
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Vet. Sci. , 24 June 2022
Sec. Veterinary Infectious Diseases
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.757504
This article is part of the Research Topic Ruminant Mastitis: A 360° View View all 21 articles
Understanding distribution of bovine mastitis pathogen Klebsiella spp. can contribute to the treatment decision and the control within programs of bovine mastitis, we conducted a meta-analysis to investigate the epidemiology and antimicrobial resistance rates of Klebsiella spp. associated with bovine mastitis in China. Three databases, namely, PubMed, Google scholar, and China National Knowledge Infrastructure database, were utilized to obtain relevant publications. According to PRISMA reporting standards, a total of 38 publications were included in the research, among them, 7 papers included an AMR test. The pooled prevalence of Klebsiella spp. was 5.41% (95% CI: 3.87–7.50%). Subgroup analysis revealed that the prevalence was higher in South China (8.55%, 95% CI: 3.57–19.09%) than in North China (4.22%, 95% CI: 2.46–7.14%), in 2010–2020 (7.45%, 95% CI: 5.29–110.40%) than in 2000–2010 (3.14%, 95% CI: 1.90–15.14%), and in the clinical bovine mastitis cases (7.49%, 95% CI: 3.71–14.54%) than in the subclinical cases (4.03%, 95% CI: 1.55–10.08%). The pooled AMR rate revealed that Klebsiella spp. were most resistant to sulfonamides (45.07%, 95% CI: 27.72–63.71%), followed by tetracyclines (36.18%, 95% CI: 23.36–51.34%), aminoglycosides (27.47%, 95% CI: 17.16–40.92%), β-lactams (27.35%, 95% CI: 16.90–41.05%), amphenicol (26.82%, 95% CI: 14.17–44.87%), lincosamides (21.24%, 95% CI: 7.65–46.75%), macrolides (20.98%, 95% CI: 7.20–47.58%), polypeptides (15.51%, 95% CI: 6.46–32.78%), and quinolones (7.8%, 95% CI: 3.25–17.56%). The climate difference between South and North China and the natural pathogenicity of Klebsiella spp. may be the primary reasons for its distribution, and the prevalence of Klebsiella spp. indicated that the genus is an increasing hazard to the dairy industry. The prevalence of AMR in China is commonly higher than in the European countries and Canada, this is a very important concern for strategy programs to control bovine mastitis caused by Klebsiella spp. in China.
Mastitis is one of the costliest diseases in the dairy industry due to the discarding of milk and expenses of treatments, including the culling of cows (1–3). Klebsiella spp. are the major gram-negative pathogens that cause mastitis (4–7), and the concern for their pernicHiousness to the dairy industry in China has increased in recent years (8). In recent research, Klebsiella spp. was isolated from 13% of clinical bovine mastitis samples collected from dairy farms in China (9). Klebsiella spp. mastitis is prolonged with a severe and long-lasting duration of intramammary infection and is often accompanied by a considerable decrease in milk production (10); this condition shows no desirable response to antimicrobial treatments (5, 11). Consequently, cows with Klebsiella spp. mastitis are more likely to be culled compared with cows with other types of mastitis (12–15).
Antimicrobials are still the major option for the treatment of mastitis (16). However, the abuse of antimicrobials increases the risks of antimicrobial resistance (AMR) in bacteria, which is a worldwide public health concern (17, 18). Alvarez-Uria et al. predicted that a considerable proportion of Klebsiella pneumoniae will likely be resistant to carbapenems and third-generation cephalosporin in most parts of the world by 2030 (19). The “National action plan to combat animal resources antimicrobial resistance (2017–2020). Beijing: China Ministry of Agriculture and Rural Affairs; 2017,” is one of the national protocols for standardizing veterinary medication, along with strict biosecurity, sterile standard, and the prudent use of antimicrobials to release the pressure of transmission of antimicrobial-resistant pathogens. Wang et al. (20) reported that the policy and decreased use of colistin in agriculture had a significant effect on the reduction of colistin resistance in animals and humans in China.
Investigation of the epidemiology and AMR profiles of Klebsiella spp. can contribute to treatment decisions and optimization of Klebsiella spp. control programs (21). Numerous publications focused on the AMR of other major bovine mastitis pathogens in China, including S. aureus and E. coli (22, 23), whereas the meta-analysis can overcome the insufficient spatial and temporal distribution of Klebsiella spp.
Figure 1 illustrates the relevant steps and results of the literature retrieval. For a previously published review, a comprehensive and systematic literature search was conducted by two independent reviewers on 23 May 2021, utilizing the PubMed (http:// www.pubmed.gov), Google scholar (https://scholar.google.com), and China National Knowledge Infrastructure (CNKI) databases (https://www.cnki.net/) to identify the literature focusing on Klebsiella mastitis in cows. The subject heading “bovine mastitis AND bacteria” was used to find all trials on this topic written in the English or Chinese language. The time was set from 2000 to 2021 to assure the timeliness of the subsequent meta-analytic investigation.
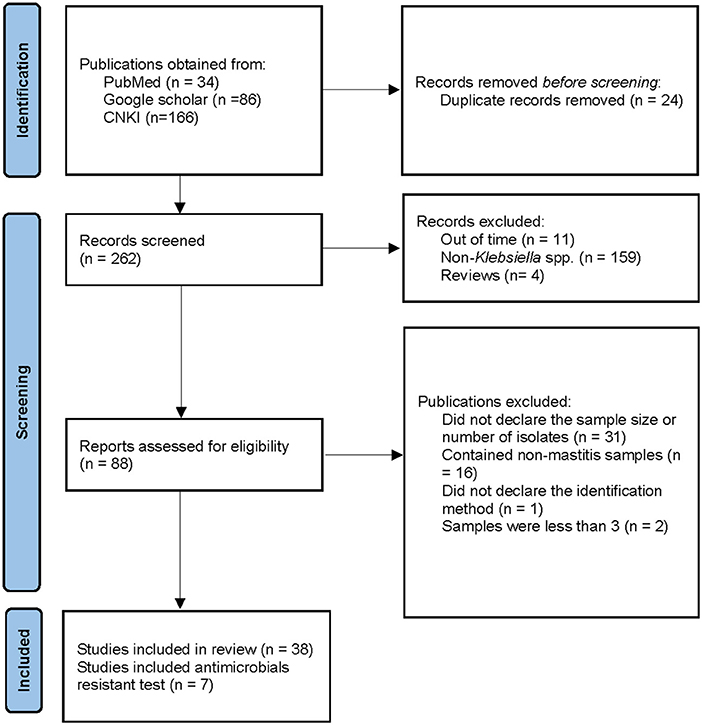
Figure 1. Search and selection criteria for literatures. CNKI means Chinese National Knowledge Infrastructure.
As reported previously (24), our study was in accordance with PRISMA reporting standards (25), specific exclusion criteria were defined to exclude articles that did not describe clinical trials (e.g., descriptive, or in vitro studies). Two authors reviewed all abstracts and then performed a full-text review of articles for eligibility independently, the agreement between the two reviewers for inclusion of articles was good (κ = 0·86). The excluded publications included review articles, articles did not meet the inclusion criteria due to wrong indexation (“off topic”), out of the considered time period, small sample size (less than three samples), exclusion of Klebsiella, undeclared bacterial identification method, samples containing non-mastitis diseases, undeclared sample size or number of bacterial isolates, and unobtainable through the internet. Then, the two reviewers extracted the data from included articles independently. Retrieval and management of references were performed with Excel (Office 16 for Windows, Microsoft Office, New York, USA) (Table 1).
Data were extracted from individual studies using a predesigned form obtaining data on the author, year, province, the number of samples, the number of Klebsiella isolates, mastitis grade (clinical and subclinical mastitis criterion: Laboratory handbook on bovine mastitis and National Mastitis Council), bacterial identification methods, the number of antibiotic-resistant isolates, and laboratory procedures. The same two reviewers independently, and in duplicate, assessed the methodological quality of each individual study based on the prespecified study quality indicators adapted from the Downs and Black checklist.
The numbers of Klebsiella spp., antimicrobial-resistant isolates, and mastitis milk samples within individual studies were calculated for their proportion. Resistance was considered a dichotomous outcome, as classified by individual primary studies. Isolates with intermediate susceptibility were classified as susceptible.
Meta-analyses were performed separately for Klebsiella spp. prevalence and their AMR rates. This procedure was performed by using the “meta” and “metafor” package in R (Version 4.0.5) and only conducted if four or more studies were considered because between-study variance cannot be estimated accurately when it is less than this number and may result in biased pooled estimates after the meta-analysis.
We pooled the prevalence of Klebsiella spp. using random effects models. Subgroup meta-analyses were conducted for isolation time, isolate regions, and mastitis grade to illustrate the heterogeneity between the included studies.
For the AMR studies, we pooled analyses within nine groups: β-lactams, quinolones, aminoglycosides, tetracyclines, polypeptides, sulfonamides, amphenicol, macrolides, and lincosamides.
A publication bias test was performed by using “Egger” test, and the funnel plot was created. Sensitivity analysis was conducted by using “leave-one-out” analyses. Both of them were conducted by using the “meta” and “metafor” packages in R (version 4.0.5).
A total of 34, 86, and 166 articles were obtained from PubMed, Google scholar, and CNKI, respectively. Among them, 24 publications were duplicated, a total of 31 publications were excluded because they did not declare the sample size nor the number of bacterial isolates, and two publications used a small sample size, which were excluded. In addition, 16 articles contained non-mastitis cases, 159 publications did not contain Klebsiella spp. cases, 11 articles were beyond the considered period (before 2000), four articles were reviews, and one article did not declare the identification method. Therefore, these publications were denied. Finally, a total of 38 full-textt publications were included in our research, of which 7 covered the AMR test (Figure 1).
As for the 38 publications, two publications did not describe the sample collected location exactly, they were included and given our focus on the prevalence of Klebsiella throughout the country. A total of seven publications obtained clinical and subclinical samples, and 14 did not describe the grade of mastitis. However, we still included them in our research because we focused on the whole condition of bovine mastitis (Table 1).
The pooled prevalence of Klebsiella spp. was 5.41% (95% CI: 3.87–7.50%). An evident heterogeneity was observed (I2 = 95%, τ2 = 0.965, P <0.01). Therefore, a subgroup analysis was conducted to explore the sources of heterogeneity (Figure 2).
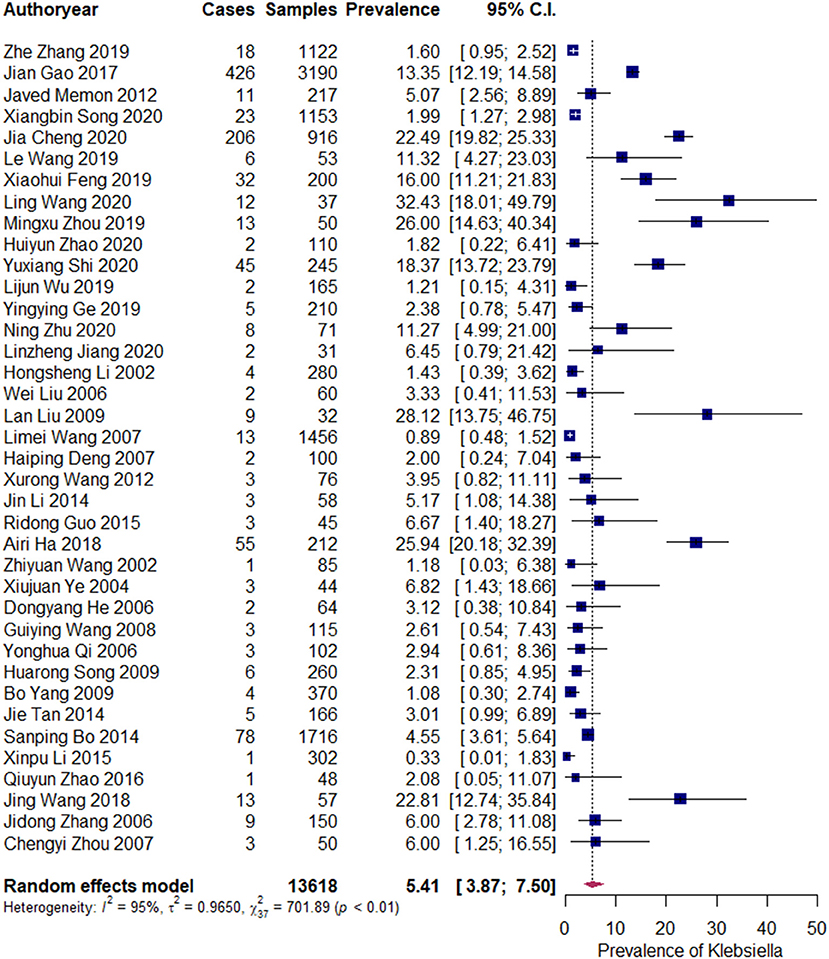
Figure 2. Prevalence of Klebsiella spp. Each navy blue square represents the mean prevalence for a study. The upper and lower limit of the line connected to the square represents the upper and lower 95% CI for the prevalence. The size of the square reflects the relative weighting of the study to the overall prevalence estimate, with larger squares representing greater weight. The broken vertical line represents overall prevalence and the diamond at the bottom represents the overall prevalence (location of the diamond) and its 95% CI (width of the diamond). Cases refers to the Klebsiella spp. containing samples. “Author year” refers to the first author and publication year of the publication (the same below).
We divided the research articles into subgroups based on the research period (2000–2010 vs. 2010–2020), sample sites (North China vs. South China), and mastitis grade (clinical mastitis vs. subclinical mastitis). The pooled prevalence values of Klebsiella spp. were 3.14 and 7.45% (2000–2010 vs. 2010–2020, Figure 3); 7.49 and 4.03% (clinical mastitis vs. subclinical mastitis, Figure 4); 4.22 and 8.55% (North China vs. South China, Figure 5), respectively.
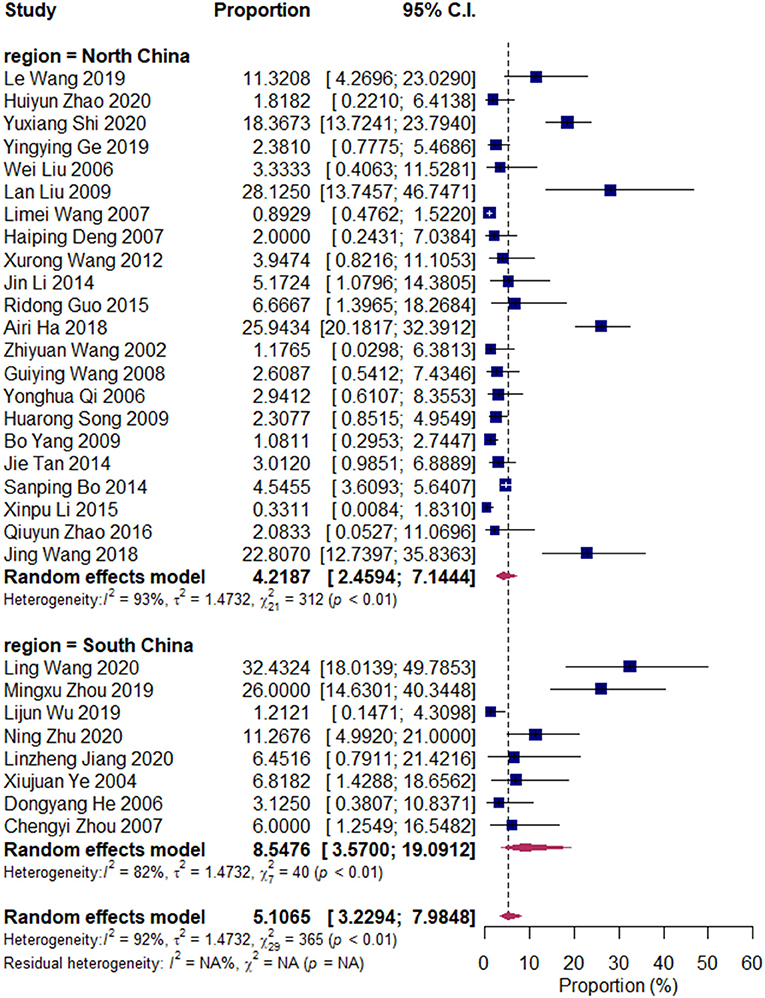
Figure 3. Prevalence of Klebsiella spp. in North and South China. Subgroups were created according to the sampling region in China (North China/South China). The two upper diamonds represent the overall prevalence within the respective subgroups, whereas the diamond at the bottom represents the overall prevalence of all studies (the same below).
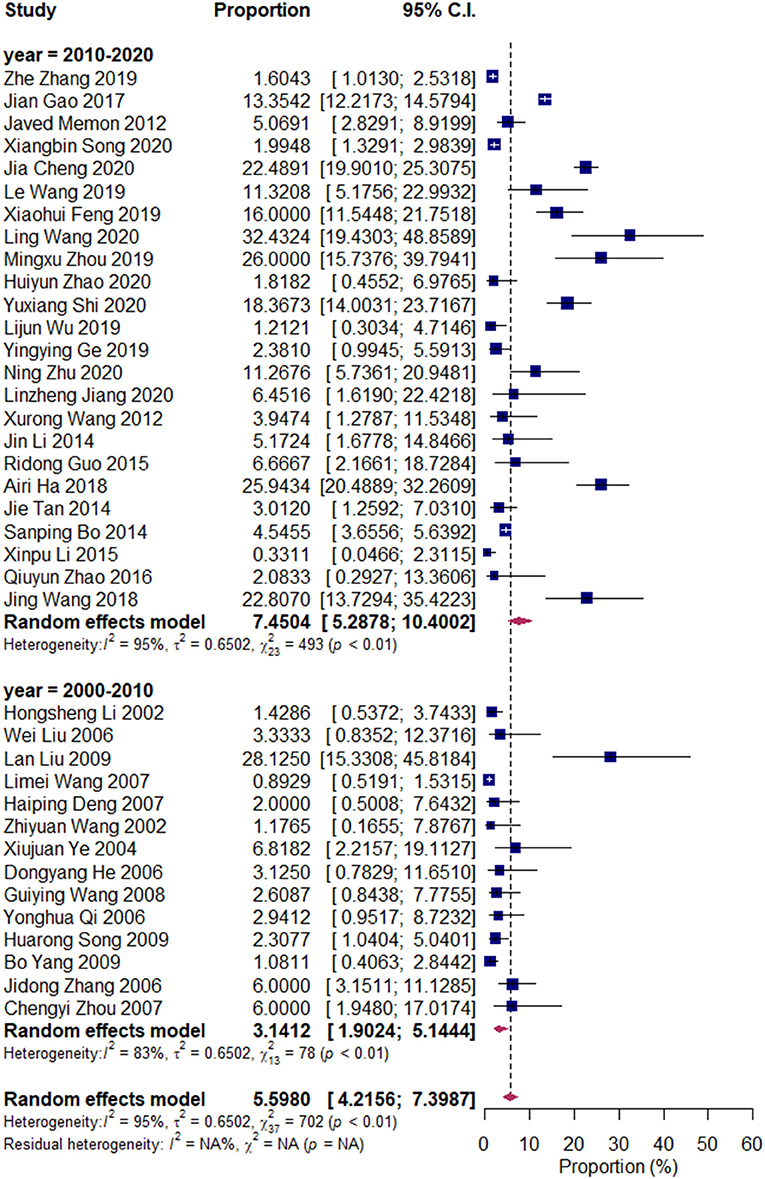
Figure 4. Prevalence of Klebsiella spp. in the period of 2000–2010 and 2010–2020. Subgroups were created according to the publication year (2000–2010/2010–2020).
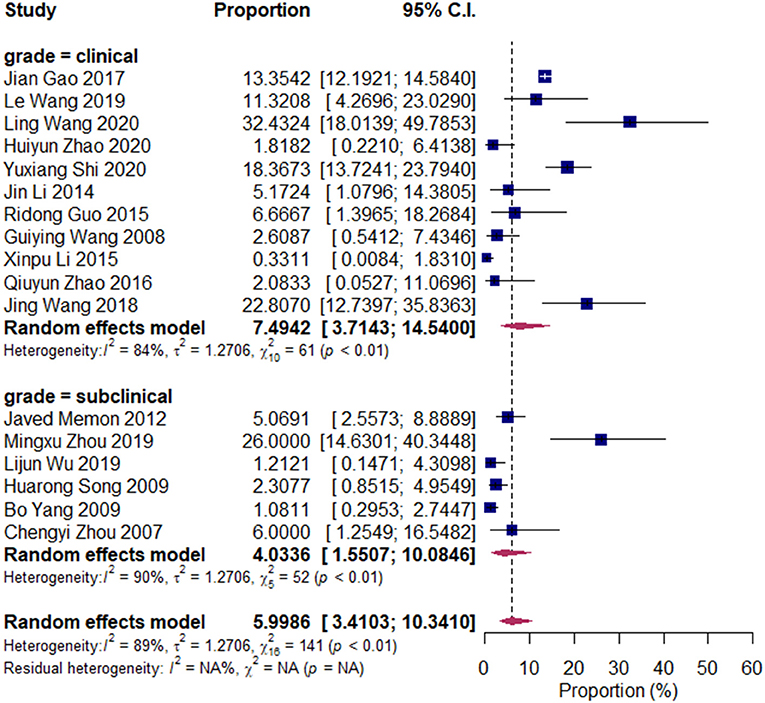
Figure 5. Prevalence of Klebsiella spp. isolated in clinical mastitis and subclinical mastitis cases. Subgroups were created according to the bovine mastitis grade (clinical/subclinical).
The funnel plot (Figure 6) exhibited an even distribution of the studies around the mean effect size, which suggested that the publication bias was not evident.
The pooled resistant rates were as follows: β-lactams, 27.35% (95% CI: 11.73–24.79%); quinolones, 7.8% (95% CI: 3.25–17.56%); aminoglycosides, 27.47% (95% CI: 17.16–40.92%); tetracyclines, 36.18% (95% CI: 23.36–51.34%); polypeptides, 15.51% (95% CI: 6.46–32.78%); sulfonamides, 45.07% (95% CI: 27.72–63.71%); amphenicol, 26.82% (95% CI: 14.17–44.87%); macrolides, 20.98% (95% CI: 7.20–47.58%); lincosamides, 22.24% (95% CI: 7.65–46.75%) (Figure 7).
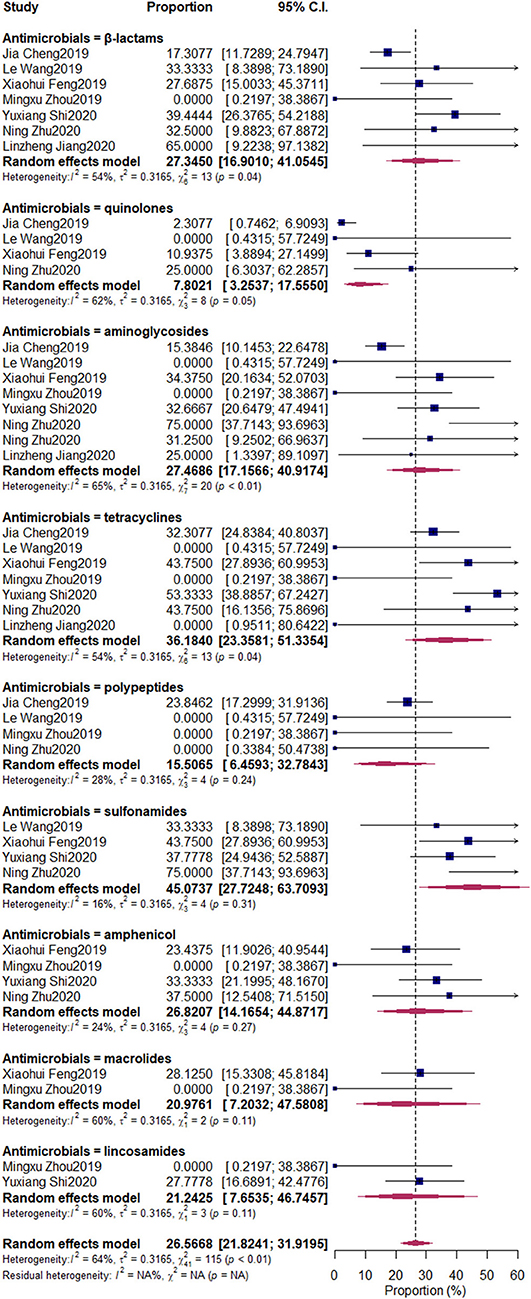
Figure 7. Antimicrobial-resistant rate of Klebsiella spp. Subgroups were created according to the different kinds of antimicrobials. The nine upper diamonds represent the overall antimicrobial resistance (AMR) rate within the respective subgroups, whereas the diamond at the bottom represents the overall AMR rate of all studies.
The funnel plot (Figure 8) exhibited an even distribution of the studies around the mean effect size, which suggested a negligible publication bias.
Bovine mastitis is the costliest disease in the dairy industry (26). Klebsiella spp. is important pathogens causing bovine mastitis and human infection (27, 28). Understanding the prevalence and AMR profiling of bovine mastitis, Klebsiella spp. may contribute to therapeutic interventions and preventive strategies.
A total of 38 publications, 13,618 samples, and 1,037 isolates were pooled in our study. The pooled prevalence of Klebsiella spp. was 5.41% (95% CI: 3.87–7.50%). Subgroup meta-analysis indicated that the prevalence of Klebsiella spp. in South China was higher than that in North China, that in subclinical mastitis was lower than that in clinical mastitis, and that in 2000–2010 was lower than that in 2010–2020.
Our results revealed that the pooled prevalence of Klebsiella spp. was relatively lower than those of previous studies conducted in China (13 and 9.78%) (9, 29). Meanwhile, the prevalence of Klebsiella spp. in South China (8.5%) was twice that in North China (4.2%). Environmental sources, such as alleyways, holding pens, and sawdust and shavings in bedding, are important sources of Klebsiella spp. (National Mastitis Council, 1999; (28, 30). Our results were consistent with those of Gao et al., whose results have revealed that the prevalence of Klebsiella spp. in Northwest China is lower than that in South China, and that in winter was lower than that in summer; this finding is attributed to the dry and cold weather in North China, which is unsuitable for environmental microorganisms (9).
In recent years, Klebsiella spp. mastitis, which is attributed to the fecal shedding of Klebsiella spp., increased the concern for herds that use inorganic bedding (31). Klebsiella pneumoniae is an endophyte of several plants, such as wheat, corn, and alfalfa, and it can act as milk cow feed; bacteria can be found inside the plants without external fecal contamination (32–34). Consequently, the oral intake of Klebsiella spp. can be due to the plants used for feed or to fecal contamination of feed and water, whereas fecal shedding of Klebsiella spp. results in the contamination of the environment of cows. Such sources provide dairy herd managers and veterinarians with additional control points for the prevention of Klebsiella spp. mastitis. The increased prevalence of Klebsiella from 2000 to 2020 raised the concern for this important mastitis pathogen.
Cheng et al. (35) revealed that Klebsiella spp. can induce severe and long-term infection in the bovine milk gland, and can give rise to clinical bovine mastitis more than the subclinical version. Our results also revealed that the prevalence of Klebsiella spp. in clinical mastitis is higher than that in subclinical bovine mastitis.
Klebsiella spp. can induce bovine mastitis; antimicrobial treatment is normally used for mastitis prevention and control (7).
The misuse of antimicrobials can increase the risk of AMR and threaten public health. (36). In our research, the AMR of Klebsiella spp. against nine kinds of frequently used antimicrobials (β-lactams, quinolones, aminoglycosides, tetracyclines, polypeptides, sulfonamides, amphenicol, macrolides, and lincosamides) was determined. We first pooled nationwide studies that were conducted to determine the AMR of Klebsiella spp. isolated from bovine mastitis in China. For these nine antimicrobials, the resistance rate of sulfonamides was the highest (45.07%), followed by tetracyclines (36.18%), aminoglycosides (27.47%), β-lactams (27.34%), amphenicol (26.82%), lincosamides (21.24%), macrolides (20.98%), polypeptides (15.51%), and quinolones (7.80%).
In a previous study conducted by Saini et al. (36), the resistance to sulfonamides (11.7%) and β-lactams (17.3%) was the highest, but the values were still lower than that in our research. In another study conducted in Canada, Klebsiella spp. was the main pathogen resistant to tetracyclines (19%) and streptomycin (38%) (37). The high percentages of AMR of Klebsiella spp. against sulfonamides, β-lactams, amphenicol, lincosamides, macrolides, and polypeptides in that study were not observed, similar to the observations in European countries (38), in which the resistance to streptomycin was higher than that in our research; however, the resistance to tetracyclines was lower than that in our study. The use of sulfonamides and tetracyclines in husbandries had been forbidden by the Chinese government. However, the observed AMR rate was still high, which indicated that the AMR mechanism was still harbored by Klebsiella spp. aminoglycosides and β-lactams should raise the most concern when used in treating bovine mastitis. Tetracyclines are one of the extensively used antimicrobials among dairy farms. Its AMR has been a serious problem before, but with the increased awareness of public health in society, the Chinese government imposed a ban on the use of antimicrobials as growth promoters in the husbandry industry, which restricted the AMR of pathogens.
Fuenzalida and Ruegg (39) indicated that the cure rate for Klebsiella pneumoniae mastitis was 21% greater in 8-day than in 2-day intramammary ceftiofur group. A previous study conducted in China by Cheng et al. (35) reported that Klebsiella spp. were also highly resistant to amoxiclav (38%), with a value higher than that in the study of Schukken et al. (11) in the USA. Our results also indicated that the AMR of bovine mastitis-associated Klebsiella spp. against β-lactams in China was as high as 27.34%. Yang et al. (40) reported that the β-lactam resistance gene blaCTM-M-1 located on pC5-like plasmids can be responsible for the resistance against ceftiofur for bovine mastitis treatment. Schukken et al. (11) suggested that the antimicrobial treatment of Klebsiella spp. bovine mastitis has a minimal value, and heteropathy for clinical symptoms should be the primary goal. A recent study indicated that third-generation cephalosporin and carbapenems will be ineffective against a large proportion of Klebsiella spp. in most parts of the world by 2030 (19), which should raise the concern for the AMR of Klebsiella spp. associated with bovine mastitis.
The results in our study were consistent with those of Cheng et al. (35), whose results indicated that the AMR occurrence rates of five common bovine mastitis pathogens, including Klebsiella spp., in China were higher than those in the European countries (41). The occurrence rate of AMR among bovine mastitis pathogens differs among various countries (42), and this condition can be attributed to complex reasons, such as the national guidelines for proper antibiotics usage, veterinarian prescription patterns, and pharmaceutical marketing strategies (43, 44). Hence, our results should raise the concern about the AMR of bovine mastitis Klebsiella spp. in Chinese dairy herds. There are still limitations including few databases retrieval and publication time substituting sample time in our manuscript, which should make improvements in the future.
The pooled prevalence of Klebsiella spp. was 5.41% (95% CI: 3.87–7.50%). Subgroup analysis revealed that the incidence was higher in South China, from 2010 to 2020, and in clinical bovine mastitis cases, and the reason is attributed to the climate between South and North China and the natural pathogenicity of Klebsiella spp. The pooled AMR rates showed that Klebsiella spp. were most resistant to sulfonamides, followed by tetracyclines, aminoglycosides, β-lactams, amphenicol, lincosamides, macrolides, polypeptides, and quinolones, which should raise the most concern when used in treating bovine mastitis.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
KL designed the study, analyzed the data, and wrote the article. LZ and XG performed the literature research and review. WQ critically reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This study was financially supported by the National Natural Science Foundation of China (No. 31260629 and No. 31660730), the Yunnan Provincial Science and Technology Department-Yunnan Expert Workstation (No. 202005AF150041), Veterinary Public Health Innovation Team of Yunnan Province (No. 202105AE160014), and Science Research Foundation of Yunnan Education Bureau (grant number: No. 2020y134).
1. Shaheen M, Tantary HA, Nabi SU. A treatise on bovine mastitis: Disease and disease economics, etiological basis, risk factors, impact on human health, therapeutic management, prevention and control strategy. J. Adv. Dairy Res. (2016) 1:1000150. doi: 10.4172/2329-888X.1000150
2. Gussmann M, Denwood M, Kirkeby C, Farre M, Halasa T. Associations between udder health and culling in dairy cows. Prev. Vet. Med. (2019) 171:104751. doi: 10.1016/j.prevetmed.2019.104751
3. Krömker V, Leimbach S. Mastitis treatment-reduction in antibiotic usage in dairy cows. Reprod Domest Anim. (2017) 3:21–9. doi: 10.1111/rda.13032
4. Wenz JR, Barrington GM, Garry FB, McSweeney KD, Dinsmore RP, Goodell G, et al. Bacteremia associated with naturally occurring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. (2001) 219:976–981. doi: 10.2460/javma.2001.219.976
5. Roberson JR, Warnick LD, Moore G. Mild to moderate clinical mastitis: Efficacy of intramammary amoxicillin, frequent milk-out, a combined intramammary amoxicillin, and frequent milk-out treatment versus no treatment. J Dairy Sci. (2004) 87:583–92. doi: 10.3168/jds.S0022-0302(04)73200-2
6. Olde Riekerink RGM, Barkema HW, Kelton DF, Scholl DT. Incidence rate of clinical mastitis on Canadian dairy farms. J Dairy Sci. (2008) 91:1366–77. doi: 10.3168/jds.2007-0757
7. Oliveira L, Hulland C, Ruegg PL. Characterization of clinical mastitis occurring in cows on 50 large dairy herds in Wisconsin. J Dairy Sci. (2013) 96:7538–49. doi: 10.3168/jds.2012-6078
8. Cheng J, Zhang J, Han B, Barkema HW, Cobo ER, Kastelic JP. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cells. J Dairy Sci. (2020) 103:3493–504. doi: 10.3168/jds.2019-17458
9. Gao J, Barkema HW, Zhang L, Liu G, Deng Z, Cai L. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J Dairy Sci. (2017) 100:4797–806. doi: 10.3168/jds.2016-12334
10. Gröhn YT, Wilson DJ, Gonzalez RN, Hertl JA, Schulte H, Bennett G. Effect of pathogen-specific clinical mastitis on milk yield in dairy cows. J Dairy Sci. (2004) 87:3358–74. doi: 10.3168/jds.S0022-0302(04)73472-4
11. Schukken Y, Chuff M, Moroni P, Gurjar A, Santisteban C, Welcome F. The “other” gram-negative bacteria in mastitis: Klebsiella, Serratia, and more Vet Clin North Am Food Anim Pract. (2012) 28:239–56. doi: 10.1016/j.cvfa.2012.04.001
12. Erskine RJ, Bartlett PC, VanLente JL, Phipps CR. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J. Dairy Sci. (2002) 85:2571–5. doi: 10.3168/jds.S0022-0302(02)74340-3
13. Gröhn YT, Gonzalez RN, Wilson DJ, Hertl JA, Bennett G, Schulte H. (2005). Effect of pathogen-specific clinical mastitis on herd life in two New York State dairy herds Prev Vet Med. (2005) 71:105–25. doi: 10.1016/j.prevetmed.2005.06.002
14. Wilson DJ, Gröhn YT, Bennett GJ, Gonzalez RN, Schukken YH, Spatz J. Comparison of J5 vaccinates and controls for incidence, etiologic agent, clinical severity, and survival in the herd following naturally occurring cases of clinical mastitis. J Dairy Sci. (2007) 90:4282–8. doi: 10.3168/jds.2007-0160
15. Podder MP, Rogers L, Daley PK, Keefe GP, Whitney HG. Klebsiella species associated with bovine mastitis in Newfoundland. PLoS ONE. (2014) 9:e106518. doi: 10.1371/journal.pone.0106518
16. Keefe G. Update on control of Staphylococcus aureus and Streptococcus agalactiae for management of mastitis. Vet Clin North Am Food Anim Pract. (2012) 28:203–16. doi: 10.1016/j.cvfa.2012.03.010
17. Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges, and responses. Nat Med. (2004) 10:S122–9. doi: 10.1038/nm1145
18. Sarmah AK, Meyer MT. Boxall AB. A global perspective on the use, sales, exposure pathways, occurrence, fate, and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. (2006) 65:725–59. doi: 10.1016/j.chemosphere.2006.03.026
19. Alvarez-Uria G, Gandra S, Mandal S. Laxminarayan R. Global forecast of antimicrobial resistance in invasive isolates of Escherichia coli and Klebsiella pneumoniae. Int J Infect Dis. (2018) 68:50–3. doi: 10.1016/j.ijid.2018.01.011
20. Wang Y, Xu C, Zhang R, Chen Y, Shen Y, Hu F. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: an epidemiological comparative study. Lancet Infect Dis. (2020) 20:1161–71. doi: 10.1016/S1473-3099(20)30149-3
21. Kaczorek E, Małaczewska J, Wójcik R, Rekawek W, Siwicki AK. Phenotypic and genotypic antimicrobial susceptibility pattern of Streptococcus spp. isolated from cases of clinical mastitis in dairy cattle in Poland. J Dairy Sci. (2017) 100:6442–53. doi: 10.3168/jds.2017-12660
22. Gao J, Ferreri M, Yu F, Liu X, Chen L, Su J. Molecular types and antibiotic resistance of Staphylococcus aureus isolates from bovine mastitis in a single herd in China. Vet J. (2011) 192:550–2. doi: 10.1016/j.tvjl.2011.08.030
23. Ali T, Rahman SU, Zhang L, Shahid M, Han D, Gao J. Characteristics and genetic diversity of multi-drug resistant extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from bovine mastitis. Oncotarget. (2017) 8:90144–63. doi: 10.18632/oncotarget.21496
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
25. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
26. Aghamohammadi M, Haine D, Kelton DF, Barkema HW, Hogeveen H, Keefe GP. Herd-level mastitisassociated costs on Canadian dairy farms. Front Vet Sci. (2018) 5:100. doi: 10.3389/fvets.2018.00100
27. Munoz MA, Bennett GJ, Ahlstrom C, Griffiths HM, Schukken YH, Zadoks RN. Cleanliness scores as indicator of Klebsiella exposure in dairy cows. J Dairy Sci. (2008) 91:3908–16. doi: 10.3168/jds.2008-1090
28. Zadoks RN, Griffiths HM, Munoz MA, Ahlstrom C, Bennett GJ, Thomas E. Sources of Klebsiella and Raoultella species on dairy farms: be careful where you walk. J Dairy Sci. (2011) 94:1045–51. doi: 10.3168/jds.2010-3603
29. Yang Y, Peng Y, Jiang J, Gong Z, Zhu H, Wang K. Isolation and characterization of multidrug-resistant Klebsiella pneumoniae from raw cow milk in Jiangsu and Shandong provinces, China. Transbound Emerg Dis. (2021) 68:1033–9. doi: 10.1111/tbed.13787
30. Kristula MA, Dou Z, Toth JD, Smith BI, Harvey N, Sabo M. Evaluation of free-stall mattress bedding treatments to reduce mastitis bacterial growth. J Dairy Sci. (2008) 91:1885–92. doi: 10.3168/jds.2007-0603
31. Munoz MA, Ahlstrom C, Rauch BJ, Zadoks RN. Fecal shedding of Klebsiella pneumoniae by dairy cows. J Dairy Sci. (2006) 89:3425–30. doi: 10.3168/jds.S0022-0302(06)72379-7
32. Chelius MK, Triplett EW. Immunolocalizatoin of dinitrogenase reductase produced by Klebsiella pneumoniae in association with Zea mays L. Appl Environ Microbiol. (2000) 66:783–7. doi: 10.1128/AEM.66.2.783-787.2000
33. Dong Y, Iniguez AL, Ahmer BM, Triplett EW. Kinetics and strain specificity of rhizosphere and endophytic colonization by enteric bacteria on seedlings of Medicago sativum and Medicago truncatula. Appl Environ Microbiol. (2003) 69:1783–90. doi: 10.1128/AEM.69.3.1783-1790.2003
34. Iniguez AL, Dong Y, Triplett EW. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol Plant Microbe Interact. (2004) 17:1078–85. doi: 10.1094/MPMI.2004.17.10.1078
35. Cheng J, Qu W, Barkema HW, Nobrega DB, Gao J, Liu G. Antimicrobial resistance profiles of 5 common bovine mastitis pathogens in large Chinese dairy herds. J Dairy Sci. (2019) 102:2416–26. doi: 10.3168/jds.2018-15135
36. Saini V, McClure JT, Leger D, Dufour S, Sheldon AG, Scholl DT, et al. Antimicrobial use on Canadian dairy farms. J Dairy Sci. (2012) 95:1209–21. doi: 10.3168/jds.2011-4527
37. Massé J, Dufour S, Archambault M. Characterization of Klebsiella isolates obtained from clinical mastitis cases in dairy cattle. J Dairy Sci. (2020) 103:3392–400. doi: 10.3168/jds.2019-17324
38. de Jong A, El Garch F, Simjee S, Moyaert H, Rose M, Youala M, et al. Monitoring of antimicrobial susceptibility of udder pathogens recovered from cases of clinical mastitis in dairy cows across Europe: VetPath results. Vet. Microbiol. (2018) 213:73–81. doi: 10.1016/j.vetmic.2017.11.021
39. Fuenzalida MJ, Ruegg PL. Negatively controlled, randomized clinical trial to evaluate use of intramammary ceftiofur for treatment of nonsevere culture-negative clinical mastitis. J Dairy Sci. (2019) 102:3321–38. doi: 10.3168/jds.2018-15497
40. Yang Y, Higgins CH, Rehman I, Galvao KN, Brito IL, Bicalho ML. Genomic diversity, virulence, and antimicrobial resistance of Klebsiella pneumoniae strains from cows and humans. Appl Environ Microbiol. (2019) 85:e02654–e02618. doi: 10.1128/AEM.02654-18
41. Thomas V, de Jong A, Moyaert H, Simjee S, El Garch F, Morrissey I, et al. Antimicrobial susceptibility monitoring of mastitis pathogens isolated from acute cases of clinical mastitis in dairy cows across Europe: VetPath results. Int J Antimicrob Agents. (2015) 46:13–20. doi: 10.1016/j.ijantimicag.2015.03.013
42. Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. (2014) 69:827–34. doi: 10.1093/jac/dkt443
43. ECDC EFSA. ENA. ECDC/EFSA/EMA first joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food producing animals. EFSA J. (2015) 13:4006. doi: 10.2903/j.efsa.2015.4006
Keywords: bovine mastitis, Klebsiella spp., epidemiology, antimicrobial resistance, meta-analysis
Citation: Liu K, Zhang L, Gu X and Qu W (2022) The Prevalence of Klebsiella spp. Associated With Bovine Mastitis in China and Its Antimicrobial Resistance Rate: A Meta-Analysis. Front. Vet. Sci. 9:757504. doi: 10.3389/fvets.2022.757504
Received: 12 August 2021; Accepted: 30 May 2022;
Published: 24 June 2022.
Edited by:
Sohail Naushad, Canadian Food Inspection Agency, CanadaReviewed by:
Faham Khamesipour, Shahid Beheshti University of Medical Sciences, IranCopyright © 2022 Liu, Zhang, Gu and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijie Qu, MTQwMTA3NTAxMEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.