- 1Department of Veterinary Clinical Sciences, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 2Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA, United States
- 3Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, Rehovot, Israel
Objective: Compare characteristics and clinical outcomes of dogs with infectious keratitis from Staphylococcus pseudintermedius considered to be multidrug-resistant (MDR) or not.
Procedures: Staphylococcus pseudintermedius isolated as the primary pathogen from canine patients with ulcerative keratitis were considered MDR if resistant to at least one agent in three or more classes of antibiotics. Medical records were reviewed for history, patients' characteristics, clinical appearance, therapeutic interventions, and clinical outcomes.
Results: Twenty-eight dogs (28 eyes) were included. Compared to non-MDR cases, MDR diagnosis was significantly more common in dogs with recent (≤30 days) anesthesia (7/15 vs. 1/13, P = 0.038) and more common in non-brachycephalic dogs (8/15 vs. 2/13, P = 0.055). Clinical appearance (ulcer size/depth, anterior chamber reaction, etc.) did not differ significantly between groups (P ≥ 0.055). Median (range) time to re-epithelialization was longer in MDR vs. non-MDR eyes [29 (10–47) vs. 22 (7–42) days] but the difference was not significant (P = 0.301). Follow-up time was significantly longer in dogs with MDR keratitis [47 (29–590) vs. 29 (13–148) days, P = 0.009]. No other significant differences were noted between MDR and non-MDR eyes in regard to time for ulcer stabilization [4 (1–17) days vs. 4 (1–12), P = 0.699], number of eyes requiring surgical stabilization (7/15 vs. 7/13, P = 0.246) or enucleation (1/15 vs. 2/13, P = 1.000), success in maintaining globe (14/15 vs. 11/13, P = 0.583) or success in maintaining vision (12/15 vs. 10/13, P = 1.000).
Conclusions: MDR infections may prolong corneal healing time but did not appear to affect overall clinical outcomes in dogs with bacterial keratitis. Further research is warranted in a larger canine population and other bacterial species.
Introduction
Bacterial keratitis is a major global cause of ocular discomfort and visual impairment in dogs and other species (1, 2). Following an injury to the eye from trauma or other causes, corneal wounds have a high tendency for infection given the presence of indigenous microflora on the corneal and conjunctival surfaces, including Staphylococcus species, Streptococcus species, Pseudomonas species, and gram-positive bacilli. More specifically, Staphylococcus pseudintermedius represents the most common bacteria causing infectious keratitis in many geographical locations around the world including North America (3–5), South America (6), Europe (7, 8), and Asia (9, 10). A component of normal flora in canines, Staphylococcus pseudintermedius is increasingly recognized as a major pathogen in various body systems of dogs including eyes, skin, ears, respiratory tract, reproductive tract, and urinary tract (11, 12). Staphylococcus pseudintermedius is also an emerging pathogen in humans (13) with reported transmission from infected dogs to the home environment and owners (14), as well as cross-contaminations to other veterinary patients in clinical settings (15).
An alarming, escalating trend of resistant Staphylococcus pseudintermedius strains has been described in the human and veterinary literature, including methicillin-resistant strains (MRSP) (10, 16, 17) and multidrug-resistant strains (MDR) (5, 18), that is, bacteria with “acquired resistance to at least one agent in three or more antimicrobial categories” (19). In a recent canine study, MDR represented 20% of all corneal isolates (including 33% of all Staphylococcus spp.) with an incidence that increased from 5% (2016) to 34% (2020) during the period examined (5). The presence of resistant bacterial strains is known to affect clinical outcomes in human patients with infectious keratitis, but to the authors' knowledge, the same has not been evaluated in dogs. In humans, corneal infections from relatively ciprofloxacin-resistant bacteria (i.e., higher minimal inhibitory concentrations) were shown to respond more slowly to therapy (20), while corneal infections caused by relatively moxifloxacin-resistant bacteria resulted in larger corneal scars (21).
The main purpose of this study was to compare the clinical outcomes of canine eyes infected by Staphylococcus pseudintermedius isolates classified as MDR or non-MDR. A secondary objective was to identify risk factors for dogs to develop infectious keratitis involving resistant Staphylococcus pseudintermedius strains.
Materials and methods
Inclusion and exclusion criteria
A retrospective review of electronic medical records (March 2014 to May 2020) was performed searching for dogs diagnosed with culture-confirmed bacterial keratitis (Staphylococcus pseudintermedius) at Iowa State University's Lloyd Veterinary Medical Center (ISU-LVMC). Cases were excluded if they involved the identification of multiple potential pathogens on culture, or if there was incomplete clinical data in the patient's medical records. Cases that presented prior to March 2014 were not included in our study as an ophthalmic-specific susceptibility profile was not available at that time.
Ophthalmic examination
At the initial visit and subsequent rechecks, each canine patient underwent a complete ophthalmic examination by a board-certified veterinary ophthalmologist or an ophthalmology resident, including: neuro-ophthalmic assessment (menace response, dazzle reflex, pupillary light reflexes, palpebral reflex), slit lamp biomicroscopy (SL-17; Kowa Company, Ltd., Tokyo, Japan), indirect ophthalmoscopy (Keeler Vantage; Keeler Instruments, Inc., Broomall, PA, USA), rebound tonometry (TonoVet; Icare Finland Oy, Espoo, Finland), Schirmer tear test-1 (STT-1; Eye Care Product Manufacturing LLC, Tucson, AZ, USA), and fluorescein staining (Flu-Glo, Akorn, Inc., Buffalo Grove, IL, USA).
Sample collection, bacterial isolation, and identification
At the ISU-LVMC, samples were collected with sterile culturette swabs (BBL™ CultureSwab™, Becton Dickinson and Company, Sparks, MD) that were pre-moistened with one drop of sterile saline prior to contact with the corneal wound. Samples were submitted to the Iowa State University Veterinary Diagnostic Laboratory (ISU-VDL) and processed for standard aerobic microbiologic assessment using tryptic soy agar with 5% sheep blood (blood agar) and MacConkey medium. The blood agar was incubated at 35 ± 2°C with 5–10% CO2 for a total length of 4 days while the MacConkey agar was incubated 35 ± 2°C without CO2 for a total length of 2 days. Both agar plates were observed for growth every 24 h. Organisms were then identified using Matrix-Assisted Laser Desorption Ionization Time-of-Flight mass-spectrometry (MALDI-TOF MS, Bruker).
Antimicrobial susceptibility testing
Minimum inhibitory concentration (MIC) susceptibility testing was performed using an automated broth microdilution system (Sensititre AIM, Trek Diagnostic System Inc.) and the following susceptibility panels from Thermo Fischer Scientific: JOEYE2 (ophthalmic panel, used 2015–2020), COMPAN1F (systemic panel, used 2015–2016) and COMPGP1F (systemic panel, used 2017–2020).
The MIC data was then used to establish clinical interpretations for each isolate/antibiotic (susceptible, intermediate, and resistant) based on canine breakpoints for systemic antimicrobials (22) or, when not available, extrapolated from human clinical breakpoints for systemic antimicrobials (23, 24) following veterinary guidelines for appropriate data extrapolation (Supplementary Table 1) (25). While recommendations from Sweeney et al. (26) were considered for veterinary MDR definition based on available canine-specific breakpoints, MDR recommendations for Staphylococcus spp. from Magiorakos et al. (19) were utilized due to the drugs tested on the panels used and the more recent CLSI VET09 guidance, which allows for use of extrapolated human breakpoints in dogs for multiple additional antibiotics. Then, each Staphylococcus pseudintermedius isolate was classified as MDR (or not) if checking one or both criteria as follows:
(i) Oxacillin resistance (23): Oxacillin resistance is used to test for resistance to methicillin, which is typically conferred through acquisition of the mecA gene; methicillin resistance is considered to also predict resistance to all subclasses of beta-lactam antibiotics including penicillinase labile and stabile penicillins, first and third generation cephalosporins, and carbapenems.
(ii) Acquired resistance to at least one agent in three or more antimicrobial categories (19). Of note, although MICs for two polypeptides (bacitracin and polymyxin) were also described in the susceptibility profiles, these antibiotics were not included in the MDR classification given the lack of clinical breakpoints reported in human or veterinary guidelines. Therefore, MDR status was determined based on demonstrated resistance to the following drug classes in addition to beta-lactams: aminoglycosides, phenicols, fluoroquinolones, lincosamides, tetracyclines, macrolides, ansamycins, folate pathway antagonists, and glycopeptides.
Medical records review
Using ISU-LVMC's electronic medical records system, multiple parameters were recorded for each case. Patient characteristics included: breed, age, sex, skull conformation, concurrent ocular disease, systemic disease, history of ocular trauma, recent anesthesia (within 30 days), number of medications prior to referral, number of eyedrops per day prior to referral, and duration of clinical signs prior to diagnosis. Clinical findings included: affected eye(s), Schirmer tear test-1 results, corneal ulcer location/size/depth, presence/absence of corneal cellular infiltrates or hypopyon, and aqueous flare grade. Clinical outcomes included: number of medications prescribed, number of eyedrops administered per day, number of susceptible infections to empirical antibiotic(s) prescribed at initial visit, systemic antibiotic use, time to corneal ulcer stabilization, time to ulcer re-epithelialization, time to discontinuing topical medications, whether hospitalization for intensive medical management was required, whether surgical stabilization was required (and if so, time from presentation to surgical stabilization), whether an end-stage procedure was required, number of follow-up days, corneal stromal thinning at last visit, success in maintaining globe, and success in maintaining vision (intact menace response at last follow up visit).
Data analysis
Normality of the data was assessed with the Shapiro–Wilk test. Statistical comparison of canine eyes affected by Staphylococcus pseudintermedius considered MDR vs. non-MDR was performed with Mann-Whitney tests for continuous data (e.g., Schirmer tear test, number of medications or eyedrops per day, etc.) and Fisher's exact tests for categorical data (e.g., sex, skull conformation, affected eye, etc.). Statistical analysis was performed with SigmaPlot 14.5 (Systat software, Point Richmond, CA), and P-values < 0.05 were considered statistically significant.
Results
Data was not normally distributed (P ≤ 0.047) therefore results are presented as median and range (minimum-maximum). Overall n = 124 isolates of Staphylococcus pseudintermedius were identified, but 96 isolates were excluded from further analysis owing to the presence of other potential pathogens or incomplete medical records. As such, a total of 28 eyes (16 left eyes, 12 right eyes) involving 28 canine patients were included, with a median (range) age of 8 years (1–15 years), 11 spayed female and 17 neutered male dogs. Of the 28 eyes, 8 cases were determined to be oxacillin-resistant, 7 were resistant to three or more classes of antibiotics, and 13 cases were either susceptible to all antibiotics tested or resistant to one class of antibiotics. Breeds represented in this study included Shih Tzu (n = 7), Boston Terrier (n = 4), Chihuahua (n = 2), French Bulldog (n = 2), Maltese (n = 2), Pomeranian (n = 2), Pug (n = 2), mixed breed (n = 2), and one dog of each: Cavalier King Charles Spaniel, Cocker Spaniel, Dachshund, Miniature Pinscher, and Yorkshire Terrier.
Patient characteristics
A history of recent general anesthesia (<30 days) was significantly more common in dogs diagnosed with MDR vs. non-MDR bacterial keratitis (46.7 vs. 7.7%, respectively; P = 0.038). Further, non-brachycephalic breeds were more commonly affected by MDR vs. non-MDR bacterial keratitis (53.3 vs. 15.4%), although this difference was not statistically significant (P = 0.055). Before referral, dogs diagnosed with MDR bacterial keratitis received a greater number of medications and daily eye drops when compared to non-MDR cases [4 (1–6) vs. 2 (0–10) and 14 (2–16) vs. 6 (0–53), respectively], although these differences were not statistically significant (P ≥ 0.095). Other parameters pertinent to “patient characteristics” are summarized in Table 1.
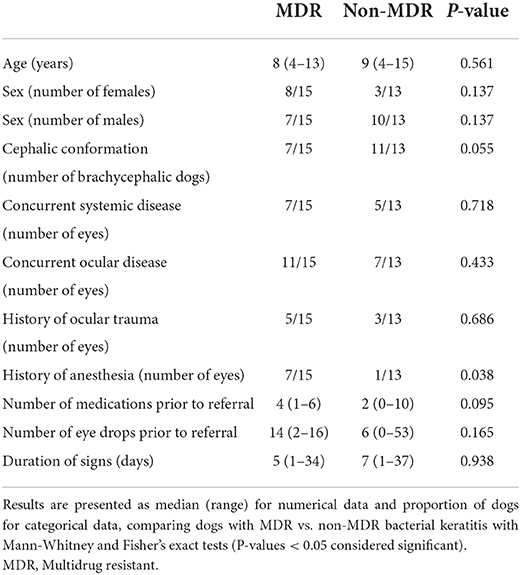
Table 1. Summary of selected characteristics from 28 canine patients and associated corneal ulcers infected with Staphylococcus pseudintermedius.
Clinical findings
Median depth of corneal ulcers was smaller in MDR vs. non-MDR bacterial keratitis (50 vs. 78%) but this difference was not statistically significant (P = 0.065). Other parameters pertinent to “clinical findings” are summarized in Table 2.
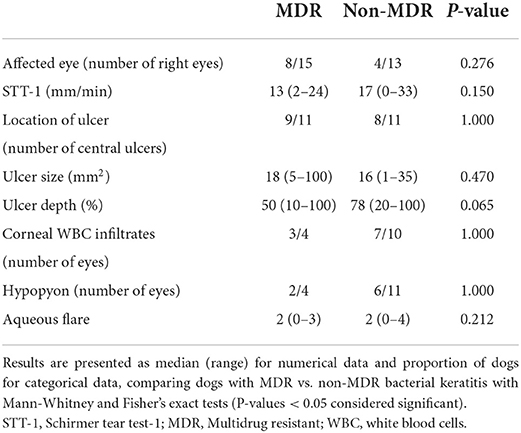
Table 2. Summary of clinical findings from 28 canine patients and associated corneal ulcers infected with Staphylococcus pseudintermedius.
Clinical outcomes
Groups did not differ in the number of eyes for which the corneal infection was considered susceptible to the empirical antibiotic(s) prescribed at the initial visit (P = 0.102). No significant differences were noted between both groups (MDR vs. non-MDR) in the need for end-stage procedure such as enucleation (6.7 vs. 15.4%, respectively; P = 1.000), nor in the likelihood of success in maintaining globe (93.3 vs. 84.6%, respectively; P = 0.583) or maintaining vision (80 vs. 76.9%, respectively; P = 1.000). Further, median follow-up time was significantly longer in dogs with MDR vs. non-MDR bacterial keratitis (47 vs. 29 days, P = 0.009). Other parameters pertinent to “clinical outcomes” are summarized in Table 3.
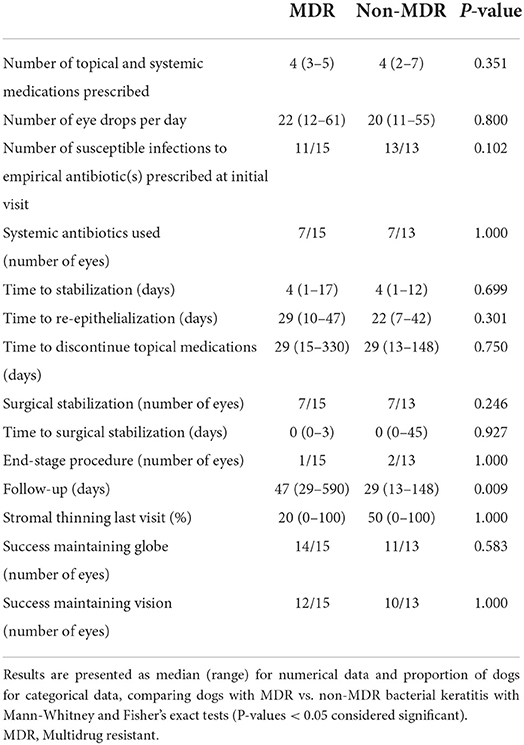
Table 3. Summary of therapeutics and clinical outcomes from 28 canine patients and associated corneal ulcers infected with Staphylococcus pseudintermedius.
Discussion
Resistant infections from Staphylococcus pseudintermedius represent a serious challenge in health care for human and veterinary patients (12, 27). The virulence of Staphylococcus pseudintermedius can be explained by several factors such as enzyme secretion (e.g., coagulase, thermonuclease, and proteases), toxin secretion (e.g., exfoliative toxin, enterotoxin, and cytotoxins) (28), and biofilm formation at the infection site (29). Specific to dogs with bacterial keratitis, a recent study showed that the biofilm-formation ability and rates of virulence gene carriage and antibiotic resistance were higher in the isolates of Staphylococcus pseudintermedius from dogs with keratitis compared with those from the healthy dogs (30). In humans, the reported detrimental effects of resistant strains include higher morbidity and mortality rates (31), limited antimicrobial treatment options for physicians, prolonged hospitalization times and increased healthcare costs (32, 33). Specific to the field of ophthalmology, corneal infections from bacteria with higher MICs were shown to cause prolonged healing time and greater scar formation (20, 21). In veterinary medicine, a similar trend toward resistant bacterial isolates has been reported in the last two decades (34–36), although the actual impact on clinical outcomes of veterinary patients is poorly understood.
In the present study, no noticeable differences were noted in the clinical appearance of bacterial keratitis from MDR or non-MDR strains of Staphylococcus pseudintermedius in dogs. Specifically, there were no significant discrepancies in the location, size or depth of the ulcer, presence/absence of corneal infiltrates or severity of the anterior chamber reaction (aqueous flare and hypopyon). In the absence of clinical cues, MDR strains can only be recognized by collecting samples for culture and susceptibility testing, however results are often delayed by several days to weeks. Clinicians could also increase their level of suspicion for MDR strains by identifying risk factors in their canine patients. Here, a major risk factor for MDR infections was recent anesthesia prior to developing corneal ulceration, possibly related to anesthesia-associated reductions in lacrimation (37)—lowering corneal protection and levels of natural defenses in the tear film (e.g., antimicrobial peptides)—as well as the presence of environmental contaminants in the vicinity of the anesthetized dog that could result in nosocomial infection (15). Another risk factor for MDR infections is a higher number of daily ophthalmic medications/eyedrops prior to referral, likely owing to natural selection of resistant bacteria from repeated antibiotic use (38) and the influence of corticosteroid use on bacterial keratitis (39, 40). Of note, recent anesthetic event and prior topical therapy were also described as predisposing factors for infectious keratitis in previous canine studies (39, 40), but the present study further emphasizes the potential for resistant infections with these two risk factors. Interestingly, while brachycephalic dogs are often considered at higher risk to develop corneal ulceration when compared to non-brachycephalic dogs (39–41), the present study showed a non-significantly higher rate of MDR infections in corneas of non-brachycephalic dogs. This finding is consistent with a recent report by Hewitt et al. who showed that two non-brachycephalic breeds (Pomeranian, Saint Bernard) were significantly more likely to yield a MDR isolate when compared to mixed breed dogs (5).
Ultimately, MDR diagnosis in dogs with bacterial keratitis did not seem to influence the overall clinical outcome, with no significant differences between MDR and non-MDR eyes in terms of successful vision-preservation or successful globe-preservation. Nonetheless, time to re-epithelialization and follow-up times were longer in dogs with MDR infections, two findings that possibly translate into more labor intensive treatment and increased financial burden from the owners' perspective. On the same note but in another clinical field (dermatology), the outcome of dogs with skin infections did not differ whether the responsible pathogen (Staphylococcus pseudintermedius) was methicillin-resistant or methicillin-susceptible, although some cases of MRSP pyoderma took longer to treat (42). In ophthalmology and dermatology, antibiotics can be administered directly at the site of infection, providing higher concentrations than ones that which can be achieved systemically. All of the clinical breakpoint interpretations used in this study were for systemic antimicrobial use; there are no clinical breakpoints that have been established for topical antibiotic use in humans or animals. Because of this, CLSI does not recommend the routine use of AST to determine topical therapies. The higher concentrations achieved with topical therapy could be therapeutic in vivo despite antimicrobial resistance demonstrated in vitro, but further work is needed to support this hypothesis. In a recent canine study, one third of corneal ulcers infected with fluoroquinolone-resistant β-hemolytic Streptococcus achieved a good outcome despite initial treatment with a topical fluoroquinolone (40).
Importantly, the authors continue to strongly advise corneal culture and susceptibility testing for canine patients with suspected infectious keratitis. First, corneal cultures help identify the causative pathogen and therefore provide clinicians with valuable insight on drug(s) of choice and clinical prognosis. Second, MDR identification provides critical data for healthcare professionals to reduce the risk of nosocomial infections (e.g., disinfection and sterilization of contact areas) (15) and to ensure surveillance of any emergent antibacterial resistance. Last, owners of canine patients with MDR strains should ideally be kept informed given the zoonotic potential of Staphylococcus pseudintermedius (13, 14).
The present study was limited to cases where Staphylococcus pseudintermedius was determined to be the only primary pathogen identified to reduce variability and better focus on the variable of interest (i.e., MDR status). However, results of this work cannot be extrapolated to canine patients with polymicrobial infections (up to 21% of cases) (3, 43) or dogs with infectious keratitis from Streptococcus spp., Pseudomonas spp., or other common bacterial pathogens reported for this condition. A recent study by Tsvetanova et al. (40) showed that melting corneal ulcers associated with Pseudomonas spp. were significantly more likely to result in globe loss than other bacterial pathogens (40), and it would be interesting to know whether MDR further complicated clinical outcomes or not. Owing to the retrospective nature of the work, another study limitation is the lack of information on other factors that may have influenced clinical outcomes, such as owner compliance, albumin levels in tear film (44), or virulence of specific bacterial strains (45).
In conclusion, resistant strains of Staphylococcus pseudintermedius did not appear to negatively impact clinical outcomes in dogs with bacterial keratitis, although treatment duration and healing time may be prolonged when compared to non-resistant strains.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the animal study because the work was a retrospective analysis of bacterial cultures collected in dogs as part of the patients' standard of care. Written informed consent for participation was not obtained from the owners because the work was a retrospective analysis of bacterial cultures collected in dogs as part of the patients' standard of care.
Author contributions
LS conceptualized and designed the study in consultation with AM, AK, and RA. AM and LS performed the experiments. LS and AK analyzed the data. All authors wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1083294/full#supplementary-material
References
1. Kern TJ. Ulcerative keratitis. Vet Clin North Am Small Anim Pract. (1990) 20:643–66. doi: 10.1016/S0195-5616(90)50055-8
2. Ollivier FJ. Bacterial corneal diseases in dogs and cats. Clin Tech Small Anim Pract. (2003) 18:193–8. doi: 10.1016/S1096-2867(03)90016-8
3. Tolar EL, Hendrix DV, Rohrbach BW, Plummer CE, Brooks DE, Gelatt NK, et al. Evaluation of clinical characteristics and bacterial isolates in dogs with bacterial keratitis: 97 cases (1993-2003). J Am Vet Med Assoc. (2006) 228:80–5. doi: 10.2460/javma.228.1.80
4. Auten CR, Urbanz JL, Dees DD. Comparison of bacterial culture results collected via direct corneal ulcer vs. conjunctival fornix sampling in canine eyes with presumed bacterial ulcerative keratitis. Vet Ophthalmol. (2020) 23:135–40. doi: 10.1111/vop.12698
5. Hewitt JS, Allbaugh RA, Kenne DE, Sebbag L. Prevalence and antibiotic susceptibility of bacterial isolates from dogs with ulcerative keratitis in Midwestern United States. Front Vet Sci. (2020) 7:583965. doi: 10.3389/fvets.2020.583965
6. Prado MR, Rocha MF, Brito EH, Girão MD, Monteiro AJ, Teixeira MF, et al. Survey of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Fortaleza, Ceará, Brazil. Vet Ophthalmol. (2005) 8:33–7. doi: 10.1111/j.1463-5224.2005.04061.x
7. Ventrella G, Moodley A, Grandolfo E, Parisi A, Corrente M, Buonavoglia D, et al. Frequency, antimicrobial susceptibility and clonal distribution of methicillin-resistant Staphylococcus pseudintermedius in canine clinical samples submitted to a veterinary diagnostic laboratory in Italy: a 3-year retrospective investigation. Vet Microbiol. (2017) 211:103–6. doi: 10.1016/j.vetmic.2017.09.015
8. Suter A, Voelter K, Hartnack S, Spiess BM, Pot AS. Septic keratitis in dogs, cats, and horses in Switzerland: associated bacteria and antibiotic susceptibility. Vet Ophthalmol. (2018) 21:66–75. doi: 10.1111/vop.12480
9. Wang L, Pan Q, Zhang L, Xue Q, Cui J, Qi C, et al. Investigation of bacterial microorganisms in the conjunctival sac of clinically normal dogs and dogs with ulcerative keratitis in Beijing, China. Vet Ophthalmol. (2008) 11:145–9. doi: 10.1111/j.1463-5224.2008.00579.x
10. Ekapopphan D, Srisutthakarn A, Moonarmart W, Buddhirongawatr R, Bangphoomi N. Identification and antimicrobial susceptibility of microorganisms isolated from severe corneal ulcers of dogs in Thailand. J Vet Med Sci. (2018) 80:1259–65. doi: 10.1292/jvms.18-0045
11. Bannoehr J, Guardabassi L. Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity. Vet Dermatol. (2012) 23:253–66. doi: 10.1111/j.1365-3164.2012.01046.x
12. Lynch SA, Helbig KJ. The complex diseases of Staphylococcus pseudintermedius in canines: where to next? Vet Sci. (2021) 8:11. doi: 10.3390/vetsci8010011
13. Bhooshan S, Negi V, Khatri KP. Staphylococcus pseudintermedius: an undocumented, emerging pathogen in humans. GMS Hyg Infect Control. (2020) 15:Doc32. doi: 10.3205/dgkh000367
14. Røken M, Iakhno S, Haaland AH, Wasteson Y, Bjelland MA. Transmission of methicillin-resistant Staphylococcus spp. from Infected Dogs to the Home Environment and Owners. Antibiotics. (2022) 11:11050637. doi: 10.3390/antibiotics11050637
15. Gentile D, Allbaugh RA, Adiguzel MC, Kenne DE, Sahin O, Sebbag L, et al. Bacterial cross-contamination in a veterinary ophthalmology setting. Front Vet Sci. (2020) 7:571503. doi: 10.3389/fvets.2020.571503
16. Starlander G, Börjesson S, Grönlund-Andersson U, Tellgren-Roth C, Melhus A. Cluster of infections caused by methicillin-resistant Staphylococcus pseudintermedius in humans in a tertiary hospital. J Clin Microbiol. (2014) 52:3118–20. doi: 10.1128/JCM.00703-14
17. Viegas FM, Santana JA, Silva BA, Xavier RGC, Bonisson CT, Câmara JLS, et al. Occurrence and characterization of methicillin-resistant Staphylococcus spp. in diseased dogs in Brazil. PLoS ONE. (2022) 17:e0269422. doi: 10.1371/journal.pone.0269422
18. Varges R, Penna B, Martins G, Martins R, Lilenbaum W. Antimicrobial susceptibility of Staphylococci isolated from naturally occurring canine external ocular diseases. Vet Ophthalmol. (2009) 12:216–20. doi: 10.1111/j.1463-5224.2009.00701.x
19. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18:268–81. doi: 10.1111/j.1469-0691.2011.03570.x
20. Wilhelmus KR, Abshire RL, Schlech AB. Influence of fluoroquinolone susceptibility on the therapeutic response of fluoroquinolone-treated bacterial keratitis. Arch Ophthalmol. (2003) 121:1229–33. doi: 10.1001/archopht.121.9.1229
21. Chen A, Prajna L, Srinivasan M, Mahalakshmi R, Whitcher JP, McLeod S, et al. Does in vitro susceptibility predict clinical outcome in bacterial keratitis? Am J Ophthalmol. (2008) 145:409–12. doi: 10.1016/j.ajo.2007.11.004
22. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacterial Isolated from Animals. Wayne, PA: Clinical and Laboratory Standards Institute Supplement VET08 (2018).
23. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests for Bacteria Isolated from Animals: CLSI Supplement VET01S; Replaces VET01-S2. Wayne, PA: Clinical and Laboratory Standards Institute (2015).
25. Clinical and Laboratory Standards Institute. Understanding Susceptibility Test Data as a Component of Antimicrobial Stewardship in Veterinary Settings. CLSI Report VET09. Wayne, PA: Clinical and Laboratory Standards Institute (2019).
26. Sweeney MT, Lubbers BV, Schwarz S, Watts JL. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J Antimicrob Chemother. (2018) 73:1460–3. doi: 10.1093/jac/dky043
27. Carroll KC, Burnham CD, Westblade FL. From canines to humans: clinical importance of Staphylococcus pseudintermedius. PLoS Pathog. (2021) 17:e1009961. doi: 10.1371/journal.ppat.1009961
28. Maali Y, Badiou C, Martins-Simões P, Hodille E, Bes M, Vandenesch F, et al. Understanding the Virulence of Staphylococcus pseudintermedius: a major role of pore-forming toxins. Front Cell Infect Microbiol. (2018) 8:e00221. doi: 10.3389/fcimb.2018.00221
29. Singh A, Walker M, Rousseau J, Weese SJ. Characterization of the biofilm forming ability of Staphylococcus pseudintermedius from dogs. BMC Vet Res. (2013) 9:93. doi: 10.1186/1746-6148-9-93
30. Wang Z, Guo L, Li J, Li J, Cui L, Dong J, et al. Antibiotic resistance, biofilm formation, and virulence factors of isolates of staphylococcus pseudintermedius from healthy dogs and dogs with keratitis. Front Vet Sci. (2022) 9:e903633. doi: 10.3389/fvets.2022.903633
31. Karam G, Chastre J, Wilcox MH, Vincent LJ. Antibiotic strategies in the era of multidrug resistance. Crit Care. (2016) 20:136. doi: 10.1186/s13054-016-1320-7
32. Cerceo E, Deitelzweig SB, Sherman BM, Amin NA. Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb Drug Resist. (2016) 22:412–31. doi: 10.1089/mdr.2015.0220
33. Medina E, Pieper DH. Tackling threats and future problems of multidrug-resistant bacteria. Curr Top Microbiol Immunol. (2016) 398:3–33. doi: 10.1007/82_2016_492
34. Morris DO, Rook KA, Shofer FS, Rankin CS. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003-04). Vet Dermatol. (2006) 17:332–7. doi: 10.1111/j.1365-3164.2006.00536.x
35. Jones RD, Kania SA, Rohrbach BW, Frank LA, Bemis AD. Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1,772 samples (2001-2005). J Am Vet Med Assoc. (2007) 230:221–7. doi: 10.2460/javma.230.2.221
36. Ruscher C, Lübke-Becker A, Semmler T, Wleklinski CG, Paasch A, Soba A, et al. Widespread rapid emergence of a distinct methicillin- and multidrug-resistant Staphylococcus pseudintermedius (MRSP) genetic lineage in Europe. Vet Microbiol. (2010) 144:340–6. doi: 10.1016/j.vetmic.2010.01.008
37. Herring IP, Pickett JP, Champagne ES, Marini M. Evaluation of aqueous tear production in dogs following general anesthesia. J Am Anim Hosp Assoc. (2000) 36:427–30. doi: 10.5326/15473317-36-5-427
38. Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology. (2012) 119:1420–4. doi: 10.1016/j.ophtha.2012.01.016
39. Guyonnet A, Desquilbet L, Faure J, Bourguet A, Donzel E, Chahory S, et al. Outcome of medical therapy for keratomalacia in dogs. J Small Anim Pract. (2020) 61:253–8. doi: 10.1111/jsap.13118
40. Tsvetanova A, Powell RM, Tsvetanov KA, Smith KM, Gould JD. Melting corneal ulcers (keratomalacia) in dogs: A 5-year clinical and microbiological study (2014-2018). Vet Ophthalmol. (2021) 24:265–78. doi: 10.1111/vop.12885
41. Hindley KE, Groth AD, King M, Graham K, Billson MF. Bacterial isolates, antimicrobial susceptibility, and clinical characteristics of bacterial keratitis in dogs presenting to referral practice in Australia. Vet Ophthalmol. (2016) 19:418–26. doi: 10.1111/vop.12325
42. Bryan J, Frank LA, Rohrbach BW, Burgette LJ, Cain CL, Bemis AD, et al. Treatment outcome of dogs with meticillin-resistant and meticillin-susceptible Staphylococcus pseudintermedius pyoderma. Vet Dermatol. (2012) 23:361–8.e365. doi: 10.1111/j.1365-3164.2012.01034.x
43. McKeever JM, Ward DA, Hendrix HDV. Comparison of antimicrobial resistance patterns in dogs with bacterial keratitis presented to a veterinary teaching hospital over two multi-year time periods (1993-2003 and 2013-2019) in the Southeastern United States. Vet Ophthalmol. (2021) 24:653–8. doi: 10.1111/vop.12897
44. Sebbag L, Broadbent V, Kenne D, Perrin A, Mochel J. Albumin in tears modulates bacterial susceptibility to topical antibiotics in ophthalmology. Front Med (Ophthalmology). (2021) 8:663212. doi: 10.3389/fmed.2021.663212
Keywords: bacterial keratitis, canine, corneal ulceration, clinical outcome, multi-drug resistance, risk factors, Staphylococcus spp.
Citation: Mauer AN, Allbaugh RA, Kreuder AJ and Sebbag L (2022) Impact of multi-drug resistance on clinical outcomes of dogs with corneal ulcers infected with Staphylococcus pseudintermedius. Front. Vet. Sci. 9:1083294. doi: 10.3389/fvets.2022.1083294
Received: 28 October 2022; Accepted: 09 November 2022;
Published: 24 November 2022.
Edited by:
Ping Yang, Nanjing Agricultural University, ChinaReviewed by:
Francesca Paola Nocera, Università degli Studi di Napoli Federico II, ItalyJuan Alberto Corbera, University of Las Palmas de Gran Canaria, Spain
Copyright © 2022 Mauer, Allbaugh, Kreuder and Sebbag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachel A. Allbaugh, YWxsYmF1Z2hAaWFzdGF0ZS5lZHU=; Lionel Sebbag, bGlvbmVsLnNlYmJhZ0BtYWlsLmh1amkuYWMuaWw=
 Ashley N. Mauer
Ashley N. Mauer Rachel A. Allbaugh1*
Rachel A. Allbaugh1* Amanda J. Kreuder
Amanda J. Kreuder Lionel Sebbag
Lionel Sebbag