- 1Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, China Agricultural University, Beijing, China
- 2China Institute of Veterinary Drug Control, Beijing, China
- 3Key Laboratory of Detection for Veterinary Drug Residues and Illegal Additives, Ministry of Agriculture and Rural Affairs of the People's Republic of China, Beijing, China
- 4Shanxi Key Laboratory for Modernization of TCVM, College of Veterinary Medicine, Shanxi Agricultural University, Jinzhong, China
The pharmacokinetic profiles and bioequivalence of two cyclosporine oral solutions were investigated in cats. Twenty-four cats were randomly allocated to two equally sized treatment groups in a randomized four-cycle, and dual-sequence cross-over design. Test and reference articles were orally administered in a single dose of 7 mg/kg Bodyweight. Serial blood samples were collected, and blood cyclosporine concentration was determined by ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS). No significant differences were present in the major pharmacokinetic parameters (Cmax, AUC0−last,) between the two formulations. The blood profiles of cyclosporine following the administration of both formulations were similar. The findings of the study suggested that the two articles were bioequivalent for cyclosporine oral solution.
Introduction
Atopic dermatitis (AD) is a T cell-dependent common, chronic, relapsing inflammatory skin disease; however, therapeutic options for patients with the moderate-to-severe disease are limited (1–3). Moreover, affected individuals typically have pruritic erythematous lesions, as well as secondary skin lesions in curved and rubbed areas (4, 5). Cyclosporine A (CyA) is a calcineurin inhibitor. It is a powerful immunosuppressant drug that acts by inhibiting the proliferation of T-lymphocytes (6, 7). CyA's direct effect is via inhibition of calcineurin and exhibits an immunosuppressive effect by inhibiting cytokines, which are secreted by T lymphocytes (8–10). Cyclosporine is lipophilic, distributes widely, and is stored in the skin and adipose tissue. Its concentration in the epidermis and dermis is about 10 feet higher than in blood (11–13). Cyclosporine was proven efficacious in the treatment of feline hypersensitivity dermatitis (14). Cyclosporine oral solution was approved by the US Food and Drug Administration as ATOPICA for Cats® (Cyclosporine oral solution, USP) for the control of feline hypersensitivity dermatitis in cats.
CyA is a narrow therapeutic index drug, and in individuals, there are differences in pharmacokinetics and bioavailability of cyclosporine in large parts (15, 16). Due to the extreme variability in absorption and metabolism, monitoring the concentrations of CyA in the blood has been recommended to reduce the occurrence of adverse drug events and maximize the treatment effect (17). CyA concentration should be evaluated in the whole blood rather than just plasma because the drug concentrates within blood cells (18). Ideally, testing should be carried out after 2 weeks of treatment and, where available, high-performance liquid chromatography is a better method than immunoassay for evaluating CyA whole blood concentrations (19).
In recent years, very few studies have been published specifically addressing the pharmacokinetics of CyA in feline species. This study was conducted to compare the pharmacokinetic profiles of generic cyclosporine manufactured by Shanghai Hanwei Biomedical Technology Co., Ltd. (Shanghai, China) with Atopica™ (Elanco Australasia Pty Ltd.) to evaluate their bioequivalence and, consequently, the possibility of substitution between the two drugs in cats.
Materials and methods
Materials
The cyclosporine oral solution (Shanghai Hanwei Biomedical Technology Co., Ltd, 30 mL/bottle, 100 mg/mL) was the Test Product, while Atopica™ was used as the reference formulation (Elanco Australasia Pty Ltd., 17 ml/bottle, 100 mg/ml). Cyclosporine Standard product was provided from Shanghai Hanwei Biomedical Technology Co., Ltd. (purity: ≥99%).
Study design
Twenty-four domesticated shorthair cats (aged 2–3 years and weighing between 3 and 4.5 kg, provided by the Experimental Animal Center of China Agricultural University) were enrolled in this study. Cats fasted for 16 h before and 8 h following drug administration. Before the initiation of the study, all procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the China Agricultural University (No. 13303-21-E-001).
The study was conducted in a single dose, a four-way fully replicated, and crossover design. A 2-week washout period was scheduled between each phase. Twenty-four cats were randomly blocked into two groups. Cats were monitored for other potential adverse effects during the study. The oral solution was administered via a dosing syringe to the back of the tongue in four phases. In brief, cyclosporine PK data were collected as follows:
• In phases 1 and 3, the 12 cats in Reference-Test- Reference-Test (RTRT) groups were administrated with 7 mg/kg Bodyweight (BW) reference formulation, while administrated with 7 mg/kg BW test formulation in Test-Reference-Test-Reference (TRTR) groups.
• In phases 2 and 4, cats in the RTRT group were administrated with a 7 mg/kg BW test formulation, and the TRTR group was administrated 7 mg/kg BW reference formulation.
Blood samples of about 0.8 mL were collected via the brachial cephalic vein at 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, 6, 8, 12, 24, 36, 48, and 72 h after dosing. Whole blood samples were immediately placed in an anticoagulation blood collection tube and stored at −20°C until analysis.
Drug analysis
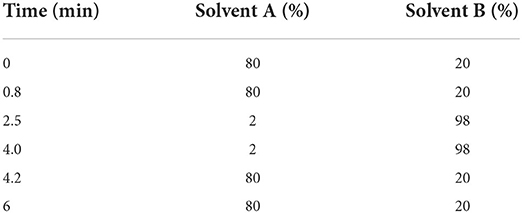
Cyclosporine concentrations in plasma samples were measured using a validated UPLC-MS/MS analytic method as previously described (20). In brief, 200 μl of blood was mixed with 20 μl methanol and 400 μl acetonitrile: methanol (1:1), vibrated for 2 min. After centrifugation at 12,000 rpm at 4°C for 20 min, 300 μl of the supernatant was centrifugated at 12,000 rpm at 4°C for 10 min. The supernatant was analyzed via UPLC-MS/MS (Waters Acquity UPLC and Water Quattro Premier, Waters Co, USA). The mobile phase consisted of 2-mm ammonium acetate 0.1% formic acid (solvent A) and methanol containing 2-mm ammonium 0.1% formic acid (solvent B) with a flow rate of 0.30 ml/min (The mobile phase ratio is shown in Table 1). The lower limit of quantification (LLOQ) was 10 ng/ml. Both inter- and intra-assay coefficients of variation were <15%. The mean recoveries ranged from 93.39 to 110.72%. Calibration curves showed satisfactory linearity through a concentration range of 10–2,000 ng/ml (r2 > 0.99) (21).
Data analysis
CVM advocates the use of 90% confidence intervals (CI), as the best available method for evaluating bioequivalence study data. The pivotal variables for bioequivalence are AUClast, AUCINF_obs,, and Cmax. Mixed model analysis was used to estimate upper and lower bounds for the two pivotal bioequivalence parameters, AUClast, AUCINF_obs,, and Cmax. The recommended BE limit is 80–125% (22, 23). In this study, blood pharmacokinetic parameters were calculated using the non-compartmental analysis model 200 (intravenous or extravascular dosing, linear/log trapezoidal method) in the WinNonlin™ software (version 8.1; Certara USA) and WinNonlin 8.1 was used for bioequivalence analysis. Analysis of variance (ANOVA) was used to calculate a 90% CI for the ratio of the two treatments (24, 25).
Results
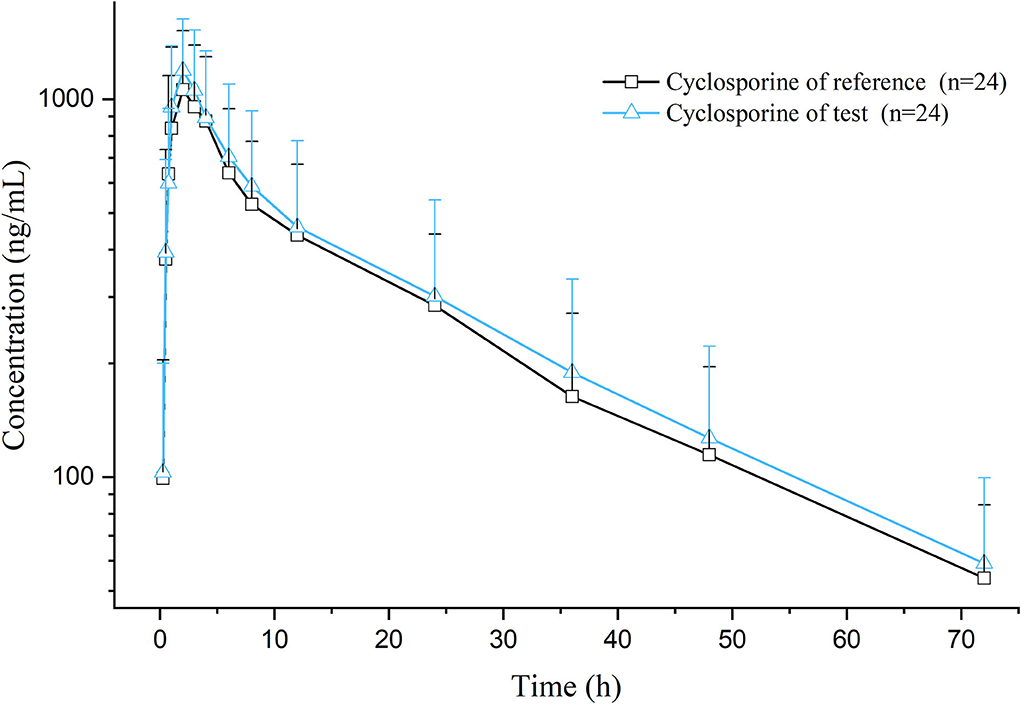
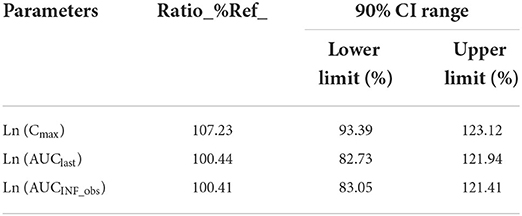
After a single oral dose of 7 mg/kg BW of cyclosporine reference and test formulations in cats (after administration and throughout the experimental process, the cats were in good condition, and no adverse reactions occurred), the average blood concentration-time curve corresponding to the test and the reference formulations measured is presented in Figure 1. The pharmacokinetic parameters were calculated using non-compartmental analysis, and the results are presented in Table 2. The geometric mean ratios of the test formulation/reference formulation Cmax, AUClast, AUCINF_obs,, and their 90% CI are presented in Table 3, which indicates that the test and reference formulations are bioequivalent.
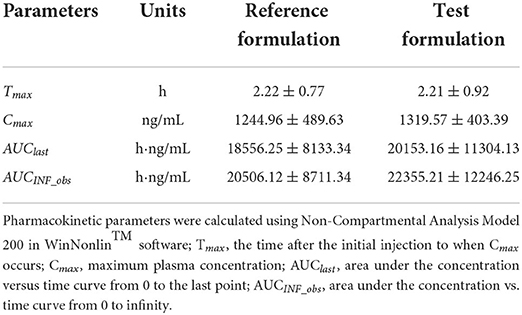
Table 2. Pharmacokinetic variables obtained for two formulations of cyclosporine in cats (n = 24) after a single dose of 7 mg/kg orally.
Discussion
After oral administration of the test and the reference formulations, the pharmacokinetic parameters of the test and the reference formulations: Tmax, Cmax, AUC0−t, and T1/2 were not significantly different. After bioequivalence analysis of AUC0−t, AUC0−∞, and Cmax, the CI 90 ranges of the test formulation compared with the reference formulation was between 80 and 125%. The test formulation is bioequivalent to the reference formulation.
When administered to cats, the plasma kinetic profile of CyA was apparently different from that of other domestic animals, and different formulations had different results. In this study, Tmax was achieved after 2.21 ± 0.92 h, which is shorter than what was reported for beagle dogs (6 ± 0.00) that were administered at 75-mg sustained-release pellets (26), rats (4 ± 2.40) after being administered with 37.8 mg/kg BW (27). Also, it is slightly longer than what was reported for dogs (1.40 ± 0.30 h) that were administered with a capsule of 5 mg/kg BW (28), and rabbits (1.75 ± 0.76 h) after being administered with an oral solution of 10 mg/kg BW (29). Our results showed that administration of CyA at 7 mg/kg in cats had a relatively rapid absorption and distribution in contrast to some studies. When CyA was administered orally (75 mg sustained-release pellets) in dogs, the AUC0−24 was 3,205 ± 149.55 ng·h/ml (26). The AUC0−24 and AUC0−∞ of CyA at 10 mg/kg BW after oral administration to rabbits were 2,057.80 ± 778.60 ng·h/ml and 3,492.90 ± 1,449.70 ng·h/ml, respectively (29). The AUC0−∞ value following oral administration at 5 mg/kg BW in dogs was 3,997 ± 1,108 ng·h/ml (28). The AUC0−t and AUC0−∞ values in this study were 20,153.16 ± 11,304.13 ng·h/ml and 22,355.21 ± 12,246.25 ng·h/ml, respectively. These results showed that this study had a higher AUC than others, the relative accumulation of the drug in the blood is greater, and higher availability of CyA was reflected in cats than in other animals. In this experiment, the Cmax of the test formulation was 1,319.57 ± 403.39 ng/ml. In research about 75 mg sustained-release pellets of CyA in beagle dogs (26), the Cmax was 268.22 ± 15.99 ng/ml. Pharmacokinetics and efficacy of canine atopic dermatitis study of CyA in dogs, the Cmax of CyA capsule (5 mg/kg) was 577 ± 158 ng/mL (28). The Cmax of CyA at 10 mg/kg BW after oral administration to rabbits was 244.67 ± 115.87 ng/ml (29). Compared with the other study, the Cmax of this experiment is higher, indicating that its concentration in the blood is higher and the drug has a stronger effect.
Conclusion
In this experiment, the results of pharmacokinetic process analysis showed that the test formulation of CyA oral liquid had the characteristics of fast absorption and slow elimination in cats. The relative bioavailability of the test formulation of CyA oral solution was 108.61%, and the test formulation of CyA and the reference formulation were bioequivalent.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the China Agricultural University (No13303-21-E-001).
Author contributions
XC and JW contribute to revising it critically for important intellectual content and approved the version to be published. YY, JK, FZ, YL, QW, YC, JQ, LZ, and XG have participated sufficiently in the work to take public responsibility for appropriate portions of the content and made a substantial contribution to the concept and design, acquisition of data or analysis, and interpretation of data. YY drafted the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Central Funds Guiding the Local Science and Technology Development In Shanxi Province [Grant Number YDZJSX2021A034], Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province [Grant Number 20210012], Project of Scientific Research for Excellent Doctors, Shanxi Province, China [Grant Number SXBYKY2021047], Research Fund (Clinical Diagnosis And Treatment Of Pet) for Young College Teachers in Ruipeng Commonweal Foundation [Grant Number RPJJ2020021], and Project of Science and Technology Innovation Fund of Shanxi Agricultural University [Grant Number 2021BQ06].
Acknowledgments
We would like to thank Shuyuan Li for her technical assistance with the analytical determination of Cyclosporine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.940472/full#supplementary-material
References
1. El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. (2013) 172:351–6. doi: 10.1007/s00431-012-1893-3
2. Khattri S, Shemer A, Rozenblit M, Dhingra N, Czarnowicki T, Finney R, et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J Allergy Clin Immun. (2014) 133:1626–34. doi: 10.1016/j.jaci.2014.03.003
3. Beltrani VS. The clinical spectrum of atopic dermatitis. J Allergy Clin Immun. (1999) 104:S87–98. doi: 10.1016/S0091-6749(99)70050-3
4. Olivry T, Steffan J, Fisch RD, Prelaud P, Guaguere E, Fontaine J, et al. Randomized controlled trial of the efficacy of cyclosporine in the treatment of atopic dermatitis in dogs. J Am Vet Med Assoc. (2002) 221:370–7. doi: 10.2460/javma.2002.221.370
5. Griffin C, DeBoer D. The ACVD task force on canine atopic dermatitis (XIV): clinical manifestations of canine atopic dermatitis. Vet Immunol Immunopathol. (2001) 81:255–69. doi: 10.1016/S0165-2427(01)00346-4
6. Kahl AL, Kirchhof J, Petrakova L, Muller J, Laubrock J, Brinkhoff A, et al. Are adverse events induced by the acute administration of calcineurin inhibitor cyclosporine a behaviorally conditioned in healthy male volunteers? Clin Ther. (2018) 40:1868–77. doi: 10.1016/j.clinthera.2018.09.008
7. Gong ZS, Wu ZH, Xu SX, Han WN, Jiang XM, Liu HP, et al. A high-throughput LC-MS/MS method for the quantification of four immunosu- ppressants drugs in whole blood. Clin Chim Acta. (2019) 498:21–6. doi: 10.1016/j.cca.2019.07.026
8. Watanabe T, Tanaka R, Ono H, Suzuki Y, Tatsuta R, Itoh H. Sensitive, wide-range and high-throughput quantification of cyclosporine in whole blood using ultra-performance liquid chromatography coupled to tandem mass spectrometry and comparison with an antibody-conjugated magnetic immunoassay. Biomed Chromatogr. (2021) 35:e5128. doi: 10.1002/bmc.5128
9. Hinchliffe E, Adaway JE, Keevil BG. Simultaneous measurement of cyclosporin A and tacrolimus from dried blood spots by ultra high performance liquid chromatography tandem mass spectrometry. J Chromatogr B. (2012) 883:102–7. doi: 10.1016/j.jchromb.2011.05.016
10. Brandt C, Pavlovic V, Radbruch A, Worm M, Baumgrass R. Low-dose cyclosporine A therapy increases the regulatory T cell population in patients with atopic dermatitis. Allergy. (2009) 64:1588–96. doi: 10.1111/j.1398-9995.2009.02054.x
11. Palmeiro BS. Cyclosporine in veterinary dermatology. Vet Clin North Am Small Anim Pract. (2013) 43:153–71. doi: 10.1016/j.cvsm.2012.09.007
12. Forsythe P, Paterson S. Ciclosporin 10 years on: indications and efficacy. Vet Rec. (2014) 174(Suppl. 2):13–21. doi: 10.1136/vr.102484
13. Colombo S, Sartori R. Ciclosporin and the cat: current understanding and review of clinical use. J Feline Med Surg. (2018) 20:244–55. doi: 10.1177/1098612X17748718
14. Roberts ES, Vanlare KA, Strehlau G, Peyrou M, Roycroft LM, King S. Safety, tolerability, and pharmacokinetics of 6-month daily dosing of an oral formulation of cyclosporine (ATOPICA for cats(R)) in cats. J Vet Pharmacol Ther. (2014) 37:161–8. doi: 10.1111/jvp.12081
15. Zhang Y, Zhang R. Recent advances in analytical methods for the therapeutic drug monitoring of immunosuppressive drugs. Drug Test Anal. (2018) 10:81–94. doi: 10.1002/dta.2290
16. Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant. (2012) 2012:230386. doi: 10.1155/2012/230386
17. Wu L, Ye Z, Zhang X, Cheng Y, Zheng L, Qiu H, et al. Comparison of sample preparation methods, validation of an UPLC-MS/MS procedure for the quantification of cyclosporine A in whole blood sample. J Pharm Biomed Anal. (2021) 193:113672. doi: 10.1016/j.jpba.2020.113672
18. Archer TM, Boothe DM, Langston VC, Fellman CL, Lunsford KV, Mackin AJ. Oral cyclosporine treatment in dogs: a review of the literature. J Vet Intern Med. (2014) 28:1–20. doi: 10.1111/jvim.12265
19. McAnulty JF, Lensmeyer GL. The effects of ketoconazole on the pharmacokinetics of cyclosporine A in cats. Vet Surg. (1999) 28:448–55. doi: 10.1111/j.1532-950X.1999.00448.x
20. Alam MA, Al-Jenoobi FI, Al-Mohizea AM, Ahad A, Raish M. Validated UPLC-MS method for pharmacokinetic investigations of cyclosporine-A in blood. Curr Pharm Anal. (2015) 11:210–5. doi: 10.2174/1573412911666150114230011
21. FDA. Bioanalytical-Method-Validation Guidance-for Industry. (2018). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed March 9, 2022).
22. FDA. CVM GFI #35 Bioequivalence Guidance. (2006). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-35-bioequivalence-guidance (accessed July 4, 2022).
23. FDA. CVM GFI #224 (VICH GL52) Bioequivalence: Blood Level Bioequivalence Study. (2016). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-224-vich-gl52-bioequivalence-blood-level-bioequivalence-study (accessed June 25, 2022).
24. Navarro C, Séguy L, Vila M, Birckel P. Bioequivalence study between two formulations of ciclosporin A (Cyclavance® oral solution and Atopica® soft capsules) following a single oral administration to dogs. BMC Vet Res. (2016) 12:54. doi: 10.1186/s12917-016-0669-9
25. Pasloske K, Ranasinghe M, Sauer S, Hare J. The bioequivalence of a single intravenous administration of the anesthetic alfaxalone in cyclodextrin versus alfaxalone in cyclodextrin plus preservatives in cats. J Vet Pharmacol Ther. (2018) 41:437–46. doi: 10.1111/jvp.12485
26. Jiang D, Zeng J, Zhu Y, Zhou G, Deng W, Xu X, et al. Sustained-release of Cyclosporin A pellets: preparation, in vitro release, pharmacokinetic studies and in vitro-in vivo correlation in beagle dogs. Drug Dev Ind Pharm. (2016) 42:1174–82. doi: 10.3109/03639045.2015.1118492
27. Xue XP, Qin XL, Xu CS, Zhong GP, Wang Y, Huang M, et al. Effect of wuzhi tablet (Schisandra sphenanthera extract) on the pharmacokinetics of cyclosporin A in rats. Phytother Res. (2013) 27:1255–9. doi: 10.1002/ptr.4849
28. Steffan J, Strehlau G, Maurer M, Rohlfs A. Cyclosporin A pharmacokinetics and efficacy in the treatment of atopic dermatitis in dogs. J Vet Pharmacol Ther. (2004) 27:231–8. doi: 10.1111/j.1365-2885.2004.00587.x
Keywords: pharmacokinetics, bioequivalence, cyclosporine oral solution, cat, four-cycle
Citation: Yang Y, Kong J, Liu Y, Wu Q, Cao Y, Qiu J, Zhang L, Gong X, Zhao F, Cao X and Wang J (2022) Pharmacokinetics and bioequivalence of two cyclosporine oral solution formulations in cats. Front. Vet. Sci. 9:940472. doi: 10.3389/fvets.2022.940472
Received: 10 May 2022; Accepted: 15 July 2022;
Published: 10 August 2022.
Edited by:
Ronette Gehring, Utrecht University, NetherlandsReviewed by:
Cengiz Gokbulut, Balıkesir University, TurkeyRobert Paul Hunter, One Medicine Consulting, United States
Copyright © 2022 Yang, Kong, Liu, Wu, Cao, Qiu, Zhang, Gong, Zhao, Cao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingyuan Cao, Y3h5QGNhdS5lZHUuY24=; Jianzhong Wang, amlhbnpob25nd2FuZ0BjYXUuZWR1LmNu; d2p6MjAyMEBmb3htYWlsLmNvbQ==
 Yuxin Yang
Yuxin Yang Jingyuan Kong
Jingyuan Kong Yu Liu1
Yu Liu1 Jicheng Qiu
Jicheng Qiu Lu Zhang
Lu Zhang Xiaohui Gong
Xiaohui Gong Xingyuan Cao
Xingyuan Cao Jianzhong Wang
Jianzhong Wang