- 1College of Animal Science and Technology, Jiangxi Agricultural University, Nanchang, China
- 2Key Laboratory of Featured Hydrobios Nutritional Physiology and Healthy Breeding, Nanchang, China
A high-carbohydrate diet lowers the rearing cost and decreases the ammonia emission into the environment, whereas it can induce liver injury, which can reduce harvest yields and generate economic losses in reared fish species. Macroalgae Saccharina japonica (SJ) has been reported to improve anti-diabetic, but the protective mechanism of dietary SJ against liver injury in fish fed a high-carbohydrate diet has not been studied. Therefore, a 56-day nutritional trial was designed for swamp eel Monopterus albus, which was fed with the normal diet [20% carbohydrate, normal carbohydrate (NC)], a high carbohydrate diet (32% carbohydrate, HC), and a HC diet supplemented with 2.5% SJ (HC-S). The HC diet promoted growth and lowered feed coefficient (FC), whereas it increased hepatosomatic index (HSI) when compared with the NC diet in this study. However, SJ supplementation increased iodine contents in muscle, reduced HSI, and improved liver injury, such as the decrease of glucose (GLU), total bile acid (TBA), and alanine aminotransferase (ALT) in serum, and glycogen and TBA in the liver. Consistently, histological analysis showed that SJ reduced the area of lipid droplet, glycogen, and collagen fiber in the liver (p < 0.05). Thoroughly, the underlying protective mechanisms of SJ supplementation against HC-induced liver injury were studied by liver transcriptome sequencing coupled with pathway analysis. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the differentially expressed genes (DEGs), such as the acetyl-coenzyme A synthetase (acss1), alcohol dehydrogenase (adh), interferon-induced protein with tetratricopeptide repeats 1 (ifit1), aldo-keto reductase family 1 member D1 (akr1d1), cholesterol 7-alpha-monooxygenase (cyp7a1), and UDP-glucuronosyltransferase (ugt), indicated that the pathway of glycolysis/gluconeogenesis was the main metabolic pathway altered in the HC group compared with the NC group. Meanwhile, hepatitis C, primary BA biosynthesis, and drug metabolism-cytochrome P450 were the three main metabolic pathways altered by SJ supplementation when compared with the HC group. Moreover, the BA-targeted metabolomic analysis of the serum BA found that SJ supplementation decreased the contents of taurohyocholic acid (THCA), taurochenodeoxycholic acid (TCDCA), taurolithocholic acid (TLCA), nordeoxycholic acid (NorDCA), and increased the contents of ursocholic acid (UCA), allocholic acid (ACA), and chenodeoxycholic acid (CDCA). In particular, the higher contents of UCA, ACA, and CDCA regulated by SJ were associated with lower liver injury. Overall, these results indicate that the 2.5% supplementation of SJ can be recommended as a functional feed additive for the alleviation of liver injury in swamp eel-fed high-carbohydrate diets.
Introduction
Carbohydrates are regarded as the most economical energy source for aquatic animals due to their abundance and relatively low cost (1). However, it is generally acknowledged that fish have a poor capability to utilize glucose (GLU) for energy purposes compared with mammals (2). Generally, most fish species (especially carnivorous ones) have an impaired GLU tolerance and often display prolonged postprandial hyperglycemia after a GLU load or the intake of a high-carbohydrate diet (3). It has additionally been reported that a high-carbohydrate diet induces liver injury, which can result in severe health problems and reduce harvest yields, thus generating economic losses in reared fish (1, 4). Therefore, the amelioration of liver injury is strongly necessary for the healthy development of aquaculture.
Saccharina japonica (SJ), a common macroalgae cultured in the temperate coastal area of the northwest Pacific Ocean, is widely cultured and has become the most productive seaweed in China (5). In particular, SJ has been reported to exhibit various biological activities, such as improving immunity, anti-tumor, and anti-diabetic, which are ascribed to the variety of biologically active ingredients, such as polysaccharides, polyphenols, vitamins, and minerals (6, 7). In recent years, dietary SJ has been shown to ameliorate liver injury by decreasing hepatic collagen fiber and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in mammals (7, 8). Moreover, studies have demonstrated that liver injury is commonly characterized by increased levels of total bile acid (TBA) and triglyceride (TG), AST, and ALT in the plasma (8–10). However, information regarding the effects of dietary SJ on liver injury in the fish fed high-carbohydrate diet is not reported.
Swamp eel (Monopterus albus) is a high-value commercially farmed, eurythermal, and freshwater carnivorous fish with a desirable growth rate, good meat quality, and an exceptional ability to obtain oxygen from the air rather than water (11, 12). Our previous study showed that a high-carbohydrate diet resulted in liver injury in swamp eel (13).
This study aimed to investigate the effect of dietary SJ on high-carbohydrate diet-induced liver injury in swamp eel by analyzing serum and hepatic biochemistry, and morphology of the liver in the present study. The liver genes and serum bile acid profile were also studied by the transcriptomic and metabolomic analysis.
Materials and Methods
Experimental Diets and Feeding Trial
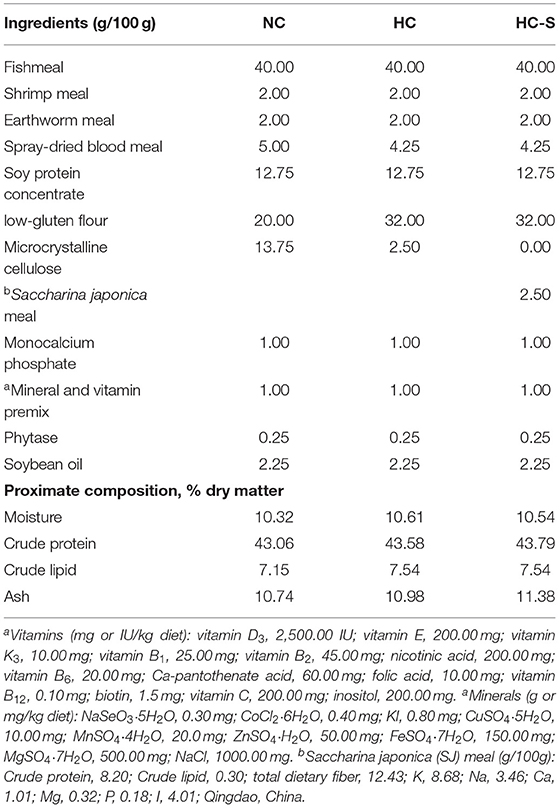
Dry macroalgae SJ meal was purchased from Qingdao Longan Biotechnology Co., Ltd (Qingdao, China). Three isonitrogenous and isolipidic diets were formulated: the normal carbohydrate diet (20% carbohydrate, NC), high carbohydrate diet (32% carbohydrate, HC), and HC diet supplemented with 2.5% SJ (HC-S) (Table 1). The NC level of 20% and HC level of 32% were obtained in our previous study (unpublished data). This SJ supplementation level was selected based on our previous study (14) and relevant publications in the field (7). The diet was prepared, and ingredients were ground, weighed, and well-mixed as previously described (15).
The 56-day feeding trial were conducted according to our previous study with some modification (11). Each diet group was randomly assigned to four replicates, and one replicate corresponded to one tank (100 cm × 60 cm × 50 cm, 20 healthy swamp eels per tank with an initial weight of 12.10 ± 0.03 g). Fish were fed with the experimental diet to apparent satiation once daily (at 17:00) referring to our previous study (14) and the feed intake was recorded. During the experiment, the water temperature ranged from 27 to 32°C, ammonia nitrogen was lower than 3 mg/L, and dissolved oxygen was above 4 mg/L.
Sample Collection
At the end of the feeding trial, all fish were counted and weighed after fasting for 12 h and anesthetized with 250 mg/L M-aminobenzoate ethyl methane sulfonate (MS-222) (Sigma-Aldrich, USA). A total of 16 fish (4 fish per tank) were sampled from four tanks in each group (n = 4). Blood was collected from the caudal vein using a 1-ml syringe. Afterward, the fish was dissected on ice to obtain dorsal muscle and liver subsequently. After dissection, the liver was cut into small pieces. After sedimentation at 4°C for 2 h, pooled blood samples were centrifuged at 3,500 g for 15 min at 4°C to obtain serum. Parts of the liver were fixed in 4% buffered neutral formalin for histological analysis. Dorsal muscle, the other parts of the liver and serum were immediately frozen in liquid nitrogen for 6 h and stored at −80°C for transcriptomic, metabolomics, and biochemical analysis. Samples were kept in 1.5 ml plastic tubes.
Survival rate (SR) = 100 × (final fish number/initial fish number). Weight gain rate (WGR) = 100 × (final body weight—initial body weight)/(initial body weight). Feed coefficient (FC) = (total dry weight of feed fed)/(final weight—initial weight). Hepatosomatic index (HSI) = 100 × liver weight/body weight.
Proximate Composition and Iodine Analysis
Moisture, ash, crude protein, and lipid were tested following the methods of the Association of Official Analytical Chemists (AOAC 2,000) with the analytical numbers 950.46, 960.39, 928.08, and 920.153, respectively. Moisture was determined by drying ground samples in a forced-air oven at 105°C for 24 h. Ash was analyzed by incinerating samples at 600°C for 24 h in a muffle furnace. Crude protein was estimated as Kjeldahl-nitrogen using a factor of 6.25, and crude lipid was analyzed by Soxhlet extraction with petroleum ether. Iodine content was determined at Guangdong Kangxin Testing Technology Co., Ltd. (Guangzhou, China) by microwave digestion with nitric acid and hydrogen peroxide (Germany Merck superior grade pure), followed by inductively coupled plasma mass spectrometry (Agilent ICPMS-7900) detection.
Biochemistry Analysis
The liver sample was accurately weighed, and 9 times the volume of physiological saline was added. Then, it was mechanically homogenized under ice-water bath conditions and centrifuged at 350 g for 10 min, and the supernatant was collected. Both the supernatants of the liver and serum were used for biochemistry analysis. AST and ALT activities, TG, TBA, GLU, insulin, and glycogen contents were determined using the diagnostic reagent kits for fish (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions. In brief, a volumetric supernatant was transferred to a 96-well plate, then the reaction solution was added to each sample. The 96-well plate was incubated at specific conditions, and then the 96-well plate was measured with the microplate spectrophotometer (Spectra Max® 190).
Histological Analysis
The fixed hepatic tissues were processed with the standard paraffin embedding method and then stained with Oil Red O (making nucleus blue and lipid red), PAS (making glycogen carmine), and Masson (making collagen fiber blue), respectively. The slides were then examined and photographed under a light microscope (Axio Imager 2, Zeiss, Oberkochen, Germany) equipped with a camera (Axiocam 506, Zeiss, Oberkochen, Germany). In addition, the relative area of lipid droplet, glycogen, and collagen fiber in the liver from 16 fish per group was measured using Image-Pro Plus 6.0 (n = 16).
Transcriptomic Assay
Total RNA was extracted from the liver tissues of the control group (NC), high carbohydrate group (HC), and HC diet supplemented with 2.5% SJ group (HC-S) using Trizol reagent (Invitrogen, USA) according to the manufacturer's instructions (12 fish per group). The Nanodrop 2,000 (Thermo Fiser Scientific, Wilmington, DE) was used to determine RNA purity and concentration. The integrity of the total RNA was assessed by the RNA Nano 6,000 assay kit of the Agilent 2,100 system (Agilent Technologies, CA, USA). Three Illumina libraries, each containing a pool of equal total RNA from four individual samples, were produced for each group (NC, HC, and HC-S). The messenger RNA (mRNA) was purified from the total RNA using poly-T oligo-attached magnetic beads, and then broken into short fragments with fragmentation buffer. First, the mRNA fragments were used as templates for the synthesis of complementary DNA (cDNA). Second, cDNA fragments of preferentially 240 bp in length, the library fragments were purified with the AMPure XP system (Beckman Coulter, Beverly, USA). At last, the suitable fragments were used for PCR amplification, and PCR products were purified (AMPure XP system). The library quality was assessed on the Agilent Bioanalyzer 2,100 system. The library was sequenced on an Illumina Hiseq X Ten platform (Illumina, CA, USA). The genome data from NCBI of swamp eel Monopterus albus were used for further analysis in this study.
Gene expression levels were estimated by fragments per kilobase of transcript per million fragments mapped (FPKM). Differentially expressed genes (DEGs) between groups were verified by DESeq2. According to fold change (FC) ≥1.5 and false discovery rate (FDR) value <0.05, DEG in the liver between different groups was identified. DEG was submitted to the KEGG database (http://www.genome.ad.jp/kegg/) to come up with pathways in KEGG pathway categories. The statistical enrichment of DEGs in the KEGG pathway was tested using KOBAS software.
Gene Expression Validation
The quantitative real-time PCR (qPCR) was performed using SYBR Green as fluorescent dye according to the manufacturer's protocol (Takara), which was used to validate and quantify genes, such as adh (alcohol dehydrogenase), cyp7a1 (cholesterol 7-alpha-monooxygenase), akr1d1 (aldo-keto reductase family 1 member D1), acss1 (acetyl-coenzyme A synthetase), ifit1 (interferon-induced protein with tetratricopeptide repeats 1), and ugt (UDP-glucuronosyltransferase) from the transcriptomic assay. The primers used for qPCR are listed in Supplementary Table 1. Each sample was run in triplicate. The 2−ΔΔCt method was used to calculate the relative expression with β-actin as a reference gene.
BA Composition by Ultra-Performance Liquid Chromatography-Mass Spectrometry Analysis
Blood samples of the NC, HC, and HC-S groups were put in the EP tube, and 600 μl methanol (−20°C) was added (16 fish per group). After the 60 s of the vortex, the samples were centrifuged at 12,000 rpm for 10 min at 4°C. A volume of 400 ul of supernatant was transferred to a fresh tube and dried in a vacuum. Then, the dried samples were dissolved with 100 μl of 30% methanol (−20°C). Finally, the supernatant was filtered through a 0.22 μm membrane for ultra-performance liquid chromatography-mass spectrometry (UPLC-MS) analysis.
In the UPLC-MS experiment, UPLC separation was performed on an Acquity UPLC system (Waters, U.K.) equipped with an Acquity UPLC® BEH C18 (1.7 μm, 2.1 mm × 100 mm, Waters) column. The temperature of the column was set at 40°C. The sample injection volume was 5 μl. Eluents consisted of 0.01% formic acid water (eluent A) and acetonitrile (eluent B). The flow rate was set at 0.25 ml/min. A 35.5-min elution gradient was performed as follows: 0–4 min, 25% B; 4–9 min, 25–30% B; 9–14 min, 30–36% B; 14–18 min, 36–38% B; 18–24 min, 38–50% B; 24–32 min, 50–75% B; 32–33 min, 75–90% B; and 33–35.5 min, 90–25% B.
The MS analysis was performed by an AB mass spectrometer (AB, USA) equipped with an ESI source in the negative-ion mode working in the multiple reaction monitoring (MRM) mode. An ion source voltage of 4,500 V and a source temperature of 500°C were used.
The calibration graphs were constructed by plotting the peak area vs. concentration for each standard. The results were shown in the report of analysis and the correlation coefficients were >0.99. The limit of quantitation (LOQ) was determined by the signal-to-noise ratio (S/N), which was calculated by comparing the signals of known samples with blank samples. Generally, the corresponding concentration was defined as LOQ when the S/N is 10:1 (S/N = 10). The precision of the analytical procedure was expressed as the relative standard deviation (RSD). The intra- and interday precision was 1.52–10.14% and 2.18–23.44%, respectively. It indicated that the instrument is of good precision.
Statistical Analysis
The data for each group were expressed as mean ± standard error of mean (SEM). SPSS 19.0 software was used for one-way ANOVA, and then Tukey's multi-range test was used to evaluate the statistical differences in growth, composition, histology, biochemistry analysis, and qPCR between treatments. The level of significance was set as p < 0.05.
Results
Growth Performance and Feed Utilization of Swamp eel Fed NC, HC, and HC-S Diet
Growth performance and feed utilization of swamp eel are presented in Table 2. The final body weight (FBI), survival rate (SR), and weight gain rate (WGR) showed non-significant differences between groups (p > 0.05). A significantly lower feed coefficient (FC) was found in the HC group compared with the NC group (p < 0.05). However, fish in HC and HC-S groups had significantly higher HSI than that of fish in the NC group (p < 0.05), and SJ supplementation inhibited the abnormal growth of the liver caused by the HC diet (p > 0.05).
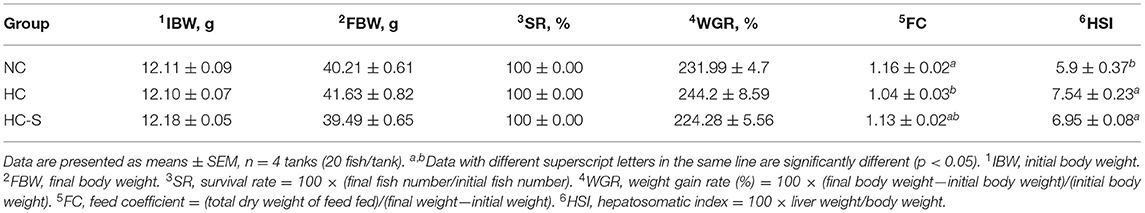
Table 2. Effects of dietary SJ on growth performance and feed utilization swamp eel fed high-carbohydrate (HC) diet.
Muscle Nutritional Values, and Hepatic and Serum Biochemistry of Swamp eel Fed NC, HC, and HC-S Diet
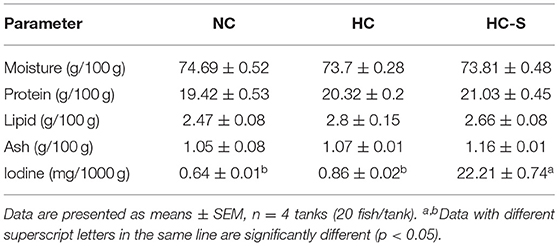
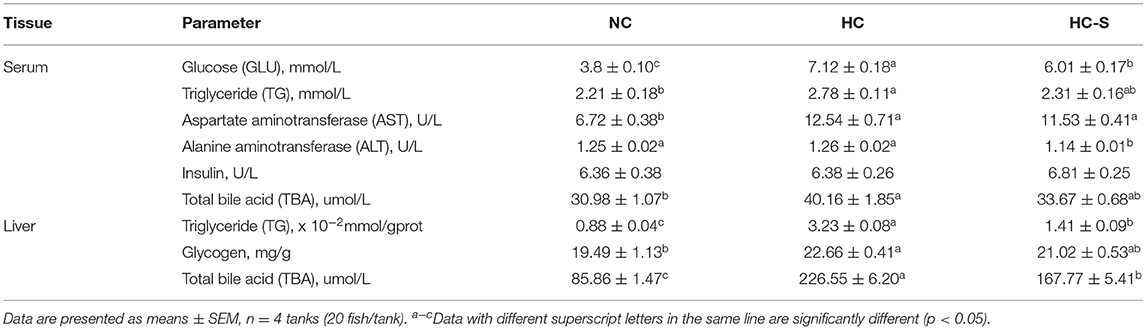
There was no significant difference in moisture, crude protein, crude lipid, and ash content between groups (p > 0.05). However, significantly higher iodine content was found in the HC-S group compared with the NC and HC groups (p < 0.05) (Table 3). Tissue biochemical parameters of swamp eel are presented in Table 4. Serum TBA, GLU, TG, and AST, and hepatic TBA, TG, and glycogen levels in the HC group were significantly higher than those in the NC group (p > 0.05), whereas the HC-S diet lower or significantly lower than those in the swamp eel fed HC diet. However, serum insulin levels showed no significant difference between groups (p > 0.05).
Histological Structure in Liver of Swamp eel Fed NC, HC, and HC-S Diet
The relative areas of lipid droplet (in Oil-Red O stained), glycogen (in PAS stained), and collagen fiber (in MASSON stained) from fish liver paraffin slice were significantly increased in the HC group compared with the NC group, whereas the HC-S group significantly reduced the relative areas of lipid droplet, glycogen, and collagen fiber compared with the HC group (p < 0.05) (Figure 1).
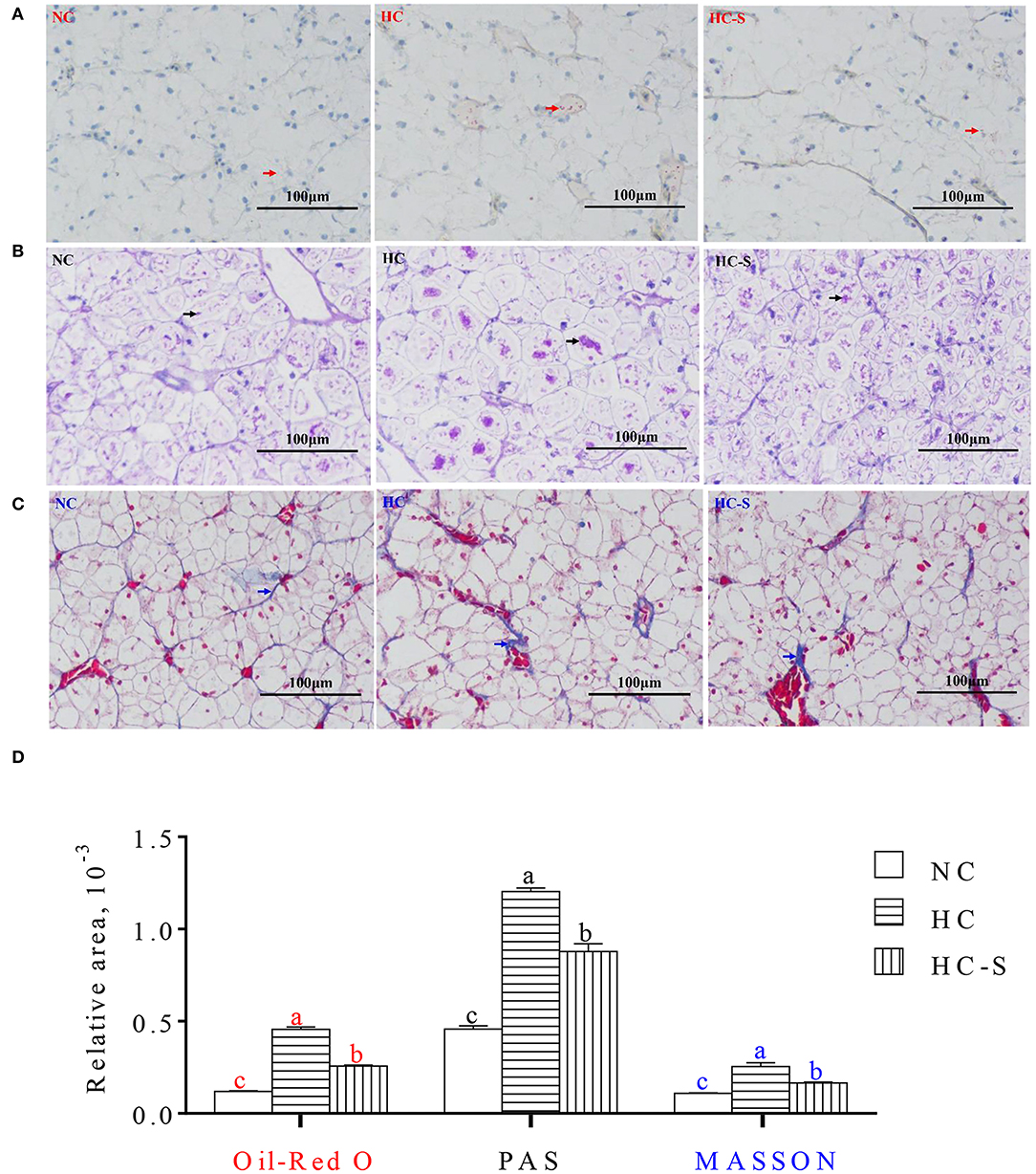
Figure 1. Influence of dietary Saccharina japonica (SJ) on hepatic histological structure of paraffin slice with Oil Red O (making nucleus blue and lipid red) staining (A), PAS (making glycogen carmine) staining (B) and Masson (making collagen fiber blue) staining (C), and the relative areas of lipid droplet with red arrows in Oil-Red O staining, glycogen with black arrows in PAS staining, and collagen fiber with blue arrows in MASSON staining of swamp eel fed high-carbohydrate (HC) diet (D) ( × 200). Values are presented as means ± SEM, n = 16 fish/diet. Means with different letters were significantly different (p < 0.05).
Liver Transcriptome Profile of Swamp eel Fed NC, HC, and HC-S Diet
RNA samples were extracted from the liver tissues of NC, HC, and HC-S groups for RNA sequencing, and generated 21.53, 21.39, and 20.88 million clean reads, respectively (Supplementary Table 2). A total of 22.88 × 103 unigenes were annotated using the Trinity assembly program (Supplementary Table 3).
There were 181 DEGs between the HC group and NC group, and 38 DEGs between the HC-S group and the HC group with the screening criteria that FC ≥1.5 and an FDR <0.05. To further functionally characterize the DEG, pathway analysis was conducted using the KEGG database. Through the KEGG pathway analysis of DEG, pathways in the top 20 significant pathways between the HC group and NC group, and the HC-S group and HC group are listed in Figures 2A,B, respectively. The changes of DEG in the top 20 significant pathways are as follows: in the aspect of carbohydrate metabolism, all the DEGs including the acss1 and adh in glycolysis/gluconeogenesis were downregulated or upregulated in the HC group compared with the NC group (Figure 2). Moreover, regarding BA metabolism, all the DEGs including the akr1d1 and cyp7a1 in primary bile acid biosynthesis were downregulated in the HC group compared with the NC group, whereas all the DEGs including the akr1d1 and cyp7a1 in that pathway were upregulated in the HC-S group compared with the HC group; SJ supplemental diet upregulated the expression of ugt, all the DEGs in the pathway of drug metabolism-cytochrome P450 (Figure 2). In addition, the SJ supplemental diet downregulated the expression of ifit1, all the DEGs in the pathway of hepatitis C, while the HC diet upregulated that DEG (Figure 2).
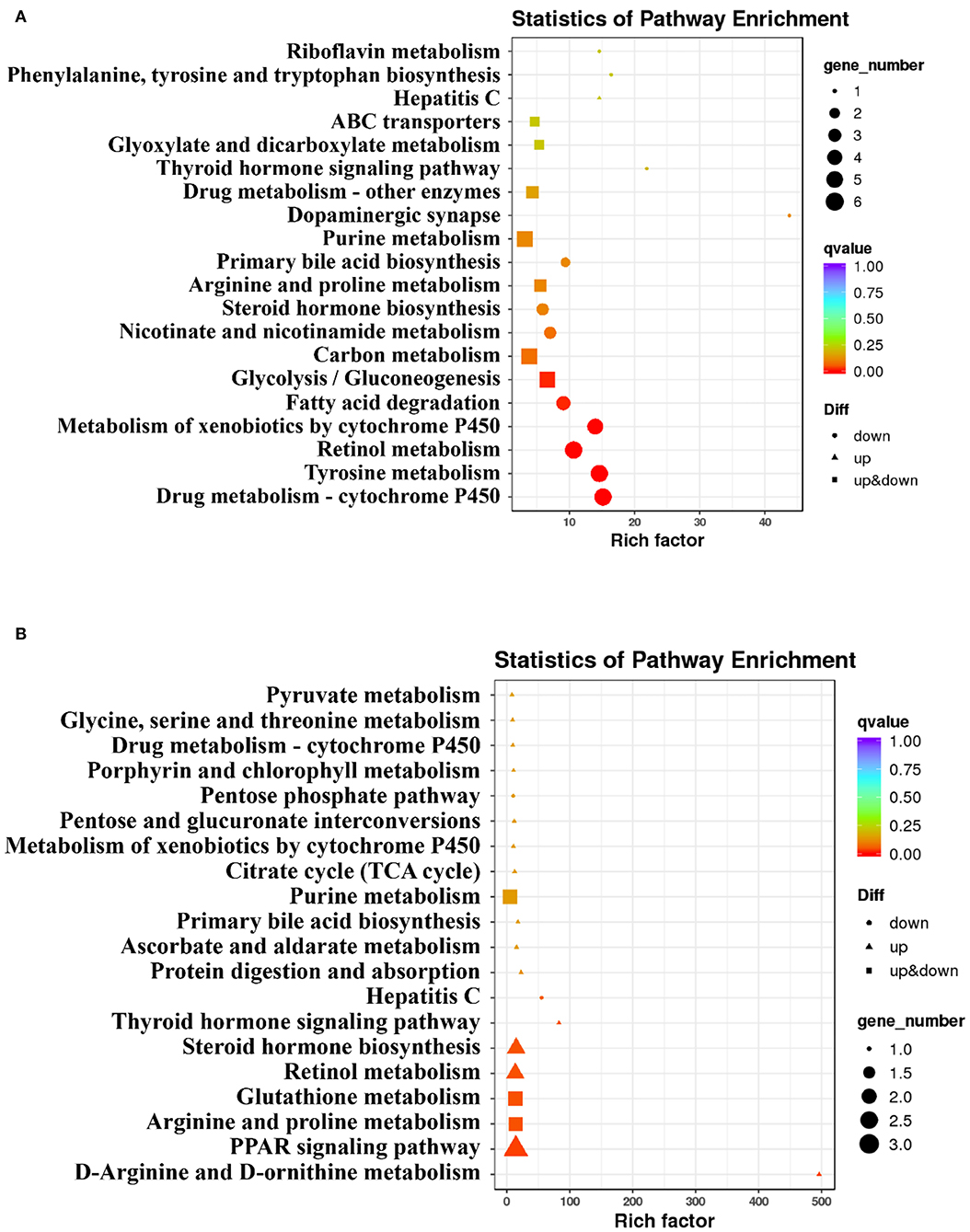
Figure 2. Analysis of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway on hepatic differentially expressed genes (DEGs) in swamp eel fed HC diet. (A) the HC group relative to the (normal carbohydrate) NC group, (B) the HC diet supplemented with 2.5% SJ (HC-S) group relative to the HC group.
Results of gene expression validation from qPCR are shown in Figure 3. Significantly lower expressions of adh, cyp7a1, and akr1d1 were found in the HC group compared with the NC group, whereas the expression of acss1 and ifit1 showed the opposite change (p < 0.05). However, the changed expression of adh, cyp7a1, akr1d1, acss1, and ifit1 in the HC group was ameliorated HC-S group. In addition, the expression of ugt was significantly upregulated in the HC-S group compared with the NC and HC groups (p < 0.05). The expression levels of DEGs (adh, cyp7a1, akr1d1, acss1, ifit1, and ugt) in qPCR showed a highly consistent trend from the results of the transcriptomic assay.
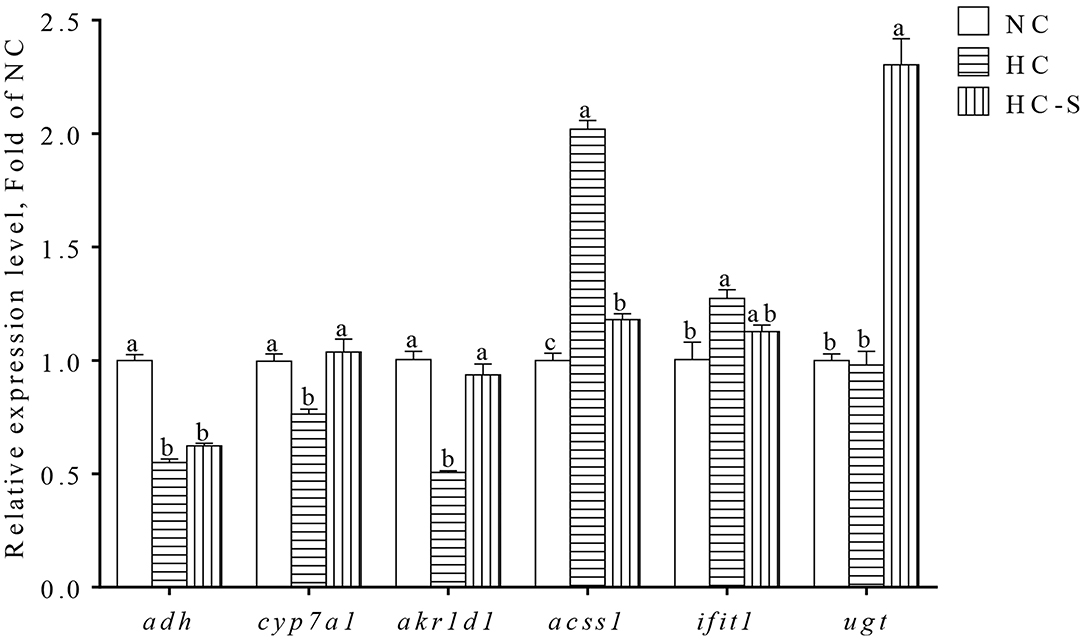
Figure 3. Quantitative real-time PCR (qPCR) analysis of hepatic DEGs in swamp eel fed HC diet. Values are presented as means ± SEM, n = 16 fish/diet. Columns with different superscripts indicated values with significant difference, p < 0.05 was significantly different.
Serum Profile of BA in Swamp eel Fed NC, HC, and HC-S Diet
To evaluate the association between BA species and liver injury, we conducted targeted serum BA profiling analyses in the NC, HC, and HC-S groups by UPLC-MS analysis. Based on UHPLC/MS data, a total of 15 BA met the quality control criteria and were quantified. The alterations of the differential BA species were then compared between groups. The taurohyocholic acid (THCA), taurocholic acid (TCA), taurochenodeoxycholic acid (TCDCA), taurolithocholic acid (TLCA), and nordeoxycholicacid (NorDCA) were significantly increased, and ursocholic acid (UCA), allocholic acid (ACA), chenodeoxycholic acid (CDCA), and glycolithocholic acid (GLCA) were significantly decreased in the HC group compared with the NC group (p < 0.05) (Figure 4A). Oppositely, the TCDCA, TLCA, and NorDCA were significantly decreased (p < 0.05), and UCA, ACA, and CDCA were increased in the HC-S group compared with the HC group (p > 0.05) (Figure 4A). Moreover, pairwise Spearman's correlation analysis showed that serum THCA, TCDCA, TLCA, and NorDCA were positively correlated with HSI and serum GLU, whereas serum UCA, ACA, CDCA, and GLCA were inversely correlated with HSI and serum GLU (p < 0.05) (Figure 4B). These results indicated that serum BA of THCA, TCDCA, TLCA, NorDCA, UCA, ACA, CDCA, and GLCA were related to GLU metabolism and regulation.
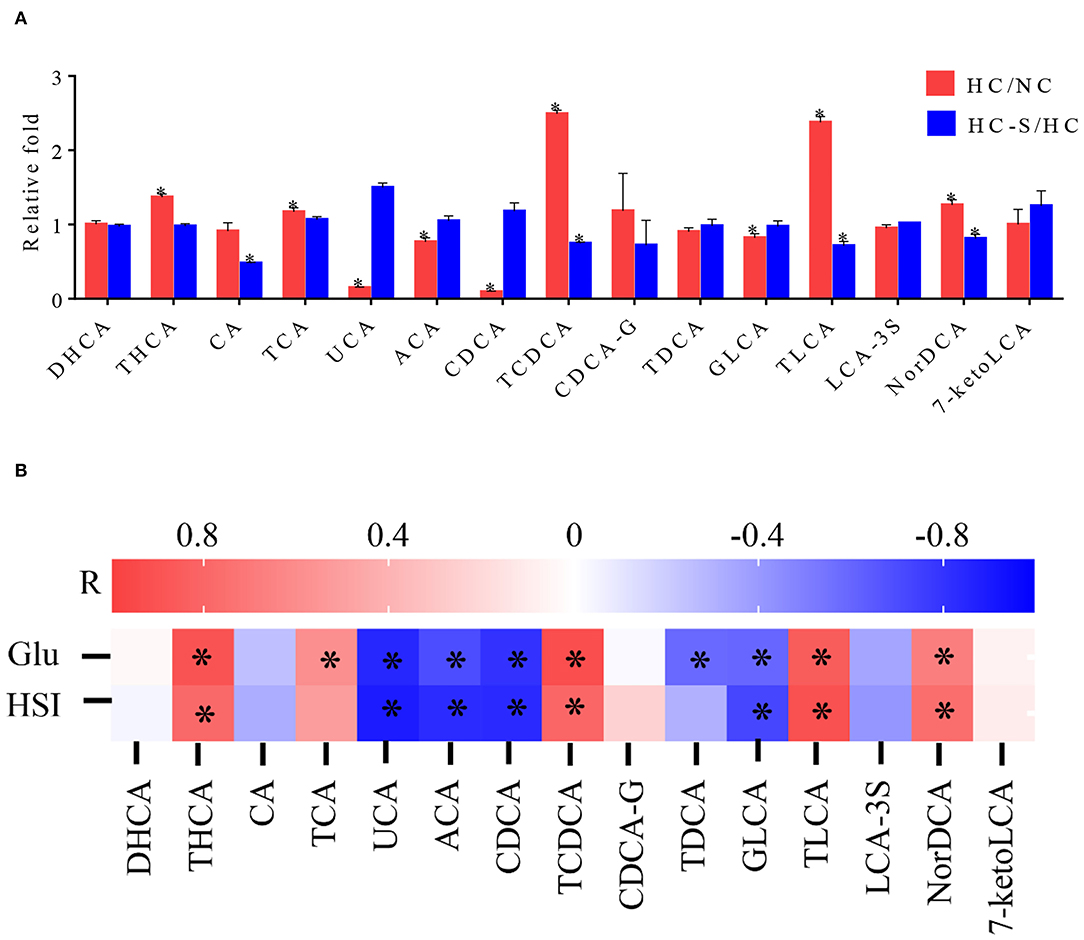
Figure 4. Influence of dietary SJ on the serum profile of bile acid (BA) in swamp eel fed HC diet. (A) Fold changes of 15 BA in the HC group relative to the mean values of the NC group and the HC-S group relative to the mean values of the HC group, (B) Heatmaps of Spearman's correlation coefficients of 15 BA with representative metabolic markers. In the bar plots (A), *indicate the statistical significance (p < 0.05) between two groups. In the heatmaps (B), R value indicates Spearman's correlation coefficient, and *indicates the statistical significance (p < 0.05) based on Spearman's correlation. Values are presented as means ± SEM, n = 16 fish/diet. THCA, taurohyocholic acid; CA, cholic acid; TCA, taurocholic acid; UCA, ursocholic acid; ACA, allocholic acid; CDCA, chenodeoxycholic acid; TCDCA, taurochenodeoxycholic acid; CDCA-G, chenodeoxycholic acid-glucuronide; TDCA, taurodeoxycholic acid; GLCA, glycolithocholic acid; TLCA, taurolithocholic acid; LCA-3S, lithocholic acid-3-sulfate; NorDCA, nordeoxycholicacid; 7-ketoLCA, 7-Ketolithocholic acid.
Discussion
Increasing dietary carbohydrates can reduce the protein percentage in the diet, thus lowering the rearing cost and decreasing the ammonia emission into the environment of aquatic animals (16). However, high dietary carbohydrate levels could decrease feed palatability and accelerate satiety, and increase the HSI and liver injury of fish (17). In this study, the feed coefficient (FC) of the HC group was lower than that of the NC group, whereas the HSI showed the opposite change. These findings implied an enhanced nutrients-sparing but the liver injury effect in this fish-fed HC diet. Importantly, the decreased HSI was observed in HC-S (HC supplemented with 2.5% SJ) group compared with the HC group. Similarly, our previous study showed that appropriate SJ supplementation in diet could sustain the liver health in black seabream, which could be linked predominantly to SJ fucoidan (14, 18).
People are still suffering from iodine deficiency disorder (IDD) in some regions around the world, so finding a cost-efficient and healthful iodine supplementation source is extremely necessary (19). It was reported that the flesh iodine content from fish could be improved by increasing dietary iodine (20–22). In addition, marine macroalgae, a potential source of natural mineral additives, meet the concept of sustainable, chemical-free, and organic farming (22). Our results showed that the HC-S diet significantly increased the muscle iodine contents in swamp eel, which is highly appealing to consumers.
Increased serum AST, ALT, and BA, and hepatic glycogen, BA, and collagen fiber are a symbol of liver injury in fish (23, 24). Since bile flow is reduced, BA accumulation in liver cells leads to oxidative stress, apoptosis, and subsequent damage to the liver parenchyma (25). In the present study, fish fed HC diet exhibited high serum GLU, AST, ALT, and BA, and hepatic glycogen, BA, and collagen fiber by biochemical and histological analysis, which were consistent with a previous study in Megalobrama Amblycephala (26) and Micropterus salmoides (4). Interestingly, our results suggest that SJ supplementation may ameliorate liver injury by reducing serum ALT, AST, and BA, and hepatic glycogen, BA, and collagen fiber in swamp eel. Consistently, several reports demonstrated that dietary SJ improved the structure and function of the liver, and decreased serum GLU and BA in mammals (7, 27). However, the effects and underlying mechanism of dietary SJ on liver injury caused by a high carbohydrate diet in fish remain elusive.
It has been reported that acetyl-coenzyme A synthetase (ACSS1), the mitochondrial form of the enzyme, converts acetate to acetyl-CoA in mitochondria, and acetate-activated intra-mitochondrially by ACSS1 can be readily oxidized to CO2 for energy derivation (28). ADH converts ethanol to the aldehyde in the liver of mammals (29). In this study, all DEGs in glycolysis/gluconeogenesis, members of the top 20 significantly enriched pathways, were adh and acss1, wherein in comparison to the NC group, the HC group upregulated the expression of acss1, and simultaneously downregulated the expression of adh by transcriptomic analysis. Accordingly, the HC diet could promote energy derivation by upregulating the expression of acss1, which was consistent with the lower FCR in the HC group. Low enzymatic activity of ADH induced hepatic fibrosis in mice (30). Moreover, activating ADH protects against acute alcohol-induced liver injury in mice (31). The results suggest that the HC diet induces hepatic fibrosis by decreasing the expression of adh.
In addition, IFIT1 is one of the proteins induced by viruses (32). SJ supplemental diet downregulated the expression of ifit1, all the DEGs in the pathway of hepatitis C (members of the top 20 significantly enriched pathway), while HC diet upregulated that DEG. This indicates the protecting role of SJ against viruses in the liver of swamp eel.
Bile acid plays important role in cholesterol, lipid, and even GLU homeostasis (33). However, a high concentration of BA is toxic, and excess BA accumulation induced hepatocyte injury and liver fibrosis in mammals (34) and fish (35). CYP7A1, the rate-limiting enzyme, is thought to be the major contributor to BA synthesis (36). However, excess BA accumulation inhibits the expression of cyp7a1 (37). In this study, the expression of all the DEGs (akr1d1 and cyp7a1, synthesizing BA) in the primary BA synthesis, members of the top 20 significantly enriched pathways, were downregulated in the HC group, whereas the expression of those DEGs was reversely upregulated in the HC-S group. This result indicates that the HC diet induces BA accumulation, and consequently inhibits the expression of cyp7a1. In addition, the accumulated BA was reduced in the HC-S group compared with the HC group, which could cause the upregulation of the expression of cyp7a1 in this study.
Glucuronide conjugates represent up to 10% of the BA circulating pool in healthy people (38), and UGT catalyzes BA detoxification with the process of glucuronidation (39). In this study, SJ supplementation upregulated the expression of ugt, all the DEGs in the pathway of drug metabolism-cytochrome P450 (members of top 20 significantly enriched pathway). This increased upregulated expression of ugt may be attributable to the high BA binding capacity of polysaccharide in SJ (40).
Bile acid toxicity decreases as the number of its hydroxyl increases (41). The BA of UCA, ACA, and CDCA is multi-hydroxy primary bile acid with lower toxicity (42, 43). Strikingly, the changing trends of UCA, ACA, and CDCA were reversely correlated with that of serum GLU and HSI in this study. Moreover, the HC-S diet increased serum UCA, ACA, and CDCA, whereas the HC diet decreased those in this study. These results suggest that UCA, ACA, and CDCA play critical role in regulating GLU homeostasis and reducing liver injury in swamp eel.
In summary, a high-carbohydrate diet resulted in liver injury through increasing the HSI, hepatic glycogen, BA, and collagen fiber, whereas those were decreased by SJ supplemented in a HC diet. Thoroughly, SJ supplementation in the HC diet could improve liver injury, which may be attributed to the suppression of BA synthesis, and decrease in BA toxicity by promoting the process of glucuronidation and increasing the multi-hydroxy BA proportion with hepatic transcriptomic and BA-targeted metabolomic analysis.
Data Availability Statement
The original contributions presented in the study are publicly available. This data can be found here: http://dx.doi.org/10.17632/4ntfwyr9x7.1.
Ethics Statement
The animal study was reviewed and approved by all experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023) in China. This study was approved by the Animal Experiment Ethics Committee of Jiangxi Agricultural University.
Author Contributions
CY contributed to the conceptualization, formal analysis, investigation, and writing—review & editing. LW involved in software. WC contributed to the investigation. WZ presented data curation. ZH involved in visualization. ZW provided resources. ZY provided data curation. MP involved in project administration. HH contributed to the supervision. QZ contributed to the conceptualization, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA (CARS-46) and the National Natural Science Foundation of China (No. 31360641).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.869369/full#supplementary-material
References
1. Xu C, Liu W, Dai Y, Jiang G, Wang B, Li X. Long-term administration of benfotiamine benefits the glucose homeostasis of juvenile blunt snout bream Megalobrama amblycephala fed a high-carbohydrate diet. Aquaculture. (2017) 470:74–83. doi: 10.1016/j.aquaculture.2016.12.025
2. Polakof S, Panserat S, Soengas JL, Moon TW. Glucose metabolism in fish: a review. J Comp Physiol B. (2012) 182:1015–45. doi: 10.1007/s00360-012-0658-7
3. Moon TW. Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B Biochem Mol Biol. (2001) 129:243–9. doi: 10.1016/S1096-4959(01)00316-5
4. Zhang W, Liu K, Tan B, Liu H, Dong X, Yang Q, et al. Transcriptome, enzyme activity and histopathology analysis reveal the effects of dietary carbohydrate on glycometabolism in juvenile largemouth bass. Micropterus salmoides. Aquaculture. (2019) 504:39–51. doi: 10.1016/j.aquaculture.2019.01.030
5. Huang L, Wen K, Gao X, Liu Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm Biol. (2010) 48:422–6. doi: 10.3109/13880200903150435
6. Rajauria G. Seaweeds: a sustainable feed source for live stock and aquaculture. (2015) 15:389–420. doi: 10.1016/B978-0-12-418697-2.00015-5
7. Zhang Q, Fan XY, Guo WL, Cao Y, Lin YC, Cheng WJ, et al. The protective mechanisms of macroalgae Laminaria japonica consumption against lipid metabolism disorders in high-fat diet-induced hyperlipidemic rats. Food Funct. (2020) 11:3256–70. doi: 10.1039/D0FO00065E
8. Lim J, Lee H, Ahn J, Kim J, Jang J, Park Y, et al. The polyherbal drug GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits hepatic steatosis and fibroinflammtion in high-fat diet-induced obese mice. J Ethnopharmacol. (2018) 225:31–41. doi: 10.1016/j.jep.2018.06.034
9. Lee JY, Shimizu H, Hagio M, Fukiya S, Watanabe M, Tanaka Y, et al. 12α-Hydroxylated bile acid induces hepatic steatosis with dysbiosis in rats, Biochim Biophys Acta Mol Cell Biol Lipids. (2020) 1865:158811. doi: 10.1016/j.bbalip.2020.158811
10. Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. (2015) 62:635–43. doi: 10.1002/hep.27744
11. Liu L, Zhou Q, Lin C, He L, Wei L. Integrative analyses of gene expression and alternative splicing to gain insights into the effects of copper on hepatic lipid metabolism in swamp eel (Monopterus albus). Aquaculture. (2022) 546:737367. doi: 10.1016/j.aquaculture.2021.737367
12. Tao T A, Lei Z A, Dy B, Peng LC, Yi HA. Evaluation of dried porcine solubles in diets of rice field eel (Monopterus albus). Aquaculture. (2020) 531:735897. doi: 10.1016/j.aquaculture.2020.735897
13. Deng Y, Wu H, Zhou J, Wu S, Che C, Hu Y, et al. Effects of dietary gelatinized starch on growth performance, glucose metabolism, oxidative status and fillet texture of rice field eel (Monopterus albus). Aquac Res. (2021) 52:5527–36. doi: 10.1111/are.15428
14. Yu CQ, Lin F, Liu GQ, Yu J, Li SK, Wen XB. The suppressive mechanism of dietary macroalgae Saccharina japonica against hepatic lipid accumulation in black seabream A. schlegelii. Aquaculture. (2021) 547:737357. doi: 10.1016/j.aquaculture.2021.737357
15. Zhou QB, Wu HD, Zhu CS, Yan XH. Effects of dietary lipids on tissue fatty acids profile, growth and reproductive performance of female rice field eel (Monopterus albus). Fish Physiol Biochem. (2011) 37:433–45. doi: 10.1007/s10695-010-9444-1
16. Watanabe T. Strategies for further development of aquatic feeds. Fisheries Sci. (2002) 68:242–52. doi: 10.1046/j.1444-2906.2002.00418.x
17. Ali MZ, Jauncey K. Optimal dietary carbohydrate to lipid ratio in African catfish Clarias gariepinus (Burchell 1822). Aquacult Int. (2004) 12:169–80. doi: 10.1023/B:AQUI.0000032065.28059.5b
18. Yu CQ, Lin F, Guo HJ, Liu GQ, He XD, Wen XB. Dietary fucoidan extracted from macroalgae Saccharina japonica alleviate the hepatic lipid accumulation of black seabream (Acanthopagrus schlegelii). Food Funct. (2021) 12:12724–33. doi: 10.1039/D1FO03490A
19. Weng HX, Liu HP, Li DW, Ye M, Pan L, Xia TH. An innovative approach for iodine supplementation using iodine-rich phytogenic food. Environ Geochem Health. (2014) 36:815–28. doi: 10.1007/s10653-014-9597-4
20. Yu CQ, Liu GQ, Yu J, Lin F, Wen XB. Dietary Saccharina japonica is a natural and effective tool to fortify marine teleost black sea bream fillets with iodine: effects on growth, flesh quality, and serum thyroid hormones. J Appl Phycol. (2020) 32:3447–56. doi: 10.1007/s10811-020-02131-2
21. Valente LMP, Rema P, Ferraro V, Pintado M, Sousa-Pinto I, Cunha LM, et al. Iodine enrichment of rainbow trout flesh by dietary supplementation with the red seaweed Gracilaria vermiculophylla. Aquaculture. (2015) 446:132–9. doi: 10.1016/j.aquaculture.2015.05.004
22. Ribeiro AR, Gon Alves A, Colen R, Nunes ML, Dinis MT, Dias J. Dietary macroalgae is a natural and effective tool to fortify gilthead seabream fillets with iodine: effects on growth, sensory quality and nutritional value. Aquaculture. (2015) 437:51–9. doi: 10.1016/j.aquaculture.2014.11.028
23. Ren S, Cai C, Cui G, Ni Q, Ye Y. High dosages of pectin and cellulose cause different degrees of damage to the livers and intestines of Pelteobagrus fulvidraco. Aquaculture. (2020) 514:734445. doi: 10.1016/j.aquaculture.2019.734445
24. Jin M, Pan T, Tocher DR, Betancor MB, Monroig Ó, Shen Y, et al. Dietary choline supplementation attenuated high-fat diet-induced inflammation through regulation of lipid metabolism and suppression of NFκB activation in juvenile black seabream (Acanthopagrus schlegelii). J Nutr Sci. (2019) 8:e38. doi: 10.1017/jns.2019.34
25. Monte MJ, Marin JJ, Antelo A, Vazquez-Tato A. Bile acids: chemistry, physiology, and pathophysiology, World J Gastroenterol. (2009) 15:804–16. doi: 10.3748/wjg.15.804
26. Prisingkorn W, Prathomya P, Jakovlić I, Liu H, Zhao Y, Wang W. Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala). Bmc Genomics. (2017) 18:856. doi: 10.1186/s12864-017-4246-9
27. Zhang Y, Zhao N, Yang L, Hong Z, Cai B, Le Q, et al. Insoluble dietary fiber derived from brown seaweed Laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet-fed mice. Food Funct. (2021) 12:587–601. doi: 10.1039/D0FO02380A
28. Moffett JR, Puthillathu N, Vengilote R, Jaworski DM, Namboodiri AM. Acetate revisited: a key biomolecule at the nexus of metabolism, epigenetics and oncogenesis-part 1: acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front Physiol. (2020) 11:580167. doi: 10.3389/fphys.2020.580167
29. Nakamura Y, Yokoyama H, Okamura Y, Ohgo H, Fukuda M, Horie Y, et al. Evidence for ethanol oxidation by Kupffer cells. Alcohol Clin Exp Res. (1999) 23:92S−5S. doi: 10.1111/j.1530-0277.1999.tb04542.x
30. Han K, Zhang Y, Yang Z. Cilostazol protects rats against alcohol-induced hepatic fibrosis via suppression of TGF-β1/CTGF activation and the cAMP/Epac1 pathway. Exp Ther Med. (2019) 17:2381–8. doi: 10.3892/etm.2019.7207
31. Shi Y, Yu F, Wu Y, Dai L, Feng Y, Chen S, et al. Identification of a novel peptide that activates alcohol dehydrogenase from crucian carp swim bladder and how it protects against acute alcohol-induced liver injury in mice. J Pharm Biomed Anal. (2022) 207:114426. doi: 10.1016/j.jpba.2021.114426
32. Li Y, Li C, Xue P, Zhong B, Mao AP, Ran Y, et al. ISG56 is a negative-feedback regulator of virus-triggered signaling and cellular antiviral response. Proc Natl Acad Sci USA. (2009) 106:7945–50. doi: 10.1073/pnas.0900818106
33. Pauli-Magnus C, Stieger B, Meier Y, Kullak-Ublick GA, Meier PJ. Enterohepatic transport of bile salts and genetics of cholestasis. J Hepatol. (2005) 43:342–57. doi: 10.1016/j.jhep.2005.03.017
34. Jansen PL, Ghallab A, Vartak N, Reif R, Schaap FG, Hampe J, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology. (2017) 65:722–38. doi: 10.1002/hep.28965
35. Cai C, Ren S, Cui G, Ni Q, Cao X. Short-term stress due to dietary pectin induces cholestasis, and chronic stress induces hepatic steatosis and fibrosis in yellow catfish, Pelteobagrus fulvidraco. Aquaculture. (2020) 516:734607. doi: 10.1016/j.aquaculture.2019.734607
36. Noshiro M, Okuda K. Molecular cloning and sequence analysis of cDNA encoding human cholesterol 7 alpha-hydroxylase. Febs Lett. (1990) 268:137–40. doi: 10.1016/0014-5793(90)80992-R
37. Chiang JY. Bile acids: regulation of synthesis, J Lipid Res. (2009) 50:1955–66. doi: 10.1194/jlr.R900010-JLR200
38. Trottier J, Caron P, Straka RJ, Barbier O. 568 fenofibrate increases bile acid glucuronidation in humans: a targeted metabolomic study. J Hepatol. (2012) 56:S225–S388. doi: 10.1016/S0168-8278(12)60581-9
39. Perreault M, Gauthier-Landry L, Trottier J, Verreault M, Caron P, Finel M, et al. The human UDP-glucuronosyltransferase UGT2A1 and UGT2A2 enzymes are highly active in bile acid glucuronidation. Drug Metab Dispos. (2013) 41:1616–20. doi: 10.1124/dmd.113.052613
40. Gao J, Lin L, Sun B, Zhao M. Comparison study on polysaccharide fractions from Laminaria japonica: structural characterization and bile acid binding capacity. J Agric Food Chem. (2017) 65:9790–8. doi: 10.1021/acs.jafc.7b04033
41. Bodin K, Lindbom U, Diczfalusy U. Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta. (2005) 1687:84–93. doi: 10.1016/j.bbalip.2004.11.003
42. Tint GS, Batta AK, Dayal B, Kovell N, Shefer S, Salen G. Metabolism of ursocholic acid in humans: conversion of ursocholic acid to deoxycholic acid. Hepatology. (1992) 15:645–50. doi: 10.1002/hep.1840150415
Keywords: dietary macroalgae, high-carbohydrate diet, fresh-water fish, liver injury, multiple omics
Citation: Yu C, Wang L, Cai W, Zhang W, Hu Z, Wang Z, Yang Z, Peng M, Huo H, Zhang Y and Zhou Q (2022) Dietary Macroalgae Saccharina japonica Ameliorates Liver Injury Induced by a High-Carbohydrate Diet in Swamp Eel (Monopterus albus). Front. Vet. Sci. 9:869369. doi: 10.3389/fvets.2022.869369
Received: 04 February 2022; Accepted: 04 May 2022;
Published: 14 June 2022.
Edited by:
Shourong Shi, Poultry Institute (CAAS), ChinaReviewed by:
Songlin Li, Shanghai Ocean University, ChinaMin Jin, Ningbo University, China
Qiaomu Hu, Chinese Academy of Fishery Sciences (CAFS), China
Copyright © 2022 Yu, Wang, Cai, Zhang, Hu, Wang, Yang, Peng, Huo, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanqi Yu, eXVjcUBqeGF1LmVkdS5jbg==; Qiubai Zhou, emhvdXFpdWJhaUAxNjMuY29t
 Chuanqi Yu
Chuanqi Yu Lu Wang1,2
Lu Wang1,2 Zirui Wang
Zirui Wang Mo Peng
Mo Peng Qiubai Zhou
Qiubai Zhou