
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 23 May 2022
Sec. Comparative and Clinical Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.875629
This article is part of the Research TopicRising Stars in Comparative and Clinical Medicine: 2021View all 21 articles
 Muhammad Fakhar-e-Alam Kulyar1,2,3
Muhammad Fakhar-e-Alam Kulyar1,2,3 Khurram Ashfaq3
Khurram Ashfaq3 Amjad Islam Aqib4
Amjad Islam Aqib4 Kun Duan5
Kun Duan5 Muhammad Asif6
Muhammad Asif6 Zeeshan Ahmad Bhutta7
Zeeshan Ahmad Bhutta7 Muhammad Shoaib8
Muhammad Shoaib8 Samina Shabbir9,10
Samina Shabbir9,10 Shah Nawaz11
Shah Nawaz11 Muhammad Aamir Naseer3
Muhammad Aamir Naseer3 Iqra Sarwar3
Iqra Sarwar3 Muhammad Akhtar2
Muhammad Akhtar2 Ayesha Safdar Chaudhry6
Ayesha Safdar Chaudhry6 Riaz Hussain12
Riaz Hussain12 Hafiz Iftikhar Hussain13
Hafiz Iftikhar Hussain13 Yi Wu1*
Yi Wu1* Kun Li1*
Kun Li1*Burns cause many significant changes in metabolism and inflammatory reactions, leading to poor regeneration in animals and humans. A list of medicines to treat burns is available in the market. But due to the high cost of these medicines, these are unaffordable, especially for farmers of middle-class families of Africa and Asia. Therefore, a low-cost complementary treatment has always been a topic of many researchers, and there is a dire need of time for the welfare of animals to save them. The current study was planned to scrutinize the therapeutic effects of Manuka honey and Nitrofurazone ointments on full-thickness burn wounds in the rabbit model. The healing efficacy was performed through wound contraction rate, hematological analysis, the thickness of dermis and epidermis, and collagen content percentage. Histopathology was performed after taking biopsy samples at the end of the research. Based on statistical analysis using wound healing time (days, D), the combination (MO + NT) resulted in a shorter period (27 D ± 1) than the average healing time of controlled (36 ± 2), Manuka ointment (31.33 D ± 1.52), and Nitrofurazone ointment (32 ± 1). A significant decrease in the count of red blood cell (RBC), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) in all treatments was noticed mainly in MO + NT. Furthermore, burns induced a significant difference (p < 0.05) in the white blood cells (WBCs) count levels in the MO-treated group. While the level of platelets (PLTs) was not significantly different from the healthy control group. Histopathological assessment (epithelialization, fibrosis, and angiogenesis) of skin showed burn healing to be better in MO and MO + NT groups. In conclusion, the composite of Manuka honey with Nitrofurazone led to the faster recovery than other treatments.
Skin is a coated barrier that protects internal organs from harsh environments. This barrier is essential for maintaining the homeostasis of body fluids, controlling temperature, and protecting the body from infection. The skin also has immunological, nervous, and metabolic functions (1, 2), devastating issues that affect such a barring ability (3). According to a study, wounds account for 30% of the total emergency caseload in veterinary healthcare centers (4). Animals (especially pets) with severe burns need immediate access to professional facilities. Such modern resuscitation and emergency burn therapies are designed to mitigate systemic changes caused by the sudden destruction of skin barriers, but unfortunately, these facilities are not available in the developing countries of Asia and Africa. The majority of burns is unintentional that occurred at veterinary clinics, relating to the use of adjunct heat to reduce the risk of developing hypothermia. Veterinarians often encounter burns that are <20% of the animal's body surface. Thus, severe metabolic disturbances, such as electrolyte levels imbalances, red blood cells destruction, and high susceptibility toward systemic infection, might not be seen (5). But the use of such animals as a model is still a viable option in overall healthcare in translational medical studies (6). A list of medicines to treat burns, i.e., Nitrofurazone and silver sulfadiazine, is available in the markets. But due to the high cost of these medicines, most people cannot afford them. Hence, more traditional herbal products have been used in African and Asian middle-class families (7). According to the WHO statistics, 265,000 deaths annually occur due to burning, most of which happen in these regions (8). The herbal products used by these people seem moderately effective, less toxic, and less expensive than other market products. Honey has been used in folk medicine for a long time, but it has only recently been discovered by medical researchers that it can be used to treat both acute and long-term wounds.
Traditionally, honey has been used to treat burns (9) due to its antimicrobial properties, especially to cover up burn wounds. It has been shown to inhibit a wide spectrum of bacteria and is as efficient against antibacterial drugs resistant bacteria (10–12). Interestingly, one of the earliest experiments of honey's efficiency against antibiotic-resistant was conducted, using Manuka honey to treat a hydroxyurea-induced leg ulcer colonized by methicillin-resistant Staphylococcus aureus (MRSA). This research established that honey has antibacterial capabilities against resistant bacteria and may also aid in the healing process of wounds (13). Such antibacterial action of honey is due to its acidity, hydrogen peroxide contents, osmotic effects, nutritional and antioxidant contents, stimulation of immunity, and unidentified compounds (14). Generally, honey has glucose, maltose, sucrose, fructose, water, and a few other things, such as amino acids, organic acids, proteins, vitamins, acetylcholine, and flavonoids (15). Many scientific studies have been urged on its medicinal properties in Ayurvedic, Chinese and Roman traditions (14). Manuka honey is the purest form of unifloral honey that is derived from Manuka trees and Leptospermum scoparium, belongs to the family Myrtaceae in New Zealand and the Eastern region of Australia (16). It is dark honey as compared to other honey types. It contains the highest concentration of phenolic and flavonoid compounds (pinobanksin, pinocembrin, and chrysin) that have been identified as having potent reactive oxygen species (ROS) scavenging activity (17). Flavonoids polyphenolic compound is a group of secondary metabolites that naturally occur in the plant kingdom that possesses numerous pharmacological activities (18).
Nitrofurazone (C6H6N4O4) is a commercially available product used as an antimicrobial agent on different wounds, especially burn wounds (19). It has been reported to show bactericidal activity against many gram-positive and gram-negative bacteria, inhibiting bacterial enzymes involved in carbohydrate metabolism. It exhibits bactericidal activity against most pathogens that commonly cause surface skin infections (20). But Nitrofurazone (NT) therapy has a limit on its low aqueous solubility. The poor solubility of NT urges a slow-release rate, causing less penetration of an effective drug concentration into the skin. Moreover, due to its high permeability through the skin, NT remains for a limited time at the applied area (21). Others include sensitivity, itching, contact dermatitis, and longer time period (8).
The current experimental study explored the effects of topical application of Manuka honey ointment on full-thickness burns, in comparison with commercially available Nitrofurazone at the thoracoabdominal regions of rabbits. The evaluation parameters, such as healing time, contraction rate, hematological, and histopathological assessment, were compared with untreated thermally injured rabbits.
The Manuka honey (batch no. GMH4765; lot no. KMH15816) was purchased from an online store, while Nitrofurazone was purchased from a local market. According to our one pilot project, all treatments were prepared according to their minimum effective levels. 1% Manuka honey ointment (MO), 1% Nitrofurazone ointment (NT), and 1% of their combination (MO + NT) were prepared by mixing them with 99 g of petroleum jelly. The quantity of both treatments was calculated on the base of their total percentage and purity. The final form of concerning ointments was then stored in the plastic bottles.
The ethics committee of the University of Agriculture Faisalabad, Pakistan approved this work (DGS/39257-60), in accordance with institutional and national guidelines. The nine rabbits from three different groups (MO, NT, MO + NT) were anesthetized by injectable anesthesia with xylazine (4 mg/kg) and ketamine hydrochloride at a dose rate of 35 mg/kg intramuscular (22). The thoracoabdominal side of the skin was shaved, and burn wounds were created using a metal plate (with a 10-mm diameter) heated at 100°C on an open flame of a Bunsen burner with equal pressure over the shaved area (23). After creating wounds, Ketoprofen (1 mg/kg) was injected as an analgesic to the rabbits (24). The administration of analgesic was performed for the next 3 days in order to attain an equal immune response for each individual.
Fifteen male healthy rabbits (8–10 weeks old) weighing 1,500 g ± 20 g were randomly allocated to five treatment groups. Each treatment group was comprised of three rabbits. The acclimatization period of 3 days was provided to rabbits with the same conditions of environment and feeding in order to attain equal immune response for each individual. Rabbits were divided as follows: NS (negative control) received no treatment, PS (positive control) was allocated to normal saline, whereas MO (Manuka honey ointment), NT (Nitrofurazone ointment), and MO + NT were treated topically twice a day (Figure 1). The healing efficacy was performed through healing time, wound contraction rate, and hematological analysis on days 0, 7, 14, 21, and 28. Histopathology was performed after taking biopsy samples at the end of the research. All rabbits were fed pelleted food with ad libitum water throughout the experiment and were housed in the animal experimentation unit.
In the preliminary test, the wound stopped increasing on the fourth day. Therefore, the observation period was set from days 0 to 4 after the injury (Figure 2A). After topical application of treatments, the wound was carefully covered with a bandage (Figure 2B).
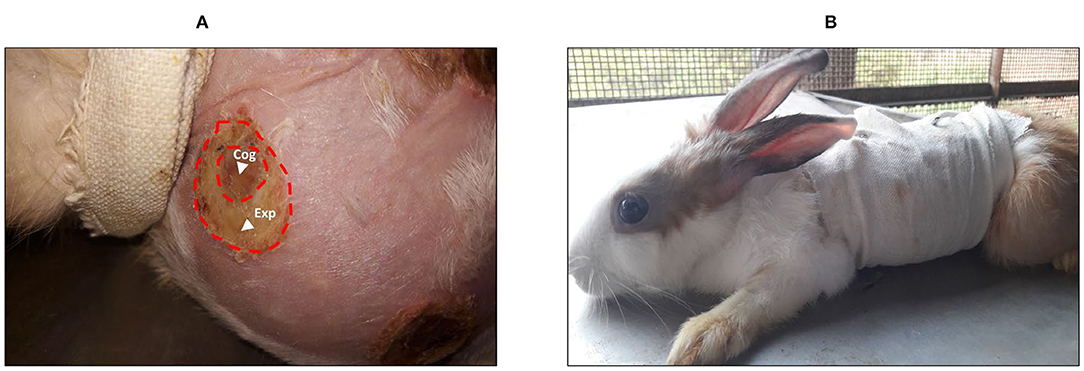
Figure 2. (A) Burn wound with the area of expansion that reveals a white color when compared with the coagulation zone. (B) A rabbit in a bandage after treatment protocol. Cog, coagulation zone; Exp, expansion zone.
Healing time was considered from the time of wound induction to re-epithelialization. All the observations were estimated randomly (7, 14, 21, and 28) until the scar fell off (25).
The wound healing was examined by digital photography every seventh day under general anesthesia (26). While the contraction rate percentage was determined by the following formula (27).
Hematological analyses were accessed (0, 7, 14, 21, and 28) to compare the relative success rate of treatment groups (28).
Histopathological analyses were performed to compare the relative healing rate for different treatments. The parameters (thickness of the epidermis and collagen content) were evaluated during the histopathological examination, in which a tissue sample was collected at the wound site (at the end of the research). The different percentages were measured to indicate the degree of compression and orientation of the collagen fibers. Cells that are part of the immune system were also examined in the tissue that had been treated.
All statistical data analyses were carried out using ANOVA through SPSS statistical computer software (version 25.00). The recovery rates observed for various groups were compared using the independent sample t-test. The statistical significance level was set < 0.05.
According to the t-test analysis, it is inappropriate to compare the values of two or more groups. Therefore, we performed ANOVA and post-hoc tests among four groups. The range of healing of full burns in the combination group was significantly different, and the frequency of treatment was also significantly improved. Rabbits were better in the first 2 weeks after that healing was gradually becoming better among different groups e.g., Manuka honey, Nitrofurazone, and the control group. The MO + NT resulted in a shorter period (27 D ± 1) than the average healing time of controlled (36 ± 2), MO (31.33 D ± 1.52), and NT (32 ± 1) (Figure 4A). Rabbits treated with the combination had significantly more prophylactic effects than untreated rabbits. Significant healing ornamentation (p < 0.05) within 14 days was noticed in two treatment groups (MO + NT and MO). There was also a significant reduction (p < 0.05) of wound size on day 7 in treated animals as compared to control group.
The frequency of wound closure was higher in the groups that were treated with combination in rabbits. On day 14, rabbits of “MO + NT” group showed better granulation tissue formation. After 21 days, “MO + NT” showed remarkable healing and scarring (Figure 3A). The significance levels of “MO + NT” among other treatments, such as Manuka honey and Nitrofurazone, were 0.026 and 0.012, respectively. While the significant level with the control group was 0.00. Similarly, in the individual comparison of Manuka honey ointment with “MO + NT” and Nitrofurazone, the significance levels were 0.026 and 0.94, respectively (Figures 3B,C).
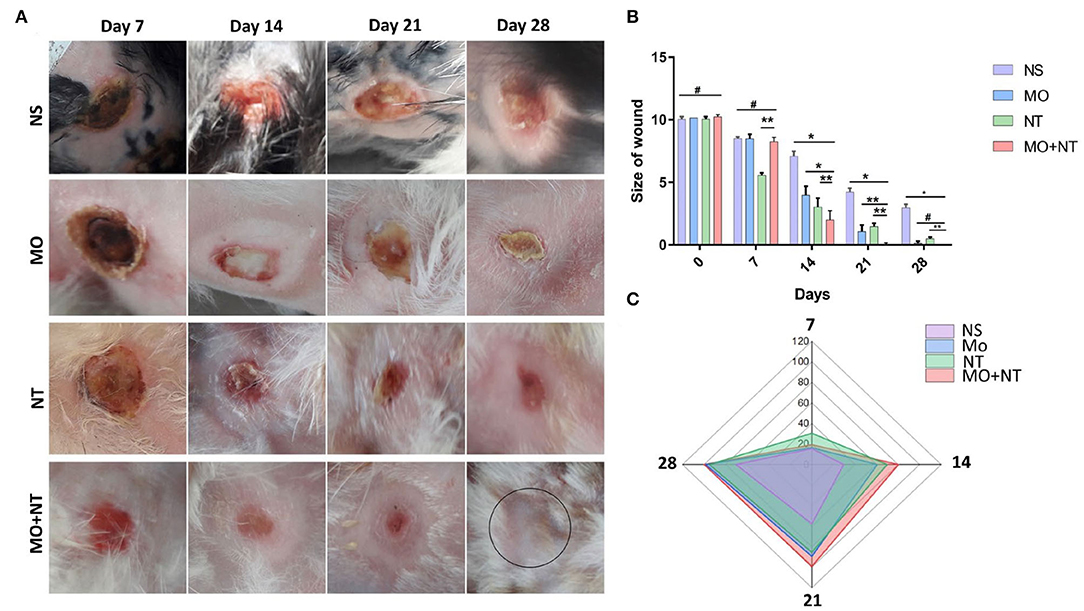
Figure 3. (A) Digital photographs of wounds taken on different days (7, 14, 21, and 28). NS, normal saline; NS, normal saline; MO, Manuka honey ointment; NT, Nitrofurazone ointment; MO + NT, Manuka honey + Nitrofurazone. (B) Size of wounds, (C) contraction rate percentage (*p < 0.05, **p < 0.01, #p > 0.05).
In the initial days, the wound area of all groups was increased. The enlarged area showed a bright white color as compared to the burned area. This area was white and became necrotic, corresponding to a stasis area. On the second day, the wound area of all groups continued to increase, and the new expansion area appeared white as compared to the coagulated site. On day 3, all groups formed a full red ring around the edges of the wound. This area corresponds to the hyperemic area, indicating increased blood flow. On the fourth day, all the wounds stopped expanding. All groups showed almost identical properties, but the MO + NT group showed intense redness at the wound's edges. The MO and the NT formed a scar at the edge of the wound.
A significant decrease in RBC count, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) on days 7, 14, 21, and 28 was noticed, except for MO + NT in all treatments. Furthermore, burns induced a significant difference (p < 0.05) in the WBCs count levels in the MO + NT treated group. The level of platelets (PLT) was not significantly different from the healthy control group (Figure 4). As it depends on heat damage and an increased level of lymphocytes. So, the number of neutrophils and eosinophils did not change as compared to the control group.
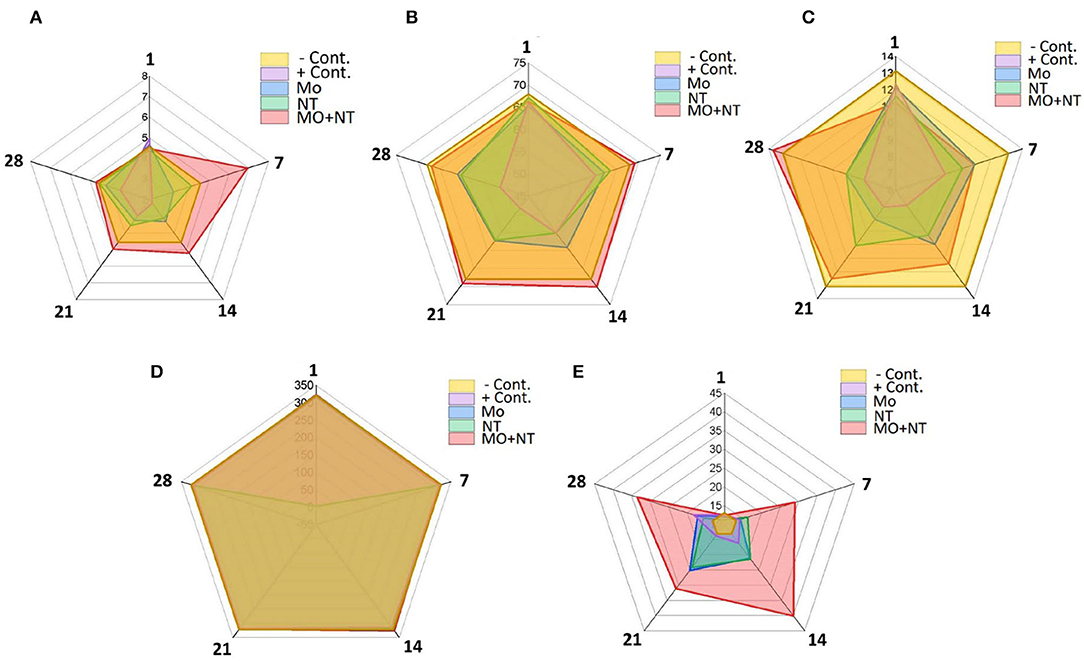
Figure 4. Hematological changes at different days (1, 7, 14, 21, and 28). (A) Ref blood cell (RBC) (1012/l), (B) MCV (Fl), (C) MCH (pg), (D) PLT (109/l), and (E) white blood cell (WBC) (109/l).
A histopathological examination of different treatments was analyzed. For this purpose, the epidermis, dermis, upper dermis, lower dermis, sweat glands, sebaceous glands, newly formed blood vessels, granular tissues or granulation, hair follicles, fibrous tissue or collagen fibers, and keratin layer were investigated. Our findings in the positive control showed that the keratin layer was absent, the epidermal closure was not firm, and dermal differentiation between the upper and lower layers was not evident. There were no glandular structures in the epidermis, only a few blood vessels and collagen fibers could be seen in the lower epidermis, which indicated a much slower repair process. In the “MO” group, the glandular structures of the skin were recovered but comparatively had fewer collagen fibers and angiogenesis than those of the group “MO + NT.” Moreover, keratinocytes and epidermal thickness were not fully restored. In the “NT” group, an incomplete keratin layer with ongoing re-epithelization was noticed. The glandular portion of the epidermis was not fully developed, but sweat and sebaceous were evident. Finally, we checked the “MO” and “NT” groups. Here, we found that all the structures were fully recovered with more collagen, angiogenesis, and keratin layer. The thickness of the epidermis was equal to the negative control group while tissue granulation was also optimum, indicating excellent treatment results (Figure 5).
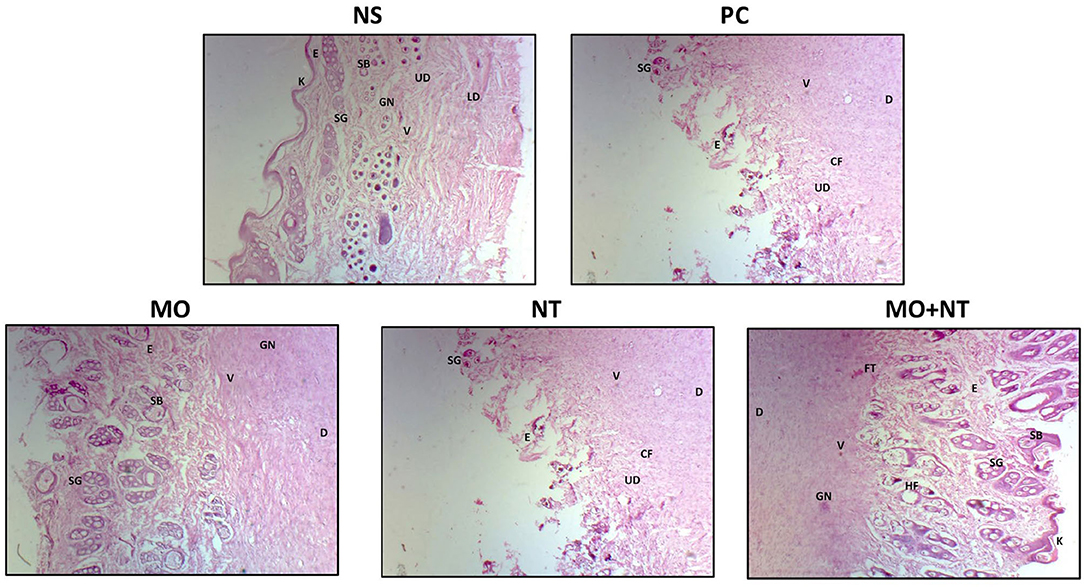
Figure 5. Histopathology of different treatments. E, epidermis; D, dermis; UD, upper dermis; LD, lower dermis; SG, sweat glands; SB, sebaceous glands; V, newly farmed blood vessels; GN, granular tissues or granulation; HF, hair follicle; FT, fibrous tissue or collagen fibers (Cf); K, keratin layer.
Wounds treated with “MO + NT” were found to have a higher epidermis thickness than other treatments. Concerning the epidermal thickness, all three treatment results were analyzed. It was concluded that the wounds treated with “MO + NT” had a better epidermal thickness of 151.92 μm. The result was much better in contrast to those who were treated with Manuka honey and Nitrofurazone alone. Therefore, the combination was proved to be more significant (p < 0.05). The honey ointment results were also better than control in epidermal thickness (Figure 6).
Tissue samples were analyzed in the laboratory on the 28th day. “MO + NT” wounds were found to have higher collagen (p < 0.05) content as compared to the other wounds treated with Manuka honey and Nitrofurazone ointments. While the wounds with no treatment had loose arrangements of collagen and minimal vascularization.
After careful examination, it was noticed that “MO + NT” has a better dermis thickness than other treatments. Statistically, G3 showed a significant (p < 0.05) improvement in the dermis as compared to the other three treatments.
The skin is the largest organ in the body that performs many important functions, e.g., homeostasis, body temperature, immunity, nervous sensations, and metabolism. The skin also acts as a physical barrier against infections (29). If this barrier is damaged, pathogens can penetrate directly into the body. Several studies have shown that burns are the leading cause of death with severe burns. Therefore, many researchers have sought to develop appropriate treatments to reduce the risk of wound infection and reduce the duration of treatment for burn injuries. These treatments include topical antimicrobials that effectively reduce mortality (30, 31), with low toxicity and sensitivity (32, 33). However, the occurrence of reactions related to allergies, toxicity, and irritation reduces skin regeneration ability, which can minimize their efficacy (29, 34). For example, the Nitrofurazone is one of the commonly used antimicrobial agents against gram-positive and gram-negative bacteria. As a consequence of its action, Nitrofurazone breaks down ribosomal proteins and some other macromolecules, which slow down the production of proteins, DNA, and RNA and stop the cellular proliferation. Additionally, it may impair bacterial cells' aerobic metabolism and carbohydrate metabolism activities (35). Meanwhile, oxidative processes produce reactive superoxide and hydroxyl radicals (36). Despite the fact that these actions take place in milliseconds or less, they have the potential to be harmful if left uncontrolled (34). As a result, the second choice is to employ natural products, which have been used since ancient times. These products are less toxic and cheaper than synthetic drugs. It has been shown that many plants and plant products effectively heal wounds. Nowadays, most drugs are a mixture of several plants or herbs, but these traditional ointments are not scientific. Using medicinal herbs has been noticed in the therapy of wounds from the very beginning due to its reduced financial load and its medical effects. Our study results are in line with the results of Bischofberger et al. (37). Where Manuka honey provided a protective barrier over the wound in preventing microbial infection. Its immunological activity accelerates wound repair and anti-inflammatory effects (38). Some other factors and bioactive components have also been involved that trigger the direct and indirect actions against the bacteria in wound repair (14). A 5.8 kDa bioactive component of Manuka honey has been identified as responsible for stimulating monocyte activity by activating the toll-like receptor 4 (TLR4). Activation of TLR4 and subsequently the intracellular signaling pathway nuclear factor kappaB (NF-κB) enhance the production of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), which are important in regulating the inflammatory response (39) (Figure 7).
Besides being able to reduce Cyclooxygenases 1 (COX-2) expression, Manuka honey can also have an antioxidant effect by changing the production of ROS and phenolic compounds (17). This is because of the production of ROS and other compounds.
Methylglyoxal is another active antibacterial ingredient of Manuka honey, which has been reported to react with lysine, arginine, and cysteine residues of structural proteins, such as collagen. Further it gives genesis to advanced glycation end products (AGEs). It promotes fibrosis in chronic tissue infections, impairs immune response microcirculation, promotes atherosclerosis and neovascularization, induces endothelial cell dysfunction, and impairs wound closure (40). Our study results are in line with the results of Bischofberger et al. (37), in which Manuka honey was found effective in experimental equine wounds in minimizing the wound area by decreasing retraction and overall healing time. The mechanism p38 is evolved during wound repair through Manuka honey. This mechanism mediates the stimulation of locomotion and proliferation by plate derivative growth factor (PDGF), the main growth factor in the early phases of wound healing (41). Our study is also in line with study of Moore et al. (42), who reviewed previous randomized controlled trials comparing honey with different treatments. Moore et al. concluded that honey is a useful treatment for burns.
The results of this study give a free hand to the companies to start the formation of medicines in the form of ointments or creams. We hope that a new burn ointment or cream can be used with herbal medicines, which will help to decrease the healing period and hypertrophic scar rate.
Manuka honey ointment may provide an alternative medication for managing extensive burns. However, there are some regulatory barriers to introducing a new product containing just honey. Therefore, changing attitudes toward clinical practice might take longer due to these barriers. Such prominent factors may decrease the acceptability of such treatment option. Hence, to address such acceptability concerns, the therapeutic options must be in combination with allopathic medications. It would place more emphasis on promoting the acceptability and would be helpful in providing a better option throughout the globe. Moreover, further research with randomized prospective is also needed to fully elucidate MO's effect on burns.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Departmental Ethical Committee.
MK: methodology and writing original draft. KA and AA: supervision. MS, MN, and IS: reagents, materials, and analysis tools. ZB, SS, HH, and MK: writing review and editing. AC, MA, and RH: visualization. KD, YW, and KL: conceptualization, funding, and resources. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the Start-up fund of Nanjing Agricultural University (804131) and the Start-up Fund for Distinguished Scholars of Nanjing Agricultural University (80900219).
KD is employed by China Tobacco Henan Industrial Co., Ltd., China.
The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
PC, positive control; NS, normal saline; MO, Manuka; NT, Nitrofurazone; MO + NT, Manuka + Nitrofurazone.
1. DeBoer S, O'Connor A. Prehospital and emergency department burn care. Crit Care Nurs Clin. (2004) 16:61–73. doi: 10.1016/j.ccell.2003.10.004
2. Wysocki AB. Evaluating and managing open skin wounds: colonization versus infection. Adv Crit Care. (2002) 13:382–97. doi: 10.1097/00044067-200208000-00005
3. World Health Organization. International Statistical Classification of Diseases and Related Health Problems: 10th Revision, Version for 2007-ICD-10. World Health Organization (2009).
4. Saito E, Rhoads C. Emergency visits to primary care veterinary hospitals. Vet Focus. (2003) 25:18–9. doi: 10.1055/s-0035-1570539
5. Pavletic MM, Trout NJ. Bullet, bite, and burn wounds in dogs and cats. Vet Clin Small Anim Pract. (2006) 36:873–93. doi: 10.1016/j.cvsm.2006.02.005
6. Volk SW, Bohling MW. Comparative wound healing—are the small animal veterinarian's clinical patients an improved translational model for human wound healing research? Wound Repair Regen. (2013) 21:372–81. doi: 10.1111/wrr.12049
7. Manafi A, Kohanteb J, Mehrabani D, Japoni A, Amini M, Naghmachi M, et al. Active immunization using exotoxin A confers protection against Pseudomonas aeruginosa infection in a mouse burn model. BMC Microbiol. (2009) 9:23. doi: 10.1186/1471-2180-9-23
8. Bagheri T, Fatemi MJ, Hosseini SA, Saberi M, Niazi M, Momeni M, et al. Comparing the effects of topical application of honey and nitrofurazone ointment on the treatment of second-degree burns with limited area: a randomized clinical trial. Matrix. (2017) 2:4.
9. Yaghoobi R, Kazerouni A. Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory anti-oxidant and anti-viral agent: a review. Jundishapur J Nat Pharm Prod. (2013) 8:100. doi: 10.17795/jjnpp-9487
10. Maddocks SE, Jenkins RE. Honey: a sweet solution to the growing problem of antimicrobial resistance? Fut Microbiol. (2013) 8:1419–29. doi: 10.2217/fmb.13.105
11. Combarros-Fuertes P, Estevinho LM, Dias LG, Castro JM, Tomás-Barberán FA, Tornadijo ME, et al. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: a screening prior to clinical application. J Agric Food Chem. (2018) 67:688–98. doi: 10.1021/acs.jafc.8b05436
12. Cooper R, Jenkins R. Are there feasible prospects for manuka honey as an alternative to conventional antimicrobials? Exp Rev Anti Infect Ther. (2012) 10:623–5. doi: 10.1586/eri.12.46
13. Natarajan S, Williamson D, Grey J, Harding K, Cooper R. Healing of an MRSA-colonized, hydroxyurea-induced leg ulcer with honey. J Dermatol Treat. (2001) 12:33–6. doi: 10.1080/095466301750163563
14. Al-Waili NS, Salom K, Butler G, Al Ghamdi AA. Honey and microbial infections: a review supporting the use of honey for microbial control. J Med Food. (2011) 14:1079–96. doi: 10.1089/jmf.2010.0161
15. Tewari J, Irudayaraj J. Quantification of saccharides in multiple floral honeys using fourier transform infrared microattenuated total reflectance spectroscopy. J Agric Food Chem. (2004) 52:3237–43. doi: 10.1021/jf035176+
16. Kato Y, Umeda N, Maeda A, Matsumoto D, Kitamoto N, Kikuzaki H. Identification of a novel glycoside, leptosin, as a chemical marker of manuka honey. J Agric Food Chem. (2012) 60:3418–23. doi: 10.1021/jf300068w
17. Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Al Jaouni SK, et al. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid Med Cell Long. (2016) 3643824. doi: 10.1155/2016/3643824
18. de Lira Mota KS, Dias GEN, Pinto MEF, Luiz-Ferreira Â, Monteiro Souza-Brito AR, Hiruma-Lima CA, et al. Flavonoids with gastroprotective activity. Molecules. (2009) 14:979–1012. doi: 10.3390/molecules14030979
19. Ahmed SW, Anwar H, Siddiqui A, Shah MR, Ahmed A, Ali SA. Synthesis and chemosensing of nitrofurazone using olive oil based silver nanoparticles (O-AgNPs). Sens Actuators B Chem. (2018) 256:429–39. doi: 10.1016/j.snb.2017.10.111
20. Shen C, Shen B, Liu X, Yuan H. Nanosuspensions based gel as delivery system of nitrofurazone for enhanced dermal bioavailability. J Drug Deliv Sci Technol. (2018) 43:1–11. doi: 10.1016/j.jddst.2017.09.012
21. Kouchak M, Ameri A, Naseri B, Boldaji SK. Chitosan and polyvinyl alcohol composite films containing nitrofurazone: preparation and evaluation. Iran J Basic Med Sci. (2014) 17:14. doi: 10.1016/j.phrp.2014.12.001
22. Henke J, Astner S, Brill T, Eissner B, Busch R, Erhardt W. Comparative study of three intramuscular anaesthetic combinations (medetomidine/ketamine, medetomidine/fentanyl/midazolam and xylazine/ketamine) in rabbits. Vet Anaesth Analg. (2005) 32:261–70. doi: 10.1111/j.1467-2995.2005.00242.x
23. Ashkani-Esfahani S, Imanieh MH, Meshksar A, Khoshneviszadeh M, Noorafshan A, Geramizadeh B, et al. Enhancement of fibroblast proliferation, vascularization and collagen synthesis in the healing process of third-degree burn wounds by topical arnebia euchroma, a herbal medicine. Galen Med J. (2013) 1:53–9. doi: 10.31661/gmj.v1i2.19
24. Liao H, Guo Y, Na MJ, Lane KB, Light RW. The short-term administration of Ketoprofen does not decrease the effect of Pleurodesis induced by talc or Doxycycline in rabbits. Respir Med. (2007) 101:963–8. doi: 10.1016/j.rmed.2006.09.007
25. Mirnezami M, Rahimi H, Fakhar HE, Rezaei K. The role of topical estrogen, phenytoin, and silver sulfadiazine in time to wound healing in rats. Ostomy Wound Manage. (2018) 64:30–4. doi: 10.25270/owm.2018.8.3034
26. Akhoondinasab MR, Akhoondinasab M, Saberi M. Comparison of healing effect of aloe vera extract and silver sulfadiazine in burn injuries in experimental rat model. World J Plast Surg. (2014) 3:29.
27. Khiati B, Ahmed M. Comparison of efficacy of unheated and heat-treated Sahara honey on wound healing in rabbits. J Coast Life Med. (2015) 3:162–5. doi: 10.12980/JCLM.3.201514J91
28. Udefa AL, Archibong AN, Akwari AA, Leilei SA. Effect of Aloe vera gel on some haematological parameters and serum electrolytes in high salt loaded Wistar rats. MicroMedicine. (2018) 6:69–77. doi: 10.5281/zenodo.1318287
29. Upadhyay NK, Kumar R, Siddiqui M, Gupta A. Mechanism of wound-healing activity of Hippophae rhamnoides L. leaf extract in experimental burns. Evid Based Comp Altern Med. (2011) 659705. doi: 10.1093/ecam/nep189
30. Hazrati M, Mehrabani D, Japoni A, Montasery H, Azarpira N, Hamidian-Shirazi A, et al. Effect of honey on healing of Pseudomonas aeruginosa infected burn wounds in rat. J Appl Anim Res. (2010) 37:161–5. doi: 10.1080/09712119.2010.9707117
31. Hosseini SV, Tanideh N, Kohanteb J, Ghodrati Z, Mehrabani D, Yarmohammadi H. Comparison between Alpha and silver sulfadiazine ointments in treatment of Pseudomonas infections in 3rd degree burns. Int J Surg. (2007) 5:23–6. doi: 10.1016/j.ijsu.2006.03.007
32. Kimura Y, Sumiyoshi M, Kawahira K, Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. (2006) 148:860–70. doi: 10.1038/sj.bjp.0706794
33. Mohajeri G, Masoudpour H, Heidarpour M, Khademi EF, Ghafghazi S, Adibi S, et al. The effect of dressing with fresh kiwifruit on burn wound healing. Surgery. (2010) 148:963–8. doi: 10.1016/j.surg.2010.02.013
34. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. (2015) 22:377–88. doi: 10.1038/cdd.2014.150
35. Karapolat S, Karapolat B, Buran A, Okatan BK, Turkyilmaz A, Set T, et al. The effects of nitrofurazone on wound healing in thoracoabdominal full-thickness skin defects. Wounds. (2020) 32:134–41.
36. Senel O, Cetinkale O, Ozbay G, Ahçioglu F, Bulan R. Oxygen free radicals impair wound healing in ischemic rat skin. Ann Plast Surg. (1997) 39:516–23. doi: 10.1097/00000637-199711000-00012
37. Bischofberger AS, Dart CM, Perkins NR, Kelly A, Jeffcott L, Dart AJ. The effect of short-and long-term treatment with manuka honey on second intention healing of contaminated and noncontaminated wounds on the distal aspect of the forelimbs in horses. Vet Surg. (2013) 42:154–60. doi: 10.1111/j.1532-950X.2012.01083.x
38. Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. (2011) 1:154. doi: 10.1016/S2221-1691(11)60016-6
39. Tsang AS, Dart AJ, Dart CM, Jeffcott LB. Mechanisms of action of manuka honey in an equine model of second intention wound healing: current thoughts and future directions. Med Res Arch. (2018) 6.
40. Kamaratos AV, Tzirogiannis KN, Iraklianou SA, Panoutsopoulos GI, Kanellos IE, Melidonis AI. Manuka honey-impregnated dressings in the treatment of neuropathic diabetic foot ulcers. Int Wound J. (2014) 11:259–63. doi: 10.1111/j.1742-481X.2012.01082.x
41. Ranzato E, Martinotti S, Burlando B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burns Trauma. (2013) 1:32–8. doi: 10.4103/2321-3868.113333
Keywords: Manuka, honey, Nitrofurazone, burns, natural remedies
Citation: Kulyar MF-e-A, Ashfaq K, Aqib AI, Duan K, Asif M, Bhutta ZA, Shoaib M, Shabbir S, Nawaz S, Naseer MA, Sarwar I, Akhtar M, Chaudhry AS, Hussain R, Hussain HI, Wu Y and Li K (2022) Enhanced Healing Activity of Manuka Honey and Nitrofurazone Composite in Full-Thickness Burn Wounds in the Rabbit Model. Front. Vet. Sci. 9:875629. doi: 10.3389/fvets.2022.875629
Received: 14 February 2022; Accepted: 16 March 2022;
Published: 23 May 2022.
Edited by:
Fazul Nabi, Lasbela University of Agriculture, Water and Marine Sciences, PakistanReviewed by:
Prerona Boruah, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2022 Kulyar, Ashfaq, Aqib, Duan, Asif, Bhutta, Shoaib, Shabbir, Nawaz, Naseer, Sarwar, Akhtar, Chaudhry, Hussain, Hussain, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Li, bGlrMjAxNEBzaW5hLmNvbQ==; Yi Wu, d3V5aTIwMDFjbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.