
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 10 August 2022
Sec. Veterinary Pharmacology and Toxicology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.882060
As the ornamental plants and traditional medicines, Rhododendron przewalskii, R. anthopogonoides, R. thymifolium, and R. capitatum are widely distributed in western China. In this paper, the essential oils from these four species were extracted by supercritical extraction and the components were analyzed using headspace solid phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS), the antibacterial, acaricidal and anti-inflammatory activities were investigated. Results showed that R. thymifolium (RTEO) contained the highest yield of 0.99% with 246 compounds, followed by R. capitatum (RCEO, 0.81%) with 290 chemicals, R. anthopogonoides (RAEO, 0.57%) with 302 compounds and R. przewalskii (RPEO, 0.30%) with 294 components. They also exhibited the safety at given doses and have the anti-inflammatory in vitro and in vivo tests via inhibiting the cytokines productions, the acaricidal and antibacterial activities also were found. 4-Hydroxy-3-methylacetophenone from RPEO, α-pinene and β-pinene from RAEO, β-farnesene and germacrone from RTEO, and benzylacetone from RCEO, as main and active components, inhibited the NO content in RAW 264.7 cells induced by LPS. These results indicated that four essential oils have certain medicinal value and laid the foundation for the development of these species as raw materials for the pharmaceutical and perfume industries.
As an important component and secondary metabolite of medicinal plants, essential oils from the stems, leaves, flowers, roots and fruits of aromatic plants play very important roles both economically and scientifically worldwide. Essential oils of various plants are rich in bioactive substances, including monoterpenes, sesquiterpenes, and their derivatives, such as aldehydes and phenols, simple phenylpropanoids and others, and these components vary for different species and in different seasons. Reports have shown that they possess biological properties, such as anti-inflammatory, antioxidant, antimicrobial, antifungal, acaricidal and insecticidal activities (1–4). Due to their low toxicity to humans, capacity for further degradation, and low environmental impact, plant essential oils have been widely applied in the pharmaceutical, perfume, an cosmetics industries and in plant protection and veterinary medicine.
Rhododendron (Ericaceae family) is one of the largest genera of vascular plants, with approximately 967 species, and is widely distributed in the Northern Hemisphere. Some species have been used in China, India, Europe and North America as traditional medicines for treating inflammation, pain, skin and alimentary canal diseases,some species are cultivated as ornamental plants for horticulture and economic crops for the pharmaceutical industry (5). Modern studies have shown that this genus consists of flavonoids, diterpenoids, iridoid glycosides and sesquiterpenoids (6). Among these, essential oils were thought to be the active components of Rhododendron species. Essential oils from R. tomentosum are thought to be a source of potential antiarthritic drugs for antiproliferative and proapoptotic activities toward CD4 and CD8 T cells, synovial-infiltrating monocytes/macrophages and fibroblast-like synovial cells (7).There are more than 700 Rhododendron species in China, most of which are widely distributed in the Himalayas (8). As the dominant species in this region, Rhododendron przewalskii Maxim., R. anthopogonoides Maxim., R. thymifolium Maxim., and R. capitatum Maxim. are distributed and planted in western China, especially in Gansu, Qinghai and Sichuan provinces (9, 10). They are widely used as traditional Tibetan medicines to treat chronic bronchitis and cough, as well as nectariferous medicines (11). From R. thymifolium, 14 chemical components were identified from the essential oil, such as germacrone (20.83%), γ-elemene (11.10%), and selina-3, 7 (11)-diene (6.18%), and their insecticidal activities against Liposcelis bostrychophila or Tribolium castaneum have been proven (9). The essential oils from R. anthopogonoides, R. thymifolium, and R. capitatum have good efficacy in relieving cough, function as expectorants, and inhibit Staphylococcus aureus (12). However, the chemical constituents and the antimicrobial and anti-inflammatory activities of the essential oils from four Rhododendron species have not been studied and compared comprehensively. In this paper, we aimed to identify the components of four kinds of Rhododendron essential oils, clarify and compare their biological activities, and to determine whether the main components are related to biological activity. This study will lay the foundation for the future development of the essential oils from four Rhododendron species in pharmacy.
The fresh leaves of R. przewalskii (5 kg), R. anthopogonoides (5 kg), R. thymifolium (5 kg) and R. capitatum (5 kg) were collected from the northern slope of a mountain near the Zhuaxixiulong region of Tianzhu County, Gansu Province, China (37°11.4′ N latitude, 102°46.1′ E longitude, 2922 m), in June 2020. The species were identified by Prof. Chaoying Luo from Lanzhou Institute of Husbandry and Pharmaceutical Sciences. All these voucher specimens were deposited at the Herbarium with the No. ZSY422-425. The collected leaves were shade-dried for the following tests.
Essential oils of four species were separated by the Spe-ed SFE-2 supercritical carbon dioxide extraction technique (Applied Separations, USA) for 2 h at following conditions: CO2 pressure: 350Bar, cooling circulation system: 5°C, oven temperature: 40°C, valve temperature: 80°C, vessel temperature: 40°C, and the oils were obtained and kept in sealed glass vials (13). The sample (50 mg) with 10 μL of 2-octanol (10 mg/L stock in dH2O) as an internal standard was placed into a 20-mL bottle for analysis in a gas chromatograph system coupled with a spectrometer (GC-MS).
In the solid-phase microextraction (SPME) cycle of the PAL rail system, the incubation temperature was 60 °C, the preheating time was 15 min, the incubation time was 30 min, and the desorption time was 4 min. GC–MS analysis was performed using an Agilent 7890 gas chromatograph system coupled with a 5977B mass spectrometer. The system utilized a DB-Wax (30 m × 250 μm × 0.25 μm) injected in Split Mode (50:1), helium was used as the carrier gas, the front inlet purge flow was 3 mL/min, and the gas flow rate through the column was 1 mL/min. The temperature of the injector was 250°C.The initial temperature was 40 °C for 4 min, raised to 245 °C at a rate of 5 °C/min, and maintained for 5 min. The injection, transfer line, ion source and quad temperatures were 250, 250, 230 and 150 °C, respectively. The energy was−70eV in electron impact mode. The mass spectrometry data were acquired in scan mode with an m/z range of 20-400 and a solvent delay of 0 min. Chroma TOF 4.3X software, produced by LECO Corporation, and the NIST database (version is 2.4, built in March 25 2020) were used for raw peak extraction, database filtering and calibration of the baseline, peak alignment, deconvolution analysis, peak identification, integration and spectrum matching of the peak area.
The antibacterial activities of the four essential oils were determined against three gram-negative bacteria, Salmonella (ATCC 14028), Escherichia coli (ATCC 25922), and Pseudomonas aeruginosa (ATCC 21625), and three gram-positive bacteria, Staphylococcus aureus (ATCC 25923) and Listeria monocytogenes (ATCC 21529), which were purchased from ATCC (U.S.A.), and Enterococcus faecalis (BNCC 102668), which was purchased from BeNa Culture Collection (BNCC, China). These bacterial strains were grown for 24 h in Mueller-Hinton broth (MHB) at 37 °C. The minimum inhibitory (MIC) values of the four essential oils against the above human pathogenic bacteria were investigated. Ampicillin sodium and Streptomycin sulfate were used as references (Solarbio Science & Technology Co., China). All tests were performed three times (14).
Psoroptes cuniculi were collected from the external auditory canals of rabbits naturally infected with mites, which were provided by a rabbit farm in Yuzhong of Lanzhou City, China. The rabbits were treated immediately after collecting all mites. Acaricidal activity was determined according to a previously described method (15). Specifically, 200 μL of four essential oils diluted in 10% DMSO were added to plates, and the excess liquid was absorbed. 10% DMSO was applied to the untreated group. Then, 20 adult mites were placed in each plate, all of which were kept at 25 °C and 75% relative humidity for 24 h. The number of dead mites was observed under a microscope. Each sample was repeated three times.
BALB/c mice (18-22 g) were obtained from the experimental animal center of Lanzhou Veterinary Research Institute, CAAS. Diets and water were provided ad libitum, with light for 12 h per 24 h. All animal tests were performed according to the guidelines of the Ethics Committee of Medical Sciences.
Mice were randomly divided into 6 groups: a model group (normal saline), four essential oil groups (200 mg/kg) and a positive control group administered dexamethasone (DEX, 2 mg/kg). Each group contained 6 mice. Before the experiment, each group was given the corresponding essential oils by gavage, and 1 h later, 25 μL of 2% carrageenan was injected into the right hind paw. The thickness levels of the left and right soles of mice were accurately measured with Vernier calipers after 5 h, and the percentage of inhibition was calculated (16).
Before the test, four essential oils were given to each group by gavage. After 1 h, the left ears of the mice were evenly coated with 30 μL of xylene. Two hours later, mice were sacrificed, both ears were cut off along the root, and patches were obtained at the same parts of the left and right auricles with a 7-mm-diameter punch. All patches were weighed accurately, and the edema inhibitory rate was calculated (16).
The primary mouse macrophage RAW 264.7 cell line was obtained from Prof. Zhang's lab., Lanzhou Institute of Husbandry and Pharmaceutical Sciences, CAAS (Lanzhou, China). RAW264.7 cells (500 μL) in the logarithmic growth stage were inoculated on 24-well plates at a concentration of 1 × 105/mL. The medium was removed after culture at 37 °C for 24 h, and then equal amounts of medium containing four essential oils (compounds) at different concentrations were added. One hour later, 5 μL of LPS (0.5 mg/mL) was added to each well except the blank control group, and the cells were cultured for another 24 h to induce inflammation. DEX (5 μg/mL) was used as a positive control. Finally, the cells were collected to measure the levels of nitric oxide (NO), interleukin-6 (IL-6) and superoxide dismutase (SOD) using enzyme-linked immunosorbent assay kits from Nanjingjiancheng Bio (NJJCBIO, China), and the absorbance was measured using a Multiskan Go Microplate Spectrophotometer (Thermo Scientific., U.S.A).
The cytotoxicities of the four essential oils against RAW 264.7 cells were evaluated using the Cell Counting Kit-8 (CCK-8, ZETA Life, U.S.A.). RAW 264.7 cells (100 μL) at a density of 1 × 105 cells/well were incubated in 96-well plates for 24 h, 10 μL of the four essential oils (250-15.63 μg/mL) or their main compounds were added and incubated for 24 h, respectively, and DMSO (0.1%) was used as a control. Then, CCK-8 agent (10 μL) was added for 30 min, and the absorbance was measured at 450 nm. Each sample was repeated three times, and cell viability was calculated.
The up-and-down method for acute toxicity testing was performed. The dose was increased from 2000 to 5000 mg/kg through the oral route of administration. The animals were observed continuously for behavioral changes for the first 4 h and then for mortality, if any presented, 24 h after the drug administration.
In this paper, the essential oils of four Rhododendron species were obtained using CO2-SFE. R. thymifolium (RTEO) contained the highest yield of 0.99% (w/w), followed by R. capitatum (RCEO, 0.81%), R. anthopogonoides (RAEO, 0.57%) and R. przewalskii (RPEO, 0.30%), as shown in Figure 1A.
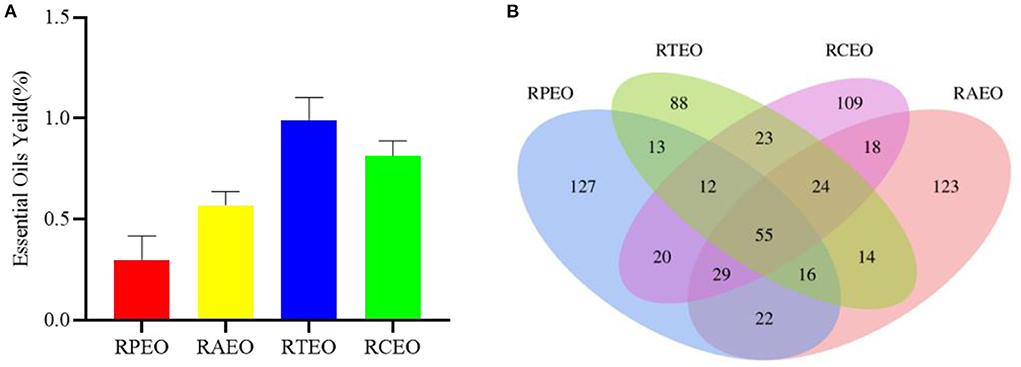
Figure 1. The yields of four essential oils (A) and venn picture of common compositions of four essential oils (B).
In this paper, HS-SPME-GC-MS analysis was performed to enrich and then analyze the components of the essential oils in four Rhododendron species, and total ion flow diagrams were obtained, as shown in Supplementary Figure S1.
After screening the components based on spectral similarity values > 700, 302 chemicals were identified from RAEO, and the main compounds were α-pinene (8.5% of the oil), followed by humulene (5.9%), β-pinene (4.1%), o-cymene (3.5%) and myrtenol (3.1%). For RPEO, 294 components were identified, and the main components were phenylethyl alcohol (12.0%), 4-hydroxy-3-methylacetophenone (5.6%), benzyl alcohol (4.1%), calarene (3.0%), and 4-phenyl-2-butanol (2.7%). RTEO has appreciable contents of cyclohexanone, 5-ethenyl-5-methyl-4-(1-methylethenyl)-2-(1-methylethylidene)-(11.0%), β-farnesene (5.0%), γ-cadinene (4.8%), selina-3,7(11)-diene (3.9%), bisabolone (3.8%), as well as other 241 chemicals; finally, 290 compounds from RCEO were found, and the major constituents were benzylacetone (11.3%), benzene, 1-ethenyl-4-ethyl- (7.9%), γ-muurolene (7.1%), and α-selinene (4.5%) (Supplementary Table S1). The top 30 compounds were list in Table 1.
Fifty-five common compounds were identified from these four essential oils, such as phenylethyl alcohol, acetic acid, hexanoic acid, 2-butanone, 4-phenyl-, linalool, and benzaldehyde (Figure 1B, Supplementary Table S2). However, although these species were collected from the same altitude and environment, more compounds were different, which was dependent on the different biosynthetic pathways and inheritances. Different chemicals may result in different activities and uses.
This study showed that among the three gram-positive bacteria, they presented an inhibitory effect on S. aureus, and the MIC values were 0.26 mg/mL for RAEO and 1.0 mg/mL for RCEO and RTEO. The activity of RPEO was weak (MIC 4.1 mg/mL). However, four essential oils showed antibacterial activity against P. aeruginosa among only three gram-negative bacteria, especially RAEO, with its MIC value of 0.06 mg/mL (Table 2).
To investigate the potential acaricidal activity of Rhododendron sp., the toxicity of the four essential oils against Psoroptes cuniculi was studied. As shown in Table 3, among the four essential oils, the acaricidal activity of the RAEO stands out, and the LC50 at 24 h was 1.59 mg/mL, followed by RPEO with an LC50 of 1.71 mg/mL. The LC50 of RTEO was 2.35 mg/mL, and RCEO had the poorest effect, with an LC50 of 4.10 mg/mL.
As shown in Figures 2A,B compared with the model group, the essential oils (200 mg/kg) significantly reduced the paw edema of mice caused by carrageenan. Among them, RAEO and RPEO had the best effects, and their inhibition rates on foot edema were 60.47 and 58.59%, respectively, with no significant difference compared with the positive control drug dexamethasone, with an inhibition rate of 57.65%. The effects of RTEO and RCEO were obviously weaker than that of DEX (P < 0.05).
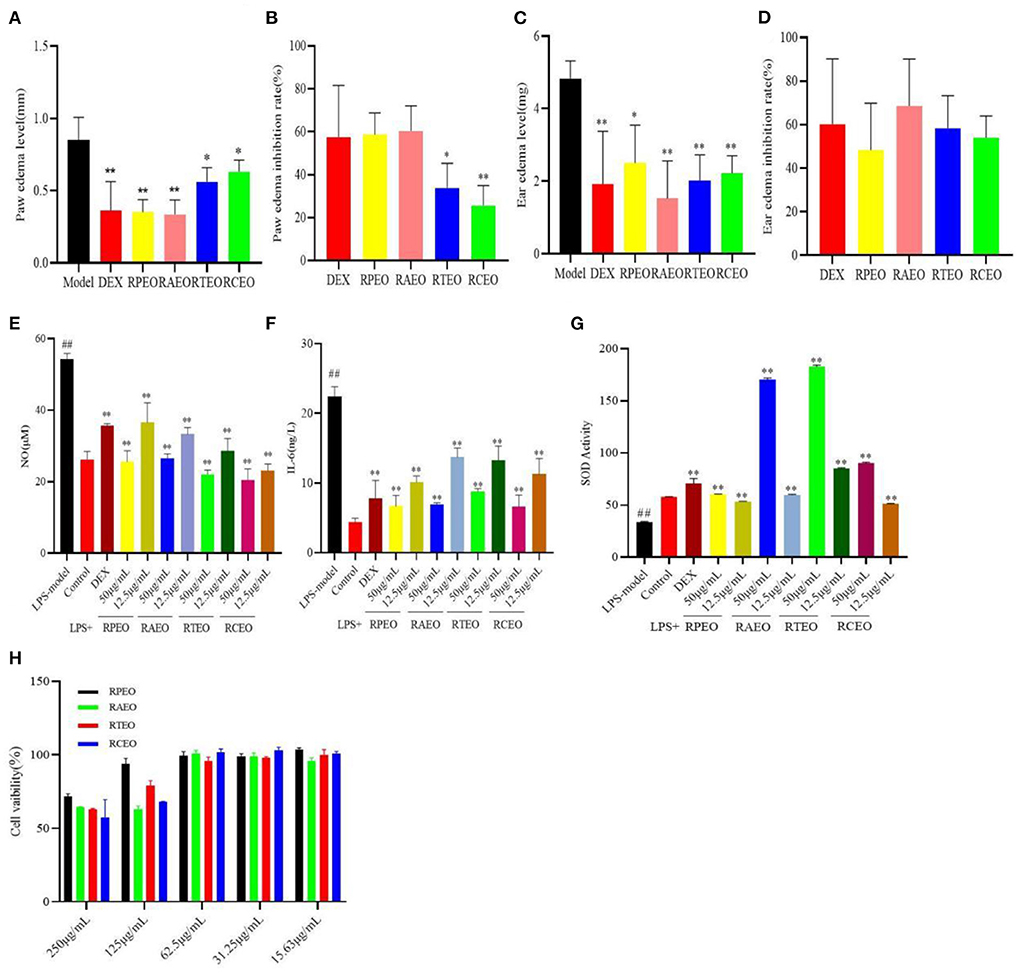
Figure 2. The paw edema level (A) and its inhibition level (B) of carrageenan-induced paw edema in mice test, and the ear edema level (C) and its inhibition level (D) of xylene induced ear edema test, the inhibitory effect of these compounds on NO production (E), IL-6 production (F) and SOD activity (G) in RAW264.7 cells induced by LPS, (H) the cell viability of four Rhododendrons species essential oils against RAW264.7 cells. (##represent the significant difference between control group and model group, p<0.01; * p<0.05 represent the significant difference between model group and drug-treated group; ** for p<0.01).
Subsequently, a xylene-induced ear edema test was used to evaluate the vascular permeability of essential oils, which was partially associated with substance P. The results showed that compared with the model group, the four essential oils notably reduced the mouse ear edema caused by xylene. The effect of RAEO was the best, with an inhibition of 68.55%, which was higher than that of 60.17% dexamethasone (Figures 2C,D). There was no significant difference in the inhibitory effects among the groups.
The results showed that the level of NO secreted in the model group was significantly higher than that in the control group (P < 0.01); however, after essential oil treatment, the NO secretion level decreased markedly in a concentration- dependent manner in the model group (P < 0.01). The activities of the essential oils were as follows: RCEO> RTEO> RAEO>RPEO at the concentration of 12.5 μg/mL (Figure 2E). Essential oils can significantly reduce the secretion level of IL-6 in RAW264.7 cells induced by LPS, RPEO has the strongest inhibitory effect (Figure 2F). The level of SOD in the cells of the four essential oils increased significantly, and RAEO (50 μg/mL) and RTEO (50 μg/mL) performed better (Figure 2G).
In the acute toxicity in mice, after oral administration of four Rhododendron species essential oils (2000-5000 mg/kg), no mice died or exhibited any acute behavior. The LD50 was calculated to be more than 5000 mg/kg. In addition, From Figure 2H, we can see that for essential oils presented the weak cytotoxicity against RAW 264.7 cells. At the concentration of 250 μg/mL, the cell viability was more than 50.00 %. This result suggested that the four essential oils were safe at the given doses or concentrations (Figure 2H).
To find the active compounds of four essential oils, the toxicity and anti-inflammatory activity of seven compounds were studied. In these, phenylethyl alcohol (PA), and 4-hydroxy-3-methylacetophenone (HA) from RPEO, α-pinene (α-PI) and β-pinene from (β-PI) RAEO, β-farnesene (FA) and germacrone (GE) from RTEO, and benzylacetone (BE) from RCEO were chose. As shown in Figure 3A, as well as the essential oils, seven compounds also have the weak cytotoxicity, and the cell viability was more than 50 % at the concentration of 250 μg/mL. Subsequently, the NO content in RAW 264.7 cells was determined to indirectly explain the anti-inflammatory of compounds. As shown in Figure 3B, except PA, other compounds presented the significant inhibitory effect on NO production compared with the model group. This result indicated that except PA, those compositions in essential oils played the synergistic effect to contribute their anti-inflammatory activity. However, the active compound of RPEO should be studied further.
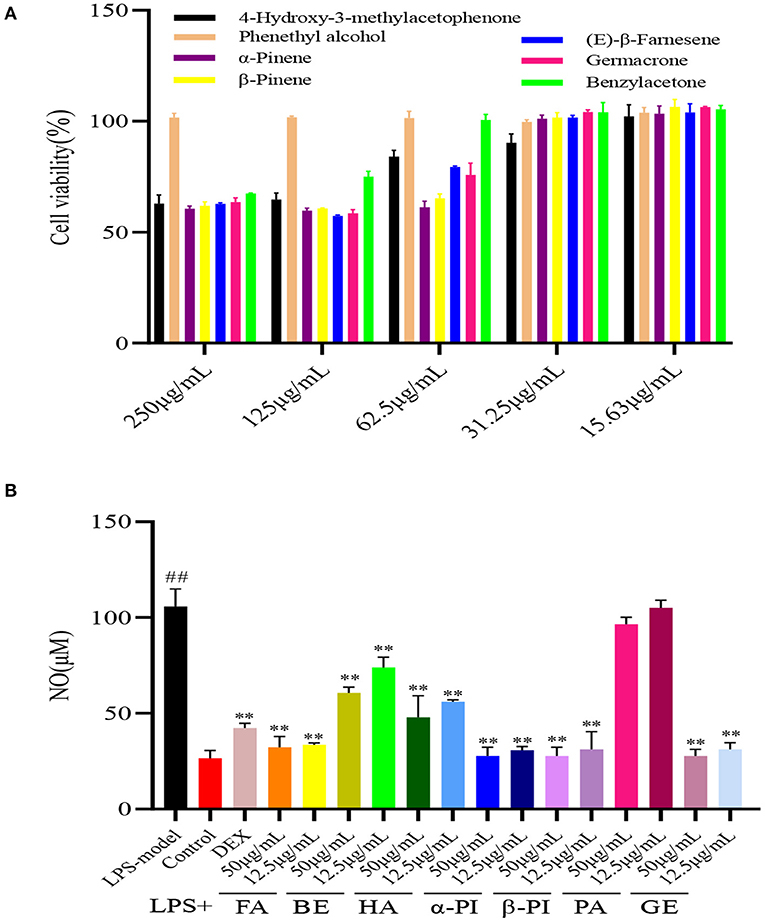
Figure 3. The cell viability of seven components from four Rhododendrons species (A) and the inhibitory effect of these compounds on NO production in RAW264.7 cells induced by LPS (B). (phenylethyl alcohol (PA), and 4-hydroxy-3-methylacetophenone (HA) from RPEO(essential of Rhododendrron przewalskii Maxim.), α-pinene (α-PI) and β-pinene (β-PI) from RAEO(essential oil of Rhododendron anthopogonoides Maxim.), β-farnesene (FA) and germacrone (GE) from RTEO(essential oil of Rhododendron thymifolium Maxim.), and benzylacetone (BE) from RCEO (essential oil of Rhododendron capitatum Maxim.) (##represent the significant difference between control group and model group, p < 0.01; ** for p < 0.01).
Currently, high-quality CO2-SFE is widely used to extract essential oils from medicinal plants. Compared with traditional hydrodistillation, this technique could improve the yield of essential oils and reduce the cost, avoid the danger of flammability and explosion during traditional solvent extraction, and maintain some thermolability components (17). Therefore, SFE using CO2 is an appropriate choice to manufacture essential oil products considering the lipophilic characteristics of essential oils (18). The essential oils components from the same plant depend on multiple factors, such as climate, soil condition, geographical location, collection time and extraction methods, as well as analytical procedures, which have been demonstrated by compelling evidence (19). Headspace solid-phase microextraction (HS-SPME) technology with simple sample pretreatment process, high sensitivity and relatively non-invasive nature, HS-SPME has an enrichment effect on substances (20, 21), and many more compounds have been identified, which may also be one of the reasons why it is different from previous studies. Hence, more compounds were identified from these plants in our study than in previous studies (9). These results were not exactly same from those reported in the published literature, for instance, essential oil collected from R.thymifolium mainly contained β-elemenone (35.2%),germacrone (20.8%), γ-elemene (11.1%), another report recorded germacrone (16.0%),myrcene (10.0%) (9), as the main components in R.thymifolium which from Qinghai Province in October (22). The main compounds of the essential oil from R. anthopogonoides were benzyl acetone (34.4%), nerolidol (10.2%), 1, 4-cineole (8.4%), β-caryophyllene (5.6%), γ-elemene (5.1%), and spathulenol (3.1%) (23). A report about essential oils from 43 species of Rhododendron showed that germacrone was the major component in five of the oils, α-Pinene was the most prominent compound in eight of the oils including R.capitatum (24). A total of 14 compounds were identified, and the main components of EO from R. anthopogonoides were 4-phenyl-2-butanone (47.7%), eudesma-3, 7(11)-diene (14.5%), curzerene (9.5%), 4-phenylbutan-2-ylacetate (6.4%), β-cadinene (5.8%), and germacrone (5.7%) (25). There were many similarities among the four species of Rhododendron EOs, but the components with the highest contents were not the same. The major components in R. capitatum and R. przewalskii EOs were cedrene (22.2%) and germacrene D (27.6%) that belonged to the class of sesquiterpenoids (26).
A previous study reported that the essential oils of R. thymifolium, R. capitatum and R. anthopogonoides isolated using the steam distillation method have strong antibacterial activity against S. aureus but have no activity against E. coli, P. aeruginosa or C. albicans (9). P. aeruginosa has high internal resistance to almost all known antibiotics and antimicrobials, even synthetic drugs, owing to a very restrictive outer membrane barrier, which has been a serious problem worldwide (27). Interestingly, different from previous studies, four essential oils showed inhibitory effect on P. aeruginosa, which indicated that RAEO has the potential to become an alternative antibiotic agent for preventing P. aeruginosa infection.
Animal acariasis can reduce the production and quality of animal products and is often fatal. Our previous study showed that R. nivale oil had in vitro acaricidal activity against adult P. cuniculi in a concentration- and time-dependent manner. As a main and active compound, γ-cadinene presented marked acaricidal activity against Psoroptes cuniculi (10). Consistent with the previous studies of other Rhododendron, the essential oils of this four have certain acaricidal activity though not very prominent.
More than 50% of medicinal rhododendron species used as folk medicines have been used to treat inflammation-related ailments such as arthritis, rheumatoid diseases, and bronchitis (5). In this study, we studied and compared the anti-inflammatory activities of the four essential oils. First, carrageenan-induced mouse paw edema was used to evaluate the inhibitory activity, which is a biphasic event, and early phase hyperemia was related to the release of histamine, serotonin and similar substances. The later phase was associated with the activation of kinin-like substances (28). Inflammation is an important part of immunopathogenesis. RAW264.7 cells, activated by LPS, release a variety of inflammatory factors, including NO and IL-6. NO is essential for host innate immune responses to pathogens, and excessive NO brings about the development of inflammatory diseases. Therefore, inhibiting the production of NO is the primary target of anti-inflammatory drug development (29). IL-6 is a pleiotropic cytokine, the role of which in the acute inflammatory response is mainly manifested in its pro-inflammatory effect on a variety of cells (30). In addition, SOD plays a protective role in various inflammatory diseases, and treatment with SOD mimetics reduces proinflammatory cytokine production (31). The results of anti-inflammatory indicated that the four essential oils presented the anti-inflammatory activity via decreasing the content of NO and IL-6, and activating SOD activity.
Meanwhile, the toxicity and anti-inflammatory activity of main compounds indicated that except PA, those compositions in essential oils played the synergistic effect to contribute their anti-inflammatory activity. However, the active compound of RPEO should be studied further.
In summary, the supercritical extraction combined HS-SPME-GC-MS analysis could be used to find more compositions from essential oils. The essential oils of four Rhododendrons species have the anti-inflammatory and acaricidal activities, and also presented the antibacterial activity against S. aureus and P. aeruginosa. The mechanism of action of these essential oils against inflammatory should be studied further. These results indicated that four essential oils have certain medicinal value and can be developed as raw materials for the pharmaceutical and perfume industries, and play a guiding role in the development and utilization of the plant.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Ethics Committee of Medical Sciences, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, Chinese Academy of Agricultural Sciences.
XS: conceptualization and writing-original draft. LD, JH, and XY: investigation. JH: soft and validation and data analysis. BL and XY: methodology. JZ and YW: supervision and project administration. All authors contributed to the article and approved the submitted version.
This work was financed by the National Natural Science Foundation of China (31772790), and the Innovation Project of the Chinese Academy of Agricultural Sciences (No. CAAS-ASTIP-2015-LIHPS).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.882060/full#supplementary-material
Supplementary Table S1. The identified compounds of the essential oils from four Rhododendron species.
Supplementary Table S2. The common compounds of the essential oils from four Rhododendron species.
Supplementary Figure S1. Total ion chromatogram of essential oils from R. anthopogonoides, R. capitatum, R. przewalskii, and R. thymifolium.
1. Costa WK, Oliveira de Oliveira JRS, da Silva Santos AM, da Cunha IB, de Freitas RX, de Menezes Lima VL, et al. Essential oil from Eugenia stipitata McVaugh leaves has antinociceptive, anti-inflammatory and antipyretic activities without showing toxicity in mice. Ind Crop Prod. (2020) 144:112059. doi: 10.1016/j.indcrop.2019.112059
2. Alves-Silva JM, Guerra I, Gonçalves MJ, Cavaleiro C, Cruz MT, Figueirinha A, et al. Chemical composition of Crithmum maritimum L. essential oil and hydrodistillation residual water by GC-MS and HPLC-DAD-MS/MS, and their biological activities. Ind Crops Prod. (2020) 149:112329. doi: 10.1016/j.indcrop.2020.112329
3. Chen Y, Luo J, Zhang N, Yu W, Jiang J, Dai G, et al. Insecticidal activities of Salvia hispanica L. essential oil and combinations of their main compounds against the beet armyworm Spodoptera exigua. Ind Crops Prod. (2021) 162:113271. doi: 10.1016/j.indcrop.2021.113271
4. Al-Ansari MM, Andeejani AMI, Alnahmi E, AlMalki RH, Masood A, Vijayaraghavan P. Insecticidal, antimicrobial and antioxidant activities of essential oil from Lavandula latifolia L and its deterrent effects on Euphoria leucographa. Ind Crops Prod. (2021) 170:113740. doi: 10.1016/j.indcrop.2021.113740
5. Popescu R, Kopp B. The genus Rhododendron: an ethnopharmacological and toxicological review. J Ethnopharmacol. (2013) 147:42–62. doi: 10.1016/j.jep.02,022.
6. Dai L, He J, Miao X, Guo X, Shang X, Wang W, et al. Multiple biological activities of Rhododendron przewalskii Maxim. extracts and UPLC-ESI-Q-TOF/MS characterization of their phytochemical composition. Front Pharmacol. (2021) 12:599778. doi: 10.3389/fphar.2021.599778
7. Jesionek A, Kokotkiewicz A, Mikosik-Roczynsk A, Ciesielska-Figlon K, Luczkiewicz P, Bucinski A, et al. Chemical variability of Rhododendron tomentosum (Ledum palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia. (2019) 139:104402. doi: 10.1016/j.fitote.2019.104402
8. Qiang Y, Zhou B, Gao K. Chemical constituents of plants from the genus Rhododendron. Chem Biodiversit. (2011) 8:792–815. doi: 10.1002/cbdv.201000046
9. Liang J, You C, Guo S, Zhang W, Li Y, Geng Z, et al. Chemical constituents of the essential oil extracted from Rhododendron thymifolium and their insecticidal activities against Liposcelis bostrychophila or Tribolium castaneum. Ind Crops Prod. (2016). 79:267–73. doi: 10.1016/j.indcrop.11,002.
10. Guo X, Shang X, Li B, Zhou XZ, Wen H, Zhang J, et al. Acaricidal activities of the essential oil from Rhododendron nivale Hook. f. and its main compund, δ-cadinene against Psoroptes cuniculi. Vet Parasitol. (2017) 236:51–54. doi: 10.1016/j.vetpar.01,028.
11. Delectis Florae Reipublicae Popularis Sinicae Agendae Academiae Sinicae Edita. Flora Reipublicae Popularis Sinicae. Beijing: Science Press. p. 57.
12. Liang J, Yang Q, Ma XM, Du XY, Zhang J. The content and antibacterial activity of volatile oil from three species of Rhododendron in Gansu Province. Chin Wild Plant Res. (2014) 33:9–10. doi: 10.3969/j.issn.1006-9690
13. Shao Q, Deng Y, Liu H, Zhang A, Huang Y, Xu G, et al. Oils extraction from Anoectochilus roxburghii using supercritical carbon dioxide and their antioxidant activity. Ind Crops Prod. (2014) 60:104–12. doi: 10.1016/j.indcrop.06,009.
14. Dai L, Li JC, Miao XL, Guo X, Shang XF, Wang WW, et al. Ultrasound-assisted extraction of five anthraquinones from Rheum palmatum water extract residues and the antimicrobial activities. Ind Crop Prod. (2021) 162:223288. doi: 10.1016/j.indcrop.2021.113288
15. Shang X, Miao X, Dai L, Wang Y, Li B, Pan H, et al. Acaricidal activity of strophanthidin derivatives against Psoroptes cuniculi and their inhibitory effect on Na+-K+-ATPase. Vet Parasitol. (2021) 296:109498. doi: 10.1016/j.vetpar.2021.109498
16. Shang X, Wang J, Li M, Miao X, Pan H, Yang Y, et al. Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia. (2011) 82:716–21. doi: 10.1016/j.fitote.03,001.
17. Fornari T, Vicente G, Vázquez E, García-Risco MR. Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J Chromatogr A. (2012) 1250:34–48. doi: 10.1016/j.chroma.04,051.
18. Anitescu G, Doneanu C, Radulescu V. Isolation of coriander oil: comparison between steam distillation and supercritical CO2 extraction. Flav Frag J. (1997) 12:173–76. doi: 10.1002/(SICI)1099-1026(199705)12:3<173::AID-FFJ630>3.0.CO;2-1
19. Zhang Q, Zhou L, Chen H, Wang CZ, Xia Z, Yuan CS, et al. Solid-phase microextraction technology for in vitro and in vivo metabolite analysis. TRAC. (2016) 80:57–65. doi: 10.1016/j.trac.02,017
20. Aziz ZAA, Ahmad A, Setapar SHM, Karakucuk A, Azim MM, Lokhat D, et al. Essential oils: extraction techniques, pharmaceutical and therapeutic potential-a review. Curr Drug Metab. (2018) 19:1100–10. doi: 10.2174/1389200219666180723144850
21. Baky MH, Farag MA, Rasheed DM. Metabolome-based analysis of herbal cough preparations via headspace solid-phase microextraction GC/MS and multivariate data analyses: a prospect for its essential oil equivalency. ACS omega. (2020) 5:31370–80. doi: 10.1021/acsomega.0c04923
22. Northwest Northwest Institute of Plateau Biology Chinese Chinese Academy of Sciences. The Chemical constituents of the essential oil of rhododendron thymifolium maxim. J Integr Plant Biol. (1978) 20:135–139.
23. Bai PH, Bai CZ, Liu QZ, Du SS, Liu ZL. Nematicidal activity of the essential oil of rhododendron anthopogonoides aerial parts and its constituent compounds against meloidogyne incognita. Z Naturforsch C. (2013) 68:307–12. doi: 10.5560./ZNC.2013.68c0307
24. Doss RP, Hatheway WH, Hrutfiord BF. Composition of essential oils of some lipidote rhododendrons. Phytochemistry. (1986) 25:1637–40. doi: 10.1016/S0031-9422(00)81225-X
25. Liang JY, Hou ZB, Wu HS, An Y, Zhang J, Wang JL, et al. Hou. QZChemical constituents of essential oil extracted from Rhododendron anthopogonoides and its bioactivities against Tribolium castaneum and Ditylenchus destructor. Biochem Syst Ecol. (2022) 103:104431. doi: 10.1016/j.bse.2022.104431
26. Bai L, Jiao ML, Zang HY, Guo SS, Wang Y, Sang YL, et al. Du. SSChemical composition of essential oils from four Rhododendron species and their repellent activity against three stored-product insects. Environ Sci Pollut R. (2019) 26:23198–205. doi: 10.1007/s11356-019-05577-1
27. Hayakawa S, Kawamura M, Sato T, Hirano T, Fujimura S. An α-lipoic acid derivative, and anti-ros agent, prevents the acquisition of multi-drug resistance in clinical isolates of Pseudomonas aeruginosa. J Infect. (2018) 25:28–33. doi: 10.1016/j.jiac.10,003.
28. Li M, Shang X, Zhang R, Jia Z, Fan P, Ying Q, et al. Antinociceptive and anti-inflammatory activities of iridoid glycosides extract of Lamiophlomis rotata (Benth.) Kudo. Fitoterapia. (2010) 81:167–72. doi: 10.1016/j.fitote.08,018.
29. Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, et al. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated raw 264.7 macrophages. J. Ethnopharmacol. (2009) 125:286–290. doi: 10.1016/j.jep.06,027.
30. Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (il-6) as an anti-inflammatory cytokine: induction of circulating il-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. (1994) 83:113–8. doi: 10.1182/blood.V83.1.113.bloodjournal831113
Keywords: Rhododendron species, essential oils, anti-inflammatory activity, acaricidal activity, α-pinene
Citation: He J, Shang X, Dai L, Yang X, Li B, Wei Y, Zhang J and Pan H (2022) Chemical constituents, antibacterial, acaricidal and anti-inflammatory activities of the essential oils from four Rhododendron species. Front. Vet. Sci. 9:882060. doi: 10.3389/fvets.2022.882060
Received: 23 February 2022; Accepted: 12 July 2022;
Published: 10 August 2022.
Edited by:
Nora Mestorino, National University of La Plata, ArgentinaReviewed by:
Douglas Siqueira Chaves, Universidade Federal Rural do Rio de Janeiro, BrazilCopyright © 2022 He, Shang, Dai, Yang, Li, Wei, Zhang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanming Wei, d2VpeW1AZ3NhdS5lZHUuY24=; Jiyu Zhang, emhhbmdqaXl1QGNhYXMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.