- 1College of Animal Science and Technology, Jilin Agricultural University, Changchun, China
- 2College of Chinese Medicine Materials, Jilin Agricultural University, Changchun, China
- 3Laboratory of Production and Product Application of Sika Deer of Jilin, Jilin Agricultural University, Changchun, China
- 4Animal Health Supervision Institute of Jilin Province, Changchun, China
- 5Key Laboratory of Animal Production, Product Quality and Security, Ministry of Education, Jilin Agricultural University, Changchun, China
Leptospirosis is an acute infectious disease caused by pathogenic bacteria from the genus Leptospira. The disease is widely distributed throughout China, causing harm to human and animal health. Murine may naturally carry a variety of pathogenic Leptospira, thus being important sources of infection by humans and livestock. The aim of this study was to assess and analyse the prevalence of Leptospira and its risk factors in murine. We collected 46 publications published between inception and 2022 through China Knowledge Network (CNKI), VIP Chinese Journal Database, Wanfang Database, PubMed, and ScienceDirect. In these studies, a total of 54,051 murine in 5 regions of China were investigated, and the prevalence of leptospirosis ranged from 1.11 to 35.29%. The prevalence of murine leptospirosis in south China was the highest, at 20.13%, and the lowest in northeast China, at 1.11% (P < 0.05). The prevalence of leptospirosis in male murine was 21.38%, which was significantly higher than that in females (17.07%; P < 0.05). Results according to detection method subgroup showed that the prevalence from serological testing was 15.94%, which was significantly higher than that of etiology and molecular biology methods (P < 0.01). In the sample subgroup, the positive rate of serum samples was 15.30%, which was significantly higher than that of tissue samples, at 7.97%. In addition, the influence of different geographical factors on prevalence was analyzed, indicating that the Yangtze River Basin was a high-incidence area for leptospirosis. The study showed that Leptospira were ubiquitous throughout the country, and factors such as environment, temperature and landform affect the murine distribution and their bacteria carrying rate. We suggest strengthening the continuous monitoring of leptospirosis and taking effective and comprehensive measures such as reducing water contact, vaccinating in high-incidence seasons, and avoiding human contamination caused by water pollution and contact with infected murine.
Introduction
Leptospirosis is a global zoonosis caused by pathogenic bacteria from the genus Leptospira. The infection occurs especially in tropical and subtropical regions and can cause symptomatic disease in humans and many species of animals (1, 2). Leptospira can be divided into three evolutionary lineages (pathogenic, intermediate, and saprophytic), with more than 300 serovars (3, 4). It is a highly heterogeneous bacterial genus, and there are great differences in the prevailing species and distribution in different places. However, because of a lack of cross-immunity among various types, preventing and controlling the resulting disease is extremely difficult (5).
Murine are important disease reservoirs, with about 90% species worldwide carrying more than 200 different pathogenic microorganisms. As many as 57 types of these microorganisms are pathogenic to humans, causing 31 viral diseases, 14 bacterial diseases, 5 rickettsial diseases, and 7 parasitic diseases (6, 7). In many natural foci, the most active diseases are murine-borne infectious diseases. Previous outbreaks of murine-borne diseases have brought destructive disasters to human society (8). Since 2001 alone, there have been 14 outbreaks of infectious diseases in the world (9). In China, murine have been confirmed to carry the rabies virus (10), Japanese encephalitis virus (11), tick-borne rickettsia (12), leptospirosis and other zoonotic pathogens. In recent years especially, a variety of subtypes and new pathogens causing murine-borne infectious diseases have been discovered, making the prevention and control of murine-borne diseases increasingly necessary (13).
Murine can naturally carry a variety of pathogenic Leptospira, and they are the most important source of infection of leptospirosis in humans and livestock. Leptospira excreted through urine can survive in contaminated water or soil for several months. Leptospirosis in humans is mostly acquired through direct contact with infected animals or indirect contact with urine-contaminated environments (14, 15). Most infected people have subclinical or mild symptoms, but if not diagnosed and treated early may progress to a severe disease characterized by liver, kidney or lung dysfunction, or bleeding manifestations. Symptoms such as pulmonary haemorrhagic syndrome may also appear in the early stage in a few infected persons (16).
Leptospirosis is widely distributed worldwide and is more prevalent in tropical and subtropical regions. At least 200 species of animals in the world have been reported as natural carriers of pathogenic Leptospira, and 67 wild and domestic species in mainland China have been proven to host pathogenic Leptospira (17). China is one of the countries with a relatively high burden of leptospirosis, in only a few provinces and autonomous regions such as Qinghai, Xinjiang and Gansu has the disease not been found. Other regions particularly affected are Guangdong and Sichuan (17). Although the overall incidence of leptospirosis in China is currently at a low level, outbreaks still occur in some areas due to factors such as climate and changes in host animal populations (18).
No systematic analysis of the prevalence of murine Leptospira has been conducted in China. This study employed a systematic review and meta-analysis to analyze the prevalence of murine leptospirosis to assess potential risk factors associated with the disease. This investigation can help to understand the conditions that may favor the infection, which will have significance in guiding risk assessments to prevent epidemics of human leptospirosis.
Methods
Search strategy and selection criteria
We used the PRISMA reporting system to report the results of our systematic review and meta-analysis (19) and retrieved articles from the following five databases: ScienceDirect, PubMed, Chinese Web of Knowledge, the VIP Chinese journal database, and Wanfang database. All English or Chinese papers on murine leptospirosis published between database inception and January 28, 2022 were included in our research scope. The search strategy was presented in Supplementary Table S2.
The following criteria were used in the selection of studies: (1) The study must have detected the prevalence of leptospirosis in murine in China. (2) It must have included the total number of tested animals and the number of positives. (3) Articles had to be published in Chinese or English; and (4) each sample must have been from a single animal (not a pooled sample). Studies inconsistent with all the above criteria were removed. Duplicate studies, review studies and data of which full-text access was not obtained were also excluded.
Data extraction and quality assessment
We collected the following information from the incorporated studies: first author, publication year, sampling year, geographic region, province, sample type, sex, breed, season, detection method, Leptospira serovars, leptospirosis prevalence and the quality of research. We further assessed the impact of geographic factors on this study, including humidity (60–70% vs. others), latitude (21–25 degrees vs. others), longitude (100–110 degrees vs. others), precipitation (500–1500 mm vs. others), altitude (0–100 m vs. others), average annual temperature (10–15°C vs. others) and topography (mountainous vs. others). The database was established using Microsoft Excel (version 16.32).
The quality of each study was assessed according to the grading criteria of the Recommended Assessment, Development and Evaluation (GRADE) method (20–22). If the study clearly described the detection method, sampling method and timing, and random sampling, and there were four or more potential risk factors, each item was awarded 1 point. The studies were divided into three grades: 0–1 point, 2–3 points, 4–5 points.
Statistical analysis
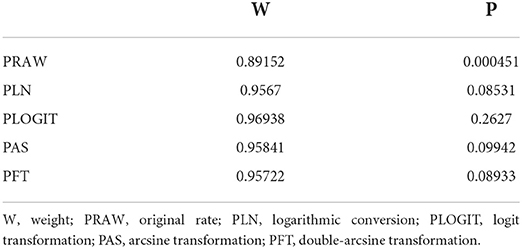
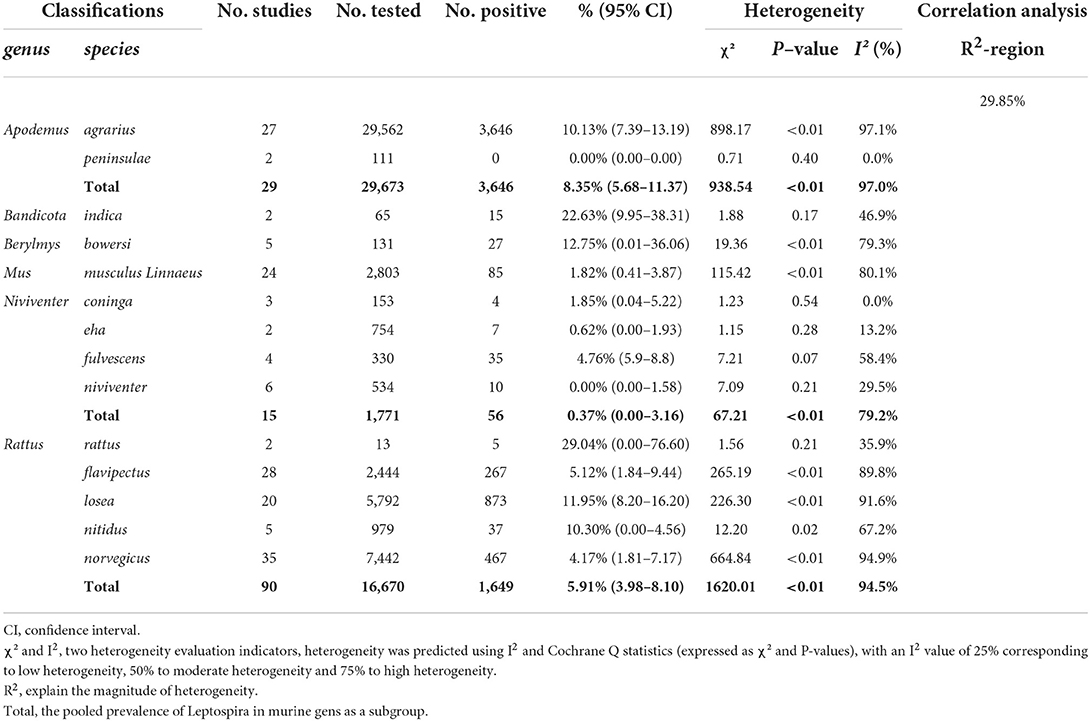
Data synthesis was performed using the “meta” package in R (version 4.0.0) software (23). On the basis of previous research, we used the double-arcsine transformation method (PFT) to perform a combined calculation of rates prior to the meta-analysis [(24–26), Table 1]. We applied a random-effects model to combine total effect size and subgroup analysis to avoid high heterogeneity owing to paired analysis. Heterogeneity was predicted using I2 and Cochrane Q statistics (expressed as χ2 and P-values), with an I2 value of 25% corresponding to low heterogeneity, 50% to moderate heterogeneity and 75% to high heterogeneity. A funnel plot and Egger's test were used to assess publication bias, and trim-and-fill analysis was used to adjust publication bias. The stability of the results was verified by sensitivity analysis. Through subgroup analysis and univariate regression analysis we identified factors contributing to the heterogeneity. Survey factors include region (eastern China and other regions), sampling year (1960 to 2009 and 2010 to 2020), detection method (serological testing methods include enzyme linked immunosorbent assay, modified agglutination test, hemagglutination test, complement fixation test; etiological testing methods include isolating culture, silver impregnation; and nucleic acid detection include polymerase chain reaction), sample (serum and tissues), sex (female vs. male), season (spring, summer and autumn), Leptospira serovars (Leptospira borgpetersenii serovar Ballum, Leptospira kirschneri serovar Pomona, etc.), murine species (Niviventer coninga, Rattus nitidus, Rattus norvegicus, etc.) and study quality (high and medium).
Results
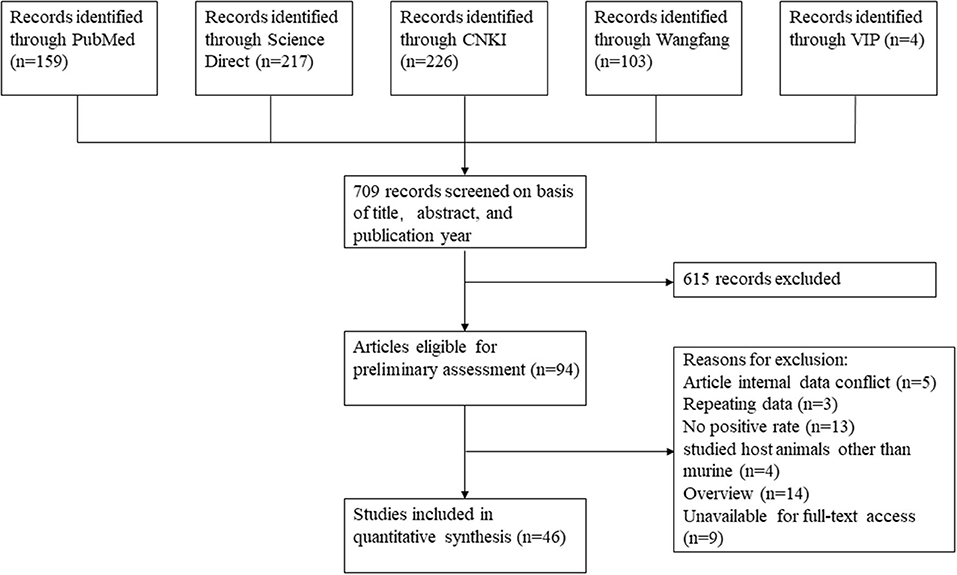
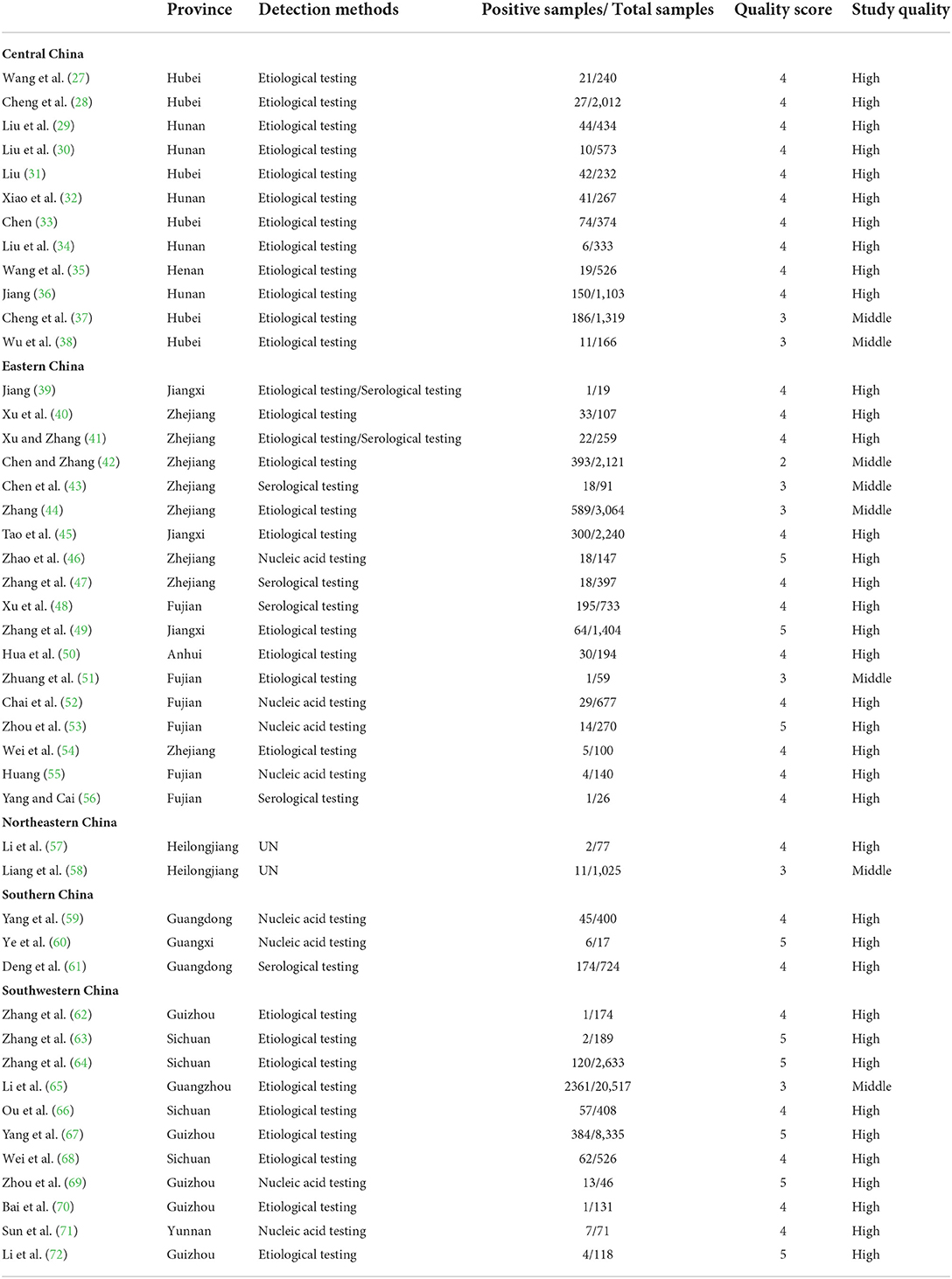
Based on our search criteria, we searched five databases and performed a meta-analysis of 46 publications, including 37 high-quality papers (4 points or 5 points) and 9 medium-quality papers (2 points or 3 points) (Figure 1). The choice of a random-effects model for the meta-analysis was appropriate. The results of the forest plot showed that the study had high heterogeneity (χ2 = 2012.16, I2 = 98%, P = 0.00; Figure 2). We used funnel plots and Egger's test to determine heterogeneity or publication bias (Figures 3, 4) and no significant publication bias was found (t = −0.489, P = 0.628) (Supplementary Table S3). Trim-and-fill analysis indicated that some studies would be included, but the effect on publication bias was not significant and the findings were relatively robust (Figure 5). In conclusion, our study was free of publication bias, but other heterogeneity or minor study effect bias may have been present. In addition, we further assessed publication bias in all subgroups using funnel plots (Supplementary Figures S1–S11). Sensitivity analyses revealed that none of the studies had a significant effect on the pooled prevalence of leptospirosis; therefore, we affirmed the reliability of our meta-analysis (Figure 6).
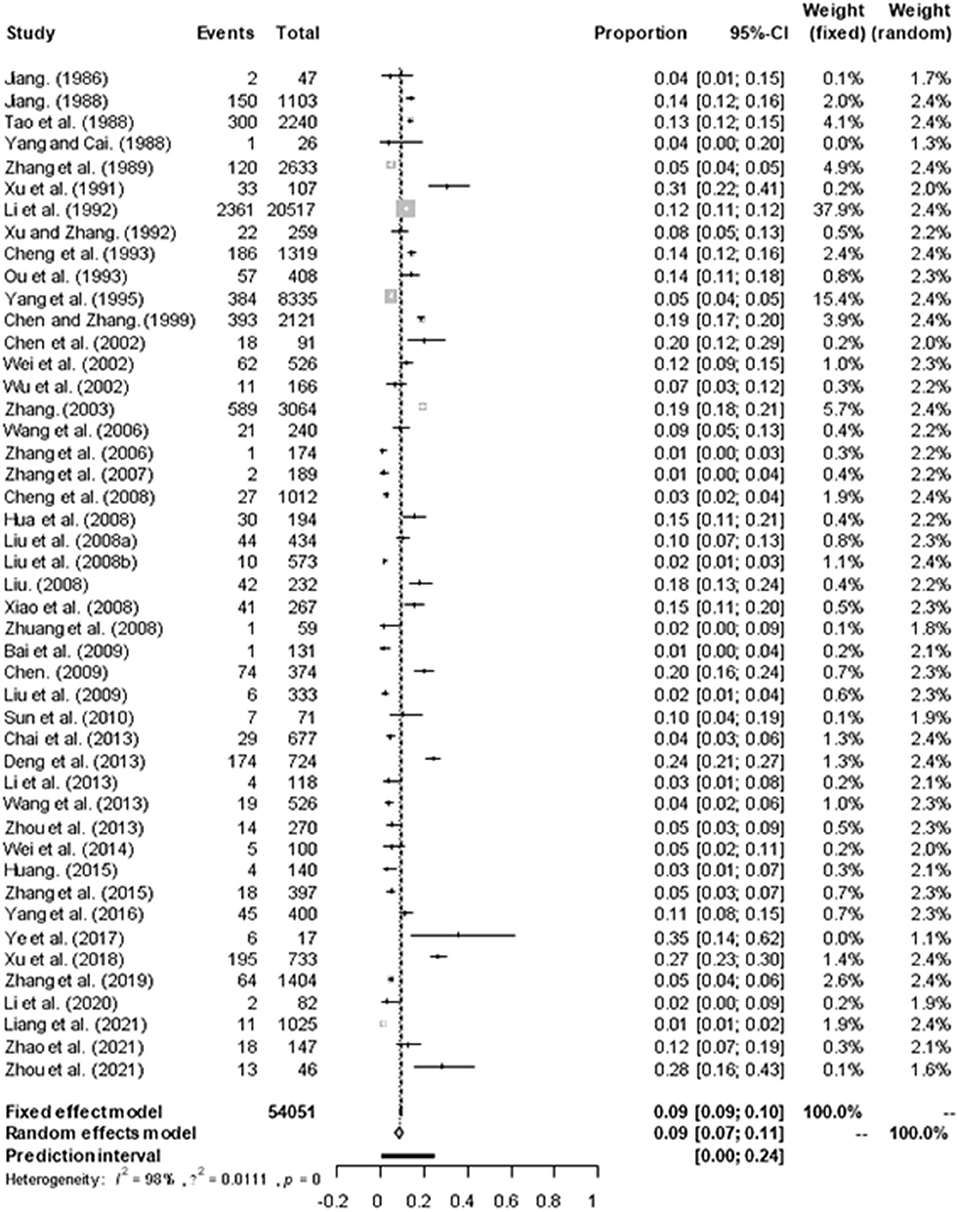
Figure 2. Forest plot of the prevalence of Leptospira in murine of China. “Study” represents the included studies; “Events” is the number of positive cases of Leptospira murine; “Total” is the total number of samples in each group; “Proportion” represents the prevalence, “CI” represents the confidence interval, “Weight” is the representative weight in the fixed and random models. The gray diamond at the bottom represents the total prevalence, the long vertical dotted line in the middle represents the meta-analysis results, and the intersection with the horizontal axis is the total OR value of 0.09, the short horizontal line represents the confidence interval of the study, the position of the short vertical line represents the OR value of each study, and the size of the short vertical line represents the weight.
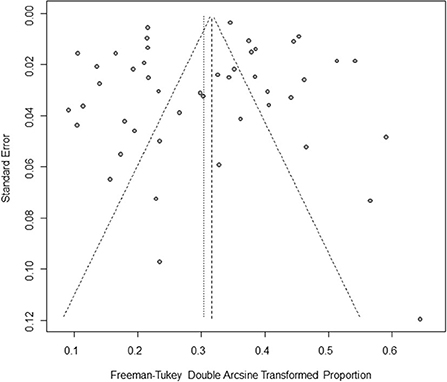
Figure 3. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias. The horizontal axis represents effect size, which is a measure of prevalence estimates (double arcsine transform). The vertical axis represents the transformed standard error. The gray origin represents the original study. The vertical line in the middle represents the combined effect (or main effect), and the slashed area on both sides of the vertical line represents the 95% confidence interval of the combined effect at different standard error scales. Since the slashes on both sides are not strictly 95% confidence intervals, they are called “pseudo 95% confidence limits”.
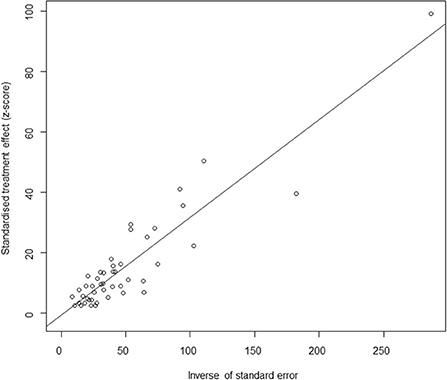
Figure 4. Egger's test for publication bias. Egger's test builds a simple linear regression model with the standardized effect estimator as the dependent variable and the precision of the effect estimator (the inverse of the standard error) as the independent variable. Circles represent each included study.
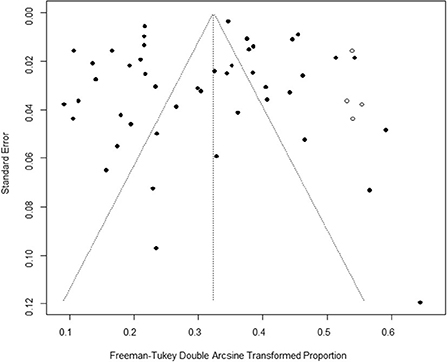
Figure 5. Trim-and-fill analysis. The vertical axis is the standard error, the horizontal axis is the Freeman-Tukey double Arcsine Transformed Proportion, the black dot represents the included studies, and the white dot represents the virtual studies.
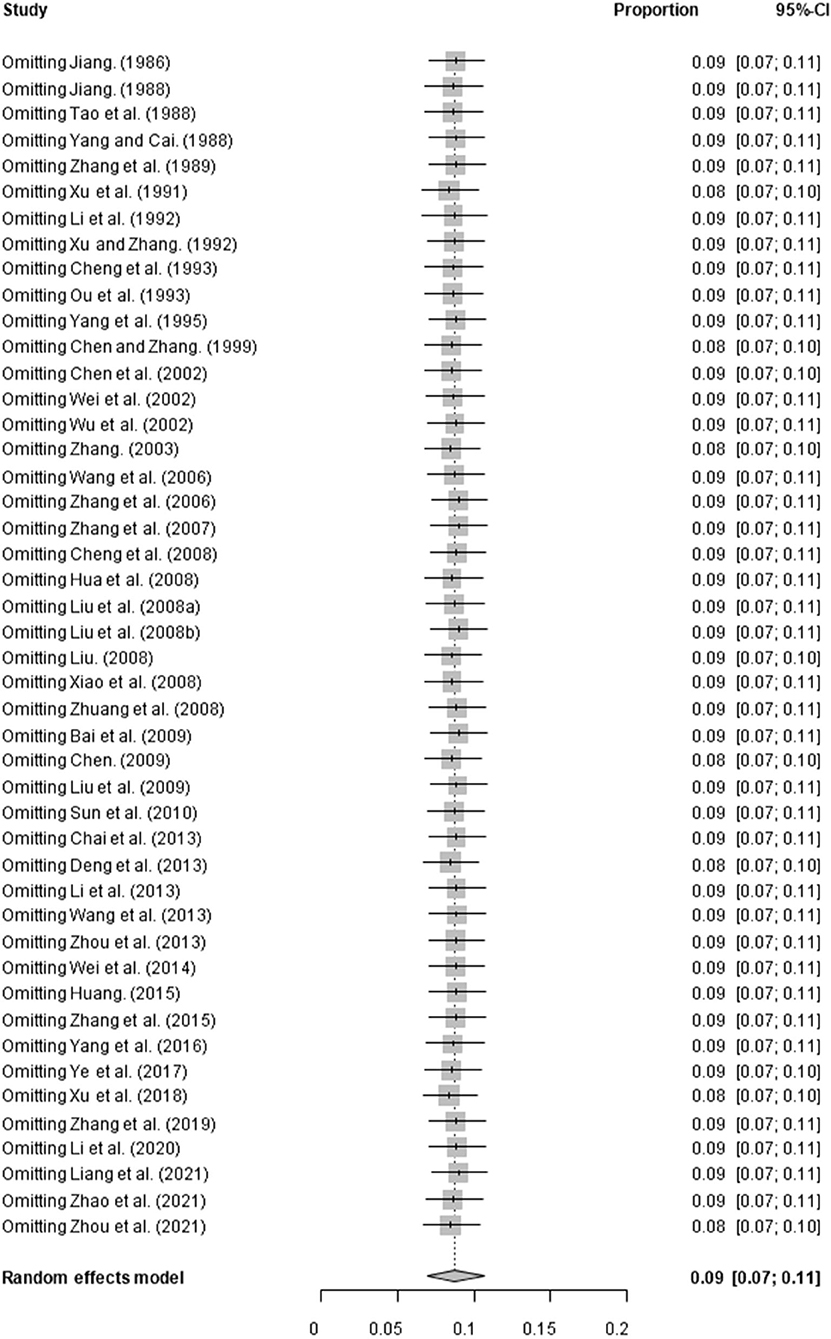
Figure 6. Sensitivity analysis. “Study” represents omitting study; “Proportion” represents pooled prevalence. The gray diamond at the bottom represents the total prevalence, the short horizontal line represents the confidence interval for the study. The short vertical line corresponding to each study represents the combined effect of the remaining studies after deleting the study, and the size of the gray square represents the size of the confidence interval.
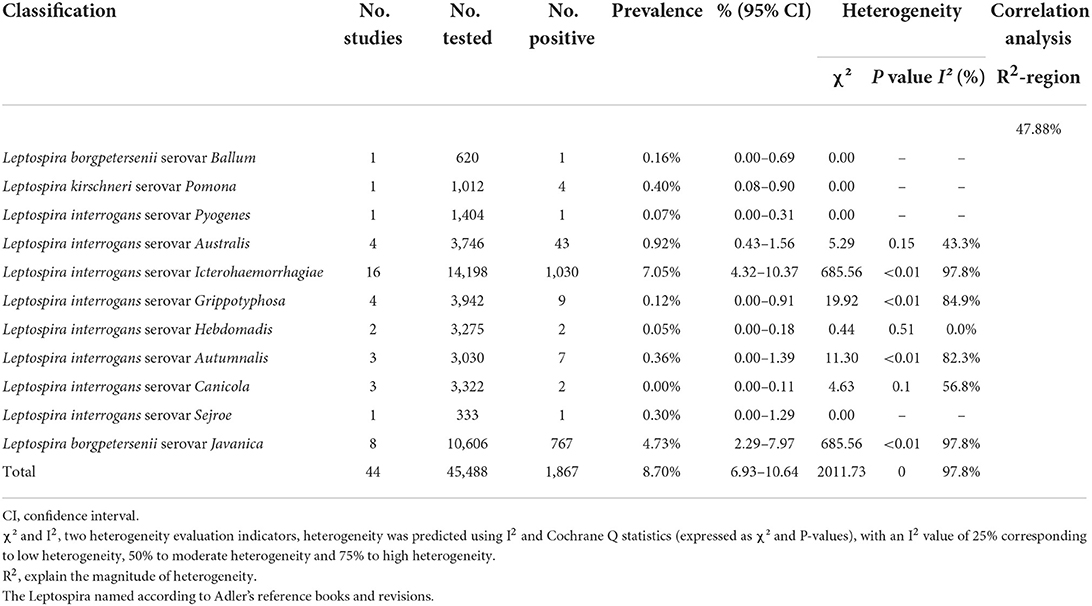
In 46 studies, a total of 54,051 murine in 5 regions of China were investigated, with leptospirosis prevalence ranging from 1.11 to 35.29% (Table 2, Figure 7). The pooled prevalence of Leptospira in murine was 8.7% (95% confidence interval [CI]: 6.93–10.64%, 5617/54051; Table 4). We performed a subgroup analysis of murine species and found that the prevalence of different murine Leptospira species ranged from 0.00 to 29.04% (P < 0.05) (Table 5), and the prevalence of different Leptospira serovars was between 0.00 to 7.05% (Table 4). Analyzed from a geographic point of view, the prevalence of leptospirosis in murine was the highest in southern China at 20.13% (95%CI: 9.36%−33.51%, 225/1141) and the lowest in northeast China at 1.11% (95%CI: 0.18%−2.61%, 13/1107); the difference was statistically significant (P < 0.05).
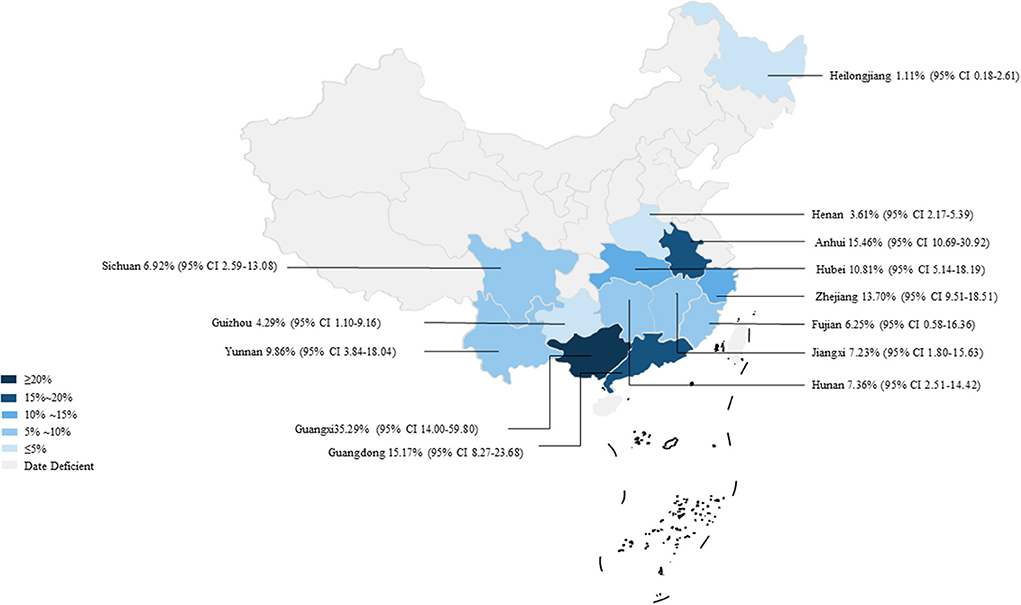
Figure 7. Map of Leptospira in murine in China. Different saturations in the HSB slider represent different infection rates.
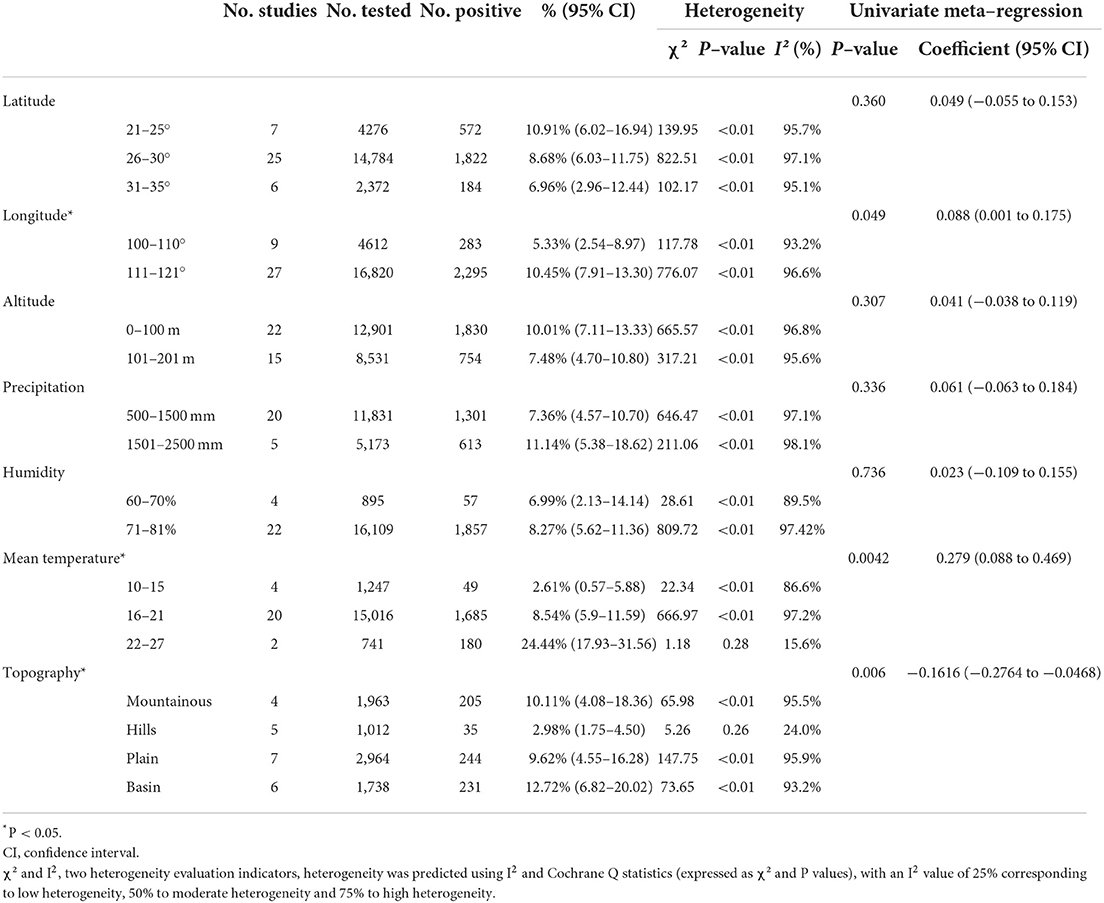
We analyzed the effects of geographic distribution and factors, sampling year, detection method, sex, sample type, season, and study quality on the prevalence of murine leptospirosis (Table 3). All estimates for each subgroup were made using a random-effects model. Among the provinces, the prevalence of murine leptospirosis was the highest in Guangxi Province, at 35.29%, and the lowest in Heilongjiang Province, at 1.11% (Figure 7). In gender subgroups, the prevalence of leptospirosis in males (21.38%, 95%CI: 17.11–26.00%; Table 3) was significantly higher than in females (17.07%, 95%CI: 15.34%−18.87%; P < 0.05). Among the three types of assays–etiological, nucleic acid and serological–the serological assay had a higher prevalence (15.94%, 95%CI: 7.94–25.91%, Table 3) and the difference was highly significant (P < 0.01). In the sample subgroup, the positive rate of serum samples (15.30%, 95%CI: 8.34–23.79%) was significantly higher than that of kidney samples (7.97%, 95%CI: 6.21–9.91%). Univariate regression analysis indicated that region, detection methods, gender and sample type subgroups were likely major sources of heterogeneity (P < 0.05).
We further assessed the prevalence of murine leptospirosis in subgroups of climatic and geographic factors and found that murine leptospirosis ranged from 111–121 degrees longitude (10.45%, 95% CI: 7.91–13.30%), 22–27°C mean temperature (24.44%, 95% CI: 17.93–31.56%) and the basin subgroup (12.72%, 95% CI: 6.82–20.02%) had a higher prevalence, suggesting that these geographic ranges may account for the heterogeneity (Table 6).
Discussion
Leptospirosis, as a zoonotic manifestation of natural foci disease, is widely distributed in the world, and is most common in tropical and subtropical regions. China has a complex ecological geography and climate, and the natural conditions in most areas are suitable for the survival of pathogenic Leptospira and its host animals (3). Therefore, leptospirosis is widely distributed throughout the country, causing great harm to human and animal health. So far, more than 80% of the provinces in China have reported leptospirosis cases, with a total of more than 2.5 million cases reported, including 20,000 deaths (73). Although the overall incidence of leptospirosis had shown a slow downward trend in recent years, there has also been an upward trend in some provinces of China (such as Fujian and Yunnan) (74). Murine have been the main source of infection, and livestock, especially pigs and cattle, and dogs are important hosts and sources of Leptospira (3). Therefore, a detailed understanding of the prevalence of murine leptospirosis is of vital importance to prevent human disease and to ensure timely preventative measures are taken.
We analyzed the sampling years first and found that the incidence of leptospirosis had decreased slightly in the past 10 years (Table 3). With the development of the economy and society and attention to and investment in zoonosis, the disease had been controlled nationwide. However, in recent years, with the warming of the global climate, changes in human activities, the variation of pathogens and increased drug resistance in murine (17), reports of the infection in many countries and regions, including some developed countries, have gradually increased, that is, re-emergence of leptospirosis has occurred (75). According to the World Health Organization, leptospirosis, with more than 1 million cases per year, is one of the major zoonotic diseases causing morbidity and mortality worldwide. Therefore, research and prevention of the disease cannot be ignored (14).
Murine are one of the main sources of Leptospira, and most of them are recessively infected—they do not get sick themselves, but discharge a large number of pathogens to contaminate the environment and infect humans and other susceptible animals (14, 75–77). Therefore, we analyzed the prevalence of leptospirosis in China and found that it ranged from 1.11 to 35.29%. The combined prevalence of Leptospira in murine was 8.7% (Table 4, Figure 7). This showed that the disease has been widespread in China, and there have been large differences across regions. At present, more than 300 serovars of Leptospira can be found in the world. The prevalent serovars and distributions differ greatly across regions, and the lack of cross-immunity among them leads to the diagnosis and prevention of the disease having certain regional specificity. Our regional distribution subgroup analysis found that the epidemic area of leptospirosis in China was widely distributed, and the epidemic range covered 5 regions and 14 provinces. However, the prevalence of the infection in murine in southern China was 20.13%, which is significantly higher than in northeast China (P < 0.05). Combined with the provincial subgroup analysis, murine leptospirosis was mainly distributed in some provinces in the Yangtze River Basin, and Guangxi Province was particularly affected, with a prevalence rate of 35.29% (Table 4). Guangxi is one of the hardest-hit areas for leptospirosis in China with deaths caused by the disease every year (78). According to reports, epidemics mainly occur in Guangxi rural areas, and the victims are mainly farmers engaged in agricultural labor in the fields for long periods, resulting in repeated exposure to contaminated water (79). In addition, the epidemic forms of leptospirosis in Guangdong and Zhejiang provinces were mainly paddy-field type and rainwater type, and the incidence was mostly in young and middle-aged farmers. Polluted water sources and bacteria-carrying murine are clearly important sources of infection (80). We suggest that murine control, environmental remediation, and publicity of prevention and control knowledge for key occupational groups such as farmers should be periodically carried out during the busy farming season. Popularized the knowledge of leptospirosis transmission routes and susceptible populations, and strengthen the vaccination of high-risk groups. At the same time, strengthen the protection of practitioners, water quality testing should be strengthened to reduce the risk of human infection. We further analyzed the reasons for the low incidence of leptospirosis in Northeast China. All samples from Northeast China collected kidney tissue samples for testing. Combining sample subgroups and detection method subgroups, we found that the results of sample types and detection methods were consistent, and the positive rate of serum samples was higher than that of tissue samples, so the detection method may be one of the reasons for the low incidence in Northeast China. In addition, Northeast China is not the main foci of leptospirosis rodent foci. The natural foci of leptospirosis in the country are mainly distributed in the Yangtze River Basin and the vast areas south of it, which reduces the possibility of disease transmission, which is another reason for its low incidence (75).
The occurrence of murine Leptospira was also closely related to climatic and geographical factors, which had important effects on the distribution and population density of host species and reservoirs. These factors determined the species and quantity distribution of host murine, which determined the distribution of pathogens, thereby determining the geographical distribution of the murine Leptospira locus. The results of this study showed that the annual average temperature, longitude, and landform characteristics were the main environmental factors affecting the occurrence of murine leptospirosis in China. In the basin area between 111- and 121-degrees east longitude, and temperature 22–27°C, the incidence is highest. These geographical conditions were mainly concentrated in Guangxi, Guangdong, Hubei, Jiangxi, Sichuan and Guizhou. Suitable temperature and altitude, and abundant rainfall determined the distribution of host animals. There are multi-species and great abundance of murine in Guangxi, which are the main source Leptospira infection (79). Hubei is the most important rice-growing area in China and, because of frequent floods, the incidence of leptospirosis was relatively high (65). We further analyzed the correlation between Leptospira serovars and provincial subgroups, and the R2 was 47.88%, indicating that different provinces have a certain influence on Leptospira serovars, which may be one of the sources of heterogeneity. Different climatic conditions, soil types and vegetation conditions in the foci, as well as differing host animals, lead to differences in the occurrence and distribution of Leptospira in natural foci. Therefore, strengthening the monitoring of environmental factors in and around the foci is an important means of effectively controlling the occurrence and prevalence of the disease.
The composition of murine species in different regions was related to the infection rate with Leptospira, and even the same species of animals had very different bacterial serovars and carrier rates in different regions. We analyzed the combined prevalence of leptospirosis infection in different species of murine and found that the combined prevalence of 29.04 and 22.63% in Rattus rattus and Bandicota indica was the highest, followed by Berylmys bowersi, Rattus losea, Rattus nitidus and Apodemus agrarius, the difference was significant (P < 0.05). In the classification of different genus, we found that the prevalence of Bandicota and Berylmys was the highest, and exhibited an extremly distinct difference (P < 0.01) (Table 5). The high merged rate for Rattus rattus and Bandicota indica might be due to the small number of collections, which may have affected the results. Secondly, these two murine species were reported in Guangdong Province, and may be endemic species that are uncommon in other regions. Most of the species in Guangxi Province were Rattus flavipectus, Apodemus agrarius and Rattus norvegicus. Rattus flavipectus is the dominant rat species. The analysis results showed that Apodemus agrarius, Rattus norvegicus, Rattus losea and Rattus flavipectus were the main host animals of Leptospira, and these were widely distributed in various regions (79). We further analyzed the correlation between the murine species and the provincial subgroup, and the R2 was 29.85%, indicating that the province had a certain influence on the murine species, which may be one of the sources of its heterogeneity. In addition, the carrier rate was also affected by factors such as gender, and season. Surveys in various places have proved that the male carrier rate is higher than that of females, which might be because the males engage in a wider range of activities, leading to an increased chance of infection. However, in this study, the factors of season did not have a significant impact on the carrier rate. In the seasonal subgroup, the positive rate in summer and autumn was higher than in spring, but the difference between the groups was not significant, which was roughly consistent with the seasonal conclusions of other studies on the incidence of leptospirosis, mainly related to the breeding season and density of rats (Table 6). The size, the chance of mutual contact, the uniformity of sampling distribution and other factors are related, and the monitoring of the murine population should be strengthened during the epidemic season.
We further analyzed the subgroups of Leptospira detection methods, and the positive rate for serological detection methods was significantly higher than that of etiological testing methods and molecular biological detection methods (Table 3). Through combined sample type analysis, we found that the sample type was consistent with the results of the detection method, and the positive rate of serum samples was higher than that of tissue samples. At present, the laboratory diagnosis of Leptospira still relies on serological methods. Cross agglutination absorption test and microscope agglutination test (MAT) were the two classic test methods used, and it's latter is also the current gold standard for diagnosis (16). MAT was very sensitive in the early stages of infection, which might be one of the reasons for the high seropositivity rate. However, MAT cannot be used for epidemiology and infection monitoring–it has high requirements for operators, as it is necessary to maintain a live culture of strains for a long time during testing, making it difficult to standardize (81). Although the etiological detection method could be used to isolate and cultivate the field isolates, the doubling time of the different Leptospira serovars has different logarithmic growth phases, making it impossible to achieve early diagnosis (82). At the same time, the typical invasion characteristics of Leptospira or the growth conditions of the respective samples were different, so isolation from different sample types requires different methods, making isolation more difficult (83, 84). Molecular diagnostic techniques such as PCR allow rapid detection of samples with higher sensitivity and specificity. In addition, PCR can quantify the amount or concentration of bacterial DNA present in the sample, which in turn can determine the degree of infection. However, this method must be carried out in the laboratory and requires professional personnel and equipment, thus it is not suitable for field applications (1, 85). In clinical practice, it is recommended that serological methods are used as the main method and a combination of multiple methods for detection, to improve the detection rate and accuracy.
Leptospirosis is listed as III categories of animal epidemic diseases that is harmful to people's health in China (86). The epidemic is the most serious in the southern provinces of China, which is mainly related to its climate, environment, and distribution of host animals. In recent years, reports of leptospirosis have gradually increased. Therefore, disease monitoring of murine should continue, not only to inform specific rodent management strategies, but to give attention also to environmental control and mobilize society as a whole to keep murine densities low. Changes in the living environment of wild animals because of human activities will inevitably lead to increased opportunities for wild animals to be in contact with domestic animals and people. Therefore, increased surveillance of the prevalence of pathogenic Leptospira in, especially, small wild mammals, is beneficial to controlling the risk of human leptospirosis outbreaks. It is of great practical significance for the prevention and control of human leptospirosis.
Our study performed a meta-analysis of 46 papers, including 37 (4–5 point) papers and 9 (2–3 point) papers. The paper did not determine the sampling time or random sampling, no factors such as four or more subgroups (Table 2) would affect the score. For further study, we should extract and analyse the specific Leptospira serovars that may be carried by the various murine species in more detail, to provide more accurate data, further scientific analysis and a more reliable theoretical basis for future research on leptospirosis.
The advantages of this study lie in the large sample size, large time span, wide regional distribution, clear and definite analysis method and comprehensive risk factor analysis, which allow for some feasible prevention and control suggestions to be made for the leptospirosis epidemic. However, our meta-analysis had some limitations that may have affected the results. First, although a search formula to comprehensively collect relevant studies had been developed, omissions may have occurred. Second, a large number of eligible studies were obtained in our systematic study, but not all data were available, and insufficient research on murine Leptospira subgroups such as age and gender would have affected the results. Thirdly, a limited number (n = 2) of the qualified studies were conducted in northeast China, which may not reflect the actual positivity rate in the areas investigated; the quality of those studies was mixed, suggesting that better surveillance and more research on Leptospira infection in those areas is required. Fourth, the data available for analysis were limited, particularly for living environment, the existence of mixed infection, and the predominant murine and prevalent strains in each region, and further, more detailed analysis has not been carried out. Despite these limitations, we believe this report is an accurate reflection of murine Leptospira infection prevalence in China.
Conclusion
Leptospira was found to be widespread in China. Region, sample type, testing method, gender and geographic factors influenced the prevalence of leptospirosis. Formulating a prevention and control strategy for murine leptospirosis that accounts for the differences in climate and environment across regions is suggested. At the same time, epidemiological surveys of Leptospira in murine are needed in more areas to further explore the risk factors and to prevent the spread of the disease in humans and animals.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
RD: idea contributions. L-ML, J-FS, TL, Q-XM, and WZ: data extraction. H-FF: database establishment. QW: data analysis. J-ML: writing original draft. FL: writing—review and editing. All authors contributed to manuscript editing and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.944282/full#supplementary-material
References
1. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. (2003) 3:757–71. doi: 10.1016/S1473-3099(03)00830-2
3. Shao JW. Distribution, Transmission Risk and Molecular Characteristics of Two Important Zoonotic Pathogens in Main Animal Hosts. Hangzhou: Zhejiang University. (2019)
4. Guglielmini J, Bourhy P, Schiettekatte O, Zinini F, Brisse S, Picardeau M. Genus-wide Leptospira core genome multilocus sequence typing for strain taxonomy and global surveillance. PLoS Negl Trop Dis. (2019) 13:e0007374. doi: 10.1371/journal.pntd.0007374
5. Adler B, Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. (2010) 140:287–96. doi: 10.1016/j.vetmic.2009.03.012
6. Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. (2015) 387:65–97. doi: 10.1007/978-3-662-45059-8_5
7. Adler B. History of leptospirosis and Leptospira. Current Topics Microbiol Immunol. (2015) 387:1. doi: 10.1007/978-3-662-45059-8_1
8. Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat Rev Microbiol. (2017) 15:297–307. doi: 10.1038/nrmicro.2017.5
9. Yang R. Plague: Recognition, treatment, and prevention. J Clin Microbiol. (2017) 56:e01519–1517. doi: 10.1128/JCM.01519-17
10. Lei YL, Wang XG, Li H, Chen XY, Ye BF, Liu FM, et al. Investigation on active agents of rabies Xinsu in mountainous areas of Zhejiang Province. Chin J Epidemiol. (2009) (04):344–7. doi: 10.3760/cma.j.issn.0254-6450.2009.04.009
11. Chen SW. Investigation of Japanese Encephalitis Virus Carried by Domestic Mice and Wild Mice and Establishment of Nih Mouse Model Infected by Japanese Encephalitis Virus [D]. Southern Medical University. (2016).
12. Kamani J, Baneth G, Gutiérrez R, Biala YN, Mumcuoglu KY, Harrus S. Coxiella burnetii and rickettsia conorii: two zoonotic pathogens in peridomestic murine and their ectoparasites in Nigeria. Ticks Tick Borne Dis. (2017) 9:86–92. doi: 10.1016/j.ttbdis.2017.10.004
13. Yu XH, Zhang XH, Ni QX, Ni CR, et al. Advances in epidemiology of mouse borne diseases. Chin J Vector Biol Control. (2015) (6):634–6. doi: 10.11853/j.issn.1003.4692.2015.06.026
14. Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. (2015) 9:e0003898. doi: 10.1371/journal.pntd.0003898
15. Monteiro MB, De Sousa IE, Piteira M, Coelho S, Freitas P. Leptospirosis, a re-emerging threat. Cureus. (2021) 13:e14295. doi: 10.7759/cureus.14295
16. Guerra MA. Leptospirosis: public health perspectives. Biologicals. (2013) 41:295–7. doi: 10.1016/j.biologicals.2013.06.010
17. Li JM. Studies On The Diversity and Function of Gut Microbes of Brandt's Vole in Inner Mongolia Based on Metagenomics and Cultureomics. Jilin Agricultural University. (2021).
18. Li ZL, Jiang LP, Zhang MT Li X, Ying KM, Lin GM. Surveillance analysis of leptospirosis after outbreak in Pan'an County, Zhejiang Province. Chin J Vector Biol Control. (2013) 3:272–4. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2013&filename=ZMSK201303031&uniplatform=NZKPT&v=2qVJUKcUrZQIGBZ1dCAWrHejLd4B3fPy1dqj7kHowFiw26Pe11wYYdgwt9e1TpSi
19. Moher D, Liberati A, Tetzlaffff J, Altman DG, PRISMA. Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicien. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
20. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y., Alonso-coello A, et al. GRADE: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BrMed J. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
21. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
22. Ni HB, Gong QL, Zhao Q, Li XY, Zhang XX. Prevalence of haemophilus parasuis “Glaesserella parasuis” in pigs in China: a systematic review and meta-analysis. Prev Vet Med. (2020) 182:105083. doi: 10.1016/j.prevetmed.2020.105083
23. Gong QL, Wang Q, Yang XY, Li DL, Du R. Seroprevalence and risk factors of the bluetongue virus in cattle in China from 1988 to 2019: a comprehensive literature review and meta-analysis. Front Vet Sci. (2021) 7:550381. doi: 10.3389/fvets.2020.550381
24. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
25. Shi JF, Gong QL, Zhao B, Ma BY, Chen ZY, Yang Y, et al. Seroprevalence of brucellosis in buffalo worldwide and associated risk factors: a systematic review and meta-analysis. Front Vet Sci. (2021) 8:553. doi: 10.3389/fvets.2021.649252
26. Liu F, Gong QL, Zhang R, Chen ZY, Wang Q, et al. Prevalence and risk factors of bluetongue virus infection in sheep and goats in China: a systematic review and meta-analysis. Microbial Pathogenesis. (2021) 3:105170. doi: 10.1016/j.micpath.2021.105170
27. Zhang GB, Ying LH, Zhang XY, Hu XX. Analysis of serological monitoring results of three mouse-borne diseases. Chin J Health Inspection. (2015) 25:3700–2. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2015&filename=ZWJZ201521038&uniplatform=NZKPT&v=lQn0mZV19cduguxfj25HZbjfP8IaUUD5zaehlJ_J6KG319jvqT6Mq6MJH9D68LTz
28. Yang DS, Zhang XP, Xu ZN, Hong WB. Epidemiological investigation of insect-borne infectious diseases in Dongguan City. Chin Vet J. (2016) 52:95–6. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2016&filename=ZSYZ201606041&uniplatform=NZKPT&v=Ok17SucvV9Z0da5WNzqiOP60fjd9xmpXx1pqpInhvpkt4tAShCmO3Ja5vkv_MBkd
29. Ye HB, Wan DZ, Xu J, Yun XY, Zhang XL, Qin FK, et al. Investigation on the pathogens of mouse-borne diseases at some ports in Guangxi. Chin J Vector Biol Control. (2017) 28:343–6. doi: 10.11853/j.issn.1003.8280.2017.04.009
30. Xu GY, Lin DH, Xiao FZ, Han TW, Liu WJ, Zhou SH, et al. Investigation on the infection status of rodent leptospirosis in Fujian Province. Chin J Dis Control. (2018) 22:599–602. doi: 10.16462/j.cnki.zhjbkz.2018.06.014
31. Zhang CC, Zhang TL, Xu JM, Jiang XG, Qiu HY. Epidemiological investigation and identification of isolated strains of leptospirosis in rodents in Jiangxi Province from 2016 to 2018. Chin J Zoonoses. (2019)35:1080–84. doi: 10.3969/j.issn.1002-2694.2019.00.185
32. Wang DJ, Wang CQ. Zhou XC, Wen LX, Zou XL, Pan HM. Report on epidemiological surveillance of leptospirosis in Xingshan County. J Public Health Prevent Med. (2006) 01:34–6.
33. Zhang RJ, Li ZY. Tang CZ, Gong CY, Ji GH. Investigation of several natural epidemic diseases along the coast of the reservoir area of the Tectic Tank Hydropower Station. J Med Pest Control. (2006) (10):724–7.
34. Zhang TS, Yan ZX, Luo G. Analysis of leptospirosis surveillance in Jiangyou City from 2002 to 2006. J Prevent Med Intelligence. (2007) 6:683–85.
35. Cheng JF, Zhang YB., Zhang LY, Fang WM, Zhou XC, Li F. Analysis of leptospirosis surveillance results in the three gorges dam area. J Public Health Prevent Med. (2008) (01):27–30.
36. Hua GR, Li Y, Yin Z, Liu J. Analysis of leptospirosis surveillance in Huaiyuan County, Anhui Province, 2005. Anhui J Prevent Med. (2008) (01):9–10
37. Liu FQ, Gao LD, Chen LZ, Zhang H, Dai DF, Wu ZG. Analysis of the results of Leptospira host animal infection status in Hunan Province in 2007. Contemporary Med. (2008) 05:153–5.
38. Liu FQ, Gao LD, Wu ZG, Guo SH, Zhang H, Dai DF, et al. A study on the status of Leptospira host animal infection in Hunan Province in 2006. Pract Prevent Med. (2008) 01:72–4.
39. Liu WD. Evaluation of the Impact of the Three Gorges Project on Leptospirosis in the Reservoir Area. Wuhan: Huazhong University of Science and Technology. (2008).
40. Xiao P, Cao XD, Xiao W, Zhu WH, Liu RQ, Wang ZL. Report on the results of leptospirosis surveillance in Shuangfeng County from 2005 to 2007. Pract Prevent Med. (2008) 15:1856–57+1840.
41. Zhuang R, Dong Y, Deng CS. Survey of rodent-borne diseases in Fuding City. Chin J Vector Biol Control. (2008) 19:601.
42. Bai M, Li X, Gao L, Yang JZ, Li XY, et al. Investigation on natural focus diseases in Jinyang new area of Guiyang. Guizhou medicine. (2009) (05):464–6.
43. Chen GJ. Investigation on Main Vectors and Related Diseases in the Three Gorges Dam Area. Wuhan: Huazhong University of Science and Technology. (2009)
44. Liu YZ, Zhan ZF, Zhang H, Gao LD, Wu ZG, Zeng K, et al. Analysis of infection status of Leptospira host animals in Hunan Province in 2008. Pract Prevent Med. (2009)16:1780–82 doi: 10.3969/j.issn.1006-3110.2009.06.031
45. Sun XH, Zhang L, Zhang JC, Wei L, Zhang XL, Gao X, et al. Investigation on the pathogens of mouse borne diseases in the border area between Hekou and Laojie. Chin J Front Health Quar. (2010) (02):73–6. doi: 10.16408/j.1004-9770.2010.02.002
46. Chai WL, Wang YP, Zhang JM, Xu Q, Zheng YP Li S, et al. Analysis on pathogens monitoring results of rodent at Fujian port from 2010 to 2012. Chin J Front Health Quar. (2013) 36:223–7. doi: 10.16408/j.1004-9770.2013.04.005
47. Deng YL, Sun XK, Chen WQ, Luo WL Li GH, Zhu JT. Serological surveillance of leptospirosis in Qingyuan. Chin J Health Lab Technol. (2013) 23:2643–6. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2013&filename=ZWJZ201312030&uniplatform=NZKPT&v=QIzXg0B8DsOy6vyxxqu-82szuRazjR70BJiSS6zMVa6-v9m5t9nds8Kb5L2xLKUP
48. Wang YX, You AG, Kang K, Sun JW. Analysis of the correlation between leptospirosis and host animals in Henan Province. Chin J Zoonoses. (2013) 29:1036–8. doi: 10.3969/cjz.j.issn.1002-2694.2013.10.020
49. Zhou TX, Wu Q, LI XN LI PH, Zheng F, Huang QF, et al. Study on pathogens and rodents on entry-exit ships and Mawei seaport. J Front Health Quar. (2013) 36:300–3. doi: 10.16408/j.1004-9770.2013.05.003
50. Wei YC, Jiang LP, Zheng BF, Zhu SR. Epidemiological survey of leptospirosis host animals in Pan'an County. Preventive Med. (2014) 26:607–9. doi: 10.19485/j.cnki.issn1007-0931.2014.06.023
51. Huang QF. Investigation of Bartonella Infection in Rodents in Fuzhou Harbor. Fuzhou: Fujian Medical University. (2015).
52. Jiang WB. A survey of rodent carriage of epidemic hemorrhagic fever and leptospirosis in Jiujiang City. Chin J Rodent Control. (1986) 3:186. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=ZMSK198603028&uniplatform=NZKPT&v=W6_Vz1IvjFPhoTYq6G_jGlvWDeEzi7yZQ6LiJz6o-w5alHrWK2wbDxOmbIWn1PQn
53. Jiang QW. Investigation of Leptospira carriage by rodents in Liuyang County. Chin J Rodent Prevent Control. (1988) 3:247. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=ZMSK198803042&uniplatform=NZKPT&v=7itkuSwJjn94aUFoJAVPf1Dn1k4uXpbmTBUAsemfEW-S7ZYxtJQQisgvLclVLESn
54. Yang BY, Cai HQ. A report on the survey of rodents and their in vitro parasitic mites in the Sandu area. J Jinan Sci Med (Special Edition on Medicine). (1988) 4:51–5. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=JNDX198804010&uniplatform=NZKPT&v=Bi_dJscKSvd8qsAkXn5B0UzbO2bSawsjmyK2LEa9VMRe4dsPNgJe0OMRj0iL9TKP
55. Zhang ZG, Yang JZ. Zhang SG. A survey on the communities of small form animals in different environments and their relationship with diseases in the Three Gorges Sichuan reservoir area of Yangtze River. Chin J Rodent J Rodent Prevent Control. (1989) 4:242–4. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=ZMSK198904018&uniplatform=NZKPT&v=-rYtWJB1D0HuNGvueAEf0ZvQVUPls5pxg7AnEQwXIjQrzNrc-0T2EAn-u0iha5pe
56. Xu J, Wu SY, Wang LY. Survey of the epidemic site of leptospirosis in Cixi City. Zhejiang Prevent Med Dis Surveillance. (1991) 0):18. doi: 10.19485/j.cnki.issn1007-0931.1991.03.011
57. Li CY, Wei XH., Zhao GH, Wang RH, Zhang WH, Lu DQ. Species of animal hosts and their epidemiological significance in the natural source of leptospirosis in Guizhou Province. Guizhou Med. (1992) (05):311–3. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9093&filename=GZYI199205028&uniplatform=NZKPT&v=YL5izNP12Y2ujIP_HYKzoVnRiXF7f7-82q3GW1QxCXED69tNg5aQ8nD0ICgXwcZg
58. Xu WJ, Zhang KuanShen. Investigation of natural leptospirosis infection in rodents. Zhejiang Prevent Med Dis Surveillance. (1992) 04:15–6. doi: 10.19485/j.cnki.issn1007-0931.1992.04.011
59. Cheng JF, Huang XH, Zhang LB, Ye YS, Xie ZX, Li J, et al. Surveillance of leptospirosis in Hubei Province from 1990 to 1992. Hubei J Prevent Med. (1993) 4:7–9. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9093&filename=FBYF199304002&uniplatform=NZKPT&v=MsPAKZciTbFftZfc9U5z_Jrdssn29H_vBtlPWv4L_0Z1D50Dsiuz_OpPCRnx2d5E
60. Ou WT, Lu QT. Xiong WF, Jing CM, Pang BP. Study on the natural carriage of Leptospira and epidemic hemorrhagic fever virus in rodents. Med Animal Control. (1993) 3:142–5. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9093&filename=YXDZ199303005&uniplatform=NZKPT&v=d5h3CW4sJU53z8r3Di0-kKixzBbFA_4fY-teVGGv-bnETk8wxqpw-yKPTx2QsdJ3
61. Yang MW, Chu ZQ., Zhang ZJ, Long ZJ, Li CS, Chen HS, et al. Epidemiological survey and study of rodent-borne diseases in Qiannan prefecture. Guizhou Med. (1995) 6:344–8. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9495&filename=GZYI199506014&uniplatform=NZKPT&v=MJyfTMw0NcD7hIrncZ6JG93VTU2St3SfTXLBk7Ytx3M46VtE3uXs2KyAJ25GgVN6
62. Chen ZM, Zhang GS. Analysis of Leptospira carriage in rodents in Xianju County. Chin J Health Inspection. (1999) 5:366. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD9899&filename=ZWJZ199905021&uniplatform=NZKPT&v=lYRJhNHrMbMzjYBSgt56J6ly7-jqXjgXcjZvVSR4p-tREkDNIyMI-SE3XI_P26is
63. Chen RF, Li JY. Zhu WG, Wang BN, Chen JF. A survey of historical epidemic sites of leptospirosis in Lishui City Zhejiang. Preventive Med. (2002) 10:12. doi: 10.19485/j.cnki.issn1007-0931.2002.10.006
64. Guo ZQ, Liu LG., Huang ZY, Yan H, Ou YB, et al. Analysis of monthly surveillance results of leptospirosis in Jiangyou City, Sichuan Province. J Prevent Med Intelligence. (2002) 4:316–8. doi: 10.3969/j.issn.1003-8507.2003.01.008
65. Wu PY, Cheng JF., Lin FR, Wang AH, Qiu DL, Su XY, et al. Survey on the prevalence factors of leptospirosis in Hubei Province. Chin J Vector Biol Control. (2002) 5:381. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2002&filename=ZMSK200205032&uniplatform=NZKPT&v=yzvtPMYYg-U4XS-gOQ0pa5bNN73djHhG3F_gqmQcyivIXdWp2Et9J_6IZcsqj-Mm
66. Zhang KS. Analysis of Leptospira carriage in rodents in Xianju County from 1987 to 2000. Chin J Vector Biol Control. (2003) 3:180. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2003&filename=ZMSK200303008&uniplatform=NZKPT&v=2–p1Wizc2C8QZEQ8Njdx6yjT5PBhGvyGwsNRqFvR_0PjN4hThAR5MAALNm8kWW9
67. Tao JH, Luo YZ. Zhang CJ, Liu TR. Investigation of Leptospira carriage in humans and animals in Longnan County, Jiangxi. Chin J Human-Vet Dis. (1988) 6:45–7. https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD8589&filename=ZRSZ198806021&uniplatform=NZKPT&v=uS7tFMac_H7klU7thmUoJ6VKyhtoETj7zV423uPlU75bMh-LNOAO_E6V2-Bm4OAN
68. Li YJ, Liang HJ, Xing TY, Lv CL, Zhang XW, Yu W. Analysis of rodent surveillance at Raohe port in Heilongjiang province. Chin J Front Health Quarantine. (2020) 43:254–56+272. doi: 10.16408/j.1004-9770.2020.04.009
69. Liang HJ, Wang DW, Wang YM, Zhang J, Shang TS. Analysis of special surveillance on rodents at border ports in Heilongjiang Province from 2019 to 2020. Chin Port Sci Technol. (2021) 3:23–9. doi: 10.3969/j.issn.1002-4689.2021.10.004
70. Zhao YS, Wu YY, Zhou HH, Lin J, Zhu GW, Chen CR. Investigation of natural infection of four pathogens in rodents in rural area of Taizhou, Zhejiang. Dise Surveillance. (2021) 36:894–7. doi: 10.3784/jbjc.202105310292
71. Zhou JZ, Liu Y, Wu YY, Tian ZZ, Wang Y, Jiang WW, et al. A preliminary study on the species of edible rodents and rodent-borne pathogens in the Qiandongnan Miao and Dong autonomous prefecture, Guizhou province, China. Chin J Vector Biol Control. (2021) 32:432–35+446. doi: 10.11853/j.issn.1003.8280.2021.04.009
72. Li SJ, Wang DM, Zhang CC Li XW, Yang HM, Tian KC, et al. Molecular typing of Leptospira spp. strains isolated from field mice confirms a link to human leptospirosis. Epidemiol Infect. (2013) 141:2278–85. doi: 10.1017/S0950268813000216
73. Zhang CL, Wang H, Yan J. Leptospirosis prevalence in Chinese populations in the last two decades. Microbes Infect. (2012) 14:317–23. doi: 10.1016/j.micinf.2011.11.007
74. Zhang H, Zhang CC, Zhu YZ, Mehmood K, Liu J, Mcdonough SP, et al. Leptospirosis trends in China, 2007-2018: a retrospective observational study. Trans Emerg Dis. (2020) 67:1119–28. doi: 10.1111/tbed.13437
75. Dai ZH, Sun Y, Xu Li. A review of leptospirosis. The 19th national symposium on canine science and technology. (2019).
76. Mayer-Scholl A, Hammerl JA, Schmidt S, Ulrich RG, Pfeffer M, Woll D, et al. Leptospira spp. in rodents and shrews in Germany. Int J Environ Res Public Health. (2014) 11:7562–74. doi: 10.3390/ijerph110807562
77. Wang YL, Zeng LB, Yang HL, Xu JM, Zhang XY, Guo XK, et al. High prevalence of pathogenic Leptospira in wild and domesticated animals in an endemic area of China. Asian Pac J Trop Med. (2011) 4:841–5. doi: 10.1016/S1995-7645(11)60205-8
78. Shi MH, Tu YR Li QJ. Geographical distribution of leptospirosis in China Study. Chin J Epidemiol. (1995) (5):259–62.
79. Deng QY. Epidemiological characteristics and influencing factors of leptospirosis in Guangxi. Chin Public Health. (2008) (2):234–234.
80. Li XP, Liang JM, Huang J, Zeng J, Jiang ZL, Yang GH, et al. Epidemiological analysis of leptospirosis in Guangxi from 2007 to 2011. Practical Prevent Med. (2013) (002):168–70.
81. Londeree WA. Leptospirosis: the microscopic danger in paradise. Hawaii J Med Public Health A J Asia Pacific Med Public Health. (2014), 73(11 Suppl. 2):21.
82. Wuthiekanun V, Chierakul W, Limmathurotsakul D, Smythe LD, Symonds ML, Dohnt MF, et al. Optimization of culture of Leptospira from humans with leptospirosis. J Clin Microbiol. (2007) 45:1363–5. doi: 10.1128/JCM.02430-06
83. Lourdault K, Aviat F, Picardeau M. Use of quantitative real-time PCR for studying the dissemination of Leptospira interrogans in the guinea pig infection model of leptospirosis. J Med Microbiol. (2009) 58:648–55. doi: 10.1099/jmm.0.008169-0
84. Wilson R, Fujioka R. Development of a method to selectively isolate pathogenic Leptospira from environmental samples. Water Sci Technol. (1995) 31:275–82. doi: 10.2166/wst.1995.0624
85. Woods K, Nic-Fhogartaigh C, Arnold C, Boutthasavong L, Phuklia W, Lim C, et al. A comparison of two molecular methods for diagnosing leptospirosis from three different sample types in patients presenting with fever in Laos. Clin Microbiol Infect. (2017) 24:17. doi: 10.1016/j.cmi.2017.10.017
86. Ministry of Agriculture Rural Affairs of the People's Republic of China. Announcement No. 573 of the Ministry of Agriculture and Rural Affairs of the People's Republic of China. (2022). Available online at: http://www.moa.gov.cn/govpublic/xmsyj/202206/t20220629_6403635.htm (accessed June 23, 2022).
Keywords: Leptospira, epidemiology, murine, Mice, rat, China, meta-analysis
Citation: Li J-M, Li L-M, Shi J-F, Li T, Wang Q, Ma Q-X, Zheng W, Feng H-F, Liu F and Du R (2022) Prevalence of Leptospira in murine in China: A systematic review and meta-analysis. Front. Vet. Sci. 9:944282. doi: 10.3389/fvets.2022.944282
Received: 15 May 2022; Accepted: 29 August 2022;
Published: 29 September 2022.
Edited by:
Fabrizio Bertelloni, University of Pisa, ItalyReviewed by:
Leila Ullmann, Universidade Federal de Mato Grosso do Sul, BrazilTatiana Rodrigues Fraga, University of São Paulo, Brazil
Copyright © 2022 Li, Li, Shi, Li, Wang, Ma, Zheng, Feng, Liu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Liu, bGYxOTg5MDNAc2luYS5jb20=; Rui Du, ZHVydWkxOTcxMDFAc2luYS5jb20=
 Jian-Ming Li
Jian-Ming Li Lian-Min Li
Lian-Min Li Jun-Feng Shi
Jun-Feng Shi Ting Li1,2,3
Ting Li1,2,3 Qi Wang
Qi Wang Fei Liu
Fei Liu Rui Du
Rui Du