagnan S, Cerquetella M, Carli E, Olivieri E, Santoro A, et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy Ticks. Tick Borne Dis. (2016) 7:853–8. doi: 10.1016/j.ttbdis.2016.04.003
21. Alho AM, Silva J, Fonseca MJ, Santos F, Nunes C, de Carvalho LM, et al. First report of Cytauxzoon spp. infection in a domestic cat from Portugal. Parasit Vectors. (2016) 10:220. doi: 10.1186/s13071-016-1506-5
22. Legroux JP, Halos L, René-Martellet M, Servonnet M, Pingret JL, Bourdoiseau G, et al. First clinical case report of Cytauxzoon spp. infection in a domestic cat in France. BMC Vet Res. (2017) 13:81. doi: 10.1186/s12917-017-1009-4
23. Nentwig A, Meli ML, Schrack J, Reichler IM, Riond B, Gloor C, et al. First report of Cytauxzoon spp. infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit Vectors. (2018) 11:292. doi: 10.1186/s13071-018-2728-5
24. Willi B, Meli ML, Cafarelli C, Gilli UO, Kipar A, Hubbuch A, et al. Cytauxzoon europaeus infections in domestic cats in Switzerland and in European wildcats in France: a tale that started more than two decades ago. Parasit Vectors. (2022) 15:19. doi: 10.1186/s13071-021-05111-8
25. Panait LC, Stock G, Globokar M, Balzer J, Groth B, Mihalca AD, et al. First report of Cytauxzoon spp. infection in Germany: organism description and molecular confirmation in a domestic cat. Parasitol Res. (2020) 119:3005–11. doi: 10.1007/s00436-020-06811-3
26. Naidenko SV, Erofeeva MN, Sorokin PA, Gershov SO, Yakovenko NP, Botvinovskaya AS, et al. The first case of Cytauxzoon spp. in Russia: the parasite conquers Eurasia. Animals. (2022) 12:593. doi: 10.3390/ani12050593
27. Panait LC, Mihalca AD, Modrý D, Juránková J, Ionică AM, Deak G, et al. Three new species of Cytauxzoon in European wild felids. Vet Parasitol. (2021) 290:109344. doi: 10.1016/j.vetpar.2021.109344
28. Grillini M, Simonato G, Tessarin C, Dotto G, Traversa D, Cassini R, et al. Cytauxzoon sp and Hepatozoon spp in domestic cats a preliminary study in North-Eastern Italy. Pathogens. (2021) 18:1214. doi: 10.3390/pathogens10091214
29. Capelli G, Ravagnan S, Montarsi F, Ciocchetta S, Cazzin S, Porcellato E, et al. Occurrence and identification of risk areas of Ixodes ricinus-borne pathogens: a cost-effectiveness analysis in north-eastern Italy. Parasit Vectors. (2012) 5:61. doi: 10.1186/1756-3305-5-61
30. Furlanello T, Tasca S, Caldin M, Carli E, Patron C, Tranquillo M, et al. Artifactual changes in canine blood following storage, detected using the ADVIA 120 hematology analyzer. Vet Clin Pathol. (2006) 35:42–6. doi: 10.1111/j.1939-165X.2006.tb00087.x
31. Carret C, Walas F, Carcy B, Grande N, Précigout E, Moubri K, et al. Babesia canis canis, Babesia canis vogeli, Babesia canis rossi: differentiation of the three subspecies by a restriction fragment length polymorphism analysis on amplified small subunit ribosomal RNA genes. J Eukaryot Microbiol. (1999) 46:298–303. doi: 10.1111/j.1550-7408.1999.tb05128.x
32. National Library of Medicine. BLAST. (2022). Available online at: http://www.ncbi.nlm.nih.gov/BLAST (accessed March 25, 2022).
33. Carli E, De Arcangeli S, Montelli S, Caldin M, Ligorio E, Furlanello T. Babesia gibsoni infection in Italy: a cross sectional study of 607 blood samples belonging to dogs that needed a molecular analysis investigation (2016-2019). Vet Parasitol Reg Stud Reports. (2021) 25:100596. doi: 10.1016/j.vprsr.2021.100596
34. Edgar RC. MUSCLE multiple sequence alignment with high accuracy and high throughput. Nucleic Nucleic Acids Res. (2004) 32:1792–7. doi: 10.1093/nar/gkh340
35. Tamura K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G+C-content biases. Mol Biol Evol. (1992) 9:678–87.
36. Ebani VV, Guardone L, Marra F, Altomonte I, Nardoni S, Mancianti F. Arthropod-borne pathogens in stray cats from Northern Italy: a serological and molecular survey. Animals. (2020) 8:2334. doi: 10.3390/ani10122334
37. Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000-2003). J Am Vet Med Assoc. (2005) 227:942–7. doi: 10.2460/javma.2005.227.942
38. Brown HM, Lockhart JM, Latimer KS, Peterson DS. Identification and genetic characterization of Cytauxzoon felis in asymptomatic domestic cats and bobcats. Vet Parasitol. (2010) 20:311–6. doi: 10.1016/j.vetpar.2010.04.041
39. Stockham SL, Scott MA. “Erythrocytes”. In:Stockham SL, Scott MA, editors. Fundamentals of Veterinary Clinical Pathology. Ames, IA: Iowa State Press (2002). p. 85–154.
40. Wikander YM, Anantatat T, Kang Q, Reif KE. Prevalence of Cytauxzoon felis infection-carriers in Eastern Kansas domestic cats. Pathogens. (2020) 9:854. doi: 10.3390/pathogens9100854
41. Reichard MV, Edwards AC, Meinkoth JH, Snider TA, Meinkoth KR, Heinz RE, et al. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol. (2010) 47:890–6. doi: 10.1093/jmedent/47.5.890
42. Shock BC, Murphy SM, Patton LL, Shock PM, Olfenbuttel C, Beringer J, et al. Distribution and prevalence of Cytauxzoon felis in bobcats (Lynx rufus), the natural reservoir, and other wild felids in thirteen states. Vet Parasitol. (2011) 175:325–30. doi: 10.1016/j.vetpar.2010.10.009
43. Allen KE, Thomas JE, Wohltjen ML, Reichard MV. Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs. Parasit Vectors. (2019) 11:28. doi: 10.1186/s13071-018-3276-8
44. Blouin EF, Kocan AA, Glenn BL, Kocan KM, Hair JA. Transmission of Cytauxzoon felis Kier, 1979 from bobcats, Felis rufus (Schreber), to domestic cats by Dermacentor variabilis (Say). J Wildl Dis. (1984) 20:241–2. doi: 10.7589/0090-3558-20.3.241
45. Santos-Silva MM, Melo P, Santos N, Antunes S, Duarte LR, Ferrolho J, et al. PCR screening of tick-borne agents in sensitive conservation areas, Southeast Portugal. Mol Cell Probes. (2017) 31:42–5. doi: 10.1016/j.mcp.2016.11.005
46. Hodžić A, Alić A, Duscher GG. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: a molecular study. Ticks Tick Borne Dis. (2018) 9:589–93. doi: 10.1016/j.ttbdis.2018.01.017
47. Di Cesare A, Veronesi F, Traversa D. Felid lungworms and heartworms in Italy: more questions than answers? Trends Parasitol. (2015) 31:665–75. doi: 10.1016/j.pt.2015.07.001
48. Reichard MV, Baum KA, Cadenhead SC, Snider TA. Temporal occurrence and environmental risk factors associated with cytauxzoonosis in domestic cats. Vet Parasitol. (2008) 152:314–20. doi: 10.1016/j.vetpar.2007.12.031
49. Crowell-Davis R, Curtis TM, Knowles RJ. Social organization in the cat: a modern understanding. J Feline Med Surg. (2004) 6:19–28. doi: 10.1016/j.jfms.2003.09.013
50. Joyner PH, Reichard MV, Meinkoth JH, Milne VE, Confer AW, Kocan AA, et al. Experimental infection of domestic cats (Felis domesticus) with Cytauxzoon manul from Pallas' cats (Otocolobus manul). Vet Parasitol. (2007) 146:302–6. doi: 10.1016/j.vetpar.2007.03.001
51. Wardrop KJ, Birkenheuer A, Blais MC, Callan MB, Kohn B, Lappin MR, et al. Update on canine and feline blood donor screening for blood-borne pathogens. J Vet Intern Med. (2016) 30:15–35. doi: 10.1111/jvim.13823
52. Pennisi MG, Hartmann K, Addie DD, Lutz H, Gruffydd-Jones T, Boucraut-Baralon C, et al. Blood transfusion in cats: ABCD guidelines for minimising risks of infectious iatrogenic complications. J Feline Med Surg. (2015) 1:588–93. doi: 10.1177/1098612X15588449
53. Lewis KM, Cohn LA, Birkenheuer AJ. Lack of evidence for perinatal transmission of Cytauxzoon felis in domestic cats. Vet Parasitol. (2012) 188:172–4. doi: 10.1016/j.vetpar.2012.02.019
Keywords: Cytauxzoonosis, feline, clinical status, laboratory findings, Europe
Citation: Carli E, Solano-Gallego L, De Arcangeli S, Ventura L, Ligorio E and Furlanello T (2022) Clinicopathological findings and risk factors associated with Cytauxzoon spp. infection in cats: A case-control study (2008–2021). Front. Vet. Sci. 9:976173. doi: 10.3389/fvets.2022.976173
Received: 23 June 2022; Accepted: 22 September 2022;
Published: 10 November 2022.
Edited by:
Münir Aktaş, Firat University, TurkeyReviewed by:
Kaori Sakamoto, University of Georgia, United StatesLeah A. Cohn, University of Missouri, United States
Copyright © 2022 Carli, Solano-Gallego, De Arcangeli, Ventura, Ligorio and Furlanello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erika Carli, ZXJpa2EuY2FybGlAc2FubWFyY292ZXQuY29t
 Erika Carli
Erika Carli Laia Solano-Gallego
Laia Solano-Gallego Stefano De Arcangeli1
Stefano De Arcangeli1 Elisa Ligorio
Elisa Ligorio