- 1Key Laboratory of Veterinary Public Health of Yunnan, College of Veterinary Medicine, Yunnan Agricultural University, Kunming, China
- 2College of Veterinary Medicine, Shanxi Agricultural University, Taigu, China
- 3State Key Laboratory of Veterinary Etiological Biology, Key Laboratory of Veterinary Parasitology of Gansu, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
- 4Hunan Provincial Key Laboratory of Protein Engineering in Animal Vaccines, College of Veterinary Medicine, Hunan Agricultural University, Changsha, China
- 5State Key Laboratory of Conservation and Utilization of Bio-Resources in Yunnan and Center for Life Science, School of Life Sciences, Yunnan University, Kunming, China
Toxoplasma gondii and Neospora caninum are two obligate intracellular protozoan parasites that can cause reproductive failure and production losses. To date, there is no data of T. gondii and N. caninum seroprevalence in black goats in Yunnan Province, southwestern China. In the present study, a total of 734 serum samples were collected from black goats in four different counties of Yunnan Province. 734 and 590 serum samples were examined for antibodies against T. gondii and N. caninum by using MAT and indirect ELISA, respectively. A total of 123 and 76 samples were T. gondii-positive and N. caninum-positive, respectively. The overall seroprevalence of T. gondii in black goats was 16.76% (123/734, 95% CI: 14.06–19.46) with the titer ranged from 1:25 to 1:3200. The seroprevalence of N. caninum was 12.88% (76/590, 95% CI: 10.18–15.58). There was significant difference in seroprevalence of N. caninum in different regions (P < 0.01, χ2 = 30.63) and age groups (P < 0.05, χ2 = 11.85). Significant differences in seroprevalence of T. gondii were observed in different regions (P < 0.05, χ2 = 9.21) and different gender groups (P < 0.01, χ2 = 12.29). Results of seroprevalence of T. gondii and N. caninum indicated that T. gondii and N. caninum were prevalent parasites in black goats in Yunnan Province. This is the first report of seroprevalence of T. gondii and N. caninum in black goats in Yunnan Province. The results of this study indicated that some measures should be taken to control these two parasites and to reduce economic losses to the livestock industry in Yunnan Province.
Introduction
Toxoplasma gondii and Neospora caninum are two obligate intracellular protozoan parasites infecting many animals. In addition, T. gondii is implicated in reproductive disorders in small ruminants, whereas N. caninum is considered an important pathogen causing abortion in dairy cows (1). Both parasites have a wide range of intermediate hosts including cattle, goats, sheep, other domestic and wild animals. Cats and dogs are the definitive hosts of T. gondii and N. caninum, respectively (1, 2). Animals can be infected by T. gondii through consumption of raw meat contained tissue cysts or ingestion of oocysts excreted by felines, and by vertical or transplacental transmission in intermediate hosts (3). In small ruminants, primary infection during pregnancy leads to serious congenital damage, resulting in abortion or stillbirth and negative economic impacts (4). A recent systematic review indicated that the global seroprevalence of T. gondii in goats was 27.49% (15,206/55,317, 95% CI: 24.15–30.95) (5). Despite goat is an important economic source of meat, fiber and milk in some countries worldwide, but a recent study indicated that there is still a higher potential to transmit T. gondii to humans by consumption of raw or undercooked meat, even small serving sizes (5 g) (6). To date, there was only one commercially available vaccine against T. gondii in sheep, but it has been discontinued due to self-limitation (3, 7). The average T. gondii seroprevalence in goats in China was 17.56% (3,260/18,556, 95% CI: 17.02–18.12) (8).
Similar to T. gondii, N. caninum has also been widely concerned and studied since it was first reported in 1984 (9). N. caninum can be transmitted horizontally and vertically in herds (10). N. caninum is considered as a major cause of abortion in cattle, particularly in dairy cattle, and studies revealed that 12% to 42% of aborted fetuses from dairy cattle were infected with N. caninum (1). Furthermore, goats would abort infectious fetuses when they were inoculated with N. caninum during pregnancy, and a meta-analysis revealed that the prevalence of N. caninum in aborted fetuses of goats was 7% worldwide (10, 11). Its zoonotic potential remains unknown because no evidence indicates that humans have been infected with N. caninum successfully (12, 13). The N. caninum seroprevalence in goats was estimated to be 5.99% (1,332/22,234, 95% CI: 4.38–7.83) worldwide (14). Because of N. caninum infection, the economic loss of beef and milk industries is approximate 1 billion US dollars annually (15).
Toxoplasmosis and neosporosis are cosmopolitan parasitic diseases and result in economic losses and reproductive reduction of the herds (1). China has the largest population of goats, and black goats are the most important economic goats in Yunnan Province, southwestern China. High density and diversity of domestic and wild animals might result in a high transmission risk of T. gondii and N. caninum in Yunnan Province. But knowledge on the seroprevalence of T. gondii and N. caninum in black goats in Yunnan Province is lacking. Therefore, the objectives of this study were to examine the seroprevalence of T. gondii and N. caninum and analyze the risk factors associated with their positivity in black goats in Yunnan Province.
Materials and methods
The investigation site and serum samples
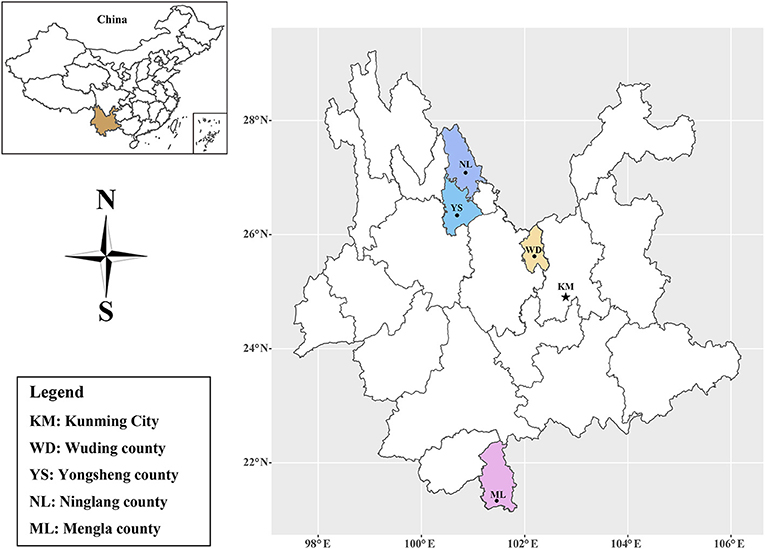
Yunnan Province (97°31′ to 106°11′E, 21°8′ to 29°15′N), located in southwestern China, has a vast territory with diverse and unique natural resources. Yunnan Province has a subtropical monsoon climate, with an average annual temperature of 5 to 24°C and over 1000 mm of annual precipitation in most areas (http://www.yn.gov.cn/yngk/). After obtaining the permission of the farm owners or managers, 734 serum samples were collected from black goats in Wuding county (n = 479), Yongsheng county (n = 90), Ninglang county (n = 100) and Mengla county (n = 65) in Yunnan Province (Figure 1) from August to September, 2017. Approximately 5 mL of blood from each goat was sampled by jugular puncture in a tube without anticoagulant and stored at 4°C for 2 h, then centrifuged at 3,000 rpm for 10 min to collect serum samples, and all serum samples were stored at -20°C freezer until use.
Serological examination
Modified agglutination test (MAT), the efficient method for diagnosis of toxoplasmosis (3), was used to detected the antibodies against T. gondii in this study. The antigen (formalin fixed tachyzoites) used in the experiment were kindly provided by Dr. Jitender P. Dubey (ARS, USDA). The MAT experiment was performed as described previously (16). Briefly, 2 μL serum sample was added to the first well of 96-well U bottomed reaction plate, then was diluted two-fold starting from 1:25 to 1:3,200. 25 μL antigen mixture was added to each well and the plates were incubated at 37°C for 12 h. The negative and positive control were contained in each plate. The serum with titer of 1:25 or higher was considered T. gondii-positive. The specific N. caninum antibodies were detected using an indirect ELISA kit (ID Screen® Neospora caninum Indirect Multi-Species kit, ID VET, Montpellier, France) following the manufacturer's instructions. The kit has a high specificity and sensitivity (17). Positive and negative controls were set in each ELISA detection. The optical density (OD) was measured at 450 nm using microplate reader. The results were expressed as the ratio of absorbance of detected sample to the absorbance of the positive control following the formula: S/P = OD sample – OD negative control / OD positive control – OD negative control. The samples with S/P % ≥50% were judged as positive.
Statistical analysis
Chi-square (χ2) tests in SPSS software (release 23.0 standard version; SPSS, Inc., Chicago) were used for analyzing the variables (region, gender and age) associated with T. gondii and N. caninum infection. The variable with P < 0.05 was considered statistically significant. Results are presented as adjusted odds ratios (OR) with 95% confidence intervals (95% CI).
Results
The seroprevalence of T. gondii and N. caninum in black goats
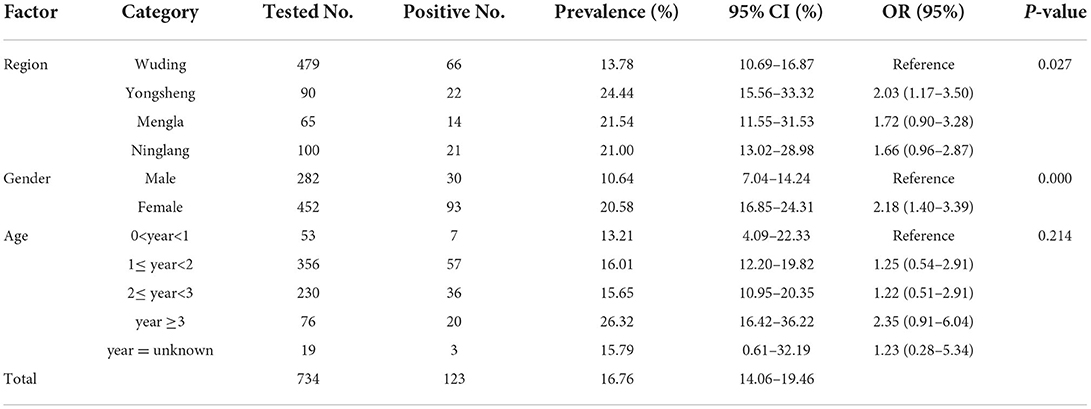
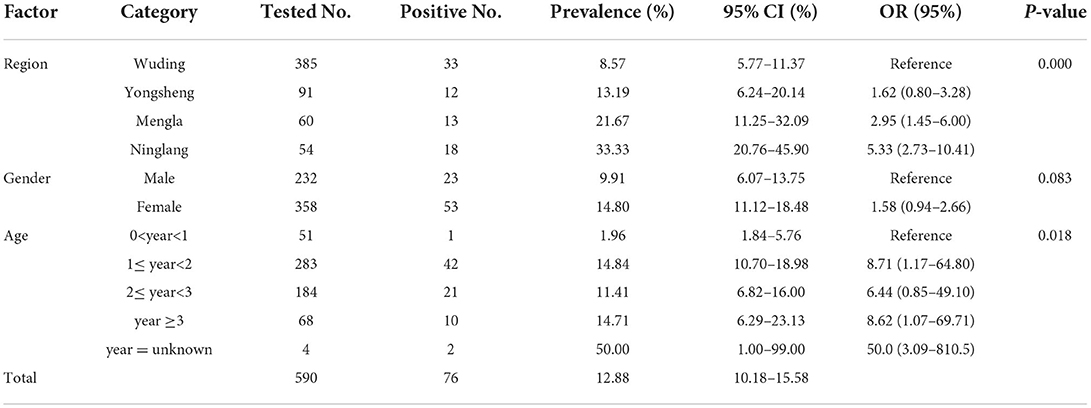
In the present study, the overall seroprevalence of T. gondii and N. caninum in black goats was 16.76% (95% CI: 14.06–19.46) (Table 1) and 12.88% (95% CI: 10.18–15.58) (Table 2), respectively. Regarding the four study regions, the goats in Wuding county has the lowest seroprevalence of both T. gondii (13.78%, 66/479) and N. caninum (8.57%, 33/385), whereas the highest seroprevalence of T. gondii and N. caninum was detected in goats in Yongsheng county (24.44%, 22/90) and Ninglang county (33.33%, 18/54), respectively. Between gender groups, higher seroprevalence of T. gondii and N. caninum was detected in female goats, with 20.58% (93/452) and 14.80% (53/358), respectively. In addition, the highest seroprevalence of T. gondii was detected in goats aged more than 3 years (26.32%, 20/76); however, the seroprevalence of N. caninum in goats older than 3 years of age (14.71%, 10/68) was close to that of goats 1 to 2 years old (14.84%, 42/283), and both were significantly higher than that in goats of <1 year old (1.96%, 1/51).
Risk factors analysis
Statistical analysis showed that the seroprevalence of T. gondii in female goats was 20.58% (93/452), which was significantly higher than that in male goats (10.64%, 30/282) (χ2 = 12.29, df = 1, P < 0.001). There was statistically significant difference in seroprevalence of T. gondii among four study regions (χ2 = 9.21, df = 3, P < 0.05); but no statistically significant difference in T. gondii seroprevalence was observed between goats of different age groups (χ2 = 5.81, df = 1, P = 0.21) (Table 1). Moreover, statistically significant difference in seroprevalence of N. caninum was observed among different counties (χ2 = 3.64, df = 3, P < 0.001) and different age groups (χ2 = 11.85, df = 1, P < 0.05); whereas no statistically significant difference in N. caninum seroprevalence was found between the two genders (χ2 = 3.00, df = 1, P = 0.083).
Discussion
Toxoplasmosis is a widely distributed zoonosis, both toxoplasmosis and neosporosis are two major causes of reproductive losses in small ruminants (18). In this study, we examined the seroprevalence of T. gondii and N. caninum in black goats in Yunnan Province, southwestern China, revealing the presence and relatively high seroprevalence of both parasites in study areas.
In the present study, the overall T. gondii seroprevalences in the examined black goats in Yunnan Province was 16.76%. A recent systematic review revealed that the seroprevalence of T. gondii in goats worldwide from 2000 to 2020 was 27.49%; of which, the highest and lowest seroprevalence of T. gondii in goats was detected in central America (62.15%) and Asia (20.74%), respectively (5). The seroprevalence of T. gondii in black goats in Yunnan Province detected in this study was higher than that in goats in Myanmar (11.39%, 32/281) (19), Korea (5.08%, 31/610) (20), Hunan Province (11.61%, 124/1,068) (21), Hubei Province (13.40%, 807/6,021) (22) and Shaanxi Province (14.11%, 106/751) (23) of China. But the T. gondii prevalence in black goats was lower than that in India (42.47%, 189/445) (24), Pakistan (42.83%, 227/530) (25), Mongolia (32.00%, 345/1,078) (26), Taiwan Province (32.22%, 203/630) (27) and Qinghai Province (29.54%, 192/650) of China (28). The seroprevalence of T. gondii detected in black goats in Yunnan Province in this study was similar to that detected in goats in Bangladesh (16.00%, 48/300) (29), and Yunnan Province (17.60%, 69/392) of China (30). The difference in T. gondii seroprevalence among difference regions may be related to different climate conditions, rearing conditions and breed variations.
The results of the present study demonstrated that there was significant difference in T. gondii seroprevalence of black goats from different geographical regions (P < 0.05) (Table 1). The difference may be caused by terrain and climate differences in Yunnan Province that has abundant rainfall and numerous lakes, and the annual average temperatures range from 5 to 24°C with humid climate. These environmental factors could be beneficial to the sporulation, viability and spread of T. gondii oocysts (31). Previous studies indicated that annual temperature and rainfall could facilitate the survival of the environmental T. gondii oocysts, and seasonally or permanently pasture goats as well as increases the contact between goats and the oocysts (32–34).
The majority of the black goats examined in this study were free-ranged, which could increase the risk of T. gondii infection via frequent contact with other free-ranged animals. The high seroprevalence of T. gondii in black goats in this study may due to the presence of cats in the farms (35, 36). Cats are the definitive host of T. gondii, and oocysts of T. gondii are shed via feces. The ruminants may be infected by ingesting oocysts (37).
In the present study, the highest T. gondii seroprevalence was found in black goats aged 3 years and more (26.32%), with seropositive rate 2.35 times higher than lambs aged lower than 1 year (95% CI = 0.91–6.04). This result is consistent with previous reports that younger goats had lower seropositivity than old goats (24, 36). In contrast, age as a significant risk factor of toxoplasmosis in older animals of ruminant species (i.e., cattle, sheep and goat) comparing to younger animals was observed in several studies (38, 39). Spišák et al. (40) found that the T. gondii prevalence in older goats (over 6 years of age) in Slovakia was 4.3 times higher than goats of up to 3 years age. Similar association between the T. gondii seroprevalence and age has also been observed in cattle, sheep, goats and pigs in Portugal (41). Furthermore, a recent meta-analysis demonstrated that goats older than 1 year of age were at higher risk of being infected with T. gondii, because long-term exposure to the pasture increases the opportunity of ingesting oocysts (5). The results of the present study indicated that T. gondii infection was common in black goats in Yunnan Province (Table 1).
A recent systematic review reported that higher T. gondii prevalence was observed in female goats than that in males (OR = 1.43; 95% CI = 1.23–1.65) (5). In this study, the seroprevalence of T. gondii in female goats was 2.18 times higher than that in male goats (Table 2) (95% CI = 1.40–3.39; P < 0.01). This result was consistent with previous studies in sheep and goats in which male animals had a lower T. gondii prevalence than the females (42, 43). Some studies inferred that higher seroprevalence in females might be associated with their longer life for milk production and reproduction, whereas males are slaughtered for meat supply at an earlier age (24, 44). In addition, a previous study indicated that hormone differences may increase their susceptibility to T. gondii (45).
In this study, the overall seroprevalence of N. caninum in black goats was 12.88% (Table 2), which was higher than that in goats in Poland (9.00%, 95/1,060) (46), Pakistan (9.15%, 13/142) (47), Argentina (6.65%, 106/1,594) (48), south America (6.35%, 25/394) (49), Spain (6.00%, 3/50) (32), Brazil (4.58%, 30/655) (50), Turkey (3.21%, 8/249) (51), Romania (2.34%, 12/512) (52), Greece (6.93%, 26/375) (18) and Jordan (1.99%, 6/302) (53). However, it was lower than that in the Czech Republic (18.57%, 13/70) (35). Growing evidences indicated that the risk of infection for N. caninum is linked to the age of the hosts, rearing system, worming, the time of exposure to the parasite and the contact with dogs around the farms and history of abortion (18, 54). Also, goat breeds, climatic conditions, feeding and management conditions might contribute to the different seroprevalence of N. caninum in black goats in Yunnan Province, and more studies are warranted to investigate the potential association. In addition, previous studies have reported that many birds (e.g., domestic chickens and many wild birds) can act as intermediate hosts of N. caninum and can transmit the pathogen after being preyed upon by dogs when they are foraging on the ground, thus facilitating the spread of the N. caninum (55–57). However, the potential role played by birds in the transmission of N. caninum in Yunnan Province needs further study in the future.
In this study, the region and age factors were significantly related to N. caninum infection in black goats (P < 0.05). The seroprevalence of N. caninum in black goats aged 0–1 years (1.96%) was significantly lower than those in 1–2 years group (14.84%) and 2–3 years group (11.41%). These results were consistent with a previous report (41). Regarding the age groups, adult black goats showed the higher N. caninum seroprevalence than lambs, which is consistent with the results that adult cattle and heifers/steers have higher infection rate than calves, due to the increasing chances in postnatal oocyst infection with age (58). Similar to our results, the lowest N. caninum seroprevalence was also found in yaks of the 0–1 year group (59). However, two previous reports indicated that no statistically significant difference was observed in N. caninum seroprevalence between cattle of different age groups, but the N. caninum seroprevalence was strongly linked with the factors of abortion, parity number, gestation number and number of lactations (60, 61). Thus, a comprehensive study should be performed in the future to elucidate the important role of age in N. caninum epidemiology. With respect to regions, the present study found that there was significant difference in N. caninum seroprevalence among different study areas (P < 0.001). We speculated that rearing system, management measures, presence of dogs, and even the history of abortion in goats in the study areas may contribute to the difference in seroprevalence. Nevertheless, the region factor is a complex of multi-subfactors, including climatic, environmental factors. In Italy, the climatic and environmental factors were determined to influence the N. caninum distribution in cattle by geographical information system (GIS) and remote sensing (RS) technology (58). Additionally, Villa et al. (62) found a correlation between the geographic distance of the sampling sites and genetic distance of N. caninum, further explaining the possible reasons for the seroprevalence difference among different regions. In our investigation, no statistically significant difference in N. caninum seroprevalence was observed between black goats of different genders (P = 0.083). Similar to our findings, Shireen et al. (63) indicated that there was no significant correlation between N. caninum and gender in small ruminants in Egypt.
In the present study, the co-infection rate of T. gondii and N. caninum in black goats was 4.44% (26/585), which was similar to the across-infection rate of T. gondii and N. caninum previous detected in black-bone sheep and goats in Yunnan Province (3.63%, 17/468) (64). Also, it was slightly lower than that in Qinghai Province, where the co-infection rate of T. gondii and N. caninum was 5.23% in goats and 6.5% in sheep (28). Sampling sizes, grazing practices, the presence of dogs and cats may be the important factors that contribute to co-infection of N. caninum and T. gondii (1, 65). However, based on an in vitro, immunological and serological experimental study, researches indicated that there is no exclusivity of infection and co-infection is a random event (66).
Conclusion
The present study examined the seroprevalence of T. gondii and N. caninum infection in black goats in Yunnan Province by using MAT and indirect ELISA methods. The overall seroprevalences of T. gondii and N. caninum in black goats were 16.76 and 12.88%, respectively. Region and gender were significantly associated with T. gondii infection in black goats, while region and age were significantly associated with N. caninum seroprevalence in black goats. The results of the present study demonstrated that T. gondii and N. caninum were highly prevalent in black goats in Yunnan Province. Therefore, integrated measures should be taken to prevent and control infection of black goats with these two parasites.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Animal Ethics and Welfare Committee of Yunnan Agricultural University.
Author contributions
J-JH, F-CZ, and X-QZ designed the study and revised the manuscript. X-HH and S-CX performed the experiments, analyzed the data, and wrote the manuscript. Q-LL, L-XS, ZL, and J-FY participated in implementation of the study. All authors read and approved the final version of the manuscript.
Funding
Project support was provided by the Yunnan Expert Workstation (Grant No. 202005AF150041), the Veterinary Public Health Innovation Team of Yunnan Province (Grant No. 202105AE160014), the Fund for Shanxi 1331 Project (Grant No. 20211331-13) and the Agricultural Science and Technology Innovation Program (ASTIP) (Grant No. CAAS-ASTIP-2016-LVRI-03). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lindsay DS, Dubey JP. Neosporosis, toxoplasmosis, and sarcocystosis in ruminants: an update. Vet Clin North Am Food Anim Pract. (2020) 36:205–22. doi: 10.1016/j.cvfa.2019.11.004
2. Miller NL, Frenkel JK, Dubey JP. Oral infections with Toxoplasma cysts and oocysts in felines, other mammals, and in birds. J Parasitol. (1972) 58:928–37. doi: 10.2307/3286588
3. Dubey JP. The history of Toxoplasma gondii–the first 100 years. J Eukaryot Microbiol. (2008) 55:467–75. doi: 10.1111/j.1550-7408.2008.00345.x
4. Ducournau C, Moire N, Carpentier R, Cantin P, Herkt C, Lantier I, et al. Effective nanoparticle-based nasal vaccine against latent and congenital toxoplasmosis in sheep. Front Immunol. (2020) 11:2183. doi: 10.3389/fimmu.2020.02183
5. Rodrigues AA, Reis SS, Moraes EDS, Do Nascimento Araujo EMA, Zanine AM, Nascimento TVC, et al. A systematic literature review and meta-analysis of Toxoplasma gondii seroprevalence in goats. Acta Trop. (2022) 230:106411. doi: 10.1016/j.actatropica.2022.106411
6. Dubey JP, Murata FHA, Cerqueira-Cezar CK, Kwok OCH. Public health and economic importance of Toxoplasma gondii infections in goats: the last decade. Res Vet Sci. (2020) 132:292–307. doi: 10.1016/j.rvsc.2020.06.014
7. Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology. (1995) 110:S11–6. doi: 10.1017/S003118200000144X
8. Dong H, Su R, Lu Y, Wang M, Liu J, Jian F, et al. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000-2017) from China. Front Microbiol. (2018) 9:2108. doi: 10.3389/fmicb.2018.02108
9. Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd. (1984) 70:271–4. doi: 10.1007/BF00942230
10. Nayeri T, Sarvi S, Moosazadeh M, Daryani A. The global prevalence of Neospora caninum infection in sheep and goats that had an abortion and aborted fetuses: a systematic review and meta-analysis. Front Vet Sci. (2022) 9:870904. doi: 10.3389/fvets.2022.870904
11. Lindsay DS, Rippey NS, Powe TA, Sartin EA, Dubey JP, Blagburn BL. Abortions, fetal death, and stillbirths in pregnant pygmy goats inoculated with tachyzoites of Neospora caninum. Am J Vet Res. (1995) 56:1176–80.
12. Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol. (2003) 41:1–16. doi: 10.3347/kjp.2003.41.1.1
13. Petersen E, Lebech M, Jensen L, Lind P, Rask M, Bagger P, et al. Neospora caninum infection and repeated abortions in humans. Emerg Infect Dis. (1999) 5:278–80. doi: 10.3201/eid0502.990215
14. Rodrigues AA, Reis SS, Sousa ML, Moraes EDS, Garcia JL, Nascimento TVC, et al. A systematic literature review and meta-analysis of risk factors for Neospora caninum seroprevalence in goats. Prev Vet Med. (2020) 185:105176. doi: 10.1016/j.prevetmed.2020.105176
15. Reichel MP, Alejandra Ayanegui-Alcerreca M, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle—the billion dollar question. Int J Parasitol. (2013) 43:133–42. doi: 10.1016/j.ijpara.2012.10.022
16. Qin SY, Zhang XX, Cong W, Zhou DH, Wang JL, Yin MY, et al. Seroprevalence and risk factors of Toxoplasma gondii infection in domestic sika deer (Cervus nippon) in northeastern China. Acta Trop. (2014) 140:184–7. doi: 10.1016/j.actatropica.2014.08.021
17. Alvarez-Garcia G, Garcia-Culebras A, Gutierrez-Exposito D, Navarro-Lozano V, Pastor-Fernandez I, Ortega-Mora LM. Serological diagnosis of bovine neosporosis: a comparative study of commercially available ELISA tests. Vet Parasitol. (2013) 198:85–95. doi: 10.1016/j.vetpar.2013.07.033
18. Diakoua A, Papadopoulos E, Panousis N, Karatzias C, Giadinis N. Toxoplasma gondii and Neospora caninum seroprevalence in dairy sheep and goats mixed stock farming. Vet Parasitol. (2013) 198:387–90. doi: 10.1016/j.vetpar.2013.09.017
19. Bawm S, Maung WY, Win MY, Thu MJ, Chel HM, Khaing TA, et al. Serological survey and factors associated with Toxoplasma gondii infection in domestic goats in Myanmar. Scientifica (Cairo). (2016) 2016:4794318. doi: 10.1155/2016/4794318
20. Jung BY, Gebeyehu EB, Lee SH, Seo MG, Byun JW, Oem JK, et al. Detection and determination of Toxoplasma gondii seroprevalence in native Korean goats (Capra hircus coreanae). Vector Borne Zoonotic Dis. (2014) 14:374–7. doi: 10.1089/vbz.2013.1452
21. Li F, Wang SP, Wang CJ, He SC, Wu X, Liu GH. Seroprevalence of Toxoplasma gondii in goats in Hunan province, China. Parasite. (2016) 23:44. doi: 10.1051/parasite/2016053
22. Luo HQ, Li K, Zhang H, Wu B, Wang J, Shahzad M, et al. Seroepidemiology of Toxoplasma gondii and Neospora caninum infections in goats in Hubei province, China. Trop Biomed. (2016) 33:285–89.
23. Zhao GH, Zhang MT, Lei LH, Shang CC, Cao DY, Tian TT, et al. Seroprevalence of Toxoplasma gondii infection in dairy goats in Shaanxi province, northwestern China. Parasit Vectors. (2011) 4:47. doi: 10.1186/1756-3305-4-47
24. Bachan M, Deb AR, Maharana BR, Sudhakar NR, Sudan V, Saravanan BC, et al. High seroprevalence of Toxoplasma gondii in goats in Jharkhand state of India. Vet Parasitol Reg Stud Reports. (2018) 12:61–8. doi: 10.1016/j.vprsr.2018.02.004
25. Ahmed H, Malik A, Arshad M, Mustafa I, Khan MR, Afzal MS, et al. Seroprevalence and spatial distribution of toxoplasmosis in sheep and goats in north-eastern region of Pakistan. Korean J Parasitol. (2016) 54:439–46. doi: 10.3347/kjp.2016.54.4.439
26. Pagmadulam B, Myagmarsuren P, Yokoyama N, Battsetseg B, Nishikawa Y. Seroepidemiological study of Toxoplama gondii in small ruminants (sheep and goat) in different provinces of Mongolia. Parasitol Int. (2020) 74:101996. doi: 10.1016/j.parint.2019.101996
27. Chiang SH, Huang HH, Chou CC, Chu CS, Shih WL, Lai JM, et al. Epidemiological survey of Toxoplasma gondii and Neospora caninum infections in dairy goats in central-southern Taiwan. J Vet Med Sci. (2020) 82:1537–44. doi: 10.1292/jvms.20-0116
28. Liu ZK, Li JY, Pan H. Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infections in small ruminants in China. Prev Vet Med. (2015) 118:488–92. doi: 10.1016/j.prevetmed.2014.12.017
29. Sah RP, Talukder MH, Rahman A, Alam MZ, Ward MP. Seroprevalence of Toxoplasma gondii infection in ruminants in selected districts in Bangladesh. Vet Parasitol Reg Stud Reports. (2018) 11:1–5. doi: 10.1016/j.vprsr.2017.10.008
30. Zou F, Yu X, Yang Y, Hu S, Chang H, Yang J, et al. Seroprevalence and risk factors of Toxoplasma gondii infection in buffaloes, sheep and goats in Yunnan province, southwestern China. Iran J Parasitol. (2015) 10:648–51.
31. Djokic V, Klun I, Musella V, Rinaldi L, Cringoli G, Sotiraki S, et al. Spatial epidemiology of Toxoplasma gondii infection in goats in Serbia. Geospat Health. (2014) 8:479–88. doi: 10.4081/gh.2014.37
32. Diaz P, Cabanelas E, Diaz-Cao JM, Vina M, Bejar JP, Perez-Creo A, et al. Seroprevalence of Toxoplasma gondii and Neospora caninum in goats from north-western Spain. Ann Agric Environ Med. (2016) 23:587–90. doi: 10.5604/12321966.1226851
33. Gazzonis A, Villa L, Manfredi M, Zanzani S. Spatial analysis of infections by Toxoplasma gondii and Neospora caninum (Protozoa: Apicomplexa) in small ruminants in northern Italy. Animals (Basel). (2019) 9:916. doi: 10.3390/ani9110916
34. Condoleo R, Musella V, Maurelli MP, Bosco A, Cringoli G, Rinaldi L. Mapping, cluster detection and evaluation of risk factors of ovine toxoplasmosis in southern Italy. Geospat Health. (2016) 11:432. doi: 10.4081/gh.2016.432
35. Bartova E, Kobedova K, Lamka J, Kotrba R, Vodicka R, Sedlak K. Seroprevalence of Neospora caninum and Toxoplasma gondii in exotic ruminants and camelids in the Czech Republic. Parasitol Res. (2017) 116:1925–29. doi: 10.1007/s00436-017-5470-6
36. Rahman M, Azad MT, Nahar L, Rouf SM, Ohya K, Chiou SP, et al. Age-specificity of Toxoplasma gondii seroprevalence in sheep, goats and cattle on subsistence farms in Bangladesh. J Vet Med Sci. (2014) 76:1257–9. doi: 10.1292/jvms.14-0171
37. Hide G, Gerwash O, Morley EK, Williams RH, Hughes JM, Thomasson D, et al. Does vertical transmission contribute to the prevalence of toxoplasmosis? Parassitologia. (2007) 49:223–6.
38. Tzanidakis N, Maksimov P, Conraths FJ, Kiossis E, Brozos C, Sotiraki S, et al. Toxoplasma gondii in sheep and goats: seroprevalence and potential risk factors under dairy husbandry practices. Vet Parasitol. (2012) 190:340–8. doi: 10.1016/j.vetpar.2012.07.020
39. Gazzonis AL, Zanzani SA, Villa L, Manfredi MT. Toxoplasma gondii infection in meat-producing small ruminants: meat juice serology and genotyping. Parasitol Int. (2020) 76:102060. doi: 10.1016/j.parint.2020.102060
40. Spišák F, Turčeková L, Reiterová K, Špilovská S, Dubinský P. Prevalence estimation and genotypization of Toxoplasma gondii in goats. Biologia. (2010) 65:670–74. doi: 10.2478/s11756-010-0070-2
41. Lopes AP, Dubey JP, Neto F, Rodrigues A, Martins T, Rodrigues M, et al. Seroprevalence of Toxoplasma gondii infection in cattle, sheep, goats and pigs from the north of Portugal for human consumption. Vet Parasitol. (2013) 193:266–9. doi: 10.1016/j.vetpar.2012.12.001
42. Gebremedhin EZ, Agonafir A, Tessema TS, Tilahun G, Medhin G, Vitale M, et al. Seroepidemiological study of ovine toxoplasmosis in east and west Shewa Zones of Oromia Regional State, Central Ethiopia. BMC Vet Res. (2013) 9:117. doi: 10.1186/1746-6148-9-117
43. Teshale S, Dumetre A, Darde ML, Merga B, Dorchies P. Serological survey of caprine toxoplasmosis in Ethiopia: prevalence and risk factors. Parasite. (2007) 14:155–9. doi: 10.1051/parasite/2007142155
44. Fortes MS, Lopes-Mori FMR, Caldart ET, Constantino C, Evers F, Pagliari S, et al. Caprine toxoplasmosis in Southern Brazil: a comparative seroepidemiological study between the indirect immunofluorescence assay, the enzyme-linked immunosorbent assay, and the modified agglutination test. Trop Anim Health Prod. (2018) 50:413–19. doi: 10.1007/s11250-017-1450-1
46. Czopowicz M, Kaba J, Szalus-Jordanow O, Nowicki M, Witkowski L, Frymus T. Seroprevalence of Toxoplasma gondii and Neospora caninum infections in goats in Poland. Vet Parasitol. (2011) 178:339–41. doi: 10.1016/j.vetpar.2011.01.039
47. Nasir A, Ashraf M, Khan MS, Javeed A, Yaqub T, Avais M, et al. Prevalence of Neospora caninum antibodies in sheep and goats in Pakistan. J Parasitol. (2012) 98:213–5. doi: 10.1645/GE-2863.1
48. Moore DP, Yaniz M, Odeón A, Ca No D, Leunda MR, Späth E, et al. Serological evidence of Neospora caninum infections in goats from La Rioja Province, Argentina. Small Ruminant Res. (2007) 73:256–8. doi: 10.1016/j.smallrumres.2006.10.019
49. Moore DP. Neosporosis in South America. Vet Parasitol. (2005) 127:87–97. doi: 10.1016/j.vetpar.2004.10.001
50. Topazio JP, Weber A, Camillo G, Vogel FF, Machado G, Ribeiro A, et al. Seroprevalence and risk factors for Neospora caninum in goats in Santa Catarina State, Brazil. Rev Bras Parasitol Vet. (2014) 23:360–6. doi: 10.1590/S1984-29612014062
51. Zhou M, Cao S, Sevinc F, Sevinc M, Ceylan O, Liu M, et al. Enzyme-linked immunosorbent assays using recombinant TgSAG2 and NcSAG1 to detect Toxoplasma gondii and Neospora caninum-specific antibodies in domestic animals in Turkey. J Vet Med Sci. (2017) 78:1877–81. doi: 10.1292/jvms.16-0234
52. Iovu A, Gyorke A, Mircean V, Gavrea R, Cozma V. Seroprevalence of Toxoplasma gondii and Neospora caninum in dairy goats from Romania. Vet Parasitol. (2012) 186:470–4. doi: 10.1016/j.vetpar.2011.11.062
53. Abo-Shehada MN, Abu-Halaweh MM. Flock-level seroprevalence of, and risk factors for, Neospora caninum among sheep and goats in northern Jordan. Prev Vet Med. (2010) 93:25–32. doi: 10.1016/j.prevetmed.2009.08.004
54. Rodrigues AA, Reis SS, Da Silva Moraes E, Do Nascimento Souza Filho JG, Dos Santos Reis MH, Martins TA, et al. Seroprevalence and risk factors for Neospora caninum and Toxoplasma gondii in goats of Maranhao State, Brazil. Vet Parasitol Reg Stud Reports. (2021) 26:100634. doi: 10.1016/j.vprsr.2021.100634
55. Darwich L, Cabezon O, Echeverria I, Pabon M, Marco I, Molina-Lopez R, et al. Presence of Toxoplasma gondii and Neospora caninum DNA in the brain of wild birds. Vet Parasitol. (2012) 183:377–81. doi: 10.1016/j.vetpar.2011.07.024
56. Gazzonis AL, Villa L, Lubian E, Ressegotti S, Grilli G, Raimondi S, et al. Molecular survey on Toxoplasma gondii and Neospora caninum infection in wild birds of prey admitted to recovery centers in Northern Italy. Microorganisms. (2021) 9:736. doi: 10.3390/microorganisms9040736
57. de Barros LD, Miura AC, Minutti AF, Vidotto O, Garcia JL. Neospora caninum in birds: a review. Parasitol Int. (2018) 67:397–402. doi: 10.1016/j.parint.2018.03.009
58. Rinaldi L, Fusco G, Musella V, Veneziano V, Guarino A, Taddei R, et al. Neospora caninum in pastured cattle: determination of climatic, environmental, farm management and individual animal risk factors using remote sensing and geographical information systems. Vet Parasitol. (2005) 128:219–30. doi: 10.1016/j.vetpar.2004.12.011
59. Meng QF, Yao GZ, Qin SY, Wu J, Zhang XC, Bai YD, et al. Seroprevalence of and risk factors for Neospora caninum infection in yaks (Bos grunniens) in China. Vet Parasitol. (2017) 242:22–3. doi: 10.1016/j.vetpar.2017.05.022
60. Villa L, Gazzonis AL, Fumagalli E, Zanzani SA, Manfredi MT. The utility of serological analysis for Neospora caninum infection in dairy cattle farms management: serological investigation and evaluation of the effects on reproductive and productive performances in two study herds in Northern Italy. Animals (Basel). (2022) 12:786. doi: 10.3390/ani12060786
61. Pfeiffer DU, Williamson NB, Reichel MP, Wichtel JJ, Teague WR, A. longitudinal study of Neospora caninum infection on a dairy farm in New Zealand. Prev Vet Med. (2002) 54:11–24. doi: 10.1016/S0167-5877(02)00011-9
62. Villa L, Maksimov P, Luttermann C, Tuschy M, Gazzonis AL, Zanzani SA, et al. Spatial distance between sites of sampling associated with genetic variation among Neospora caninum in aborted bovine foetuses from northern Italy. Parasit Vectors. (2021) 14:47. doi: 10.1186/s13071-020-04557-6
63. Aboelwafa SS, Ali AO, Hamada R, Mahmoud HY. Seroprevalence of Toxoplasma gondii and Neospora caninum in small ruminants in Luxor, Egypt. Adv Animal Vet Sci. (2021) 10:412–20. doi: 10.17582/journal.aavs/2022/10.2.412.420
64. Sun LX, Liang QL, Nie LB, Hu XH Li Z, Yang JF, et al. Serological evidence of Toxoplasma gondii and Neospora caninum infection in black-boned sheep and goats in southwest China. Parasitol Int. (2020) 75:102041. doi: 10.1016/j.parint.2019.102041
65. Dubey JP, Schares G. Neosporosis in animals–the last five years. Vet Parasitol. (2011) 180:90–108. doi: 10.1016/j.vetpar.2011.05.031
Keywords: black goats, Southwestern China, Toxoplasma gondii, Neospora caninum, seroprevalence, risk factors
Citation: Hu X-H, Xie S-C, Liang Q-L, Sun L-X, Li Z, Yang J-F, Zhu X-Q, Zou F-C and He J-J (2022) Seroprevalence and risk factors of Toxoplasma gondii and Neospora caninum infection in black goats in Yunnan Province, Southwestern China. Front. Vet. Sci. 9:975238. doi: 10.3389/fvets.2022.975238
Received: 22 June 2022; Accepted: 23 September 2022;
Published: 11 October 2022.
Edited by:
Simona Gabrielli, Sapienza University of Rome, ItalyCopyright © 2022 Hu, Xie, Liang, Sun, Li, Yang, Zhu, Zou and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Cai Zou, emZjMTIwN0B2aXAuMTYzLmNvbQ==; Jun-Jun He, aGVqdW5qdW42MTdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiao-Hui Hu
Xiao-Hui Hu Shi-Chen Xie
Shi-Chen Xie Qin-Li Liang
Qin-Li Liang Li-Xiu Sun
Li-Xiu Sun Zhao Li5
Zhao Li5 Xing-Quan Zhu
Xing-Quan Zhu Feng-Cai Zou
Feng-Cai Zou Jun-Jun He
Jun-Jun He