- 1Poultry Mineral Nutrition Laboratory, College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 2Mineral Nutrition Research Division, State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China
- 3College of Veterinary Medicine, Yangzhou University, Yangzhou, China
Understanding the underlying mechanisms that regulate the bone phosphorus (P) utilization would be helpful for developing feasible strategies to improve utilization efficiency of P in poultry. We aimed to investigate the effects of inorganic P levels on P utilization, local bone-derived regulators and bone morphogenetic protein/mitogen-activated protein kinase (BMP/MAPK) pathway in primary cultured osteoblasts of broiler chicks in order to address whether local bone-derived regulators or BMP/MAPK pathway was involved in regulating the bone P utilization of broilers using an in vitro model. The primary cultured tibial osteoblasts of broiler chicks were randomly divided into one of five treatments with six replicates for each treatment. Then, cells were respectively incubated with 0.0, 0.5, 1.0, 1.5, or 2.0 mmol/L of added P as NaH2PO4 for 24 days. The results showed that as added P levels increased, tibial osteoblastic P retention rate, number and area of mineralized nodules, the mRNA expressions of endopeptidases on the X chromosome (PHEX), dentin matrix protein 1 (DMP1), bone morphogenetic protein 2 (BMP2), and the mRNA and protein expressions of matrix extracellular phosphoglycoprotein (MEPE) increased linearly (p < 0.001) or quadratically (p < 0.04), while extracellular signal-regulated kinase 1 (ERK1) mRNA expression and c-Jun N-terminal kinase 1 (JNK1) phosphorylated level decreased linearly (p < 0.02) or quadratically (p < 0.01). Correlation analyses showed that tibial osteoblastic P retention rate was positively correlated (r = 0.452–0.564, p < 0.03) with MEPE and BMP2 mRNA expressions. Furthermore, both number and area of mineralized nodules were positively correlated (r = 0.414–0.612, p < 0.03) with PHEX, DMP1, MEPE, and BMP2 mRNA expressions but negatively correlated (r = −0.566 to −0.414, p < 0.04) with the ERK1 mRNA expression and JNK1 phosphorylated level. These results suggested that P utilization in primary cultured tibial osteoblasts of broiler chicks might be partly regulated by PHEX, DMP1, MEPE, BMP2, ERK1, and JNK1.
Introduction
Phosphorus (P) is an essential macro-mineral required for bone development, growth, and productivity (1–3) and also the third most expensive component in poultry diets (4). Dietary P deficiency can cause a reduced feed intake, growth retardation, and other skeletal deformities of broilers (5–8). Therefore, sufficient P supply in their diets is vital to meet metabolic requirements. On the other hand, excessive P over requirements is excreted through manure, leading to both economical loss and environmental pollution (9, 10). Concerns about the environmental burden caused by high P excretion and the existing status of scarce P resources make it imperative to strive for more efficient P utilization of broilers.
Approximately 80% of P absorbed from the small intestine is deposited in the bone as hydroxyapatite (11), and this deposition process is mainly carried out by osteoblasts (12). Furthermore, the uptake of P by osteoblasts is usually considered as a major factor regulating the bone development, mineralization, and remodeling (13). Therefore, primary cultured osteoblasts may be an ideal in vitro model for exploring the patterns of bone P utilization in broilers. Possible traits related to bone P utilization in vitro are tibial osteoblastic P retention rate and number and area of mineralized nodules; among them, the number and area of mineralized nodules are often used as response indicators in in vitro studies of calcium/P deposition (14–16). Local bone-derived regulators, such as fibroblastic growth factor 23 (FGF23), matrix extracellular phosphoglycoprotein (MEPE) and dentin matrix protein 1 (DMP1), and phosphate-regulating gene with homologies to endopeptidases on the X chromosome (PHEX) have been found to be involved in regulating P homeostasis and bone mineralization (17, 18). One latest study from our laboratory suggested that bone development and P retention might be partly regulated by FGF23, DMP1, and PHEX (5), implying possible contributions of local bone-derived regulators to bone P utilization of broilers. In addition to local bone-derived regulators, multiple signaling pathways are essential components during osteoblast mineralization, such as bone morphogenetic protein/mitogen-activated protein kinase (BMP/MAPK) pathway (19). As a member of the BMP family, BMP2 is a potent inducer governing the osteoblastic mineralization (20, 21). Furthermore, accumulating evidence have revealed that BMP2 exerts its osteogenic function via activating MAPK signaling pathway (22, 23). Another latest study from our laboratory further suggested that BMP/MAPK pathway might play a potential regulatory role in bone development and P retention of broilers (24). However, the results from the above latest studies in vivo with broilers might be affected by other factors like the feed intake change except for P, and the specific and direct effect of P itself on the above aspects of broilers is unknown. Therefore, it is necessary to investigate the specific and direct effect of P itself on the above aspects using the primary cultured model of osteoblasts of broiler chicks. According to the results of our above latest studies in vivo, it is reasonable to hypothesize that P utilization in an osteoblastic model in vitro might be partly regulated by local bone-derived regulators and BMP/MAPK pathway. Therefore, the objective of the current study was to investigate the effects of added inorganic P levels on P utilization parameters, local bone-derived regulators, and BMP/MAPK pathway in primary cultured tibial osteoblasts of broiler chicks to test the above hypothesis.
Materials and Methods
Isolation and Cultivation of Primary Tibial Osteoblasts of Broiler Chicks
The primary tibial osteoblasts of broiler chicks were isolated and cultivated by using a modified approach of the previous method reported in mouse (25). Tibias from both legs were obtained from 1-day-old commercial chicks (male Arbor Acres broilers, purchased from Huadu Broiler Breeding Corp., Luanping, China) after cervical dislocation. The birds were soaked in alcohol for 2 min after cervical dislocation. Legs were removed from the hip joint and metacarpal. Dissected legs were kept in Dulbecco's modified Eagle's medium (DMEM; Solarbio, Beijing, China) containing 1% penicillin-streptomycin (Gibco, Grand Island, NY, USA) until the connective tissues and muscles were completely removed. The epiphysis of the tibia was removed to expose the bone marrow cavity. Bone marrow inside the bone was flushed with washing buffer in a syringe. The tibias were split longitudinally with a scalpel and then scraped with a curved forceps to remove the hematopoietic cells adhered to the compact bones. The clean bone flakes were cut into 1 mm2 with sterile surgical scissors, plated in 60-mm cell culture dishes, and then incubated in the complete culture medium, consisting of DMEM, supplemented with 1% penicillin-streptomycin, 1% L-glutamine (Gibco, Grand Island, NY, USA), and 15% fetal bovine serum (FBS; Gibco, Auckland, New Zealand) at 37°C in a humidified atmosphere containing 5% CO2. Once the cells migrated from bone pieces and covered up to 80% of the flask bottom surface, the cells were washed twice with Dulbecco's phosphate-buffered saline (D-PBS) without CaCl2 and MgCl2 (Gibco, Grand Island, NY, USA), dissociated with 0.25% Trypsin-EDTA (Solarbio, Beijing, China) for 1 min, and subcultured in six-well plates at a density of 5 × 105 cells/well in the above complete culture medium. This passage was marked as P1. The P1 cells were identified by alkaline phosphatase (ALP), immunofluorescence, and alizarin res S (ARS) staining (26), and then used in the following experiments.
Experimental Design and Treatments
A one-factor completely randomized design with six replicates was adopted in the present study. Once the P1 osteoblasts grew to ~80–90% confluence, the cells were washed with the P-free medium (DMEM with no phosphate, Gibco, Grand Island, NY, USA), randomly divided into one of five treatments, and then incubated in the P-free medium supplemented with both 15% FBS and 0.0, 0.5, 1.0, 1.5, or 2.0 mmol/L of P as NaH2PO4, respectively, for 24 days. The total P levels in the above media were 0.568, 1.055, 1.513, 2.010, and 2.562 mmol/L by analysis, respectively, and calcium level in each medium used in the present study was 2.2 mmol/L by analysis. The reason for selecting NaH2PO4 as the P source is that the original P source in the osteoblastic DMEM culture is NaH2PO4, while the P source (CaHPO4) usually used in the diet of broilers is less soluble in DMEM. The supplemented P levels were based on the study of Zoidis et al. (27). The medium was replaced every 2 days. The optimal incubation time (24 days) was chosen based on the results of our latest study (28).
Bone ALP Staining
When the P1 cells reached 100% confluence, cells were fixed with 4% formaldehyde (Solarbio, Beijing, China) for 10 min and then washed in PBS three times for 5 min each. Subsequently, the fixed cells were stained by the ALP strain kit (Beyotime, Shanghai, China). The stained cells were observed and photographed by an optical microscope (Nikon, Japan). The cells strained in purple blue were osteoblasts.
Immunofluorescence
When the P1 cells reached 100% confluence, cells were fixed with 4% formaldehyde for 30 min and then washed in PBS three times for 5 min each. The fixed cells were then permeabilized with 0.2% Triton X-100 (Solarbio, Beijing, China) for 15 min and then blocked with normal goat serum (Solarbio, Beijing, China) for 30 min at 37°C. After blocking, the cells were incubated with a primary antibody against collagen type I alpha 1 (COL1A1; Boster, Wuhan, China) overnight at 4°C. Afterwards, the cells were then incubated with a DyLight-488 labeled goat-anti-rabbit IgG secondary antibody (Boster, Wuhan, China) for 1 h at 37°C. The cell nuclei were visualized using 4′,6-diamidino-2-phenylindole (DAPI) staining solution (Beyotime, Shanghai, China). Finally, images were captured with a fluorescence microscope (Leica, Wetzlar, Germany) by randomly selecting five fields. The COL1A1-positive cells showed green fluorescence. As for the cell purity, the percentage of COL1A1-positive cells was calculated as (number of COL1A1-positive cells)/(total number of cells in the field) × 100%.
ARS Staining
The mineralized module formation was detected by ARS straining as previously described (29). When cultured for 24 days, the cells were washed with PBS and fixed in 4% formaldehyde for 15 min at room temperature. The cells were then washed twice with D-PBS prior to addition of 1 ml of 1% ARS (pH 4.2) per well. The plates were incubated at room temperature for 20 min with gentle shaking, followed by thrice washing with D-PBS. Mineralized nodules were positive to ARS.
Tibial Osteoblastic P Retention Rate
In vivo, tibia P retention rate was used to evaluate the bone P utilization of broilers (30); therefore, tibial osteoblastic P retention rate was determined in vitro to evaluate the osteoblastic P utilization. To determine the tibial osteoblastic P retention rate, the old medium was collected and pooled at every medium replacement. The total P contents in the fresh or old medium were determined by inductively coupled plasma optical emission spectrometry [5110 ICP-OES, Agilent Technologies Australia (M) Pty Ltd, Australia]. Accurately 1.0 ml of the fresh or old medium was taken in triplicate and digested with 12.5 ml of HNO3 and 2.5 ml of HClO4 at 200°C in a 50-ml calibrated flask until the solution became clear, and it was evaporated to almost dryness and diluted to about 15 ml with 2% HNO3 before analyses. Validation of the mineral analysis was conducted using soybean meal (GBW10013; National Institute of Standards and Technology, Beijing, China) as a standard reference material. The tibial osteoblastic P retention rate was calculated as follows:
where V1 is total volume (ml) of the added fresh medium, C1 is total P content (mmol/L) in the fresh medium, V2 is total volume (ml) of the pooled old medium, and C2 is total P content (mmol/L) in the old medium.
Mineralized Nodule Formation in Tibial Osteoblasts
The mineralized nodule formation was detected by ARS staining as described in ARS Staining. After staining, the number of positively stained mineral nodules was measured by visual counting under an optical microscope. Furthermore, the area of the mineralized nodules in five randomly selected visual fileds of each replicate well was analyzed with Image Pro-Plus 6.0 software (Media Cybernetics, USA).
Quantitative Real-Time Polymerase Chain Reaction
After 24 d treatments with various P concentrations, the mRNA expression levels of local bone-derived regulator genes (FGF23, MEPE, DMP1 and PHEX) and BMP/MAPK pathway-related genes [BMP2, extracellular signal-regulated kinase 1 (ERK1), c-Jun N-terminal kinase 1 (JNK1) and p38 mitogen-activated protein kinase alpha (p38α)] were evaluated by real-time quantitative polymerase chain reaction (RT-qPCR) as described by Li et al. (31). Briefly, according to the manufacturer's instruction, PrimeScriptTM RT Master Mix kit (TaKaRa, Dalian, China) was used to reverse RNA into cDNA. Subsequently, 50 ng of total cDNA was applied for RT-qPCR analysis using SYBR-Green PCR Master Mix (Life Technologies, Carlsbad, CA, USA). Finally, the relative expression of the target genes was calculated using the 2−ΔΔCt method, and the data were normalized by the geometric mean of two internal reference genes (β-actin and GAPDH). All primer sequences are presented in Table 1.
Western Blotting
At the end of the P treatments, whole cell lysates were harvested in RIPA buffer (Beyotime, Shanghai, China). Cell lysates containing 30 μg of each protein were then subjected to a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Merck Millipore, Billerica, MA, USA). After blocking with 5% bovine serum albumin, the membrane was incubated overnight at 4°C with primary antibody against FGF23 (Abclonal, Wuhan, China), PHEX (Abclonal, Wuhan, China), DMP1 (Abcam, Cambridge, UK), MEPE (Abcam, Cambridge, UK), BMP2 (Abclonal, Wuhan, China), ERK1 (Abcam, Cambridge, UK), JNK1 (Abcam, Cambridge, UK), p38α (Abclonal, Wuhan, China), phosphorylated (p-)ERK (Abclonal, Wuhan, China), p-JNK1 (Abclonal, Wuhan, China), p- p38α (Abclonal, Wuhan, China), β-actin (Huaxingbio, Beijing, China), or β-tubulin (Huaxingbio, Beijing, China). After washing with Tris-buffered saline containing 0.02% (v/v) Tween-20 (TBST) three times for 5 min each, the membranes were developed with the secondary antibody of goat anti-rabbit (Huaxingbio, Beijing, China) or goat anti-mouse (Huaxingbio, Beijing, China). After washing with TBST three times for 5 min each, protein bands were visualized using enhanced chemiluminescence substrate (Tanon, Shanghai, China) and quantified with an ImageQuant LAS4000 scanner (GE Healthcare Life Sciences, Pittsburgh, PA), followed by analysis with TotalLab Quant Software (TotalLab, Newcastle on Tyne, UK). The β-actin or β-tubulin protein was used to normalize the expression levels of target proteins.
Statistical Analyses
Data from the present study were analyzed by SAS statistical software (version 9.4, SAS Institute Inc., Cary, NC). The data were first tested for normality and homogeneity of variance using the Shapiro–Wilk and F-tests, respectively. Then, the data were subjected to one-way ANOVA using the general liner model (GLM) procedure, and the treatment comparisons for significant differences in all figures were tested by the least significant difference (LSD) method. Orthogonal polynomials were applied for testing linear and quadratic effects of dependent variables to independent variables. Simple correlation analyses between the P utilization-related parameters and local bone-derived regulators or BMP/MAPK pathway were carried out based on replicate values from different P level treatments using the Pearson procedure (5, 32). The replicate served as the experimental unit. The statistical significance was set at p ≤ 0.05.
Results
Morphological Features and Identification of Primary Cultured Tibial Osteoblasts of Broilers
As shown in Figures 1A,B, both P0 and P1 cells appeared typical morphological features of osteoblasts, such as multi-angle or fusiform shape. With the extension of culture time, cells grew like pave road stone (Figure 1C). Moreover, P1 cells showed the osteoblastic phenotypes, such as their synthesis of ALP (Figure 1D), collagen I (Figure 1E), and formation of mineralized nodules (Figure 1F). Immunofluorescence staining with osteoblast-specific antibody COL1A1 showed that the positive rate of osteoblasts was over 95%, indicating that the purity of the primary osteoblasts was high. Taken together, the cultured cells were osteoblasts with a high purity and can be used in the next experiment.
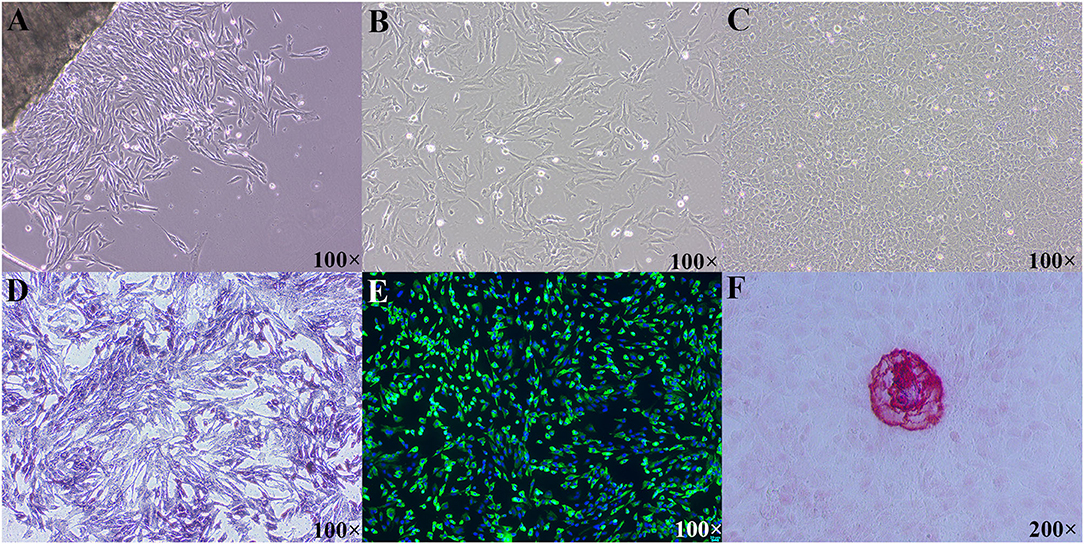
Figure 1. Morphological features and identification of primary cultured tibial osteoblasts of broiler chicks. (A) Morphological image of P0 cells, day of 3 culture; (B) morphological image of P1 cells, day of 2 culture; (C) morphological image of P1 cells, day of 10 culture; (D) primary osteoblasts were positive in ALP straining; (E) immunofluorescence of COL1A1 in the primary tibial osteoblasts; (F) mineralized nodules were positive in ARS staining. ALP, alkaline phosphatase; COL1A1, collagen type I alpha 1; ARS, alizarin res S.
Tibial Osteoblastic P Retention Rate and Number and Area of Mineralized Nodules
The added P level had an effect (p ≤ 0.038) on the tibial osteoblastic P retention rate and number and area of mineralized nodules in primary cultured tibial osteoblasts of broiler chicks (Figure 2). The tibial osteoblastic P retention rate increased linearly (p = 0.014), whereas the number and area of mineralized nodules increased linearly (p < 0.0001) and quadratically (p ≤ 0.026) as added P levels increased and reached a stabilized level (p > 0.141) at 1.0–2.0 mmol/L of added P.
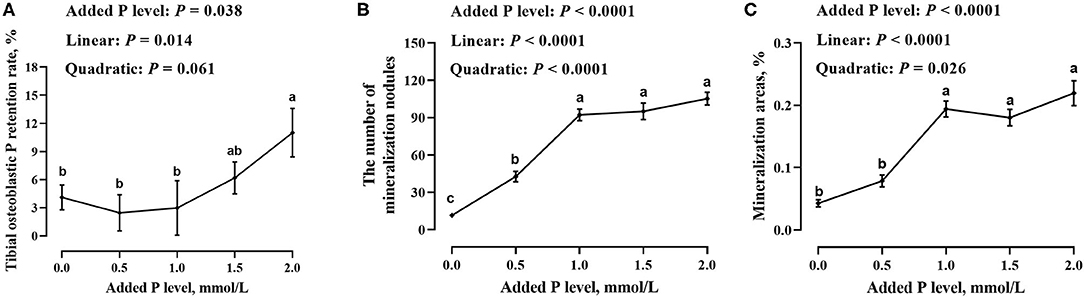
Figure 2. Effect of added P level on tibial osteoblastic P retention rate (A) and number (B) and area (C) of mineralized nodules in primary cultured tibial osteoblasts of broiler chicks. Each value was mean ± SE, n = 6. Means without a common letter differ, p ≤ 0.05.
MRNA and Protein Expressions of Local Bone-Derived Regulators
The added P level affected (p ≤ 0.0005) PHEX, DMP1, and MEPE mRNA expressions but did not affect (p = 0.360) FGF23 mRNA expression in primary cultured tibial osteoblasts of broiler chicks (Figure 3). The DMP1 and PHEX mRNA expressions increased (p ≤ 0.001) at 1.0 mmol/L of added P, followed by a decrease (p ≤ 0.038) at 1.5 mmol/L of added P and another increase (p ≤ 0.009) at 2.0 mmol/L of added P (Figures 3C,D). The MEPE mRNA expression increased linearly (p = 0.0002) and quadratically (p = 0.037) as added P levels increased (Figure 3B). The added P level affected MEPE protein expression but did not affect (p > 0.156) FGF23, PHEX, and DMP1 protein expressions (Figure 4). The protein expression of MEPE increased quadratically (p = 0.005) and reached a peak at 1.0 mmol/L of added P (Figure 4B).
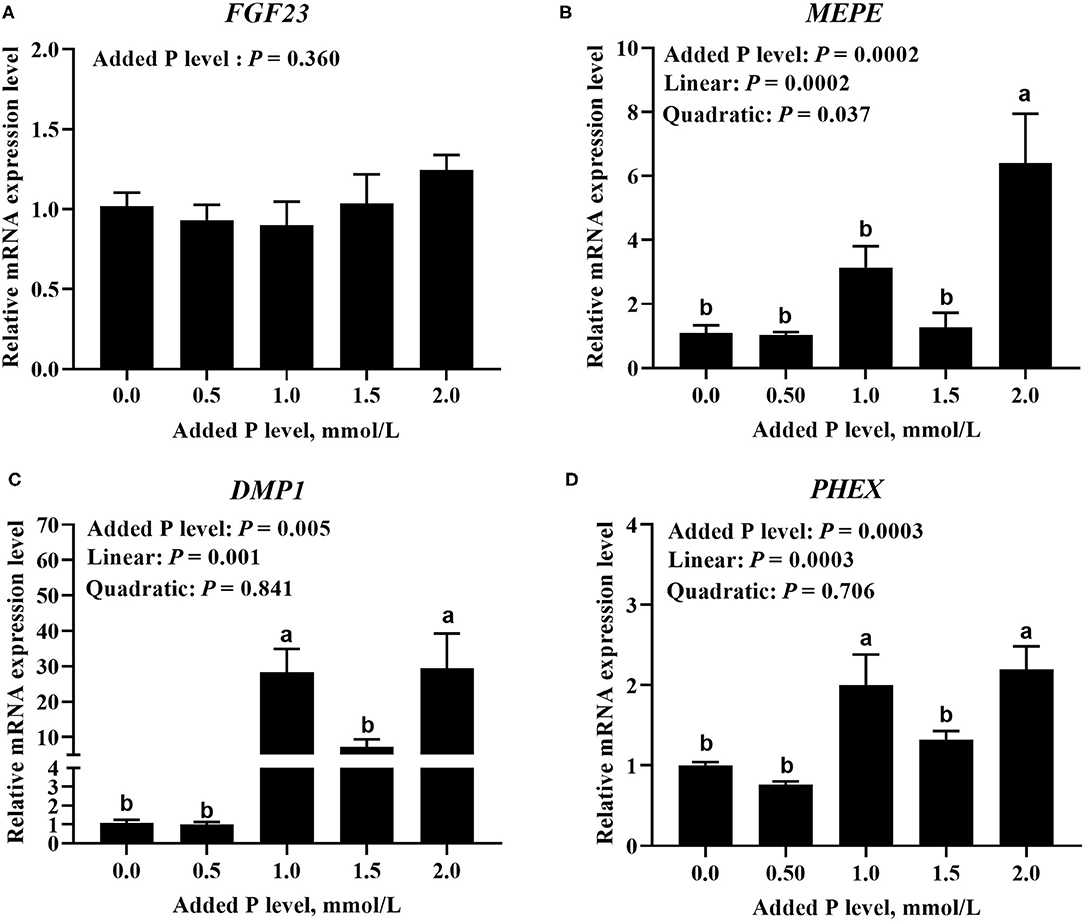
Figure 3. Effect of added P level on the mRNA expression levels of FGF23 (A), MEPE (B), DMP1 (C), and PHEX (D) in primary cultured tibial osteoblasts of broiler chicks. Each value was mean ± SE, n = 6. Means without a common letter differ, p ≤ 0.05. FGF23, fibroblast growth factor 23; MEPE, matrix extracellular phosphoglycoprotein; DMP1, dentin matrix protein 1; PHEX, phosphate-regulating gene homologous to endopeptidase on X chromosome.
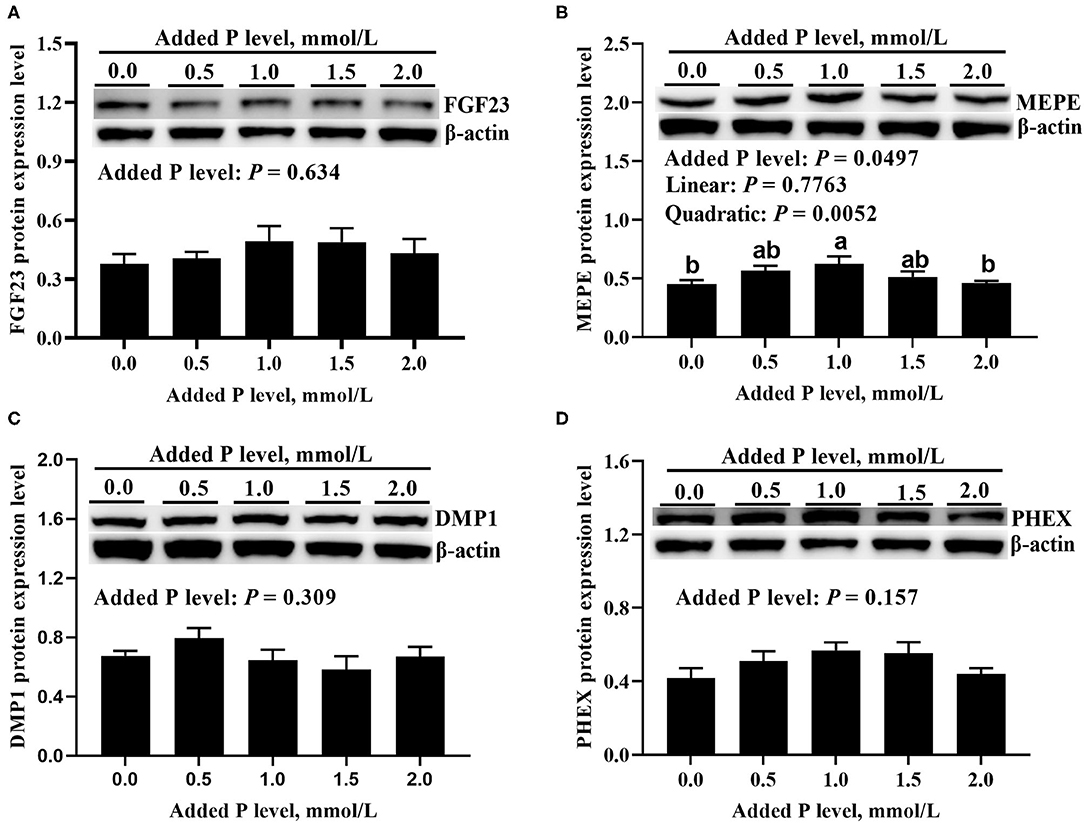
Figure 4. Effect of added P level on the protein expression levels of FGF23 (A), MEPE (B), DMP1 (C), and PHEX (D) in primary cultured tibial osteoblasts of broiler chicks. Representative western blots for these proteins were shown. Each value was mean ± SE, n = 6. Means without a common letter differ, P ≤ 0.05. FGF23, fibroblast growth factor 23; MEPE, matrix extracellular phosphoglycoprotein; DMP1, dentin matrix protein 1; PHEX, phosphate-regulating gene homologous to endopeptidase on X chromosome.
MRNA and Protein Expressions of BMP/MAPK Pathway
The added P level affected (p ≤ 0.05) BMP2 and ERK1 mRNA expressions but did not affect (p > 0.643) JNK1 and p38α mRNA expressions in primary cultured tibial osteoblasts of broiler chicks (Figure 5). The BMP2 mRNA increased linearly (p < 0.0001) and quadratically (p < 0.0001), whereas the ERK1 mRNA expression decreased linearly (p < 0.007) as added P levels increased (Figures 5A,B). The added P level affected (p = 0.046) p-JNK1 protein expression but did not affect (p > 0.290) total and phosphorylated protein expression levels of other genes (Figure 6). The p-JNK1 protein expression decreased linearly (p = 0.013) and quadratically (p = 0.009) as added P levels increased (Figure 6E).
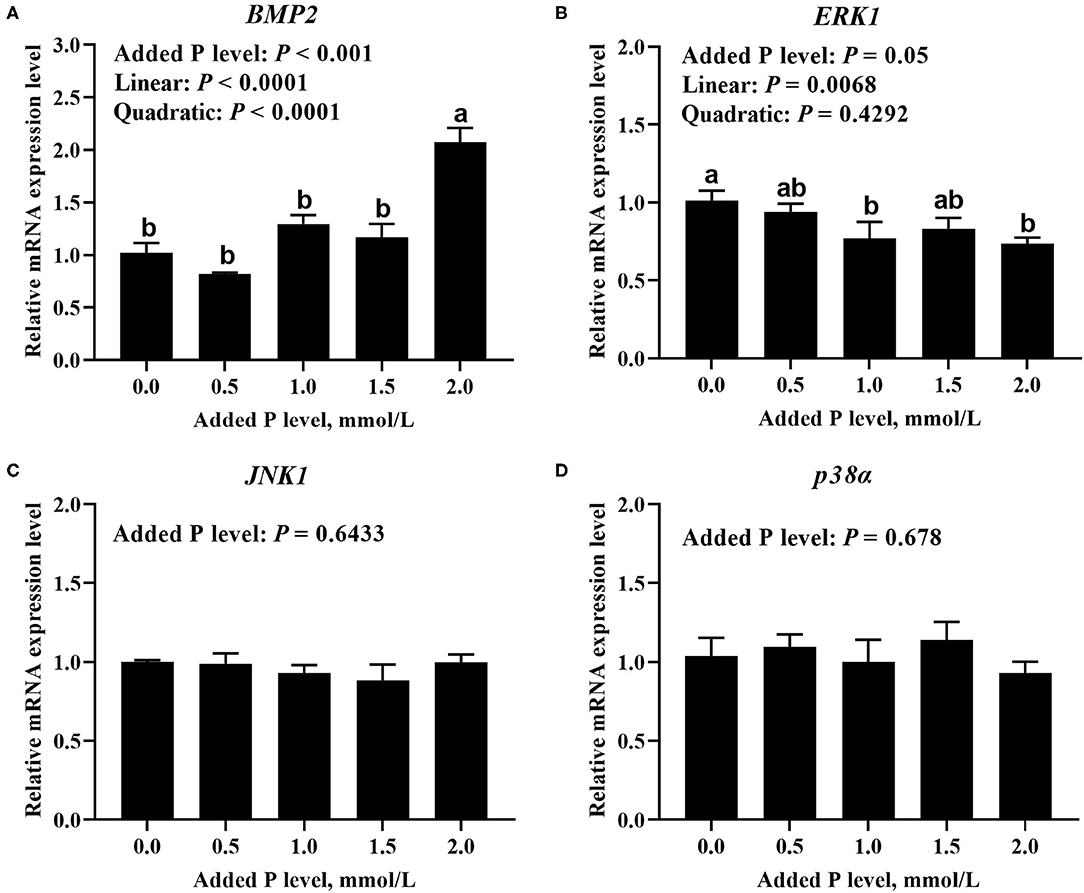
Figure 5. Effect of added P level on the mRNA expression levels of BMP2 (A), ERK1 (B), JNK1 (C), and p38α (D) in primary cultured tibial osteoblasts of broiler chicks. Each value was mean ± SE, n = 6. Means without a common letter differ, p ≤ 0.05. BMP2, bone morphogenetic protein 2; ERK1, extracellular signal-regulated kinase 1; JNK1, c-Jun N-terminal kinase 1; p38α, p38 mitogen-activated protein kinase alpha.
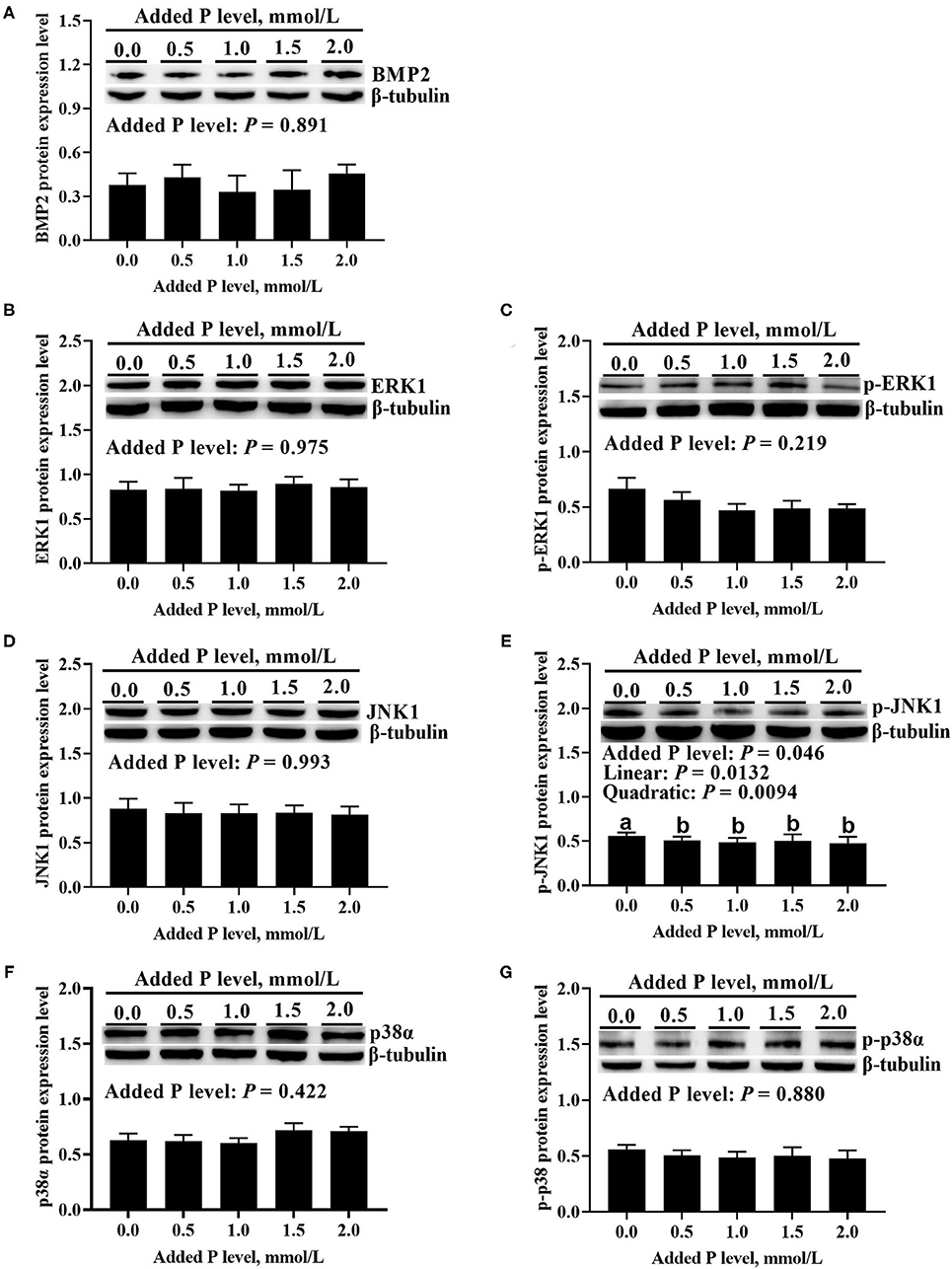
Figure 6. Effect of added P level on BMP2 protein expression level (A), total and phosphorylated protein expression levels of ERK1 (B,C), JNK1 (D,E), and p38α (F,G) in primary cultured tibial osteoblasts of broiler chicks. Representative Western blots for these proteins were shown. Each value was mean ± SE, n = 6. Means without a common letter differ, p ≤ 0.05. BMP2, bone morphogenetic protein 2; ERK1, extracellular signal-regulated kinase 1; p-ERK1, phosphorylated extracellular signal-regulated kinase 1; JNK1, c-Jun N-terminal kinase 1; p-JNK1, phosphorylated c-Jun N-terminal kinase 1; p38α, p38 mitogen-activated protein kinase alpha; p-p38α, phosphorylated p38 mitogen-activated protein kinase alpha.
Correlations Between Phosphorus Utilization Parameters and Local Bone-Derived Regulators or BMP/MAPK Pathway
The correlations between P utilization parameters (tibial osteoblastic P retention rate and number and area of mineralized nodules) and local bone-derived regulators or BMP/MAPK pathways are presented in Table 2. The tibial osteoblastic P retention rate was positively correlated (r = 0.452–0.564, p < 0.028) with MEPE and BMP2 mRNA expressions; the number and area of mineralized nodules were positively correlated (r = 0.414–0.612, p < 0.03) with PHEX, DMP1, MEPE, and BMP2 mRNA expressions but negatively correlated (r = −0.566 to 0.414, p < 0.04) with ERK1 mRNA expression and p-JNK1 protein expression; all of the P utilization parameters were not correlated (p > 0.05) with MEPE protein expression.
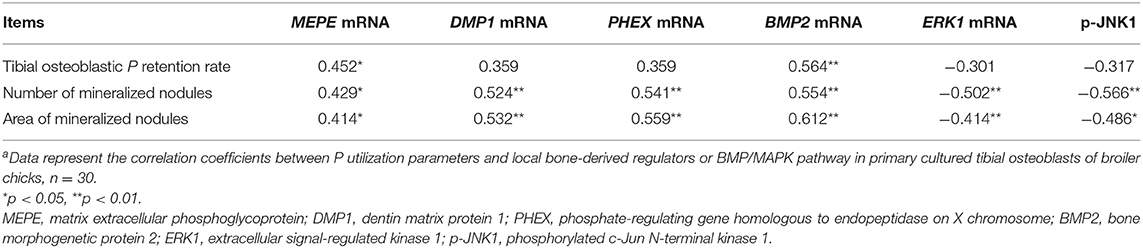
Table 2. The correlation coefficients between P utilization parameters and local bone-derived regulators or BMP/MAPK pathway in primary cultured tibial osteoblasts of broiler chicksa.
Discussion
In order to improve the bone P utilization efficiency of broilers and reduce its excretion to the environment, it is necessary to investigate the underlying regulatory mechanisms of the bone P utilization first. The hypothesis that P utilization in an osteoblastic model in vitro might be partly regulated by local bone-derived regulators and BMP/MAPK pathway has been supported by the results of the present study. The present study indicated that as added P levels increased, tibial osteoblastic P retention rate, number and area of mineralized nodules, the osteoblastic expressions of DMP1 mRNA, PHEX mRNA, MEPE mRNA, and protein, BMP2 mRNA, ERK1 mRNA, and p-JNK increased or decreased linearly or quadratically. Correlation analyses showed that the above local bone-derived regulators and BMP/MAPK pathway genes were positively or negatively associated with the above P utilization parameters, suggesting that they might partly regulate the P utilization in primary cultured tibial osteoblasts of broiler chicks and thus might be potential targets for improving the bone P utilization of broilers. These findings provide a novel insight into the regulation of the P utilization and its mechanisms in the bone of broilers and will contribute to develop feasible strategies to improve the bone P utilization efficiency of broilers so as to decrease its excretion.
Dietary P supply plays a critical role in bone development and mineralization of broiler chickens (2, 3, 5, 6), since a high proportion of P was deposited in the bone as the component of hydroxyapatite (11). Moreover, recently emerging evidence indicated that the body P utilization efficiency in birds was shown to have strong phenotypic correlations with bone development and bone P utilization parameters like the tibial P retention efficiency (33). Therefore, bone P retention and bone mineralization-associated parameters can be considered good indicators for P utilization in broilers (34). Likewise, in vitro, the hydroxyapatite has been identified as the major component in the mineralized nodules (35). Thus, number and area of mineralized nodules may be considered as the suitable indices for evaluating P utilization in vitro. A series of studies showed that bone ash, P retention, and bone mineral content and density were affected by dietary P level, and increased linearly or quadraticly as dietary P levels increased (2, 3, 5, 36). Consistent with the in vivo findings, the results of the present study indicated that tibial osteoblastic P retention rate and number and area of mineralized nodules increased linearly and quadraticly as added P levels increased. However, the underlying regulatory mechanisms of P utilization are not well known.
Local bone-derived regulators, such as FGF23, MEPE, DMP1, and PHEX, have emerged as important regulators of P homeostasis and bone mineralization (37). Of these, DMP1 and PHEX are osteogenic marker genes, whose mutations result in hypophosphatemia, low bone mineral density, and elevated FGF23 production (38, 39). Moreover, DMP1 and FGF23 have been identified as P-responsive genes. Nishino et al. (17) reported that elevated extracellular P facilitated osteoblastic differentiation by upregulating DMP1 expression. An elevation of dietary P level induced tibia FGF23 expression in broilers (40). The MEPE is a component of bone matrix, and its overexpression in mice displayed growth and bone mineralization defects characterized by low bone mass, low bone density, etc. (41). In the present study, osteoblastic DMP1, PHEX mRNA, MEPE mRNA, and protein expressions increased as added P level increased, while osteoblastic FGF23 mRNA expression and protein expressions of DMP1, PHEX, and FGF23 were not affected by added P level. In an in vivo study of broilers, Cao et al. (5) found that tibia DMP1 mRNA expression increased as dietary P level increased, while FGF23 and PHEX protein expressions were not affected by dietary P level, which is in agreement with the above in vitro results of the present study. However, Cao et al. (5) also reported that FGF23 mRNA expression increased, and PHEX mRNA expression and DMP1 protein expression decreased as dietary P level increased, while MEPE mRNA and protein expressions were not affected by dietary P level, which is not consistent with the above in vitro results of the present study. Notably, the DMP1 and PHEX mRNA expressions increased and reached a peak at 1.0 mmol/L of added P level, indicating an induced role of elevated extracellular P in DMP1 and PHEX expressions (17, 42). But interestingly, they decreased at 1.5 mmol/L of added P, followed by another increase at 2.0 mmol/L of added P, and the exact reasons are unclear and need to be further studied. Moreover, correlation analyses in the current study showed that the osteoblastic P utilization parameters were positively associated with MEPE, DMP1, and PHEX mRNA expressions. In broilers (5), DMP1 mRNA expression had positive correlation with bone P retention parameters, which is consistent with the above results of the present study; however, bone P retention parameters were positively associated with FGF23 mRNA expression but negatively associated with PHEX mRNA expression, which is not consistent with the above results of the present study. No other similar studies have been reported before. The above disparities between in vivo and in vitro findings might be due to the effects of other in vivo factors except for P, such as the feed intake and hormones. However, in vitro, P was a single impacting factor, and thus, the in vitro results might better reflect the specific and direct effect of P itself on the above local bone-derived regulators. The above results from the present study suggested that the osteoblastic P utilization might be partly regulated by these local bone-derived regulators DMP1, MEPE, and PHEX in primary cultured tibial osteoblasts of broiler chicks. However, further studies are needed to confirm whether and how DMP1, MEPE, and PHEX are involved in regulating the osteoblastic P utilization of broilers.
The BMP/MAPK pathway plays a key role in regulating osteoblastic differentiation and mineralization (19, 22, 23). The BMP is a multifunctional acidic polypeptide, which is predominantly synthesized and secreted by osteoblasts (43). Within the BMP family, BMP2 is a potent inducer of bone formation through its stimulation of osteoblast differentiation (44, 45). Sun et al. (21) found that overexpression of BMP2 in bone mesenchymal stem cells not only induced ALP activity but also promoted the formation of mineralization nodules. Moreover, BMP2 has been reported to exert its osteogenic function via MAPK pathway. Bokui et al. (46) reported that BMP2 activated the p38, ERK1/2, and JNK1/2 to promote the expression of Runx2, an osteogenic-specific transcription factor. Earlier studies also showed that p38, ERK, and JNK were essential for BMP2-induced osteoblastic differentiation and mineralization (23, 47). In the present study, osteoblastic BMP2 mRNA expression increased, and the expressions of ERK1 mRNA and p-JNK decreased as added P level increased, while the expressions of p38α and JNK1 mRNA, BMP2 protein expression, the total or phosphorylated protein expressions of ERK1 and p38α, and the total protein expression of JNK1 were not affected by added P level. In an in vivo study of broilers, Liao et al. (24) found that tibia ERK1 mRNA expression decreased as dietary P level increased, while total protein expressions of ERK1 and JNK1, and total and phosphorylated protein expressions of p38α were not affected by dietary P level, which is in agreement with the above in vitro results of the present study. However, Liao et al. (24) also observed that the expressions of p38α mRNA, BMP2, and p-ERK proteins decreased as dietary P level increased, while the expressions of BMP2 mRNA and p-JNK protein were not affected by dietary P level, which is not consistent with the above in vitro results of the present study. Furthermore, correlation analyses in the present study showed that osteoblastic P utilization parameters were positively correlated with BMP2 mRNA expression but negatively correlated with ERK1 mRNA expression and p-JNK1 protein expression. In broilers (24), bone P retention parameters were negatively correlated with ERK1 mRNA expression, which is consistent with the above results of the present study, but also negatively correlated with the expressions of JNK1 mRNA, BMP2, and p-ERK protein, which is not consistent with the above results of the present study. No other similar studies have been available so far. The above differences between in vivo and in vitro findings might be caused by other in vivo factors except for P, such as the feed intake and hormones. Therefore, the results from the above in vivo and in vitro studies have pointed out a possible role of BMP/MAPK pathway in regulating the bone P utilization in broilers.
Conclusions
The results from the present study indicated that P utilization parameters (tibial osteoblastic P retention rate and number and area of mineralized nodules) had positive correlations with MEPE, DMP1, PHEX, and BMP2 mRNA, but negative correlations with ERK1 mRNA and phosphorylated JNK1 in primary cultured osteoblasts of broiler chicks, and thus, they might be partly regulated by these local bone-derived regulators and BMP/MAPK pathway. Further studies are necessary to verify the roles of these local bone-derived regulators and BMP/MAPK pathway in regulating the osteoblastic P utilization of broilers.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare issue) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China) and performed in accordance with the guidelines. Ethical approval on animal survival was given by the animal ethics committee of IAS-CAAS.
Author Contributions
TL: data curation and writing—original draft preparation. SC, XLi, and LZ: investigation. YS: formal analysis. LL: methodology. ZL: resources. XLu: supervision and writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Program of the National Natural Science Foundation of China (project no. 31630073; Beijing, China), Initiation Funds of Yangzhou University for Distinguished Scientists (Yangzhou, China), the National Key R&D Program of China (project no. 2017YFD0502200; Beijing, China), the China Agriculture Research System of MOF and MARA (project no. CARS-41; Beijing, China), and the Agricultural Science and Technology Innovation Program (project no. ASTIP-IAS09; Beijing, China).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cowieson AJ, Perez-Maldonado R, Kumar A, Toghyani M. Possible role of available phosphorus in potentiating the use of low-protein diets for broiler chicken production. Poult Sci. (2020) 99:6954–63. doi: 10.1016/j.psj.2020.09.045
2. Jiang Y, Lu L, Li SF, Wang L, Zhang LY, Liu SB, et al. An optimal dietary non-phytate phosphorus level of broilers fed a conventional corn-soybean meal diet from 4 to 6 weeks of age. Animal. (2016) 10:1626–34. doi: 10.1017/S1751731116000501
3. Liu SB, Liao XD, Lu L, Li SF, Wang L, Zhang LY, et al. Dietary non-phytate phosphorus requirement of broilers fed a conventional corn-soybean meal diet from 1 to 21 d of age. Poult Sci. (2017) 96:151–9. doi: 10.3382/ps/pew212
4. Abbasi F, Fakhur-Un-Nisa T, Liu JB, Luo XG. I. Abbasi IHR Low digestibility of phytate phosphorus, their impacts on the environment, and phytase opportunity in the poultry industry. Environ Sci Pollut Res Int. (2019) 26:9469–79. doi: 10.1007/s11356-018-4000-0
5. Cao SM, Li TT, Shao YX, Zhang LY, Lu L, Zhang RJ, et al. Regulation of bone phosphorus retention and bone development possibly by related hormones and local bone-derived regulators in broiler chicks. J Anim Sci Biotechnol. (2021) 12:88. doi: 10.1186/s40104-021-00610-1
6. Li TT, Xing GZ, Shao YX, Zhang LY, Li SF, Lu L, et al. Dietary calcium or phosphorus deficiency impairs the bone development by regulating related calcium or phosphorus metabolic utilization parameters of broilers. Poult Sci. (2020) 99:3207–14. doi: 10.1016/j.psj.2020.01.028
7. Shao YX, Xing GZ, Zhang LY, Lu L, Li SF, Liao XD, et al. Effects of Dietary calcium or phosphorus deficiency on growth performance, rickets incidence characters and tibia histological structure of broilers during 1 to 21 days of age. Chinese J Anim Nutr. (2019) 31:2107–18. doi: 10.3969/j.issn.1006-267x.2019.05.017
8. Yang YF, Xing GZ, Li SF, Shao YX, Zhang LY, Lu L, et al. Effect of dietary calcium or phosphorus deficiency on bone development and related calcium or phosphorus metabolic utilization parameters of broilers from 22 to 42 days of age. J Integr Agr. (2020) 19:2775–83. doi: 10.1016/S2095-3119(20)63302-0
9. Liu SB, Li SF, Lu L, Xie JJ, Zhang LY, Luo XG. Estimation of standardized phosphorus retention for corn, soybean meal, and corn-soybean meal diet in broilers. Poult Sci. (2012) 91:1879–85. doi: 10.3382/ps.2011-02061
10. Liu SB, Xie JJ, Lu L, Li SF, Zhang LY, Jiang Y, et al. Estimation of standardized phosphorus retention for inorganic phosphate sources in broilers. J Anim Sci. (2013) 91:3766–71. doi: 10.2527/jas.2012-5729
11. Li XK, Wang JZ, Zhang CH, Li X, Tang CH. Content and distribution of different phosphorus forms in broiler chickens. J Anim Physiol Anim Nutr. (2016) 100:271–8. doi: 10.1111/jpn.12364
12. Serna J, Bergwitz C. Importance of dietary phosphorus for bone metabolism and healthy aging. Nutrients. (2020) 12:3001. doi: 10.3390/nu12103001
13. Hasegawa T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem Cell Biol. (2018) 149:289–304. doi: 10.1007/s00418-018-1646-0
14. Liu TL, Zou WW, Shi GD, Xu J, Zhang F, Xiao JR, et al. Hypoxia-induced MTA1 promotes MC3T3 osteoblast growth but suppresses MC3T3 osteoblast differentiation. Eur J Med Res. (2015) 20:10. doi: 10.1186/s40001-015-0084-x
15. Song M, Huo H, Cao Z, Han YF, Gao L. Aluminum trichloride inhibits the rat osteoblasts mineralization in vitro. Biol Trace Elem Res. (2017) 175:186–93. doi: 10.1007/s12011-016-0761-9
16. Wu Y, Jiang Y, Liu Q, Liu CZ. lncRNA H19 promotes matrix mineralization through up-regulating IGF1 by sponging miR-185-5p in osteoblasts. BMC Mol Cell Biol. (2019) 20:48. doi: 10.1186/s12860-019-0230-3
17. Nishino J, Yamazaki M, Kawai M, Tachikawa K, Yamamoto K, Miyagawa K, et al. Extracellular phosphate induces the expression of dentin matrix protein 1 through the FGF receptor in osteoblasts. J Cell Biochem. (2017) 118:1151–63. doi: 10.1002/jcb.25742
18. Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. (2003) 285:E1–9. doi: 10.1152/ajpendo.00016.2003
19. Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. (2002) 17:101–10. doi: 10.1359/jbmr.2002.17.1.101
20. Halloran D, Durbano HW, Nohe A. Bone morphogenetic protein-2 in development and bone homeostasis. J Dev Biol. (2020) 8:19. doi: 10.3390/jdb8030019
21. Sun J, Li JY, Li CC, Yu YC. Role of bone morphogenetic protein-2 in osteogenic differentiation of mesenchymal stem cells. Mol Med Rep. (2015) 12:4230–7. doi: 10.3892/mmr.2015.3954
22. Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, et al. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. (2001) 28:491–8. doi: 10.1016/S8756-3282(01)00415-X
23. Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. (2003) 18:2060–8. doi: 10.1359/jbmr.2003.18.11.2060
24. Liao XD, Cao SM, Li TT, Shao YX, Zhang LY, Lu L, et al. Regulation of bone phosphorus retention and bone development possibly by BMP and MAPK signaling pathways in broilers. J Integr Agr. (2021). Available online at: https://www.chinaagrisci.com/Jwk_zgnykxen/EN/abstract/abstract373631.shtml
25. Bakker AD, Klein-Nulend J. Osteoblast isolation from murine calvaria and long bones. Methods Mol Biol. (2012) 816:19–29. doi: 10.1007/978-1-61779-415-5_2
26. Liu BY, Lu YQ, Wang Y, Ge LN, Zhai NX, Han JX, et al. protocol for isolation and identification and comparative characterization of primary osteoblasts from mouse and rat calvaria. Cell Tissue Bank. (2019) 20:173–82. doi: 10.1007/s10561-019-09751-0
27. Zoidis E, Ghirlanda-Keller C, Gosteli-Peter M, Zapf J, Schmid C. Regulation of phosphate (Pi) transport and NaPi-III transporter (Pit-1) mRNA in rat osteoblasts. J Endocrinol. (2004) 181:531–40. doi: 10.1677/joe.0.1810531
28. Cao SM, Li TT, Shao YX, Zhao YZ, Zhang LY, Lu L, et al. Establishment and evaluation of the primary cultured tibial osteoblasts model of broiler chicks. J Integr Agr. (2022). Available online at: https://www.chinaagrisci.com/Jwk_zgnykxen/EN/abstract/abstract423704.shtml
29. Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. (2004) 329:77–84. doi: 10.1016/j.ab.2004.02.002
30. Shao YX, Wen Q, Zhang SM, Lu L, Zhang LY, Liao XD, et al. Dietary supplemental vitamin D3 enhances phosphorus absorption and utilisation by regulating gene expression of related phosphate transporters in the small intestine of broilers. Br J Nutr. (2019) 121:9–21. doi: 10.1017/S0007114518002763
31. Li TT, He WG, Liao XD, Lin X, Zhang LY, Lu L, et al. Zinc alleviates the heat stress of primary cultured hepatocytes of broiler embryos via enhancing the antioxidant ability and attenuating the heat shock responses. Anim Nutr. (2021) 7:621–30. doi: 10.1016/j.aninu.2021.01.003
32. Shao YX, Sun GM, Cao SM, Lu L, Zhang LY, Liao XD, et al. Bone phosphorus retention and bone development of broilers at different ages. Poult Sci. (2019) 98:2114–21. doi: 10.3382/ps/pey565
33. Künzel S, Bennewitz J, Rodehutscord M. Genetic parameters for bone ash and phosphorus utilization in an F2 cross of Japanese quail. Poult Sci. (2019) 98:4369–72. doi: 10.3382/ps/pez398
34. Wang WW, Cai HY, Zhang AR, Chen ZM, Chang WH, Liu GH, et al. Enterococcus faecium modulates the gut microbiota of broilers and enhances phosphorus absorption and utilization. Animals. (2020) 10:1232. doi: 10.3390/ani10071232
35. Zhang ZL, Chen XR, Bian S, Huang J, Zhang TL, Wang K. Identification of dicalcium phosphate dihydrate deposited during osteoblast mineralization in vitro. J Inorg Biochem. (2014) 131:109–14. doi: 10.1016/j.jinorgbio.2013.11.006
36. Cardoso EF, Donzele JL, de Oliveira Donzele RFM, Sufiate BL, Silva AD, Tizziani T. Non-phytate phosphorus requirement for broilers from 8 to 21 days of age under heat stress conditions. Trop Anim Health Prod. (2018) 50:317–25. doi: 10.1007/s11250-017-1434-1
37. Rowe PS. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr. (2012) 22:61–86. doi: 10.1615/CritRevEukarGeneExpr.v22.i1.50
38. Ichikawa S, Gerard-O'Riley RL, Acton D, McQueen AK, Strobel IE, Witcher PC, et al. A mutation in the Dmp1 gene alters phosphate responsiveness in mice. Endocrinology. (2017) 158:470–6. doi: 10.1210/en.2016-1642
39. Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, et al. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. (2011) 25:2551–62. doi: 10.1096/fj.10-177816
40. Wang RM, Zhao JP, Wang XJ, Jiao HC, Wu JM, Lin H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poult Sci. (2018) 97:2258–66. doi: 10.3382/ps/pey092
41. David V, Martin A, Hedge AM, Rowe RS. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. (2009) 150:4012–23. doi: 10.1210/en.2009-0216
42. Ito N, Findlay DM, Anderson PH, Bonewald LF, Atkins GJ. Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J Steroid Biochem Mol Biol. (2013) 136:183–6. doi: 10.1016/j.jsbmb.2012.09.029
43. Smith DM, Cooper GM, Mooney MP, Marra KG, Losee JE. Bone morphogenetic protein 2 therapy for craniofacial surgery. J Craniofac Surg. (2008) 19:1244–59. doi: 10.1097/SCS.0b013e3181843312
44. Hang QL, Zhou Y, Hou SC, Zhang DM, Yang XY, Chen JP, et al. Asparagine-linked glycosylation of bone morphogenetic protein-2 is required for secretion and osteoblast differentiation. Glycobiology. (2014) 24:292–304. doi: 10.1093/glycob/cwt110
45. Shibasaki S, Kitano S, Karasaki M, Tsunemi S, Sano H, Iwasaki T. Blocking c-Met signaling enhances bone morphogenetic protein-2-induced osteoblast differentiation. FEBS Open Bio. (2015) 5:341–7. doi: 10.1016/j.fob.2015.04.008
46. Bokui N, Otani T, Igarashi K, Kaku J, Oda M, Nagaoka T, et al. Involvement of MAPK signaling molecules and Runx2 in the NELL1-induced osteoblastic differentiation. FEBS Lett. (2008) 582:365–71. doi: 10.1016/j.febslet.2007.12.006
Keywords: BMP/MAPK pathway, broiler, local bone-derived regulator, phosphorus utilization, tibial osteoblast
Citation: Li T, Cao S, Liao X, Shao Y, Zhang L, Lu L, Liu Z and Luo X (2022) The Effects of Inorganic Phosphorus Levels on Phosphorus Utilization, Local Bone-Derived Regulators, and BMP/MAPK Pathway in Primary Cultured Osteoblasts of Broiler Chicks. Front. Vet. Sci. 9:855405. doi: 10.3389/fvets.2022.855405
Received: 15 January 2022; Accepted: 03 February 2022;
Published: 22 March 2022.
Edited by:
Shiping Bai, Sichuan Agricultural University, ChinaReviewed by:
Yongwen Zhu, South China Agricultural University, ChinaYanling Huang, Southwest Minzu University, China
Zhouzheng Ren, Northwest A&F University, China
Copyright © 2022 Li, Cao, Liao, Shao, Zhang, Lu, Liu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xugang Luo, d2x5c3pAMjYzLm5ldA==
†These authors have contributed equally to this work
 Tingting Li
Tingting Li Sumei Cao2†
Sumei Cao2† Xiudong Liao
Xiudong Liao Yuxin Shao
Yuxin Shao Zongping Liu
Zongping Liu