
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 22 July 2022
Sec. Veterinary clinical, anatomical, and comparative pathology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.946702
This article is part of the Research Topic Current Knowledge on Camelids Infectious and Parasitic Diseases View all 11 articles
 Ahmed Gareh1
Ahmed Gareh1 Dalia Hassan2
Dalia Hassan2 Asmaa Essa3
Asmaa Essa3 Saber Kotb2
Saber Kotb2 Mohammed Karmi4
Mohammed Karmi4 Abou El-Hamd H. Mohamed5
Abou El-Hamd H. Mohamed5 Abeer Mousa Alkhaibari6
Abeer Mousa Alkhaibari6 Elzahara Elbaz7
Elzahara Elbaz7 Nagwa M. Elhawary8
Nagwa M. Elhawary8 Eman A. A. Hassanen9
Eman A. A. Hassanen9 Maha S. Lokman10,11
Maha S. Lokman10,11 Fatma A. El-Gohary12
Fatma A. El-Gohary12 Ehab Kotb Elmahallawy13*
Ehab Kotb Elmahallawy13*Tick infestation remains one of the major health problems that affect the productivity and comfort of camels. The control of ticks mainly relies on using chemical acaracides. Limited information is available on the potential benefits and activity of various neem extracts on Hyalomma ticks. The present study investigated the acaricidal activity of neem seed extracts at different concentrations against developmental stages of the camel tick Hyalomma dromedarii in comparison to Butox and diazinon. The acaricidal activity of three extracts, namely, hexane extract (HE), methyl chloride extract (MCE), and methanol extract (ME), of neem seeds (Azadirachta indica) were tested at varying concentrations of 5, 10, 15, and 20% on engorged H. dromedarii female ticks at days 1, 3, 5, 7, 12, 16, 20, 28, 37, and 43 after treatment (DPT). Interestingly, results of applying different neem seed extracts to engorged H. dromedarii female ticks showed that the most effective extract was hexane at concentration 20%, causing 100% mortality at 1st day post-application, while methanol extract at 20% and dichloromethane extract at 20% caused the death of all ticks at 28th day posttreatment as compared to Butox® 5.0 and Diazinon-60, which resulted in mortality of all ticks at 3 and 5 DPT, respectively. In addition, no mortality was reported with the application of aqueous extract (AE), which served as the control group. Furthermore, the neem hexane extract exhibited high efficacy against reproductive performance of female ticks, whereas no fertility or oviposition was reported at all of their concentrations. Additionally, no hatchability occurred using all neem extracts, except the aqueous extract, which showing no effect. In the present study, larvae responded more rapidly to the plant extracts, whereas mortality of all larvae was recorded at 24 h after treatment with 5% hexane. Taken together, this study pointed out that the acaricidal effect of hexane extract of neem seeds was more effective and could be economically used for controlling H. dromedarii ticks.
Camels are considered elemental part in the sophistication and farming of many countries around the world, being a good source of milk and meat, and serve as a means of transportation (1, 2). However, their productivity is hindered by a wide range of external and internal parasites, resulting in considerable economic losses (1, 3–6). Ticks are destructive blood-sucking ectoparasites of worldwide distribution and of a greater economic importance in tropical and subtropical areas (7, 8). Ticks have been considered potent vectors for the transmission of various bacterial, viral, and parasitic diseases of veterinary and medical importance. Therefore, ticks are considered a major contributor for several emerging and re-emerging diseases (9, 10). Given its important economic impacts, tick infestation in camels might result in a series of symptoms, ranging from mild to severe anemia; intense pruritus and deterioration the skin of affected animals; loss of appetite, leading to a reduction in growth rate and decreased productivity; and occasional mortalities in untreated and young animals, and therefore, they result in considerable economic losses (11, 12). To the authors' knowledge, Hyalomma dromedarii is considered the most reported Hyalomma spp. parasitizing camels in Egypt (13). The camel is considered the primary host containing the adult stage of Hyalomma spp.; however, it also infests other domestic animals, including cattle, sheep, goats, and equids. The remaining stages, including larvae and nymphs, feed on birds and small burrowing animals, but nymphs can infest large animals such as adults and complete its life cycle on two or three hosts (14).
The control of ticks is mainly based on the direct application or injecting of acaricides to animals. Several acaricides have been extensively used and recommended for the control of ticks including organophosphates, carbamates, pyrethroids, and amidines (15). However, several major drawbacks were reported for the majority of acaricides including alarming reports of tick resistance, residues in foods, and environmental pollution (16, 17). It is therefore not surprising to mention that there is an urgent need to develop eco-friendly effective alternatives for these chemicals. Interestingly, the use of herbal medications becomes a promising alternative approach for the treatment of various infectious agents because of their biodegradability, target efficiency, and cost-effectiveness, and therefore, they gained a considerable interest in tropical and subtropical regions (18–26). Among others, plant-derived materials and their bioactive substances were proposed as substitutes for synthetic acaricides due to their activity against ticks (27). As compared to synthetic ones, several previous reports revealed that herbal acaricides caused little environmental pollution and a low toxicity level to non-target organisms including humans, apart from the rapid biodegradation of their residues and their role in prevention of resistance development (28, 29).
Neem is considered one of the most reliable botanical sources of biopesticides with a wide range of biological activities (30). The neem tree, Azadirachta indica A. Juss, is an evergreen tree that originates from India and other neighboring countries (31–33). The functional ingredients of neem, including neem oil, bark, leaves, and their purified biochemical products, exhibited promising therapeutic effects and showed anticancer, anti-inflammatory, and antimicrobial properties (32–35). It is noteworthy to state that neem and its extracts showed potent activity against all the stages (adult, nymph, and larva) of ticks (29, 36, 37). Azadirachtin analogs, including azadirachtin A and its B counterpart, are among the most potent constituents isolated from the crude extracts of neem (38, 39). These analogs exhibited a wide range of biological properties, which include antifeedant, larvicidal, ovicidal, repellent action, growth deregulation, reduction in ecdysone levels, alterations in development and reproduction, sterility, and damage in molting processes (40). In accordance with its mechanisms of action against insects, neem is structurally analogous to insect hormones known as “ecdysone,” which are responsible for metamorphosis in insects. It is therefore not surprising to mention that the larvicidal and acaricidal properties of neem are the underlying bases for the use of neem products for control of agricultural pests.
Revising the available literature, limited information is available about the use of A. indica (neem) extracts against H. dromedarii. A previous study (41) showed the in vitro acaricidal activity of the methanolic extract of neem leaves against engorged adult female ticks, egg hatchability, and larvae of camel ticks (H. dromedarii) using the immersion method and in mortality rates of engorged ticks from 1st to 15th DPT (up to 100%), with some changes in morphology. Also, there was a potent activity of the methanolic extract of neem leaves on hatchability of H. dromedarii eggs (100%) from 1st to 15th DPT, and it induced 100% mortality on the newly hatched larvae of H. dromedarii ticks. Agreed with the aforementioned information, this study aimed to investigate the effect of neem extract application on ticks infesting one-humped camels in Aswan Governorate, Egypt, which might provide new insights into the control of hard ticks in camels.
The ethical approval of the present study was obtained from the Research, Publication, and Ethics Committee of the Faculty of Veterinary Medicine, Aswan University, Egypt, and the Institutional Review Approval Board Number is 2020/5.
Engorged adult, larvae, and nymphs of hard ticks were collected from naturally infested camels (5- to 15-year-olds) from Daraw market, Daraw city, Aswan Governorate, Egypt. In order to minimize damage to the mouthparts and cuticle, the ticks were manipulated by rotating for easy removal with a pair of soft forceps. The collected ticks were then placed in a clean plastic container with perforated lids to allow ventilation, then immediately transported to the laboratory at the Department of Parasitology, Faculty of Veterinary Medicine, Aswan University, for identification using standardized international keys and bioassays, as described elsewhere (14, 42, 43).
Hexane, diethyl ether, dichloromethane, and methanol solvents, Tween 20, Tween 80, TLC plates 20 × 20 cm, and vanillin spray reagent were purchased from Sigma Chemical Company, Cairo, Egypt. Synthetic chemical acaricides such as deltamethrin (Butox 5%) and Diazinon-60 were purchased from Arab Chemical Industrial Company, Cairo, Egypt.
Neem seeds were collected from an old neem tree located at Aswan University during July 2020 at 10 a.m. and identified and authenticated by Department of Botany, College of Science, Aswan University. The collected seeds were then transported to the Laboratory of Parasitology, Faculty of Veterinary Medicine, Aswan University, Egypt, and stored at 4°C until analysis.
The seeds were cleaned to remove any sticks, unwanted leaves, bad seeds, sand and dirt in order to ensure the oil produced is not contaminated and of high quality. The cleaned neem seeds were dried at 55°C for 72 h until constant weight and moisture content determined, as described elsewhere (44) using Equation 1:
where W1 is the weight of neem seeds before drying and W2 is the weight of neem seeds after drying.
The dried clean neem seeds were roasted about 5 min to enhance extraction of oil, then crushed in a blender, and sieved to obtain particles ranging from 425 to 710 μm in size. The sieved neem powder was then stored under vacuum in an airtight container at 4°C prior to use (45).
To obtain extracts from the neem seeds, about 9,000 ml of hexane was added to 3,000 g of the dried grounded neem seeds in a conical flask, which is then allowed to stand at room temperature for a period of 3 days with intermittent agitation (stirring) until the soluble matter dissolved. This mixture was filtered by gravity filtration, producing a hexane extract mixture as the filtrate, which was then concentrated using a rotary evaporator and stored at 4°C until further use (46). For the study, three more consecutive extracts, including dichloromethane, methanol, and aqueous extracts from residues have been collected using the same method. The yielded neem oil was calculated elsewhere (47) using the following equation (Equation 2).
where M1 is the mass of the neem seed before extraction and M2 is the mass of the neem seed after extraction.
The emulsion was prepared by mixing neem oil and two different non-ionic surfactants (Tween 20 and Tween 80) using each emulsifier separately, at rates of 1:5 and 1:3, respectively. Surfactants and deionized water were first mixed using a stirrer, and then neem oil was added. This step was followed by preparation of the different concentrations (5, 10, 15, and 20%) from each extracts as described elsewhere (48) with slight modification in which Tween 20 and Tween 80 were replaced by soap as a surfactant for complete blending of neem oil with water.
In this step, the plant extract was spotted on the plate with the aid of a capillary tube. The spot was applied 1 cm upward from the lower end of the TLC plate, then placed in a beaker consisting hexane:ether [(1:1), (2:1), (3:1), (5:1), and (6:1)] with drops of methanol and dichloromethane:methanol (7:1/4) as the mobile phase. Run spots were performed to separate the compounds. Later on, when the mobile phase reached the upper end of the TLC plate, it was removed from a beaker and was air dried. The TLC run plates were observed by using a UV spectrophotometer, and the separated spots were marked. Then, the spots were visualized by vanillin spray reagent. After spraying, the plates were immediately placed in the oven maintained at 1,100°C for 5–10 min (49). A preparative thin-layer chromatographic separation of the dichloromethane extract was applied.
Four concentrations (5, 10, 15, and 20%) of neem extract emulsions were used, while in case of aqueous emulsion, only two concentrations of 5 and 10% were tested. Also, 5% deltamethrin (Butox 5%) and Diazinon-60 were applied. The tested groups included 15 adult female ticks (three replicates for each concentration) that were weighed and immersed in their respective dilutions for 5 min. The adult immersion test was then performed by placing each group of ticks in separate petri dishes, and all plates were incubated at 27–30°C and 70%−80% relative humidity (RH). A negative control composed of the surfactant and distilled deionized water was included along the study. The number of live and dead ticks was counted during the period of 43 days (posttreatment period). The mortality rate of adult female ticks was calculated according to the following equation (Equation 3):
The acaricidal efficacy was calculated using the following equation (50), and the index of egg laying was calculated after completed oviposition (36 days), using the following formula (Equation 4), as described elsewhere (51):
The eggs were weighted and then incubated in test tubes at 27–30 °C and 70–80% RH. Later on, the percentage inhibition of egg laying was calculated after hatching 21 days by using the following formula (Equation 5):
The neem extract emulsions with the different concentrations (5, 10, 15, and 20%) were applied to its corresponding group of eggs, which were laid by a control group. The eggs (5-day-old eggs) were immersed in neem seed extract emulsions for 5 min and then were placed in open test tubes until drying emulsions, and then the test tubes were closed with cotton plugs to avoid contamination. All tubes were also incubated at 27–30°C and RH of 70%−80%. The control group eggs consisted of filter paper envelops immersed in the surfactant, and distilled deionized water was also used. After 21 days, all tubes were incubated at 40°C for counting, and hatchability was determined, as described elsewhere (52).
The eggs of the adult control group were placed in test tubes and incubated at 27–30°C and 70%−80% RH until they hatched into larvae. The larval packet test was performed using about 300–400 larvae (at 10-day-old larvae) which were then placed in filter paper envelopes immersed in neem seed extract emulsions with the different concentrations (5, 10, 15, and 20%) (53). All envelopes were kept under the same incubating conditions. Reading of the results was recorded under UV light with a magnifying glass, and larvae with no movement were considered dead. Furthermore, the mortality rate was calculated as described elsewhere (54) according to the following equation (Equation 6):
This step involved collection of nymphs from camel tail and ears. Each group of 15 nymphs was immersed in the various concentrations (5, 10, 15, and 20%) of neem extract emulsions for 5 min. Then, the nymphs were placed in separate petri dishes, and all plates were incubated at 27–30°C and 70%−80% RH. A negative control consisted of the surfactant, and distilled deionized water was also included. The number of live and dead nymphs of ticks was counted posttreatment during the whole time of the test, and the acaricidal efficacy was determined (50), using the following equation (Equation 7), and this step was performed on three replicates for each concentration.
The data related to effects of treatment using different concentrations of various neem seed extracts against the different stages of ticks (eggs, larva, nymph, and adult) and non-hatching eggs and antifeedant activity of the ticks were analyzed by one-way ANOVA. This was followed by using of Duncan multiple range tests (55) to determine the significant differences between treatments, and the least significant difference (LSD) test was used to separate the mean values, which were significant at the 95% level with Statistical Package for the Social Sciences (SPSS version 25). The level of significant association between treatments was considered at p < 0.05. Curves and the lethal concentrations (LC50 and LC90) were obtained at 95% using probit analysis with LDP line software (56).
Data presented in Table 1 show the effect of neem seed extracts, hexane extract (HE), dichloromethane extract (DE), methanol extract (ME), and aqueous extract (AE), used at a concentration of 5% as compared to the effect of Butox 5% and Diazinon-60 against H. dromedarii infestation in camels. As shown, the 5% hexane extract was the most effective extract, which showed evident changes and caused death of Hyalomma ticks from the first day of application with a percentage of 46.7%, and caused complete mortality of ticks at day 20. The methanol extract (ME) followed HE in its efficacy since and caused 100% mortality at day 37. On the other hand, little effect was obtained by uding dichloromethane, compared to that recorded with Butox® 5.0 and Diazinon-60, which caused mortality of all ticks at day 3 and day 5, respectively. By contrast, no mortality was recorded among control groups and following treatment with aqueous extracts. The data presented in Table 2 show that hexane extract 10% was the most effective extract, causing mortality to 90% of hard ticks on the first day of application and the death of all ticks at day 5, followed by methanol extract which resulted in 100% mortality at day 28, while little effect was obtained by dichloromethane extract. On the other hand, no mortality was recorded among control groups and because of treatment with aqueous extracts. Data presented in Table 3 indicate that 15% concentration of hexane extract of neem seeds revealed significantly higher mortality (90%) of ticks at the first day of application and 100% mortality at the third day, followed by 15% methanol extract which caused 100% mortality at day 28, while little effect was obtained by 15% dichloromethane extract. As shown in Table 4, 20% concentration of hexane extract of neem seeds was the most toxic extract and triggered 100% mortality of ticks at the first day of application, while the same concentration of methanol and dichloromethane extracts evoke a similar effect but at day 28.
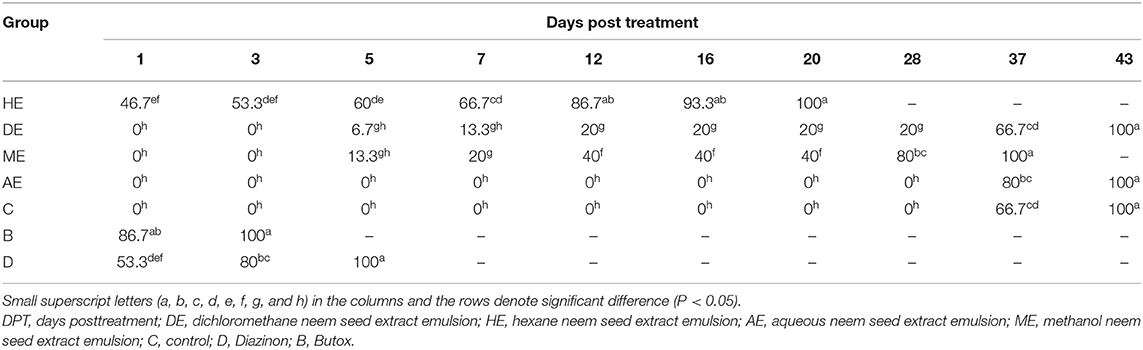
Table 1. In vitro mortality rates of engorged Hyalomma dromedarii treated with 5% hexane, methanol, dichloromethane, and aqueous extracts of neem seeds in comparison to some synthetic chemical materials (Butox 5%, Diazinon-60).
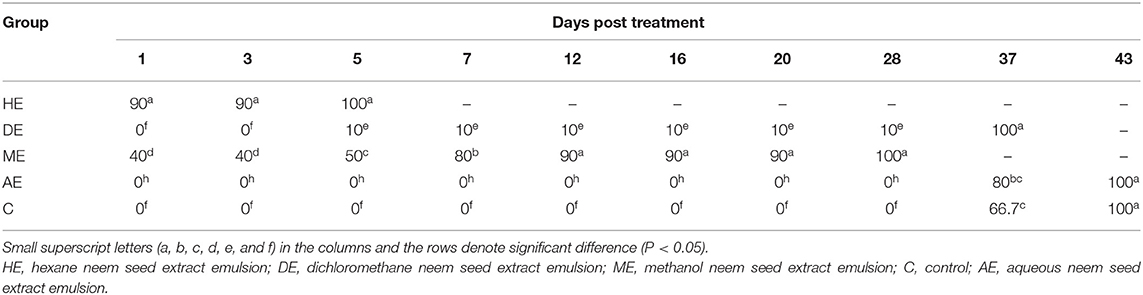
Table 2. In vitro mortality rates of engorged Hyalomma dromedarii treated with 10% hexane, methanol, and dichloromethane extracts of neem seeds.
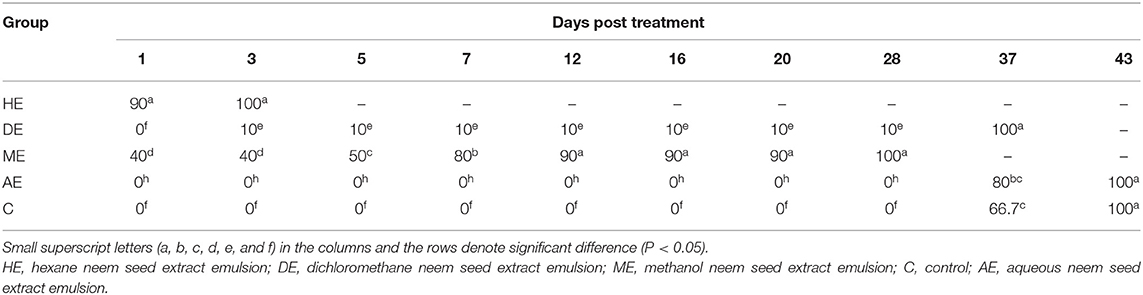
Table 3. In vitro mortality rates of engorged Hyalomma dromedarii treated with 15% hexane, methanol, and dichloromethane extracts of neem seeds.
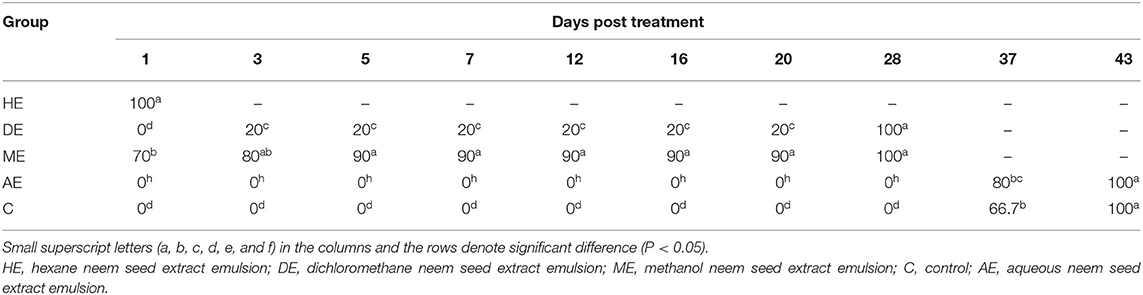
Table 4. In vitro mortality rates of engorged Hyalomma dromedarii treated with 20% hexane, methanol, and dichloromethane extracts of neem seeds.
Table 5 shows the reproductive performance, including oviposition and fertility percentage, of H. dromedarii engorged female ticks exposed to the effect of various concentrations of neem seed extracts. The results indicated that there was no oviposition with all concentrations of hexane extract, while oviposition percentages with application of methanol extract at concentrations of 5, 10, 15, and 20% were 18.8, 12.5, 7.5, and 2.5%, respectively. Furthermore, a little effect was obtained with the application of dichloromethane as the oviposition percentages were 87.5, 81.3, 75, and 68.8% at concentrations of 5, 10, 15, and 20%. The results also indicated that there was a significant effect for hexane extract on the fertility of H. dromedarii at all concentrations. In this regard, the fertility rates were 100, 95, 91.6, and 75%, and their corresponding concentrations of methanol extract concentrations were 5, 10, 15, and 20%, respectively. On the other hand, no effect was obtained upon application of aqueous extracts on oviposition and fertility of ticks. Data presented in Table 5 also show that the hatching rates reached 0% with the application of all extract concentrations, except aqueous extract that has no inhibitory effect.
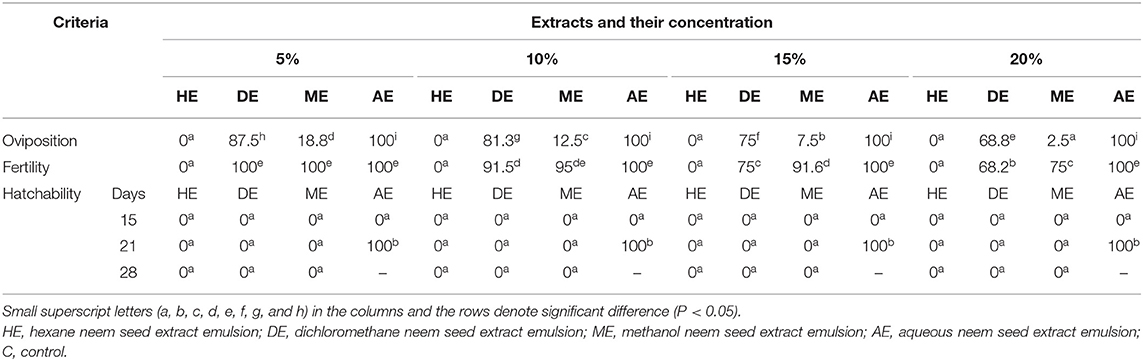
Table 5. Effect of 5, 10, 15, and 20% neem seed extracts on reproductive performance of Hyalomma dromedarii including oviposition, fertility, and hatchability.
Data presented in Table 6 show that the hexane extract of neem seeds, at all concentrations, induced high mortality rates of newly hatched larvae at the first day posttreatment, followed by methanol extract which caused death of all hatched larvae using concentrations of 10 and 15% first day posttreatment, and the mortality rate was 80% at 1st DPT and reached 100% at the third day of application of 5% methanol extract, while the lowest effect was attained by dichloromethane extract (concentration 5%) which resulted in 65% and 100% mortality of newly hatched larvae at 3rd and 5th days, respectively. Regarding 10% DE, it resulted in 75 and 100% mortality at 1st and 3rd days posttreatment, respectively, while at the concentration of 15%, death of all hatched larvae at 1st DPT occurred. In accordance with nymphs, as shown in Table 7, there was a significant effect of hexane extract and methanol extract on the mortality of H. dromedarii nymphs. In this regard, hexane extract at a concentration of 5% induced a mortality rate of nymphs reaching 80 and 100% at the first day posttreatment and 3rd day posttreatment, respectively. Furthermore, the mortality rate of nymphs reached 100% at concentrations of 10 and 15% of hexane extract at first day posttreatment. In the case of methanol extracts, the mortality of nymphs reached to 40 and 100% at the first day posttreatment and 3rd day posttreatment, respectively, while the mortality reached 100% at concentrations of 10 and 15% using the same extract on the first day posttreatment. On the other hand, the lowest effect was observed with dichloromethane, and no effect gained using the aqueous extract.
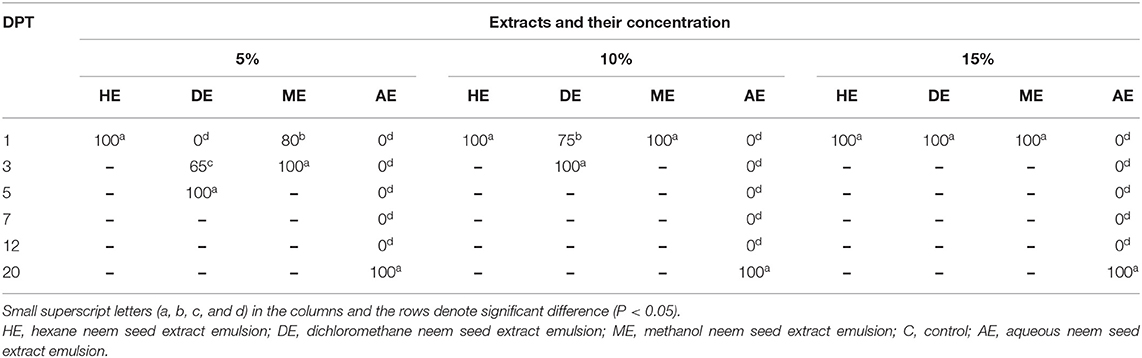
Table 6. In vitro mortality rates of Hyalomma spp. larvae treated with 5, 10, and 15% of hexane, methanol, dichloromethane, and aqueous extracts emulsions of neem seeds.
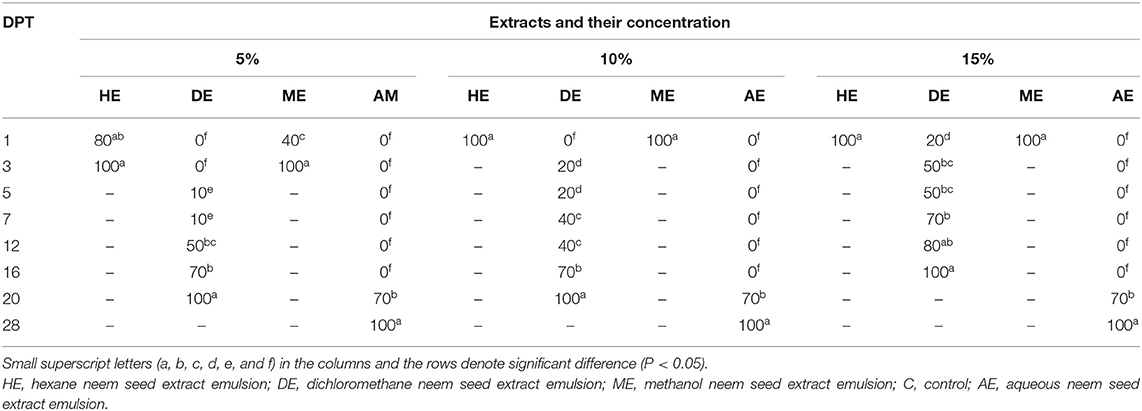
Table 7. In vitro mortality rates of Hyalomma spp. nymphs treated with 5, 10, and 15% of hexane, methanol, dichloromethane, and aqueous extract emulsions of neem seeds.
Chemical acaricides have been considered the main tick control strategy in domestic animals (57). However, the inappropriate use of acaricides and their wider application resulted in an emerging problem of the development of tick resistance to these acaricides (16). Clearly, there is an urgent need for developing environmentally friendly, safe, effective anti-tick natural products that can interrupt the life cycle and all biological processes of insects and dispersal as a herbal acaricide (58, 59). The present study revealed potent acaricidal activity of different concentrations of neem seed extracts against different stages of H. dromedarii collected from camels, and their activity seem to depend on the used concentration. As shown in Tables 1–4, the immersion of different concentrations (5, 10, 15, and 20%) of hexane extract (HE) of neem seeds (neem oil) for 5 min resulted in 100% mortality of adult female ticks at 20, 5, 3, and 1 days posttreatment (DPT), respectively. Meanwhile, Butox® 5.0 and Diazinon-60 induced in mortality of all ticks at 3 and 5 DPT, respectively. Furthermore, application of the aqueous extract (AE) of neem seeds caused the mortality of adult ticks 43 DPT, which also occurred in the control groups. The same effect was also observed in previous studies on Rhipicephalus microplus (60, 61). Furthermore, another previous report (62) revealed that azadirachtin causes a significant increase in the mortality rate of unfed adults, which reached to 100% on 15th DPT. A previous study documented the potent activity of neem against eggs, immature and adult stages of Hyalomma anatolicum excavatum at concentrations of 1.6, 3.2, 6.4, and 12.8%. In the same study, a significant increase in the hatching rate was observed during the first 7 days posttreatment, followed by incompletely developed and dead larvae, and then after 15 days, neem resulted in hatching failure and induced a significant increase in mortality rates of newly hatched larvae, unfed larvae, and unfed adults (63).
As shown in Tables 5–7, the present study revealed that the application of HE induced hatching failure and a high acaricidal effect on oviposition, egg hatchability larvae, and nymphs. Similarly, Al-Rajhy et al. (64) investigated the effects of neem on Hyalomma anatolicum ticks and revealed a high acaricidal effect of azadirachtin at low concentrations against larvae and nymphs. Another previous study concluded that various concentrations (10%−100%) of neem seed oil were able to kill all Boophilus decoloratus larvae in cattle after a period of 24–27 h (65). By contrast, the obtained results disagree with those of a previous study (64) which reported that azadirachtin had no effects on egg production with a significant reduction in the feeding activity of larvae and a 60% reduction in molting. Another previous study (62) documented the different effects of commercial neem seed oil (Neem Azal F) on H. anatolicum excavatum ticks that included an increased hatching rate and earlier hatching before the larvae were fully viable. As shown in our results (Tables 1–4), the used concentrations (5, 10, 15, and 20%) of the dichloromethane extract (DE) of neem seeds exhibited a low acaricidal effect on engorged adult ticks of H. dromedarii from the 5th day of application and continued up to an increase at 43th, 37th, 37th, and 28th DPT, respectively, resulting in 100% mortality. The possible explanation is the absence of azadirachtin in DE. Regarding the effect of DE against egg hatchability, it had a highly acaricidal effect on egg hatchability (Table 5) at all concentrations and a highly acaricidal effect on larvae at 15% concentration (Table 6), resulting in 100% mortality the 1st DPT. Similar effects were observed by Choudhury (66). The possible explanation might be attributed to the lethal effect of salannin compound (67). Salannin is one of the active components of neem with insect growth-regulating and antifeedant activity since it increases the larval stage duration and causes delayed molt, leading to decreased pupal weight that results in larval and pupal mortality (68). On the other hand, a low acaricidal effect of DE at 15% concentration was reported in nymphs, from the 1st day of application and continued up to 16th DPT, resulting in 100% mortality (Table 7). These findings are consistent with those of a previous study (62) which pointed out that DE contained a large amount of nimbin and salannin (69); nimbin had no significant effect on insects, but salannin had moderate antifeedant and growth-disrupting properties (67). Moreover, DE impaired oviposition at 5, 10, 15, and 20% concentrations by 87.5, 81.25, 75, and 68.75%. In addition, DE impaired the fertility by 100, 91.5, 75, and 68.25% at 5, 10, 15, and 20% concentrations, respectively.
Regarding the effect of methanol extract (ME) of neem seeds on ticks, which is shown in Tables 2–4, ME, at 10, 15, and 20% concentrations, exhibited a highly acaricidal effect against engorged adult ticks of H. dromedarii from the 1st day of application and continued up to 28th DPT, reaching 100% mortality. The present results are in agreement with those of a previous study which revealed the acaricidal effect of ME of neem leaves against H. dromedarii ticks. Moreover, all concentrations of ME had a lethal effect on oviposition and fertility, with a high acaricidal effect on egg hatchability without influence on hatching rate (Table 5). Our result is in agreement with that reported in several previous studies (41, 60, 70). Moreover, ME exhibited a high acaricidal effect at 10% concentration on larvae and nymphs from 1st DPT (Tables 6, 7), which is consistent with some previous reports (62). Importantly, the present study showed that the AE of neem seeds at all concentrations (Tables 1, 5–7) had no effect on the adult tick of H. dromedarii, egg hatchability, oviposition, larvae, and nymphs. These findings are in harmony with data reported by Tamirat et al. (61). This possible explanation of these findings could be attributed to the hypothesis that several polar compounds, like sugars and proteins, are eliminated in the aqueous extract (71). In the present work, the statistical analysis revealed a significant difference in the efficacy and effects of application of Butox (5%), diazinon, 10% hexane, and control group (p < 0.05), as well as between 10% hexane extract and Butox (5%) and diazinon (p > 0.05), while there is no significant relationship between Butox 5% and diazinon (p > 0.05). The tabulated data concluded that the efficiency of Butox 5% and diazinon was more than that of 10% hexane extract against infested ticks. Despite the fact that the data of our current study indicated that synthetic chemical insecticides were more efficient in controlling ticks than neem extract oil, the application of neem at higher doses on affected animals might offer many advantages for the control of ectoparasites without the risk of toxicity to them (72).
The study concluded that the in vitro application of neem extracts showed high efficacy against camel ticks. More importantly, the hexane extract exhibited a highly acaricidal effect on adult ticks of camels from the first day of application and continued up to 20 days after treatment, resulting in 100% mortality. The present data provide a platform for the development of environment-friendly, non-toxic, non-accumulating medicines against ectoparasites, which could be carried out in a large scale in animal farms.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Ethics Committee of the Faculty of Veterinary Medicine, Aswan University, Egypt, and the Institutional Review approval Board Number is 2020/5.
AG, DH, AE, SK, MK, and AM designed the idea of the conception, performed the methodology, formal analysis, data curation and supervision, and revised the manuscript. AA, EE, NE, EH, ML, FE-G, and EKE participated in the methodology, formal analysis, data curation, and scientific advice. AG, AE, SK, EE, and EKE drafted the manuscript, prepared the manuscript for publication, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the veterinarians for their support with the present work.
1. Kotb S, Abdel-Rady A. Sarcoptic mange of camel in upper Egypt: prevalence, risk assessment, and control measures. J Adv Vet Anim Res. (2015) 2:410–7. doi: 10.5455/javar.2015.b109
2. Burger PA, Ciani E, Faye B. Old world camels in a modern world - a balancing act between conservation and genetic improvement. Anim Genet. (2019) 50:598–612. doi: 10.1111/age.12858
3. Volpato G, Lamin Saleh SM, Di Nardo A. Ethnoveterinary of Sahrawi pastoralists of Western Sahara: camel diseases and remedies. J Ethnobiol Ethnomed. (2015) 11:1–22. doi: 10.1186/s13002-015-0040-4
4. Ahmed MA, Elmahallawy EK, Gareh A, Abdelbaset AE, El-Gohary FA, Elhawary NM, et al. Epidemiological and histopathological investigation of sarcoptic mange in camels in Egypt. Animals. (2020) 10:1485. doi: 10.3390/ani10091485
5. Gareh A, Soliman M, Saleh AA, El-Gohary FA, El-Sherbiny HMM, Mohamed RH, et al. Epidemiological and histopathological investigation of Sarcocystis spp. in slaughtered dromedary camels (Camelus dromedarius) in Egypt. Vet Sci. (2020) 7:162. doi: 10.3390/vetsci7040162
6. Gareh A, Saleh AA, Moustafa SM, Tahoun A, Baty RS, Khalifa RMA, et al. Epidemiological, morphometric, and molecular investigation of cystic echinococcosis in camel and cattle from upper Egypt: current status and zoonotic implications. Front Vet Sci. (2021) 8:750640. doi: 10.3389/fvets.2021.750640
7. El Tigani MA, Mohammed A. Ticks (Acari: Ixodidae) infesting camels in El Butana area mid-central sudan. Sudan J Vet Res. (2010) 25:51–4.
8. El-Gohary FA, Shokier KA, Elbably MA. Prevalence and risk determinants of ixodid tick infestation of cattle in Beni- suef governorate, Egypt. Ann Vet Anim Sci. (2016) 42–55.
9. Karesh WB, Cook RA, Bennett EL, Newcomb J. Wildlife trade and global disease emergence. Emerg Infect Dis. (2005) 11:1000. doi: 10.3201/eid1107.050194
10. Oguntomole O, Nwaeze U, Eremeeva ME. Tick-, flea-, and louse-borne diseases of public health and veterinary significance in Nigeria. Trop Med Infect Dis. (2018) 3:3. doi: 10.3390/tropicalmed3010003
11. Constantin T, Paraschiv I, Ionita M, Mitrea IL. The efficacy of different acaricides against the hard tick Dermacentor marginatus on infested sheep. Vet Med. (2012) 58:359−66.
12. Champour M, Mohammadi GR, Chinikar S, Razmi GR, Mostafavi E, Jalali T. Frequency of hard-ticks and the influence of age and sex of camel on ticks' infestation rates in one-humped camel (Camelus dromedarius) population in the northeast of Iran. Sci Parasitol. (2013) 14:89–93.
13. Alsarraf M, Mierzejewska EJ, Mohallal EM, Behnke JM, Bajer A. Genetic and phylogenetic analysis of the ticks from the Sinai Massif, Egypt, and their possible role in the transmission of Babesia behnkei. Exp Appl Acarol. (2017) 72:415–27. doi: 10.1007/s10493-017-0164-4
14. Walker AR. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Edinburgh: Bioscience Reports (2003).
15. De Meneghi D, Stachurski F, Adakal H. Experiences in tick control by acaricide in the traditional cattle sector in Zambia and Burkina Faso: possible environmental and public health implications. Front Public Health. (2016) 4:239. doi: 10.3389/fpubh.2016.00239
16. Ghosh S, Tiwari SS, Srivastava S, Sharma AK, Kumar S, Ray D, et al. Acaricidal properties of Ricinus communis leaf extracts against organophosphate and pyrethroids resistant Rhipicephalus (Boophilus) microplus. Vet Parasitol. (2013) 192:259–67. doi: 10.1016/j.vetpar.2012.09.031
17. Gill HK, Garg H. Pesticide: environmental impacts and management strategies. In: Larramendy ML, Soloneski S, editors. Pesticides-Toxic Aspects, Vol. 8. London: IntechOpen (2014). p. 187.
18. Elmahallawy EK, Jiménez-Aranda A, Martínez AS, Rodriguez-Granger J, Navarro-Alarcón M, Gutiérrez-Fernández J, et al. Activity of melatonin against Leishmania infantum promastigotes by mitochondrial dependent pathway. Chem Biol Interact. (2014) 220:84–93. doi: 10.1016/j.cbi.2014.06.016
19. Alkazzaz MA, Adel Aziz A, Elmahalawy E, Hassan A. Hematological profile in Schistosoma mansoni infected mice treated with Commiphora molmol extract compared with praziquantel. PSM Biol Res. (2018) 3:77−84.
20. Elmahallawy EK, Elshopakey GE, Saleh AA, Agil A, El-Morsey A, EL-shewehy DMM, et al. S-Methylcysteine (SMC) ameliorates intestinal, hepatic, and splenic damage induced by Cryptosporidium parvum infection via targeting inflammatory modulators and oxidative stress in swiss albino mice. Biomedicines. (2020) 8:423. doi: 10.3390/biomedicines8100423
21. Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complementary Altern Med. (2020) 2020:2560645. doi: 10.1155/2020/2560645
22. Elmahallawy EK, Mohamed Y, Abdo W, El-Gohary FA, Ahmed Awad Ali S, Yanai T. New insights into potential benefits of bioactive compounds of bee products on COVID-19: a review and assessment of recent research. Front Mol Biosci. (2021) 7:618318. doi: 10.3389/fmolb.2020.618318
23. Ramez AM, Elmahallawy EK, Elshopakey GE, Saleh AA, Moustafa SM, Al-Brakati A, et al. Hepatosplenic protective actions of spirulina platensis and matcha green tea against Schistosoma mansoni infection in mice via antioxidative and anti-inflammatory mechanisms. Front Vet Sci. (2021) 8:650531. doi: 10.3389/fvets.2021.650531
24. Tahmasbi SF, Revell MA, Tahmasebi N. Herbal medication to enhance or modulate viral infections. Nurs Clin North Am. (2021) 56:79–89. doi: 10.1016/j.cnur.2020.10.007
25. Zheoat AM, Alenezi S, Elmahallawy EK, Ungogo MA, Alghamdi AH, Watson DG, et al. Antitrypanosomal and antileishmanial activity of chalcones and flavanones from Polygonum salicifolium. Pathogens. (2021) 10:175. doi: 10.3390/pathogens10020175
26. Elmahallawy EK, Fehaid A, El-Shewehy DMM, Ramez AM, Alkhaldi AAM, Mady R, et al. S-methylcysteine ameliorates the intestinal damage induced by Eimeria tenella infection via targeting oxidative stress and inflammatory modulators. Front Vet Sci. (2022) 8:754991. doi: 10.3389/fvets.2021.754991
27. Abdel-Ghany H, Abdel-Shafy S, Abuowarda M, El-Khateeb R, Hoballah E, Fahmy M. Acaricidal activity of some medicinal plant extracts against different developmental stages of the camel tick Hyalomma dromedarii. Adv Anim Vet Sci. (2021) 9:722–33. doi: 10.17582/journal.aavs/2021/9.5.722.733
28. Rattan RS. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. (2010) 29:913–20. doi: 10.1016/j.cropro.2010.05.008
29. Singh NK, Vemu B, Nandi A, Singh H, Kumar R, Dumka V. Acaricidal activity of Cymbopogon winterianus, Vitex negundo and Withania somnifera against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Parasitol Res. (2014) 113:341–50. doi: 10.1007/s00436-013-3660-4
30. Raizada R, Srivastava M, Kaushal R, Singh R. Azadirachtin, a neem biopesticide: subchronic toxicity assessment in rats. Food Chem Toxico. (2001) 39:477–83. doi: 10.1016/S0278-6915(00)00153-8
31. Nisbet AJ. Azadiractina do nim, Azadirachta indica: sua ação contra insetos. An Soc Entomol Brasil. (2000) 29:615–32. doi: 10.1590/S0301-80592000000400001
32. Paul R, Prasad M, Sah NK. Anticancer biology of Azadirachta indica L (neem): a mini review. Cancer Biol Ther. (2011) 12:467–76. doi: 10.4161/cbt.12.6.16850
33. Avinash B, Venu R, Prasad TN, Raj MA, Rao KS, Srilatha C. Synthesis and characterisation of neem leaf extract, 2, 3-dehydrosalanol and quercetin dihydrate mediated silver nano particles for therapeutic applications. IET Nanobiotechnol. (2017) 11:383–9. doi: 10.1049/iet-nbt.2016.0095
34. Raut RR, Sawant AR, Jamge BB. Antimicrobial activity of Azadirachta indica (Neem) against pathogenic microorganisms. J Acad Ind Res.(2014) 3:327–9.
35. Kumar S, Vandana UK, Agrwal D, Hansa J. Analgesic, anti-inflammatory and anti-pyretic effects of Azadirachta indica (Neem) leaf extract in albino rats. Int J Sci Res. (2015) 4:713–21.
37. Ghosh S, Rawat A, Srivastava S, Rastogi S, Sharma A, Kumar S, et al. Identification of plant extract for ecofriendly development of phytoacaricides for controlling chemical resistant tick infestations in animals. Biochem Pharmacol. (2013) 2:226. doi: 10.4172/2167-0501.S1.004
38. Rembold H. Azadirachtins - their structures and mode of action. In: Arnason JT, Philogine BJR, Morand P, editors. Insecticides of Plant Origin. American Chemical Symposium Series No. 387. Washington DC: American Chemical Society (1989), pp. 150–63. doi: 10.1021/bk-1989-0387.ch011
39. Isman M. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. (2006) 51:45–66. doi: 10.1146/annurev.ento.51.110104.151146
40. Vietmeyer N. Neem: A Tree For Solving Global Problems. Report of an ad hoc Panel of the Board on Science and Technology for International Development. Washington, DC: National Academy Press (1992), p. 141.
41. Mahran M, Wahba A, Mansour K. In vitro acaricidal effect of Neem leaves (Azadirachta indica) and Citrullus Colocynthis extracts against the camel ticks, Hyalomma dromedarii (Acari: Ixodidae). J Ecosystem Ecography. (2020) 10:264–300.
42. Hoogstraal H. African Ixodoidea. Volume I. Ticks of the Sudan. Cairo: NAMRU-3 (1956). doi: 10.5962/bhl.title.6870
43. Essa AM, Kotb SA, Hussein MK, Dyab AK, Abdelazeem AG. Epidemiological and morphological studies on Hyalomma Species infesting dromedary camels In Aswan governorate, Egypt. J Egypt Soc Parasitol. (2022) 52:123–32. doi: 10.21608/jesp.2022.235828
44. Tesfaye B, Tefera T. Extraction of essential oil from neem seed by using soxhlet extraction methods. Int J Adv Eng Manag Sci. (2017) 3:239870. doi: 10.24001/ijaems.3.6.5
45. El Shafie H, Almahy A. Effect of storage conditions and duration on the potency of Neem (Azadirachta indica A. Juss) seeds as a home-made insecticide. Agric Biol J North Am. (2012) 3:385–90. doi: 10.5251/abjna.2012.3.10.385.390
46. Jadeja G, Maheshwari R, Naik S. Extraction of natural insecticide azadirachtin from neem (Azadirachta indica A. Juss) seed kernels using pressurized hot solvent. J Supercrit Fluids. (2011) 56:253–8. doi: 10.1016/j.supflu.2011.01.004
47. Adewoye TL, Ogunleye OO. Optimization of neem seed oil extraction process using response surface methodology. J Nat Sci Res. (2012) 2:66–75.
48. Swathy JS, Mishra P, Thomas J, Mukherjee A, Chandrasekaran N. Nanometric neem oil emulsification through microfluidization, and its therapeutic potential against Aeromonas culicicola infection in Cyprinus carpio. Flavour Fragr J. (2018) 33:340–50. doi: 10.1002/ffj.3453
50. Drummond R, Ernst S, Trevino J, Gladney W, Graham OH. Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Econ Entomol. (1973) 66:130–3. doi: 10.1093/jee/66.1.130
51. Sabatini G, Kemp D, Hughes S, Nari A, Hansen J. Tests to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick, Boophilus microplus. Vet Parasitol. (2001) 95:53–62. doi: 10.1016/S0304-4017(00)00406-4
52. Bicalho KA, Ferreira F, Borges LMF, Ribeiro MFB. In vitro evaluation of the effects of some acaricides on life stages of Rhipicephalus sanguineus (Acari: Ixodidae). Arq Bras Med Vet Zootec. (2001) 53:548–52. doi: 10.1590/S0102-09352001000500006
53. FAO. Resistance Management and Integrated Parasite Control in Ruminants Guidelines. Module 1. Ticks: Acaricide Resistance: Diagnosis, Management and: Prevention. Rome: FAO, Animal Production and Health Division (2004), p. 25–77.
54. Luguru S, Banda D, Pegram R. Susceptibility of ticks to acaricides in Zambia. Trop Anim Health Prod. (1984) 16:21–6. doi: 10.1007/BF02248923
55. Duncan DB. Multiple range and multiple F tests. Biometrics. (1955) 11:1–42. doi: 10.2307/3001478
56. Bakr E,. LDP Line software. (2010). Available online at: http://www.ehabsoft.com/ldpline (accessed March 15, 2022).
57. Rajput ZI, Hu S-H, Chen W-J, Arijo AG, Xiao C-W. Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci B. (2006) 7:912–21. doi: 10.1631/jzus.2006.B0912
58. Habeeb SM. Ethno-veterinary and medical knowledge of crude plant extracts and its methods of application (traditional and modern) for tick control. World Appl Sci J. (2010) 11:1047–54.
59. Zaman MA, Iqbal Z, Abbas RZ, Khan MN, Muhammad G, Younus M, et al. In vitro and in vivo acaricidal activity of a herbal extract. Vet Parasitol. (2012) 186:431–6. doi: 10.1016/j.vetpar.2011.11.018
60. Giglioti R, Forim M, Oliveira HN, Chagas A, Ferrezini J, Brito L, et al. In vitro acaricidal activity of neem (Azadirachta indica) seed extracts with known azadirachtin concentrations against Rhipicephalus microplus. Vet Parasitol. (2011) 181:309–15. doi: 10.1016/j.vetpar.2011.03.053
61. Tamirat S, Basu A, Getachew T, Bersisa K. Study on the acaricidal effects of Azadirachta indica and Phytolacca dodecandra on Amblyomma ticks in Ethiopia. Ethiop Vet J (2014) 18:1–14.
62. Abdel-Shafy S, Zayed A. In vitro acaricidal effect of plant extract of neem seed oil (Azadirachta indica) on egg, immature, and adult stages of Hyalomma anatolicum excavatum (Ixodoidea: Ixodidae). Vet Parasitol. (2002) 106:89–96. doi: 10.1016/S0304-4017(02)00023-7
63. Atawodi SE, Atawodi JC. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev. (2009) 8:601–20. doi: 10.1007/s11101-009-9144-6
64. Al-Rajhy DH, Alahmed AM, Hussein HI, Kheir SM. Acaricidal effects of cardiac glycosides, azadirachtin and neem oil against the camel tick, Hyalomma dromedarii (Acari: Ixodidae). Pest Manag Sci. (2003) 59:1250–4. doi: 10.1002/ps.748
65. Choudhury M. Toxicity of neem seed oil against the larvae of Boophilus decoloratus, a one-host tick in cattle. Indian J Pharm Sci. (2009) 71:562–3. doi: 10.4103/0250-474X.58191
66. Choudhury M. Toxicity of neem seed oil (Azadirachta indica) against the larvae of Rhipicephalus sanguineus a three-host tick in dog. J Parasitic Dis. (2001) 25:46–7.
67. Morgan E, Wilson I. Insect Hormones and Insect Chemical Ecology, Vol. 8. Basle, Switzerland: Comprehensive Natural Products Chemistry Elsevier (1999), p. 324. doi: 10.1016/B978-0-08-091283-7.00053-9
68. Govindachari T, Narasimhan N, Suresh G, Partho P, Gopalakrishnan G. Insect antifeedant and growth-regulating activities of salannin and other C-seco limonoids from neem oil in relation to azadirachtin. J Chem Ecol. (1996) 22:1453–61. doi: 10.1007/BF02027724
69. Jarvis AP, Johnson S, Morgan ED, Simmonds MS, Blaney WM. Photooxidation of nimbin and salannin, tetranortriterpenoids from the neem tree (Azadirachta indica). J Chem Ecol. (1997) 23:2841–60. doi: 10.1023/A:1022523329255
70. Srivastava R, Ghosh S, Mandal D, Azhahianambi P, Singhal P, Pandey N, et al. Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol Res. (2008) 104:149–53. doi: 10.1007/s00436-008-1173-3
71. Sinha S, Murthy P, Rao C, Ramaprasad G, Sitaramaiah S, Kumar D, et al. Simple method for Enrichment of Azadirachtin From Neem Seeds. Rajahmundry (1999).
Keywords: acaricidal properties, neem seed extracts, eggs, immature, adult, camel tick
Citation: Gareh A, Hassan D, Essa A, Kotb S, Karmi M, Mohamed AE-HH, Alkhaibari AM, Elbaz E, Elhawary NM, Hassanen EAA, Lokman MS, El-Gohary FA and Elmahallawy EK (2022) Acaricidal Properties of Four Neem Seed Extracts (Azadirachta indica) on the Camel Tick Hyalomma dromedarii (Acari: Ixodidae). Front. Vet. Sci. 9:946702. doi: 10.3389/fvets.2022.946702
Received: 17 May 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
Alireza Sazmand, Bu-Ali Sina University, IranReviewed by:
Asadollah Hosseini-Chegeni, Lorestan University, IranCopyright © 2022 Gareh, Hassan, Essa, Kotb, Karmi, Mohamed, Alkhaibari, Elbaz, Elhawary, Hassanen, Lokman, El-Gohary and Elmahallawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ehab Kotb Elmahallawy, ZWVoYWFAdW5pbGVvbi5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.