- 1Istituto Zooprofilattico Sperimentale della Lombardia e dell' Emilia Romagna “Bruno Ubertini”, Brescia, Italy
- 2Consiglio per la ricerca in agricoltura e l'analisi dell'economia agraria, Centro di ricerca Agricoltura e Ambiente, Bologna, Italy
- 3Azienda Unità Sanitaria Locale di Bologna, Dipartimento di Sanità Pubblica, Bologna, Italy
- 4Azienda Unità Sanitaria Locale della Romagna, Dipartimento di Sanità Pubblica, Ravenna, Italy
- 5Agenzia di Tutela della Salute di Bergamo-Dipartimento di Prevenzione Veterinario, Bergamo, Italy
American Foulbrood (AFB) is a contagious and severe brood disease of honey bees caused by the spore-forming bacterium Paenibacillus larvae. The identification of honey bee colonies infected by P. larvae is crucial for the effective control of AFB. We studied the possibility of identifying the infection levels by P. larvae in honey bee colonies through the examination of powdered sugar samples collected in the hives. The powdered sugar was dusted on the top bars of honeycombs and collected from a sheet paper placed at the bottom of the hive. Three groups of honey bee colonies were examined: Group A1 - colonies with clinical symptoms of AFB (n = 11); Group A2 – asymptomatic colonies located in apiaries with colonies showing symptoms of AFB (n = 59); Group B – asymptomatic colonies located in apiaries without cases of the disease (n = 49). The results showed that there was a significant difference in spore counting between Groups and that the spore load in sugar samples was always consistent with the clinical conditions of the colonies and with their belonging to AFB-affected apiaries or not. Based on the obtained results the cultural examination of powdered sugar samples collected from hives could be an effective tool for the quantitative non-destructive assessment of P. larvae infections in honey bee colonies.
Introduction
American Foulbrood (AFB), which is caused by spore-forming bacterium Paenibacillus larvae (1), is the most widespread and severe bacterial disease affecting honey bee brood and causes considerable economic losses to beekeepers worldwide (2).
Clinically, the disease is characterized by darkened brood combs with a mottled appearance; a sour smell, greasy, sunken and perforated cell caps, but the pathognomonic symptom is the transformation of dead larvae into a ropy mass that forms a characteristic viscous thread when a matchstick is inserted into the cell and then pulled out.
If not removed, the dead larvae dries into a blackish scale tightly adhering to the wall of the cells (3).
The diagnosis of AFB is based on the recognition of typical symptoms and on the identification of P. larvae by means of laboratory techniques (4).
The molecular characterization of P. larvae strains via repetitive element PCR (rep-PCR) using enterobacterial repetitive intergenic consensus (ERIC) primers allows for the identification of five different ERIC genotypes designated as ERIC I-V (1, 5).
Genotype ERIC V was isolated only once from a Spanish honey sample (5), while the genotypes ERIC III and ERIC IV have been isolated very rarely and in the past and have little practical importance (6). The genotypes ERIC I and ERIC II instead are those regularly isolated from AFB outbreaks in Europe and worldwide (1, 7–11). These two genotypes have different phenotypic characteristics and differ, above all, in the morphology/pigmentation of colonies and in virulence (1).
The colonies of P. larvae genotype ERIC II are characterized by a typical orange pigmentation that is never observed in the colonies of genotype ERIC I (1, 10, 12).
Regarding virulence, strains of P. larvae genotype ERIC II are more virulent at the larval level than strains of genotype ERIC I, and all infected larvae are killed within 6-7 days instead of within 13–15 days. Therefore, most larvae die before cell capping, and adult bees can easily remove dead larvae from their hives. There are few larvae that die after cell capping; consequently, the darkened and perforated caps can be very low in number. Identifying decaying larvae is more difficult than in the case of ERIC I genotype infections, and the risk of overlooking the disease, especially in the early stages, is considerable (13, 14).
Križanová et al. (15) and Drobniková et al. (16) described outbreaks of AFB with atypical symptoms caused by orange-pigmented Bacillus larvae strains in the Czech and Slovak Republics. In these outbreaks, the following atypical symptoms were observed: (a) the caps of diseased brood cells were not darkly colored; (b) decaying larvae were light brown or gray in color without a glue-like consistency but instead with a watery consistency; (c) light brown or gray scales were easily removed from the cells; (d) white larvae protruding from uncapped cells could be seen; (e) sometimes the larvae had an appearance similar to what is observed in Sacbrood disease.
Outbreaks of AFB caused by P. larvae genotype ERIC II with the same or very similar symptoms are sometimes observed also today in northern Italy (personal observation).
When the darkened and perforated caps are very low in number or atypical symptoms are present the clinical diagnosis of AFB is, of course, more difficult.
American Foulbrood generally occurs when a certain level of contamination by P. larvae spores in honey bee colonies is reached (17); nevertheless, some colonies can contain even a relatively large number of spores over several seasons without showing clinical symptoms (18–21).
These colonies contribute, first of all, to the transmission of P. larvae spores from one hive to another (22); furthermore, they can more or less quickly develop the disease.
The identification of infected but asymptomatic colonies can be performed through a search for P. larvae spores in materials taken from hives, and standard methods based on microbiological and biomolecular techniques are available for the detection and quantification of the spore load in adult bees, honey from super, honey from the brood chamber, wax and wax debris (4, 23, 24).
The culture-based techniques for the detection of P. larvae in materials taken from the hive (honey, bees and debris) are a good alternative to the PCR methods. The results obtained with the cultural methods are often more accurate than those obtained with PCR. This may depend on a variety of factors, such as the presence of polymerase inhibitors in some hive materials and/or failures in the DNA extraction from P. larvae spores (25).
We studied the possibility of identifying honey bee colonies infected by P. larvae at various levels using the powdered sugar dusting technique.
The powdered sugar is frequently used in beekeeping practices for various purposes.
The powdered sugar roll method performed on samples of adult bees is used to monitor Varroa infestation levels (26, 27). This approach can be used to determine further treatment needs or to assess past treatment effectiveness.
The dusting of bees with powdered sugar, removing mites from adult bees, has been used also for the control of Varroa infestations (28, 29).
Another use of this material occurs in the therapy of some diseases where the drugs are mixed with powdered sugar before the dusting on the tops of the combs.
Icing sugar is not toxic to adult bees and bee brood (30) moreover is practice to use and inexpensive.
In this work we have dusted the powdered sugar on the top bars of the combs and the sugar collected at the hive bottom, was examined by a culture method for P. larvae spore counting.
The results of cultural examinations were compared to the outcomes of clinical inspections performed at the time of sugar sampling to study relationships between the number of spores in the sugar samples and the clinical status of the colonies or apiaries.
Materials and Methods
Apiaries and Clinical Inspection
The work was carried out on 119 colonies belonging to 10 apiaries located in the Emilia-Romagna and Lombardy regions of northern Italy. The number of hives ranged from 4 to 17 per apiary (Tables 1, 2).
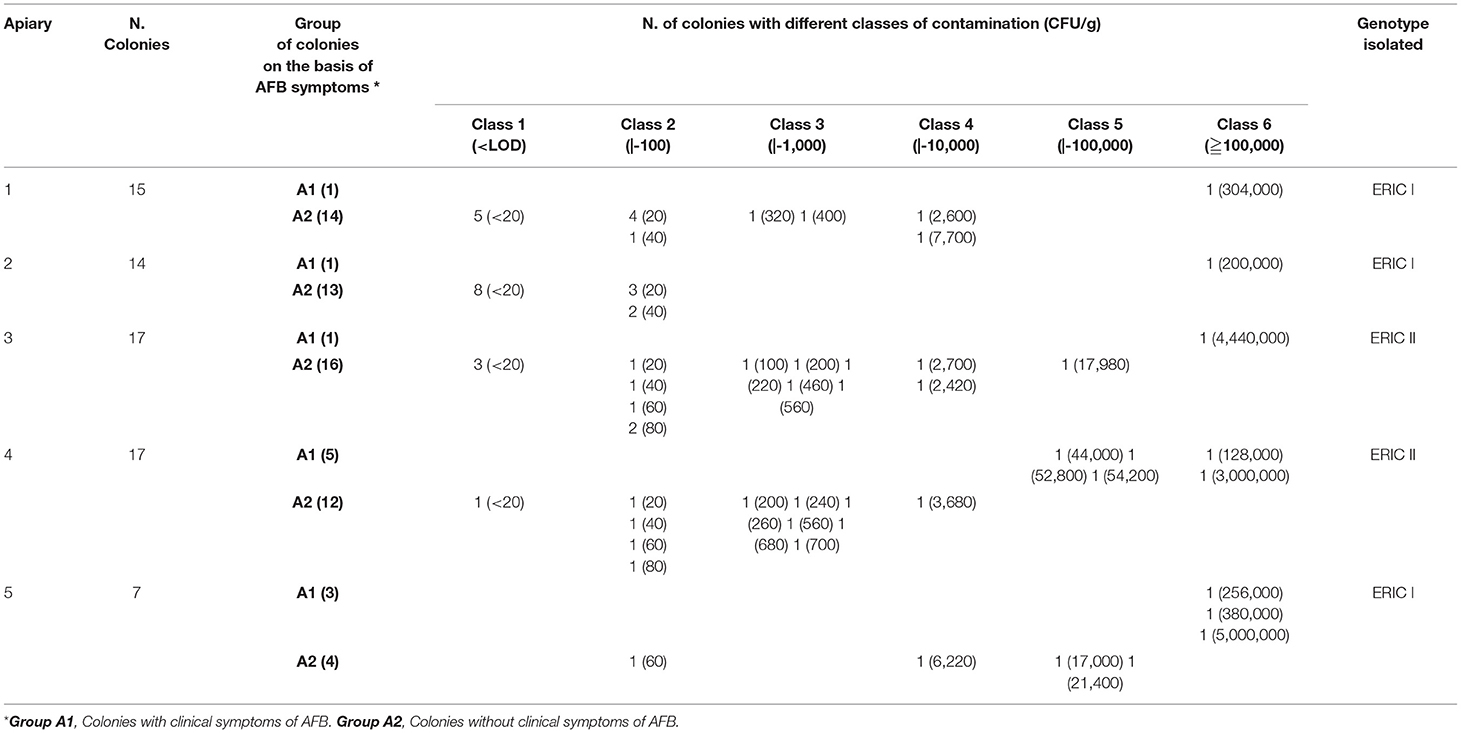
Table 1. Apiaries (Groups A1 and A2) with recurrent cases of overt AFB reported in recent years: results of clinical inspections, cultural examinations and genotype of P. larvae isolates.
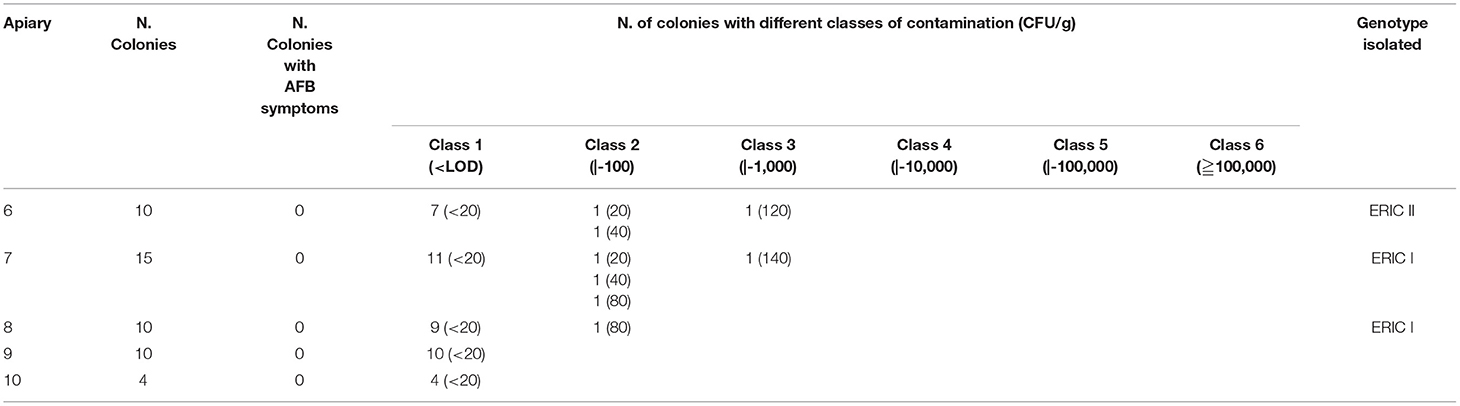
Table 2. Apiaries (Group B) without cases of AFB reported in recent years: results of clinical inspections, cultural examinations and genotype of P. larvae isolates.
The apiaries were characterized by different histories in terms of the presence of AFB. In five apiaries (Groups A1 and A2), cases of recurring AFB were reported in years previous to this work, whereas in the other five apiaries (Group B), which were tested as a control group, no cases of AFB had been reported in the last years.
Before sampling, each colony was checked via clinical examination. Visual inspection was carried out on all brood combs, and the suspected cells were inspected to detect ropy larval remains or scales. Additionally, dead larvae in capped or uncapped cells with atypical lesions (e.g., a watery consistency) were examined. The clinical diagnosis was confirmed after the laboratory isolation of P. larvae from larval remains or scales. The results of clinical examinations were recorded as denoting the presence or absence of disease symptoms.
Sampling Procedures
In each hive, 50 g of powdered sugar containing 3% corn starch was dusted on the top bars of the combs in the brood chamber and immediately brushed between the frames. After 20–25 min, the sugar was collected from a sheet paper previously placed on the hive bottom and transferred into a plastic jar with a screw cap. The jars were sent to the laboratory and stored at 3°C until analysis.
After powdered sugar sampling, all the diseased colonies were killed and burned in accordance with national law.
Microbiological Analysis
In brief, 1 g of sugar was placed in a 15 ml test tube with a screw cap containing 9 ml of sterile distilled water. The test tube was shaken by hand until the sugar dissolved and then heated in a water bath at 85°C for 15 min to inactivate the thermosensitive contaminants. The solution was plated on five Petri dishes (100 μl/plate) of MYPGP (Mueller-Hinton broth, yeast extract, potassium phosphate, glucose and pyruvate) agar supplemented with nalidixic and pipemidic acid.
The plates were incubated at 37 °C in an atmosphere with 10% CO2 and examined after 3 and 8 days.
When counting was not possible due to the presence of very large numbers of colonies or to the rapid growth of spore-forming bacteria, ten-fold dilutions from the initial solution were prepared and then cultured with the previously described procedures.
For each sample, one to five colonies with P. larvae-like morphology were tested for catalase reactions and subjected to Gram staining, always resulting in catalase negativity with Gram-positive rods.
One or two strains for each sample were cultured on TSYEA (tryptic soy yeast extract agar) slant and stored at 2 °C for further molecular analysis.
After the numeration of P. larvae colonies, the number of viable spores was calculated and expressed as colony forming units (CFU). The limit of detection (LOD) of the method was 20 CFU/g.
Molecular Analysis
The strains stored on TSYEA slant were tested by PCR according to Dobbelaere et al. (31), and all strains were identified as P. larvae.
In the sugar samples positive for P. larvae, the colony morphology was always uniform, and only one isolate per sample was genotyped by ERIC-PCR using primers applied by [Genersch and Otten (32)] and following the PCR protocol described by Bassi et al. (10).
Data Analysis
Descriptive statistics (absolute frequencies and percentages) were obtained regarding the number of colonies with different contamination levels. Data “ < LOD” were treated as 10 CFU/g for calculation purposes. The Kruskal-Wallis test was used to compare spore counts in Groups identified with clinical inspection. R 4.0.2 (33) and Microsoft Excel for Windows were used to manage the data.
Results
The results of clinical inspections, spore counting carried out on the powdered sugar samples, and ERIC genotyping are reported in Tables 1, 2.
The clinical controls in the five apiaries with recurrent AFB outbreaks highlight 11 colonies with AFB symptoms and 59 asymptomatic colonies (Table 1).
The clinical controls in five apiaries with no cases of AFB in recent years showed no symptoms of AFB (Table 2).
The diagnostic controls allowed us to divide the 119 colonies into 3 groups: Group A1-colonies with clinical symptoms of AFB (n = 11); Group A2-colonies without clinical symptoms located in apiaries with colonies showing symptoms of AFB (n = 59); and Group B-colonies without symptoms of AFB and located in apiaries where no cases of the disease were reported in the last years (n = 49).
In the AFB-diseased apiaries, the spore load in symptomatic colonies (Group A1) ranged from 4.4 x 104 to 5.0 x 106 CFU/g with a mean value of 1,259,909 (sd = 1,913,607). In the non-symptomatic colonies (Group A2), the presence of P. larvae spores was not demonstrated in 17 samples, and in the remaining 42 samples (71.2, 95% CI 57.9 – 82.2), the spore load ranged from 2.0 x 101 to 2.1 x 104 CFU/g with an average number of spores per sugar sample of 2,081 CFU/g (sd = 4,993) (Table 1).
In the apiaries of Group B, the presence of P. larvae spores was not observed in 41 sugar samples; in the remaining 8 samples (16.3, 95% CI 7.3–29.7), the spore load ranged from 2.0 x 101 to 1.4 x1 02 CFU/g, and the mean number of spores per sugar sample reached 67.5 CFU/g (sd=45.3) (Table 2).
The distribution of P. larvae spore contamination levels (CFU/g) in these Groups is reported in Figure 1. The Kruskal-Wallis test shows a significant difference in spore counts between the Groups (H = 59.2, p < 0.01).
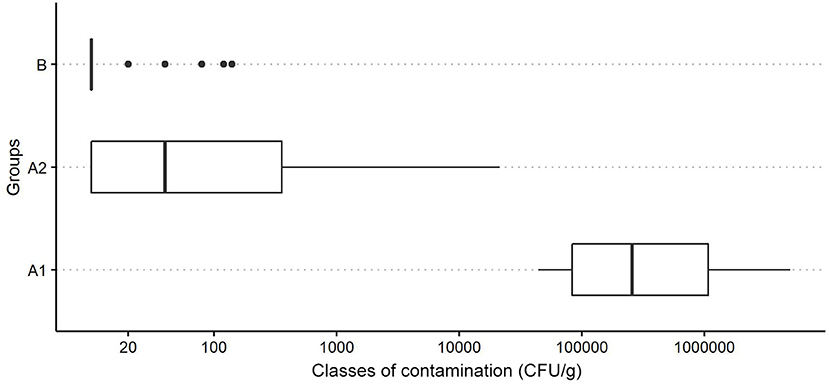
Figure 1. Boxplot of the distribution of P. larvae spore contamination classes (CFU/g) in powdered sugar samples for different groups (A1, colonies with clinical symptoms of AFB; A2, colonies without clinical symptoms of AFB located in apiaries with colonies showing symptoms of AFB; and B: colonies from apiaries with no cases of AFB in the last years-control group).
In Figure 2, the percentage distributions of samples/colonies are grouped into six classes of contamination (class 1: < LOD; class 2: <100; class 3: <1,000; class 4: <10,000; class 5: <100,000, and class 6: ≤ 1,000,000 CFU/g), and the number of samples/colonies belonging to each Group is reported inside each bar.
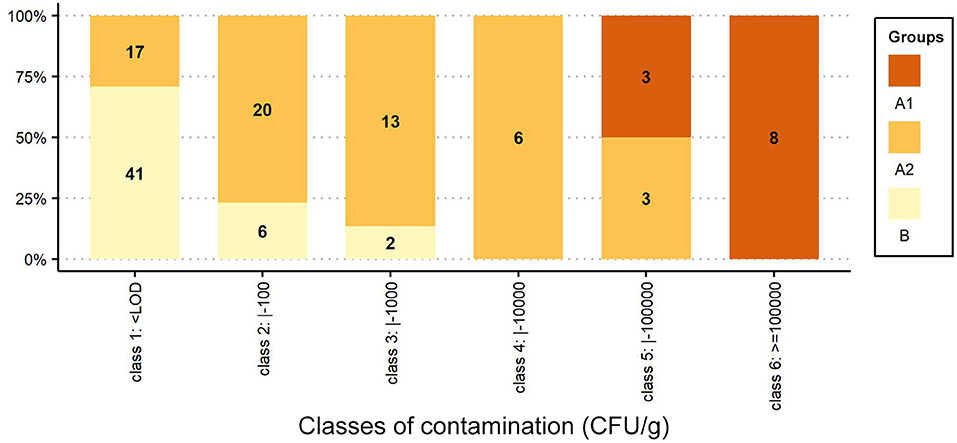
Figure 2. Percentage distribution of samples/colonies grouped by classes of contamination (CFU/g). The number of samples/colonies is shown inside each bar.
The distribution of the results in terms of contamination classes (Figure 2) highlights the differences, reported in Figure 1, between the Groups: all symptomatic colonies of the AFB-diseased apiaries (Group A1) fall under classes 5 and 6 (3 and 8 colonies, respectively); the asymptomatic colonies (Group A2) span different size classes (for classes 1 to 5, we find 17, 20, 13, 6 and 3 colonies, respectively); the asymptomatic colonies belonging to the control group (Group B) are mainly of the first class (41 colonies), and the 8 positive samples are of classes 2 and 3 (6 and 2 colonies, respectively).
The ERIC genotyping of the selected strains shows that in the AFB-diseased apiaries, P. larvae genotype ERIC I was isolated in three apiaries (1–2–5), and P. larvae genotype ERIC II was isolated in two apiaries (3–4). No mixed infections were observed in the same hive or apiary (Table 1).
In the apiaries without cases of AFB, genotype ERIC I was isolated from the powdered sugar in two apiaries (7–8), and genotype ERIC II was isolated from one apiary (6). In the remaining two (9–10), P. larvae were not isolated (Table 2).
Discussion and Conclusion
The aim of this work was to study the performance of powdered sugar samples collected from hives in detecting P. larvae spore load in honey bee colonies.
Powdered sugar samples were taken from apiaries with different clinical conditions and history.
The results show that the P. larvae spore numbers in the sugar samples were clearly related to the presence or absence of the disease in the colonies or apiaries.
As highlighted by our boxplot graph (Figure 1), the difference in the spore loads of the AFB-diseased apiaries and non-diseased apiaries is notable.
In the AFB-diseased apiaries, the number of P. larvae spores in sugar samples collected from colonies with overt AFB (Group A1) was always greater than for the asymptomatic colonies (Group A2), although some (3/42) asymptomatic colonies showed spore numbers very close to those of symptomatic colonies (Table 1) and fell under size class 5 together with diseased colonies (Figure 2).
The spore loads observed in the symptomatic colonies (Group A1) were significantly higher than those found in the colonies of the control group (Group B), and the results always fall under different size classes (Figure 2).
Taking into consideration only colonies without symptoms, the comparison between the Group A2 (apiaries including diseased colonies) and the Group B (healthy apiaries) shows a significant higher spores level in colonies of the Group A2, in addition to a much higher proportion of positive values (71 vs. 14%). This can be explained by the fact that in the apiaries with AFB cases the spread of P. larvae spores to healthy colonies inevitably takes places, as common beekeeping practices facilitate horizontal transfer of pathogens, especially if numerous hives are managed in close proximity (34).
The bacterial spore load in the sugar samples is always consistent with the clinical conditions of colonies and with whether they belong to AFB diseased apiaries or not.
These results show that the cultural examination of powdered sugar samples collected from hives allows us to detect infection levels by P. larvae spores of colonies.
Knowledge of infection levels allows for the adoption of appropriate management strategies to prevent both AFB onset and the transmission of infection between colonies and apiaries (35).
The identification of colonies with high P. larvae spore levels can be useful not only for preventive purposes but also for diagnostic purposes.
The clinical diagnosis of AFB is typically based on the recognition of symptoms by visual inspection. However, field inspection, particularly for large beekeeping operations, is laborious and time consuming; moreover, the obtained results are highly operator dependent (36).
It should be added that by visual inspection in early stages, the disease may remain undetectable (22), and in any case, clinical examination does not enable the detection of colonies with asymptomatic infections.
The identification of colonies with significant spore loads used as a screening/diagnostic tool allows for clinical checks only of suspected colonies, reducing field labor requirements.
Moreover, targeted checks improve clinical examination performance, facilitating the identification of colonies with very few suspected cells or with atypical lesions that can escape a “routine” clinical examination.
Powdered sugar has some advantages over other materials used to monitor P. larvae infections in honey bee colonies. The sampling is not destructive and is very easy to do, the packaging, the transportation to the laboratory and the sample handling in laboratory are very practical to perform.
Furthermore, the powdered sugar examination, as well as the examination of adult bees (25), provides a “snapshot” of the actual infection status unlike that occurs by examining other materials, such as the debris collected during the wintering period or the honey, that reflect instead the historical accumulation of bacterial spores over time.
Based on the results of our trial, the cultural examination of powdered sugar samples collected from hives seems to be an effective tool for the quantitative non-destructive assessment of P. larvae infections in honey bee colonies.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
SB: conceptualization, methodology, investigation, data collection and analysis, writing the original draft, review and editing, and supervision. GL, FB, and SP: investigation and supervision. ECarp: conceptualization, review and editing the original draft, and supervision. GG: data analysis and review and editing the original draft. ECarr: investigation, data collection and analysis, review and editing the original draft, and supervision. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Genersch E, Forsgren E, Pentikäinen J, Ashiralieva A, Rauch S, Kilwinski J, et al. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp larvae as Paenibacillus larvae without subspecies classification. Int J Syst Evol Microbiol. (2006) 56:501–11. doi: 10.1099/ijs.0.63928-0
2. Ellis JD, Munn PA. The worldwide health status of honey bees. Bee World. (2005) 86:88–101. doi: 10.1080/0005772X.2005.11417323
3. Hansen H, Brødsgaard CJ. American foulbrood: a review of its biology, diagnosis and control. Bee World. (1999) 80:5–23. doi: 10.1080/0005772X.1999.11099415
4. Anonymous. American foulbrood of honey bees (Infection of honey bees with Paenibacillus larvae). In: OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.2.2. (2018). p. 719–35. Available online at: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.02.02_AMERICAN_FOULBROOD.pdf.
5. Beims H, Bunkc B, Erlerd S, Mohre K, Spröerc C, Pradellac S, et al. Discovery of Paenibacillus larvae ERIC V: Phenotypic and genomic comparison to genotypes ERIC I-IV reveal different inventories of virulence factors which correlate with epidemiological prevalences of American Foulbrood. Int J Med Microbiol. (2020) 310:151394. doi: 10.1016/j.ijmm.2020.151394
6. Genersch E. American foulbrood in honey bees and its causative agent, Paenibacillus larvae. J Invert Pathol. (2010) 103:10–9. doi: 10.1016/j.jip.2009.06.015
7. Pentikäinen J, Kalliainen E, Pelkonen S. Molecular epidemiology of Paenibacillus larvae infection in Finland. Apidologie. (2009) 40:73–81. doi: 10.1051/apido:2008061
8. Loncaric I, Derakhshifar I, Oberlerchner JT, Köglberger H, Moosbeckhofer R. Genetic diversity among isolates of Paenibacillus larvae from Austria. J Invertebr Pathol. (2009) 100:44–6. doi: 10.1016/j.jip.2008.09.003
9. Schäfer MO, Genersch E, Fünfhaus A, Poppinga L, Formella N, Bettin B, et al. Rapid identification of differentially virulent genotypes of Paenibacillus larvae, the causative organism of American foulbrood of honey bees, by whole cell MALDI-TOF mass spectrometry. Vet Microbiol. (2014) 170:291–7. doi: 10.1016/j.vetmic.2014.02.006
10. Bassi S, Formato G, Milito M, Trevisiol K, Salogni C, Carra E. Phenotipic characterization and ERIC-PCR based genotyping of Paenibacillus larvae isolates recovered from American foulbrood outbreaks in honey bees from Italy. Vet Q. (2015) 35:27–32. doi: 10.1080/01652176.2014.993095
11. Biová G, Bzdil J, Dostálková S, Pet?rivalský N, Brus J, Carra E, et al. American foulbrood in the Czech Republic: ERIC II genotype of Paenibacillus larvae is prevalent. Front Vet Sci. (2021) 8:698976. doi: 10.3389/fvets.2021.698976
12. Hirai Y, Suzuki T, Inaba N, Minoguchi N, Takamatsu D. Existence of Paenibacillus larvae genotypes ERIC I-ST2, ERIC I-ST15 and ERIC II-ST10 in the western region of Aichi prefecture, Japan. J Vet Med Sci. (2016) 78:1195–9. doi: 10.1292/jvms.16-0041
13. Genersch E. Paenibacillus larvae and American Foulbrood long since known and still surprising. J Verbr Lebensm. (2008) 3:429–34. doi: 10.1007/s00003-008-0379-8
14. Genersch E. Amerikanische Faulbrut: oft anders als im Lehrbuch. Deutsches Bienen J. (2009) 8:4–6.
15. KriŽanová H, Halaša N, Roško L, Zubaj J. Results of studies on the causative agent of American foulbrood. Vet Med. (1988) 33:633–40.
16. Drobniková V, Richter V, Häusler J, Pytelová I. Characterization of Bacillus larvae and related bacilli by chromatography of cell fatty acids. J Apic Res. (1994) 33:69–74. doi: 10.1080/00218839.1994.11100852
17. Anonymous. Report of the Meeting of the OIE ad hoc Group on Diseases of Honey Bees.- January 31 - February 2, Paris, France. The World Organisation for Animal Health, OIE (2012). Available online at: https://www.oie.int/fileadmin/Home/eng/Internationa_Standard_Setting/docs/pdf/SCAD/A_SCAD_Feb2012.pdf.
18. Hansen H, Rasmussen B. The investigation of honey from bee colonies for Bacillus larvae. Dan J Plant Soil Sci. (1986) 90:81–6.
19. Hornitzky MAZ, Clark S. Culture of Bacillus larvae from bulk honey samples for the detection of American foulbrood. J Apic Res. (1991) 30:13–6. doi: 10.1080/00218839.1991.11101228
20. Steinkraus KH, Morse RA. American foulbrood incidence in some US and Canadian honeys. Apidologie. (1992) 23:497–501. doi: 10.1051/apido:19920601
21. Fries I, Lindström A, Korpela S. Vertical transmission of American foulbrood (Paenibacillus larvae) in honey bees (Apis mellifera). Vet Microbiol. (2006) 114:269–74. doi: 10.1016/j.vetmic.2005.11.068
22. Lindström A, Fries I. Sampling of adult bees for detection of American foulbrood (Paenibacillus larvae subsp larvae) spores in honey bee (Apis mellifera) colonies. J Apic Res. (2005) 44:82–6. doi: 10.1080/00218839.2005.11101154
23. Von der Ohe W, Dustmann JH. Efficient prophylactic measures against 8 American foulbrood by bacteriological analysis of honey for spore contamination. Am Bee J. (1997) 137:603–6.
24. de Graaf DC, Alippi AM, Antúnez K, Aronstein KA, Budge G, De Koker D, et al. Standard methods for American foulbrood research. In: Dietemann V, Ellis JD, Neumann P, Editors. The Coloss Beebook, Volume II: Standard Methods For Apis Mellifera Pest And Pathogen Research. (2013). p. 52. doi: 10.3896/IBRA.1.52.4.16
25. Forsgren E, Laugen AT. Prognostic value of using bee and hive debris samples for the detection of American foulbrood disease in honey bee colonies. Apidologie. (2014) 45:10–20. doi: 10.1007/s13592-013-0225-6
26. Macedo PA, Wu J, Ellis Marion D. Using inert dusts to detect and assess varroa infestations in honey bee colonies. J Apic Res. (2002) 40:3–7. doi: 10.1080/00218839.2002.11101062
27. Gregorc A, Knight PR, Adamczyk J. Powdered sugar shake to monitor and oxalic acid treatments to control varroa mites (Varroa destructor Anderson and Trueman) in honey bee (Apis mellifera) colonies. J Apic Res. (2017) 56:71–5. doi: 10.1080/00218839.2017.1278912
28. Aliano NP, Ellis MD. A strategy for using powdered sugar to reduce varroa populations in honey bee colonies. J Apic Res. (2005) 44:54–7. doi: 10.1080/00218839.2005.11101148
29. Stanimirovic Z, Aleksi N, Stevanovi J, Cirkovic D, Mirilovic Djelic N, et al. The influence of pulverised sugar dusting on the degree of infestation of honey bee colonies with Varroa destructor. Acta Veterinaria. (2011) 61:309–25. doi: 10.2298/AVB1103309S
30. Pettis JS, Kochansky J, Feldlaufer MF. Larval Apis mellifera L. (Hymenoptera: Apidae) mortality after topical application of antibiotics and dusts. J Econ Entomol. (2004) 97:171–6. doi: 10.1603/0022-0493-97.2.171
31. Dobbelaere W, de Graaf DC, Peeters JE, Jacobs FJ. Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie. (2001) 32:363–70. doi: 10.1051/apido:2001136
32. Genersch E, Otten C. The use of repetitive element PCR fingerprinting (rep-PCR) for genetic subtyping of German field isolates of Paenibacillus larvae subsp. larvae Apidologie. (2003) 34:195–206. doi: 10.1051/apido:2003025
33. Core Team R. R: A language environment for statistical computing. Vienna: R Foundation for Statistical Computing (2018). Available online at: URL https://www.R-project.org/.
34. Fries I, Camazine S. Implications of horizontal and vertical pathogen transmission for honey bee epidemiology. Apidologie. (2001) 32:199–214. doi: 10.1051/apido:2001122
35. Locke B, Low M, Forsgren E. An integrated management strategy to prevent outbreaks and eliminate infection pressure of American foulbrood disease in a commercial beekeeping operation. PrevVet Med. (2019) 167:48–52. doi: 10.1016/j.prevetmed.2019.03.023
36. Lyall D, Hansbro P, Horvat J, Stanwell P. Quantitative nondestructive assessment of paenibacillus larvae in apis mellifera hives. In: Ahram T, Karwowski W, Pickl S, Taiar R, editors. Human Systems Engineering and Design II: Proceedings of the 2nd International Conference on Human Systems Engineering and Design (IHSED2019): Future Trends and Applications. Munich: Springer International Publishing (2019). p. 579–83. doi: 10.1007/978-3-030-27928-8_88
Keywords: Paenibacillus larvae, American Foulbrood, powdered sugar, assessment of infection levels, American Foulbrood control
Citation: Bassi S, Galletti G, Carpana E, Palminteri S, Bosi F, Loglio G and Carra E (2022) Powdered Sugar Examination as a Tool for the Assessment of Paenibacillus larvae Infection Levels in Honey Bee Colonies. Front. Vet. Sci. 9:853707. doi: 10.3389/fvets.2022.853707
Received: 12 January 2022; Accepted: 23 March 2022;
Published: 14 April 2022.
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Julius Mugweru, University of Embu, KenyaOlimpia Lai, University of Bari Aldo Moro, Italy
Copyright © 2022 Bassi, Galletti, Carpana, Palminteri, Bosi, Loglio and Carra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Carra, ZWxlbmEuY2FycmFAaXpzbGVyLml0
 Stefano Bassi1
Stefano Bassi1 Giulio Loglio
Giulio Loglio Elena Carra
Elena Carra