
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci. , 13 September 2022
Sec. Veterinary Infectious Diseases
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.938601
This article is part of the Research Topic Tick-borne Viruses of Domestic Livestock: Epidemiology, Evolutionary Trends, Biology and Climate Change Impact View all 5 articles
Crimean-Congo hemorrhagic fever (CCHF) is an important tick-borne viral infection with a fatality rate of up to 50% during outbreaks. Crimean-Congo hemorrhagic fever virus (CCHFV) is sustained in the ecosystem in benign form through vertical and horizontal transmission cycles involving tick vectors, wildlife, and livestock. Hyalomma ticks are considered the major source of human infection. CCHF occurs most often among butchers, slaughterhouse workers, and farmworkers through infected tick bites or/and contact with blood and tissues of infected livestock. The nosocomial transmission can occur in auxiliary nurses and physicians through contact with the infected patients. The widespread distribution of CCHFV most probably occurred by ticks on migratory birds, or through international travel and trade of livestock and wildlife. During co-infections of ticks and vertebrates, reassortment among genome segments could play a significant role in generating diversity, and hence, a potential risk for the emergence of novel variants. In this systematic review, we aimed to determine the epidemiology, transmission, distribution, mortality, and clinical features of CCHF in 22 Arab countries, comprising the Arab world. Based on the analysis of 57 studies published from 1978 to 2021, we found 20 tick species that could be associated with CCHFV transmission. During the 43-year period, 321 cases of CCHF were reported from 9/22 Arab countries, Iraq, Kuwait, UAE, Saudi Arabia, Oman, Sudan, Egypt, Tunisia, and Mauritania. The mean case fatality rate was 29% during various outbreaks. Individuals working in abattoirs/slaughter houses, livestock farms, and healthcare were most at risk. Contact with blood or body secretions from infected animals and patients was the most common mode of transmission. A number of different animals, including cattle, goats, sheep, and camels were reported to be seropositive for CCHFV. The highest seroprevalence was observed in camels (29%), followed by cattle (21%), goats (15%), and sheep (14%). We discuss these results in the context of policy-making and potential preventative measures that can be implemented to reduce the burden of CCHF in the Arab world.
Emergence or re-emergence of vector-borne zoonotic diseases across the world exhibit the association among pathogens, vectors, animals, and humans, that can lead to health challenges and economic losses (1, 2). Furthermore, vector-borne disease transmission and perseverance mostly rely on overlapping areas/movements of hosts, circulation of competent vectors, and favorable environmental conditions for vector-borne pathogens (3). Ticks are ectoparasites of livestock, wildlife, and humans, and an important vector of viral pathogens. Many tick-borne viral diseases such as Alkhurma hemorrhagic fever (ALKF), Crimean–Congo hemorrhagic fever (CCHF), and Tick-Borne Encephalitis (TBE) have been reported in the Middle East and North Africa (MENA) region (4), where the control of tick vectors continues to be a challenge.
CCHF is a severe tick-borne zoonosis caused by Crimean-Congo hemorrhagic fever virus (CCHFV) (5). It is a biosafety level 4 pathogen with a case fatality rate of up to 50% (6). CCHF has been reported in many countries from Asia, Africa, South-East Europe, and the Middle East (7). In the MENA region, CCHF has been reported from numerous countries (4, 8, 9) and in some of these countries it is endemic (8, 10). Indeed, the incidence of CCHF in the WHO Eastern Mediterranean Region (WHO EMR) appears to have increased in the last decade (8). However, accurate data is lacking, most probably due to the unavailability of comprehensive surveillance systems, and poor understanding of the epidemiology of virus and risk factors of transmission. CCHF is mostly asymptomatic in many animals such as camels, cattle, goats, and sheep (11, 12). Ticks, mainly belonging to the genus Hyalomma, act as reservoirs and vectors. Infection in humans occurs through tick bites or by contact with a CCHF infected patient, or by contact with tissues/body fluid or blood of viremic persons and animals (7, 13). CCHF outbreak in the UAE, Oman, and Saudi Arabia with high fatality rate (14, 15) was considered to be associated with the Hyalomma tick. Furthermore, human cases were mostly in individuals working in the agriculture and livestock industry (7, 13). Although CCHF/CCHFV has been reported in the Arabian Peninsula (8, 15–25), a detailed and comprehensive picture of the epidemiology, prevalence, mortality rate and clinical features, remains limited. Therefore, we conducted a record-based systematic review and analysis of CCHF in 22 countries of the Arab world from 1978 to 2021 with the aim of filling this gap. We describe the epidemiological characteristics of the disease, provide a record of circulating tick vectors and host species in the region, determine the main routes of transmission of the virus and outline the clinical picture reported in infected cases. Based on our analysis, we suggest potential policies that can be instituted and preventative actions that can be implemented to reduce the burden of CCHF in the region.
In this study, we systemically searched for relevant literature published on CCHF/CCHFV in humans, animals, and ticks in the 22 Arab countries using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol (26) (Figure 1). Our search strategy was based on searching different databases such as Google Scholar, Science Direct, Web of Science, Scopus, and PubMed for retrieving relevant articles published in the Arab countries, from 1978 to 2021. Search terms, “CCHF,” “CCHFV,” “humans” or “patients,” “tick” or “ticks,” “tick vectors,” “animals,” “livestock,” “wildlife,” “small mammals,” and the name of the concerned country were used for retrieving data. The filters were used such as time line (1978–2021) and Arab world/Arab countries.
The CCHF/CCHFV detection, prevalence, and distribution studies on animals, tick vectors and human case reports with fatality rate were analyzed carefully and reviewed systematically. NP retrieved the data, screened each record/report. GK checked and verified each record/report on the basis of inclusion criteria to avoid duplications and errors to enhance the quality of extracted data. We did not use automation tool. Details are given in PRISMA flow chart diagram (Figure 1). Non-duplicate published records were identified and titles and abstracts were screened. A total of 57 relevant studies specifically on CCHF/CCHFV in Arab countries were identified. For data extraction and qualitative assessment, CCHF case reports, CCHFV screening, prevalence, and distribution studies were analyzed, and duplicates removed. For documentation, we used original research studies and case reports. Letters to the editor and commentaries were excluded. All articles with relevant data according to our searches are summarized in Table 1. Out of all published papers, studies on human cases/clinical reports were more common compared to studies on tick vectors, and serological detection of CCHFV in animals. Some studies were multidimensional and included CCHFV detection from more than one source, for example, ticks, animals, and humans, and these studies were included in data.
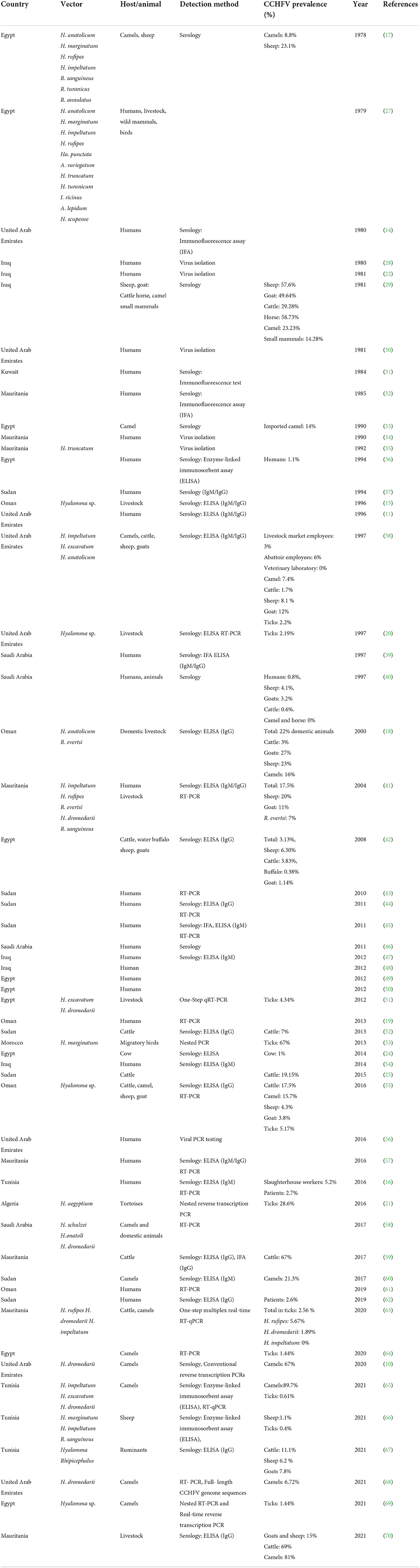
Table 1. Chronological reporting of CCHFV vectors, hosts, and human cases in the Arab world from 1978–2021.
In this study, we reviewed literature from 22 Arab countries in the MENA Region, namely Algeria, Bahrain, Comoros, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Mauritania, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates, and Yemen (71) (Figure 2). Approximately 427 million people belong to Arab nations across the world (72). Genus Hyalomma is considered the main vector of CCHFV and is found in almost all countries of the region (Figure 2) (4, 73). Anthropogenic changes to the environment in the Arab world, both at small and at large scales, abiotic and biotic factors affect the distribution and abundance of Hyalomma ticks and transmission dynamics of the virus. We have documented the presence of tick vectors, serological evidence of CCHFV, its prevalence, and reporting of human cases in different counties of the Arab world from 1978 to 2021 (Table 1). After analysis of 57 studies, CCHFV serological evidence has been recorded from 11 Arab countries including Iraq, Kuwait, UAE, Saudi Arabia, Oman, Sudan, Egypt, Tunisia, Algeria, Mauritania, and Morocco. However, deaths were reported in only seven counties, Mauritania, Oman, UAE, Saudi Arabia, Egypt, Iraq, and Sudan (Figure 2). Fatality rate ranged from 24 to 61% (mean: 29%) during the different outbreaks. Figure 3 illustrates the number of studies published on CCHFV from 1978 to 2021. The mean prevalence of CCHFV antibodies in different hosts and vectors from the published data is given in Table 1. For example, the prevalence of CCHFV antibodies in camels was 29%, in cattle 22%, in buffaloes 0.4%, in sheep 14%, in goats 15%, and in small mammals 14%. Thus, our analysis indicates that camels had the highest seroprevalence of CCHFV in the Arab world. In ticks, the seroprevalence rate was ~10%, as compared to Europe, where CCHFV antibodies were reported as 11.76% in ticks (74).
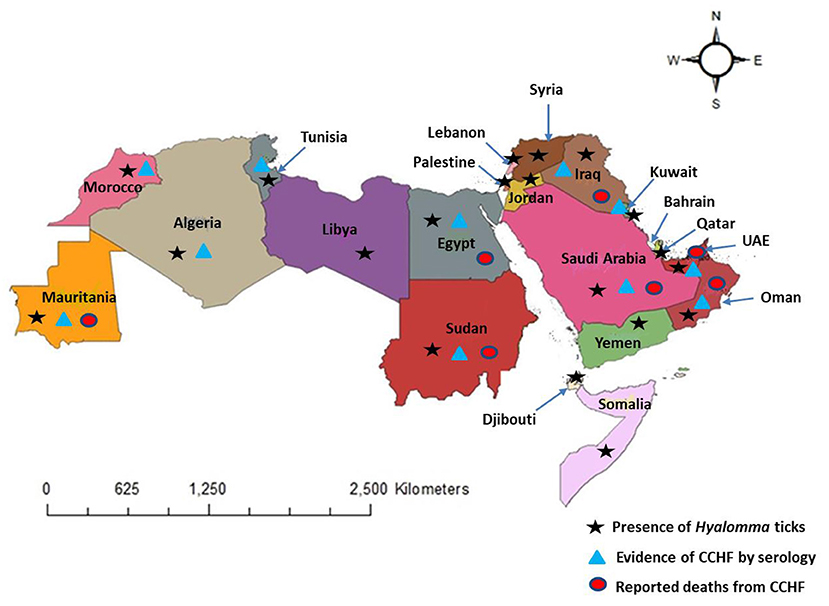
Figure 2. Distribution of Hyalomma ticks, evidence of CCHF, and reported deaths from CCHF in the Arab world (map is reproduced published under the Creative Commons Attribution 4.0 International (CC BY 4.0) License in our study, Perveen et al. (4).
We established the record of five tick genera, Amblyomma, Haemaphysalis, Hyalomma, Ixodes, and Rhipicephalus, including 20 species, Hyalomma aegyptium, Hyalomma anatolicum, Hyalomma excavatum, Hyalomma dromedarii, Hyalomma marginatum, Hyalomma rufipes, Hyalomma impeltatum, Haemaphysalis punctata, Amblyomma variegatum, Hyalomma truncatum, Hyalomma turanicum, Ixodes ricinus, Amblyomma lepidum, Hyalomma scupense, Hyalomma schulzei, Hyalomma onatoli, Rhipicephalus annulatus, Rhipicephalus evertsi, Rhipicephalus sanguineus, and Rhipicephalus turanicus from published data that could be associated with CCHFV transmission (Table 1). Out of the 20 tick species, twelve belong to the genus Hyalomma, four belong to the genus Rhipicephalus and two belong to the genus Amblyomma and one of each belong to Haemaphysalis, and Ixodes. Hyalomma species overlap in the area where other species were found. Focusing on screening of CCHFV, Egypt conducted the greatest number of studies followed by UAE and Sudan (Table 1). However, in some countries of the Arab world, published data on CCHFV was scarce. We found only one published record on CCHFV in Algeria, Kuwait and Morocco. This may be due to a lack of focus of research, low prevalence of the disease in these countries, or poor funding and infrastructure for conducting research. The complex dynamics of host-tick-pathogen system highlights the need for strong interdisciplinary collaborations and teamwork to explain the reasons for recent changes in tick vectors and the virus distribution and abundance. In the following sections, we will discuss CCHFV classification and genome structure, transmission, epidemiology, mortality rate, clinical picture and policy making, relevant to the Arab countries.
CCHFV is a Nairovirus belonging to the family Bunyaviridae that also includes genera Orthobunyavirus, Hantavirus, Phlebovirus, and Tospovirus (75). All these genera are known to include human pathogens except Tospovirus which infects plants (76). Nairoviruses are tick-borne viruses (77, 78) and they are distinguished from other bunyaviruses by their large genome L segments (75, 79). CCHFV is an RNA enveloped virus with a diameter of ~80–100 nm (79) (Figure 4). Its lipid envelope is specked with spikes comprising of the glycoproteins (Gn and Gc), which are responsible for the binding of the virus to cellular receptors. The genome consists of single-stranded RNA with negative polarity, contains three segments, small (S), medium (M), and large (L), encapsidated by the nucleoprotein (NP), and the RNA-dependent RNA polymerase (RdRp), which is required for transcription and genome replication in the host cell (75, 79) (Figure 4).
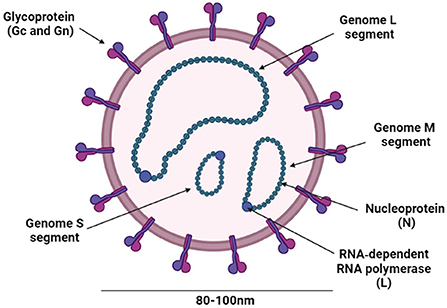
Figure 4. Schematic representation of the CCHFV structure. The figure was created with BioRender (https://Biorender.Com/).
CCHFV is genetically diverse arbovirus and analysis of complete and partial S segment sequences revealed the seven genetic lineages of CCHFV correlated with the geographical area of virus detection including Asia 1, Asia 2, Africa 1, Africa 2, Africa 3, Europe 1, and Europe 2 (80–82). Reassortment and recombination of segments that occurs with concurrent infections of vectors with viral strains of different lineages may lead to the emergence of new genetic variants of CCHFV (83–85).
Ticks become infected with CCHFV during their blood meal on an infected animal. The virus replicates in the tick midgut, disseminates to the hemocoel, and then spreads to the salivary glands to be transmitted to the next host through saliva. As compared to mosquitoes, ticks feed for a longer period on the host and ingest a greater volume of blood. Ticks digest blood in the acidic intracellular compartments of the gut epithelium (86). Therefore, the virus does not need to bind to a receptor in the tick's midgut to infect and replicate in the cells of the midgut and to spread to different parts of the body such as the salivary glands and reproductive organs (87). The virus passes through several barriers within ticks during the process of replication and transmission. Viral replication is stimulated by the attachment of the tick to the host during the feeding period (6). CCHFV can be associated with the vector for an extended period by persistent infection through the trans-ovarial transmission to the next generation and trans-stadial transmission to the next life stage (6). However, the frequency of both of these transmission processes requires further investigations. For example, ticks can survive long periods without feeding; consequently, tick vectors serve as reservoirs of CCHFV infection even in the absence of vertebrate hosts. For example, in H. marginatum, CCHFV was detectable up to 700 days after an infectious blood meal (88). Moreover, ticks have been reported to transmit the virus by biting the vertebrates even after storage at 4 °C for up to 10 months (88).
CCHF infections are enzootic and mostly asymptomatic in various animals (89). The CCHF virus can be transmitted to humans via contact with infected humans and animal tissues/blood or by tick bites (90) (Figure 5). Nosocomial outbreaks in hospitals are associated with resource-poor settings (43). For example, a nosocomial outbreak was reported in Al-Fulah, Kordufan, Sudan in 2008 when a 60 years old male patient who had worked as a butcher was admitted to hospital. Due to the lack of personal protective equipment (PPE) and implementation of stringent infection control measures, the virus was transmitted to nurses who had provided care to the index patient (43). The majority of CCHF cases however, have occurred in people associated with the livestock industry, slaughterhouse/abattoir, and veterinary practice (91, 92). In the Arab world, the virus has been shown to circulate in many tick genera (Table 1). However, ticks belonging to the genus Hyalomma are the main source of human infections, perhaps due to both immature and adult ticks feeding on host blood that they requires at each stage of their maturation (75). Hyalomma ticks act as both reservoirs and vectors for CCHFV (93). The Hyalomma tick larvae and nymphs feed on small mammals or/and birds, or reptiles whereas the adults feed on ungulates, and maintain CCHFV in nature through trans-ovarian and trans-stadial transmission (6, 27, 35). The role of reptiles as competent host for CCHFV transmission and as reservoirs needs to be determined. The transmission of CCHFV to animals occurs through a bite of an infected tick. Subsequently, the virus transmits to non-infected ticks while taking blood meal from the infected host. Ticks can also acquire infection during co-feeding of infected and non-infected ticks on same host and viral substances present in the saliva of ticks accelerate the viral transmission (94). However, all mammals are equally susceptible to CCHFV infection (95). Birds are considered poor hosts for CCHFV replication and transmission because birds are commonly resistant to becoming viremic (96). Humans are generally considered as incidental, dead-end host for CCHFV. People predominately get infected through tick bites, contact with tissues and blood of viremic animals, and though tissues and body fluid/blood of infected humans (75). Travel and trade of infected livestock from infected areas to new areas can also lead to CCHFV transmission (97). The threat of CCHFV transmission can be reduced through changes in land use, and by controlling the travel and trade of infected livestock. In the Arab world, during various outbreaks, the most common mode of CCHFV transmission was found to be contact with contaminated blood of carcasses through wounds or mucous membranes of infected animals and patients (Table 1).
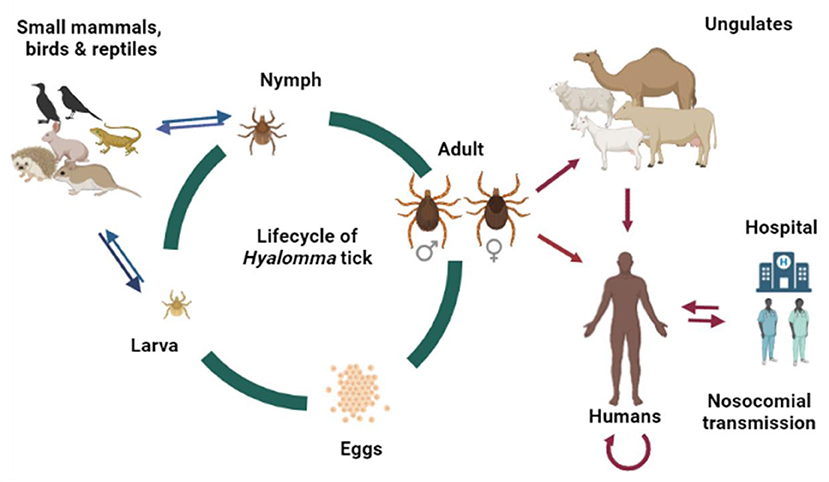
Figure 5. Lifecycle of Hyalomma tick and potential transmission route of CCHFV in the Arab world. The original figure was created with BioRender (https://Biorender.Com/).
CCHF first caught attention during an outbreak in Crimea in 1944 when 200 Soviet military personnel were infected while assisting farmers (27). It later emerged that the same virus infected a 13-year-old male patient in Congo in 1956, giving the virus its current name (27). It was designated as arbovirus in 1962 (98, 99). CCHF is endemic in Africa, Asia, Eastern Europe, and the Middle East (8, 100). The CCHFV distribution covers the maximum geographic range of any tick-borne virus in the MENA Region. Many Arab countries of the MENA Region have reported CCHF cases. The CCHF geographic distribution overlaps with that of Hyalomma ticks (Figure 2). These ticks feed on several animals including livestock and wildlife that could serve as asymptomatic reservoirs of CCHFV in the transmission cycle in endemic areas (89). A wide range of hosts and favorable climatic and ecological conditions in several Arab countries bordering each other could upsurge the incidence of CCHF in the region in the future. Ecological settings and human behavior can also play a crucial role in the maintenance and occurrence of CCHFV within region (101). Furthermore, changes in land-use, urbanization, transportation and trade of infected livestock can also impact the risk of CCHFV transmission. Limitations in surveillance and diagnostic capacities are important impediments in the estimation of the CCHF burden in many countries (102). In the following section we will discuss the epidemiology of CCHFV in specific countries in the Arab world.
In 2009–2010, a study was conducted in Laghouat Province of Algeria to determine the biological role of Hyalomma aegyptium ticks in the epidemiology of CCHF. CCHFV has been detected in H. aegyptium ticks collected from tortoises with a prevalence of 28.6% (21). Hyalomma aegyptium adults feed mostly on tortoises (103) and less often on hedgehogs and hares. However, larvae and nymphs feed on a wide range of hosts including humans, birds, reptiles, and mammals that increase this species' role as a possible bridge vector for linking wildlife, livestock, and humans to transmit CCHFV (21). No human case has been reported in Algeria (Figure 2).
Egypt is positioned between numerous foci of CCHFV in Eurasia and Africa. In our data set, Egypt was found to have the highest number of tick species associated with the CCHFV virus (Table 1). Migrating birds during their spring and fall passages (northward- and southward) linked to transport of large numbers of ticks through Egypt from and within the African and Eurasian ranges of CCHFV (17, 27). In 1978, a serological study from Egypt provided the first evidence that antibodies to CCHFV were present in several wild and domestic animals, including camels (8.8%) and sheep (23.1%), and hence, the virus was circulating in country (17). The study also suggested that H. anatolicum, H. marginatum H. rufipes, H. impeltatum, R. sanguineous, R. turanicus, and R. annulatus were the most common tick species associated with CCHFV. In 2004–2005, to find the role of ruminants as a reservoir host for CCHFV, a serological survey indicated that 3.13% of animals tested were positive for CCHFV antibodies (42). In 1986–1987, 14% of sera of imported camels tested positive for CCHFV antibodies (33). CCHFV antibodies have been reported in other animals including cows (1%) (24), sheep (6.30%) cattle (3.83%), buffaloes (0.38%), and goats (1.14%) (42). Ticks have also been shown to be positive for CCHFV antibodies (51, 64, 69). Human cases, including healthcare workers have also been reported (Table 1). Thus, vector control, continuous screening of domestic animals and strict implementation of infection control measures in healthcare facilities is essential to avoid such outbreaks.
In 1979, a 24 year-old lady was diagnosed with CCHF at Al-Yarmouk hospital, Baghdad, making her the first confirmed case of CCHF in the country (22). Later two close contacts, one physician and one health worker also contracted the infection and subsequently died (22). Thereafter, CCHF cases were reported in Iraq in different periods from 1980 to 2014 (22, 28, 47, 48, 54) and most of the cases had a history of contact with animals and others were physicians/health workers. Tantawi et al. carried out a study in 1980 to determine the prevalence of CCHFV in animals and most of the animals tested positive with high prevalence for antibodies to the virus (29) (Table 1).
From 1979 to 1982, a total of 502 sera samples were collected from two hospitals in Kuwait. Only 18 human cases were found to be positive for CCHFV antibodies (31). Furthermore, Hyalomma ticks in Kuwait have been reported in different studies (4, 104), implying that they could be involved in the transmission of CCHFV.
In 1983, a 48 year-old male who lived in Selibaby, Mauritania was admitted to a hospital and subsequently confirmed to be positive for CCHFV (32). Five years later, another case was detected (34). In 1992, the sexual and trans-ovarial transmission of CCHFV was determined in Hyalomma truncatum ticks, indicating that tick populations could contribute to the maintenance of CCHFV transmission in nature (35). In 2003, there was an urban outbreak of CCHF in which 28.6 % of the cases were fatal (41). Additionally, CCHFV has also been detected in livestock and ticks in different periods (41, 59, 63, 70) (Table 1). In Mauritania, 5–49 cases of CCHF have been reported per year (73).
In 2013, CCHFV was detected in H. marginatum ticks collected from migratory birds in Zouala, Morocco. A total of 546 birds were captured and investigated for ticks. Fifty-two ticks including 19 larvae and 33 nymphs were collected and screened for the virus. Using nested PCR assays (using Eecf primers) 4/6 pools were found positive. All nucleotide sequences showed 100% similarity with the CCHFV strains from Mauritania and Sudan (53). The presence of H. marginatum ticks and reservoir of the virus, such as livestock, provide optimal conditions for the establishment of CCHFV in the country. The risk could be increased due to presence of CCHFV in the neighboring country, Mauritania (Figure 2).
A 37 year-old male from Buraimi, Oman, near the UAE border, was diagnosed with CCHF in 1995 (15). On further investigation, this person was staying at the farm having sheep and goats and a few Hyalomma ticks were also found on the animals (15). Another case was reported in the same year, but this time in an individual working in a farm in Sur, Muscat, Oman (15). Once again, Hyalomma spp. ticks were recovered from livestock on the farm. In 2000, to evaluate the circulation and prevalence of CCHFV in Oman, samples were screened for CCHFV antibodies from workers and animals from different locations, farms, livestock markets, and abattoirs (18). Screening revealed that 30.3% of workers and 22% of animals were positive (18). Tick analysis showed H. anatolicum to be the most abundant. In 2011, after 15 years, a 37 years old man was admitted to the Sultan Qaboos University Hospital and diagnosed with CCHFV (19). The patient was started on ribavirin and his condition improved dramatically (19). High prevalence of CCHFV antibodies was reported in cattle (17.5%), camels (15.7 %), goats (4.8%), sheep (4.3%), and ticks (5.1%) (55). From 2011 to 2017, human cases gradually increased and the major risk for CCHFV infections was contact with animals and/or butchering (61). However, no spread in families or healthcare-associated infections were reported (61).
CCHF was reported for the first time in the country in 1990 (39) when seven individuals were infected with CCHFV in the city of Mecca. This prompt a study to determine the reason for the outbreak and to understand the epidemiology of CCHF in the region (39). Ticks were collected from livestock and 10/13 ixodid tick species were found to be capable of transmitting the virus. CCHF confirmed and suspected cases had a history of contact with fresh mutton and slaughtering sheep. Twelve fatalities were reported in a serological survey of 40 abattoir workers (confirmed or suspected cases) in Mecca from 1989 to 1990 (39). However, it was suspected that the CCHF virus may have been introduced into the country by infected ticks on imported sheep arriving via Jeddah seaport (39, 105). Another survey of CCHFV antibodies was carried out in imported livestock at Jeddah seaport, as well as in humans who had contact with imported animals on farms and in quarantine stations. CCHFV was detected in humans (0.8%), sheep (4.1%), goats (3.2%), and cattle (0.6%) (40), suggesting that the virus was introduced into Saudi Arabia through imported animals. Recently, during an investigation of hemorrhagic fever viruses in the tick populations, H. schulzei, H. onatoli, and H. dromedarii were found to be positive for CCHFV (58).
In 1989 an outbreak of acute febrile illness was reported in Northern Sudan coinciding with the presence of phlebotomine sandflies in high density areas. Five human cases tested positive for CCHFV antibodies, along with other viruses (37). During 2008–2009, an outbreak involving seven cases of CCHF was reported in South Sudan (45), indicating both sporadic and nosocomial transmission (43–45). During a seroepidemiological survey to determine the prevalence of CCHFV in North Kordufan State, 7% of cattle tested positive for CCHFV antibodies (52). More recent studies have confirmed the presence of CCHFV in livestock animals, including camels and cattle (25, 60). Recently, human cases of CCHF have been reported from Khashm el Girba, Eastern Sudan (62).
Samples from acute febrile patients and slaughterhouse workers were collected in 2014 to investigate the circulation of CCHFV in Tunisia. Ticks were also collected from Northern and Southern Tunisia and examined for the presence of CCHFV. Slaughterhouse workers (5.2%) and patients (2.7%) tested positive with CCHFV antibodies (16). However, no CCHFV infection was detected in ticks. Seroprevalence of CCHFV infection has been reported in one-humped camels (89.7%) in Southern Tunisia (65). Recently, CCHFV infections have also been reported in cattle, sheep, goats, and ticks in different studies (66, 67) (Table 1).
A nosocomial CCHF outbreak was reported in Dubai, UAE in 1979 when an index case died just after admission to hospital. Five secondary cases were also identified amongst hospital staff, two of whom died (14). Autopsies on the two fatal cases, confirmed the diagnosis of CCHF (30). Another outbreak of CCHF was reported in UAE during 1994–1995 (11, 38). Investigations revealed CCHFV antibodies in the serum of livestock market employees (3%), abattoir employees (6%), camels (7.4%), cattle (1.7%), sheep (8.1%), goats (12%), and in ticks (2.2%) (38). In 2010, two human cases of CCHF were reported in Dubai (56). More recently, CCHFV antibodies were detected in dromedary camels in two different studies (10, 68) (Table 1). Hyalomma ticks are the most prevalent vector species reported on camels, cows, sheep, and goats in the UAE (106–108). Therefore, continued surveillance, monitoring, and screening of tick vectors, animals, and associated people are required to prevent any future CCHF outbreak.
There is no published record of CCHF infections in humans and animals. However, several tick species have been reported in Yemen that could be potential vectors for CCHFV (27, 109). This creates a huge risk and screening of CCHFV in animal and tick populations is crucial to managing any future infections.
CCHFV infection can be broadly grouped into four phases: Incubation period, pre-hemorrhagic, hemorrhagic, and convalescent (110). The incubation period is the asymptomatic phase, which persists for 3–7 days after infection. The second is a pre-hemorrhagic phase that lasts for 4–5 days and is characterized by symptoms such as high fever, headache, abdominal pain, myalgia, and hypotension (27). The third phase involves severe symptoms, such as epistaxis, hemoptysis, ecchymosis, diarrhea, neuropsychiatric and cardiovascular changes (7). Severely ill patients can progress to multi-organ failure and death. Those who survive, recovery starts around 10–20 days after the onset of the illness (78). Full recovery can take almost a year in CCHF survivors (13). However, some patients were reported with dramatic recovery in much shorter time (14).
CCHFV causes severe disease in humans with a high fatality rate, up to 50% (111) and up to 80% for nosocomial transmission (112). In Arab countries, mortality rate varied from 24–61% during different outbreaks. Early diagnosis is critical for patient support and for preventing the spread of infection through well-documented human-to-human transmission (113). Ribavirin has been used extensively as an antiviral treatment (113). Table 2 documents CCHF cases and fatality rates reported in different countries in the Arab world over the last four decades.
CCHF is a disease with a high potential of an outbreak with high fatality rate. Hyalomma ticks are present across the Arab countries. Climate change and anthropogenic factors could contribute to an extension of the geographic range of CCHFV. Continuous surveillance of tick vectors and animals is required to monitor the CCHF burden and epidemiological trends. Considering the high case fatality rate of CCHF, early detection and diagnosis are critical to allow quick interventions at all levels, including patient, hospital, and community level. Further, the development of a vaccine and new drugs against CCHFV is of major importance. Ribavirin efficacy should be evaluated through well-designed clinical protocols. Awareness about the mode of transmission of CCHF to the general public is essential to curtail the spread in the area. In many rural areas in Arab countries, backyard slaughtering is common practice and this can result in transmission of the virus to humans (8). Similarly, auxiliary staff should be well trained to recognize and act accordingly to avoid nosocomial spread of the infection. High biosafety level laboratories (BLS4) are crucial for rapid confirmation of suspected cases. Tests need to be reliable and affordable. Climate change and anthropogenic factors that may affect the epidemiology of CCHF should be further studied. Risk assessment in CCHF endemic areas is important for devising tick-control strategies. Therefore, multidisciplinary collaboration is required at the local and regional levels to identify relevant gaps and work in an integrated fashion for the prevention and control of CCHF.
This study has a number of limitations. We only included studies/reports published in English. It is possible that some studies may have been published in Arabic which we did not include. Furthermore, we could not find published data on CCHF/CCHFV for some countries. Moreover, reliable and good quality data on CCHF, such as demographics, clinical data, and incidence/fatality rates are not always available or accessible in some of the countries in the region. These limitations can clear impact the analysis.
CCHF is a zoonotic disease and a public health menace in the Arab world. The geographic range of the disease is mushrooming due to the change in climatic conditions, and travel and trade of livestock. Furthermore, due to alteration in distribution pattern of host range, the distribution of Hyalomma ticks is expanding and consequently CCHFV infection risk is increasing. In this systematic review, we have provided a detailed descriptive epidemiology of CCHF in 22 Arab countries. We have discussed the patterns of CCHF at regional as well as country level and suggested strategies which could be implemented to reduce the burden of the disease. Only 9/22 countries, namely, Iraq, Kuwait, UAE, Saudi Arabia, Oman, Sudan, Egypt, Tunisia, and Mauritania have reported cases of CCHF in the literature. Not all countries in the region have the same level of resources or robust surveillance and reporting systems. Thus, the 321 cases of CCHF with 105 deaths reported in the region over a period of 43 years are likely to be underestimates. Outbreaks continue to occur on regular basis. This year in Iraq, there has been an upsurge in the disease to epidemic levels not seen since it was first recorded in 1979; 23 cases and 8 deaths have been reported in just 4 months (https://promedmail.org/ accessed on 24.04.2022). Individuals working in slaughterhouses and veterinarians were found to be most affected in this outbreak. This further highlights the urgent need for establishing effective policies and the strict enforcement of preventative and control measures in countries in the region where they are underdeveloped.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
GK checked the record on the basis of inclusion criteria, provided intellectual inputs and shared ideas, conceived, and designed the study. NP searched the literature, screened, and organized the data. NP and GK wrote the manuscript, prepared illustrations, and revised the manuscript. All authors read and approved the final manuscript.
We are thankful to all authors whose articles are included in this study. We also appreciate the library facility provided by the United Arab Emirates University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Harrus S, Baneth G. Drivers for the emergence and re-emergence of vector-borne protozoal and bacterial diseases. Int J Parasitol. (2005) 35:1309–18. doi: 10.1016/j.ijpara.2005.06.005
2. Collinge SK, Ray C. Disease Ecology: community strusture and pathogen dynamics. Oxford University Press (2006).
3. Cunningham AA, Daszak P, Wood JLN. One health, emerging infectious diseases and wildlife: two decades of progress? Philos Trans R Soc B Biol Sci. (2017) 372:1–8. doi: 10.1098/rstb.2016.0167
4. Perveen N, Muzaffar SB, Al-Deeb MA. Ticks and tick-borne diseases of livestock in the Middle East and North Africa: a review. Insects. (2021) 12:1–34. doi: 10.3390/insects12010083
5. Temur AI, Kuhn JH, Pecor DB, Apanaskevich DA, Keshtkar-Jahromi M. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa—Underestimated for Decades. Am J Trop Med Hyg. (2021) 104:1978–90. doi: 10.4269/ajtmh.20-1413
6. Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies HHS Public Access. Antiviral Res. (2017) 144:93–119. doi: 10.1016/j.antiviral.2017.05.010
7. Whitehouse CA. Crimean-congo hemorrhagic fever. Antiviral Res. (2004) 64:145–60. doi: 10.1016/j.antiviral.2004.08.001
8. Al-Abri SS, Abaidani I Al, Fazlalipour M, Mostafavi E, Leblebicioglu H, Pshenichnaya N, et al. Current status of Crimean-Congo haemorrhagic fever in the World Health Organization Eastern Mediterranean Region: issues, challenges, and future directions. Int J Infect Dis. (2017) 58:82–9. doi: 10.1016/j.ijid.2017.02.018
9. Shahhosseini N, Wong G, Babuadze G, Camp J V, Ergonul O, Kobinger GP, et al. Crimean-congo hemorrhagic fever virus in Asia, Africa and Europe. Microorganisms. (2021) 9:1–24. doi: 10.3390/microorganisms9091907
10. Camp J V, Kannan DO, Osman BM, Shah MS, Howarth B, Khafaga T, et al. Crimean-congo hemorrhagic fever virus endemicity in United Arab Emirates, 2019. Emerg Infect Dis. (2020) 26:2019–21. doi: 10.3201/eid2605.191414
11. Schwarz TF, Nsanze H, Ameen AM. Clinical features of Crimean-Congo haemorrhagic fever in the United Arab Emirates. Infection. (1997) 25:364–7. doi: 10.1007/BF01740819
12. Wernery U. Zoonoses in the Arabian Peninsula. Saudi Med J. (2014) 35:1455–62. Available online at: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc4362160/
13. Ergönül O. Crimean-congo haemorrhagic fever. Lancet Infect Dis. (2006) 6:203–14. doi: 10.1016/S1473-3099(06)70435-2
14. Suleiman MNEH, Muscat-Baron JM, Harries JR, Satti AGO, Platt GS, Bowen ETW, et al. Congo/Crimean Haemorrhagic Fever in Dubai: An Outbreak at the Rashid Hospital (1980).
15. Scrimgeourl EM, Zaki A, Mehta FR, Abraham AK, Al-Busaidy S, El-Khatim H, et al. Crimean-Congo in Oman haemorrhagic fever in Oman. Trans R Soc Trop Med Hyg. (1996) 90:290–1. doi: 10.1016/S0035-9203(96)90254-0
16. Wasfi F, Dowall S, Ghabbari T, Bosworth A, Chakroun M, Varghese A, et al. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite. (2016) 23:1–5. doi: 10.1051/parasite/2016010
17. Darwish MA, Imam IZE, Omar FM, Hoogstraal H. Results of a preliminary seroepidemiological survey for Crimean-Congo hemorrhagic fever virus in Egypt. Acta Virol. (1978) 22:77.
18. Williams RJ, Al-Busaidy S, Mehta FR, Maupin GO, Wagoner KD, Al-Awaidy S, et al. Crimean-Congo haemorrhagic fever: a seroepidemiological and tick survey in the Sultanate of Oman. Trop Med Int Heal. (2000) 5:99–106. doi: 10.1046/j.1365-3156.2000.00524.x
19. Al-Zadjali M. Al-hashim H, Al-gh M, Balkha A. A Case of Crimean-Congo Hemorrhagic Fever in Oman. Oman Med J. (2013) 28:210–2. doi: 10.5001/omj.2013.57
20. Rodriguez LL, Maupin GO, Ksiazek TG, Rollin PE, Khan ALS, Schwarz TEF, et al. Molecular investigation of a multisource outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates. Am J Trop Med Hyg. (1997) 57:512–8. doi: 10.4269/ajtmh.1997.57.512
21. Kautman M, Tiar G, Papa A, Široký P. AP92-like crimean-congo hemorrhagic fever virus in hyalomma aegyptium Ticks, Algeria. Emerg Infect Dis. (2016) 22:354–6. doi: 10.3201/eid2202.151528
22. Al-Tikriti SK, Al-Ani F, Jurji FJ, Tantawi H, Mahmud MIA, Habib H, et al. Congo / Crimean haemorrhagic fever in Iraq. Bull World Health Organ. (1981) 59:85–90.
23. Cecaro M, Isolani L, Cuteri V. European Monitoring plans for the management of outbreak of crimean congo haemorrhagic fever (CCHF). Occup Med Heal Aff . (2013) 01:123. doi: 10.4172/2329-6879.1000123
24. Horton KC, Wasfy M, Samaha H, Abdel-Rahman B, Safwat S, Abdel Fadeel M, et al. Serosurvey for zoonotic viral and bacterial pathogens among slaughtered livestock in Egypt. Vector-Borne Zoonotic Dis. (2014) 14:633–9. doi: 10.1089/vbz.2013.1525
25. Ibrahim AM, Adam IA, Osman BT, Aradaib IE. Ticks and tick-borne diseases epidemiological survey of crimean congo hemorrhagic fever virus in cattle in East Darfur State, Sudan. Ticks Tick Borne Dis. (2015) 6:439–44. doi: 10.1016/j.ttbdis.2015.03.002
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:1–9. doi: 10.1186/s13643-021-01626-4
27. Hoogstraal H. The Epidemiology of Tick-borne crimean-congo hemorrhagic fever in Asia, Europe and Africa. J Med Entomol. (1979) 15:307–417. doi: 10.1093/jmedent/15.4.307
28. Tantawi HH, Al-Moslih MI, Al-Janabi NY, Al-Bana AS, Mahmud MI, Jurji F, et al. Crimean-Congo haemorrhagic fever virus in Iraq: isolation, identification and electron microscopy. Acta Virol. (1980) 24:464–7.
29. Tantawi HH, Shony MO, Al-Tikriti SK. Antibodies to Crimean-Congo haemorrhagic fever virus in domestic animals in Iraq: a seroepidemiological survey. Int J Zoonoses. (1981) 8:115–20.
30. Baskerville A, Satti AGO, Murphy FA, Simpson DIH. Congo-Crimean haemorrhagic fever in Dubai: histopathological studies. J Clin Pathol. (1981) 34:871–4. doi: 10.1136/jcp.34.8.871
31. Al-Nakib W, Lloyd G, El-Mekki A, Platt G, Beeson A, Southee T. Preliminary report on arbovirus-antibody prevalence among patients in Kuwait: evidence of congo/crimean virus infection. Trans R Soc Trop Med Hyg. (1984) 78:474–6. doi: 10.1016/0035-9203(84)90065-8
32. Saluzzo JF, Aubry P, Digoutte JP. Haemorrhagic fever caused by crimean congo haemorrhagic fever virus in Mauritania. Trans R Soc Trop Med Hyg. (1985) 79:268. doi: 10.1016/0035-9203(85)90356-6
33. Morrill JC, Soliman AK, Imam IZ, Botros BAM, Moussa MI, Watts DM. Serological evidence of Crimean-Congo haemorrhagic fever viral infection among camels imported into Egypt. J Trop Med Hyg. (1990) 93:201–4.
34. Gonzalez JP, LeGuenno B, Guillaud M, Wilson ML. A fatal case of Crimean-Congo haemorrhagic fever in Mauritania: virological and serological evidence suggesting epidemic transmission. Trans R Soc Trop Med Hyg. (1990) 84:573–6. doi: 10.1016/0035-9203(90)90045-G
35. Gonzalez JP, Camicas JL, Cornet JP, Faye O, Wilson ML. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Res Virol. (1992) 143:23–8. doi: 10.1016/S0923-2516(06)80073-7
36. Darwish MA, Buck A, Faris R, Gad A, Moustafa A, El-Khashab T, et al. Antibodies to certain arboviruses in humans from a flooded village in Egypt. J Egypt Public Health Assoc. (1994) 69:239–60.
37. Watts DM, El-Tigani A, Botros BA, Salib AW, Olson JG. Arthropod-borne viral infectious associated with a fever outbreak in the Northern Province of Sudan. Dep Med Zool Nav Med Res Unit No 3, Cairo, Egypt (1994)
38. Khan AS, Maupin GO, Rollin PE, Noor AM, Shurie HHM, Shalabi AGA, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994-1995. Am J Trop Med Hyg. (1997) 57:519–25. doi: 10.4269/ajtmh.1997.57.519
39. El-Azazy OME, Scrimgeour EM. Crimean-Congo haemorrhagic fever virus infection in the Western Province of Saudi Arabia. Trans R Soc Trop Med Hyg. (1997) 91:275–8. doi: 10.1016/S0035-9203(97)90072-9
40. Hassanein KM, El-Azazy OME, Yousef HM. Detection of Crimean-Congo haemorrhagic fever virus antibodies in humans and imported livestock in Saudi Arabia. Trans R Soc Trop Med Hyg. (1997) 91:536–7. doi: 10.1016/S0035-9203(97)90014-6
41. Nabeth P, Cheikh DO, Lo B, Faye O, Ould I, Vall M. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg Infect Dis. (2004) 10:2143–9. doi: 10.3201/eid1012.040535
42. Mohamed M, Said A-R, Murad A, Graham R. A serological survey of Crimean-Congo haemorrhagic fever in animals in the Sharkia Governorate of Egypt. Vet Ital. (2008) 44:513–7.
43. Aradaib IE, Erickson BR, Mustafa ME, Khristova ML, Saeed NS, Elageb RM, et al. Nosocomial outbreak of crimean-congo hemorrhagic fever, Sudan. Emerg Infect Dis. (2010) 16:837–9. doi: 10.3201/eid1605.091815
44. Elata AT, Karsany MS, Elageb RM, Hussain MA, Eltom KH, Elbashir MI, et al. nosocomial transmission of crimean-congo hemorrhagic fever to an attending physician in north kordufan, Sudan. Virol J. (2011) 8:1–7. doi: 10.1186/1743-422X-8-303
45. Aradaib IE, Erickson BR, Karsany MS, Khristova ML, Elageb RM, Mohamed MEH, et al. Multiple Crimean-Congo hemorrhagic fever virus strains are associated with disease outbreaks in Sudan, 2008-2009. PLoS Negl Trop Dis. (2011) 5:2008–9. doi: 10.1371/journal.pntd.0001159
46. Memish ZA, Albarrak A, Almazroa MA, Al-Omar I, Alhakeem R, Assiri A, et al. Seroprevalence of Alkhurma and other hemorrhagic fever viruses, Saudi Arabia. Emerg Infect Dis. (2011) 17:2316–8. doi: 10.3201/eid1712.110658
47. Abul-Eis ES, Mohammad NA, Wasein SM. Crimean-congo hemorrhagic fever in iraq during 2010. In: Proceeding of the Eleventh Veterinary Scientific Conference. (2010), p. 99–103.
48. Majeed B, Dicker R, Nawar A, Badri S, Noah A, Muslem H. Morbidity and mortality of Crimean-Congo hemorrhagic fever in Iraq: cases reported to the National Surveillance System, 1990–2010. Trans R Soc Trop Med Hyg. (2012) 106:480–3. doi: 10.1016/j.trstmh.2012.04.006
49. El-Bahnasawy MM, Sabah AA, Saleh HA, Morsy TA. The tick-borne Crimean-Congo hemorrhagic fever in Africa, Asia, Europe, and America: what about Egypt. J Egypt Socirty Parasitol. (2012) 42:373–84. doi: 10.12816/0006324
50. WHO. Report on the consultation to develop guidelines for infection prevention and control for viral haemorrhagic fevers in health care facilities, Cairo, Egypt, 20–21 June 2012 (No. WHO-EM/CSR/051/E). (2012).
51. Chisholm K, Dueger E, Fahmy NT, Samaha HAT, Zayed A, Abdel-Dayem M, et al. Crimean-Congo hemorrhagic fever virus in ticks from imported livestock, Egypt. Emerg Infect Dis. (2012) 18:181. doi: 10.3201/eid1801.111071
52. Adam IA, Mahmoud MAM, Aradaib IE. A seroepidemiological survey of Crimean Congo hemorrhagic fever among Cattle in North Kordufan State, Sudan. Virol J. (2013) 10:1–6. doi: 10.1186/1743-422X-10-178
53. Palomar AM, Portillo A, Santibáñez P, Mazuelas D, Arizaga J, Crespo A, et al. Crimean-congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. (2013) 19:260–3. doi: 10.3201/eid1902.121193
54. Ibrahim AS, Ibrahim KS, Mohammed MO, Al-Shaikhani MA, Barzanji AA, Saeed SJ, et al. Crimean Congo hemorrhagic fever management in Erbil during 2010-2011. Eur Sci Journal, ESJ. (2014) 10:14. doi: 10.19044/esj.2014.v10n24p%25p
55. Body MHH, ALrawahi AH, Hussain HM, Ahmed MS, ALHabsi SS, Al-Maklady S, et al. Cross-sectional survey of Crimean-Congo hemorrhagic fever virus in the Sultanate of Oman. J Vet Med Anim Heal. (2016) 8:44–9. doi: 10.5897/JVMAH2016.0472
56. Al-Dabal LM, Reza M, Shahmirzadi R, Baderldin S, Abro A, Dessi Z, Eassa E Al, Khan G, Shuri H, Alwan AM. Crimean-Congo Hemorrhagic Fever in Dubai, United Arab Emirates, 2010: Case Report. Iran Red Crescent Med J. (2016) 18:74. doi: 10.5812/ircmj.38374
57. Kleib AS, Salihy SM, Ghaber SM, Sidiel BW, Sidiya KC, Bettar ES. Crimean-Congo hemorrhagic fever with acute subdural hematoma, Mauritania, 2012. Emerg Infect Dis. (2016) 22:1305–6. doi: 10.3201/eid2207.151782
58. Mohamed RAEH, Mohamed N, Aleanizy FS, Alqahtani FY, Khalaf A Al, Al-Keridis LA. Investigation of hemorrhagic fever viruses inside wild populations of ticks: One of the pioneer studies in Saudi Arabia Asian Pacific. J Trop Dis. (2017) 7:299–303. doi: 10.12980/apjtd.7.2017D6-371
59. Sas MA, Mertens M, Isselmou E, Reimer N, El Mamy BO, Doumbia B, et al. Crimean-congo hemorrhagic fever virus-specific antibody detection in Cattle in Mauritania. Vector-Borne Zoonotic Dis. (2017) 17:582–7. doi: 10.1089/vbz.2016.2084
60. Suliman HM, Adam IA, Saeed SI, Abdelaziz SA, Haroun EM, Aradaib IE. Crimean Congo hemorrhagic fever among the one-humped camel (Camelus dromedaries) in Central Sudan. Virol J. (2017) 14:1–7. doi: 10.1186/s12985-017-0816-3
61. Al-Abri SS, Hewson R, Al-kindi H, Al-abaidani I AlA, Id AA, Almahrouqi S. Clinical and molecular epidemiology of Crimean-Congo hemorrhagic fever in Oman. PLoS Negl Trop Dis. (2019) 19:1–15. doi: 10.1371/journal.pntd.0007100
62. Rahden P, Adam A, Mika A, Jassoy C. Elevated human crimean-congo hemorrhagic fever virus seroprevalence in Khashm el Girba, Eastern Sudan. Am J Trop Med Hyg. (2019) 100:1549–51. doi: 10.4269/ajtmh.18-0977
63. Schulz A, Barry Y, Stoek F, Pickin MJ, Ba A, Chitimia-Dobler L, et al. Detection of Crimean-Congo hemorrhagic fever virus (CCHFV) in Hyalomma ticks collected from Mauritanian livestock. Res Sq Priprint. (2020) 20:1–20. doi: 10.21203/rs.3.rs-45301/v1
64. Abdulall AK, Raslan F, Zaki AM. Molecular detection of crimean—congo hemorrhagic fever virus in Ticks Isolated from Camels in Egypt. J Appl Pharm. (2020) 12:163–4.
65. Bouaicha F, Eisenbarth A, Elati K, Schulz A, Ben Smida B, Bouajila M, et al. Epidemiological investigation of Crimean-Congo haemorrhagic fever virus infection among the one-humped camels (Camelus dromedarius) in southern Tunisia. Ticks Tick Borne Dis. (2021) 12:601. doi: 10.1016/j.ttbdis.2020.101601
66. Khamassi Khbou M, Romdhane R, Bouaicha Zaafouri F, Bouajila M, Sassi L, Appelberg SK, et al. Presence of antibodies to Crimean Congo haemorrhagic fever virus in sheep in Tunisia, North Africa. Vet Med Sci. (2021) 7:2323–9. doi: 10.1002/vms3.597
67. Zouaghi K, Bouattour A, Aounallah H, Surtees R, Krause E, Michel J, et al. First serological evidence of crimean-congo hemorrhagic fever virus and rift valley fever virus in ruminants in tunisia. Pathogens. (2021) 10:1–19. doi: 10.3390/pathogens10060769
68. Khalafalla AI Li Y, Uehara A, Hussein NA, Zhang J, Tao Y, Bergeron E, et al. Identification of a novel lineage of Crimean-Congo haemorrhagic fever virus in dromedary camels, United Arab Emirates. J Gen Virol. (2021) 102:1–4. doi: 10.1099/jgv.0.001473
69. Bendary HA, Rasslan F, Wainwright M, Alfarraj S, Zaki AM, Abdulall AK. Crimean-congo hemorrhagic fever virus in ticks collected from imported camels in Egypt. Saudi J Biol Sci. (2021) 21:43. doi: 10.1016/j.sjbs.2021.12.043
70. Schulz A, Barry Y, Stoek F, Ba A, Schulz J, Haki ML, et al. Crimean-congo hemorrhagic fever virus antibody prevalence in mauritanian livestock (Cattle, goats, sheep and camels) is stratified by the animal's age. PLoS Negl Trop Dis. (2021) 15:1–12. doi: 10.1371/journal.pntd.0009228
72. WBG. Total Population-Arab World. Arab World Data. (2020). Available online at: https//data.worldbank.org/country/1A (accessed 19092020)
73. WHO. Crimean-Congo Haemorrhagic Fever. (2017). Available online at: https://www.who.int/health-topics/crimean-congo-ha (accessed 22032022).
74. Albayrak H, Ozan E, Kurt M. Serosurvey and molecular detection of Crimean-Congo hemorrhagic fever virus (CCHFV) in northern Turkey. Trop Anim Health Prod. (2012) 44:1667–71. doi: 10.1007/s11250-012-0122-4
75. Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. (2013) 100:159–89. doi: 10.1016/j.antiviral.2013.07.006
76. Olaya C, Adhikari B, Raikhy G, Cheng J, Pappu HR. Identification and localization of Tospovirus genus-wide conserved residues in 3D models of the nucleocapsid and the silencing suppressor proteins. Virol J. (2019) 16:1–15. doi: 10.1186/s12985-018-1106-4
77. Elliott RM, Bouloy M, Calisher CH, Goldbach R, Moyer JT, Nichol S. “Family Bunyaviridae,” in Virus Taxonomy: Classification and Nomenclature of Viruses. Seventh Report of the International Committee on Taxonomy of Viruses., ed. I. van R. MHV (San Diego: Academic Press) (2000), p. 599–621.
78. Appannanavar SB, Mishra B. An update on crimean congo hemorrhagic fever. J Glob Infect Dis. (2011) 3:285–92. doi: 10.4103/0974-777X.83537
79. Marriott AC, Nuttall PA. Molecular biology of nairoviruses. In: Elliott RM, editors. The Bunyaviridae. The Viruses. Boston, MA: Springer (1996). doi: 10.1007/978-1-4899-1364-7_4
80. Hewson R. Molecular epidemiology, genomics, and phylogeny of crimean-congo hemorrhagic fever virus. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever. Dordrecht: Springer (2007). p. 45–55. doi: 10.1007/978-1-4020-6106-6_5
81. Deyde VM, Khristova ML, Rollin PE, Ksiazek TG, Nichol ST. Crimean-congo hemorrhagic fever virus genomics and global diversity. J Virol. (2006) 80:8834–42. doi: 10.1128/JVI.00752-06
82. Volynkina A, Lisitskaya Y, Kolosov A, Shaposhnikova L, Pisarenko S, Dedkov V, et al. Molecular epidemiology of Crimean-Congo hemorrhagic fever virus in Russia. PLoS One. (2022) 17:e0266177. doi: 10.1371/journal.pone.0266177
83. Zhou Z, Deng F, Han N, Wang H, Sun S, Zhang Y, et al. Reassortment and migration analysis of crimean-congo haemorrhagic fever virus. J Gen Virol. (2013) 94:2536–48. doi: 10.1099/vir.0.056374-0
84. Lukashev AN. Evidence for recombination in crimean-congo hemorrhagic fever virus. J Gen Virol. (2005) 86:2333–8. doi: 10.1099/vir.0.80974-0
85. Hewson R, Gmyl A, Gmyl L, Smirnova SE, Karganova G, Jamil B, et al. Evidence of segment reassortment in Crimean-Congo haemorrhagic fever virus. J Gen Virol. (2004) 85:3059–70. doi: 10.1099/vir.0.80121-0
86. Sojka D, Franta Z, Horn M, Caffrey CR, Mareš M, Kopáček P. New insights into the machinery of blood digestion by ticks. Trends Parasitol. (2013) 29:276–85. doi: 10.1016/j.pt.2013.04.002
87. Dickson DL, Turell MJ. Replication and tissue tropisms of Crimean-Congo hemorrhagic fever virus in experimentally infected adult Hyalomma truncatum (Acari: Ixodidae). J Med Entomol. (1992) 29:767–73. doi: 10.1093/jmedent/29.5.767
88. Turell MJ. Role of ticks in the transmission of Crimean-Congo hemorrhagic fever virus. In: Ergonul O, Whitehouse CA, editors. Crimean-Congo Hemorrhagic Fever. Dordrecht: Springer (2007). p. 143–54. doi: 10.1007/978-1-4020-6106-6_121
89. Spengler JR, Bergeron É, Rollin PE. Seroepidemiological studies of crimean-congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. (2016) 10:1–28. doi: 10.1371/journal.pntd.0004210
90. Papa A, Tsergouli K, Tsioka K, Mirazimi A. Crimean-Congo hemorrhagic fever: tick-host-virus interactions. Front Cell Infect Microbiol. (2017) 7:1–7. doi: 10.3389/fcimb.2017.00213
91. Sharifi-Mood B, Metanat M, Alavi-Naini R. Prevalence of crimean-congo hemorrhagic feveramong high risk human groups. Int J High Risk Behav Addict. (2014) 3:5–8. doi: 10.5812/ijhrba.11520
92. Nasirian H. Crimean-Congo hemorrhagic fever (CCHF) seroprevalence: a systematic review and meta-analysis. Acta Trop. (2019) 196:102–20. doi: 10.1016/j.actatropica.2019.05.019
93. Sorvillo TE, Rodriguez SE, Hudson P, Carey M, Rodriguez LL, Spiropoulou CF, et al. Towards a sustainable one health approach to crimean-congo hemorrhagic fever prevention: focus areas and gaps in knowledge. Trop Med Infect Dis. (2020) 5:1–28. doi: 10.3390/tropicalmed5030113
94. Jones LD, Davies CR, Steele GM, Nuttall PA. A novel mode of arbovirus transmission involving a nonviremic host. Science. (1987) 237:775–7. doi: 10.1126/science.3616608
95. Shepherd AJ, Leman PA, Swanepoel R. Viremia and antibody response of small African and laboratory animals to Crimean-Congo hemorrhagic fever virus infection. Am J Trop Med Hyg. (1989) 40:541–7. doi: 10.4269/ajtmh.1989.40.541
96. Shepherd AJ, Swanepoel R, Leman PA, Shepherd SP. Field and laboratory investigation of Crimean-Congo haemorrhagic fever virus (Nairovirus, family Bunyaviridae) infection in birds. Trans R Soc Trop Med Hyg. (1987) 81:1004–7. doi: 10.1016/0035-9203(87)90379-8
97. Alam MM, Khurshid A, Sharif S, Shaukat S, Rana MS, Angez M, et al. Genetic analysis and epidemiology of Crimean Congo hemorrhagic fever viruses in Baluchistan province of Pakistan. BMC Infect Dis. (2013) 13:201. doi: 10.1186/1471-2334-13-201
98. Taylor RM. Purpose and Progress in cataloguing and exchanging information on arthropod-borne viruses. (The Twenty-Sixth Charles Franklin Craig Lecture.). Am J Trop Med Hyg. (1962) 11:169–74. doi: 10.4269/ajtmh.1962.11.169
99. Taylor RM. Catalogue of Arthropod-borne Viruses of the World. US Dep Heal Educ Welfare, Public Heal Serv. (1967). p. 898.
100. Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. (2015) 109:503–13. doi: 10.1093/trstmh/trv050
101. Leblebicioglu H. Crimean-Congo haemorrhagic fever in Eurasia. Int J Antimicrob Agents. (2010) 36:43–6. doi: 10.1016/j.ijantimicag.2010.06.020
102. Maltezou HC, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, et al. Crimean-congo hemorrhagic fever in Europe: current situation calls for preparedness. Eurosurveillance. (2010) 15:48–51. doi: 10.2807/ese.15.10.19504-en
103. Bizhga B, Sönmez B, Bardhaj L, Sherifi K, Gündemir O, Duro S. Hyalomma aegyptium the dominant hard tick in tortoises Tesdudo hermanni boettgeri found in different regions of Albania. Int J Parasitol Parasites Wildlife. (2022) 17:199–204. doi: 10.1016/j.ijppaw.2022.02.002
104. Converse JD, Moussa MI. Virus From Hyalomma dromedarii (Acari : Ixodoidea) collected in Kuwait, Iraq and Yemen. J Med Entomol. (1982) 19:209–10. doi: 10.1093/jmedent/19.2.209
105. Memish ZA. Infection control in Saudi Arabia: meeting the challenge. Am J Infect Control. (2002) 30:57–65. doi: 10.1067/mic.2002.120905
106. Perveen N, Muzaffar SB, Al-Deeb MA. Population dynamics of Hyalomma dromedarii on camels in the United Arab Emirates. Insects. (2020) 11:1–9. doi: 10.3390/insects11050320
107. Perveen N, Muzaffar SB, Al-deeb MA. Prevalence, Distribution, and Molecular Record of Four Hard Ticks from Livestock in the United Arab Emirates. Insects. (2021) 12:1016. doi: 10.3390/insects12111016
108. Perveen N, Muzaffar SB, Al-Deeb MA. Four Tick-borne microorganisms and their prevalence in hyalomma ticks collected from livestock in United Arab Emirates academic editors: Sergio. Pathogens. (2021) 10:1005. doi: 10.3390/pathogens10081005
109. Pegram RG, Hoogstraal H, Wassef HY. Ticks (Acari: Ixodoidea) of the Yemen Arab Republic. I Species infesting livestock Bull Entomol Res. (1982) 72:215–27. doi: 10.1017/S0007485300010518
110. Aslam S, Latif MS, Daud M, Rahman ZU, Tabassum B, Riaz MS, et al. Crimean-congo hemorrhagic fever: risk factors and control measures for the infection abatement (Review). Biomed Reports. (2016) 4:15–20. doi: 10.3892/br.2015.545
111. Morikawa S, Saijo M, Kurane I. Recent progress in molecular biology of Crimean-Congo hemorrhagic fever. Comp Immunol Microbiol Infect Dis. (2007) 30:375–89. doi: 10.1016/j.cimid.2007.07.001
112. Atkinson B, Chamberlain J, Logue CH, Cook N, Bruce C, Dowall SD, et al. Development of a Real-Time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector-Borne Zoonotic Dis. (2012) 12:786–93. doi: 10.1089/vbz.2011.0770
113. Ergonul O. Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. (2012) 2:215–20. doi: 10.1016/j.coviro.2012.03.001
114. Boushab BM, Kelly M, Kébé H, Bollahi MA, Basco LK, Leonardo K. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg Infect Dis. (2020) 26:817–8. doi: 10.3201/eid2604.191292
115. Kebede S, Duales S, Yokouide A, Alemu W. Trends of major disease outbreaks in the African region, 2003–2007. East Afr J Public Heal. (2010) 7:20–9. doi: 10.4314/eajph.v7i1.64672
116. Abdelhakam HAA, Taha MA. Crimean-Congo hemorrhagic fever (CCHF) in Southern Kordofan. Sudan J Paediatr. (2014) 14:81–4.
117. Kohl C, Eldegail M, Mahmoud I, Schrick L, Radonic A, Emmerich P, et al. Crimean congo hemorrhagic fever, 2013 and 2014 Sudan. Int J Infect Dis. (2016) 53:9. doi: 10.1016/j.ijid.2016.11.027
118. Bower H, El Karsany M, Alzain M, Gannon B, Mohamed R, Mahmoud I, et al. Detection of Crimean-Congo Haemorrhagic Fever cases in a severe undifferentiated febrile illness outbreak in the Federal Republic of Sudan: a retrospective epidemiological and diagnostic cohort study. PLoS Negl Trop Dis. (2019) 13:1–15. doi: 10.1371/journal.pntd.0007571
Keywords: CCHF, CCHFV, prevalence, distribution, epidemiology, Arab world
Citation: Perveen N and Khan G (2022) Crimean–Congo hemorrhagic fever in the Arab world: A systematic review. Front. Vet. Sci. 9:938601. doi: 10.3389/fvets.2022.938601
Received: 07 May 2022; Accepted: 25 August 2022;
Published: 13 September 2022.
Edited by:
Solomon Olawole Odemuyiwa, University of Missouri, United StatesReviewed by:
Gaurav Kumar Sharma, Indian Veterinary Research Institute (IVRI), IndiaCopyright © 2022 Perveen and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gulfaraz Khan, Z19raGFuQHVhZXUuYWMuYWU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.