- 1Department of Veterinary Epidemiology and Public Health, University of Gondar, Gondar, Ethiopia
- 2Department of Veterinary Biomedical Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Veterinary Paraclinical Studies, University of Gondar, Gondar, Ethiopia
A cross-sectional study was undertaken in four (4) districts of the West Amhara sub-region of Ethiopia with the aim of assessing the diversity and distribution of serotypes of Pasteurella species, their seroprevalence, and associated risk factors, and knowledge, attitude, and practice of farmers toward ovine pasteurellosis. A total of 600 sheep sera were collected using multistage cluster sampling. Each sample was examined for the presence of six (6) serotype-specific antibodies using an indirect haemagglutination test. We are reporting a higher seroprevalence of 90.17% (541/600) in which all seropositive animals were shown to have been co-infected with multiple serotypes. Individual serotype prevalence showed that serotype A7 has the highest prevalence of 77.83% followed by A2 (74.33%), T15 (64%), T4 (62%), PA (60%), and A1 (39.17%). In this study, being female [odds ratio (OR): 2.45, 95% CI (1.09–5.52), p = 0.031] and living in high altitude areas [OR: 20.29, 95% CI (2.54–161.95), p = 0.004] were found to be significantly associated with sero-positivity. A questionnaire survey (n = 384) employed in a face-to-face interview was used to assess the knowledge, attitude, and practice of farmers related to ovine pasteurellosis. Accordingly, the majority (72.4%) of respondents had an inadequate knowledge level of the disease. The proportion of farmers with a favorable attitude and good practices toward the disease was 50.26 and 77.6%, respectively. This study is highly indicative that ovine pasteurellosis is a ubiquitous disease in the study area challenging the sheep production sector. The existence of diverse serotypes reported to lack cross-protective immunity is likely to explain why the current vaccination practice with the mono-serotype Pasteurella multocida biotype A vaccine is not providing adequate protection against outbreaks of the disease. Prioritization of one or more serotypes for inclusion in a multivalent vaccine should be dictated by the abundance and distribution of a particular serotype, its clinical importance, and its resultant economic impact. Furthermore, training farmers on key aspects of the disease is vital in the implementation of effective disease management strategies through a participatory approach. Data from the remaining regions of the country could help realize the development of an effective vaccine that works best at the national level.
Introduction
Sheep production is a key agricultural activity throughout Ethiopia, a country with an enormous sheep population of about 43 million by the year 2020 (1). Several attributes such as adaptability to harsh environmental conditions, less feed and space requirement, high fertility, short reproductive interval, high off-take rate, and low initial investment cost make this segment of livestock a viable option for resource-limited farmers in the country. Sheep are multiple purpose animals offering their owners a wide range of products and services such as meat, milk, skin, hair, wool, bones, manure, security, gifts, and religious rituals (2, 3). Currently, the Ethiopian population is growing at an alarming rate potentially worsening the already existing food insecurity in the country (4). Small ruminant production is vital in ensuring food security (5).
According to investigations in household surveys in Ethiopia, infectious diseases are consistently reported to be the major hurdles constraining smallholder livestock activities (6). Owing to its substantial morbidity and mortality (7, 8), pasteurellosis is ranked as one of the priority diseases threatening sheep production in various regions of the country (6). This is corroborated by various reports of the disease in different parts of Ethiopia (9–13) which underscored the need to understand the epidemiology of the disease and devise effective control strategies. In addition, Ayelet et al. (2004) (9) in their year-long longitudinal study on multifactorial respiratory diseases of sheep, concluded that pneumonic pasteurellosis is a disease of concern to sheep production as well as smallholder farmers.
The etiological agents for ovine pasteurellosis are Pasteurella multocida, Mannheimia hemolytica, and Bibersteinia trehalosi, normal inhabitants of the tonsil and oropharynx of healthy animals. The disease is always initiated when animals are exposed to environmental stressors such as adverse weather (hot or cold), inadequate ventilation, shipping, feed/water shortage, and concurrent infection (14). A remarkable feature of these bacterial agents is the presence of abundant serotypes of clinical and immunological significance (15–17). Based on lipopolysaccharide antigens, P. multocida, can be classified into 16 serotypes which can be regrouped into five (5) serogroups (A, B, D, E, and F) based on passive haemagglutination testing of capsular antigens (18). Several works on P. haemolytica lend to the naming of M. haemolytica and B. trehalosi (19). First, P. haemolytica was classified into two (2) biotypes, A and T, depending on the tendency to ferment arabinose and trehalose, respectively (19). Further re-classification of these biotypes based on their capsular antigens by indirect haemagglutination (IHA) test resulted in 17 serotypes, i.e., A1, A2, A5–A9, A11–A14, A16, and A17 belonging to the P. haemolytica biotype A, and T3, T4, T10, and T15 belonging to P. haemolytica biotype T. Later, P. haemolytica biotype A and P. haemolytica biotype T was renamed as M. haemolytica and B. trehalosi, respectively (20). The classification gets broader with the re-categorization of serotype A11 (due to its unique biochemical features) into a new genus, M. glucosida (21). Therefore, M. haemolytica, B. trehalosi, and M. glucosida represent a collection of 12, 4, and 1 (1) serotypes, respectively. It's worth noting that there are also reports of additional Non-typeable isolates of M. haemolytica further depicting the complexity of these pathogens (17).
Identification of serotypes and their distribution may serve various purposes. Clinical, postmortem, and microbiological evaluation endpoints have shown that different serotypes have varying degrees of virulence and pathogenicity. In addition, they exhibited variations in host preference and adaptability with potential inter-species transmissibility, antigenicity, immunogenicity, and drug resistance pattern potentially impacting the course of the disease as well as a choice of treatment and its outcome (22). Of particular concern to the management of the disease through vaccination, is the lack of cross-protection among the serotypes, i.e., immunity against a given serotype may provide no benefit against infection with other serotypes (23–25). Despite this, the status of circulating serotypes of M. haemolytica, P. multocida, and B. trehalosi in the sheep population in the Amhara region remains largely unknown. Nowadays, a vaccine based on killed P. multocida biotype A (NVI, Bishoftu, Ethiopia) coupled with other management strategies is used on farms throughout Ethiopia to help control the disease, and avoid its drastic consequences (26). However, ovine pasteurellosis remains a major concern with several reports of cases and outbreaks in the Amhara region. A mismatch between the circulating field and vaccinal serotypes is a critical factor impacting vaccine effectiveness particularly when cross-immunity is partial or Non-existent among the different serotypes (27). The other important factor that facilitates the success of control of the disease is farmer/community engagement through a participatory approach (28) which in turn is influenced by the level of knowledge, attitude, and practices (KAP) of farmers toward the disease. Therefore, in this study, we aimed to determine the diversity and distribution of circulating serotypes of M. haemolytica, P. multocida, and B. trehalosi, their prevalence, the predisposing factors, and the KAP of farmers toward ovine pasteurellosis.
Materials and Methods
Study Area
This study was carried out in selected zones of the Western Amhara sub-region, namely, West Gojam, Central Gondar, South Gondar, and North Gondar zones located in Northwest Ethiopia (Figure 1). The sub-region is situated between 10–14° North latitude and 35.1–38.35° East longitude with a total annual rainfall ranging from 878 mm to 2,100 mm, and annual average maximum and minimum temperature of 30.7 and 22°C, respectively. The area is characterized by different agro-climates with subsistence crop-livestock production systems (29) and ranked first in its small ruminant population in the Amhara regional state (1). A significant number of people live through agricultural activities including small-scale farming, supplying food animals, and animal products to the communities and slaughterhouses. Amhara region shares extensive internal boundaries with Benshangul Gumuz, Oromia, and Tigray regional states of Ethiopia, and is bordered by Sudan where transboundary livestock movement is common.
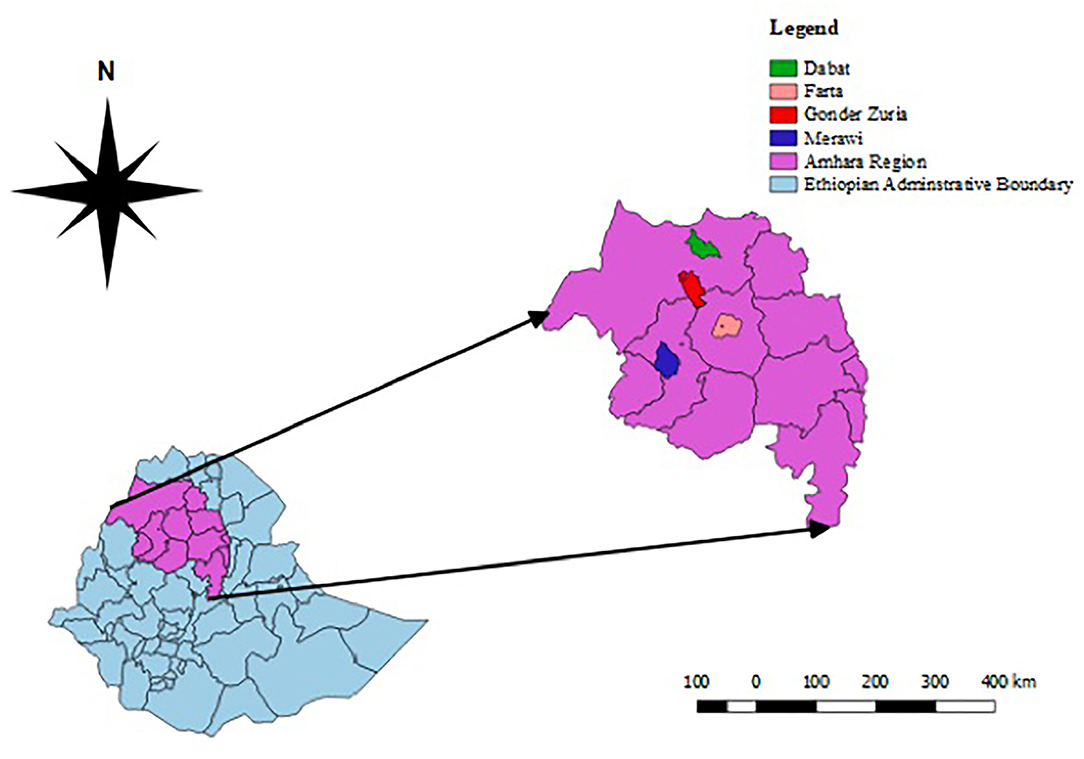
Figure 1. Map depicting the different districts of the study area in the West Amhara sub-region. The map was created by QGIS version 2.18.1 Las Palmas.
Study Design and Study Animals
A cross-sectional study design was used to collect blood samples and questionnaire data from October 2019 to June 2021. The study animals were indigenous breeds of sheep kept under an extensive, semi-intensive, and intensive management system. Sheep were considered for sampling when they were found above 4 months of age and with no history of vaccination against pasteurellosis.
Sampling Technique
Multi-stage cluster sampling method was employed, in which four (4) districts from the Western Amhara sub-region were considered. The selection of districts was based on representativeness in terms of agroecology, an abundance of sheep population, and prior complaints of respiratory diseases. Kebeles (lowest administrative divisions) and villages (sub-divisions of a kebele), and individual animals were selected using simple random and systematic sampling, respectively. Three (3) kebeles from each of two (2) districts and two (2) kebeles from the other two (2) districts were selected based on the proportion of kebeles within the districts. Kebeles with a history of vaccination were excluded from the sampling frame. Finally, three (3) villages from two (2) kebeles and two (2) villages from the remaining eight (8) kebeles were selected proportional to the number of villages available. In this study clusters were villages. Thus, blood samples were collected from a total of 22 villages.
Sample Size Determination
The sample size for the sero-epidemiology was determined using the formula described by Bennett et al. (30).
Where “n” is the sample size, “p” is the prevalence as a percentage, “D” is the design effect, “SE” is the standard error, “g” is the average number of individuals sampled per cluster, and “c” is the number of clusters.
The estimate of intracluster correlation coefficient (ICC) for most infectious diseases does not exceed 0.2 (31). So, taking 0.2 for the cluster (village) and considering the possibility to collect about 30 serum samples (g) in a village, D equals 6.8. Sampling 30 animals per village with an expected prevalence of 50% (as no previous studies were conducted in the study area) and a standard error of 0.05 gave about 22 clusters, and thus a total sample size of 680 animals. However, the total number of samples collected was 600, due to owners' consent during sampling. The clusters were proportionally distributed among 4 districts and 10 kebeles, i.e., 7 clusters (from 6 kebeles) were considered from each of the two districts having 41 and 44 kebeles (Merawi and Gondar zuria districts), and 4 clusters (from 4 kebeles) from each of the other two districts having 32 and 34 kebeles (Farta and Dabat districts).
For the questionnaire survey, sheep owners who consented to participate were recruited for the interview. The sample size was calculated based on the formula described previously (32).
Where “t” is the value for a selected alpha level of 0.025 in each tail which equals 1.96, “p” is the proportion of the population that had knowledge about the disease which is 0.5, “q” proportion of the household who did not have knowledge about the disease which is 0.5, “(p)(q)” is an estimate of variance across the population, and “d” the acceptable margin of error which is equal to 0.05. Thus, 384 households were interviewed.
Data Collection Methods
Blood Sample Collection and Laboratory Analysis
About 8 ml of blood was collected aseptically from the jugular vein of each animal using a plain vacutainer tube. Blood containing tubes were kept horizontally and rendered to clot without agitation for 2 h at room temperature, then samples were transported in an icebox to the microbiology laboratory of the college of veterinary medicine and animal sciences at the University of Gondar, where they were placed tilted overnight at 4°C. Serum was then separated from the clot by centrifugation at 3,000 rpm for 10 min, transferred into labeled cryovials, and kept frozen at −20°C until shipment to the National Veterinary Institute (NVI), Bishoftu, Ethiopia for serotyping. An indirect haemagglutination (IHA) test was performed to determine the presence of serotypes-specific antibody reactions as per the methods described by (33, 34). The presence of a dense clot at the bottom of the microtiter well was considered a positive agglutination reaction while the presence of uniform suspension of the red blood cells indicated a negative reaction. An agglutination rate of >50% was taken as positive (35), and those showing a hemagglutination reaction in 1/20 dilution and above were taken as positive samples (9). Each sample was tested for six (6) different serotypes, namely, A1, A2, A7 of M. haemolytica; T4 and T15 of B. trehalosi; and biotype A of P. multocida. Controls used were sera containing high titer of antibodies against each serotype (positive controls) and sera with no antibodies (negative controls), all obtained from NVI, Bishoftu, Ethiopia.
Questionnaire Survey
A semi-structured questionnaire was used to collect information on the KAP of sheep owners and the predisposing factors favoring the occurrence of the disease in the study areas. The questionnaire comprised three (3) parts: part-I, designed to collect socio-demographic information about the respondents; part-II, intended to assess predisposing factors for the disease; and part-III, purposed to assess the KAP of farmers toward ovine pasteurellosis (Supplementary Material 1). The data were collected through face-to-face interviews using the local language (Amharic). Before starting an actual data collection, the questionnaire was pretested to identify gaps. When assessing KAP, the disease's local name “Gororsa” was used to communicate with farmers, and only those aware of it were considered for the whole interview.
Assessment of Predisposing Factors
Data relevant to evaluating risk factors for the occurrence of ovine pasteurellosis were collected along with blood specimens. The individual animal details such as sex and age were recorded. Disease determinants like flock size, presence/absence of concurrent infection, contact with other flocks, husbandry system, and agro-climatic zones (such as midland and highland) were registered. The ages of individual sheep were estimated according to Abegaz et al. (36) and were categorized into three age-groups: <1 year (young), 1–2 years (adult), and >2 years (old). Regarding geographical attributes, areas with an altitude <1,500 m.a.s.l were categorized as lowland while areas ranging from 1,500 to 2,300 m.a.s.l and >2,300 m.a.s.l as midland and highland, respectively. Sheep flock sizes were categorized as a flock of <5 sheep, 5–10 sheep, and >10 sheep. In addition, location (zones, districts, kebeles, and villages), name of household, and global positioning system point (longitude and latitude altitude) data were taken from the sampling area.
Assessment of Knowledge, Attitude, and Practices of Farmers
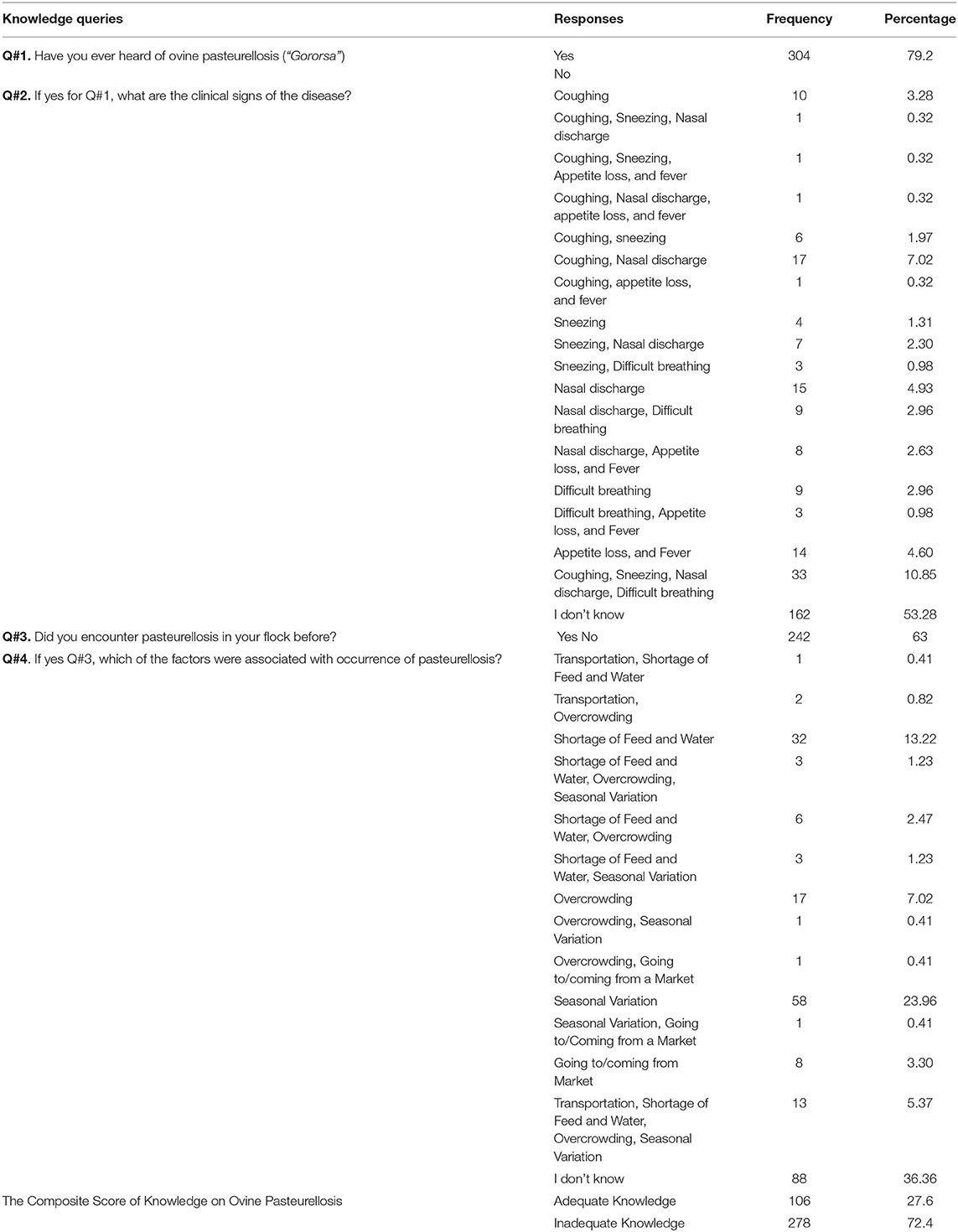
Knowledge was assessed using four (4) questions, two (2) of which were answered as “yes” or “no”, and the remaining two (2) asked farmers to list clinical signs related to the disease, and environmental stressors driving the occurrence of ovine pasteurellosis. The maximum attainable knowledge score was 11 (maximum of one point for each of Q#1 and Q#3, 5 points for Q#2, and 4 points for Q#4) while the minimum score was 0, by giving a score of 1 for correctly answered and 0 for wrongly answered questions. As the knowledge score of respondents was normally distributed, the mean is a good central value, which was then taken as a cut-off value for knowledge level. The knowledge level of respondents was categorized as “good” if the knowledge score was greater or equal to the knowledge mean score and as “poor” if the score was less than the mean knowledge score. The total sum of the knowledge scores was changed into binary outcome as adequate knowledge (for knowledge score ≥50%) and inadequate knowledge (for knowledge score <50%).
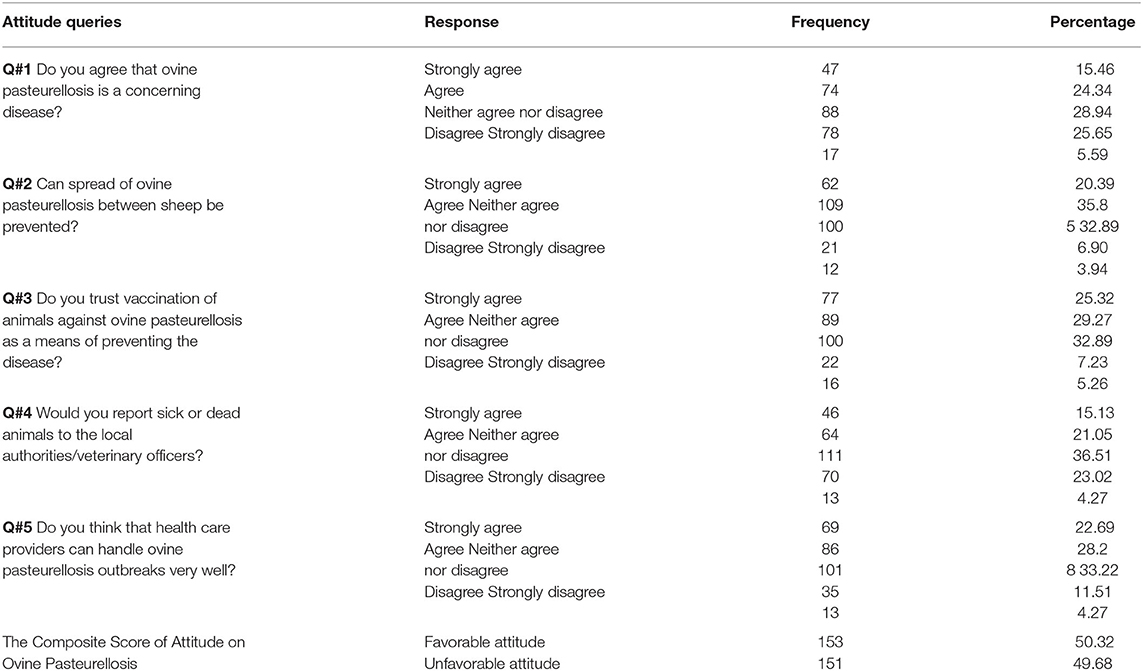
The attitude was assessed using five (5) questions developed as Likert's scale having five components valued as 1 = strongly agree, 2 = agree, 3 = neither agree nor disagree, 4 = disagree, and 5 = strongly disagree (37). As the attitude score was not normally distributed, the median is a good central value, which was then taken as a cut-off value for attitude delineation. Respondents who had scored points greater than or equal to the attitude median score were considered as having desirable/favorable attitudes and those who did score less than the median were considered as having undesirable/unfavorable attitudes toward the disease (38).
The practice was assessed using five (5) questions answered as “yes” or “no” (by scoring 1 for correct answers and 0 for incorrect answers). The maximum and minimum attainable practice scores were 5 and 0, respectively. Practice score was normally distributed and thus mean a good central value which was then taken as a cut-off value for practice level. Respondents who scored greater or equal to the mean practice score were classified as having “good practice” and those who scored below the median practice score were considered as having “poor practice”.
Data Management and Analysis
Raw data collected were entered into a Microsoft Excel spreadsheet (Microsoft Excel 2010, Microsoft Corporate, USA) coded, and then imported into STATA version 14 (Stata Corp., College Station, TX, USA) for statistical analysis. Descriptive statistics involving frequency and percentage were computed to illustrate the characteristics of the study population, KAP of respondents, and the seroprevalence and serotypes distribution in the study areas. Three separate multivariable models were developed to investigate the association of KAP with the socio-demographic characteristics of respondents. Initially, the cumulative score obtained for questions pertaining to the three response criteria (knowledge, attitudes, and practices related to ovine pasteurellosis) was converted into binomial outcomes by categorizing the respondents as having scored above or below the mean or median score for each response criteria. Binary logistic regression was performed with a 95% CI to determine associations between categorical dependent and independent variables. All explanatory variables with a p < 0.25 were included in the multivariable logistic regression models. The reduced subset models were developed using backward elimination based on the AIC (Akaike Information Criteria) score for each model. The final multivariable logistic regression models were evaluated using Pearson's and Deviance residuals and their goodness-of-fit was assessed by the Hosmer-Lemeshow test. Variables with p < 0.05 were retained in the final model.
For analysis of risk factors associated with the sero-positivity, mixed-effect logistic regression was performed. First, the potential risk factor variables were screened using univariable analysis, and then variables with p < 0.25 were subjected to multivariable analysis considering village as a random effect. Those variables screened for the multivariable analyses were checked for co-linearity and none were found collinear. A factor or independent variable of interest was considered to have an influence on sero-positivity at a significance level of below 5%. Nested models were performed by the use of the forward addition method and changes in the beta value (coefficient) of risk factor variables were computed to detect the presence of a confounder. If the change in the beta value of a certain risk factor variable following the addition of a Non-significant variable to the nested model is ≥30%, the added variable is considered a confounder and should be included in the final model. Animal level apparent prevalence was calculated based on sampled population by percentage as:
Results
Seroprevalence of Ovine Pastuerellosis
Of all sheep sampled (n = 600), 541 [90.17%, (95% CI: 88–93)] tested positive for at least one serotype of M. haemolytica, P. multocida, or B. trehalosi. The highest individual animal level apparent prevalence was recorded in North Gondar zone, Dabat district (100%) followed by South Gondar zone, Farta district (98%), West Gojam zone, Merawi district (96.25%) and Central Gondar zone, Gondar zuria district (78.75%) (Figure 2). Seroprevalence was shown to vary significantly among districts (p = 0.001).

Figure 2. Seroprevalence (%) of ovine pasteurellosis in the four zones of West Amhara region. Serum was obtained from those zone as followed, West Gojam (n = 160), South Gondar (n = 100), Central Gondar (n = 240), and North Gondar (n = 100). IHA test was performed in each serum to determine the presence of serotype-specific antibody reactions. An animal is considered sero-positive if it tested positive for at least one of the six (6) serotypes examined (A1, A2, A7, T4, T15, and PA). The district-level prevalence was 96.25% (West Gojam), 98% (South Gondar), 78.75% (Central Gondar), and 100% (North Gondar) (p = 0). PA = Pasteurella multocida biotype A.
Serotyping
As mentioned in the method, each serum sample was tested against six (6) serotypes using the IHA test i.e., A1, A2, A7 of M. haemolytica; P. multocida A; and T4 and T15 of B. trehalosi. Of all, the prevalence of A7 and A2 were the highest (77.83 and 74.33%, respectively), followed by T15 (64%), T4 (62%), P. multocida A (60%), and A1 (39.17%) (Figure 3). The district-level prevalence of each serotype is indicated in Figure 4.
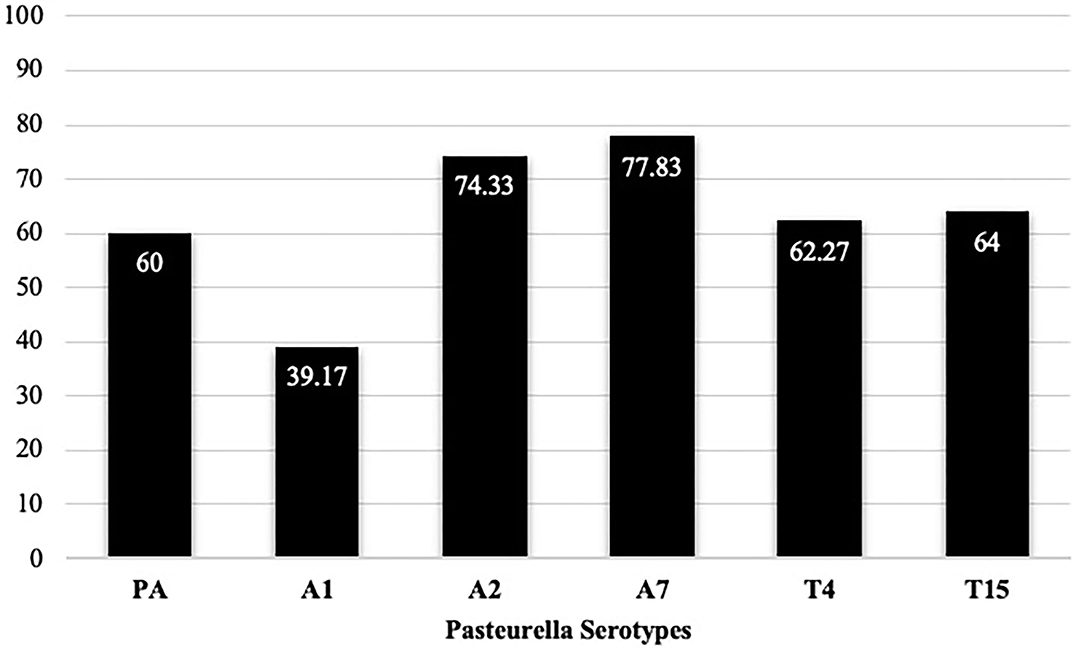
Figure 3. Individual-level serotype prevalence, in which 600 serum samples were collected from the study areas and each sample was subjected to IHA testing against six (6) serotypes. PA = P. multocida biotype A. Percentage of positive samples out of 600 is provided.
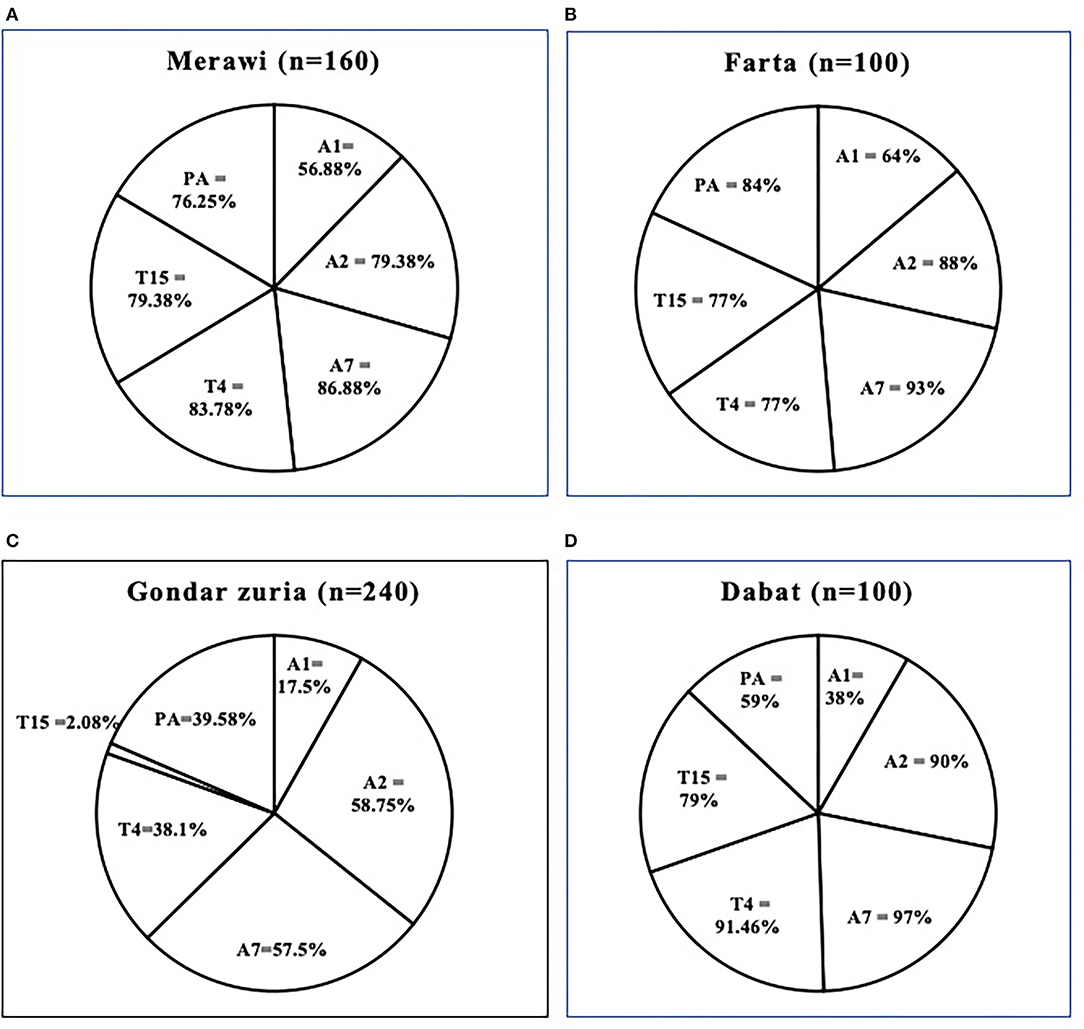
Figure 4. Prevalence and percentage of various serotypes of M. haemolytica, P. multocida, and B. trehalosi for each district: Merawi (A), Farta (B), Gondar Zuria (C), and Dabat (D). All the districts have been shown to have a high prevalence of the six (6) serotypes except for the Gondar Zuria district where T15 had an unusually low prevalence as compared to the other districts. Infection with multiple serotypes is apparent in all the sampled animals. PA = P. multocida biotype A.
Risk Factors Associated With Ovine Pasteurellosis
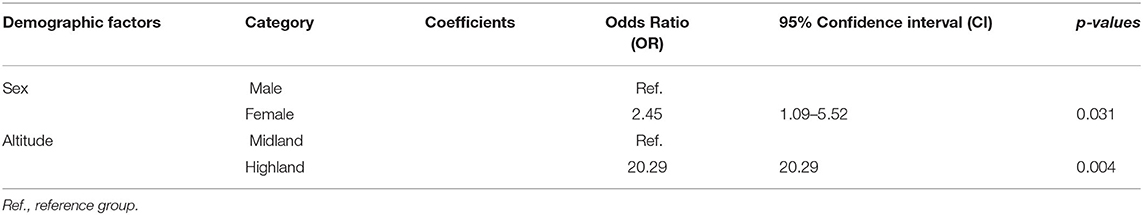
Host and environment-related factors like sex, age, and altitude were included in the multivariable model (p < 0.25). The results of the univariable mixed-effect logistic regression model are provided in Table 1. The final model showed that female sex [OR: 2.45, 95% CI (1.09–5.52), p = 0.031] and higher altitude [OR: 20.29, 95% CI (2.54–161.95), p = 0.004] had a statistically significant association with sero-positivity while there was no interaction effect among sex and altitude (Table 2). The remaining factors appeared Non-significant in the multivariable analysis and had no confounding effect hence omitted from the final model.
Demographic Characteristics of Respondents
A total of 384 respondents were considered from four (4) districts with the following distribution: 96 (25%)—Merawi; 95 (24.74%)—Farta; 100 (26.04%)—Gondar zuriya; and 93 (24.22%)—Dabat. In this survey, the majority of the respondents were males (84.4%, 324/384). Age-wise, respondents were categorized into three (3) groups as: 19–36 years (20.3%; 78/384), 37–55 years (47.1%; 181/384); and above 55 years (32.6%; 125/384). A relatively high number of respondents (44.5%, 171/384) owned a flock of 5 to 10 sheep while 34.6% (133/384) and 20.8% (80/384) had an average flock size of 1–5 and > 10 sheep, respectively. Regarding the respondents' literacy level, 85.9% did not have formal education, 7 and 5.2% had completed primary school and secondary school, respectively, while the remaining 1.8% had a college diploma (Table 3).
Respondents' Knowledge, Attitude, and Practices Pertinent to Ovine Pasteurellosis
Assessment of Knowledge
Questions about the local name, seasonal occurrence, clinical signs, and predisposing factors of ovine pasteurellosis were used to assess knowledge. Of all respondents, most (79.2%) knew ovine pasteurellosis by the local name “Gororsa”, of which more than half (53.28%) were not able to mention any of the clinical signs of the disease. On the other hand, 63% of respondents claimed to have had the disease occurred in their flocks, of which 63.64% knew one or more of the environmental stressors such as transportation, confinement, and shortage of feed or water contributing to the occurrence of the disease. However, comprehensive understanding of the disease was low as most of the respondents (72.4%) had inadequate knowledge about ovine pasteurellosis as shown by the low knowledge composite score (Table 4). Univariable and multivariable logistic regression analyses were carried out to assess the associations of demographic variables with knowledge about the disease as presented in Tables 5, 6. Among these, age and flock size were selected for the multivariable model (p < 0.25). In the final model, age-groups: 37–55 years-old [OR: 2.92, 95% CI (1.43–5.98), p = 0.003] and >55 years-old [OR: 2.44, 95% CI (1.15–5.19), p = 0.02], had an adequate knowledge level about ovine pasteurellosis than the age-group 19–36 years-old. Similarly, having higher numbers of sheep (>10) was significantly associated with a good knowledge level [OR: 2.59, 95% CI (0.39–4.84), p = 0.003] about the disease (Table 6).
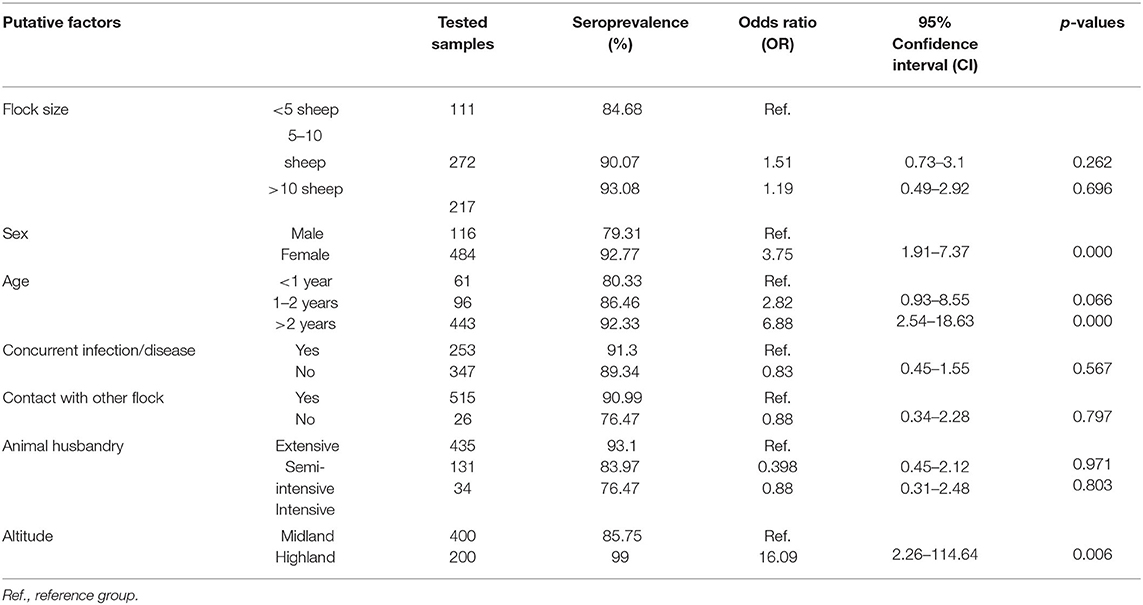
Table 4. Univariable analysis of putative risk factors in relation to serological status using mixed effect logistic regression models including village as a random effect.
Assessment of Attitude
Taking five (5) attitude assessing questions into consideration, the composite score of the respondents having a favorable attitude regarding ovine pasteurellosis was about 50.26% (Table 7). The association between the demographic variables and the attitude of participants toward the disease is presented in Table 8. Both univariable and multivariable logistic regression models were fitted for demonstrating the attitude about the disease. Only sex was found to likely affect the attitude of farmers in the final multivariable logistic regression model. Being a male [OR: 0.56, 95% CI (0.32–0.99), p = 0.046] was significantly associated with having a favorable attitude toward the disease as compared to females.
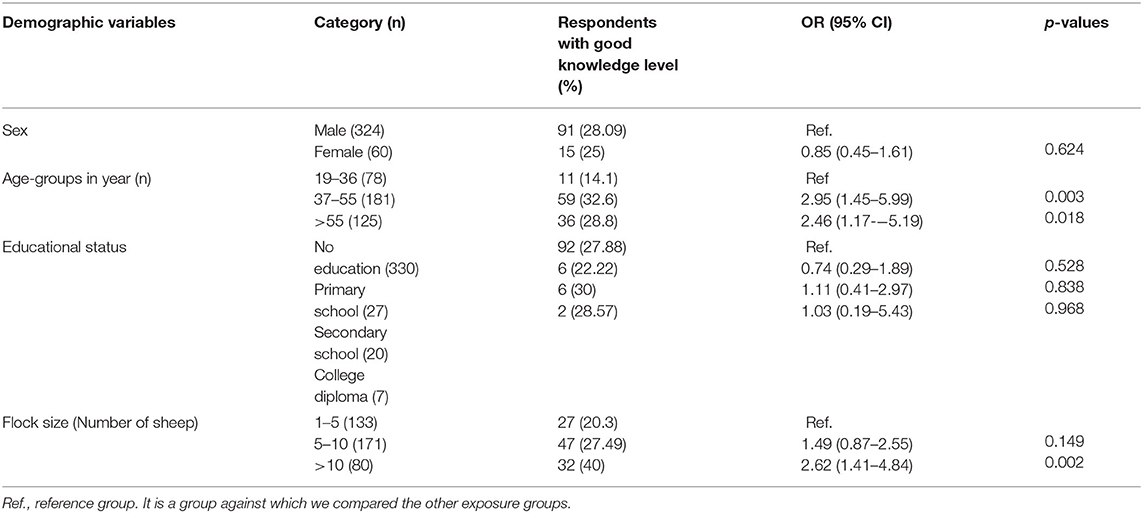
Table 7. Association of demographic variables with knowledge level of participants about ovine pasteurellosis (n = 384).
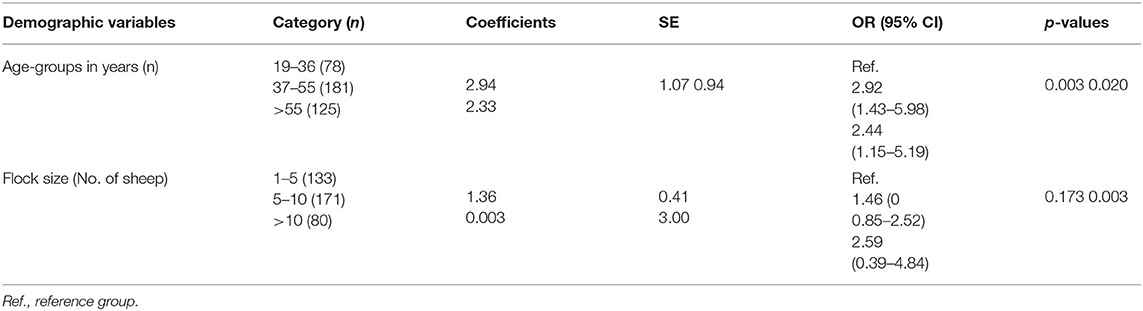
Table 8. Final multivariable logistic regression model of factors associated with respondents' knowledge about ovine pastuerellosis (n = 384).
Assessment of Practice
Taking five (5) practice assessing questions into consideration, the composite score of the respondents having a good practice level was about 77.6% (Table 9). About one-fifth (19.4%) of respondents had to walk their animals in long distances to search for market and veterinary services. In this survey, 93.8% of respondents had the practice of taking sick animals to veterinary clinics for treatment purposes. In addition to seeking veterinary services, 86.5% reported that they drench their sheep with drugs for various diseases including ovine pasteurellosis. Similarly, the majority (88.5%) of them did not practice isolation, i.e., they tend to manage healthy and sick sheep mixed up together. The association between socio-demographic factors and the practice of participants is presented in Table 10. Of all the factors, only age (age-group >55 years old) was found to have a significant association with good practice toward the disease [OR: 2.05, 95% CI (1.01–4.18), p = 0.048] in the final multivariable logistic regression model.
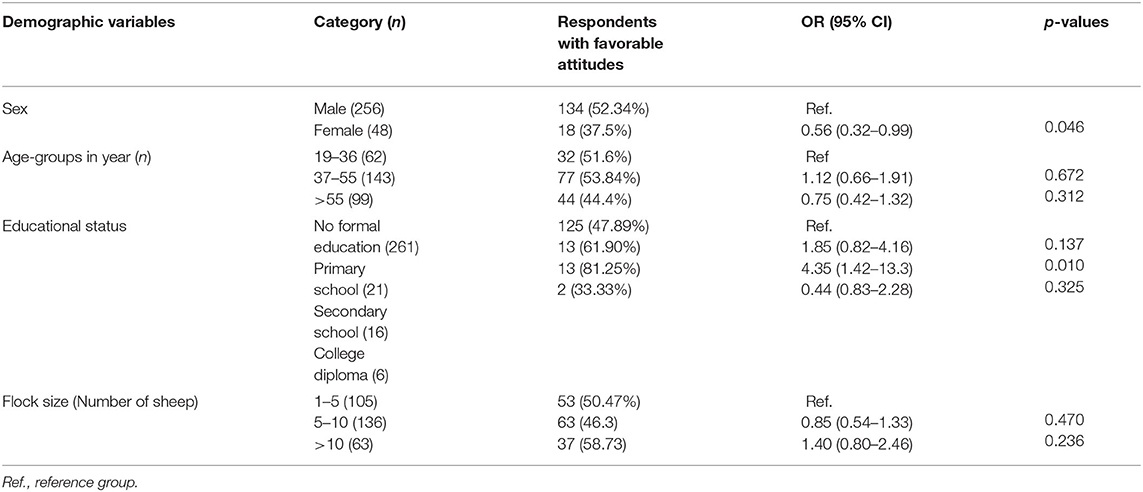
Table 9. Association of demographic variables with attitudes of respondents toward ovine pasteurellosis (n = 304).
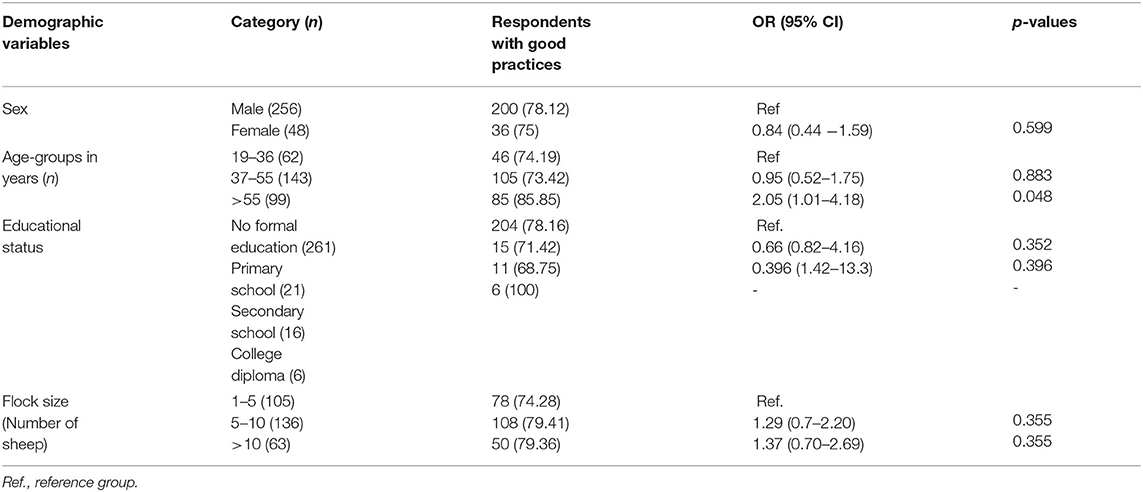
Table 10. Association of demographic variables with practice of animal owners toward ovine pasteurellosis (n = 304).
Discussion
Ruminant production is one of the potential sectors identified by the livestock master plan of the Ethiopian government (LMP, 2015–2020) to achieve food security (39). However, this is constrained by multiple factors. Ovine pasteurellosis due to M. haemolytica, B. trehalosi, and P. multocida, is a priority infectious disease for the sheep production sector causing a substantial economic loss through high mortality and morbidity (6–9). Despite the current prevention and control attempts through vaccination, the problem remains to be a major challenge in Ethiopia including in the Amhara region (26).
In this study, we determined the seroprevalence and distribution of various serotypes of M. haemolytica, P. multocida, and B. trehalosi in the Western Amhara sub-region which informs the dominant serotypes for inclusion in a multivalent ovine Pasteurella vaccine. We also assessed the predisposing factors of ovine pasteurellosis which helps better understand and control the disease in the study area. In addition, we investigated the KAP of farmers/animal caretakers toward the disease which could enable identify and address major gaps for implementation of effective disease management strategies through a participatory approach.
The overall seroprevalence of ovine pasteurellosis in this study was 90.17% which is comparable to the 100 and 83% seroprevalence reported by Berhe et al. (11), and Sisay and Zerihun (8). This high prevalence is an indirect indication that the disease is negatively impacting sheep productivity as well as the livelihood of smallholder farmers which calls for urgent and effective interventions. Despite this, the study could not rule out if this enormous seroprevalence is due to recent infections or the cumulative effect of chronic exposure to the disease. Serotyping of Pasteurella species is vital to inform/dictate the composition of ovine Pasteurella vaccines that best suits the field situation (40). In the current study, we reported six (6) serotypes at different levels of occurrence as followed: A1 (39.17%), A2 (74.83%), A7 (77.83%), P. multocida serotype A (60%), T4 (62%), and T15 (64%). Higher or lower seroprevalence of distinct serotypes could be reported at varied frequencies in different areas (8, 11, 12, 17, 41–43) due to variation in sample size, laboratory methods used, management practices, level of environmental stressors, or exacerbating factors, climatic factors, and occurrence of concurrent pathogens (9). Different serotypes can be associated with a wide range of clinical diseases. Isolates of biotype A of M. haemolytica are associated with respiratory disease in sheep and septicaemia in young lambs, while biotype T isolates have been associated with septicaemia in young adult sheep. All serotypes can be involved in pneumonic pasteurellosis in sheep, but serotype A2 is the most commonly isolated serotype from cases of ovine pneumonic pasteurellosis (17). Serotypes of B. trehalosi are most commonly associated with septicemia and pneumonic pasteurellosis in sheep <2-months-old (44). Young lambs are reported to be affected by polyarthritis due to P. multocida (44). The regular use of mono-serotype vaccine composed of inactivated P. multocida biotype A vaccine (9) in areas intricate with widespread distribution of various serotypes that lack cross-protection (23, 40) may explain why the disease remains a challenge with the current vaccination practices. In addition, co-infection with two or more serotypes on a single animal has been well recorded in almost all sampled (90.17%) animals. Similar findings have been reported by (11, 45) in Northern Ethiopia. Whether multiple serotype infection exhibits a different clinical or immunological outcome as opposed to mono-serotype infections is an area of further investigation.
In this study, the sero-prevalence of Pasteurella was found to be higher in female animals than in males [OR: 2.45, 95% CI (1.09–5.52), p = 0.031] which does not comply with the findings of other studies that observed no association between Pasteurella infection and sex (11, 12, 46). However, it has been reported that female animals are at high risk of succumbing to Pasteurella infections and other diseases during gestation, lambing, and lactation (47). In addition to sex, altitude was shown to influence the prevalence of the disease with high altitude favoring the occurrence of the latter which could be due to the windy, cold and unstable nature of the weather. Nevertheless, flock size, age, husbandry system, and maternal status did not show a statistically significant association with seropositive.
In the questionnaire survey, the proportion of animal owners who heard about ovine pasteurellosis was high (79.2%). Similarly, Mengstie et al. (48) reported that 81% of respondents in their study were aware of ovine pasteurellosis. Given the ubiquity of the disease, and the country having the largest sheep population in Africa, it is likely that most sheep owners could hear/learn about the disease at some point in their life. However, only 46.72% were able to list one or more of the clinical signs of the disease. Similarly, the proportion of respondents who claimed to encounter the disease in their flock was also relatively higher (63%), of which 63.63% were aware that the disease is initiated in the presence of one or more of the stress factors, i.e., mingling with other flocks in market areas, transportation, feed and water shortage, overcrowding, and seasonal variations. Seasonal variations associated with feed availability and weather fluctuation have been reported to drive the occurrence of diseases such as pasteurellosis (47). Collectively, the comprehensive understanding of sheep owners about ovine pasteurellosis was inadequate as indicated by the low composite knowledge score of the majority (74.2%). In this study, respondents age-group 37–55 years old [OR: 2.92, 95% CI (1.43–5.98), p = 0.003] and >55 years old [OR: 2.44, 95% CI (1.15–5.19) p = 0.02] had a good knowledge level about ovine pasteurellosis than age-group, 19–35 years old, which is likely to be associated with the make-up of the families largely comprising adults older than 35 years who may have more experience with managing animals. Likewise, farmers having a relatively higher number of sheep (>10) had a good knowledge level [OR: 2.59, 95% CI (0.39–4.84), p = 0.003] about the disease. Ovine pasteurellosis is favored by overcrowding which induces stress, compromising the immunity of animals thus creating an ideal condition for the occurrence of the disease (47). Large flock size is susceptible to the disease unless sufficiently managed (which is the case with these farmers having less space), thus, frequent encounters with the disease could have increased owners' awareness about the latter.
In this study, a comparable number (50.26%) of participants had a favorable attitude toward ovine pasteurellosis as shown by positive answers on reporting the disease to local veterinary experts, the importance of the disease, its transmissibility, and vaccination of animals as a means to combat it. The fact that farmers trusted vaccination as a means to control and prevent the disease is expected to increase vaccine uptake thus increasing vaccination coverage in the area.
Of the socio-demographic factors, only sex was found to have a significant association with attitude [OR: 0.56, 95% CI (0.32–0.99) p = 0.046]. Being a male is associated with having a favorable attitude toward the disease. This could result from the presence of a larger number of male respondents than females in the questionnaire (84.21%). Nonetheless, it can be anticipated that males are most likely to manage or monitor sheep flocks both in-door as well as in the field which could increase the level of awareness of the disease. In addition, they are the family members that manage to participate in various training and professionals-led community discussions relevant to animal disease. In this study, 77.6% of the respondents followed good practices in relation to ovine pasteurellosis (as shown by the composite practice score). Nevertheless, the majority (86.5%) of them attempt to treat diseased sheep by themselves at their home in addition to seeking veterinary services. Administration of antibiotics by un-professionals is malpractice highly linked to antimicrobial resistance (49, 50) and drug toxicity (51) which requires prompt intervention. Similarly, the majority (88.5%) of the farmers did not practice animal isolation, i.e., they tend to manage healthy and sick sheep mixed up together.
From the demographic variables, only age (>55 years old) was found to seemingly influence practice toward the disease [OR: 2.05, 95% CI (1.01–4.18), p = 0.048]. In the study areas, activities such as consultation with veterinarians /animal health officers, participation in awareness-raising programs, and engagement in disease management are likely to be handled by old family members.
Conclusion
This study showed that ovine pasteurellosis is an important disease of sheep in the study area. An overall seroprevalence of 90.17% was reported with widespread distribution of six (6) serotypes, i.e., A1 (39.17%), A2 (74.83%), A7 (77.83%), P. multocida serotype A (60%), T4 (62%), and T15 (64%). The existence of such diverse serotypes that exhibited little-to-none cross-protection in the study areas where the mono-serotype vaccine is regularly used, is likely to provide no adequate protection against the disease. The judgment of the inclusion of one or more serotypes should be dictated by composite criteria that involve the abundance of individual serotypes, the clinical outcome, and the resultant economic impact. In addition, this study reported the presence of multiple serotype co-infection in the majority (90.17%) of the study animals. The clinical or immunological implication of mixed serotypes co-infection as compared to mono-serotype infection is a subject of further studies. Assessment of risk factors indicated that sex and agro-ecological zones are likely to affect the prevalence of the disease. Female animals need to be managed with precaution particularly during physiologically stressing conditions such as pregnancy, lambing, and lactation to avoid the incidence of the disease. Similarly, flocks in highland areas may require appropriate and timely management during and prior to seasons of weather fluctuation to prevent outbreaks of the disease. Moreover, this study highlighted that farmers in the study areas had low levels of knowledge about key aspects of ovine pasteurellosis. This in turn may call for the provision of various awareness-raising training to improve the knowledge, attitude, and practice of farmers toward the disease for implementation of effective disease control strategy through a participatory approach engaging sheep owners.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Institutional Review Board of University of Gondar. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
SI responsible for the conception of the study and prepared the initial draft of the manuscript. KG, BA, and HG collected the blood samples and the questionnaire data. WM, SM, and KG analyzed the data. SI, WM, SM, BM, and AA supervised the conduct of the study. All authors have read, revised, improved, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work is part of a mega research project funded by the University of Gondar, the office of the vice president for research and community service (Budget code: VPRCS 6223, 2019/20-2020/21) to SI as PI and the co-authors as co-I of the project. We declare that the funding body did not contribute to the execution of any part of the study, interpretation of the results, and write-up of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to laboratory technicians at UoG, CVMAS, and NVI, animal owners, and district veterinarians for their invaluable contribution to the success of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.866206/full#supplementary-material
References
2. Tibbo M. Productivity and Health of Indigenous Sheep Breeds and Crossbreds in Central Ethiopian Highlands. Swedish University of Agricultural Sciences (2006).
3. Getachew T, Haile A, Tibbo M, Sharma A, Sölkner J, Wurzinger M. Herd management and breeding practices of sheep owners in a mixed crop-livestock and a pastoral system of Ethiopia. Afr J Agric Res. (2010) 5:685–91. Availale online at: https://hdl.handle.net/10568/1858
4. Ayenew MM, Kopainsky B editors. Food insecurity in Ethiopia: Population, food production and market. In: 32nd International Conference of the System Dynamics Society. Delft, The Netherlands (2014).
5. Wodajo HD, Gemeda BA, Kinati W, Mulem AA, van Eerdewijk A, Wieland B. Contribution of small ruminants to food security for Ethiopian smallholder farmers. Small Rumin Res. (2020) 184:106064. doi: 10.1016/j.smallrumres.2020.106064
6. Alemu B, Desta H, Kinati W, Mulema AA, Gizaw S, Wieland B. Application of mixed methods to identify small ruminant disease priorities in Ethiopia. Front Vet Sci. (2019) 6:417. doi: 10.3389/fvets.2019.00417
7. Bell S. Respiratory disease in sheep: 1. Differential diagnosis and epidemiology. In Pract. (2008) 30:200–7. doi: 10.1136/inpract.30.4.200
8. Sisay T, Zerihun A. Diversity of Mannheimia haemolytica and Pasteurella trehalosi serotypes from apparently healthy sheep and abattoir specimens in the highlands of Wollo, North East Ethiopia. Vet Res Commun. (2003) 27:3–14. doi: 10.1023/a:1022088005887
9. Ayelet Gelagay LY, Esayas Gelaye, Selam Tariku, Kassahun Asmare. Epidemiologic and serologic investigation of multifactorial respiratory disease of sheep in the central highland of Ethiopia. Int J Appl Res Vet Med. (2004) 2:274–8. Available online at: http://www.jarvm.com/articles/Vol2Iss4/AyeletIJARVMVol2No4.pdf
10. Yimer N, Asseged B. Aerobic bacterial flora of the respiratory tract of healthy sheep slaughtered in Dessie municipal abattoir, northeastern Ethiopia. Revue de médecine vétérinaire. (2007) 158:473. links/599d415b0f7e9b892bb17d17/Aerobic-bacterial-flora-of-the-respiratory- tract-of-healthy-sheep-slaughtered-in-Dessie-municipal-abattoir-northeastern -Ethiopia.pdf
11. Berhe K, Weldeselassie G, Bettridge J, Christley RM, Abdi RD. Small ruminant pasteurellosis in Tigray region, Ethiopia: marked serotype diversity may affect vaccine efficacy. Epidemiol Infect. (2017) 145:1326–38. doi: 10.1017/S095026881600337X
12. Haji H, Abunna F. Epidemiology of Ovine Pasteurollosis in Lume District, East Shewa Zone of Oromiya Region, Ethiopia. Int Know Share Plat. (2016) 6:12–20. Available online at: https://medwinpublishers.com/OAJVSR/OAJVSR16000115.pdf
13. Deressa A, Asfaw Y, Lubke B, Kyule M, Tefera G, Zessin K. Molecular detection of Pasteurella multocida and Mannheimia haemolytica in sheep respiratory infections in Ethiopia. J Appl Res Vet Med. (2010) 8:101. Available online at: http://www.jarvm.com/articles/Vol8Iss2/Deressa.pdf
14. Mohamed R, Abdelsalam E. A review on pneumonic pasteurellosis (respiratory mannheimiosis) with emphasis on pathogenesis, virulence mechanisms and predisposing factors. Bulgarian J Vet Med. (2008) 11:139–60. Available online at: http://www.uni-sz.bg/bjvm/vol11-no3-01.pdf
15. Blanco-Viera F, Trigo F, Jaramillo-Meza L, Aguilar-Romero F. Serotypes of Pasteurella multocida and Pasteurella haemolytica isolated from pneumonic lesions in cattle and sheep from México. Rev Latinoam Microbiol. (1995) 37:121–6. Available online at: https://pubmed.ncbi.nlm.nih.gov/8552871/
16. Fodor L, Varga J, Hajtos I, Molnar T. Serotypes of Pasteurella haemolytica and Pasteurella trehalosi isolated from farm animals in Hungary. Zentralblatt fur Veterinarmedizin Reihe B J Vet Med Series B. (1999) 46:241–7. doi: 10.1111/j.0931-1793.1999.0_224.x
17. Ilhan Z, Keleş I. Biotyping and serotyping of Mannheimia (Pasteurella) haemolytica isolated from lung samples of slaughtered sheep in the Van region. Turk J Vet Anim Sci. (2007) 31:137–41. Available online at: https://dergipark.org.tr/tr/download/article-file/132530
18. Carter G. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. (1955) 16:481−4. Available online at: https://pubmed.ncbi.nlm.nih.gov/13238757/
19. Haig S-J. Adherence of Mannheimia haemolytica to ovine bronchial epithelial cells. Biosci Horizons. (2011) 4:50–60. doi: 10.1093/biohorizons/hzr007
20. Biberstein E, Gills M. The relation of the antigenic types to the A and T types of Pasteurella haemolytica. J Comp Pathol Ther. (1962) 72:316–20. doi: 10.1016/S0368-1742(62)80036-8
21. Villard L, Gauthier D, Lacheretz A, Abadie G, Game Y, Maurin F, et al. Serological and molecular comparison of Mannheimia haemolytica and Pasteurella trehalosi strains isolated from wild and domestic ruminants in the French Alps. Vet J. (2006) 171:545–50. doi: 10.1016/j.tvjl.2005.02.018
22. Odugbo MO, Odama LE, Umoh JU, Lombin LH. The comparative pathogenicity of strains of eight serovars and untypable strains of Mannheimia haemolytica in experimental pneumonia of sheep. Vet Res. (2004) 35:661–9. doi: 10.1051/vetres:2004044
23. Fodor L, Pénzes Z, Varga J. Coagglutination test for serotyping Pasteurella haemolytica. J Clin Microbiol. (1996) 34:393–7. doi: 10.1128/jcm.34.2.393-397.1996
24. Purdy CW, Cooley JD, Straus DC. Cross-protection studies with three serotypes of Pasteurella haemolytica in the goat model. Curr Microbiol. (1998) 36:207–11. doi: 10.1007/s002849900295
25. Gilmour N. Pasteurella haemolytica infections in sheep. Vet Q. (1980) 2:191–8. doi: 10.1080/01652176.1980.9693780
26. Legesse A, Abayneh T, Mamo G, Gelaye E, Tesfaw L, Yami M, et al. Molecular characterization of Mannheimia haemolytica isolates associated with pneumonic cases of sheep in selected areas of Central Ethiopia. BMC Microbiol. (2018) 18:1–10. doi: 10.1186/s12866-018-1338-x
27. Alfelali M, Khandaker G, Booy R, Rashid H. Mismatching between circulating strains and vaccine strains of influenza: effect on Hajj pilgrims from both hemispheres. Hum Vaccin Immunother. (2016) 12:709–15. doi: 10.1080/21645515.2015.1085144
28. Alders RG, Ali SN, Ameri AA, Bagnol B, Cooper TL, Gozali A, et al. Participatory epidemiology: principles, practice, utility, and lessons learnt. Front Vet Sci. (2020) 7:1–19. doi: 10.3389/fvets.2020.532763
29. Adugna A. Amhara Demography Health. Bulletin. (2014). http://www.ethiodemographyandhealth.org/ (accessed 11 August, 2019).
30. Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat Q. (1991) 44:98–106. Available online at: https://apps.who.int/iris/handle/10665/47585
31. Otte M, Gumm I. Intra-cluster correlation coefficients of 20 infections calculated from the results of cluster-sample surveys. Prev Vet Med. (1997) 31:147–50. doi: 10.1016/S0167-5877(96)01108-7
32. Kotrlik J, Higgins C. Organizational research: determining appropriate sample size in survey research appropriate sample size in survey research. Inf Tech Learn Perform J. (2001) 19:43. Available online at: https://www.opalco.com/wp-content/uploads/2014/10/Reading-Sample-Size1.pdf
33. Biberstein E, Gills M, Knight H. Serological types of Pasteurella hemolytica. Cornell Vet. (1960) 50:283–300. Available online at: https://pubmed.ncbi.nlm.nih.gov/13800458/
34. Biberstein E, Thompson D. Epidemiological studies on Pasteurella haemolytica in sheep. J Comp Pathol. (1966) 76:83–94. doi: 10.1016/0021-9975(66)90050-8
35. Frank GH, Wessman G. Rapid plate agglutination procedure for serotyping Pasteurella haemolytica. J Clin Microbiol. (1978) 7:142–5. doi: 10.1128/jcm.7.2.142-145.1978
36. Abegaz S, Awgichew K. Estimation of Weight and Age of Sheep and Goats. ESGPIP (Ethiopia Sheep and Goat Productivity Improvement Program). Technical Bulletin (2009).
37. Chyung SY, Roberts K, Swanson I, Hankinson A. Evidence-based survey design: the use of a midpoint on the Likert scale. Perform Improv. (2017) 56:15–23. doi: 10.1002/pfi.21727
38. Ahmed T, Hussain S, Zia U-u-R, Rinchen S, Yasir A, Ahmed S, et al. Knowledge, attitude and practice (KAP) survey of canine rabies in Khyber Pakhtunkhwa and Punjab Province of Pakistan. BMC Public Health. (2020) 20:1–12. doi: 10.1186/s12889-020-09388-9
39. Shapiro BI, Gebru G, Desta S, Negassa A, Negussie K, Aboset G, et al. Ethiopia Livestock Master Plan: Roadmaps for Growth and Transformation. Addis Ababa, Ethiopia: ILRI Editorial and Publishing Services (2015).
40. Gilmour N, Gilmour J. Pasteurellosis of sheep. In: Adlam C, Rutter JM, editors. Pasteurella and Pasteurellosis. London, UK: Academic Press (1989).
41. Ferede Y, Mekuriaw S, Mazengia H, Amane A. Sero-typing and evaluation of the level of protective antibody titer in Northwest Ethiopian sheep before and after ovine Pasteurollosis vaccination. Int J Pharm Med Bio Sc. (2013) 2:58–64. Available online at: http://www.ijpmbs.com/uploadfile/2015/0412/20150412033816770.pdf
42. Hussein A, Mohamed OE. A serological survey of sheep sera for antibodies to Pasteurella haemolytica serotypes in the Sudan. Revue d'élevage et de médecine vétérinaire des pays tropicaux. (1984) 37:418–21. Available online at: https://pubmed.ncbi.nlm.nih.gov/6545836/
43. Thompson D, Fraser J, Gilmour N. Serotypes of Pasteurella haemolytica in ovine pasteurellosis. Res Vet Sci. (1977) 22:130–1. doi: 10.1016/S0034-5288(18)33329-0
44. Fleming S. Overview of Pasteurellosis in sheep and goats. MSD Vet Man. (2018). Available online at: https://www.msd-animal-health.ie/species/sheep/pasteurellosis/
45. Assefa GA, Kelkay MZ. Goat pasteurellosis: serological analysis of circulating Pasteurella serotypes in Tanqua Aberegelle and Kola Tembien Districts, Northern Ethiopia. BMC Res Notes. (2018) 11:1–5. doi: 10.1186/s13104-018-3606-0
46. Marru HD, Anijajo TT, Hassen AA. A study on Ovine pneumonic pasteurellosis: Isolation and Identification of Pasteurellae and their antibiogram susceptibility pattern in Haramaya District, Eastern Hararghe, Ethiopia. BMC Vet Res. (2013) 9:1–8. doi: 10.1186/1746-6148-9-239
47. Quinn P, Markey BK, Carter M, Donnelly W, Leonard F. Veterinary Microbiology and Microbial Disease. Blackwell science (2002).
48. Mengstie F. Serotyping and identification of predisposing factors for the occurrence and distribution of ovine pasteurellosis in Bonga sheep breed. Asian J Med Biol Res. (2015) 1:175–81. doi: 10.3329/ajmbr.v1i2.25608
49. Geta K, Kibret M. Knowledge, attitudes and practices of animal farm owners/workers on antibiotic use and resistance in Amhara region, north western Ethiopia. Sci Rep. (2021) 11:1–13. doi: 10.1038/s41598-021-00617-8
50. Mutua F, Sharma G, Grace D, Bandyopadhyay S, Shome B, Lindahl J, et al. A review of animal health and drug use practices in India, and their possible link to antimicrobial resistance. Antimicrob Resist Infect Control. (2020) 9:1–13. doi: 10.1186/s13756-020-00760-3
Keywords: B. trehalosi, indirect haemagglutination, M. haemolytica, ovine pasteurellosis, P. multocida, predisposing factors, seroprevalence
Citation: Getnet K, Abera B, Getie H, Molla W, Mekonnen SA, Megistu BA, Abat AS, Dejene H, Birhan M and Ibrahim SM (2022) Serotyping and Seroprevalence of Mannheimia haemolytica, Pasteurella multocida, and Bibersteinia trehalosi and Assessment of Determinants of Ovine Pasteurellosis in West Amhara Sub-region, Ethiopia. Front. Vet. Sci. 9:866206. doi: 10.3389/fvets.2022.866206
Received: 31 January 2022; Accepted: 28 March 2022;
Published: 19 May 2022.
Edited by:
Julio Alvarez, VISAVET Health Surveillance Centre (UCM), SpainReviewed by:
Chengming Wang, Auburn University, United StatesRahat Zaheer, Agriculture and Agri-Food Canada, Canada
Copyright © 2022 Getnet, Abera, Getie, Molla, Mekonnen, Megistu, Abat, Dejene, Birhan and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saddam Mohammed Ibrahim, c2FkaW1vaGFtZWQ2QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Kalkidan Getnet1†
Kalkidan Getnet1† Wassie Molla
Wassie Molla Sefinew Alemu Mekonnen
Sefinew Alemu Mekonnen Anmaw Shite Abat
Anmaw Shite Abat Haileyesus Dejene
Haileyesus Dejene Saddam Mohammed Ibrahim
Saddam Mohammed Ibrahim