- 1Department of Husbandry and Development of Animal Wealth, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 2Department of Animal Production, Faculty of Agriculture, Zagazig University, Zagazig, Egypt
- 3Food Hygiene and Control Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
- 4Department of Animal Production, Faculty of Agriculture, Al-Azhar University, Cairo, Egypt
- 5Department of Biology, Turabah University College, Taif University, Taif, Saudi Arabia
- 6Biotechnology Department, College of Science, Taif University, Taif, Saudi Arabia
- 7Clinical Laboratory Sciences Department, Turabah University College, Taif University, Taif, Saudi Arabia
- 8Physiology Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt
- 9Physiology Department, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt
The current research sought to assess the effects of paulownia leaves extract (PLE) on performance, blood hematological, antioxidant activity, and immunological response of broiler chicken. In total, two hundred 1-day-old male Cobb500 chicks were allocated randomly into four equal treatments with 5 replicates. The first treatment served as a control (CNT) and was fed the basal diet only, while the other treated treatments were fed on the basal diet supplemented with 0.1, 0.3, and 0.5 g/kg diet of PLE, respectively. The performance results showed significant increments (P < 0.05) in live body weight (LBW), weight gain (WG), and European production efficiency factors (EPEIs) (linearly; p < 0.001) in cooperated with increasing PLE levels in broiler diets. At the same time, feed conversion ratio (FCR) and livability percentages were numerically enhanced under the effects of PLE supplementation. Moreover, a notable increase (P < 0.05 or 0.01) in oxidative remarks activity (GSH, glutathione; SOD, super oxide-dismutase and CAT, catalase) and elevated levels of immunoglobulin (IgM, immunoglobulin M and IgG, immunoglobulin G) were noted (P < 0.05) for treatments fed with PLE in a dose-dependent manner. Also, a dramatic linear increase was observed in mRNA expression of IGF-1, GHR, IL-1β, and IL-10 genes of broiler chickens. This study concluded that enriched broiler feeds with 0.5 g/kg PLE might be a beneficial strategy to promote broiler health and production.
Introduction
For many decades, the poultry production industry has been under growing pressure to enhance productivity to offer high-quality meat products to feed the world's rising population while dealing with limited natural resources. Disease outbreaks may affect poultry growth and feed efficiency directly by reducing the digestive tract's capability of absorbing all nutrients and indirectly by limiting microbiota diversification and development (1). Therefore, employing antibiotic products as a feed treatment in poultry diets to alleviate infectious disorders or as growth promoters has resulted in the development of a large number of antibiotic-resistant bacterial strains that may threaten consumer health (2). Thus, because of consumer health concerns, certain antimicrobial growth promoters (AGPs) have been totally restricted internationally, particularly, in the European Union, since 2006 (3). Eventually, the ban of AGP in poultry production intensified the search for alternatives to improve the health and productive efficiency of broiler chickens (2, 4). Recently, some feed additives such as prebiotics, probiotics, essential oils, enzymes, and organic acids have been applied to replace AGP in poultry diets (5). The natural feed additives could enhance appetite and consumed feed, elevate higher nutrient digestibility, increase digestive enzyme activity and secretion, maintain the intestinal microvilli, antimicrobial activity, antifungal activity, antiviral activity, antioxidant effects, and immune stimulant action (6, 7).
Specifically, supplemented poultry feed with several phytogenic molecules extracted from plants improved performance and boosted general health status (1), for instance, Prunus armeniaca (8) and Hippophae rhamnoides (9). In addition, enriched animal and poultry diets with phytogenics promoted performance, feed efficiency, appetite, carcass meat quality, and health status (10, 11).
Paulownia (P. tomentosa) is one of the most exploited medicinal plants that grow natively in China, Japan and the Far East Asian countries (12, 13). Besides, it has been used in traditional Chinese medicine to treat or prevent a wide range of infectious diseases (14). He et al. (15) indicated that each of paulownia parts (seeds, roots, wood, fruits, flowers, bark, and leaves) had been exhibited to have one or more bioactive molecules, for instance, matteucinol and ursolic acid (leaves), dsesamin and paulownia (wood), catalpinoside, and syringin (bark). Furthermore, the high-flavonoid concentration of paulownia elongate leaf extracts, both dried and fresh, suggests that this plant has the potential for novel therapeutic applications against a broad range of oxidative disorders (12). Therefore, paulownia leaves, fruits, and flowers (a wood by-product) are the most important plant parts employed in the folk herbal medicine (16, 17). According to a Chinese medical book edited by Li Shizhen in 1578, paulownia bark might be applied to treat hemorrhoids and worms infections, while the flowers could be used to reduce swelling and promote hair development (16).
Also, paulownia leaves might be employed as an alternate feed component for a variety of animals due to their diverse biochemical features (12). The main bioactive compounds found in paulownia (such as phenolic compounds, glycosides, flavonoids, lignans, saponins, syringin, and triterpenoids) exhibited several health benefits to consumers and animals (16, 18). In addition, paulownia and its extract showed several therapeutic properties as antibacterial, anti-inflammatory, thirst-quenching, diuretic, antihypertensive, hemostatic, and insecticidal agents (13, 19, 20). Furthermore, it could be considered a growth promoter and immunostimulant agent at various concentrations in animal diets (12, 21). Also, several recent investigations have shown the antibacterial and antioxidant characteristics of PLE-derived compounds loaded with chitosan or calcium alginate, and also their specific applicability in meat preservation (22, 23).
Paulownia leaves as a potential feed ingredient for domestic animals is a relatively new approach and research field (4). In the same context, a wood by-product of the paulownia tree has been utilized as an alternative feed ingredient for different animals due to its good nutritional value present in leaves and other parts (11). In addition, their varied biochemical properties are recognized for medical applications (24, 25). Still, too little research has focused on the bioactive chemicals derived from paulownia leaves and their positive impact on animal health. We hypothesized that owing to their effective antioxidant activity, PLE can effectively reduce the oxidative stress, boost immunity, and promote growth of broiler chicks. Thus, it is essential to determine bioactive components in PLE and evaluate the feasibility of supplemented broiler chicken diets with PLE as a natural growth promoter. Therefore, this study was designed to identify common bioactive components extracted from paulownia leaves and analyze their biological effects as feed additives on growth performance, blood hematology, antioxidant activity and immune response of broiler chickens.
Materials and Methods
Animal Ethical Approval Statement
All in-vivo trials were followed the practical guidelines of the Local Experimental Animal Care Committee and approved by the Ethics Committee of Animal Use in Research Committee of Mansoura University (Code No: R/87).
Prepartion and Characterization of Paulownia Leaf Extracts (PLEs)
Plant Source and Collection
The leaf parts of paulownia (Paulownia tomentosa) was picked and gathered directly from trees (6 months old) from a wood tree farm located in Bani–Salama village, Wadi El-Natroun district, Beheira Governorate, Egypt. Fresh paulownia leaves were transported to laboratory for further processing. The collected leaves were washed, cleaned, air-dried, ground, then kept in an airtight container to avoid the effect of humidity and then stored at room temperature until further extraction and isolation.
Samples Preparation and Extraction
The dried leaves were made as a fine powder for extraction uses. Before extraction, about 20 g of the paulownia leaf powder was defatted by soaking in 120 ml methanol for 24 h in a rotatory shaker. The defatted dry plant material was then extracted in an ultrasonic bath (Sonicator-10 L, India MART, Inter MESH Ltd.) for 20 min with 300 ml of aqueous ethanol (50%, v/v). After extraction, the solution was filtered and concentrated through Whatman No. 1 filter paper to separate the extract of plant leaves. The extract was stored in the refrigerator for further phytochemical analysis followed Nety et al. (26) procedure.
Gas Chromatography–Mass Spectroscopy Analysis
In total, 1 mg of the dried leaf extract residue was dissolved in 1 ml of methanol and the extract was analyzed with Gas Chromatography–Mass Spectroscopy analysis (GC–MS). About 1 μl of the methanol extract was injected into the GC–MS using a micro-syringe. GC–MS analysis was carried out on the GC–MS-5975C Agilent system comprising an autosampler and gas chromatograph interfaced to a mass spectrometer utilizing the following condition. The sample was inoculated into the injected port of the gas chromatography (GC) device. The GC apparatus vaporizes the injected sample and after that, isolates and investigates the various components. Each component produces a certain spectral peak which will be recorded on a paper chart electronically. The time elapsed between elution and injection is called the “retention time.”
Before testing the extract using gas chromatography and mass spectroscopy, the oven temperature, the flow rate of the gas used and the electron gun were programmed initially. The identity of the components in the extracts was accomplished by the comparison of their retention times (RTs) and mass spectra fragmentation patterns with the help of a commercial standard mass spectral database. Only the components with a similarity index of 90% and above according to databases were considered (27, 28). The total phenolic content and total anthocyanin content were determined, followed Chattuwatthana and Okello (29) and Zakaria et al. (30) procedures, respectively.
Experimental Design, Management, and Feeding Regime
The present study was conducted at the Poultry Research Farm belonging to the Faculty of Veterinary Medicine, Mansoura University, Egypt. A total number of 200 1-day-old male broiler chicks Cobb500 were purchased from a local commercial hatchery. At an average 53.8 ± 0.54 g initial weight, 1-day-old chicks were randomly distributed into four equal experimental treatments (n = 50) with five replicates (10 × 5). The first treatment was fed the basal diet only without any PLE supplementation (served as a control), while the second, third, and fourth treatments were fed on a basal diet supplemented with 0.1, 0.3, and 0.5 mg g/kg diet of PLE, respectively. The concentrations examined were selected based on the results of Yang et al. (17).
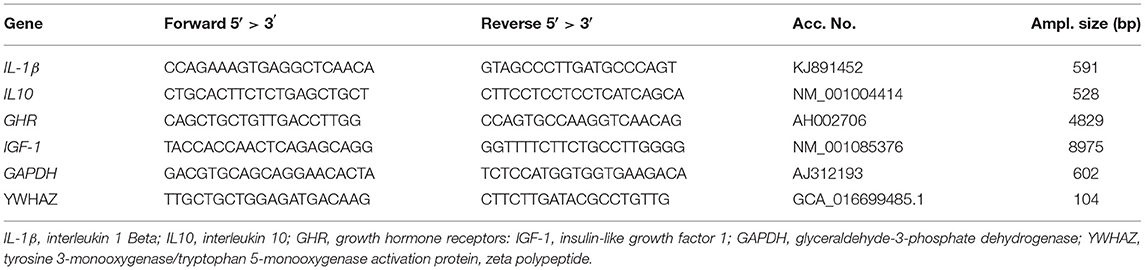
All the birds were housed and reared under identical environmental, managerial, and hygienic conditions. A routine vaccination schedule against the most common viral diseases in Egypt was administered and necessary medication when needed based on diagnoses and symptoms shown by the birds (Table 1). The birds had free access to feed and water for ad-libtum consumption during the experimental period. The ambient temperature was gradually decreased from 32°C at placement to 21°C at 42 d and the light schedule decreased from continuous light (24 h) for the first 3 d to 16L:8D thereafter. For the first 3 weeks, chicks were fed on a starter ration (2,900 Kcal. ME/kg, 23% C.P.) and followed by a finisher ration (3,050 Kcal. ME/kg, 22% C.P.) for the remaining period. The basal diets were formulated to meet the requirements of the broiler strain (31) (Table 2).
Growth Performance and Blood Biochemical Traits
During the experimental period, all the birds were subjected to the same strategy of data collection. Chicks were independently weighed at a weekly interval until the 5th week of age then the mean body weight of the pen was cumulatively calculated as an experimental unit. Feed consumption was recorded weekly until the marketing age on a replicate basis and dead birds (if any) were recorded. Consequently, body weight gain (BWG), total feed intake (TFI), feed conversion ratio (FCR), and mortality percentage were estimated from these data during the experimental period. The performance index for broilers (European Production Efficiency Factors; EPEF) was used as ascribed by Aviagen (32). EPEF were calculated according to the following formula:
Blood Biochemical Parameters, Oxidative Remarks, and Immune Activity
At the end of the experiment (35th day), blood samples (2 birds/replicate) were collected from wing veins into clean tubes without coagulating. The samples were coagulated and centrifuged at 3,000 rpm for 15 min and the separated sera were collected in Eppendorf, frozen and stored at −20°C until further analysis. The following serum biochemical parameters were determined: total protein (g/dl), albumin (g/dl), globulin (g/dl), total cholesterol (mg/dl), triglyceride (mg/dl), high-density lipoprotein (HDL), low-density lipoprotein (LDL) (mg/dl), reduced glutathione (GSH), super oxide dismutase (SOD), lipid peroxidation malondialdehyde (MDA), and catalase (CAT) and immunoglobulins, namely, IgG and IgM were estimated in the blood by using commercial diagnostic kits provided by the Bio Diagnostic Co. (29 El-Tahrir St. Dokki, Giza, Egypt) and a spectrophotometer (Shimadzu, Japan).
RNA Extraction and cDNA Synthesis
RNA was extracted using 30–50 mg of liver and spleen tissues from experimental chickens using the QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH) following the manufacturer's protocol. The integrity and concentration of the obtained RNA were determined with spectrophotometric NanoDrop® ND-1000. The synthesis of the first strand of cDNA from the obtained RNA was achieved through the use of QuantiTect Reverse Transcription kit (Qiagen, Heidelberg, Germany) and the manufacturing procedures were applied.
Quantitative Real-Time PCR
Quantitative real-time PCR was determined using Rotor-Gene Q cycler (Qiagen, Heidelberg, Germany) by SYBR Green QuantiTect PCR kits (Qiagen, Germany). Relative expression of mRNA level was carried out for each gene. The sequence of the used primers is illustrated in Table 3. Two possible housekeeping genes (GAPDH and YWHAZ) were selected as they are commonly applied as based controls in target tissue expression investigations. The reaction mixture consisted of 12.5 μl of 2x SYBR Green PCR Mastermix, 0.25 μl of RevertAid Reverse Transcriptase (200 U/μl) (Thermo Fisher), 0.5 μl of each primer of 20 pmol concentration, 8.25 μl of water, and 3 μl of RNA template. The reaction was performed in a Stratagene MX3005P real-time PCR machine. The thermal cycling conditions were: initial denaturation at 95°C for 15 min for a number of 40 cycles followed by initial heat activation at 94°C for 15 s; primers annealing at 59°C for 1 min for IL-10 gene, 60°C for 1 min for IL-1β and GHR, 65°C for 1 min for IGF-1; finally, elongation at 72°C for 30 s. The relative fold changes in the mRNA expression of the studied genes were calculated as recorded by Yuan et al. (33) through the comparative 2−ΔΔCt method (Ct: cycle threshold).
Statistical Analysis
The data obtained were subjected to the one-way ANOVA using IBM SPSS Ver. 24. The GLM statistical model was as follows: Xijkl = μ + Aj + ei.
Where, Xij = an observational data, μ = overall mean, Aj = effect of PLE supplementation level, ei = random error. The main effect of the PLE supplementation was the experimental unit. Tukey's multiple range test was used to compare means when a significant difference (p < 0.05) was detected. For broiler performance, five replicates pen per treatment (10 broiler chicks per replicate pen) served as the experimental unit followed Hernández-Ramírez et al. (34).
Results
The GC–MS Analysis of Paulownia Leaf Extracts
The results of the GC–MS analysis of main bioactive compounds found in paulownia leaf extract with their retention time (RT), and area percentage are presented in Table 4. Chromatograms GC–MS result indicates that the tested extract of paulownia leaf has a variety of bioactive compounds. The presence of 5 main peaks determined was as follows: the first main bioactive compound was thymol which was identified in the shortest RT (7.86) with the highest peak area (82.37%) and the last compound Octasiloxane was identified in much longest RT (38.59) and low percentage peak area (2.78) was observed. Among those 2 bioactive compounds, too many phytocompounds having different biological activities were confirmed such as α-Tocospiro (RT:26.59; 3.18%), Phytol (RT:19.04; 9.28), and Pentadecanoic acid observed at (RT:16.82; 2.785).
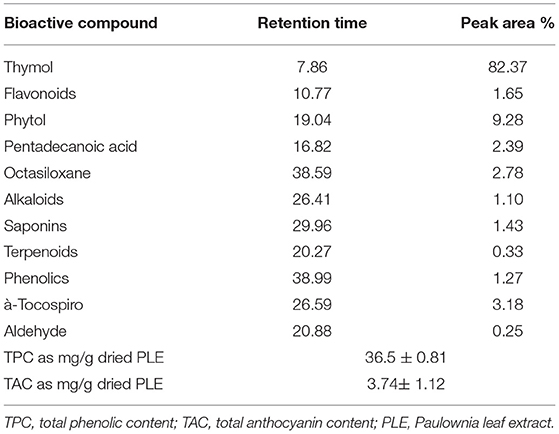
Table 4. The gas chromatography/mass spectrometry analysis of the main bioactive compounds found in paulownia leaf extracts (PLEs).
The phytochemical characterization of the PLE of phytochemicals molecules indicated the presence of saponins and phenolics, namely, flavonoids, with total phenolic content (TPC) and total anthocyanin content (TAC) values of 36.5 ± 0.81 and 3.74 ± 1.12 mg/g dried PLE, respectively (Table 4).
Growth Performance
The PLE supplementation on the growth performance of broiler chicks during the experimental period is presented in Table 5. Clearly, results showed that there were significant differences (P < 0.05) in final body weight (FBW) and body weight gain (BWG) due to PLE supplementation. Furthermore, increasing the inclusion level of PLE from 0.1 up to 0.5 g/kg within the broiler diet increased the positive effect on LBW and BWG (linearly; p < 0.002). On the contrary, TFI, FCR, and mortality rates showed no significant differences (P > 0.05) under the effects of PLE dietary supplementation. Whereas, values of FCR were numerically improved in PLE-treated treatments (PLE0.1, PLE0.3, and PLE0.5) in comparison with the non-treated treatment (CNT). Besides, mortality percentage reduced from 2.96% in the CNT treatment to 1.48, 0.00, and 0.00 for PLE-treated treatments (PLE0.1, PLE0.3, and PLE0.5), respectively. Herein, the EPEI level was significantly (linearly; p < 0.001) increased gradually by about 10.96, 12.24 and 17.19% for PLE supplemented treatments (PLE0.3 and PLE0.5), respectively, in comparison with the control treatment.
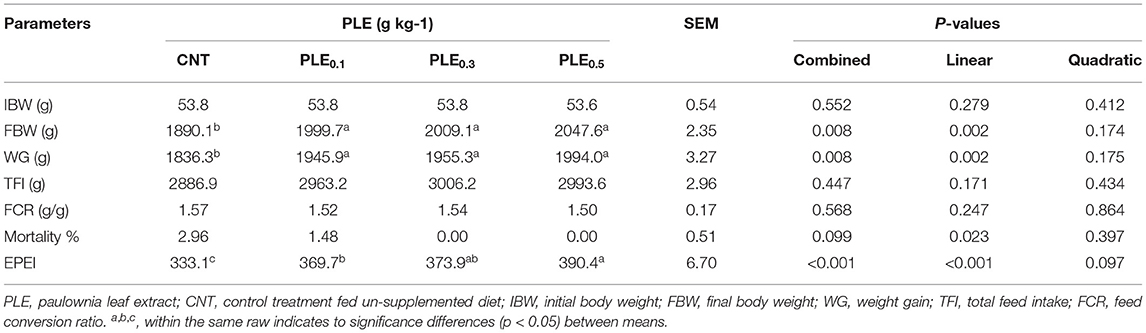
Table 5. Productive performance parameters of broilers as affected by PLE supplemented diets at different levels.
Blood Biochemical Parameters
The effects of PLE supplementation on blood biochemical parameters of broiler chicks are presented in Table 6. Generally, results showed no significant differences (P > 0.05) in blood biochemical parameters (total protein, albumin, globulin, total cholesterol, HDL, and LDL) in all the experimental treatments. While, lipid triglycerides values showed a significant (P < 0.05) reduction under the effects of PLE dietary supplementation. Blood triglyceride values reduced from 208.3 in the CNT treatment to 194.5 and 148.8 for PLE0.1 and PLE0.3, respectively.
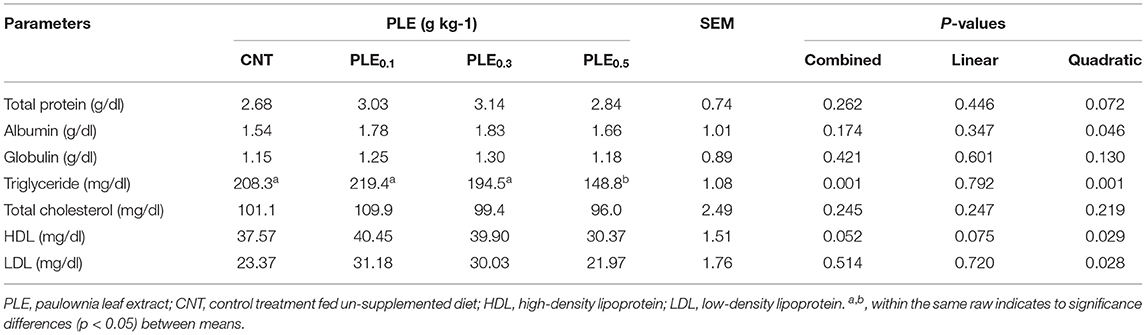
Table 6. Blood biochemical measurements of broilers as affected by PLE supplemented diets at different levels.
Oxidative Remarks and Immune Status
Oxidative remarks parameters such as GSH, SOD, MDA, and CAT are indicators for assessing the oxidative stress status of birds. Results in Table 7 illustrated the antioxidant parameters of broiler chicks at 35 days of age as affected by different levels of PLE supplementation. Data demonstrated that PLE supplementation significantly (P < 0.01) affects antioxidant parameters (GSH, SOD, and CAT) of different broiler treatments. On the other hand, lipid peroxidation activity (MDA) was not significantly (P > 0.05) affected by the different levels of PLE (PLE0.1, PLE0.3, and PLE0.5) in comparison with the non-treated treatment (CNT). Whereas, the MDA values showed slightly decreased in all the PLE-supplemented treatments than in the control treatment.
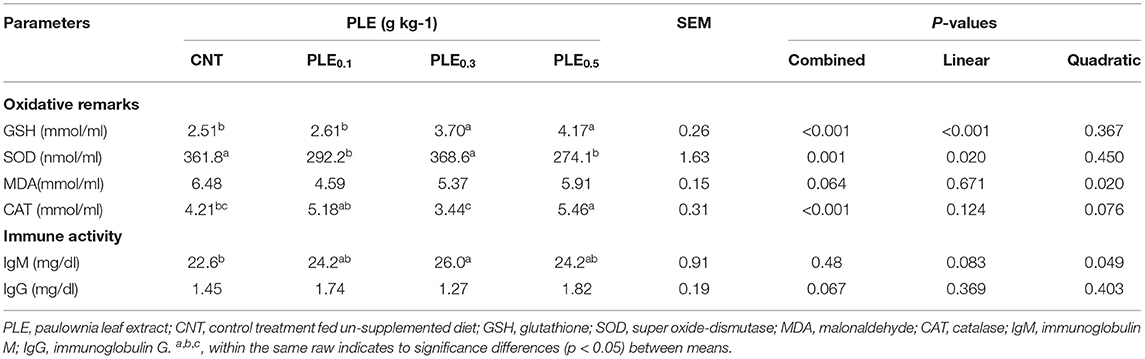
Table 7. Oxidative remarks and immune status of broilers as affected by PLE supplemented diets at different levels.
Glutathione (GSH) concentration was increased significantly (P < 0.05) and gradually (linearly; p < 0.001) from 2.51 in the CNT treatment to 2.61, 3.70, and 4.17 for PLE supplemented treatments (PLE0.1, PLE0.3, and PLE0.5), respectively. However, a similar pattern was not seen for SOD and CAT, where values for the SOD parameter tended to be higher in the PLE0.3 supplemented treatment than in the other treated treatments (PLE0.1 and PLE0.5). An opposite trend was found in CAT values, where it was tended to be higher in PLE0.1 and PLE0.5 treatments than in other treated treatments (PLE0.3) and non-treated treatments (CNTs).
In addition, in regards to immune activity parameters (IgM and IgG), results showed that dietary supplementation of PLE had a significant (P < 0.05) positive effect on IgM. The immune activity of IgM increased from 22.6 in the CNT treatment to 24.2, 26.0, and 24.2 for PLE0.1, PLE0.3, and PLE0.5, respectively. Furthermore, IgG values showed numerically improved for the broiler treatments that received PLE0.1 and PLE0.5 (1.74 and 1.82, respectively) compared with the control treatment fed un-supplemented diets (1.45).
Gene Expression
The effects of dietary supplementation of graded levels of PLE on mRNA expression of hepatic (IGF-1 and GHR) and splenic (IL-1β and IL-10) of the broiler chickens are presented in Figure 1. The results showed a significant (P < 0.01) interaction between supplemented graded levels of PLE and the mRNA expression of all the assessed genes. A dramatic linear increase (P < 0.01) was observed in mRNA levels in hepatic IGF-I and GHR of birds fed with PLE in a dose-dependent manner. Compared with the un-supplemented treatment (CONT), the relative transcript levels of mRNA expression (IGF-1 and GHR) in the liver were duplicated about 1, 2, and 3 times in treated broiler treatments supplemented with PLE0.1, PLE0.3, and PLE0.5, respectively. In the same manner, regarding splenic genes expression level, a significant upregulation in mRNA levels of splenic IL-1β and IL-10 was observed with increasing the dietary level of PLE supplementation compared with the control treatment. Briefly, mRNA expression of all the evaluated genes was more prominent in broiler treatments with increasing PLE supplementation levels.
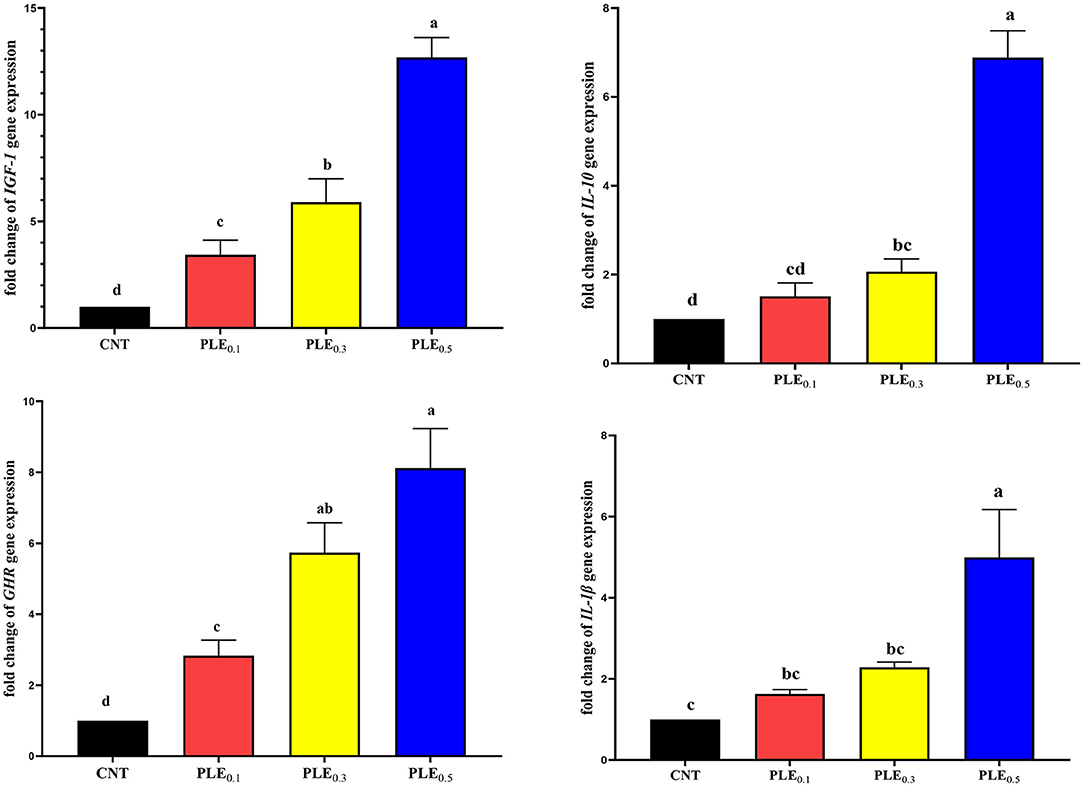
Figure 1. The effect of PLE dietary supplementation on the mRNA expression of the broiler chicken hepatic (IGF-1 and GHR) and splenic (IL-1β and IL-10) genes (mean ± MSE). Columns with different superscript letters are significantly different (P < 0.05).
Discussion
Paulownia is one of the traditional Chinese medical (13), which contains a variety of active ingredients such as flavonoids, phenolic acids, saponins, phenylethanoid glycosides, lignans, and triterpenoids in its leaf, fruit, and flower (12, 19, 35), with multiple pharmacological and economical values (18). Our GC mass analysis demonstrated that the methanolic extract of P. tomentosa included large amounts of thymol, phytol, flavonoids, and other bioactive compounds, which indicates the great efficacy of methanol as an organic solvent in extracting the primary bioactive molecules found within leaves. In addition, methanol is highly effective in extracting essential oils from plant material, particularly, the leaves (16, 19). Thus, this research studied the incorporation of P. tomentosa leaf extract into broiler diets and has shown that it is a viable strategy for improving performance and general health status.
Our feeding trial results showed that increasing supplemental levels of PLE resulted in noticeable linear increases in both final body weight and weight gain. The obtained results were found to be inconsistent with Agah et al. (36) findings that enriched broiler diets with olive leaf extract significantly promoted the growth and efficiency of consumed diets. In addition, Acamovic and Cross (37) demonstrated that supplementing broiler chicks' diet with a 1,000 mg/kg thyme essential oil significantly increased body weight growth as well as remarkedly reduced total feed intake. Thyme essential oils isolated from paulownia leaves showed higher antibacterial activities, which are particularly effective in promoting digestion through maintaining intestinal microbial balance (38) and stimulating the release of internal digestive enzymes (39), consequently enhancing overall growth performance in chicken.
In the same context, aromatic herbs and their extracts are well-known to enhance the flavor and palatability of feed, hence increasing growth and feed efficiency (40). In particular, thyme essential oil has a significant influence on feed conversion ratio because it could improve beneficial microbial population development and increase nutrient absorption. Hence, the dietary inclusion of thymol into chicken diets stimulated lipase activity by 29% and trypsin activity by 18% in the digestive tract. Despite the fact that our feed efficiency parameters results revealed no significant variations in feed efficiency (TFI and FCR) because of the dietary supplementation of PLE on the broiler diets, but the lowest FI and FCR values were reported in the broiler-fed diets supplemented with higher PLE levels. In the same context, the numerically decreasing FCR values and mortality percentage highly correlated with the higher EPEI values in the broiler treatments supplemented with graded amounts of PLE extract. This finding was reported to be in agreement with Olaifa et al. (41) and Elbaz et al. (42), who stated that phytogenic feed additives could significantly improve all performance and feed efficiency parameters, namely, live body weight, weight gain, feed consumption, and feed conversion ratio of the broiler chickens. In addition, El-Ashram and Abdelhafez (43) reported that dietary inclusion of phytogenic feed additives such as thymol into the broiler diet significantly improved values of EPEF for the broiler compared with the untreated treatment. The antibacterial features of thymol and other flavonoids compounds found in PLE extract (14), as well as its capability to promote nutrient digestion (15), enhance intestinal structure, and increase epithelial absorption activity (12), may be responsible for the improvement in all feed efficiency indicators.
On the other hand, the blood biochemical components are usually correlated to the health conditions of birds (6). Furthermore, the alteration of biochemical parameters is a useful indicator of the nutritional, physiological, and pathological condition of the birds (44). In addition, it might be a valuable technique for determining the impact of feed additives on bird health and productivity (45). In the current study, all the biochemical blood markers were shown to be unaffected with PLE dietary administration, with the exception of triglyceride concentrations. The unchanged serum parameters were in line with the results of Al-Sagheer et al. (21) and Kovitvadhi et al. (46), who mentioned that dietary phytogenic supplementation had no significant effect on the biochemical parameters (total protein, globulin, and albumin) in growing rabbits. The absence of variations in these parameters suggests that dietary paulownia leaves extract supplementation has health advantages. Conversely, increasing the PLE level in the broiler diet significantly lowered the triglyceride levels in the blood. Recent studies have proven that thyme can decrease triglyceride and total cholesterol levels (47, 48). Yu et al. (49) reported that thymol possesses a lipid-reducing function by altering hepatic triglyceride secretion. As a consequence, our findings indicated that PLE dietary inclusion had no harmful effects on biochemical blood markers and could be applied safely.
Moreover, oxidative stress remarks such as GSH, SOD, MDA, and CAT are frequently used as indicators for assessing oxidative stress status (50). Regarding antioxidative biomarkers, supplementation of PLE improved the activity of antioxidant enzymes, namely, GSH, SOD, and CAT and decreased MDA levels in comparison with the unsupplemented treatment. These findings are consistent with several earlier studies which documented the antioxidant properties of P. tomentosa and its phenolic treatment constituents (15, 16, 35). Moreover, Alagawany et al. (12) showed that the dietary supplementation of phytogenic constituents significantly increased serum antioxidant enzyme activities and decreased MDA concentration. Herbal extract such as paulownia is a good source of many phytochemicals such as thymol and phytol and other bioactive components (28). These bioactive compounds found in P. tomentosa possess potent anti-inflammatory and antioxidant effects (4, 12, 16, 35). From these findings, it could be proposed that supplements with natural antioxidants could be practical in the future to enhance the health status of the broiler.
During evolution, ontogeny and immune responses, immunoglobulin (Ig) M is found to be the first isotype of antibodies that significantly stimulate (42). IgM not only acts as the first defensive line for the host against infectious pathogens, but it also plays a crucial role in inflammatory processes and innate immune responses (45). IgG antibodies are present in extracellular fluid and blood stream, where they might absorb pollutants, phagocytic viruses, and bacteria and stimulate the complement pathways (20). Our findings showed that supplementing birds' rations with PLE significantly stimulated immunoglobulin activity. In full agreement with our results, Yang et al. (17) and Wang et al. (20) reported that paulownia tomentosa flower (PTFP) as a new immunostimulant enhanced the humoral and cellular responses in chickens as well as promoted specific immunoglobulin (IgGs) antibodies responses in rats (16). The enhancement of immunoglobulin (IgM and IgG) in PLE supplemented treatments might be due to the antibacterial, antioxidant, and anti-inflammatory properties of bioactive components found in PLE (21). Thymol is the main component of PLE and it has been known for its positive immune effects such as an increase in lymphocyte proliferation rate, phagocytic rate as well as an increase in immunoglobulins such as IgA and IgM in the blood (51, 52).
Owing to our results, the expression of hepatic IGF-I and GHR mRNA significantly improved in birds fed supplemented diets with graded amounts of PLE. Similar results have been reported by Hosseini et al. (53), who concluded that using phytogenic feed additives (such as Thymolina) in the broiler chicken's diet causes improvements in the immune system by increasing the gene expression of hepatic IGF-1. Thus, the upregulation of hepatic IGF-I and GHR mRNA levels of birds fed PLE supplemented treatments might be due to the growth-promoting properties of the bioactive components found in PLE, specifically thymol and phytol (51). For the same reason, anti-inflammatory and immunomodulatory properties of bioactive components found in paulownia extract have the ability to upregulate mRNA expression of spleen pro-inflammatory interleukins (IL-1β and IL-10) (53). It is well-known that the expressions of interleukin IL-1β and IL-6 are highly associated with the immune status of poultry and livestock (6). Consequently, thymol as the major compound found in paulownia leaves extracts significantly upregulated the expression of pro-inflammatory cytokines, namely, IL-1β, IL-6, IL-10, IL-12α, and IL-18 (54).
Conclusion
Dietary supplementation of PLE significantly improved FBW, WG, FCR, while remarkedly enhanced EPEI values. However, the treatment increases the activity of oxidative remarks (GSH, SOD, and CAT). Besides, our study revealed the dose-dependent effect of PLE on serum immunoglobulin (IgM and IgG) activity in broiler. Also, a dramatic linear increase was observed in mRNA expression of hepatic (IGF-1 and GHR) and splenic (IL-1β and IL-10) of broiler chickens. The current research revealed that PLE at 0.5 g per kg diet is the most acceptable dosage for supplementation in broilers to optimize performance, improve oxidative remarks activity, and boost immunity without affecting productivity. However, larger cohort studies are needed to provide mechanistic insights into the role of PLE in alleviating oxidative stress, modulating intestinal microbial biodiversity, and mediating metabolic activities associated with gut function activity in the broiler chicks.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by all in-vivo trials were followed the practical guidelines of the Local Experimental Animal Care Committee and approved by the Ethics Committee of Animal Use in Research Committee of Mansoura University (Code No: R/87).
Author Contributions
SS, HE-E, NW, ME, YA, SA, MSo, and HZ carried out broiler maintenance and sample collection and contributed to conception and design of the study. MN, MA, YA, SA, MSo, and MSh organized the database and performed the statistical analysis. MN and SA drafted the manuscript and prepared it for publishing. SS, HE-E, MN, NW, HZ, MA, YA, SA, MSo, MSh, and ME read, agreed, and equally established the final published version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Taif University Researchers Supporting Project (TURSP-2020-258), Taif University, Taif, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seidavi A, Tavakoli M, Asroosh F, Scanes CG, Abd El-Hack ME, Naiel MAE, et al. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ Sci Pollut Res Int. (2022) 29:5006–31. doi: 10.1007/s11356-021-17401-w
2. Suliman GM, Alowaimer AN, Al-Mufarrej SI, Hussein EO, Fazea EH, Naiel MA, et al. The effects of clove seed (Syzygium aromaticum) dietary administration on carcass characteristics, meat quality, and sensory attributes of broiler chickens. Poultry Sci. (2021) 100:100904. doi: 10.1016/j.psj.2020.12.009
3. Oliveira N, Gonçalves B, Lee S, Oliveira C, Corassin C. Use of antibiotics in animal production and its impact on human health. J Food Chem Nanotechnol. (2020) 6:40–7. doi: 10.17756/jfcn.2020-082
4. Barreto M, Menten J, Racanicci A, Pereira P, Rizzo P. Plant extracts used as growth promoters in broilers. Brazil J Poultry Sci. (2008) 10:109–15. doi: 10.1590/S1516-635X2008000200006
5. Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. (2011) 187:182–8. doi: 10.1016/j.tvjl.2010.03.003
6. Abou-Elkhair R, Abdo Basha H, Slouma Hamouda Abd El Naby W, Ajarem JS, Maodaa SN, Allam AA, et al. Effect of a diet supplemented with the Moringa oleifera seed powder on the performance, egg quality, and Gene expression in Japanese laying quail under heat-stress. Animals. (2020) 10:809. doi: 10.3390/ani10050809
7. Naiel MAE, Ismael NEM, Shehata SA. Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile Tilapia (Oreochromis niloticus). Aquaculture. (2019) 511:734264. doi: 10.1016/j.aquaculture.2019.734264
8. Kalia S, Bharti VK, Giri A, Kumar B. Effect of Prunus armeniaca seed extract on health, survivability, antioxidant, blood biochemical and immune status of broiler chickens at high altitude cold desert. J Adv Res. (2017) 8:677–86. doi: 10.1016/j.jare.2017.08.005
9. Kalia S, Bharti VK, Giri A, Kumar B, Arora A, Balaje S. Hippophae rhamnoides as novel phytogenic feed additive for broiler chickens at high altitude cold desert. Sci Rep. (2018) 8:1–12. doi: 10.1038/s41598-018-24409-9
10. Abo Ghanima MM, Elsadek MF, Taha AE, El-Hack A, Mohamed E, Alagawany M, et al. Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals. (2020) 10:245. doi: 10.3390/ani10020245
11. Dhama K, Latheef SK, Mani S, Samad HA, Karthik K, Tiwari R, et al. Multiple beneficial applications and modes of action of herbs in poultry health and production-a review. Int J Pharmacol. (2015) 11:152–76. doi: 10.3923/ijp.2015.152.176
12. Alagawany M, Farag MR, Sahfi ME, Elnesr SS, Alqaisi O, El-Kassas S, et al. Phytochemical characteristics of Paulownia trees wastes and its use as unconventional feedstuff in animal feed. Animal Biotechnol. (2022) 33:586–93. doi: 10.1080/10495398.2020.1806074
13. Móricz ÁM, Ott PG, Knaś M, Długosz E, Krüzselyi D, Kowalska T, et al. Antibacterial potential of the phenolics extracted from the Paulownia tomentosa L. leaves as studied with use of high-performance thin-layer chromatography combined with direct bioautography. J Liquid Chromatogr Relat Technol. (2019) 42:282–9. doi: 10.1080/10826076.2019.1585604
14. Zima A, Hošek J, Treml J, Muselík J, Suchý P, Pražanová G, et al. Antiradical and cytoprotective activities of several C-geranyl-substituted flavanones from Paulownia tomentosa fruit. Molecules. (2010) 15:6035–49. doi: 10.3390/molecules15096035
15. He T, Vaidya BN, Perry ZD, Parajuli P, Joshee N. Paulownia as a medicinal tree: traditional uses and current advances. Euro J Med Plants. (2016) 14:1–15. doi: 10.9734/EJMP/2016/25170
16. Chen X, Jin J, Hao F, Yang H, Sun H, Jiang C. Paulownia tomentosa flower polysaccharide as an effective immunopotentiator to enhance immune responses for Newcastle disease vaccine in mice. Italian J Food Sci. (2021) 33:11–20. doi: 10.15586/ijfs.v33i4.2107
17. Yang H, Zhang P, Xu X, Chen X, Liu Q, Jiang C. The enhanced immunological activity of Paulownia tomentosa flower polysaccharide on Newcastle disease vaccine in chicken. Biosci Rep. (2019) 39:BSR20190224. doi: 10.1042/BSR20190224
18. Adach W, Zuchowski J, Moniuszko-Szajwaj B, Szumacher-Strabel M, Stochmal A, Olas B, et al. Comparative phytochemical, antioxidant, and hemostatic studies of extract and four fractions from paulownia clone in vitro 112 leaves in human plasma. Molecules. (2020) 25:4371. doi: 10.3390/molecules25194371
19. Dzugan M, Miłek M, Grabek-Lejko D, Heclik J, Jacek B, Litwińczuk W. Antioxidant activity, polyphenolic profiles and antibacterial properties of leaf extract of various Paulownia spp. Clones. Agron. (2021) 11:2001. doi: 10.3390/agronomy11102001
20. Wang Q, Meng X, Zhu L, Xu Y, Cui W, He X, et al. A polysaccharide found in Paulownia fortunei flowers can enhance cellular and humoral immunity in chickens. Int J Biol Macromol. (2019) 130:213–9. doi: 10.1016/j.ijbiomac.2019.01.168
21. Al-Sagheer AA, El-Hack A, Mohamed E, Alagawany M, Naiel MA, Mahgoub SA, et al. Paulownia leaves as a new feed resource: chemical composition and effects on growth, carcasses, digestibility, blood biochemistry, and intestinal bacterial populations of growing rabbits. Animals. (2019) 9:95. doi: 10.3390/ani9030095
22. Zhang H, Li X, Kang H. Chitosan coatings incorporated with free or nano-encapsulated Paulownia tomentosa essential oil to improve shelf-life of ready-to-cook pork chops. LWT. (2019) 116:108580. doi: 10.1016/j.lwt.2019.108580
23. Zhang D, Ivane NMA, Haruna SA, Zekrumah M, Elysé FKR, Tahir HE, et al. Recent trends in the micro-encapsulation of plant-derived compounds and their specific application in meat as antioxidants and antimicrobials. Meat Sci. (2022) 191:108842. doi: 10.1016/j.meatsci.2022.108842. [Epub ahead of print].
24. El-Showk S, El-Showk N. The Paulownia Tree. An Alternative for Sustainable Forestry, Crop Development. Morocco (2003). p. 1–8. Retrived from http://www.cropdevelopment.org/docs/PaulowniaBrochure_print.pdf
25. Ganchev G, Ilchev A, Koleva A. Digestibility and energy content of Paulownia (Paulownia elongata SY Hu) leaves. Agric Sci Technol. (2019). 11:307–10. doi: 10.15547/ast.2019.04.051
26. Nety S, Koley K, Chourasia D, Sharma K, Bhandeker S. Study of phytochemical and immunomodulatory activity of methanolic extract of Andrographis paniculata in broiler birds. J Animal Res. (2018) 8:27–31. doi: 10.30954/2277-940X.2018.00150.05
27. Agustika DK, Mercuriani IS, Ariyanti NA, Purnomo CW, Triyana K, Iliescu DD, et al. Gas chromatography-mass spectrometry analysis of compounds emitted by pepper yellow leaf curl virus-infected chili plants: a preliminary study. Separations. (2021) 8:136. doi: 10.3390/separations8090136
28. Al-Marzoqi AH, Hameed IH, Idan SA. Analysis of bioactive chemical components of two medicinal plants (Coriandrum sativum and Melia azedarach) leaves using gas chromatography-mass spectrometry (GC-MS). Afr J Biotechnol. (2015) 14:2812–30. doi: 10.5897/AJB2015.14956
29. Chattuwatthana T, Okello E. Anti-collagenase, anti-elastase and antioxidant activities of Pueraria candollei var. mirifica root extract and Coccinia grandis fruit juice extract: an in vitro study. Euro J Med Plants. (2015) 5:318–27. doi: 10.9734/EJMP/2015/14129
30. Zakaria N, Okello E, Howes MJ, Birch-Machin M, Bowman A. In vitro protective effects of an aqueous extract of Clitoria ternatea L. flower against hydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother Res. (2018) 32:1064–72. doi: 10.1002/ptr.6045
31. C. Cobb Broiler Performance and Nutrient Supplement Guide. Siloam Springs, AR: Cobb-Vantress (2012).
33. Yuan JS, Reed A, Chen F, Stewart CN. Statistical analysis of real-time PCR data. BMC Bioinformatics. (2006) 7:1–12. doi: 10.1186/1471-2105-7-85
34. Hernández-Ramírez J, Nava-Ramírez M, Merino-Guzmán R, Téllez-Isaías G, Vázquez-Durán A, Méndez-Albores A. The effect of moderate-dose aflatoxin B1 and Salmonella Enteritidis infection on intestinal permeability in broiler chickens. Mycotoxin Res. (2020) 36:31–9. doi: 10.1007/s12550-019-00367-7
35. Ashour EA, Abd El-Hack ME, Swelum AA, Osman AO, Taha AE, Alhimaidi AR, et al. Does the dietary graded levels of herbal mixture powder impact growth, carcass traits, blood indices and meat quality of the broilers? Ital J Animal Sci. (2020) 19:1228–37. doi: 10.1080/1828051X.2020.1825998
36. Agah M, Mirakzehi M, Saleh H. Effects of olive leaf extract (Olea europea L.) on growth performance, blood metabolites and antioxidant activities in broiler chickens under heat stress. J Animal Plant Sci. (2019) 29:657–66.
37. Acamovic T, Cross D. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in young chickens from 7-28 days of age. Br Poultry Sci. (2007) 48:496–506. doi: 10.1080/00071660701463221
38. Hussein EO, Ahmed SH, Abudabos AM, Suliman GM, El-Hack A, Mohamed E, et al. Ameliorative effects of antibiotic-, probiotic-and phytobiotic-supplemented diets on the performance, intestinal health, carcass traits, and meat quality of clostridium perfringens-infected broilers. Animals. (2020) 10:669. doi: 10.3390/ani10040669
39. Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Animal Sci. (2008) 86(Suppl_14):E140–8. doi: 10.2527/jas.2007-0459
40. Gholami-Ahangaran M, Ahmadi-Dastgerdi A, Azizi S, Basiratpour A, Zokaei M, Derakhshan M. Thymol and carvacrol supplementation in poultry health and performance. Vet Med Sci. (2022) 8:267–88. doi: 10.1002/vms3.663
41. Olaifa R, Sogunle O, Obileye L, Ibitoye S, Ayodeji T, Odutayo O, et al. Effect of oral administration of three different phytobiotics on growth performance of locally-adapted turkeys. EC Vet Sci. (2019) 4:656–62.
42. Elbaz AM, Ibrahim NS, Shehata AM, Mohamed NG, Abdel-Moneim A-ME. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop Animal Health Prod. (2021) 53:1–10. doi: 10.1007/s11250-021-02554-0
43. El-Ashram S, Abdelhafez GA. Effects of phytogenic supplementation on productive performance of broiler chickens. J Appl Poultry Res. (2020) 29:852–62. doi: 10.1016/j.japr.2020.07.005
44. Khafaga AF, Naiel MA, Dawood MA, Abdel-Latif HM. Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquatic Toxicol. (2020) 228:105624. doi: 10.1016/j.aquatox.2020.105624
45. Mohamed SH, Attia AI, Reda FM, Abd El-Hack ME, Ismail IE. Impacts of dietary supplementation of Boswellia serrata on growth, nutrients digestibility, immunity, antioxidant status, carcase traits and caecum microbiota of broilers. Ital J Animal Sci. (2021) 20:205–14. doi: 10.1080/1828051X.2021.1875336
46. Kovitvadhi A, Gai F, Dabbou S, Ferrocino I, Rotolo L, Falzone M, et al. Rabbit dietary supplementation with pale purple coneflower. 2. Effects on the performances, bacterial community, blood parameters and immunity of growing rabbits. Animal. (2016). 10:1110–7. doi: 10.1017/S1751731115002980
47. Mehdipour Z, Afsharmanesh M, Sami M. Effects of supplemental thyme extract (Thymus vulgaris L.) on growth performance, intestinal microbial populations, and meat quality in Japanese quails. Compar Clin Pathol. (2014) 23:1503–8. doi: 10.1007/s00580-013-1813-6
48. Popović S, Puvača N, Kostadinović L, Džinić N, Bošnjak J, Vasiljević M, et al. Effects of dietary essential oils on productive performance, blood lipid profile, enzyme activity and immunological response of broiler chickens. Poult Sci. (2016) 80:1–12. doi: 10.1399/eps.2016.146.CORR
49. Yu Y-M, Chao T-Y, Chang W-C, Chang MJ, Lee M-F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J Food Drug Anal. (2016) 24:556–63. doi: 10.1016/j.jfda.2016.02.004
50. Abdel-Moneim A-ME, Shehata AM, Mohamed NG, Elbaz AM, Ibrahim NS. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol Trace Element Res. (2022) 200:768–79. doi: 10.1007/s12011-021-02662-w
51. Hashemipour H, Kermanshahi H, Golian A, Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poultry Sci. (2013) 92:2059–69. doi: 10.3382/ps.2012-02685
52. Abd El-Hack ME, Alagawany M, Farag MR, Tiwari R, Karthik K, Dhama K. Nutritional, healthical and therapeutic efficacy of black cumin (Nigella sativa) in animals, poultry and humans. Int J Pharmacol. (2016) 12:232–48. doi: 10.3923/ijp.2016.232.248
53. Hosseini S, Chamani M, Seidavi A, Sadeghi A, Ansari-Pirsaraei Z. Effect of feeding Thymolina® powder on the gene expression IGF-1 in Ross 308 broiler chickens. J Livestock Sci. (2016) 7:274–9.
Keywords: paulownia extract, growth performance, immunity, broiler, antioxidant
Citation: Sakr SA, EL-Emam HA, Naiel MAE, Wahed NM, Zaher HA, Abougabal MSh, Alghamdi YS, Albogami S, Soliman MM, Shukry M and Elghareeb MM (2022) The Impact of Paulownia Leaves Extract on Performance, Blood Biochemical, Antioxidant, Immunological Indices, and Related Gene Expression of Broilers. Front. Vet. Sci. 9:882390. doi: 10.3389/fvets.2022.882390
Received: 23 February 2022; Accepted: 27 May 2022;
Published: 05 July 2022.
Edited by:
Arda Yildirim, Gaziosmanpaşa University, TurkeyReviewed by:
Sahil Kalia, Cornell University, United StatesMaghsoud Besharati, University of Tabriz, Iran
Copyright © 2022 Sakr, EL-Emam, Naiel, Wahed, Zaher, Abougabal, Alghamdi, Albogami, Soliman, Shukry and Elghareeb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed A. E. Naiel, bW9oYW1tZWRuYWllbC4xOTg0QGdtYWlsLmNvbQ==
 Shimaa A. Sakr1
Shimaa A. Sakr1 Mohammed A. E. Naiel
Mohammed A. E. Naiel