- 1Department of Zoonoses, Faculty of Veterinary Medicine, Assiut University, Asyut, Egypt
- 2Department of Surgery, Anesthesiology and Radiology, Faculty of Veterinary Medicine, Assiut University, Asyut, Egypt
- 3Department of Microbiology and Immunology, Faculty of Medicine, Assiut University, Asyut, Egypt
- 4Department of Biotechnology, College of Science, Taif University, Taif, Saudi Arabia
- 5Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
Bartonellosis is a vector-borne zoonotic disease caused by the intracellular bacterium of genus Bartonella. The disease has a worldwide distribution and cats represent the major reservoir of this disease. Despite its global distribution, very limited previous studies have investigated the occurrence of bartonellosis in cats and their owners in Egypt. In an endeavor to explore this topic, we investigated the occurrence of Bartonella henselae (B. henselae) infection in 225 samples (blood, saliva, and claw) obtained from 75 healthy cats in Upper Egypt. These samples were routinely obtained during veterinary clinic visits. This study also involved an examination of 100 humans, including cat owners and people with a history of contact with cats. Attempted isolation and identification of B. henselae in cats were also performed. Furthermore, PCR was performed for molecular identification of B. henselae in blood samples from cats. Meanwhile, an immunofluorescent assay was performed to study the seroprevalence of B. henselae infection in humans. In this study, B. henselae could not be isolated from any of the examined blood, saliva, or claw samples from cats. Interestingly, B. henselae was identified molecularly in 8% (6/75) of blood samples from cats. The seroprevalence of B. henselae in humans was 46% and its occurrence was higher in females (46.6%) than in males (41.7%) (P = 0.748). B. henselae infection was higher among cat owners [51.4% (19/37)] than among people with a history of contact with cats [42.9% (27/63)] (P = 0.410). Infection was higher in rural regions [79.5% (31/39)] than in urban regions [24.6% (15/61)] (P < 0.001). Collectively, this data provide interesting baseline information about the occurrence of B. henselae in cats and humans in Upper Egypt, which reflects the potential zoonotic transmission of this bacterium. Future study is mandatory to explore the occurrence of B. henselae in major reservoirs in Egypt.
Introduction
Bartonellosis is a vector-borne zoonotic disease with a worldwide distribution (1–3). This disease is mainly caused by the Gram-negative facultative intracellular bacterium of genus Bartonella (4). Among others, infection by Bartonella henselae (B. henselae) is considered the most common species of the bacterium, which is associated with cat-scratch disease (4, 5). The epidemiological profile of this disease includes humans and a wide range of mammalian hosts, mainly companion animals. Cats, particularly kittens, represent the major reservoir of B. henselae (6). Furthermore, a wide range of blood-sucking arthropods, such as fleas, biting flies, sandflies, mosquitos, lice, and ticks, have been considered as competent vectors. In addition, some studies have revealed that rodent-associated Bartonella species and their ectoparasites in different regions of the world can cause bartonellosis in humans (7, 8). Exposure to infected flea feces appears to be the main route of infection in cats, while some other reports have indicated that ingestion of infected fleas or infected feces could facilitate transmission (9, 10). Another previous study (11) illustrated that ticks (mainly Ixodes ricinus) may play a role in the transmission of B. henselae and other Bartonella species among cats through trans-stadial transmission. Humans can contract bartonellosis through bites or scratches from cats, which act as mechanical methods of transmission, in addition to skin lesions infected by inanimate objects, such as thorns or pins (12).
In accordance with its worldwide distribution and presence as a public health concern, the etiological agent is distributed on all the continents among major reservoirs. For example, ~12,000 Americans contract bartonellosis each year and around 500 of these are admitted to hospitals (13). In accordance with its clinical impact, B. henselae has been implicated as a cause of acute bacterial disease, which primarily affects the lymph nodes, skin, and internal organs. The reported series of symptoms includes fever, erythematous papules, pustules, ulcers, and unilateral lymphadenitis (14). Naturally infected cats are mostly asymptomatic, but some animals develop cardiac syndromes, such as endocarditis or myocarditis, in addition to ocular complications in some cases (15). Although the prevalence of B. henselae infection in cats significantly fluctuates, the highest rates of infection occur in temperate regions where conditions are most favorable for the development of Ctenocephalides felis (16). Meanwhile, in humans, B. henselae can result in a series of diseases, such as lymphadenopathy, bacteremia, bacillary angiomatosis, and bacillary peliosis (17). Revising the available literature, very limited previous studies have investigated the occurrence of B. henselae in Egypt. Thus, this study aimed to explore the potential role of cats as a major reservoir of B. henselae infection. Moreover, this study explored the seroprevalence of B. henselae infection in humans who come into contact with infected animals.
Materials and Methods
Ethical Considerations
This study was ethically reviewed and approved by the Scientific Research Committee and Ethics Board of Assiut University, Egypt (institutional review board ethics approval number: 17300307).
Sampling
Cat Samples
A total of 225 samples were collected from 75 apparently healthy cats from Asyut Governorate, Egypt, during the period from March 2016 to March 2018. These samples were routinely collected during veterinary clinic visits. The full details of the study cohort of cats are shown in Table 1. Three types of sample, namely, blood, saliva, and claw, were collected from each cat. For blood samples, 1 ml of blood was collected from the cephalic vein of each cat under complete aseptic conditions in sodium citrate vacuum tubes before being stored at −20°C for culture examination. Salivary and oral swabs were taken using a sterile cotton applicator placed against the inside surface of each cat's cheek. These swabs were then suspended in 5 ml of brain heart infusion (BHI) broth with Brucella growth supplement, as previously described (18). Meanwhile, claw samples were collected in 5 ml of BHI broth (Biolife, code: 1230) with Brucella growth supplement (18).

Table 1. The full details of the study cohort for each of the enrolled cat's sex, age, and living habits.
Human Samples
A total of 100 blood samples were collected from human participants. Specifically, 37 samples were collected from owners of cats (N = 37) and 63 samples were collected from people with a history of contact with cats (N = 63) that means those people had a history who raised cats in their homes. All the participants were healthy and the full details of the study cohort and their results are shown in Table 2. Blood samples were collected without anticoagulation from humans and collection tubes were left in a standing position for 20–30 min before being centrifuged at 3,000 rpm for 15 min. Sera samples were stored at −20°C until further examination.
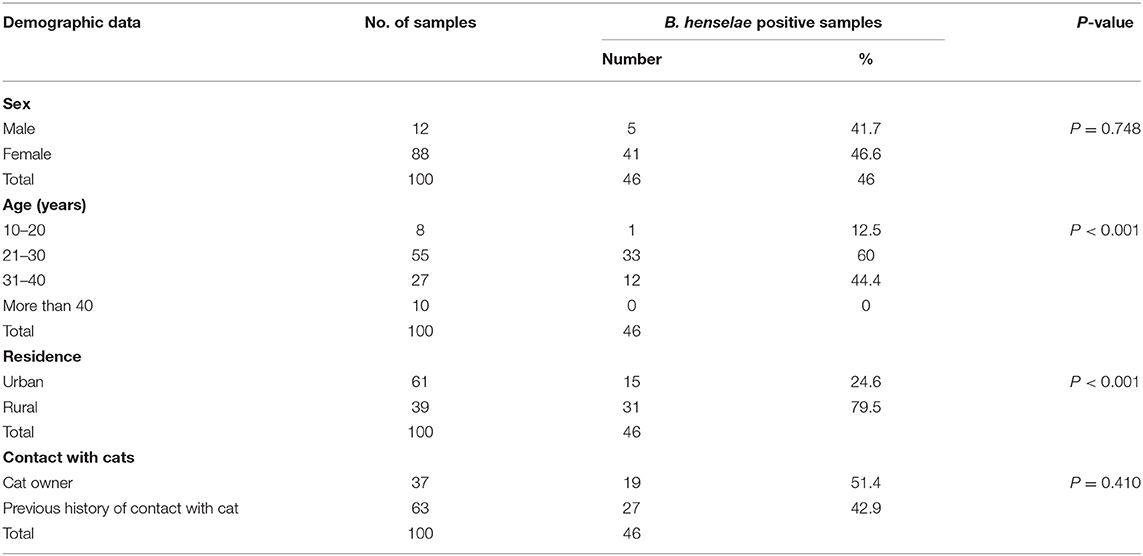
Table 2. Seroprevalence and epidemiological data associated with the occurrence of Bartonella henselae (B. henselae) infection in humans.
Isolation of Bartonella henselae From Cat Samples
Blood samples from cats were defrosted and centrifuged at 3,800 rpm for 70 min, as described previously (19). The pellets were then inoculated on BHI agar (Biolife, Ref. 4012352) containing 5% sheep blood that was confirmed to be Bartonella negative (20, 21) and 2% Brucella growth supplement (HiMedia, FD005) and then incubated at 35°C in an atmosphere of 5% carbon dioxide (CO2) for 4–8 weeks (18, 22). Oral swabs and claw samples were enriched in 5 ml of BHI broth containing 5% sheep blood and Brucella growth supplement, before being incubated at 35°C in an atmosphere of 5% CO2 for 10 days (21, 23, 24). A loopful of each sample was plated on BHI agar with 5% sheep blood and 2% Brucella growth supplement, before being incubated at 35°C in an atmosphere of 5% CO2 for 4–8 weeks (18, 22). The cultured plates were continuously checked following inoculation for any colony formation. If any, the pure colonies were then subjected to identification through colony morphology, Gram staining, and certain biochemical tests, including the oxidase and catalase tests (25).
Molecular Detection of Bartonella henselae Among Cats
This step involved molecular detection of B. henselae in cat samples, which was performed as described below.
Deoxyribonucleic Acid Extraction From Blood Samples
Deoxyribonucleic acid extraction from the blood samples of cats was performed using the QIAamp DNA Mini Kit (Ref. 51403) according to the manufacturer's instructions.
Polymerase Chain Reaction Amplification
This step involved targeted amplification of the citrate synthase gene (gltA) of B. henselae, as described previously (26) with slight modifications. The following species-specific primers were used: BartogltA forward: 5′-TTCCGYCTTATGGGTTTTGG-3′ and Bartohenselae: 5′-CATTTCTGTTGGAAATCCTAG-3' with an amplicon size of 246 bp. A thermal cycler (Biometria, T Professional) was used for DNA amplification. Briefly, the PCR reaction mixture volume was 25 μl, which comprised 13 μl of master mix, 1 μl of forward primer (0.40 p/mol), 1 μl of reverse primer (0.40 p/mol), and 100 pg/μl to 100 ng/μl DNA template and deionized distilled water was added to the reaction to make up the final volume. The amplification included an initial denaturation step of 5 min at 95°C, followed by 35 cycles of 45 s at 95°C, 45 s at 56°C, and 45 s at 72°C, with a final extension step at 72°C for 7 min. Five microliter of each of the resultant amplified PCR products was analyzed in 1.6% (w/v) agarose gels in Tris-acetate-ethylenediaminetetraacetic acid (EDTA) buffer stained with ethidium bromide, transilluminated under ultraviolet light, and photographed. The AMPLIRUN® B. henselae DNA Control (Vircell, Ref MBC005) was used as the positive control, while purified water was used as the negative control. The controls were processed in parallel with the experimental samples to detect possible contamination.
Serological Detection of Bartonella henselae in Human Sera Samples
In this step, an indirect immunofluorescence immunoglobulin G (IgG) kit of B. henselae (Vircell, lot 16 BHQ307) was used to examine human sera samples according to the manufacturer's instructions. A B. henselae IgG antibody titer of ≥1:64 was considered as an indicator of infection.
Statistical Analysis
All the data were analyzed using Statistical Package for the Social Sciences (SPSS) software version 17. The data were subjected to ANOVA using the chi-squared procedure of SPSS software. A probability value (P-value) of < 0.05 was considered as statistically significant.
Results and Discussion
Detection of Bartonella spp. in Samples From Cats
The diagnosis of zoonotic pathogens in major reservoirs remains one of the main methods to control this category of diseases (27–31). This study provides a novel contribution by investigating the occurrence of B. henselae in cats and humans in Egypt. Attempts were also made to isolate and identify B. henselae from cats bacteriologically. B. henselae was identified in cats using molecular methods. Serological detection of B. henselae IgG from human sera samples was also achieved using indirect immunofluorescence.
In this study, B. henselae could not be isolated on BHI agar from any of the examined blood, saliva, or claw samples from cats, which is consistent with several previous studies (18, 32). The inability to culture B. henselae may be due to the difficulty in isolating B. henselae or due to the presence of inactive bacteria (non-culturable) in the examined samples, as reported in a previous study (33). However, it should be taken into consideration the incubation time or the medium used and the level of bacteremia that might influence the isolation of Bartonella on culture, in addition to the fastidious nature of this bacteria, making it problematic to isolate from culture media and lower the sensitivity of this method (23, 34–36). In contrast, Bartonella spp. were previously cultured from blood samples obtained from 7% (7/100) of cats in Spain (37). It is noteworthy that molecular methods remain among the most accurate for the detection of pathogens in their major reservoirs (38). In accordance with the molecular detection of B. henselae in blood samples from cats (Table 3), a total of 75 blood samples from cats were examined. The results showed that 8% (6/75) of the samples were positive for B. henselae. Similar results were reported in cats from the Czech Republic (8%) (39), Turkey (8.2%) (40), and Switzerland (8.3%) (41). However, lower prevalences of B. henselae were reported in previous studies in different countries, including Portugal (6.7%) (42) and Sweden (2.2%) (43), using PCR. In stark contrast, a previous serological study in Egypt reported a prevalence rate of 59.6 in cats from Cairo, Egypt (44). Likewise, higher rates of B. henselae infection were detected in Japan (9.1%) (45), the United Kingdom (9.4%) (46), Germany (13%) (22), South Brazil (17.02%) (47), Italy (18%) (48), South Korea (33.3%) (49), and Poland (40.48%) (50). The differences in the reported rates of infection among cats in different countries might be attributed to the influence of climatic conditions because the gradient of infection increases from cold climates (0% in Norway) to warm and humid climates (68% in the Philippines) (51). However, the influence of flea infestation, which is more likely in warm and humid areas than in cold areas, should also be considered (52, 53). Furthermore, other factors, including sample size, environmental conditions, hygienic practices and socioeconomic level, type of diagnostic method (either serological or molecular), type of PCR and primers used, and the studied population (stray vs. household cats), might influence the differences in the reported rates of infection by B. henselae among cats in different countries (35, 54–58). It is evident that the molecular detection of B. henselae is more reliable and sensitive than culture techniques to identify the microorganism, as documented in several previous studies (47, 59, 60).
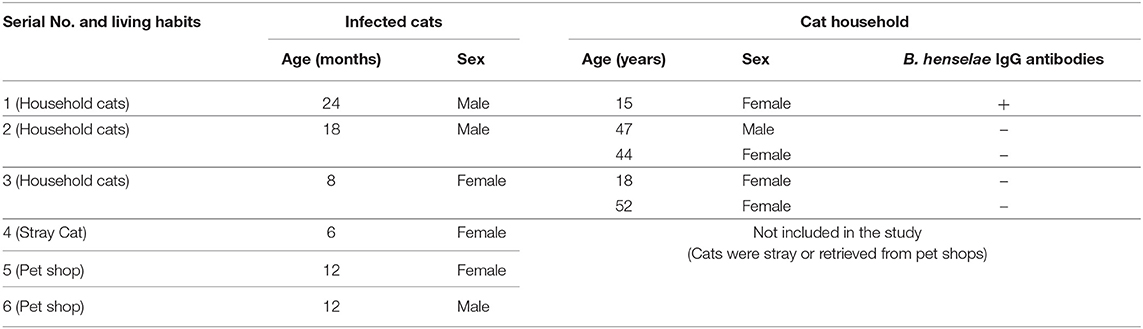
Table 3. Correlation between the results B. henselae infected cats and their corresponding household.
Seroprevalence of B. henselae Infection in Humans
Several serological techniques have been developed as cheap and rapid methods to diagnose many infectious agents. Given the fact that it is difficult to isolate Bartonella spp. from major reservoirs, serological tests (mainly immunofluorescent assay) confer many advantages in the diagnosis of bartonellosis in humans (61). In this study (Table 2), the overall seroprevalence of B. henselae IgG in human sera samples was 46%, which is higher than that reported in a previous study in China (9.68%) (62). However, the seroprevalence in this study was lower than that recorded in veterinarians and cat owners in Poland (53.3%) (63). This variation in the reported seroprevalence might be associated with the risk of Bartonella infection in humans, which is lower at northern latitudes than in countries with warm climates (6).
In accordance with the studied epidemiological pattern and potential risk factors for infection, which are shown in Table 2, this study revealed that the rate of infection in females (46.6%) was higher than in males (41.7%), although this difference was not statistically significant (P = 0.748). Similar results were reported in a previous study in the United States (13). However, no significant difference in the seroprevalence of B. henselae between males (7%) and females (4.7%) was reported in Thailand, which is in harmony with our present findings (54). This difference in the prevalence of B. henselae between males and females in various studies might reflect that sex is not a risk factor for B. henselae infection. Importantly, as shown in Table 2, the highest rate of B. henselae infection in humans (60%) was observed in the 21–30-year age group, followed by the 31–40-year age group (44.4%), while the lowest seroprevalence (12.5%) was detected in the 10–20-year age group. None of the groups aged >40 years demonstrated B. henselae infection, but the differences between the age groups were significant (P < 0.001). Similar results were reported in a previous study by Maruyama et al. (54) conducted in Thailand. On the contrary, no difference in IgG seropositivity was found between children and adults in another study in Croatia (64). Several previous studies revealed that bartonellosis occurs more frequently among children (65–67). The differences in prevalence rates and its association with age as a potential individual variable factor could be attributed to the development of immunity with age, specific host–pathogen interactions, and history of previous exposure to infection, which results in an acquired immunity following exposure (64, 68, 69).
In terms of the seroprevalence of B. henselae infection in humans in relation to residence (Table 2), a significantly higher rate (P < 0.001) of B. henselae infection was observed in humans in rural (79.5%) compared with urban (24.6%) areas. The present results are in harmony with those reported in another study (70). In stark contrast, no significant difference in IgG positivity was recorded among Croatian patients living in urban areas (44.1%) and others living in rural areas (44.8%) (64). The possible explanation for this high seroprevalence of B. henselae in rural areas could be the lower socioeconomic level and poorer hygienic measures in these regions, lack of awareness on dealing with cat scratches and bites, and a higher incidence of flea infestation in these regions than in urban areas (71). Table 2 depicts that the seroprevalence of B. henselae in cat owners was higher (51.4%) than in people with a history of contact with cats (42.9%), although the difference was not significant (P = 0.410). The high seroprevalence of B. henselae reported among cat owners was expected and it is related to continuous exposure to the same source of infection because cat owners are at a high risk of scratches and bites. Along the same line, the high rate of seropositivity for B. henselae was estimated in cat owners in Korea (72). The data of the correlation between the infected cats and their households are shown in Table 3. As depicted in this Table, six cats were found infected with B. henselae and their age ranged from 6 to 24 months. In accordance with households, only three households of those corresponding infected cats were included in this study. Meanwhile, the households of the remaining three positive cats were not included, since these three positive cats were either stray or retrieved from pet shops. It is noteworthy to state that among other positive cats, there was one cat (cat number 1) and its owner was found infected with B. henselae. On the other hand, the remaining households did not carry antibodies against B. henselae. Although households in these latter cases were serologically negative, they remain exposed to infection from their infected cats. Collectively, the present serological data provide novel contributions about the high occurrence of B. henselae among humans in Egypt.
Conclusion
This study reports that the occurrence of B. henselae infection is high among cats and high exposure of cat owners and people with a history of exposure to cats to the pathogen, reflecting the potential zoonotic transmission cycle between cats and humans. Clearly, this data highlight the benefits of the use of serological and molecular methods in the diagnosis of B. henselae infection in major reservoirs. The present findings also prompt local health authorities to take measures to increase the awareness of the public and immunocompromised individuals of the role of cats in transmitting bartonellosis and the importance of keeping cats indoors and controlling flea infestation. Future study is recommended to explore the occurrence and epidemiological pattern of B. henselae in Egypt on a large scale, followed by genetic characterization of the circulating species in the country.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The study was ethically reviewed and approved by the Scientific Research Committee and Ethics Board of Assiut University, Egypt. The ethical approval number is IRB No: 17300307. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AS, RMA, MA, and RFA designed the idea of the conception, performed the methodology, formal analysis, data curation, and supervision besides revision of the manuscript. RB and EE participated in the methodology, formal analysis, data curation, and contributed their scientific advice. AS, RMA, and EE drafted the manuscript and prepared the manuscript for publication and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Taif University Researchers Supporting Program (Project number: TURSP-2020/269), Taif University, Saudi Arabia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Assiut University for their support of this study. We are thankful to Vircell Company, Granada, Spain for providing B. henselae DNA positive control and immunofluorescence IgG kits of B. henselae.
References
1. Solano-Gallego L, Bradley J, Hegarty B, Sigmon B, Breitschwerdt E. Bartonella henselae IgG antibodies are prevalent in dogs from southeastern USA. Vet Res. (2004) 35:585–95. doi: 10.1051/vetres:2004034
2. Chomel BB, Kasten RW. Bartonellosis, an increasingly recognized zoonosis. J Appl Microbiol. (2010) 109:743–50. doi: 10.1111/j.1365-2672.2010.04679.x
3. Pennisi MG, Marsilio F, Hartmann K, Lloret A, Addie D, Belak S, et al. Bartonella species infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg. (2013) 15:563–9. doi: 10.1177/1098612X13489214
4. Zangwill KM. Cat Scratch disease and Bartonellaceae: the known, the unknown and the curious. Pediatr Infect Dis J. (2021) 40:S11–5. doi: 10.1097/INF.0000000000002776
5. Iannino F, Salucci S, Di Provvido A, Paolini A, Ruggieri E. Bartonella infections in humans dogs and cats. Vet Ital. (2018) 54:63–72. doi: 10.12834/VetIt.398.1883.2
6. Chomel BB, Boulouis H-J, Maruyama S, Breitschwerdt EB. Bartonella spp. in pets and effect on human health. Emerg Infect Dis. (2006) 12:389. doi: 10.3201/eid1203.050931
7. Gutierrez R, Krasnov B, Morick D, Gottlieb Y, Khokhlova IS, Harrus S. Bartonella infection in rodents and their flea ectoparasites: an overview. Vector Borne Zoonotic Dis. (2015) 15:27–39. doi: 10.1089/vbz.2014.1606
8. Klangthong K, Promsthaporn S, Leepitakrat S, Schuster AL, McCardle PW, Kosoy M, et al. The distribution and diversity of Bartonella species in rodents and their ectoparasites across Thailand. PLoS ONE. (2015) 10:e0140856. doi: 10.1371/journal.pone.0140856
9. Foil L, Andress E, Freeland RL, Roy AF, Rutledge R, Triche PC, et al. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis (Siphonaptera: Pulicidae) feces. J Med Entomol. (1998) 35:625–8. doi: 10.1093/jmedent/35.5.625
10. Telford SR III, Wormser GP. Bartonella spp. transmission by ticks not established. Emerg Infect Dis. (2010) 16:379. doi: 10.3201/eid1603.090443
11. Cotté V, Bonnet S, Le Rhun D, Le Naour E, Chauvin A, Boulouis H-J, et al. Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. (2008) 14:1074. doi: 10.3201/eid1407.071110
12. Jurja S, Stroe AZ, Pundiche MB, Docu Axelerad S, Mateescu G, Micu AO, et al. The clinical profile of cat-scratch disease's neuro-ophthalmological effects. Brain Sci. (2022) 12:217. doi: 10.3390/brainsci12020217
13. Nelson CA, Saha S, Mead PS. Cat-scratch disease in the United States, 2005–2013. Emerg Infect Dis. (2016) 22:1741. doi: 10.3201/eid2210.160115
14. Lins KA, Drummond MR, Velho P. Cutaneous manifestations of bartonellosis. An Bras Dermatol. (2019) 94:594–602. doi: 10.1016/j.abd.2019.09.024
15. Guptill L, Wu C-C, HogenEsch H, Slater L, Glickman N, Dunham A, et al. Prevalence, risk factors, and genetic diversity of Bartonella henselae infections in pet cats in four regions of the United States. J Clin Microbiol. (2004) 42:652–9. doi: 10.1128/JCM.42.2.652-659.2004
16. Lappin MR, Griffin B, Brunt J, Riley A, Burney D, Hawley J, et al. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg. (2006) 8:85–90. doi: 10.1016/j.jfms.2005.08.003
17. Im J-H, Baek JH, Lee H-J, Lee J-S, Chung M-H, Kim M, et al. First case of Bartonella henselae bacteremia in Korea. Infect Chemother. (2013) 45:446–50. doi: 10.3947/ic.2013.45.4.446
18. Oskouizadeh K, Zahraei-Salehi T, Aledavood S. Detection of Bartonella henselae in domestic cats' saliva. Iran J Microbiol. (2010) 2:80.
19. Maruyama S, Izumikawa K, Miyashita M, Kabeya H, Mikami T, Yamanouchi H, et al. First isolation of Bartonella henselae type I from a cat-scratch disease patient in japan and its molecular analysis. Microbiol Immunol. (2004) 48:103–9. doi: 10.1111/j.1348-0421.2004.tb03495.x
20. Drummond MR, Pitassi LH, Lania BG, Dos Santos SR, Gilioli R, Velho PE. Detection of Bartonella henselae in defibrinated sheep blood used for culture media supplementation. Braz J Microbiol. (2011) 42:430–2. doi: 10.1590/S1517-83822011000200003
21. Satranarakun P, Maruyama S, Kabeya H, Sato S, Jitapalapong S, Jitchum S, et al. Prevalence of Bartonella infection in well-cared cats in Bangkok metropolitan. Thai J Vet Med. (2016) 46:555.
22. Sander A, Bühler C, Pelz K, Von Cramm E, Bredt W. Detection and identification of two Bartonella henselae variants in domestic cats in Germany. J Clin Microbiol. (1997) 35:584–7. doi: 10.1128/jcm.35.3.584-587.1997
23. Maggi RG, Duncan AW, Breitschwerdt EB. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol. (2005) 43:2651–5. doi: 10.1128/JCM.43.6.2651-2655.2005
24. Masci JR, Wormser GP. (2005). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. In: Mandell GL, Bennett JE, Dolin R, editors. Philadelphia: Elsevier Churchill Livingstone; The University of Chicago Press (2005). p. 3661.
25. Baron E, Peterson LR, Finegold BM. Bairly and Scott's Diagnostic Microbiology. 9th ed. Louis, MI: Mosby (1994).
26. Fard RMN, Vahedi SM, Ashrafi I, Alipour F, Sharafi G, Akbarein H, et al. Molecular identification and phylogenic analysis of Bartonella henselae isolated from Iranian cats based on gltA gene. In: Veterinary Research Forum: Faculty of Veterinary Medicine. Urmia: Urmia University (2016). p. 69.
27. Tomley FM, Shirley MW. Livestock infectious diseases and zoonoses. Philos Trans R Soc Lond B Biol Sci. (2009) 364:2637–42. doi: 10.1098/rstb.2009.0133
28. Allen T, Murray KA, Zambrana-Torrelio C, Morse SS, Rondinini C, Di Marco M, et al. Global hotspots and correlates of emerging zoonotic diseases. Nat Commun. (2017) 8:1124. doi: 10.1038/s41467-017-00923-8
29. Drummond MR, Lania BG, Diniz P, Gilioli R, Demolin DMR, Scorpio DG, et al. Improvement of Bartonella henselae DNA detection in cat blood samples by combining molecular and culture methods. J Clin Microbiol. (2018) 56:e01732-17. doi: 10.1128/JCM.01732-17
30. Elmahallawy EK, Zanet S, Poggi M, Alsharif KF, Agil A, Trisciuoglio A, et al. Feline Leishmaniosis in Northwestern Italy: current status and zoonotic implications. Vet Sci. (2021) 8:215. doi: 10.3390/vetsci8100215
31. Gareh A, Saleh AA, Moustafa SM, Tahoun A, Baty RS, Khalifa RMA, et al. Epidemiological, morphometric, and molecular investigation of cystic Echinococcosis in camel and cattle from upper Egypt: current status and zoonotic implications. Front Vet Sci. (2021) 8:750640. doi: 10.3389/fvets.2021.750640
32. Tierno PMJr, Inglima K, Parisi MT. Detection of Bartonella (Rochalimaea) henselae bacteremia using BacT/Alert blood culture system. Am J Clin Pathol. (1995) 104:530–6. doi: 10.1093/ajcp/104.5.530
33. Duncan AW, Maggi RG, Breitschwerdt EB. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment liquid culture followed by PCR and subculture onto agar plates. J Microbiol Methods. (2007) 69:273–81. doi: 10.1016/j.mimet.2007.01.010
34. Brouqui P, Raoult D. New insight into the diagnosis of fastidious bacterial endocarditis. FEMS Immunol Med Microbiol. (2006) 47:1–13. doi: 10.1111/j.1574-695X.2006.00054.x
35. Srisanyong W, Takhampunya R, Boonmars T, Kerdsin A, Suksawat F. Prevalence of Bartonella henselae, Bartonella clarridgeiae, and Bartonella vinsonii subsp. berkhoffii in pet cats from four provincial communities in Thailand. Thai J Vet Med. (2016) 46:663.
36. Okaro U, Addisu A, Casanas B, Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. (2017) 30:709–46. doi: 10.1128/CMR.00013-17
37. Pons I, Sanfeliu I, Quesada M, Anton E, Sampere M, Font B, et al. Prevalence of Bartonella henselae in cats in Catalonia, Spain. Am J Trop Med Hyg. (2005) 72:453–7. doi: 10.4269/ajtmh.2005.72.453
38. Galluzzi L, Magnani M, Saunders N, Harms C, Bruce IJ. Current molecular techniques for the detection of microbial pathogens. Sci Prog. (2007) 90(Pt 1):29–50. doi: 10.3184/003685007780440521
39. Melter O, Hercik K, Weyant R, Janeček J, Nemec A, Mecera J, et al. Detection and characterization of feline Bartonella henselae in the Czech Republic. Vet Microbiol. (2003) 93:261–73. doi: 10.1016/S0378-1135(03)00032-4
40. Celebi B, Kilic S, Aydin N, Tarhan G, Carhan A, Babur C. Investigation of Bartonella henselae in cats in Ankara, Turkey. Zoonoses Public Health. (2009) 56:169–75. doi: 10.1111/j.1863-2378.2008.01170.x
41. Glaus T, Hofmann-Lehmann R, Greene C, Glaus B, Wolfensberger C, Lutz H. Seroprevalence of Bartonella henselae infection and correlation with disease status in cats in Switzerland. J Clin Microbiol. (1997) 35:2883–5. doi: 10.1128/jcm.35.11.2883-2885.1997
42. Childs J, Olson J, Wolf A, Cohen N, Fakile Y, Rooney J, et al. Prevalence of antibodies to Rochalimaea species (cat-scratch disease agent) in cats. Vet Rec. (1995) 136:519–20. doi: 10.1136/vr.136.20.519
43. Olsson Engvall E, Fasth C, Brändström B, Fermer C, Blomqvist G, Englund L. Prevalence of Bartonella henselae in young, healthy cats in Sweden. Vet Rec. (2003) 152:366–9. doi: 10.1136/vr.152.12.366
44. Al-Kappany YM, Lappin MR, Kwok OC, Abu-Elwafa SA, Hilali M, Dubey JP. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp. feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis infections in Egyptian cats. J Parasitol. (2011) 97:256–8. doi: 10.1645/GE-2654.1
45. Maruyama S, Hiraga S, Yokoyama E, Naoi M, Tsuruoka Y, Ogura Y, et al. Seroprevalence of Bartonella henselae and Toxoplasma gondii infections among pet cats in Kanagawa and Saitama Prefectures. J Vet Med Sci. (1998) 60:997–1000. doi: 10.1292/jvms.60.997
46. Birtles R, Laycock G, Day M, Kenny M, Shaw S. Prevalence of Bartonella species causing bacteraemia in domesticated and companion animals in the United Kingdom. Vet Rec. (2002) 151:225–9. doi: 10.1136/vr.151.8.225
47. Staggemeier R, Venker CA, Klein DH, Petry M, Spilki FR, Cantarelli VV. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats in the south of Brazil: a molecular study. Memórias Instit Oswaldo Cruz. (2010) 105:873–8. doi: 10.1590/S0074-02762010000700006
48. Fabbi M, De Giuli L, Tranquillo M, Bragoni R, Casiraghi M, Genchi C. Prevalence of Bartonella henselae in Italian stray cats: evaluation of serology to assess the risk of transmission of Bartonella to humans. J Clin Microbiol. (2004) 42:264–8. doi: 10.1128/JCM.42.1.264-268.2004
49. Kim Y, Seo K, Lee J, Choi E., Lee H, Hwang C, et al. Prevalence of Bartonella henselae and Bartonella clarridgeiae in cats and dogs in Korea. J Vet Sci. (2009 10:85–87. doi: 10.4142/jvs.2009.10.1.85
50. Mazurek Ł, Carbonero A, Skrzypczak M, Winiarczyk S, Adaszek Ł. Epizootic situation of feline Bartonella infection in eastern Poland. J Vet Res. (2020) 64:79. doi: 10.2478/jvetres-2020-0019
51. Boulouis H-J, Chang C, Henn JB, Kasten RW, Chomel BB. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet Res. (2005) 36:383–410. doi: 10.1051/vetres:2005009
52. Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasites Vec. (2013) 6:59–59. doi: 10.1186/1756-3305-6-59
53. Kreppel KS, Telfer S, Rajerison M, Morse A, Baylis M. Effect of temperature and relative humidity on the development times and survival of Synopsyllus fonquerniei and Xenopsylla cheopis, the flea vectors of plague in Madagascar. Parasites Vec. (2016) 9:82. doi: 10.1186/s13071-016-1366-z
54. Maruyama S, Boonmar S, Morita Y, Sakai T, Tanaka S, Yamaguchi F, et al. Seroprevalence of Bartonella henselae and Toxoplasma gondii among healthy individuals in Thailand. J Vet Med Sci. (2000) 62:635–7. doi: 10.1292/jvms.62.635
55. Inoue K, Maruyama S, Kabeya H, Kawanami K, Yanai K, Jitchum S, et al. Prevalence of Bartonella infection in cats and dogs in a metropolitan area, Thailand. Epidemiol Infect. (2009) 137:1568–73. doi: 10.1017/S095026880900257X
56. Müller A, Walker R, Bittencourt P, Machado RZ, Benevenute JL, Do Amaral RB, et al. Prevalence, hematological findings and genetic diversity of Bartonella spp. in domestic cats from Valdivia, Southern Chile. Parasitology. (2017) 144:773–82. doi: 10.1017/S003118201600247X
57. Oteo JA, Maggi R, Portillo A, Bradley J, Garcia-Alvarez L, San-Martin M, et al. Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasit Vec. (2017) 10:553. doi: 10.1186/s13071-017-2483-z
58. Ansil B, Mendenhall IH, Ramakrishnan U. High prevalence and diversity of Bartonella in small mammals from the biodiverse Western Ghats. PLoS Negl Trop Dis. (2021) 15:e0009178. doi: 10.1371/journal.pntd.0009178
59. Mitchell BM, Font RL. Molecular detection of Bartonella Henselae for the diagnosis of cat scratch disease and bacillary angiomatosis of the conjunctiva. Cornea. (2011) 30:807–14. doi: 10.1097/ICO.0b013e318201440c
60. Zumla A, Keiser J. Tropical Diseases, An Issue of Infectious Disease Clinics-E-Book. Amsterdam: Elsevier Health Sciences (2012). doi: 10.1016/j.idc.2012.03.012
61. Hoey JG, Valois-Cruz F, Goldenberg H, Voskoboynik Y, Pfiffner J, Tilton RC, et al. Development of an immunoglobulin M capture-based enzyme-linked immunosorbent assay for diagnosis of acute infections with Bartonella henselae. Clin Vacc Immunol. (2009) 16:282–4. doi: 10.1128/CVI.00305-08
62. Song XP, Zhang HB, Liu QY, Sun JM, Lei X, Gu SH, et al. Seroprevalence of Bartonella henselae and identification of risk factors in China. Biomed Environ Sci. (2020) 33:72–5. doi: 10.3967/bes2020.011
63. Chmielewski T, Podsiadly E, Tylewska-Wierzbanowska S. Presence of Bartonella spp. in various human populations. Polish J Microbiol. (2007) 56:33.
64. Cavlek TV, Martinkovic DK, Sternak SL, Tabain I, Persic Z, Galinovic GM. High prevalence of Bartonella henselae and Bartonella quintana antibodies in Croatian patients presenting with lymphadenopathy. Polish J Microbiol. (2012) 61:315–8. doi: 10.33073/pjm-2012-043
65. Jackson LA, Perkins BA, Wenger JD. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. (1993) 83:1707–11. doi: 10.2105/AJPH.83.12.1707
66. Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, et al. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. (1993) 329:8–13. doi: 10.1056/NEJM199307013290102
67. Anderson BE, Neuman MA. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. (1997) 10:203–19. doi: 10.1128/CMR.10.2.203
68. Chamberlin J, Laughlin LW, Romero S, Solórzano N, Gordon S, Andre RG, et al. Epidemiology of endemic Bartonella bacilliformis: a prospective cohort study in a Peruvian mountain valley community. J Infect Dis. (2002) 186:983–90. doi: 10.1086/344054
69. Picascia A, Pagliuca C, Sommese L, Colicchio R, Casamassimi A, Labonia F, et al. Seroprevalence of Bartonella henselae in patients awaiting heart transplant in Southern Italy. J Microbiol Immunol Infect. (2017) 50:239–44. doi: 10.1016/j.jmii.2015.05.001
70. Nadal D, Zbinden R. Serology to Bartonella (Rochalimaea) henselae may replace traditional diagnostic criteria for cat-scratch disease. Eur J Pediatr. (1995) 154:906–8. doi: 10.1007/BF01957503
71. Farkas R, Gyurkovszky M, Solymosi N, Beugnet F. Prevalence of flea infestation in dogs and cats in Hungary combined with a survey of owner awareness. Med Vet Entomol. (2009) 23:187–94. doi: 10.1111/j.1365-2915.2009.00798.x
Keywords: serology, molecular, Bartonella, cats, humans, Egypt
Citation: Sayed ASM, Alsaadawy RM, Ali MM, Abd El-Hamid RF, Baty RS and Elmahallawy EK (2022) Serological and Molecular Detection of Bartonella henselae in Cats and Humans From Egypt: Current Status and Zoonotic Implications. Front. Vet. Sci. 9:859104. doi: 10.3389/fvets.2022.859104
Received: 20 January 2022; Accepted: 11 March 2022;
Published: 14 April 2022.
Edited by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomReviewed by:
Marina Drummond, State University of Campinas, BrazilLucas Blanton, University of Texas Medical Branch at Galveston, United States
Copyright © 2022 Sayed, Alsaadawy, Ali, Abd El-Hamid, Baty and Elmahallawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amal S. M. Sayed, YW1hbHNheWVkNzNAYXVuLmVkdS5lZw==; Ehab Kotb Elmahallawy, ZWVoYWFAdW5pbGVvbi5lcw==
 Amal S. M. Sayed1*
Amal S. M. Sayed1* Reem M. Alsaadawy
Reem M. Alsaadawy Rawhia F. Abd El-Hamid
Rawhia F. Abd El-Hamid Roua Sami Baty
Roua Sami Baty Ehab Kotb Elmahallawy
Ehab Kotb Elmahallawy