
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 22 July 2022
Sec. Veterinary Pharmacology and Toxicology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.924373
This article is part of the Research Topic Phytotherapeutic Alternatives in Veterinary Medicine: Vol II View all 5 articles
This study aimed to determine the potential effects of alfalfa saponins on the production performance, serum biochemical factors, and immune factors in sheep. Twenty Small-Tailed Han sheep were equally and randomly divided into Groups 1–4, fed with diets containing 0, 5, 10, and 20 g alfalfa saponins per kg, respectively, for 40 consecutive days. During the treatments, the body weight change was recorded for each sheep. Before, during, and after the treatments of alfalfa saponins, serum was collected from each group to compare the levels of biochemical and immune factors. All sheep were killed after the treatments, and the longissimus dorsi muscle was collected to compare the meat quality. The results validated the effects of alfalfa saponins on the growth performance and meat quality in Small-Tailed Han sheep, and the supplementation level of 10 g/kg was the best. Alfalfa saponins also had effects on the levels of biochemical factors in serum. However, both dose- and time-dependent effects were observed. After a shorter feeding period (14 days), the concentrations of cholesterol (CHOL) and low-density lipoprotein (LDL) in Groups 2, 3, and 4 were all lower than those in the control group; however, when alfalfa saponins were continuously fed, this effect was not apparent or even gone. Supplying alfalfa saponins increased serum concentrations of IgA, IgG, IgE, IgM, IL-1, IFN-α, and IFN-β. And this effect was distinctly observed in Groups 3 and 4. Based on the current results, the alfalfa saponins concentration of 10 g/kg (for 14 consecutive days) could be suggested as the optimum ratio for good health conditions of Small-Tailed Han sheep.
Alfalfa (Medicago sativa) is cultivated worldwide as an essential legume for grazing, hay, silage, green manure, and cover crop (1). It contains characteristic secondary metabolites such as saponins, coumarins, isoflavones, and alkaloids. Among them, alfalfa saponins have been shown to have various biological and pharmaceutical functions. Their lipid-soluble core and steroid or triterpene structures confer the membrane-solubilizing activity of alfalfa saponins and explain their antibacterial, antitumor, and anti-inflammatory properties in animals (2–4). Alfalfa saponins can act on cholesterol and regulate lipid metabolism (5, 6). Due to their excellent antioxidant properties, alfalfa saponins have been shown to excel in promoting growth characteristics in weaned piglets (4).
These various biological and pharmaceutical functions of alfalfa saponins have been demonstrated in different animal species, including ruminants (5, 7–9), pigs (4), poultry (10, 11), and aquaculture species (12). However, there are few reports about their applications in Small-tailed Han sheep, a well-known local sheep breed in China. Farmers favor the Small-tailed Han sheep because of their rapid growth and development, high reproductive performance, and strong adaptability (13). To our knowledge, this breed has become one of the most commonly farmed sheep species in China. With the rapid development of intensive sheep farming, however, Small-Tailed Han sheep are suffering from many pathogens because of the high breeding density, which has caused substantial economic losses. It has been demonstrated that the lipid-soluble extract from alfalfa, including alfalfa saponins, could modulate the host immunity in mice infected with Citrobacter rodentium (14). Similar immunity-enhancing activity has also been found for alfalfa saponins in the crossbred lambs (15). However, it is unknown whether there is a similar immune-enhancing effect in the Small-Tailed Han sheep. Therefore, the present study aimed to investigate the potential effects of alfalfa saponins on the production performance, serum biochemical indexes, and immune function in the Small-Tailed Han sheep.
The alfalfa saponins extract (Lot No. 201405102) with a purity of 518.5 g/kg dry matter was purchased from Xi'an Realin Biotechnology Co., Ltd. (Xi'an, Shaanxi, China). This commercial product was extracted from the leaves and roots of alfalfa (Medicago sativa), purified through high-performance liquid chromatography, and quantified with oleanolic acid as the standard substance (5).
Animal experiments were conducted on the sheep farm of Xianda Animal Husbandry Development Co., Ltd. (Luoyang, Henan, China) under the protocols approved by the Institutional Animal Care and Use Committee (IACUC) of Henan University of Science and Technology. Twenty healthy male Small-Tailed Han sheep were selected, with an average body weight of 36.08 ± 2.68 kg. Five sheep were randomly kept in pen (5 m × 8 m) equipped with a bamboo slatted floor and water taps. All sheep were acclimated for at least 10 days and fed commercial feed twice daily in the morning and evening, supplemented with silage corn stover at other times and water ad libitum.
The sheep in each pen were grouped as Groups 1, 2, 3, and 4, respectively. Group 1 (Control group) received the basal diet (Table 1), and Groups 2, 3, and 4 received different doses of alfalfa saponins extract powder which was mixed into the basal diet (5, 10, 20 g/kg basal diet, respectively). All sheep were fed the corresponding diets for 40 days.
The entire feeding period lasted 40 days. Before alfalfa saponins treatments, the fasting body weight of each sheep was weighed and recorded as the initial weight. Then the body weight was recorded prior to the morning feeding on days 8, 15, 25, 35, and 41 days. The initial and final body weights were recorded. Average daily gain (ADG, kg/day) was calculated between weighing intervals.
After the 40-day feeding period, sheep in each group were slaughtered. Approximately 200 g of the longissimus dorsi muscle was collected to determine meat quality traits, including cooking loss, drip loss, and shear force. For cooking loss determination, a meat sample with 2.5 cm thickness was heated in an 80°C water bath for 30 min. The proportion of weight lost during heating is the cooking loss (%). Re-weighting was done when the meat cooled down for 20 min at room temperature. For the drip loss determination, a 2.0 cm thick meat sample was weighed and then hung in a refrigerator at 4°C. The meat sample was weighed again after 48 h. The proportion of weight loss was determined as the drip loss. The shear force was determined by a digital texture analyzer (C-LM3B; Beijing Tianxiang Feiyu Instrument & Equipment Co., Ltd.).
Approximately 10 ml of blood samples were collected from sheep before morning feeding on days 0 (before treatment), 15, 25, and 41 (before the slaughter). After the collection, blood samples were quickly stored at 4°C for 3 h, followed by centrifugation at 2,000 g for 10 min. Then the serum was collected and stored at −20°C for further determination of biochemical and immune factors. The automatic biochemical analyzer AU5400 (Beckman Coulter Commercial Enterprise, Shanghai, China) was used to determine the levels of serum biochemical factors, including alanine transaminase (ALT), aspartate aminotransferase (AST), total protein (TP), albumin (ALB), globulin (GLO), Urea-N, creatinine (CRT), glucose (GLU), calcium, phosphorus, triglyceride (TRIG), cholesterol (CHOL), high-density lipoprotein (HDL), low-density lipoprotein (LDL), creatine kinase (CK), lactate dehydrogenase (LDH), glutamyltransferase (GT), and alkaline phosphatase (ALP). Based on our previous studies (16, 17), the commercial enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) were used to determine immunoglobulins, interleukins, and interferons levels, including IgA, IgG, IgE, IgM, IL-1, IL-6, IFN-α, IFN-β, and IFN-γ.
All data were expressed as mean ± SE (standard error) or SD (standard deviation). Normality and homogeneity of variance were checked with the Kolmogorov-Smirnov test. Statistical analysis was performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A two-way analysis of variance (two-way ANOVA) was used for serum biochemical factors to evaluate the interaction between feeding periods and doses. And least significant difference (LSD) test was used to compare the differences between the four groups or periods. Differences in the other parameters were processed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test, considering P < 0.05 as the significance level.
There were differences, but not significant, in the body weights of the four groups before alfalfa saponins treatments. After different treatment periods, the body weight changes in each group are shown in Table 2. After 7 and 14 days of continuous feeding, the average body weight of Small-Tailed Han sheep in the three treatment groups was significantly higher than that of the control group (P < 0.05). However, at the last three weighing points, the average body weight in Group 3 was significantly higher than those in Groups 1 and 4 (P < 0.05). After different feeding periods, the average daily gain in each group was also compared in Table 2. During the first four feeding periods, the ADGs in the three treatment groups were significantly higher than those in the control group (P < 0.05). However, Group 3 had the best ADG considering the 40-day feeding period, which was significantly higher than Groups 1 and 4 (P < 0.05), indicating that the supplementation level of 10 g/kg was the best for growth performance.
After 40 days of treatment with alfalfa saponins, all sheep were slaughtered, and their longissimus dorsi muscles were collected for meat quality comparison. The results are shown in Table 3. The drip loss in the three treatment groups decreased compared to the control group, but without a significant difference. The lowest cooking loss (31.74%) was observed in Group 3 (P < 0.05). Regarding shear force, Group 3 also had the smallest values (2.95 ± 0.58 kgF), significantly different from Groups 1 and 4, indicating that the supplementation of alfalfa saponins at 10 g/kg might be the best one.
No interaction between feeding time and feeding dose was observed for all serum biochemical factors after the two-way ANOVA. Before treatments with alfalfa saponins, no significant differences were found in the serum biochemical factors among the four groups (Table 4). After 14, 24, and 40 days of continuous feeding, the changes among different treatment groups were observed (Table 4). In addition to the comparison of dose-dependent effect, the time-dependent effects were also compared for the 4 groups (Table 5).
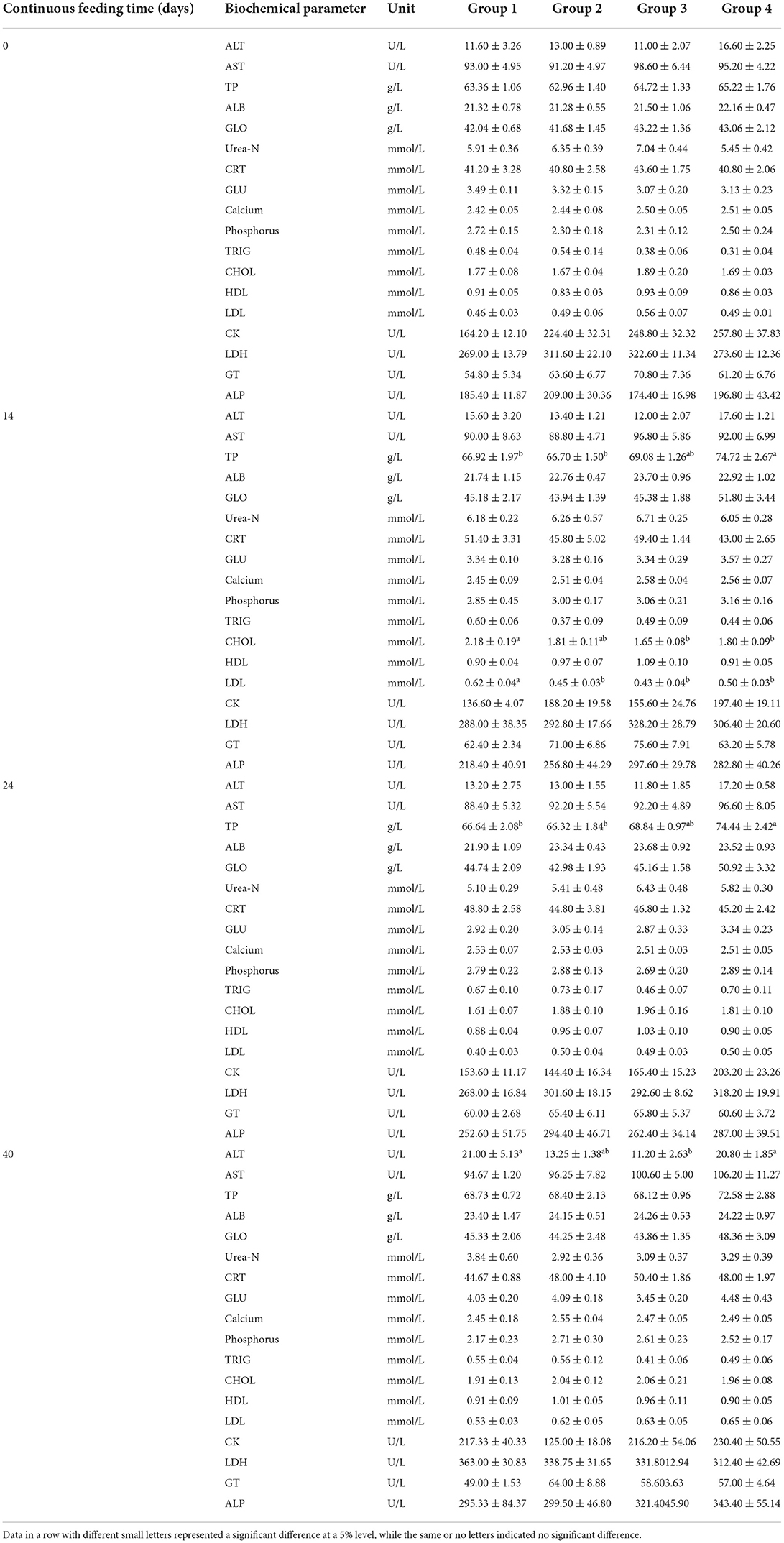
Table 4. Comparison of serum biochemical parameter levels (mean ± SE) among four treatment groups (n = 5 in each group) after 0 (before feeding), 14, 24, and 40 consecutive days of feeding.
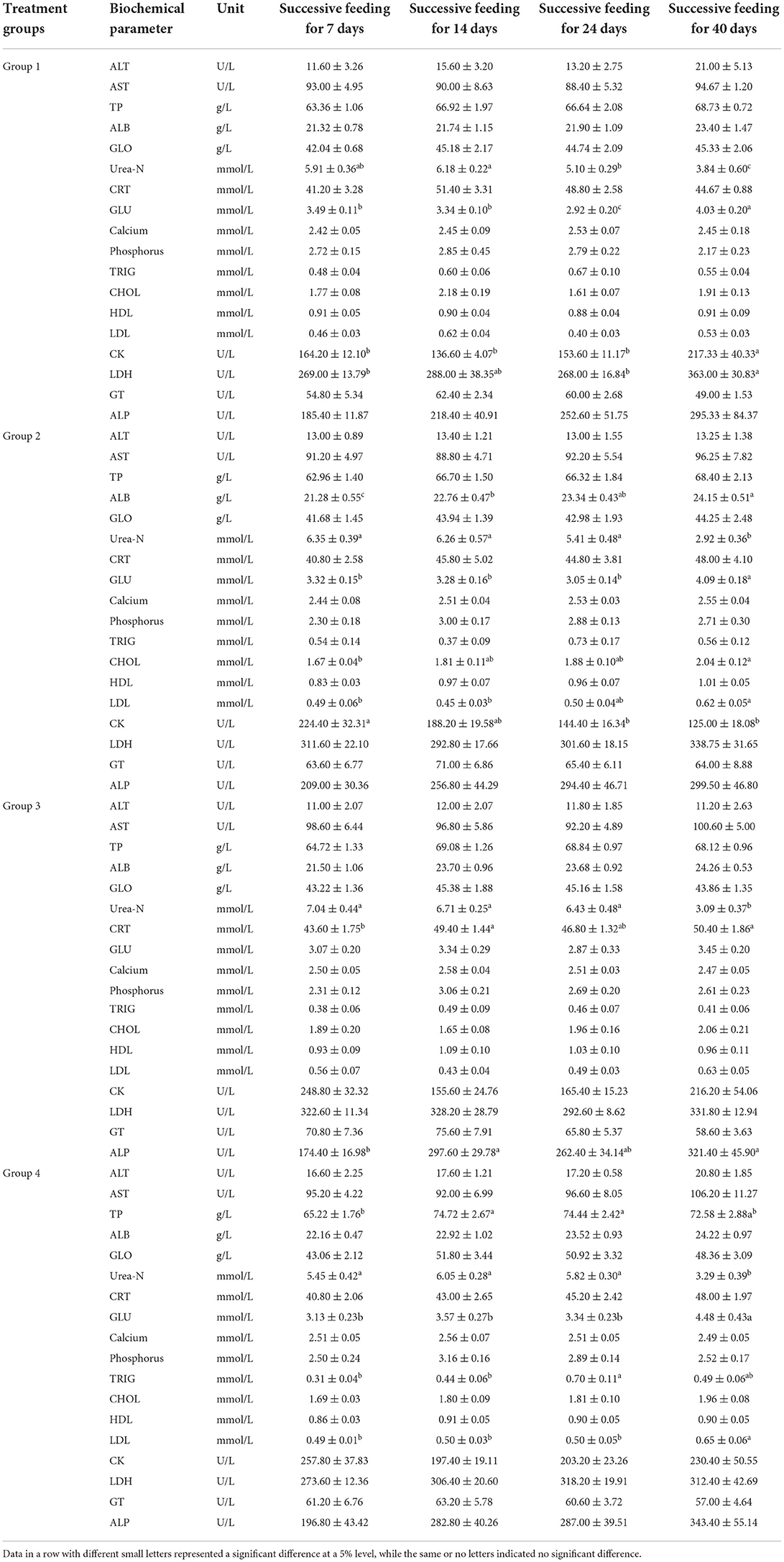
Table 5. Comparisons of serum biochemical parameter levels (mean ± SE) at different sampling time points in four groups (n = 5).
Commercial ELISA kits were used to determine the levels of immune factors in serum. The levels of immunoglobulin, interleukin, and interferon in different treatment groups are compared in Tables 6–8, respectively. Unlike serum biochemical factors, the time-dependent effect comparisons were not performed for these immune factors. Before the alfalfa saponins treatments, the levels of all tested immune factors were not significantly different among the four groups. However, after different feeding periods, the level of immunoglobulin increased to a certain extent compared with the control group (Table 6). Alfalfa saponins had no significant effect on IL-6 levels, and the impact on IL-1 was only observed in Groups 3 and 4 (Table 7). The levels of serum IFN-α and IFN-β in the alfalfa saponins-treated groups were also increased to a certain extent compared with the control group, but a similar effect on IFN-γ was not observed (Table 8).
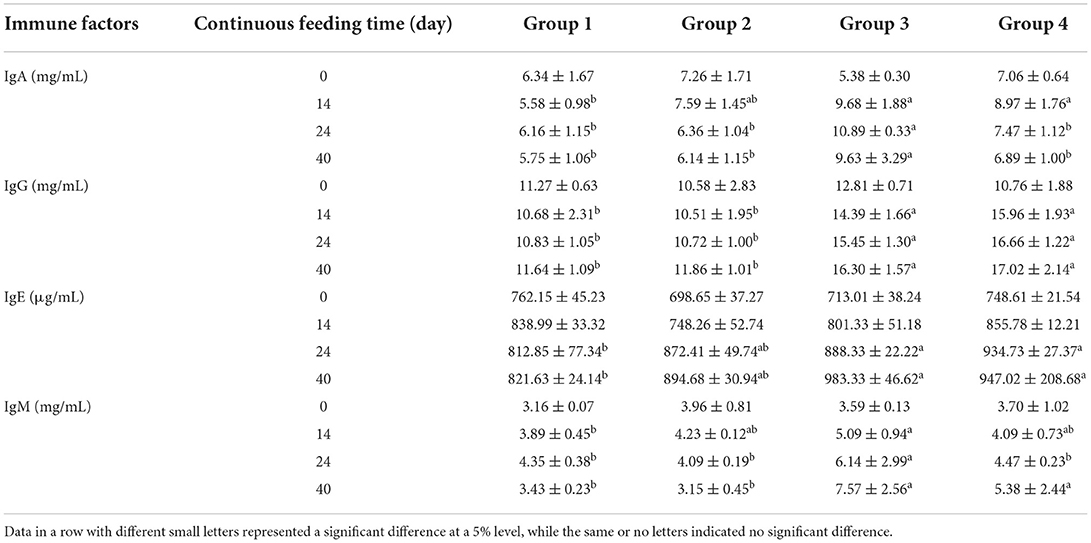
Table 6. Comparison of serum immunoglobulin levels (mean ± SD) among four treatment groups (n = 5 in each group).
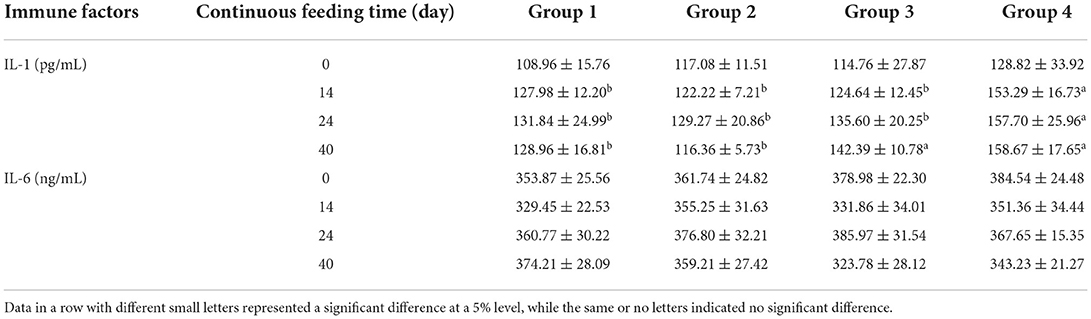
Table 7. Comparison of serum interleukins levels (mean ± SD) among four treatment groups (n = 5 in each group).
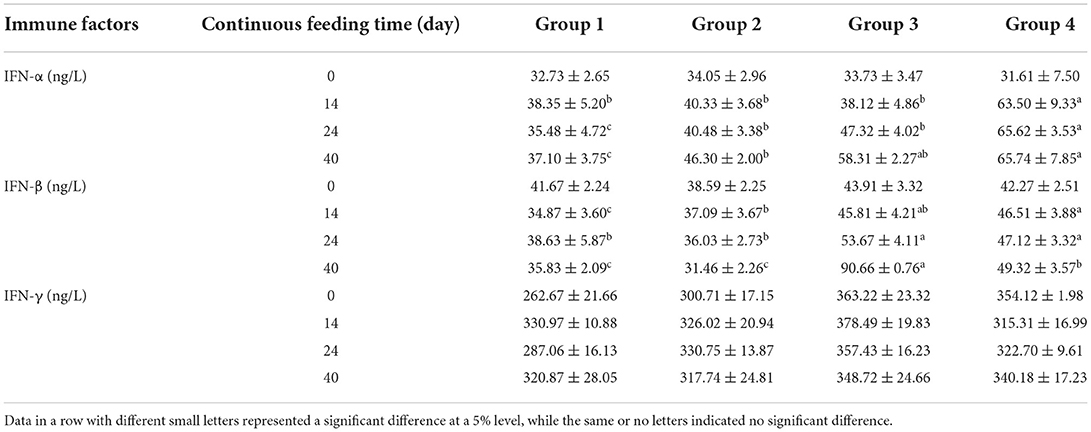
Table 8. Comparison of serum interferons levels (mean ± SD) among four treatment groups (n = 5 in each group).
The dose-dependent effects of alfalfa saponins on the growth performance of Small-Tailed Han sheep were observed in the current study. Small-Tailed Han sheep in the 10 g/kg supplemental dose group had the highest ADG (0.258 ± 0.071 kg/day), which was significantly higher (P < 0.05) in comparison to the control group and group receiving 20 g/kg saponins (0.090 ± 0.031 and 0.168 ± 0.072 kg/day, respectively). Initially, saponins were generally considered the main antinutritional factors in alfalfa because of their bloat effects in the rumen (7, 18). However, lately, several studies have shown the beneficial effects of alfalfa saponins (5, 9, 14, 19). Alfalfa saponins would reduce microbial fermentation and nutrient degradation in the rumen; however, the nutrient absorption was increased in the small intestine (18). The authors did not examine the species and abundances of microbes in the rumen in the present study; however, the sheep in the highest supplementation dose group (20 g/kg) had a lower ADG value than the 5 and 10 g/kg supplementation groups, also confirming the antinutritional properties of alfalfa saponins at relatively higher levels.
A previous study has demonstrated that alfalfa saponins could improve meat quality in Hu lambs (9), including bringing a bright red meat color, lowering intramuscular fat content, and decreasing the drip loss of muscle. Similar results were also found in the current Small-Tailed Han sheep. The drip loss, cooking loss, and shear force decreased in the alfalfa saponins treatment groups (Table 3). The existing results have confirmed that alfalfa saponins could improve the meat quality, especially by reducing the shear force and further improving the meat tenderness. In the previous study in Hu sheep (9), lower supplemental doses were used, ranging from 0.5 to 4 g/kg; however, a more extended feeding period was used, which lasted 90 days. Such an extended feeding period may be worth exploring further because the results on serum biochemical factors showed that the effect of long-term supplementation was not ideal. The sheep used in the current study were in the breeding stage, and their body weights were close to the slaughter weight, so long-term feeding experiments were not carried out. In the future, similar studies should be performed in Small-Tailed Han lambs to explore the optimal supplementation dose for lambs.
The levels of Urea-N and GLU are susceptible to many uncontrolled factors during feeding trials. The present results showed that alfalfa saponins might not affect the metabolism of protein and glucose in plasma with no differences in Urea-N and GLU levels. Cholesterol is known as a cell membrane stabilizer. Loss of cholesterol leads to membrane instability and membrane lysis. In the present study, the decrease in cholesterol after alfalfa saponins treatments was inconsistent with the previous results in Hu lambs (5), which might result from different supplementation doses, different feeding periods or different sheep breeds. In addition to the dose-dependent effects of alfalfa saponins, this study also compared the time-dependent effects of alfalfa saponins supplementation on the serum biochemical factor levels. Although the sheep in the control group had not been exposed to alfalfa saponins during the 40-day feeding period, as the sheep grew, the serum levels of Urea-N, GLU, CK, and LDH were all changed (Table 5). Excluding these changes in biochemical factors caused by the growth of sheep, the time-dependent effects were indeed observed in the treatment groups. In Group 2, with the prolongation of feeding time, the concentrations of ALB and CHOL showed a significant upward trend, while the contents of Urea-N and CK showed a significant downward trend (Table 5). In Group 3, a changing trend in Urea-N was consistent with Group 2; significant differences were also found in CRT and ALP, although no clear trends were found (Table 5). In Group 4, the concentration of TRIG first increased (reaching the peak concentration of 0.70 ± 0.11 mmol/L on Day 25) and then decreased with the prolongation of feeding time (Table 5). The influence of alfalfa meal on the serum biochemical profile in broilers has also been identified in a previous study (20). In that study, alfalfa meal was supplemented at four levels from 0 to 75 g/kg diet. And the serum levels of AST and CHOL were both decreased by the supplement of alfalfa meal. One previous study has confirmed that N intake and the type of N source can affect serum Urea-N concentrations (21). Additionally, a high correlation has been found in ruminants between serum Urea-N concentration and rumen ammonia concentration (21). However, the rumen ammonia levels were not determined in the present study. Therefore, the potential correlation could not be determined.
After continuous feeding of alfalfa saponins, almost all serum immunoglobulin levels were elevated compared with the control group. After 14 days of feeding, IgA, IgG, and IgM concentrations were significantly higher than those of the control group (Table 6). Similar results were also found for IL-1 (Table 7), IFN-α, and IFN-β (Table 8). Immunity can be divided into innate and acquired ones. The former is formed by the body innately and inherited from parents, while the acquired immunity is the immunity acquired in fighting against pathogenic microorganisms (22). Innate immunity mainly involves cytokines, such as immunoglobulin, interferon, and interleukin (14, 23). Currently, there is no research on the effect of alfalfa saponins on the immune function of ruminants. The researchers compared the effects of betaine and alfalfa saponins on IgA, IgG, and IgM in the serum of weaned piglets (24). They found that both compounds could increase the serum IgG levels, with alfalfa saponins being more effective than betaine. There are few studies on the effects of alfalfa saponins on immune function, instead of focusing on alfalfa meal or fermented alfalfa (25–28). These studies have confirmed the immune-enhancing effects of alfalfa, but whether these effects are derived from alfalfa saponins needs to be further explored.
The present study has determined the potential effects of alfalfa saponins on the growth performance, meat quality, serum biochemical, and immune factors in Small-Tailed Han sheep. The results validated the effects of alfalfa saponins on the growth performance and meat quality in Small-Tailed Han sheep, and the supplementation level of the 10 g/kg diet was the best. Alfalfa saponins also had effects on the levels of biochemical factors in serum. Additionally, the dose- and time-dependent effects were observed. After a 14-day feeding period, the concentrations of cholesterol (CHOL) and low-density lipoprotein (LDL) in the three treatment groups were lower than those in the control group. But when continued feeding, the effect became less noticeable or even disappeared. Supplying alfalfa saponins increased serum concentrations of IgA, IgG, IgE, IgM, IL-1, IFN-α, and IFN-β. Based on the current results, the alfalfa saponins concentration of 10 g/kg diet (for 14 consecutive days) could be suggested as the optimum ratio for good health conditions of Small-Tailed Han sheep.
The raw data supporting the conclusions of this article will be made available by the authors, without unduereservation.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Henan University of Science and Technology.
FanY and Y-QW conceived this project. FangY, Z-HZ, and S-QW performed animal experiments. LZ and B-LZ determined the serum immune factor levels. J-CC performed the serum biochemical factors analysis. FanY performed the statistical analysis and wrote this manuscript with support from FangY. All authors read and approved this final manuscript.
This study was financially supported by the China Agriculture Research System of MOF and MARA (Grant No. CARS-38), the Natural Science Foundation of Henan (Grant No. 212300410037), and the Foundation for University Young Key Teacher Program of Henan Province (Grant No. 2021GGJS044).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hadidi M, Ibarz A, Pagan J. Optimisation and kinetic study of the ultrasonic-assisted extraction of total saponins from alfalfa (Medicago sativa) and its bioaccessibility using the response surface methodology. Food Chem. (2020) 309:125786. doi: 10.1016/j.foodchem.2019.125786
2. Wojciechowski K, Orczyk M, Gutberlet T, Geue T. Complexation of phospholipids and cholesterol by triterpenic saponins in bulk and in monolayers. Biochim Biophys Acta. (2016) 1858:363–73. doi: 10.1016/j.bbamem.2015.12.001
3. Cui YL, Li F, Zhu XY, Xu JY, Muhammad A, Chen YY, et al. Alfalfa saponins inhibit oxidative stress-induced cell apoptosis through the MAPK signaling pathway. Redox Rep. (2021) 27:1–8. doi: 10.1080/13510002.2021.2017681
4. Shi YH, Wang J, Guo R, Wang CZ, Yan XB, Xu B, et al. Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets. Livest Sci. (2014) 167:257–62. doi: 10.1016/j.livsci.2014.05.032
5. Liu C, Qu YH, Guo PT, Xu CC, Ma Y, Luo HL. Effects of dietary supplementation with alfalfa (Medicago sativa L.) saponins on lamb growth performance, nutrient digestibility, and plasma parameters. Anim Feed Sci Tech. (2018) 236:98–106. doi: 10.1016/j.anifeedsci.2017.12.006
6. Shi Y, Guo R, Wang X, Yuan D, Zhang S, Wang J, et al. The regulation of alfalfa saponin extract on key genes involved in hepatic cholesterol metabolism in hyperlipidemic rats. PLoS ONE. (2014) 9:e88282. doi: 10.1371/journal.pone.0088282
7. Lindahl IL, Cook AC, Davis RE, Maclay WD. Preliminary investigations on the role of alfalfa saponin in ruminant bloat. Science. (1954) 119:157–8. doi: 10.1126/science.119.3083.157
8. Klita PT, Mathison GW, Fenton TW, Hardin RT. Effects of alfalfa root saponins on digestive function in sheep. J Anim Sci. (1996) 74:1144–56. doi: 10.2527/1996.7451144x
9. Liu C, Xu CC, Qu YH, Guo PT, Ma Y, Wang B, et al. Effect of alfalfa (Medicago sativa L.) saponins on meat color and myoglobin reduction status in the longissimus thoracis muscle of growing lambs. Anim Sci J. (2021) 92:e13556. doi: 10.1111/asj.13556
10. Fan WN, Zhang XL, Shi PF, Li J, Wang CZ, Li DF, et al. Effects of dietary alfalfa saponins on laying performance, egg cholesterol concentration, and ATP-binding cassette transporters G5 and G8 expression in laying hens. J Appl Anim Res. (2018) 46:1051–8. doi: 10.1080/09712119.2018.1454323
11. Yildiz AO, Senturk ET, Olgun O. Use of alfalfa meal in layer diets - a review. World Poultry Sci J. (2020) 76:134–43. doi: 10.1080/00439339.2020.1708839
12. Song HM, Wei MX, Mou XD, Liu Y, Wang XJ, Liu C, et al. Efficacy of alfalfa saponins on promoting pigmentation by astaxanthin in blood parrot fish (Vieja synspila female x Amphilophus citrinellus male). Isr J Aquacult Bamid. (2016) 68:1274. doi: 10.46989/001c.20821
13. Wang CX, Zhao YH, Yuan ZY, Wu YJ, Zhao Z, Wu CL, et al. Genome-wide identification of mRNAs, lncRNAs, and proteins, and their relationship with sheep fecundity. Front Genet. (2022) 12:750947. doi: 10.3389/fgene.2021.750947
14. Fries-Craft K, Anast JM, Schmitz-Esser S, Bobeck EA. Host immunity and the colon microbiota of mice infected with Citrobacter rodentium are beneficially modulated by lipid-soluble extract from late-cutting alfalfa in the early stages of infection. PLoS ONE. (2020) 15:e0236106. doi: 10.1371/journal.pone.0236106
15. Lisonbee LD, Villalba JJ, Provenza FD. Effects of tannin on selection by sheep of forages containing alkaloids, tannins and saponins. J Sci Food Agr. (2009) 89:2668–77. doi: 10.1002/jsfa.3772
16. Wang H, Yang F, Song ZW, Shao HT, Bai DY, Ma YB, et al. The influence of immune stress induced by Escherichia coli lipopolysaccharide on the pharmacokinetics of danofloxacin in broilers. Poult Sci. (2022) 101:101629. doi: 10.1016/j.psj.2021.101629
17. Wang H, Yang F, Song ZW, Shao HT, Zhang M, Ma YB, et al. Influence of Escherichia coli endotoxemia on danofloxacin pharmacokinetics in broilers following single oral administration. J Vet Pharmacol Ther. (2022) 45:220–5. doi: 10.1111/jvp.13035
18. Lu CD, Jorgensen NA. Alfalfa saponins affect site and extent of nutrient digestion in ruminants. J Nutr. (1987) 117:919–27. doi: 10.1093/jn/117.5.919
19. Wang B, Ma MP, Diao QY, Tu Y. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front Microbiol. (2019) 10:356. doi: 10.3389/fmicb.2019.00356
20. He G, Zhao L, Shishir MSR, Yang Y, Li Q, Cheng L, et al. Influence of alfalfa meal, as a source of dietary fibre, on growth performance, development, pH of gastrointestinal tract, blood biochemical profile, and meat quality of broilers. J Appl Anim Res. (2021) 49:431–9. doi: 10.1080/09712119.2021.2000417
21. Abdullah DN, Halim RA, Jalaludin S, Ho YW. Rumen parameters and urea kinetics in goats and sheep. Asian Austral J Anim. (2000) 13:922–8. doi: 10.5713/ajas.2000.922
22. McReynolds JL, Genovese KJ, He H, Swaggerty CL, Byrd JA, Ricke SC, et al. Alfalfa as a nutritive modulator in maintaining the innate immune response during the molting process. J Appl Poult Res. (2009) 18:410–7. doi: 10.3382/japr.2008-00044
23. Landers KL, Moore RW, Dunkley CS, Herrera P, Kim WK, Landers DA, et al. Immunological cell and serum metabolite response of 60-week-old commercial laying hens to an alfalfa meal molt diet. Bioresour Technol. (2008) 99:604–8. doi: 10.1016/j.biortech.2006.12.036
24. Wu Z-M. Effects of Betaine and Alfalfa Saponins on the Growth Performance and Immune Function of Weaned Piglets (Dissertation/Master's Thesis). Daqing Heilongjiang Bayi Agricultural University (2010).
25. Jiang JF, Song XM, Wu JL, Jiang YQ. Effects of alfalfa meal on the intestinal microbial diversity and immunity of growing ducks. J Anim Physiol Anim Nutr. (2014) 98:1039–46. doi: 10.1111/jpn.12167
26. Hall JA, Bobe G, Vorachek WR, Hugejiletu Gorman ME, Mosher WD, et al. Effects of feeding selenium-enriched alfalfa hay on immunity and health of weaned beef calves. Biol Trace Elem Res. (2013) 156:96–110. doi: 10.1007/s12011-013-9843-0
27. Wallace LG, Bobe G, Vorachek WR, Dolan BP, Estill CT, Pirelli GJ, et al. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn calves. J Anim Sci. (2017) 95:2408–20. doi: 10.2527/jas.2017.1377
Keywords: alfalfa saponins, Small-Tailed Han sheep, production performance, serum biochemical factors, immune factors
Citation: Yang F, Yang F, Zhai Z-H, Wang S-Q, Zhao L, Zhang B-L, Chen J-C and Wang Y-Q (2022) Effects of alfalfa saponins on the production performance, serum biochemical factors, and immune factors in Small-Tailed Han sheep. Front. Vet. Sci. 9:924373. doi: 10.3389/fvets.2022.924373
Received: 20 April 2022; Accepted: 30 June 2022;
Published: 22 July 2022.
Edited by:
Nora Mestorino, National University of La Plata, ArgentinaReviewed by:
Małgorzata Majewska, Polish Academy of Sciences (PAN), PolandCopyright © 2022 Yang, Yang, Zhai, Wang, Zhao, Zhang, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Qin Wang, d2FuZ3lxNjgzNkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.