
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci., 04 August 2022
Sec. Veterinary Neurology and Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.912253
 Daniel Engelsman1†
Daniel Engelsman1† Tamara Sherif2†
Tamara Sherif2† Sebastian Meller2
Sebastian Meller2 Friederike Twele2
Friederike Twele2 Itzik Klein1‡
Itzik Klein1‡ Anna Zamansky3*‡
Anna Zamansky3*‡ Holger A. Volk2,4‡
Holger A. Volk2,4‡Ataxia is an impairment of the coordination of movement or the interaction of associated muscles, accompanied by a disturbance of the gait pattern. Diagnosis of this clinical sign, and evaluation of its severity is usually done using subjective scales during neurological examination. In this exploratory study we investigated if inertial sensors in a smart phone (3 axes of accelerometer and 3 axes of gyroscope) can be used to detect ataxia. The setting involved inertial sensor data collected by smartphone placed on the dog's back while walking in a straight line. A total of 770 walking sessions were evaluated comparing the gait of 55 healthy dogs to the one of 23 dogs with ataxia. Different machine learning techniques were used with the K-nearest neighbors technique reaching 95% accuracy in discriminating between a healthy control group and ataxic dogs, indicating potential use for smartphone apps for canine ataxia diagnosis and monitoring of treatment effect.
Neurological disorders are among the most severe and difficult-to-treat pathological conditions in human and veterinary medicine. In a random sample of dogs attending 89 clinics in the United Kingdom between 2009 and 2013, every 10th patient was diagnosed with a neurological disorder (1). Often, they progress with no prospect of cure and diagnosis and treatment occurring too late to make a difference, ending only with euthanasia (2, 3). Ataxia dramatically impairs the quality of life not only of the affected animals but also of their caretakers (4). Ataxia is a greek term describing a ‘lack of order'. Canine ataxia is defined as an impairment of the coordination of movement or the interaction of associated muscles and is accompanied by a disturbance of the gait pattern (5). Since ataxia is a clinical sign and not a disease (5), a precise assessment of the neuroanatomical localization of the lesion causing the gait disorder is necessary for appropriate diagnosis and therapy (6, 7). Ataxia is a sensory phenomenon causing the aforementioned disturbance of coordination of movement, and may occur as a sensory (proprioceptive), cerebellar or vestibular ataxia (or a combination of these) (6, 8–10). Sensory ataxia causes a loss of sense of limb and body position, often seen as wide-based stance, swaying gait, increased (Upper Motor Neuron, UMN) or decreased (Lower Motor Neuron, LMN) stride length, and dragging or scuffing of the digits. It is caused by a lesion of the afferent sensory (proprioceptive) pathways in the peripheral nerves or centrally in the spinal cord, brainstem or forebrain. Cerebellar ataxia (11) is characterized by an inability to control the rate and range of movement, truncal swaying, resulting in dysmetria (often hypermetria), and intention tremor. Cerebellar ataxia occurs with lesions of the cerebellum or spinocerebellar tracts within the spinal cord. Vestibular ataxia is seen as leaning, falling or rolling to one side. Head tilt and abnormal nystagmus may be present. Vestibular ataxia results from lesions of the vestibular system peripherally (receptors in the inner ear, vestibulocochlear nerve) or centrally (brainstem, cerebellum).
These types of ataxia and their associated clinical signs are usually recognized during a neurological examination and subjectively assessed. However, manual assessment by experts has serious limitations. Regardless of the degree of the observer's experience, the human eye is not capable of processing the full complexity of all components of the movement patterns (12). In dogs, it has been also shown that obvious irregularities in gait, such as orthopedically caused lameness, cannot reliably be quantified when observed by experienced orthopedic surgeons (13, 14). Ataxia in animals can also be difficult to quantify with poor interobserver agreement, despite using raters with high clinical competence levels [see, e.g., Olsen et al. (15) for horses]. Thus, there is the urgent need to use objective gait analysis systems, especially when quantifying levels of ataxia.
An objective gait assessment would be an essential tool for clinics not only to diagnose different neurological conditions but also to monitor disease progression and therapeutic effects without human biases. There are well-established methods and models for data collection and interpretation within different pathologies in people (16), highlighting that instrumentation of gait using digital technologies provides objective and subtle information that is not possible to detect from clinical observation alone. For quadrupeds, objective assessment of ataxia gait patterns was studied in Olsen et al. (17). It was found that motion capture can objectively aid the assessment of horses with ataxia; moreover, blindfolding of horses facilitates the discrimination of ataxic patterns.
Smartphone inertial sensors are commonly used in human activity recognition tasks (18, 19), as well as smartphone location recognition (20), both essential for human gait analysis as part of pedestrian dead reckoning application (21, 22). Yahalom et al. (23) and Tchelet et al. (24) used the EncephaLogTM (Mon4t) platform for collecting internal motion data for conducting motor evaluation in the context of human neurology, which utilizes smartphones' internal motion unit (IMU) sensors. It was validated against motion capture cameras, pressure mat and wearable sensors used in motion labs in instrumented Completion-time of the Timed-Up-and-Go (TUG) test, a well-accepted clinical biomarker for rating mobility and prediction of falls risk. TUG Completion Time and nine additional biomarkers were validated against in Yahalom et al. (23) and Tchelet et al. (24) respectively, suggesting that EncephaLog (Mon4t) can provide an accurate, yet simpler, instrumented TUG platform than existing alternatives, offering a solution for clinics that cannot afford the cost or space required for a dedicated motion lab and for monitoring patients at their homes. In the iTUG test the smartphone device running EncephaLog is strapped to the subject's sternum. In the current study, adapting the data collection protocol to dogs, we placed the smartphone device on the dog's back, securing it with an adjustable harness and bracket, as shown in Figure 1.
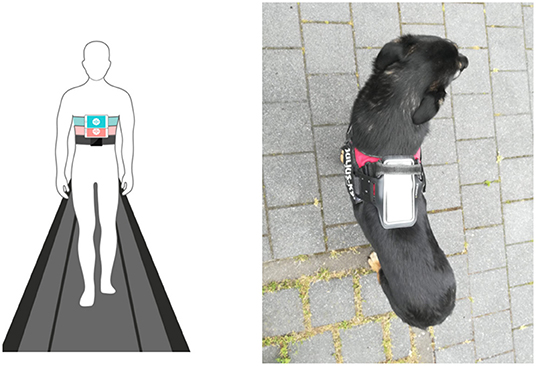
Figure 1. Left: placement of smartphone in Encephalog (Mon4t) iTUG test strapped to the subject's sternum; Right: placement of smartphone strapped to the dog's back in our study.
Gait analysis of dogs is commonly performed using pressure walkways (25), force plates (26), treadmills (27), computer-assisted motion capture (28), and kinematic gait analysis (29). These methods require expensive equipment to collect and analyze data and are time-intensive. They are therefore rarely used as a day-to-day clinical tool. Thus, body-worn devices may provide an attractive simpler and cheaper alternative. Yet, in contrast to the human domain, canine gait analysis using body-worn devices remains underexplored.
To close this scientific gap, the current study investigated whether IMU data obtained from a body-worn smartphone can be used to automatically classify between dogs diagnosed with ataxia, and a healthy control group.
Data collection was performed at the Department for Small Animal Medicine and Surgery of the University of Veterinary Medicine Hannover, Germany. The Lower Saxony State Office for Consumer Protection and Food Safety in Oldenburg, Germany has approved the study (reference number for this project: 20A555). Owners of participating dogs received a fact sheet prior to the day of data collection and were informed about the procedure in a personal conversation. All owners provided written consent for study participation, as well as signed a form about our privacy and data protection policy.
Prior to objective gait assessment patient history evaluation was performed and during general examination orthopedic diseases were excluded in order to ensure only neurologically caused gait incoordination were included. In addition, neurological examination was performed by board certified diplomates as well as residents of the European College of Veterinary Neurology (ECVN). They agreed upon a neuroanatomical localization of the lesion and differentiated the aforementioned forms of ataxia. During data collection the dogs simultaneously underwent continuous inspection by the same investigator and any gait abnormalities were recorded.
The smartphone used for gait assessment was an iPhone SE (Apple Inc.) on which a customized version of the EncephalogClinic® (Mon4t) app was installed. Modern smartphones contain an electronic device called IMU containing two types of inertial sensors—accelerometers and gyroscopes (30) the recordings of which are captured by the EncephalogClinic® (Mon4t) app upon its actication. An accelerometer measures the specific force vector and a gyroscope measures the angular rate vector. In the collected dataset, a single data point is a pair of three-dimensional accelerometer and gyroscopes readings. The duration of a single sample (several data points) is determined by a fixed window size (WS), thus, the data signal can be viewed as a multivariate time series, and we apply standard techniques of feature extraction and feature selection (31) to it, as illustrated in Figure 3.
The smartphone was fixed on the dog's body using an adjustable elastic harness (Julius-K9®, Hungary) with a bracket on top in which the smartphone was securely placed on the participant's back, as shown in Figure 2.
An individual adaptation phase to the gait analysis laboratory as well as the surrounding technical equipment and the harness with the smartphone on the dogs' dorsum was implemented in order to guarantee a smooth and relaxed, most possibly natural walking. Participating dogs were then led on a 150 cm long, loose leash to walk back and forth on a 140 x 400 cm, skid resistant felt grid mat with regularly arranged crisscross marks. Walking was performed in a slow pace in a straight line in the center of the grid mat. While orthopedic disease usually causes more severely affected gait abnormalities when the pace is increased, the opposite is true for ataxia. The integration of movement is best observed in slower pace. Observations at a slow walking pace for gait evaluation in neurological disorders ensure that the clinician does not miss neurological gait deficits. Walking pace was selected by the dog and only artificially corrected by the owner if the dog started trotting.
This protocol was repeated for at least five times, in order to reach at least a minimum of 50 strides, which is based on the experience from our former studies into ataxia evaluation (32, 33).
To be able to retrospectively reconstruct each gait assessment session the procedure was filmed from five perspectives (each side and bird's eye view).
Walking sessions were performed during routine neurological examination and could be easily implemented to clinical engagement. Owners of participating dogs were informed prior to the procedure and provided written consent.
This section presents the functional flow of a sample signal starting from dataset, through preprocessing, ending with model evaluation, as demonstrated in Figure 3.
Data was collected from 507 walking sessions of 55 healthy control group dogs (39 females and 16 males) and samples from 263 walking sessions of 23 dogs with ataxia (9 females and 14 males). Median age of dogs was 3 years (range 1–12 years) and 6 years (range 1–13 years) for the control group and the ataxic group, respectively. Median weight of dogs was 15.5 kg (range 7–32 kg) and 20 kg (range 4.4–41.4 kg) for the control group and the ataxic group, respectively. Ataxia was caused by spinal cord lesions in 11 dogs, by cerebellar disorders in 4 dogs, and 8 dogs had ataxia secondary to phenobarbitone treatment. Ataxia of one dog was of uncertain origin. The patient data is included in the Supplementary Material.
The recordings were made by the Mon4t app at a sampling rate of 100Hz; the Mon4t company made them available for us for downloading. Preprocessing, data analysis and modeling were performed using Python. Supplementary Figure 1 presents two different samples of healthy (blue) and ataxic (red) dogs, measured by the accelerometer (left) and the gyroscopes (right).
Since the samples had stagnant “tails” at the beginning and end, where the dog was recorded prior to walking start (when the tester pushed the start button on the app), the following cleaning procedure was conducted to guarantee that a given time window captures only gait: (i) Minimum acceleration threshold, to remove stationary segments. (ii) K-means clustering, to remove further outliers.
Next, an heuristic search was performed over the original dataset, to examine the influence of a given sample length, namely window size (WS), over the accuracy of a trained model. Using a logistic regression (LR) model, Supplementary Table 1 shows how an optimal window size could be determined, since its duration affects the number of available samples for each class. The tradeoff is evident: the longer the WS, the slower the periodicities that can be captured; in contrast, shorter WS yields more samples from each raw measurement, thus improving the model generalizability. As can be seen below, different WS elicits different datasets, characterized by a different number of class samples and a different Healthy/Ataxic ratio. Next, each dataset is evaluated by the LR model, to allow an empirical benchmarking, following the traditional 80:20 train-test split ratio. As marked in the table, the optimal window size was chosen to be three seconds, with a total of 1210 samples.
To further evaluate machine learning approaches, 15 different statistical and frequency features, presented in Supplementary Table 2, were extracted for each of the six time axes making a total of 90 features.
Using random forest classifier as a meta-estimator (34), standard feature selection (FS) methods were applied (35), using a scoring function that measures their “importance” in terms of target predictability [here, Gini impurity; (36)]. After sorting in descending order, redundant variables are identified by their weak contribution to the model accuracy (left y-axis), as shown in Supplementary Figure 5.
After setting a threshold value of 0.025 relative magnitude (right y-axis), the model achieved 95% accuracy, with only twelve most correlated features, thus reducing the curse of dimensionality (37). This threshold value was chosen heuristically using the 'knee of a curve' method, where the optimum point is defined as the accuracy level from which changes in slope (accuracy) are significantly reduced, such that adding further features becomes redundant.
We experimented with several models: Extra trees classifier (ET), AdaBoost (AB), Gaussian Naive Bayes (GNB), gradient boosting (GB), logistic regression (LR), K-nearest neighbors (KNN), support vector machine (SVM), random forest (RF) and decision trees (DT). Table 1 presents a comparison in terms of the evaluation metrics of these models. The best performance of over 95% accuracy was achieved by the KNN method, a non-parametric model which performs classification based on semantic similarity between neighboring data points.
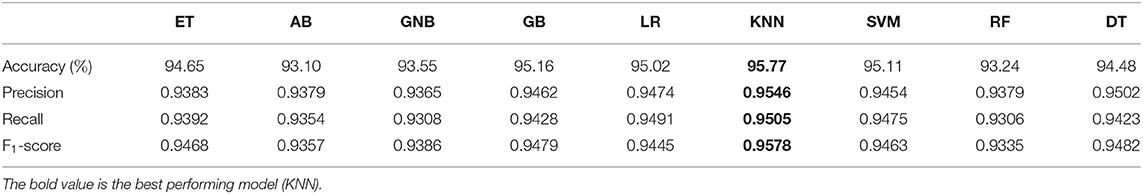
Table 1. Model performance comparison between ET, Extra trees classifier; AB, AdaBoost; GNB, Gaussian Naive Bayes; GB, gradient boosting; LR, logistic regression; KNN, K-nearest neighbors; SVM, support vector machine RF, random forest; DT, decision trees (DT).
Several studies applied smartphone IMUs for gait analysis in the human domain (38–40), and specifically to quantify ataxia (41). However, to the best of our knowledge, our study is the first to show the use of smartphone IMUs to quantify neurological canine gait.
This pilot study showed that inertial data obtained from smartphone IMUs can be used with classical machine learning algorithms to detect ataxia with 95% accuracy. While this accuracy of detection of ataxic gait in dogs is high, further data collection is important to further strengthen the validity of our approach in a clinical setting. These preliminary results highlight the future clinical potential of body-worn smartphone sensors. Only few manipulations of the animal are necessary for analysis, resulting in reduced disturbance of the animals' behavior. In addition, the analysis can be conducted flexibly without increased technical effort on site. Further studies will be needed to investigate potential clinical use of the devices in ataxic dogs.
We have shown here the feasibility of using data obtained by a standard smartphone fixed on the dog's back—a setting that can be easily replicated in any consultation room or home, enabling data acquisition anytime, anywhere. Although large and medium-sized dogs tolerate the placement of smartphone on their backs, smaller dogs might require other solutions. In our study, only 9 dogs with a bodyweight of less than 10 kilogram were included. Safe and stable positioning of the smartphone on top of the dogs' back as well as avoiding artificial alterations of the walking pattern was more challenging in small breeds. For small dogs smartphones might not be the solution, but IMU sensors can be potentially fixed on a collar, or other places on the dog, although it may affect accuracy of detection and requires further research to establish optimal locations.
Using a regular smartphone, with an appropriate recoding application, puts pet owners in a position to contribute in a participatory way to the large-scale collection of data to promote their pet's health. This opens the door to unprecedented opportunities for collecting datasets for gaining understanding into canine neurological disorders in order to enhance detection of pathological alterations. It can also lead to the development of smartphone apps for diagnosing ataxia digitally and remotely. The aspect of domestic data acquisition has another great advantage. Stress in cats and dogs in a clinical setting represents a burden to the animals and the owners, often masking disease and complicating examination and therapy, leading to even more stress (42). Domestic data sampling minimizes stress factors and allows for longitudinal and temporally high-resolution assessment of conditions and its treatments, which add valuable aspects to the on-site visits to the veterinarian.
Here, we have provided a proof of concept study, indicating that the accelerometers and gyroscopes readings obtained from smartphone on a dog's back contain a strong signal related to the characteristics of ataxic gait. One limitation of our study is that we had a limited data set. More data are needed with a higher number of ataxic dogs. It should be noted, that although the KNN algorithm produces good accuracy in our, the classifier is expected to perform slower and costlier in terms of time and memory on larger datasets. However, other simple and more robust algorithms such as SVM performed well on our data, and thus can be also potentially used in future studies. In any case, this provides a starting point for further exploration of larger datasets, which will hopefully lead to digital biomarkers for ataxic gait. Such biomarkers, which are objective, quantifiable data collected by means of digital devices, such as wearables, are increasingly used in human medicine and are typically used to explain, assess and/or predict health-related outcomes (43).
Another limitation is that the low-level statistical features that we used in this study answering if ataxia was present or not, do not provide yet adequate guidance for clinical decision making, nor for monitoring of treatment. Thus, another immediate direction for further studies is exploring the explainability of the resulting models, looking also at individual differences on ataxic patients, and correlating IMU data characteristic with clinical data of the patients.
As initially described, neurological issues can persist for a long time and healing processes can be subtle and long lasting. Extending the presented method from binary classification (ataxic/healthy) to assessing the severity of the ataxia condition will provide a strong basis for a continuous and objective monitoring of the respective condition, probably revealing subtle improvements or deteriorations of ataxic gait. In addition, longitudinal studies about drug impact on ataxic gait patterns could be performed easily.
Finally, in this study we only considered classical machine learning techniques. Obtaining more data samples will allow us to further experiment with deep learning approaches that capture temporal dimensions, which may provide additional insights into the dynamics of ataxic walking patterns.
Using a simple testing protocol of walking the dogs in a straight line on a leash, we collected IMU data samples from 23 dogs with ataxia, and sampled 55 healthy control group dogs. We experimented with different machine learning classification techniques on the IMU data (3 axes of accelerometer and 3 axes of gyroscope), reaching above 95% accuracy. This indicates potential use for smartphone apps for canine ataxia diagnosis and treatment monitoring in a clinical as well as domestic setting. Future research is needed to optimize and further develop this new diagnostic tool.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by University of Veterinary Medicine Hannover. Written informed consent was obtained from the owners for the participation of their animals in this study.
TS, SM, FT, and HV performed data acquisition. TS, SM, FT, HV, and AZ conceived the experiment(s). DE, IK, and AZ ran the experiment and analyzed the data. All authors participated in manuscript writing.
The research was partially funded by Israel Ministry of Science (MOST) according to the research project No. 19-57-06007 and by the Israeli Ministry of Agriculture.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Nareed Farhat for her help with data analysis. This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) - 491094227 Open Access Publication Funding and the University of Veterinary Medicine Hannover, Foundation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.912253/full#supplementary-material
1. Vetcompass Resources Royal Veterinary College UK. Available online at: https://www.rvc.ac.uk/vetcompass/audio-visual-resources/interactive-resources/canine.
2. Coates JR, Wininger FA. Canine degenerative myelopathy. Vet Clin. (2010) 40:929–50. doi: 10.1016/j.cvsm.2010.05.001
3. Monteiro R, Adams V, Keys D, Platt S. Canine idiopathic epilepsy: prevalence, risk factors and outcome associated with cluster seizures and status epilepticus. J Small Anim Pract. (2012) 53:526–30. doi: 10.1111/j.1748-5827.2012.01251.x
4. Wessmann A, Volk H, Packer R, Ortega M, Anderson T. Quality-of-life aspects in idiopathic epilepsy in dogs. Vet Rec. (2016) 179:229. doi: 10.1136/vr.103355
7. Platt SR, Olby NJ. BSAVA Manual of Canine and Feline neurology, 4 Edn. Quedgeley. British Small Animal Veterinary Association. (2014).
9. Cochraine S. Practical approach to the abnormal gait: neurologic or orthopedic? In: OVMA Conference. (2005). p. 158–62.
10. Krasovsky T, Levin M. Toward a better understanding of coordination in healthy and poststroke gait. Neurorehabil Neural Repair. (2010) 24:213–24. doi: 10.1177/1545968309348509
11. Stolze H, Klebe S, Petersen G, Raethjen J, Wenzelburger R, Witt K, et al. Typical features of cerebellar ataxic gait. J Neurol Neurosurg Psychiatry. (2002) 73:310–2. doi: 10.1136/jnnp.73.3.310
12. Attinger D. Subjektive gangbeobachtung im vergleich zu gemessener asymmetrie. Swiss Med. (1987) 9:58–61.
13. Quinn M, Keuler N, Lu Y, Faria M, Muir P, Markel M. Evaluation of agreement between numerical rating scales, visual analogue scoring scales, and force plate gait analysis in dogs. Vet Surg. (2007) 36:360–7. doi: 10.1111/j.1532-950X.2007.00276.x
14. Waxman A, Robinson D, Evans R, Hulse D, Innes J, Conzemius M. Relationship between objective and subjective assessment of limb function in normal dogs with an experimentally induced lameness. Vet Surg. (2008) 37:241–6. doi: 10.1111/j.1532-950X.2008.00372.x
15. Olsen E, Dunkel B, Barker W, Finding E, Perkins J, Witte T, et al. Rater agreement on gait assessment during neurologic examination of horses. J Vet Internal Med. (2014) 28:630–8. doi: 10.1111/jvim.12320
16. Celik Y, Stuart S, Woo WL, Godfrey A. Gait analysis in neurological populations: progression in the use of wearables. Med Eng Phys. (2021) 87:9–29. doi: 10.1016/j.medengphy.2020.11.005
17. Olsen E, Fouchè N, Jordan H, Pfau T, Piercy R. Kinematic discrimination of ataxia in horses is facilitated by blindfolding. Equine Vet J. (2018) 50:166–71. doi: 10.1111/evj.12737
18. Elhoushi M, Georgy J, Noureldin A, Korenberg MJ. A survey on approaches of motion mode recognition using sensors. IEEE Trans Intell Transport Syst. (2016) 18:1662–86. doi: 10.1109/TITS.2016.2617200
19. Sousa Lima W, Souto E, El-Khatib K, Jalali R, Gama J. Human activity recognition using inertial sensors in a smartphone: an overview. Sensors. (2019) 19:3213. doi: 10.3390/s19143213
20. Klein I. Smartphone location recognition: a deep learning-based approach. Sensors. (2019) 20:214. doi: 10.3390/s20010214
21. Qian J, Ma J, Ying R, Liu P, Pei L. An improved indoor localization method using smartphone inertial sensors. In: International Conference on Indoor Positioning and Indoor Navigation. Montbeliard: IEEE (2013). p. 1–7.
22. Klein I, Solaz Y, Ohayon G. Pedestrian dead reckoning with smartphone mode recognition. IEEE Sens J. (2018) 18:7577–84. doi: 10.1109/JSEN.2018.2861395
23. Yahalom G, Yekutieli Z, Israeli-Korn S, Elincx-Benizri S, Livneh V, Fay-Karmon T, et al. AppTUG-A smartphone application of instrumented ‘timed up and go'for neurological disorders. EC Neurol. (2018) 10:689–95.
24. Tchelet K, Stark-Inbar A, Yekutieli Z. Pilot study of the EncephaLog smartphone application for gait analysis. Sensors. (2019) 19:5179. doi: 10.3390/s19235179
25. Gordon-Evans WJ, Evans RB, Conzemius MG. Accuracy of spatiotemporal variables in gait analysis of neurologic dogs. J Neurotrauma. (2009) 26:1055–60. doi: 10.1089/neu.2008.0805
26. Brown D, Boston R, Farrar J. Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. J Vet Internal Med. (2013) 27:22–30. doi: 10.1111/jvim.12004
27. Jeffery N, Hamilton L, Granger N. Designing clinical trials in canine spinal cord injury as a model to translate successful laboratory interventions into clinical practice. Vet Rec. (2011) 168:102–7. doi: 10.1136/vr.d475
29. Drüen S, Böddeker J, Meyer-Lindenberg A, Fehr M, Nolte I, Wefstaedt P. Computer-based gait analysis of dogs: evaluation of kinetic and kinematic parameters after cemented and cementless total hip replacement. Vet Compar Orthopaedics Traumatol. (2012) 25:375–84. doi: 10.3415/VCOT-10-02-0026
30. Groves PD. Principles of GNSS, inertial, and multisensor integrated navigation systems, [Book review]. IEEE Aerospace Electr Syst Mag. (2015) 30:26–7. doi: 10.1109/MAES.2014.14110
31. Sonni A, Kurdziel LB, Baran B, Spencer RM. The effects of sleep dysfunction on cognition, affect, and quality of life in individuals with cerebellar ataxia. J Clin Sleep Med. (2014) 10:535–43. doi: 10.5664/jcsm.3706
32. Olsen E, Suiter E, Pfau T, McGonnell M, Matiasek K, Giejda A, et al. Cavalier king charles spaniels with chiari-like malformation and syringomyelia have increased variability of spatio-temporal gait characteristics. BMC Vet Res. (2017) 13:1–7. doi: 10.1186/s12917-017-1077-5
33. Suiter E, Packer R, Volk H. Comparing the effects of first-line antiepileptic drugs on the gait of dogs with idiopathic epilepsy. Vet Rec. (2016) 178:652–2. doi: 10.1136/vr.103736
34. Saeys Y, Abeel T, Peer YVd. Robust feature selection using ensemble feature selection techniques. In: Joint European Conference on Machine Learning and Knowledge Discovery in Databases. Antwerp: Springer (2008). p. 313–25.
35. Chandrashekar G, Sahin F. A survey on feature selection methods. Comput Electr Eng. (2014) 40:16–28. doi: 10.1016/j.compeleceng.2013.11.024
36. Menze BH, Kelm BM, Masuch R, Himmelreich U, Bachert P, Petrich W, et al. A comparison of random forest and its Gini importance with standard chemometric methods for the feature selection and classification of spectral data. BMC Bioinform. (2009) 10:1–16. doi: 10.1186/1471-2105-10-213
37. Kramer MA. Nonlinear principal component analysis using autoassociative neural networks. AIChE J. (1991) 37:233–43. doi: 10.1002/aic.690370209
38. LeMoyne R, Mastroianni T, Cozza M, Coroian C, Grundfest W. Implementation of an iPhone as a wireless accelerometer for quantifying gait characteristics. Annu Int Conf IEEE Eng Med Biol Soc. (2010) 2010:3847–51. doi: 10.1109/IEMBS.2010.5627699
39. LeMoyne R, Mastroianni T, Cozza M, Coroian C. iPhone wireless accelerometer application for acquiring quantified gait attributes. BioMed. (2010) 32067:19–20. doi: 10.1115/BioMed2010-32067
40. LeMoyne R, Mastroianni T, Cozza M, Coroian C. Quantification of gait characteristics through a functional iPhone wireless accelerometer application mounted to the spine. BioMed. (2010) 49453:87–8. doi: 10.1115/BioMed2010-32043
41. LeMoyne R, Heerinckx F, Aranca T, De Jager R, Zesiewicz T, Saal H. Wearable body and wireless inertial sensors for machine learning classification of gait for people with Friedreich's ataxia. In: 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN). San Francisco, CA: IEEE (2016). p. 147–51.
42. Lloyd JK. Minimising stress for patients in the veterinary hospital: why it is important and what can be done about it. Vet Sci. (2017) 4:22. doi: 10.3390/vetsci4020022
Keywords: ataxia, inertial measurement unit (IMU), smartphone and IoT services, neurology, canis, gait analysis, wearable and mobile computing
Citation: Engelsman D, Sherif T, Meller S, Twele F, Klein I, Zamansky A and Volk HA (2022) Measurement of Canine Ataxic Gait Patterns Using Body-Worn Smartphone Sensor Data. Front. Vet. Sci. 9:912253. doi: 10.3389/fvets.2022.912253
Received: 04 April 2022; Accepted: 09 June 2022;
Published: 04 August 2022.
Edited by:
George M. Strain, Louisiana State University, United StatesReviewed by:
Rebecca Parkes, City University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2022 Engelsman, Sherif, Meller, Twele, Klein, Zamansky and Volk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Zamansky, YW5uYXphbUBpcy5oYWlmYS5hYy5pbA==
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.