- 1College of Veterinary Medicine, Shanxi Agricultural University, Jinzhong, China
- 2College of Veterinary Medicine, Jilin Agricultural University, Changchun, China
- 3College of Life Science, Changchun Sci-Tech University, Changchun, China
- 4Marine College, Shandong University, Jinan, China
- 5Key Laboratory of Veterinary Public Health of Higher Education of Yunnan Province, College of Veterinary Medicine, Yunnan Agricultural University, Kunming, China
Anisakidosis, caused by anisakid larvae, is an important fish-borne zoonosis. This study aimed to summarize the prevalence of anisakid infection in fish in China. A systematic review and meta-analysis were performed using five bibliographic databases (PubMed, CNKI, ScienceDirect, WanFang, and VIP Chinese Journal Databases). A total of 40 articles related to anisakid infection in fish in China were finally included. Anisakid nematodes were prevalent in a wide range of fish species, and the overall pooled prevalence of anisakid nematodes in fish in China was 45.5%. Fresh fish had the highest prevalence rate (58.1%). The highest prevalence rate was observed in Eastern China (55.3%), and fish from East China Sea showed the highest prevalence of anisakid nematodes (76.8%). Subgroup analysis by sampling year suggested that the infection rate was higher during the years 2001–2011 (51.0%) than the other periods. Analysis of study quality revealed that the middle-quality studies reported the highest prevalence (59.9%). Compared with other seasons, winter had the highest prevalence (81.8%). The detection rate of anisakid nematodes in muscle was lower (7.8%, 95% CI: 0.0–37.6) than in other fish organs. Our findings suggested that anisakid infection was still common among fish in China. We recommend avoiding eating raw or undercooked fish. Region, site of infection, fish status and quality level were the main risk factors, and a continuous monitoring of anisakid infection in fish in China is needed.
Introduction
Anisakidosis is a parasitic zoonosis caused by any member of the family Anisakidae, including the genera Anisakis, Contracaecum, and Pseudoterranova (1–3). The first case of anisakiasis was reported in the Netherlands around 1960, and the total number of anisakiasis cases up to December 2017 was estimated to be about 76,000 throughout the world (4, 5). The pathogenic effects of infection by anisakid nematodes are due mainly to two mechanisms, direct tissue damage and allergic reactions (6). The clinical syndromes can be categorized into gastric anisakiasis, intestinal anisakiasis, ectopic anisakiasis, and allergic anisakiasis (7, 8). Gastric anisakiasis represents about 95% of cases in Japan, and the typical symptom is acute and severe epigastric pain (6, 9). The symptoms of intestinal anisakiasis include intermittent or constant abdominal pain and/or intestinal obstruction, and treatment often requires surgery to remove the worm (7, 10). Moreover, infection with anisakids can lead to life-threatening anaphylaxis (6).
Anisakid nematodes have an indirect life cycle, and crustaceans are intermediate hosts while fish (and mollusks) are paratenic hosts (7, 11, 12). The larvae of anisakid nematodes, especially when located in the musculature, can affect the commercial value of fish (13). Furthermore, anisakid nematodes can lead to disease in fish (13). Humans act only as an accidental host in the life cycle of anisakid nematodes, and the infection can be obtained through consumption of raw or incompletely cooked fish infected with the third-stage larvae of the nematode (14, 15). Hence, infection of fish with anisakid nematodes should be given high priority not only because of anisakiasis in humans, but also because of the economic losses to the fishing industry (13, 16).
Fish are one of the most important food sources in China, and a number of individual studies have reported the prevalence of anisakid nematodes in fish in China. Meanwhile, the first human case of anisakiasis in China has been reported (17). Herein, a systematic review and meta-analysis was performed to analyze the prevalence of anisakid nematodes in fish in China, and the potential related factors were also investigated.
Materials and Methods
Search Strategy
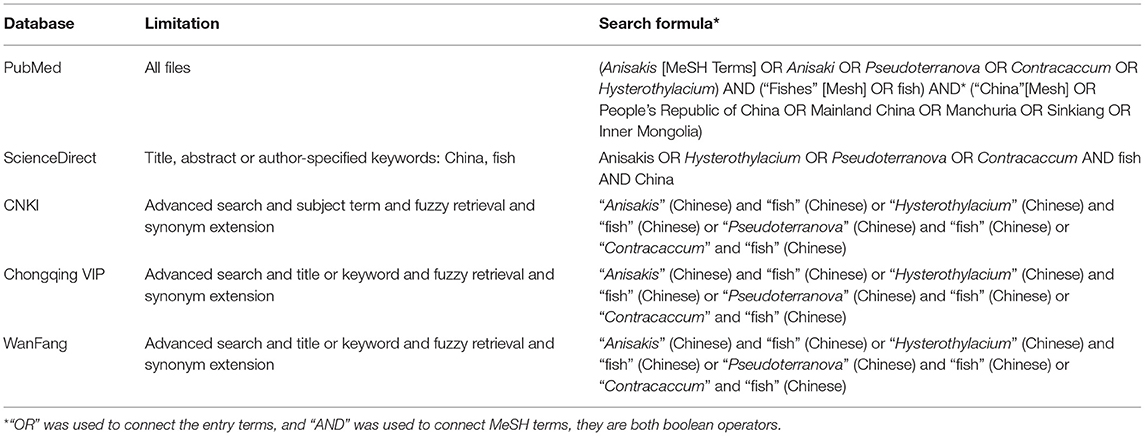
This study was performed following the PRISMA guideline (Supplementary Table 1) (18). Five bibliographic databases (VIP Chinese Journal Databases, WanFang, ScienceDirect, CNKI, and PubMed) were used to identify published articles regarding anisakid infection in fishes in China in both Chinese and English up to August 2020. The detailed search strategy and restriction information are recorded in Table 1. Meanwhile, the reference lists of retrieved articles and recent reviews were reviewed. Additionally, we did not contact the original investigators for additional data, and unpublished reports were not retrieved. Endnote X9.3.1 software was utilized to collate information for all studies.
Study Selection
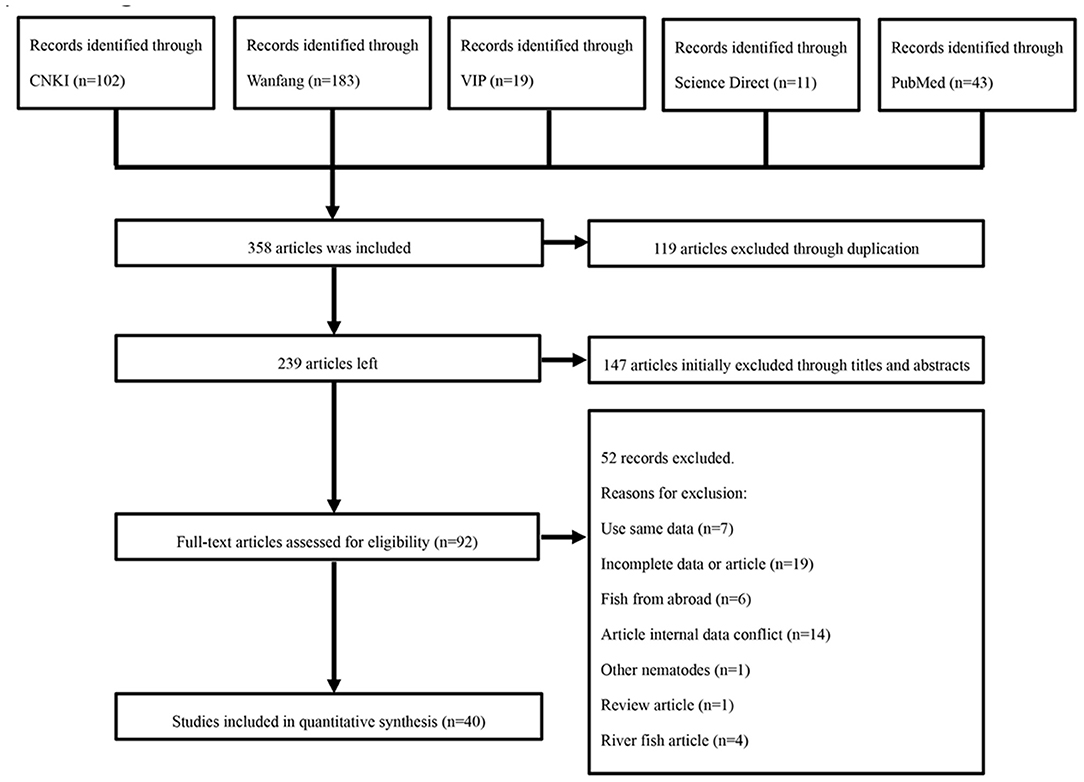
After removing duplicates, the relevant articles were selected through an initial screen of identified abstracts and/or titles and a second screen of full-text articles. Qualified studies needed to meet all of the following criteria: (i) targeted objects must be fish (ii) selected fishing sites within China; (iii) cross-sectional study; (iv) the content of the studies must include the prevalence of anisakid nematodes; and (v) natural infection. Studies with the following characteristics were excluded: using the same data; incomplete data or article; fish from abroad; having internal data conflict; other nematodes; review article; river fish article (Figure 1). Eligibility for inclusion for all studies was evaluated by two independent reviewers (QL and QW). Any disagreements were resolved by the primary reviewer's (QLG) opinion as necessary.
Data Extraction and Quality Assessment
Two reviewers (QW and JYM) independently extracted the following variables from each included study: Year of sampling, first author, publication year, study region, province, detection method, site of infection, collection season, sea, the total number of fishes, the number of positive samples, fish status, and fish category. The statistical geographic factor data (longitude range, latitude range, annual average rainfall, altitude, annual average temperature, and annual average humidity) were acquired from the National Meteorological Information Center of China Meteorological Administration. The primary reviewer (QLG) confirmed all the extracted data. A “quality” assessment of each included study was made by using criteria derived from the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach (19–21). The scoring method was used for grading, and each of the below mentioned criteria was determined as 1 point: (i) randomly sampled; (ii) clear detection method; (iii) provide a detailed description of sampling method; (iv) clear sampling time; and (v) contained four or more risk factors. Studies with total score of four or five points were considered to be of high quality, studies with total scores of 2–3 points were considered to be of moderate quality, whereas studies with lower scores were marked as low quality.
Statistical Analysis
We performed meta-analysis using the package “meta” (version 4.11-0) in R software (version 3.5.2) (22). Prior to meta-analysis, we tried different methods to fit the data to a Gaussian distribution: double-arcsine transformation (PFT), loga-rithmic conversion (PLN), logit transformation (PLOGIT) and arcsinetransformation (PAS). As indicated by previous studies, PFT has better variance stabilization performance (Table 2) (23–25). The formulas for PFT were as follows:
t, transformed prevalence; n, sample size; r, positive number; se, standard error.
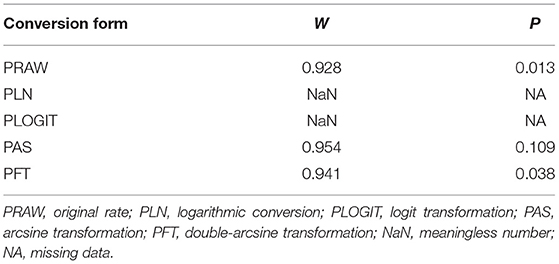
Table 2. Normal distribution test for the normal rate and the different conversion of the normal rate.
Hence, PFT was used for rate conversion in this study. Heterogeneity across all eligible studies was tested by using the Cochran Q-test and I-squared statistic. A P < 0.05 was considered to indicate statistically significant heterogeneity, and I2-values of ≥25, ≥50, and ≥75% correspond to low, moderate, and high heterogeneity, respectively (26). Heterogeneity was present, and hence the random effect pooled measure was selected. Forest plots were generated for overall assessment of the results of each included study and the heterogeneity between studies. A funnel plot, trim and fill method and an Egger's test were used to evaluate the publication bias of studies. In addition, the stability of our study was determined by using a sensitivity analysis (27).
Meanwhile, we performed subgroup analysis stratified by the potential risk factors to explore the potential sources of heterogeneity in our meta-analysis (28). The factors included the region (eastern China vs. other regions), the year of collection (2001–2011 vs. other periods), site of infection (others vs. muscle), season (winter vs. spring, summer, and autumn), seas (Bohai Sea vs. East China Sea, South China Sea, and Yellow Sea), fish status (Fresh fish vs. frozen fish, and live fish), and quality level (middle vs. high). In the meta-analysis of prevalence, regional factor is usually the source of heterogeneity. Hence, meta-regression analysis with other risk factors using the provinces as a covariate was conducted to explain the heterogeneity caused by the provinces. The explained heterogeneity of the covariates is expressed in R2.
Also, potential sources of heterogeneity were explored by subgroup analysis based on geographical factors. We evaluated latitude (30–35° vs. other latitudes), longitude (>120° vs. other longitudes), altitude (>500 m vs. other altitudes), precipitation (1,000–1,500 mm vs. other precipitation categories), humidity (<70% vs. other humidity categories), mean temperature (15–20°C vs. mean temperature of other groups), lowest average temperature (10–15°C vs. lowest average temperature in other groups) and highest average temperature (>25°C vs. highest average temperature in other groups). The R software code for meta-analysis is shown in Supplementary Table 2.
Results
Included Studies
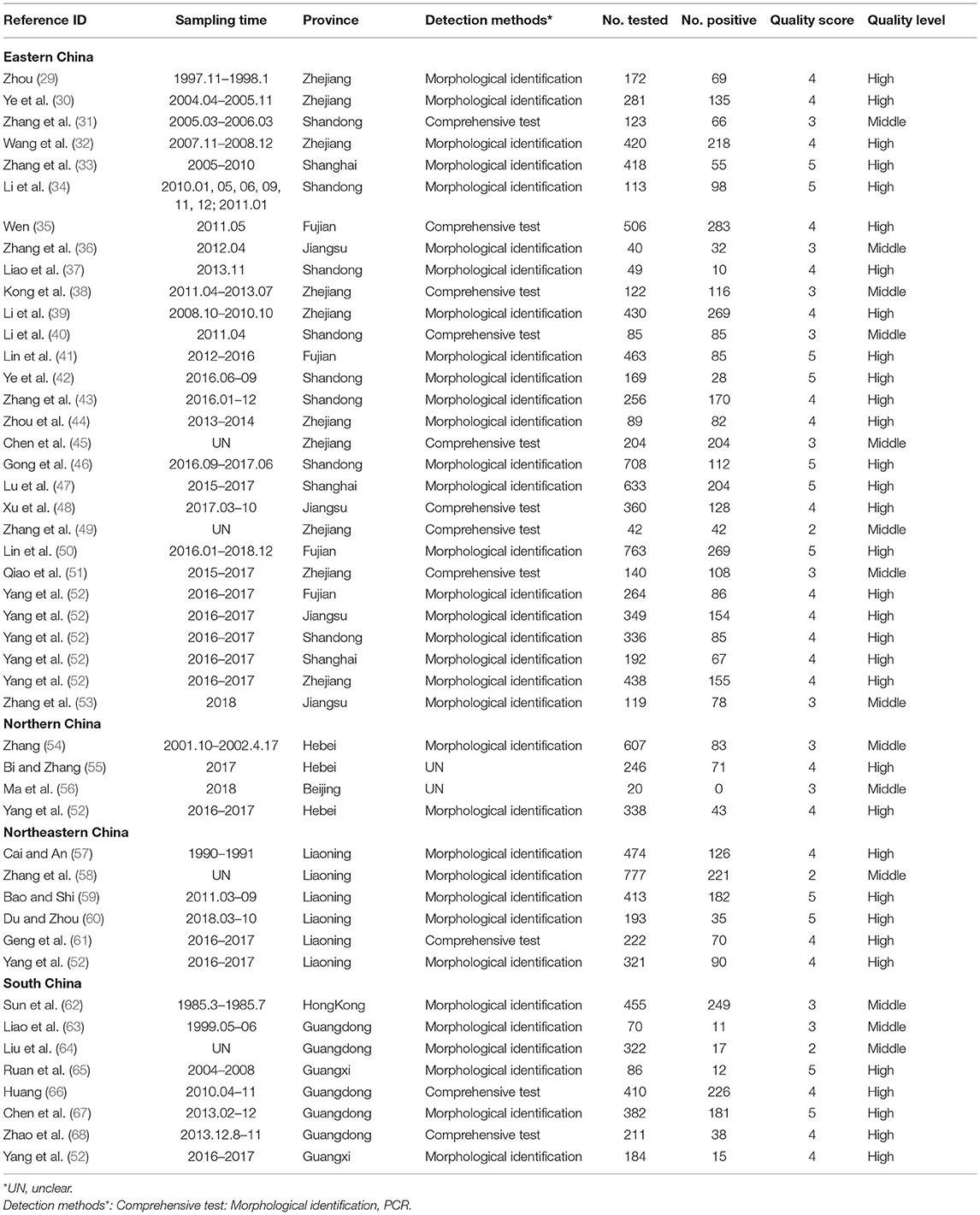
In this study, a total of 358 relevant articles were found. Following initial screening and removal of duplicates, 92 articles were identified. Following full text review, 52 articles were further excluded. A further search was carried out based on the reference lists of relevant studies. However, no additional qualified articles were found. Finally, 40 full-text studies published between 2000 and 2020 were included in the quantitative analysis (Figure 1). Of which, eight articles were published in English. According to our quality criteria, 26 publications were of high quality (four or five points), 14 publications showed moderate quality (two or three points), and no publications were of low quality (Table 3, Supplementary Table 3).
Pooling and Heterogeneity Analysis
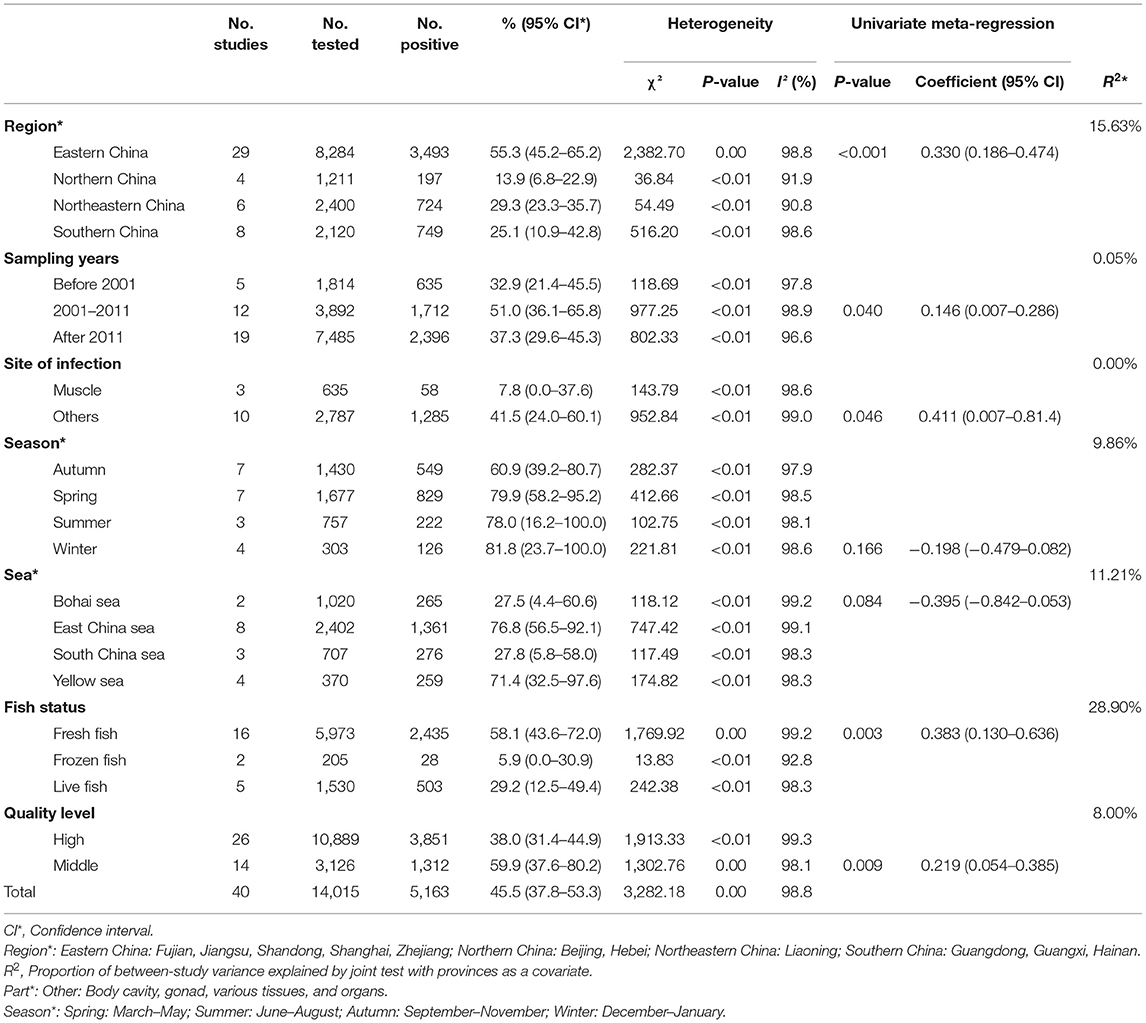
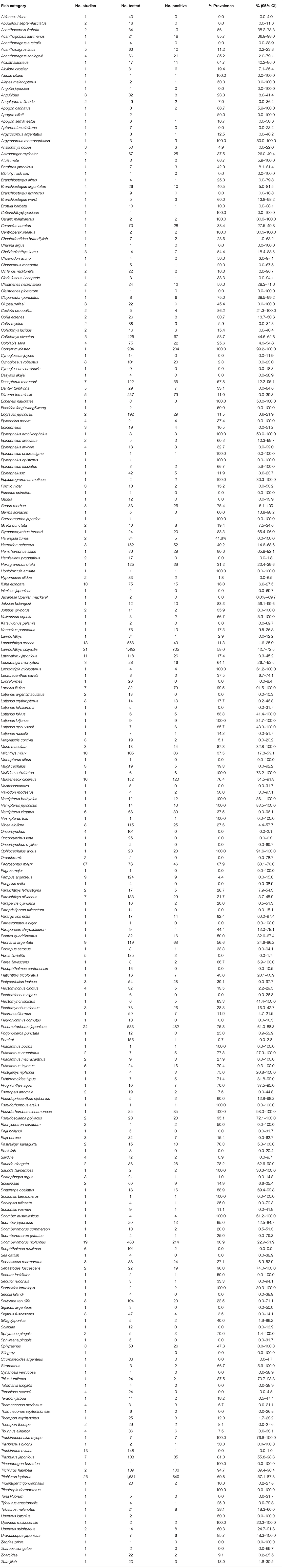
A total of 40 studies involving 14,015 fish were included in this meta-analysis. However, high heterogeneity (I2 = 98.8%, P < 0.001) in the selected studies was observed (Table 4, Figure 2). Hence, a random effects model was adopted for the analysis. The overall pooled prevalence of anisakid nematodes in fish in China was 45.5% (95% CI: 37.8–53.3) (Table 4). The included studies covered a variety of fish species, and the prevalence of anisakid nematodes ranged from 0 to 100% (Table 5).
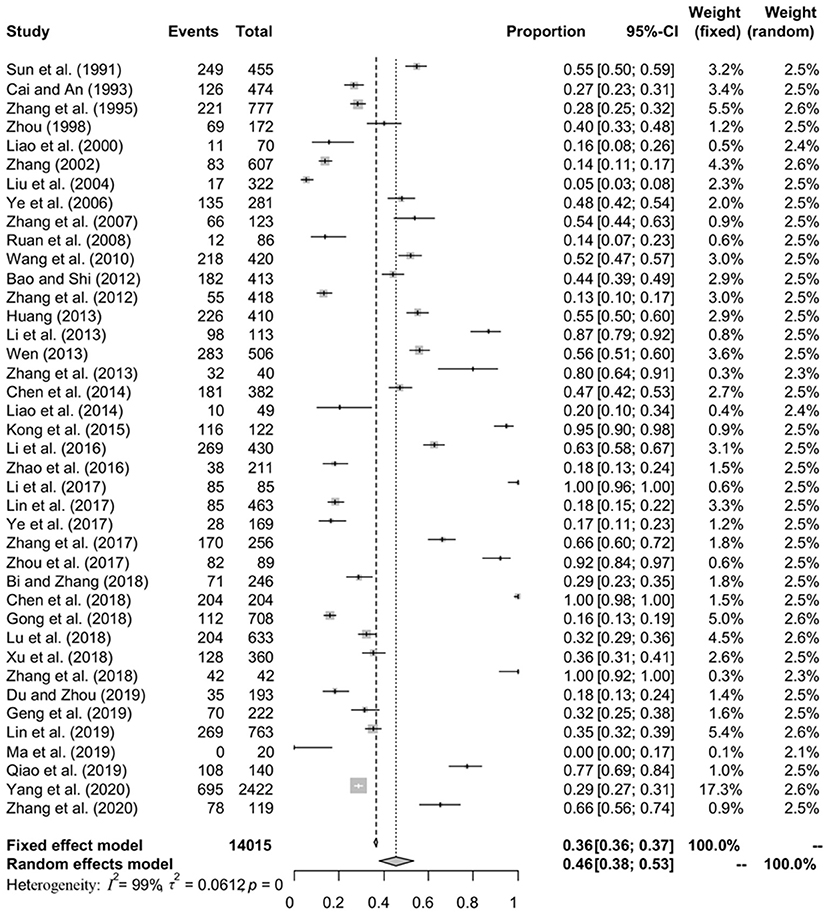
Figure 2. Forest plot of prevalence of anisakids in fish amongst studies conducted in China. The length of the horizontal line represents the 95% confidence interval, and the diamond represents the summarized effect.
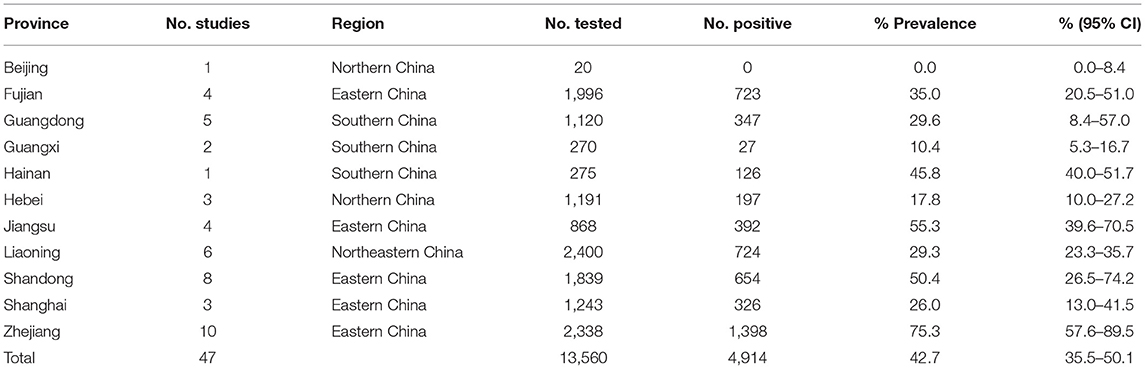
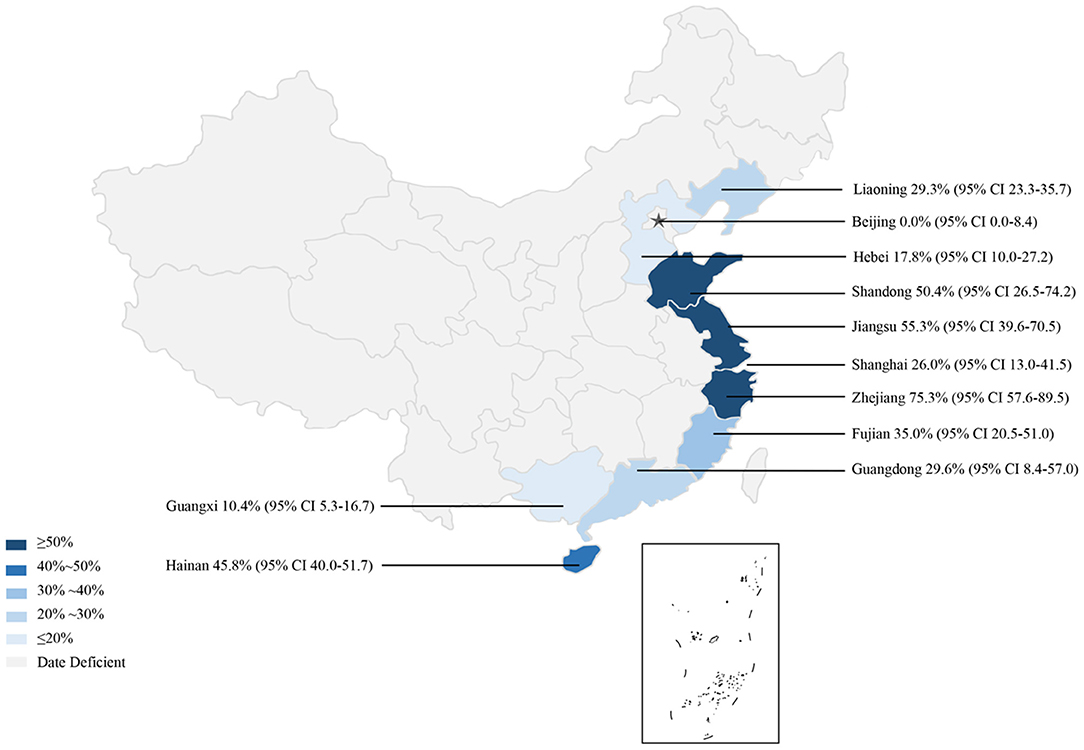
In the subgroup analysis, a random effect model was selected due to the fact that significant heterogeneity was observed (Table 4). The subgroup analysis based on geographical areas suggested that eastern China had the highest prevalence rate (55.3%, 95% CI: 45.2–65.2), and fish in East China Sea showed the highest point estimate of prevalence of anisakid nematodes (76.8%, 95% CI: 56.5–92.1). At the single province level, Zhejiang Province had the highest rate of 75.3% (1,398/2,338; 95% CI: 57.6–89.5) (Table 6). No anisakid nematodes were found in fish in Beijing City (Table 6, Figure 3).
The subgroup analysis by sampling years demonstrated that the infection rate was higher during 2000–2011 (51.0%, 95% CI: 36.1–65.8) than other periods. Compared with other seasons, autumn had the lowest prevalence rate (60.9%, 95% CI: 39.2–80.7) (Table 4).
Analysis of study quality indicated that the middle-quality studies reported the highest prevalence rate (59.9%, 95% CI: 37.6–80.2). The detection rate of anisakid nematodes in muscle was lower (7.8%, 95% CI: 0.0–37.6) than in other fish organs. The meta-regression analysis showed that the heterogeneity can be explained by the province ranges from 0.00 to 31.93% after joint analysis with province (Table 4).
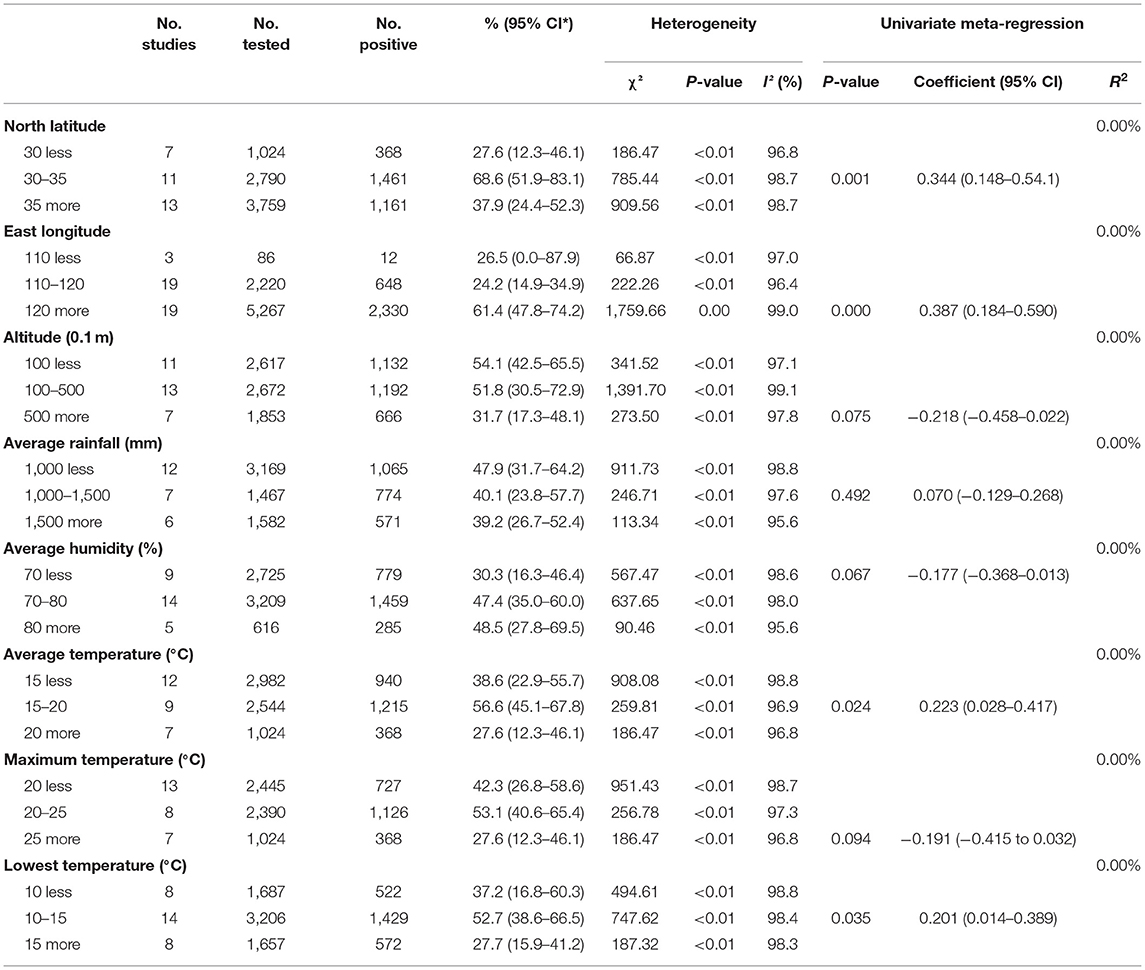
We also evaluated the impact of geographical and climatic parameters on prevalence and calculated the latitude range (30–35°; 68.6%, 95% CI: 51.9–83.1), the longitude range (>120°; 61.4%, 95% CI: 47.8–74.2), and altitude (<100; 54.1%, 95% CI: 42.5–65.5). Compared with other groups, the prevalence of anisakid nematodes in fish in these geographic ranges was significantly higher (P < 0.05), which may account for the heterogeneity (Table 7).
Publication Bias and Sensitivity Analysis
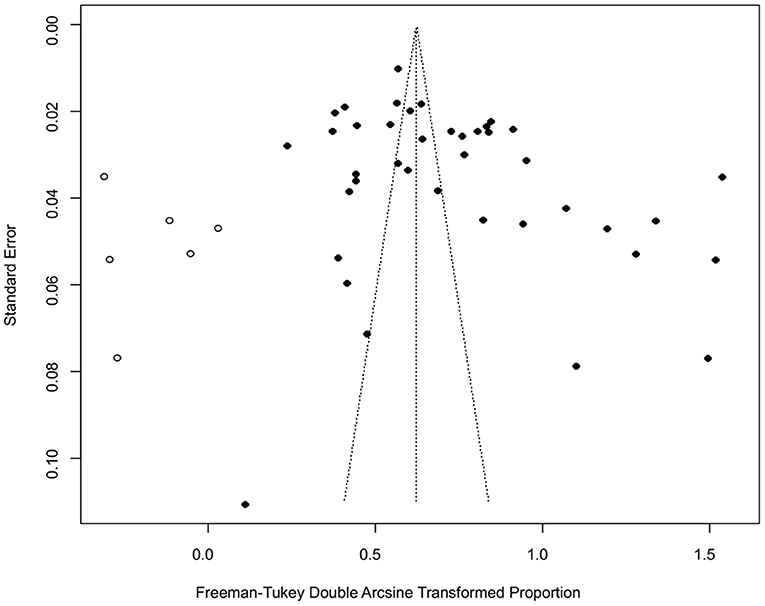
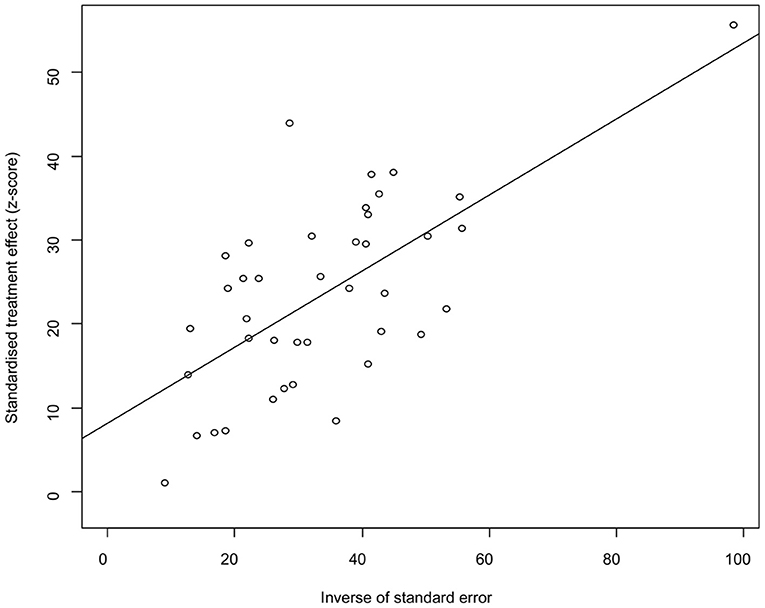
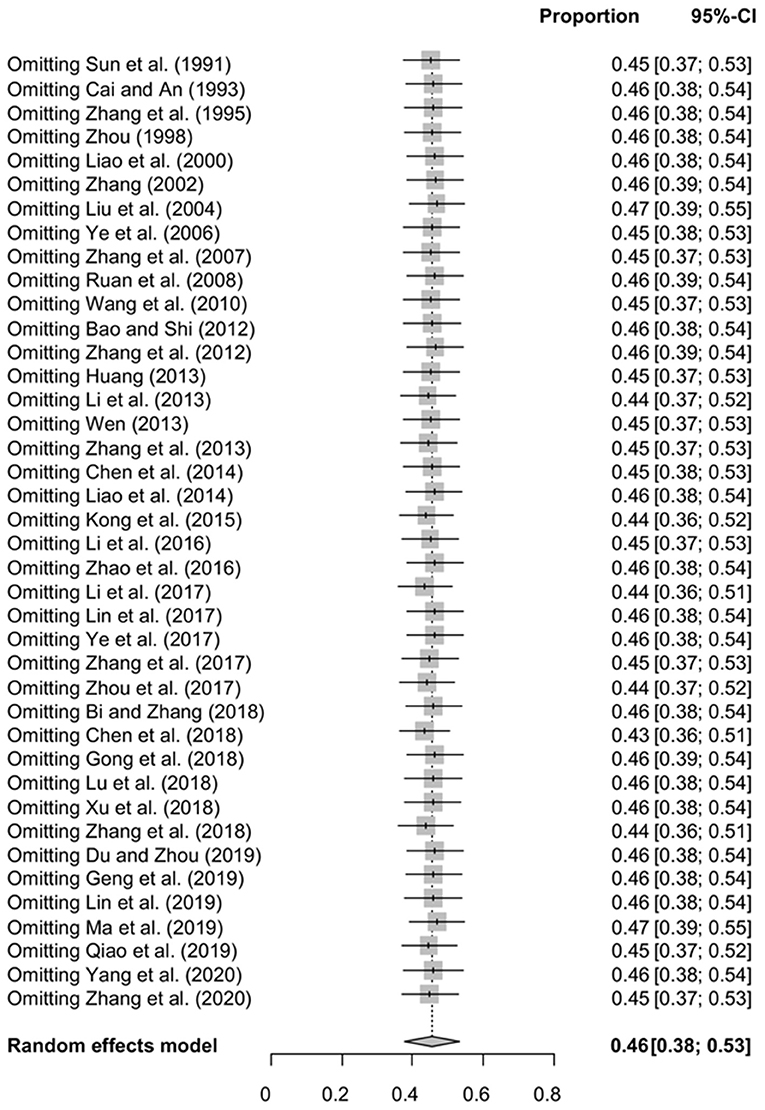
The funnel plot was asymmetric, suggesting that the included studies might have publication bias or small-study effect bias (Figure 4). Meanwhile, the trim and fill analysis showed six studies with negative results (white circles in Figure 5), indicating that there was potential publication bias in the present study. Additionally, Egger's test suggested that there might be publication bias among the studies selected for our analysis (P < 0.05) (Supplementary Table 4, Figure 6). We also used funnel plots (Supplementary Figures 1–9) and forest plots (Supplementary Figures 10–16) for all subgroups to test for the presence of publication bias and heterogeneity. However, the sensitivity analysis showed that the pooled data were basically the same after omitting one study at a time, indicating that our results were statistically robust (Figure 7).
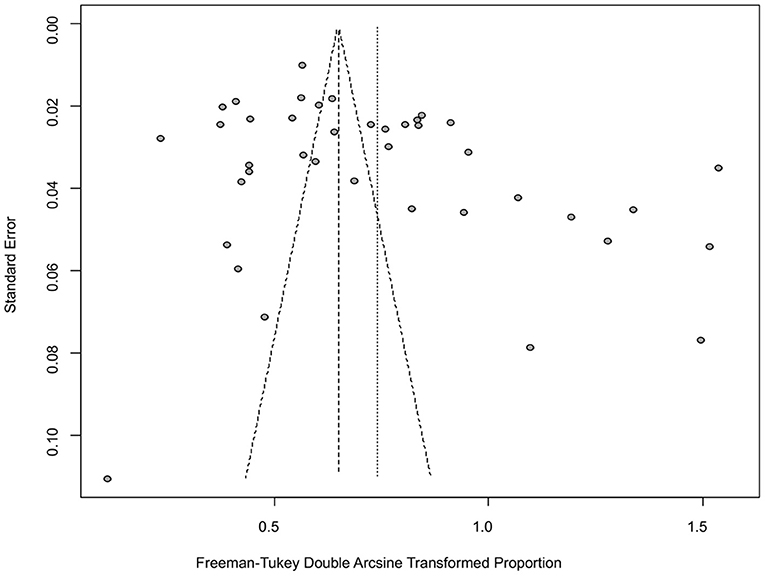
Figure 4. Funnel plot with pseudo 95% confidence interval limits for the examination of publication bias.
Discussion
Human anisakiasis is caused by consumption of raw or poorly cooked fish parasitized by anisakid nematodes (69, 70). Hence, detailed knowledge of the epidemiological status of anisakid nematodes in fish is central for the prevention and control of human anisakiasis. Our meta-analysis revealed that the pooled estimate of Anisakidae larvae prevalence among fish in China was 45.5%, and the prevalence varied by sea areas. East China Sea and Yellow Sea had high prevalence. Fish species may contribute to such high prevalence, such as hairtail (Trichiurus haumela), chub mackerel (Pneumatophorus japonicus), yellow croaker (Pseudosciaena polyactis) and whitespotted conger (Conger myriaster) in East China Sea, and chub mackerel (P. japonicus) in Yellow Sea. Several previous studies showed that they were highly infected species (52, 71–73). Additionally, the relationship between the lowest prevalence in Bohai Sea and fish species needs to be further studied, because only two studies were included for analysis, and one did not disclose the 23 fish species which were tested negative for anisakid nematodes (74). A previous investigation using fish collected from three sea areas of the Republic of Korea also showed that the infection rate was higher in East Sea than that in Yellow Sea (71). However, fish from South Sea, Republic of Korea had higher prevalence rate than that from South China Sea (71). This may be due to the fact that fat greenling (Hexagrammos otakii) and Korean rockfish (Sebastes schlegeli) from South Sea with high infection rate were not included in fish species sampled from South China Sea (71). In addition to fish species, differences in prevalence may be associated with fishing grounds (15). For example, previous studies demonstrated that the distribution of Anisakis spp. and the infection levels in the same fish species varied among different fishing grounds (15, 75).
Among five provinces within eastern China, Zhejiang province had the highest prevalence. This may be due to the fish species, such as hairtail (Trichiurus lepturus) and yellow croaker (Larimichthys polyactis) which were reported to be highly infected species of marine fish (52). Previous studies showed that the high incidence of anisakidosis was significantly associated with living on the coast, where the habit of consuming raw fish is higher compared to inland regions (76, 77). Considering that consumption of raw or undercooked fish is a common practice in the coastal areas of China, there should be some potential cases of anisakiasis in eastern China, especially in Zhejiang province (17, 40). However, no cases of human infection by anisakid nematodes have been reported in eastern China. To date, only one case of anisakiasis has been reported in other areas of China (17). This may be due to misdiagnosis and missed diagnosis (78). Infection by anisakid nematodes should be considered in patients who had a history of ingestion of raw fish with associated symptoms, such as vomiting and frequent mucous diarrhea (17).
The method of examining fish for anisakid infection include routine visual inspection, digesting the fish filet using a pepsin/HCl solution, and incubation of internal organs (79). In all of the included studies, prevalence of anisakid nematodes in fish in China was determined by routine visual inspection. Additional species identification using PCR method was performed only in several studies. Hence, detection method as the risk factor was not included.
China released the National Agricultural and Rural Economic Development in the Tenth Five-Year Plan implemented from June 2001 (2001–2005). Of which, speeding up the development of the aquaculture industry was included. Meanwhile, establishing and perfecting a system for monitoring the safety and quality of aquatic products was mentioned. Hence, 2001 was used to be a first cut-off point for subgroup analysis. The 12th Five-Year Plan on Fishery Development and the 13th Five-Year Plan on Fishery Development were released in June 2011 and December 2016, respectively, each gives a higher priority for epidemic prevention and control of aquatic animals as well as safety and quality of aquatic products than before. Thus, we chose 2011 as the cut-off point to analyze the prevalence of anisakid nematodes. It is worth noting that we found 19 studies published after 2011, but only 5 studies before 2001. Hence, we speculated that the pooled estimates after 2011 was more likely to reflect prevalence of anisakid nematodes in fish in China.
Additionally, the rareness of anisakiasis in China may be associated with anisakid nematode species. Previous studies showed that the majority of human cases of anisakiasis were caused by Anisakis simplex, Anisakis pegreffii, and Pseudoterranova decipiens (10, 80, 81). However, A. simplex and A. pegreffii were reported only in 12 and 11 articles, respectively. The PCR approach proved to be cost-effective and reliable for the identification of the species of the genus Anisakis (82). However, PCR approach was not used in all studies related to species identification, which may lead to species misidentification. Moreover, only one article reported the presence of P. decipiens in fish in China.
Parasites were detected in muscle, intestine, mesentery and gonads. Although the point estimate of anisakid nematodes in muscle was low, larval migration to the muscles may occur after the death of the fish, which can increase the risk of anisakiasis (83, 84). Moreover, the differences between the two sibling species (A. simplex and A. pegreffii) in migration to the muscles of fish and to penetrate into the tissue of accidental hosts were found in several studies (38, 85, 86). From the perspective of food safety, further studies are needed to reveal the species composition of Anisakis and their geographical distribution in China.
The included studies covered a variety of fish species, and the prevalence of anisakid nematodes ranged from 0 to 100%. The results can serve as a guideline associated with food safety. Yellow goosefish (Lophius litulon) is a commercially important marine fish, and its stomach, intestine and liver are considered to be a delicacy in China (49). Also, cinnamon flounder (Pseudorhombus cinnamoneus) is a frequently consumed marine fish in China (40). Our analysis showed that L. litulon and P. cinnamoneus had a high prevalence, respectively. The high prevalence may be due to the fact that they eat crustaceans and small fishes, which are intermediate or paratenic hosts of anisakid nematodes (7, 11, 12). Additionally, several fish species, such as banded sergeant (Abudefduf septemfasciatus), sablefish (Anoplopoma fimbria), and skipjack tuna (Katsuwonus pelamis) tested negative for anisakid nematodes. This may be due to the small sample size for each of these fish species, because infection of K. pelamis by Anisakis larvae has been reported (12). Hence, further studies employing a larger number of sampled fish are needed to determine the prevalence in several fish species.
The advantages of the present study include the wide coverage, large total sample size, valid analysis method, large time span, and a comprehensive risk factor analysis. This is the first meta-analysis of the prevalence of anisakid nematodes in China. In the present study, most of the articles of medium quality reached the score of three. In addition, four or more potential risk factors were explored in the majority of articles. We believe that the study can reflect the prevalence of anisakid nematodes in fish in China during the last two decades. However, there are some limitations in this meta-analysis as follows: (i) five databases were used to identify publications, which may exclude some qualified articles from other databases; (ii) parts of the subgroups (such as sites of infection) have included fewer articles, which may lead to unstable results; (iii) this study was not registered in Cochrane, however, our meta-analysis was carried out strictly in accordance with the steps of PRISMA; and (iv) the range of environmental temperatures in the sea area where fish live is quite different from that of the land area, and analysis based on different regions of land areas may only serve as a reference. It is suggested that the researchers should clarify the sampling locations and fishing sites (such as the latitude and longitude of the specific sea area), which can contribute to the assessment of the environmental factor.
Conclusion
This study has shown that anisakid infection in fish was widespread in China, and the pooled prevalence varied among different fish species and provinces. Region, site of infection, fish status and quality level were the main factors affecting the prevalence rate. There is a need for continuous monitoring of anisakid infection in fish in China. Meanwhile, it is necessary to educate people, especially those living in coastal regions, about the risk of infection with anisakid nematodes and to avoid consumption of raw or undercooked fish.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Q-LG and JJ contributed to conception and design of this analysis. QL, QW, and J-YM collected the data and built the database. QW and Q-LG analyzed the results. QL prepared the manuscript. Q-LG and X-QZ revised the manuscript. All authors contributed to manuscript editing and approved the final manuscript.
Funding
Project support was provided by the Fund for Shanxi 1331 Project (Grant No. 20211331-13), the Research Fund for Introduced High-level Leading Talents of Shanxi Province, the Special Research Fund of Shanxi Agricultural University for High-level Talents (Grant No. 2021XG001), and Yunnan Expert Workstation (Grant No. 202005AF150041).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.792346/full#supplementary-material
References
1. Mohandas N, Jabbar A, Podolska M, Zhu XQ, Littlewood DT, Jex AR, et al. Mitochondrial genomes of Anisakis simplex and Contracaecum osculatum (sensu stricto)–comparisons with selected nematodes. Infect Genet Evol. (2014) 21:452–62. doi: 10.1016/j.meegid.2013.10.026
2. Carrascosa MF, Mones JC, Salcines-Caviedes JR, Román JG. A man with unsuspected marine eosinophilic gastritis. Lancet Infect Dis. (2015) 15:248. doi: 10.1016/S1473-3099(14)70892-8
3. Liu SS, Liu GH, Zhu XQ, Weng YB. The complete mitochondrial genome of Pseudoterranova Azarasi and comparative analysis with other anisakid nematodes. Infect Genet Evol. (2015) 33:293–8. doi: 10.1016/j.meegid.2015.05.018
4. Zanelli M, Ragazzi M, Fiorino S, Foroni M, Cecinato P, Del Mar Jordana Sanchez M, et al. An Italian case of intestinal anisakiasis with a presurgical diagnosis: could this parasite represent an emerging disease? Pathol Res Pract. (2017) 213:558–64. doi: 10.1016/j.prp.2017.01.027
5. Kołodziejczyk L, Szostakowska B, Sobecka E, Szczucki K, Stankiewicz K. First case of human anisakiasis in Poland. Parasitol Int. (2020) 76:102073. doi: 10.1016/j.parint.2020.102073
6. Caramello P, Vitali A, Canta F, Caldana A, Santi F, Caputo A, et al. Intestinal localization of anisakiasis manifested as acute abdomen. Clin Microbiol Infect. (2003) 9:734–7. doi: 10.1046/j.1469-0691.2003.00660.x
7. Nieuwenhuizen NE. Anisakis - immunology of a foodborne parasitosis. Parasite Immunol. (2016) 38:548–57. doi: 10.1111/pim.12349
8. Mitsuboshi A, Yamaguchi H, Ito Y, Mizuno T, Tokoro M, Kasai M. Extra-gastrointestinal anisakidosis caused by Pseudoterranova azarasi manifesting as strangulated inguinal hernia. Parasitol Int. (2017) 66:810–2. doi: 10.1016/j.parint.2017.09.008
9. Kochanowski M, González-Muñoz M, Gómez-Morales MÁ, Gottstein B, Dabrowska J, Rózycki M, et al. Comparative analysis of excretory-secretory antigens of Anisakis simplex, Pseudoterranova decipiens and Contracaecum osculatum regarding their applicability for specific serodiagnosis of human anisakidosis based on IgG-ELISA. Exp Parasitol. (2019) 197:9–15. doi: 10.1016/j.exppara.2018.12.004
10. Hochberg NS, Hamer DH. Anisakidosis: perils of the deep. Clin Infect Dis. (2010) 51:806–12. doi: 10.1086/656238
11. Mattiucci S, Nascetti G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv Parasitol. (2008) 66:47–148. doi: 10.1016/S0065-308X(08)00202-9
12. Anshary H, Sriwulan, Freeman MA, Ogawa K. Occurrence and molecular identification of Anisakis Dujardin, 1845 from marine fish in southern Makassar Strait, Indonesia. Korean J Parasitol. (2014) 52:9–19. doi: 10.3347/kjp.2014.52.1.9
13. Shamsi S. Recent advances in our knowledge of Australian anisakid nematodes. Int J Parasitol Parasites Wildl. (2014) 3:178–87. doi: 10.1016/j.ijppaw.2014.04.001
14. Guardone L, Armani A, Nucera D, Costanzo F, Mattiucci S, Bruschi F. Human anisakiasis in Italy: a retrospective epidemiological study over two decades. Parasite. (2018) 25:41. doi: 10.1051/parasite/2018034
15. Mattiucci S, Cipriani P, Levsen A, Paoletti M, Nascetti G. Molecular epidemiology of Anisakis and anisakiasis: an ecological and evolutionary road map. Adv Parasitol. (2018) 99:93–263. doi: 10.1016/bs.apar.2017.12.001
16. Bao M, Pierce GJ, Strachan NJ, Pascual S, González-Muñoz M, Levsen A. Human health, legislative and socioeconomic issues caused by the fish-borne zoonotic parasite Anisakis: challenges in risk assessment. Trends Food Sci Tech. (2019) 86:298–310. doi: 10.1016/j.tifs.2019.02.013
17. Qin Y, Zhao Y, Ren Y, Zheng L, Dai X, Li Y, et al. Anisakiasis in China: the first clinical case report. Foodborne Pathog Dis. (2013) 10:472–4. doi: 10.1089/fpd.2012.1325
18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
19. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
20. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
21. Ni HB, Gong QL, Zhao Q, Li XY, Zhang XX. Prevalence of Haemophilus parasuis“Glaesserella parasuis” in pigs in China: a systematic review and meta-analysis. Prev Vet Med. (2020) 182:105083. doi: 10.1016/j.prevetmed.2020.105083
22. Wei XY, Gong QL, Zeng A, Wang W, Wang Q, Zhang XX. Seroprevalence and risk factors of Toxoplasma gondii infection in goats in China from 2010 to 2020: a systematic review and meta-analysis. Prev Vet Med. (2021) 186:105230. doi: 10.1016/j.prevetmed.2020.105230
23. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
24. Li X, Ni HB, Ren WX, Jiang J, Gong QL, Zhang XX. Seroprevalence of Toxoplasma gondii in horses: a global systematic review and meta-analysis. Acta Trop. (2020) 201:105222. doi: 10.1016/j.actatropica.2019.105222
25. Wang W, Gong QL, Zeng A, Li MH, Zhao Q, Ni HB. Prevalence of Cryptosporidium in pigs in China: a systematic review and meta-analysis. Transbound Emerg Dis. (2021) 68:1400–13. doi: 10.1111/tbed.13806
26. Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. (2005) 28:123–37. doi: 10.1002/gepi.20048
27. Gong QL, Wang Q, Yang XY, Li DL, Zhao B, Ge GY, et al. Seroprevalence and risk factors of the Bluetongue virus in cattle in China from 1988 to 2019: a comprehensive literature review and meta-analysis. Front Vet Sci. (2021) 7:550381. doi: 10.3389/fvets.2020.550381
28. Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, et al. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. (2017) 4:e177–88. doi: 10.1016/S2352-3018(17)30005-X
29. Zhou XF. Investigation of anisakis larvae infection in marine fish in Ningbo market. Parasit Infect Dis. (1998) 6:54.
30. Ye LP, Sun F, Xu GZ, Sun YW, Chen ZH, Lu F, et al. Investigation on anisakis infection of East China Sea fish and study on the tolerance of larvae to Wasabi. China Trop Med. (2006) 6:1345–6.
31. Zhang LP, Hu M, Shamsi S, Beveridge I, Li HM, Xu Z, et al. The specific identification of anisakid larvae from fishes from the yellow sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Mol Cell Probes. (2007) 21:386–90. doi: 10.1016/j.mcp.2007.05.004
32. Wang JY, Zhang JH, Lin Q, Zhang QT, He WX, Li KF, et al. Infection and physico—chemical characteristics of Anisakis among marine fish caught in Zhoushan Fisher. Chin J Epidemiol. (2010) 30:1001–4. doi: 10.3760/cma.j.issn.0254-6450.2010.09.010
33. Zhang XP, Jiang SF, Hong GB, Fu YH, He YY, Ma XJ, et al. Investigation on food contamination with parasites in Shanghai market. Chin J Schistosom Control. (2012) 24:404–9. doi: 10.16250/j.32.1374.2012.04.006
34. Li J, Guo JN, Zhou JB, Shi W, Li WW, Fang F, et al. A preliminary investigation on the third stage larvae of heteromynchus japonicus infected with mackerel in the yellow sea. Chin J Food Hyg. (2013) 25:56–61. doi: 10.3969/j.issn.1673-7555.2010.13.125
35. Wen Q. Morphological and Molecular Characterization of Anisakidae Larvae From in Taiwan Strait and the Analysis of Infection Status. Shijiazhuang: Hebei Normal University (2013).
36. Zhang ZJ, Zhang WB, Zhao RM, Shen MX, Jiang WC, Jin F, et al. Status of Clonorchis sinensis and nematoda in common fishes in Nantong city. Chin J Prevent Med. (2013) 47:669. doi: 10.3760/cma.j.issn.0253-9624.2013.07.022
37. Liao F, Zhang BG, Liu X, Chen XX, Fei YK. Investigation on the infection of Isoapex nematode in aquatic products in yellow sea area of Shandong Province. Paras Infect Dis. (2014) 12:189–90.
38. Kong Q, Fan L, Zhang J, Akao N, Dong K, Lou D, et al. Molecular identification of Anisakis and Hysterothylacium larvae in marine fishes from the East China sea and the Pacific coast of central Japan. Int J Food Microbiol. (2015) 199:1–7. doi: 10.1016/j.ijfoodmicro.2015.01.007
39. Li XJ, Shen YY, Bai J, Chen LM, Zhou Y, Shen CL, et al. Investigation of anisakis larvae infection in marine fish entering and leaving the Zhoushan Port. Anim Husb Vet Med. (2016) 48:119–22.
40. Li L, Zhao JY, Chen HX, Ju HD, An M, Xu Z, et al. Survey for the presence of ascaridoid larvae in the cinnamon flounder Pseudorhombus cinnamoneus (Temminck and Schlegel) (Pleuronectiformes: Paralichthyidae). Int J Food Microbiol. (2017) 241:108–16. doi: 10.1016/j.ijfoodmicro.2016.10.018
41. Lin CX, Lin SH, Chen WW, Huang SL, Jiang DW. Parasite pollution in aquatic products marketed in Fujian Province. Chin J Zoo. (2017) 33:564–8. doi: 10.3969/j.issn.1002-2694.2017.06.018
42. Ye B, Sun ZQ, Song XY, Shi XX, Li DD, Xiao N, et al. Investigation of anisakis larvae infection in commercial marine fish in Qingdao City, Shandong. J Med Pest Control. (2017) 33:985–6. doi: 10.7629/yxdwfz201709024
43. Zhang QW, Ren XT, Zhao YQ, Gai XX, Zhang YM, Bai XL. Investigation of Anisakis spp. larva infection in marine fish for sale in Yantai City. Chin J Parasitol Parasit Dis. (2017) 35:472–7.
44. Zhou JY, Lin Q, Zhang H, Zhang JH, Gu ZX. Investigation and molecular identification of anisakis infection in marine fishes in Zhoushan fishing ground. Prevent Med. (2017) 29:694–701. doi: 10.19485/j.cnki.issn1007-0931.2017.07.010
45. Chen HX, Zhang LP, Gibson DI, Lv L, Xu Z, Li HT, et al. Detection of ascaridoid nematode parasitesin the important marine food-fish Congermyriaster (Brevoort) (Anguilliformes:Congridae) from the Zhoushan Fishery, China. Parasit Vect. (2018) 11:274. doi: 10.1186/s13071-018-2850-4
46. Gong CB, Wang ZX, Dong FG, Xing YF, Sun YL. Infection status of third stage larvae of heteronema in 140 fresh marine fish sold in Yantai from 2016 to 2017. Mod Prevent Med. (2018) 45:1766–8.
47. Lu L, Jiang SF, He YY, Zhang XP, Ma XJ, Han YJ, et al. Investigation on parasite infection of animal food sold in Huangpu district, Shanghai from 2015 to 2017. J Trop Dis Parasitol. (2018) 16:23–5. doi: 10.3969/j.issn.1672-2302.2018.01.007
48. Xu Y, Zeng QY, Sun ZH, Zhang LX, Wang N, Liu XL, et al. Preliminary investigation on the infection of anemone elegans in sea fish in Lianyungang. Chin J Vet Sci. (2018) 38:2343–7. doi: 10.16303/j.cnki.1005-4545.2018.12.20
49. Zhang K, Xu Z, Chen HX, Guo N, Li L. Anisakid and raphidascaridid nematodes (Ascaridoidea) infection in the important marine food-fish Lophius litulon (Jordan) (Lophiiformes: Lophiidae). Int J Food Microbiol. (2018) 284:105–11. doi: 10.1016/j.ijfoodmicro.2018.08.002
50. Lin CX, Huang SL, Lin SH, Jiang DW, Xie HG. Investigation on the infection of anisoderma larvae and identification of the species in Fujian coastal fishes. Chin J Parasitol Parasit Dis. (2019) 37:417–21. doi: 10.12140/j.issn.1000-7423.2019.04.008
51. Qiao Y, Zhou QJ, Li XJ, Miao L, Cheng T. The establishment of a method for detecting simple Anisakis/Anisakis spp. with loop-mediated isothermal amplification and lateral flow test strips. Oceanol Limnol Sin. (2019) 50:324–35. doi: 10.11693/hyhz20180800207
52. Yang SR, Pei XY, Li Y, Zhan L, Tang Z, Chen WW, et al. Epidemical study of third stage larvae of anisakis spp. infection in marine fishes in china from 2016 to 2017. Food Control. (2019) 107:106769. doi: 10.1016/j.foodcont.2019.106769
53. Zhang XY, Yu M, Zhao QQ, Wang Y, Sun BC. Investigation of Anisakis infection in marine fishes in Dongtai City. Chin J Schistosom Control. (2020) 32:426–40. doi: 10.16250/j.32.1374.2019267
54. Zhang L. Preliminary investigation on the infection of simple anisakis larvae in Bohai fish. J Cangzhou Norm Univer. (2002) 18:41–7.
55. Bi HJ, Zhang YM. Results of risk monitoring of food microorganisms and their pathogenic factors in Cangzhou City in 2017. Occup Health. (2018) 34:1917–20. doi: 10.13329/j.cnki.zyyjk.2018.0532
56. Ma XM, Wang JJ, Xiao GY, Zhao J, Li J. Analysis of monitoring results of pathogenic microorganisms of animal aquatic products in fengtai district, Beijing. Chin J Health Lab Technol. (2019) 29:2798–801.
57. Cai ZX, An SR. Investigation on the transmission vector of Anisakis disease. Chin J Public Health. (1993) 11:284–5.
58. Zhang BX, Ge LM, Chen FY, Sun YH, Zhang M, Yu YL, et al. A preliminary study on the ecological distribution of anisakis in marine fish. Chin J Microecol. (1995) 7:53–5.
59. Bao M, Shi KS. Investigation on anisakis nematode infections in sea fishes sold in Jinzhou City. Chin J Zoo. (2012) 28:513–6. doi: 10.3969/j.issn.1002-2694.2012.05.027
60. Du XT, Zhou QY. Infection of the third stage larvae of heteronema in some sea fishes in Dandong City. China Trop Med. (2019) 19:40–2. doi: 10.13604/j.cnki.46-1064/r.2019.01.11
61. Geng YZ, Li F, Wang WJ, Zhang MM. Investigation and molecular identification of anisakis infection in marine fish in Liaoning Province. Chin J Food Hyg. (2019) 31:10–3. doi: 10.13590/j.cjfh.2019.01.003
62. Sun SZ, Koyama T, Kagei N. Anisakidae larvae found in marine fishes and squids from the Gulf of Tongking, the east China sea and the yellow sea. Jpn J Med Sci Biol. (1991) 44:99. doi: 10.7883/yoken1952.44.99
63. Liao YM, Li DL, Zhang XL. An investigation on the infection of the larvae of Heteromynchus spp. in the coastal waters of Nan'ao Town, Shenzhen. South China J Prevent Med. (2000) 26:46–7.
64. Liu JS, Wu SQ, Chen HH, Lin RQ, Wei DX, Deng Y, et al. Investigation of anisakis larvae infection in marine fish in Daya Bay. China Anim Livest Vet Med. (2005) 24:39. doi: 10.3969/j.issn.1005-944X.2005.07.024
65. Ruan YQ, Zhang HM, Tan YG, Huang FM, Lin R, Ou YY, et al. Preliminary investigation on the infection of Marine fish with echinodiasis in Guangxi. Appl Prevent Med. (2008) 14:147–8. doi: 10.3969/j.issn.1673-758X.2008.03.008
66. Huang GP. Molecular Identification and Genetic Relationship Analysis of the Larvae of Parasitic Heterophyllus Nematode in the Order Perciformes in the Southern China Sea. Shijiazhuang: Hebei Normal University (2013).
67. Chen JH, Xu ZX, Xu GX, Huang JY, Chen HH, Shi SZ, et al. Survey of simple anisakis larvae infection in marine fish in Shantou. Chin J Parasitol Parasit Dis. (2014) 32:212–6.
68. Zhao WT, Lv L, Chen HX, Yang Y, Zhang LP, Li L. Ascaridoid parasites infecting in the frequently consumed marine fishes in the coastal area of China: a preliminary investigation. Parasitol Int. (2016) 65:87–98. doi: 10.1016/j.parint.2015.11.002
69. Zuloaga J, Rodríguez-Bobada C, Corcuera MT, Grmez-Aguado F, González P, Rodríguez-Perez R, et al. A rat model of intragastric infection with Anisakis spp. live larvae: histopathological study. Parasitol Res. (2013) 112:2409–11. doi: 10.1007/s00436-013-3359-6
70. Baron L, Branca G, Trombetta C, Punzo E, Quarto F, Speciale G, et al. Intestinal anisakidosis: histopathological findings and differential diagnosis. Pathol Res Pract. (2014) 210:746–50. doi: 10.1016/j.prp.2014.06.022
71. Cho SH, Lee SE, Park OH, Na BK, Sohn WM. Larval anisakid infections in marine fish from three sea areas of the Republic of Korea. Korean J Parasitol. (2012) 50:295–9. doi: 10.3347/kjp.2012.50.4.295
72. Cho J, Lim H, Jung BK, Shin EH, Chai JY. Anisakis pegreffii larvae in sea eels (Astroconger myriaster) from the South Sea, Republic of Korea. Korean J Parasitol. (2015) 53:349–53. doi: 10.3347/kjp.2015.53.3.349
73. Reyes-Balaguer J, Díez-Gandía A, Serna-Andrada N. Consideraciones a la infección por Anisakis con presentación atípica [Anisakis infection with atypical presentation]. Semergen. (2016) 42:212. doi: 10.1016/j.semerg.2015.04.014
74. Zhang L. Preliminary investigation on the infection of simple anisakis larvae in Bohai fish. J Cangzhou Norm Univer. (2002) 18:41–47. doi: 10.3969/j.issn.1008-4762.2002.03.021
75. Quiazon KM, Yoshinaga T, Ogawa K. Distribution of anisakis species larvae from fishes of the Japanese waters. Parasitol Int. (2011) 60:223–6. doi: 10.1016/j.parint.2011.03.002
76. Cavallero S, Martini A, Migliara G, De Vito C, Iavicoli S, D'Amelio S. Anisakiasis in Italy: analysis of hospital discharge records in the years 2005-2015. PLoS ONE. (2018) 13:e0208772. doi: 10.1371/journal.pone.0208772
77. Mladineo I, Poljak V. Ecology and genetic structure of zoonotic Anisakis spp. from adriatic commercial fish species. Appl Environ Microbiol. (2014) 80:1281–90. doi: 10.1128/AEM.03561-13
78. Shamsi S, Sheorey H. Seafood-borne parasitic diseases in Australia: are they rare or underdiagnosed? Intern Med J. (2018) 48:591–6. doi: 10.1111/imj.13786
79. Shamsi S, Suthar J. A revised method of examining fish for infection with zoonotic nematode larvae. Int J Food Microbiol. (2016) 227:13–6. doi: 10.1016/j.ijfoodmicro.2016.03.023
80. Röser D, Stensvold CR. Anisakiasis mistaken for dientamoebiasis? Clin Infect Dis. (2013) 57:1500. doi: 10.1093/cid/cit543
81. Sohn WM, Na BK, Kim TH, Park TJ. Anisakiasis: report of 15 gastric cases caused by anisakis type I larvae and a brief review of Korean anisakiasis cases. Korean J Parasitol. (2015) 53:465–70. doi: 10.3347/kjp.2015.53.4.465
82. D'Amelio S, Mathiopoulos KD, Brandonisio O, Lucarelli G, Doronzo F, Paggi L. Diagnosis of a case of gastric anisakidosis by PCR-based restriction fragment length polymorphism analysis. Parassitologia. (1999) 41:591–3.
83. Cipriani P, Acerra V, Bellisario B, Sbaraglia GL, Cheleschi R, Nascetti G, et al. Larval migration of the zoonotic parasite Anisakis pegreffii (Nematoda: Anisakidae) in European anchovy, Engraulis encrasicolus: implications to seafood safety. Food Control. (2016) 59:148–57. doi: 10.1016/j.foodcont.2015.04.043
84. Guardone L, Nucera D, Lodola LB, Tinacci L, Acutis PL, Guidi A, et al. Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. Int J Food Microbiol. (2018) 268:10–8. doi: 10.1016/j.ijfoodmicro.2017.12.030
85. Arizono N, Yamada M, Tegoshi T, Yoshikawa M. Anisakis simplex sensu stricto and Anisakis pegreffii: biological characteristics and pathogenetic potential in human anisakiasis. Foodborne Pathog Dis. (2012) 9:517–21. doi: 10.1089/fpd.2011.1076
Keywords: anisakid nematodes, fish, prevalence, China, meta-analysis
Citation: Liu Q, Wang Q, Jiang J, Ma J-Y, Zhu X-Q and Gong Q-L (2022) Prevalence of Anisakid Nematodes in Fish in China: A Systematic Review and Meta-Analysis. Front. Vet. Sci. 9:792346. doi: 10.3389/fvets.2022.792346
Received: 19 October 2021; Accepted: 20 January 2022;
Published: 21 February 2022.
Edited by:
Elisabetta Antuofermo, University of Sassari, ItalyReviewed by:
Serena Cavallero, Sapienza University of Rome, ItalyLisa Guardone, University of Pisa, Italy
Copyright © 2022 Liu, Wang, Jiang, Ma, Zhu and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Jiang, amlhbmdqaW5neGlhb3lhb0AxNjMuY29t; Qing-Long Gong, Z29uZ3Fpbmdsb25nMTAwMUAxNjMuY29t
 Qing Liu
Qing Liu Qi Wang
Qi Wang Jing Jiang3*
Jing Jiang3* Jun-Yang Ma
Jun-Yang Ma Xing-Quan Zhu
Xing-Quan Zhu Qing-Long Gong
Qing-Long Gong