- 1Department of Diagnostics and Crisis Organization, Wageningen Bioveterinary Research, Lelystad, Netherlands
- 2Department of Bacteriology, Host Pathogen Interaction and Diagnostics, Wageningen Bioveterinary Research, Lelystad, Netherlands
Equine piroplasmosis (EP) is a tick-borne disease affecting horses, donkeys, mules and zebras, caused by the intracellular apicomplexan protozoa Babesia caballi and Theileria equi. The geographical distribution of EP is closely related to the distribution of its vector tick species belonging to the genera of Dermacentor, Rhipicephalus and Hyalomma. Since the discovery of Dermacentor reticulatus ticks in 2007 and the first reported autochthonous cases in the South of the Netherlands in 2012, no data on the (sero)prevalence of EP in horses in the Netherlands have been reported and it remains unclear whether B. caballi and T. equi have been able to establish themselves in the Netherlands. This study aims to give an update on the current status of EP in horses in the Netherlands using data from serological tests performed in the context of export and screening of 12,881 horses from 2015 through 2020. Horses were categorized as “Dutch,” “Foreign,” or “Unknown” based on microchip number. The overall seroprevalence of EP in Dutch horses was found to be 0.5% (95% exact CI [0.4–0.7]), compared to 1.9% (95% exact CI [1.3–2.6]) in horses in the category “Foreign” and 1.7% (95% exact CI [1.2–2.3]) in horses in the category “Unknown.” In addition, the seroprevalence per country in the category “Foreign” ranged from 0% (0.95% exact CI [0–2.8]) for Ireland to 6.0% (0.95% exact CI [3.5–9.3]) for Spain. In light of the reports on the seroprevalence during the outbreak of autochthonous EP reported in 2012 and on seroprevalences of EP in other countries in Northwestern Europe, the seroprevalence of EP in horses exported from the Netherlands is very low. However, the higher seroprevalence of EP in horses from abroad warrants the need for the monitoring of EP, as tick vectors are present in the Netherlands and the import of horses from endemic areas increases the chances of EP becoming more prevalent in the Netherlands.
Introduction
Equine piroplasmosis (EP), also known as equine babesiosis or biliary fever, is a tick-borne disease affecting horses, donkeys, mules and zebras, caused by the intracellular apicomplexan protozoa Babesia caballi and Theileria equi (formerly Babesia equi), either individually or in the form of a co-infection (1, 2). EP is transmitted by tick vectors or iatrogenically (for instance, through blood transfusion or contaminated needles), while transplacental transmission has also been described (1, 3). Tick species belonging to the genera of Dermacentor, Rhipicephalus and Hyalomma are considered to be competent vectors, but the process of identifying other competent tick vectors is still ongoing (1, 4). For B. caballi, infected equids as well as tick vectors act as a reservoir, while only chronically infected equids are considered as the reservoir for T. equi (2).
Clinical symptoms of EP include signs of hemolytic anemia, such as icterus and hemoglobinuria, fever and signs of systemic illness (1, 2). Apparently healthy mares can transmit T. equi to the unborn foal, leading to abortion or neonatal piroplasmosis (1, 2). The infection manifests in several forms: peracute, acute, subacute or chronic (2, 4). In the case of T. equi, the persistent infection is life-long, while for B. caballi, evidence exists that some infected horses are able to clear the parasite (2). In areas where EP is considered to be endemic, clinical manifestation during outbreaks is rare and the majority of infected horses are believed to become inapparent carriers (2). However, depending on factors like immunity, the mortality of horses in endemic areas can be as high as 5–10% (2, 5). Peracute or acute symptoms of EP usually derive from the introduction of naive horses in endemic areas, especially when ticks are abundant (2). In non-endemic areas, EP can result in the death of up to 50% of the infected horses, while most horses that survive infection, become asymptomatic carriers, similar to situations where EP is endemic (2, 5). Asymptomatic carriers serve as a source of infection for tick vectors, but are also at risk of relapse, for instance, after demanding exercise (5, 6). Apart from the impact on animal health and welfare, EP leads to substantial economic losses in the equine sector (5). Treatment of EP is difficult and often without success. Imidocarb dipropionate has proven to be effective in reducing clinical symptoms in several studies, but complete clearance of the parasite, especially T. equi remains a challenge (6–8). To date, there is no vaccine commercially available. Strategies aiming at prevention of infection include tick control measures, such as the application of tick-specific repellents and trade restrictions, mainly in the form of required health certificates stating the animals are free of EP.
The geographical distribution of EP is closely related to the distribution of its vector tick species belonging to the genera of Dermacentor, Rhipicephalus and Hyalomma which are endemic in tropical and subtropical regions, hence the majority of cases of EP are reported in Africa, Asia, Central and South America and southern parts of Europa and the USA (1, 2, 4). When tick vectors are present in a particular country or region considered to be free of EP, the introduction of an infected horse can result in the epizootic spread of EP. This is why the global movement of horses for trade and equine events poses a risk for non-endemic nations. Historically, the Netherlands were considered to be free of EP and its tick vectors were reported only sporadically or not at all. However, over the last few years, Dermacentor ticks have been found more frequently in regions with a more moderate climate (9, 10). In 2007, the presence of a population of Dermacentor reticulatus ticks in the Netherlands was described for the first time (11). Dermacentor reticulatus had previously only been detected on imported animals (11). A few years later, DNA of B. caballi and T. equi was isolated for the first time from ticks collected from horses in the Netherlands between 2008 and 2009 (12). Following the discovery of both of these agents responsible for EP, a horse with a subclinical B. caballi infection was presented to a veterinary clinic in the Netherlands in 2009 (13). The horse had never been abroad and this case led to a seroprevalence survey in an area in the South West of the Netherlands, where the B. caballi infected horse was kept (13). Twelve (4%) of the 300 randomly selected horses in the area were seropositive for EP, while five (1.6%) tested positive for T. equi DNA by performing polymerase chain reaction combined with reverse line blotting (PCR-RLB) (13). Of these positive horses, four (1.3%) had never left the country, providing evidence for autochthonous EP infections in the Netherlands. During this survey, the researchers were contacted about two indigenous horses with clinical signs of EP outside of the sampling area, which were later diagnosed with an acute T. equi infection (13). These horses with B. caballi and T. equi infections were the first reported autochthonous cases of EP in the Netherlands.
Since then, no data on the (sero)prevalence of EP in horses in the Netherlands has been reported. It remains unclear whether B. caballi and T. equi have been able to establish themselves in the Netherlands since the first reports of autochthonous cases of EP in 2012 and how the current seroprevalence of EP in apparently healthy horses compares to the 4% reported during the outbreak in 2009 and 2010 (13). EP is not notifiable in the Netherlands and there is no surveillance program in place. This study aims to give an update on the current status of EP in horses in the Netherlands using data from serological tests performed at the national reference laboratory (NRL) in the context of export and screening between 2015 and 2021. In addition, a distinction was made between Dutch horses and horses from abroad based on microchip number to be able to compare the seroprevalence of EP in the indigenous equine population to the seroprevalence of EP in imported horses.
Materials and methods
Origin of samples
Being the Dutch NRL for export diagnostics, Wageningen Bioveterinary Research (WBVR) receives sera from equine export and transport companies, either directly or through a veterinary practice. In most cases, importing countries will request one or more specific tests to clear an animal for import. As a result, some horses are tested by indirect fluorescence antibody test (IFAT) only, others by competitive enzym-linked immunosorbent assay (cELISA) and IFAT, some by cELISA, IFAT and complement fixation test (CFT), and so on. In addition, sera are sent to the NRL by equine breeding companies to maintain a certain health status. Finally, serum samples are submitted for screening purposes, for example, prior to participation in equestrian competitions. Test results are entered in a digital database and as such, were available for analysis. It should be noted that the majority (>90%) of the samples included in this study, were tested for the purpose of export of the animal. This means that the majority of the horses included in this study, resided in the Netherlands for at least the length of the period of quarantine required for export and that they did not show any clinical symptoms when the samples were collected.
Serological tests
All serological tests were performed at the NRL and in parallel for each sample. The IFAT was performed using an in-house protocol in accordance with the OIE manual and slides provided by the Faculty of Veterinary Medicine in Utrecht, The Netherlands from 2015 up to and including the first half of 2018 (14). From the second half of 2018 up to 2020, the IFAT was performed using an in-house protocol in accordance with the OIE manual and commercial slides (MegaFLUO® BABESIA caballi and MegaFLUO® THEILERIA equi, MEGACOR, Diagnostik GmbH, Hörbranz, Austria) (14). Samples with a titer of 80 or higher were considered positive. The exact sensitivity and specificity of the IFAT are unknown, but the IFAT is considered as a highly specific confirmatory test (15). For this study, the specificity of the IFAT was assumed to be 100%.
The CFT was performed using an in-house protocol in accordance with the OIE manual. Samples with a titer of 5 or higher were considered positive. The exact sensitivity and specificity of the CFT performed in-house are unknown, but the CFT in general is considered to be less sensitive than the cELISA (15). For this study, the specificity of the CFT was assumed to be 100%.
Commercial cELISA kits for B. caballi (Babesia caballi Antibody Test Kit, cELISA, VMRD, USA) and T. equi (Theileria equi Antibody Test Kit, cELISA, VMRD, USA) were used to test the submitted samples by cELISA maintaining the cut-off value of ≥40% as described by the kit manuals. The cELISA is considered to be a highly sensitive as well as a highly specific test according to the OIE manual: the specificity appears to be 99.5% for B. caballi and 99.2% for T. equi (14). However, for this study, the specificity of both cELISAs was assumed to be 100%.
Sample selection and analysis of results
For this study, data was checked and filtered based on a number of conditions. Test results deriving from samples that had been tested by IFAT, cELISA and/or CFT for both B. caballi and T. equi between 2015 and 2020 were included. Occasionally, multiple samples from one individual horse are submitted within a few weeks or months or over the years. As this study focuses on individual horses rather than samples, the decision was made to only include the (chronologically) first test result of every horse, that had been entered in the database between 2015 and 2020. Consequently, a positive result following a negative result was not taken into account (and vice versa). Furthermore, a horse was considered to be seropositive if one or more serological tests yielded a positive result, disregarding the negative results of other tests when performed. Only results from horses and no other animal species were taken into account. As this study aims to represent the (healthy) equine population in the Netherlands, the reason for submission had to be either export or screening in order to exclude any clinical cases. Finally, horses were divided into three categories based on the microchip numbers as stated on the submission forms: “Dutch” (valid microchip numbers starting with 528), “Foreign” (valid microchip numbers starting with anything other than 528 and up to and excluding 900) and “Unknown” (invalid microchip numbers, names, no information, microchip numbers starting from 900). Microchip numbers starting from 900 do not refer to a specific country, but are property of the microchip manufacturer.
Results
Seroprevalence of EP per category
A total of 52,219 serological test results were entered in the database between 2015 and 2020. Filtering as described above, resulted in a total of 12,881 unique animals tested for antibodies against both B. caballi and T. equi, of which 9,148 (71%) animals belonged to the category “Dutch,” 1,611 (13%) to the category “Foreign” and 2,122 (16%) to the category “Unknown”.
Concerning horses in the category “Dutch”, 22 out of 9,148 horses (0.2%, 95% exact CI [0.2–0.4]) tested positive according to at least one serological test for B. caballi, compared to 27 out of 9,148 (0.3%, 95% exact CI [0.2–0.4]) for T. equi (Table 1). Four horses tested positive for both B. caballi as well as T. equi antibodies resulting in an overall seroprevalence of EP of 0.5% (95% exact CI [0.4–0.7]) (Table 1).
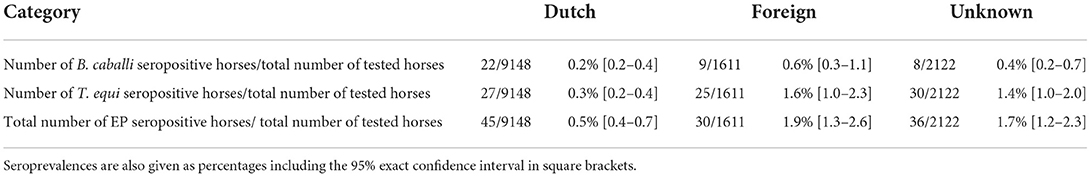
Table 1. Total numbers of seropositive horses for B. caballi, T. equi and combined (“EP seropositive”) compared to the total of horses tested between 2015 through to 2020 per category based on microchip numbers (“Dutch,” “Foreign” and “Unknown”).
In the category “Foreign,” nine out of 1,611 horses (0.6%, 95% exact CI [0.3–1.1]) tested positive according to at least one serological test for B. caballi, compared to 25 out of 1,611 (1.6%, 95% exact CI [1.0–2.3]) for T. equi (Table 1). Four horses tested positive for both B. caballi antibodies as well as T. equi antibodies resulting in an overall seroprevalence of EP of 1.9% (95% exact CI [1.3–2.6]) (Table 1).
Based on the microchip numbers of the horses stated on the submission forms, the origin of the horses in the category “Foreign” could be determined. Of the 1,611 horses in this category, 45% of the horses were microchipped with a German microchip number, 18% with a Spanish microchip number, 15% with a French microchip number, 8% with an Irish microchip number and 5% with a Danish microchip number (Figure 1). The percentages for the other countries (13 countries in total) were below 5% and were grouped together under “Other countries,” making up 9% of the horses in the category “Foreign” (Figure 1). The seroprevalences of EP in the countries making up 91% of the submissions are shown in Table 2. The highest seroprevalence (6.0%, 95% exact CI [3.5–9.3]) was found in horses originating from Spain, followed by France (1.7%, 95% exact CI [0.5–4.3]) and Germany (0.6%, 95% exact CI [0.2–1.4]). The dataset did not include any seropositive animals with a Danish or an Irish microchip number. The seoprevalence of EP for the grouped other countries was (3.3%, 95% exact CI [1.1–7.5]).
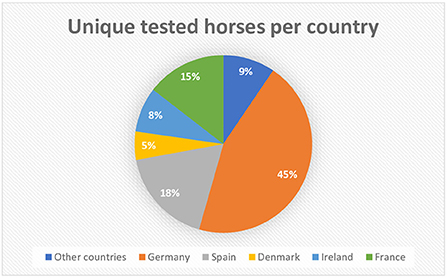
Figure 1. Distribution of the origin of horses in the category “Foreign” (n = 1,611), based on microchip numbers and country codes. 91% (n = 1,466) of the horses originate from Germany, Spain, France, Ireland, and Denmark, constituting the top five, while 9% (n = 145) of the horses in the category “Foreign” originate from other countries.
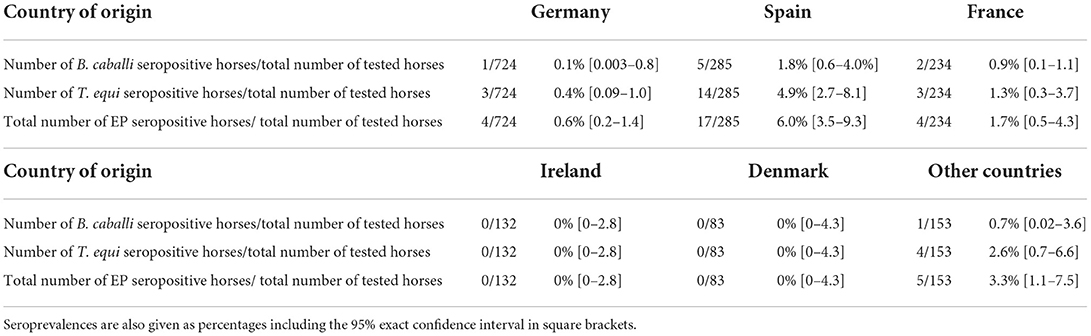
Table 2. Total numbers of seropositive horses for B. caballi, T. equi and combined (“EP seropositive”) compared to the total of horses tested between 2015 through to 2020 per category based on microchip numbers and country codes.
As for the horses in the category “Unknown,” eight out of 2,122 horses (0.4%, 95% exact CI [0.2–0.7]) tested positive according to at least one serological test for B. caballi, compared to 30 out of 2,122 horses (1.4%, 95% exact CI [1.0–2.0]) for T. equi (Table 1). Two horses tested positive for both B. caballi antibodies as well as T. equi antibodies resulting in an overall seroprevalence of EP of 1.7% (95% exact CI [1.2–2.3]) (Table 1).
Discussion
The aim of this study was to provide an update of the current status of EP in the Netherlands based on the data of routine diagnostics for export, since no data on the (sero)prevalence of EP has been published since the first infections with EP in autochthonous horses in 2009 (13). The overall seroprevalence of EP in healthy Dutch horses was calculated to be 0.5% (95% exact CI [0.4–0.7]) based on the data of routine diagnostics for export between 2015 and 2020, compared to the seroprevalence of 4% found during the survey in a local area where autochthonous cases of EP were reported in 2012 (13). Despite the differences between these studies, namely 300 horses in the vicinity of autochthonous cases of EP, having access to pasture and/or being used for outdoor recreation, and sampled in the summer months of 2010 vs. 9148 healthy (indigenous) horses sampled between 2015 and 2021 in the context of export and screening, the seroprevalence of 0.5% in Dutch horses as found by this study a decade later, does not indicate that EP is a disease of significance in the Netherlands. It may be argued that the true seroprevalence of EP in the Netherlands possibly exceeds this percentage, as the data used for this study mainly derives from horses intended for export. This presumably involves above-average care and indoor-housing, thus reducing the probability of contracting EP. However, EP is also rarely diagnosed in the field and usually involves imported horses from endemic areas (C. van Maanen, personal communication, January 10th 2022). This supports the low seroprevalence as found by this study and there is no indication that the true seroprevalence will be much higher. However, studies including horses not intended for export should be carried out to establish how the prevalence found in this study relates to the true seroprevalence.
During the survey following the first autochthonous case in 2009, two ponies from outside the sampling area were diagnosed with acute EP and were considered to be indigenous as well (13). This implies that competent tick vectors for the transmission of EP were present in at least part of the Netherlands. This was confirmed by a study that reported the detection of DNA of both B. caballi and T. equi from Ixodes ricinus ticks collected from Dutch horses in 2008–2009 (12). It should be noted that I. ricinus is not considered as a typical vector for EP, but its capability of transmitting either B. caballi or T. equi could have implications for countries where this tick species is abundant (16). Since the outbreak of EP as described by Butler et al. (13), three surveys have been published that have investigated the presence of EP tick vectors and the presence of B. caballi or T. equi in those tick vectors in the Netherlands. Jongejan et al. (17) analyzed 855 D. reticulatus ticks from various sites in the Netherlands (n = 566) and Belgium (n = 289) by PCR/RLB, of which one tick from the Netherlands and one tick from Belgium tested positive for B. caballi (17). No ticks tested positive for T. equi (17). In another study in 2019, none of 860 D. reticulatus ticks collected in the Netherlands were found to be positive for either B. caballi or T. equi by means of a high-throughput real-time PCR based array (18). In another more recent study in 2021, 17 Hyalomma ticks, of which 15 discovered on horses in the Netherlands were analyzed for the presence of pathogens, such as B. caballi and T. equi (19). All Hyalomma ticks, which were most likely introductions, tested negative for the presence of EP parasites by PCR, and an additional cross-sectional study in 202 horses in 2019 revealed that Hyalomma ticks could not be detected on these horses during the observatory period (19). The very low prevalence of B. caballi and T. equi in the Dutch tick population including introductions seems to support the low seroprevalence found in Dutch horses intended for export.
The overall seroprevalence of EP in horses from abroad (category “Foreign”) based on the data from 2015 through 2020 was calculated to be 1.9% (95% exact CI [1.3–2.6]), which is higher than the seroprevalence of EP in Dutch horses (0.5% (95% exact CI [0.4–0.7])). The highest seroprevalences of 6.0% (95% exact CI [3.5–9.3]) and 1.7% (95% exact CI [0.5–4.3]) were found in horses originating from, respectively, Spain and France, which is to be expected, as EP is considered to be endemic in Spain and (some parts of) France (4, 20). Being a neighboring country, it is not surprising that the seroprevalence of horses originating from Germany (0.6% (95% exact CI [0.2–1.4]) is more in line with the seroprevalence of Dutch horses (0.5% (95% exact CI [0.4–0.7]).
As the category “Unknown” may include both autochthonous as well as non-autochthonous horses, the percentages are difficult to interpret. However, given the probable mix in origin of the animals in this category, it seems logical that the overall seroprevalence of 1.7% (95% exact CI [1.2–2.3]) lies somewhere in between the seroprevalences of EP in the categories “Foreign” and “Dutch.”
Studies similar to this have been conducted in other countries in Northwestern Europe. A recent study from the United Kingdom (UK) revealed seroprevalences of 4.4% for B. caballi, 5.9% for T. equi and an overall seroprevalence of EP of 8.0% (21). These seroprevalences are considerably higher than our observations, while both studies make use of samples submitted for routine diagnostics in the context of export, and are more in the line of the seroprevalence reported in Switzerland (7.3%) (22). A similar study with samples submitted for screening in Ireland showed an overall seroprevalence of 3.5% (1.5% for B. caballi and 2.5% for T. equi) which is more comparable to the seroprevalences found in our study compared to the seroprevalence of 8.0% in the UK (23). Due to legislation concerning data protection, no detailed information was available on the origin of the tested horses (23). However, the seroprevalence is still at least twice as high as the seroprevalences in both Dutch horses and horses originating from abroad in our study. This also holds true for the seroprevalence of 6.1% for T. equi reported by researchers in Germany, where 314 indigenous horses were tested for EP (24). In contrast, the researchers also report a seroprevalence of 0.3% for B. caballi, which is more in line with our findings, including both the seroprevalence in Dutch horses of 0.2% (95% exact CI [0.2–0.4]) as well as the seroprevalence found in German horses of 0.1% (95% exact CI [0.003–0.8]) (24).
The difference between the results of our study and the studies conducted in the UK and Ireland can at least partially be explained by our decision to analyze at the level of individual horses rather than individual samples. While analyzing the data, it became clear to us that multiple submissions within weeks or months after the first positive test result were not uncommon. This can be explained by the nature of the samples, which were mainly submitted to meet the health requirements of importing countries. The antibody titer of a seropositive animal will likely decline over time and there is a probability that the seropositive animal will test negative at some point, which means that the animal can be exported. Some horses in our dataset were tested positive up to three times on multiple occasions following an initial positive test result. The great majority of tested horses is assumed to be exported when tested negative and there is no reason for a new sample to be submitted. Indeed, the pattern of multiple submissions was observed to a far lesser extent for horses that were tested negative the first time as opposed to horses that were tested positive. To avoid the potential bias and overestimation of the seroprevalence caused by the consecutively submitted, seropositive samples, only the (chronologically) first test result of a horse that had been entered in the database was taken into account in our study.
Another factor that might explain the differences in seroprevalences to some extent might be the application of different serological tests between the studies. As described earlier, the sensitivity differs between the serological tests. This is demonstrated by the study in Germany, where the only positive B. caballi cELISA result could not be confirmed by IFAT and only 10 out of 16 positive T. equi IFAT results could be confirmed by cELISA (24). The difference in sensitivity of the applied tests is one of the reasons that for this study a horse was considered to be seropositive if the result of at least one of the serological tests was defined as positive, disregarding the negative results of other tests for that submission when performed. In addition, the specificity of all tests was assumed to be 100%.
Another factor that may have led to a potential underestimation of the seroprevalence of EP in this study is the pre-screening of horses. For official reports (recognized by the Dutch authorities), export companies or horse owners are required to submit their samples to the NRL in the Netherlands. However, they can have their horses tested by another laboratory when an official report is not necessary, allowing them to get insight in the serological status of the animals and exclude seropositive horses from export. It is unknown at which scale this pre-screening takes place and how much this bias will have affected the results.
The very low seroprevalence of EP in Dutch horses in this study is in accordance with the very low prevalence of B. caballi and T. equi in the Dutch tick population: it seems that little over a decade after the first reported outbreak of EP infections in autochthonous horses, the seroprevalence of EP in the Netherlands is still very low. The latter is especially true for supposedly indigenous horses and compared to other countries in Northwestern Europe. Whether the very low seroprevalence in horses tested for the purpose of export is representative for the seroprevalence in the field needs to be confirmed. It is left to speculation to which extent regional differences in tick distribution, climate or other factors account for the differences between seroprevalence studies conducted in several countries in Northwestern Europe. Nevertheless, suitable tick vectors for EP are present in the Netherlands and based on the higher seroprevalence of horses imported from endemic areas, as shown by the results of this study, it is recommended to continue monitoring the status of EP in both tick vectors and horses in the Netherlands.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study because the study used data on previously tested animals of which samples were submitted in the context of export and screening.
Author contributions
HG initiated and designed the study and wrote the first draft of the manuscript. PK performed the analysis of the data. JG supervised the design of the study, data analysis, and statistical analysis of the data. HG, PK, JG, and MK were actively involved in the interpretation of the data. JG and MK reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was primarily financed by the Ministry of Agriculture, Nature and Food Quality through an ongoing project on equine diseases within the framework of statutory tasks, Grant Number WOT-01-002-005.03.
Acknowledgments
The authors would like to thank Eefke Weesendorp for her feedback on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Waal DT. Equine piroplasmosis: a review. Br Vet J. (1992) 148:6–14. doi: 10.1016/0007-1935(92)90061-5
2. Wise LN, Kappmeyer LS, Mealey RH, Knowles DP. Review of equine piroplasmosis. J Vet Intern Med. (2013) 27:1334–46. doi: 10.1111/jvim.12168
3. Allsopp MT, Lewis BD, Penzhorn BL. Molecular evidence for transplacental transmission of Theileria equi from carrier mares to their apparently healthy foals. Vet Parasitol. (2007) 148:130–6. doi: 10.1016/j.vetpar.2007.05.017
4. Onyiche TE, Suganuma K, Igarashi I, Yokoyama N, Xuan X, Thekisoe O. A review on equine piroplasmosis: epidemiology, vector ecology, risk factors, host immunity, diagnosis and control. Int J Environ Res Public Health. (2019) 16:e101736. doi: 10.3390/ijerph16101736
5. Rothschild CM. Equine piroplasmosis. J Equine Vet Sci. (2013) 33:497–508. doi: 10.1016/j.jevs.2013.03.189
6. Grause JF, Ueti MW, Nelson JT, Knowles DP, Kappmeyer LS, Bunn TO. Efficacy of imidocarb dipropionate in eliminating Theileria equi from experimentally infected horses. Vet J. (2013) 196:541–6. doi: 10.1016/j.tvjl.2012.10.025
7. Kumar S, Gupta AK, Pal Y, Dwivedi SK. In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. J Vet Med Sci. (2003) 65:1171–7. doi: 10.1292/jvms.65.1171
8. Schwint ON, Ueti MW, Palmer GH, Kappmeyer LS, Hines MT, Cordes RT, et al. Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob Agents Chemother. (2009) 53:4327–32. doi: 10.1128/AAC.00404-09
9. Dautel H, Dippel C, Oehme R, Hartelt K, Schettler E. Evidence for an increased geographical distribution of Dermacentor reticulatus in Germany and detection of Rickettsia sp. RpA4. Int J Med Microbiol. (2006) 296 Suppl 40:149–56. doi: 10.1016/j.ijmm.2006.01.013
10. Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip Perspect Infect Dis. (2009) 2009:593232. doi: 10.1155/2009/593232
11. Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. (2007) 7:585–95. doi: 10.1089/vbz.2007.0130
12. Catherine M. Butler MM, Stout TE, Jongejan F, Werners AH, Houwers DJ. Classification of ticks collected from horses in the Netherlands in 2008–2009 and identification of the (zoonotic) agents they contain. Pferdeheilkunde. (2016) 32:6. doi: 10.21836/PEM20160405
13. Butler CM, Sloet van Oldruitenborgh-Oosterbaan MM, Stout TA, van der Kolk JH, Wollenberg L, Nielen M, et al. Prevalence of the causative agents of equine piroplasmosis in the South West of The Netherlands and the identification of two autochthonous clinical Theileria equi infections. Vet J. (2012) 193:381–5. doi: 10.1016/j.tvjl.2011.12.014
14. OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (2021). Available online at: https://www.oie.int/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/
15. Kappmeyer LS, Perryman LE, Hines SA, Baszler TV, Katz JB, Hennager SG, et al. Detection of equine antibodies to babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. (1999) 37:2285–90. doi: 10.1128/JCM.37.7.2285-2290.1999
16. Scoles GA, Ueti MW. Vector ecology of equine piroplasmosis. Annu Rev Entomol. (2015) 60:561–80. doi: 10.1146/annurev-ento-010814-021110
17. Jongejan F, Ringenier M, Putting M, Berger L, Burgers S, Kortekaas R, et al. Novel foci of dermacentor reticulatus ticks infected with babesia canis and babesia caballi in the Netherlands and in Belgium. Parasit Vectors. (2015) 8:232. doi: 10.1186/s13071-015-0841-2
18. Sprong H, Fonville M, Docters van Leeuwen A, Devillers E, Ibanez-Justicia A, Stroo A, et al. Detection of pathogens in Dermacentor reticulatus in northwestern Europe: evaluation of a high-throughput array. Heliyon. (2019) 5:e01270. doi: 10.1016/j.heliyon.2019.e01270
19. Uiterwijk M, Ibanez-Justicia A, van de Vossenberg B, Jacobs F, Overgaauw P, Nijsse R, et al. Imported hyalomma ticks in the Netherlands 2018-2020. Parasit Vectors. (2021) 14:244. doi: 10.1186/s13071-021-04738-x
20. Tirosh-Levy S, Gottlieb Y, Fry LM, Knowles DP, Steinman A. Twenty years of equine piroplasmosis research: global distribution, molecular diagnosis, and phylogeny. Pathogens. (2020) 9:e110926. doi: 10.3390/pathogens9110926
21. Coultous RM, Phipps P, Dalley C, Lewis J, Hammond TA, Shiels BR, et al. Equine piroplasmosis status in the UK: an assessment of laboratory diagnostic submissions and techniques. Vet Rec. (2019) 184:95. doi: 10.1136/vr.104855
22. Sigg L, Gerber V, Gottstein B, Doherr MG, Frey CF. Seroprevalence of Babesia caballi and Theileria equi in the Swiss horse population. Parasitol Int. (2010) 59:313–7. doi: 10.1016/j.parint.2010.02.005
23. Coultous RM, Leadon DP, Shiels BR, Sutton D, Weir W. Investigating the presence of equine piroplasmosis in Ireland. Vet Rec. (2020) 187:e97. doi: 10.1136/vr.105937
Keywords: equine piroplasmosis, Babesia caballi, Theileria equi, seroprevalence, horses
Citation: Graham H, van Kalsbeek P, van der Goot J and Koene MGJ (2022) Low seroprevalence of equine piroplasmosis in horses exported from the Netherlands between 2015 and 2021. Front. Vet. Sci. 9:954046. doi: 10.3389/fvets.2022.954046
Received: 26 May 2022; Accepted: 20 September 2022;
Published: 10 October 2022.
Edited by:
Münir Aktaş, Firat University, TurkeyReviewed by:
Lachhman Das Singla, Guru Angad Dev Veterinary and Animal Sciences University, IndiaHeba Alzan, Washington State University, United States
Karla Georges, The University of the West Indies St. Augustine, Trinidad and Tobago
Copyright © 2022 Graham, van Kalsbeek, van der Goot and Koene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heather Graham, aGVhdGhlci5ncmFoYW1Ad3VyLm5s
 Heather Graham
Heather Graham Paul van Kalsbeek1
Paul van Kalsbeek1 Miriam G. J. Koene
Miriam G. J. Koene