- 1Pigeon Breeding Laboratory, Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China
- 2College of Life Science and Food Engineering, Hebei University of Engineering, Handan, China
Introduction: It is of great importance to find antibiotic alternatives that can improve poultry performance and enhance immunity. Plant-derived extracts and their concentrates are natural bioactive compounds that are widely and effectively applied as the antibiotic alternatives in animal industries. This study was conducted to investigate the effects of Yucca schidigera extract (YSE) on growth performance, serum biochemical parameters, immune function, intestinal morphology, and microbiota diversity of young pigeons.
Methods: A total of 120 healthy White King pigeons (28 days old) with similar weight were randomly assigned to 4 treatments with six replicate cages. Each of the pigeons from 4 treatments was orally administrated with 0 (control), 5, 10, and 15 mg YSE per day, respectively.
Results: The results showed that orally supplemental YSE had no significant effects (P > 0.05) on the growth performance and immune organ index of pigeons. The serum total protein and IgM contents in the 10 mg YSE group were significantly higher (P < 0.05) than those in the control group. Supplemental 10 and 15 mg YSE significantly lowered the level of serum total cholesterol (P < 0.05) and increased (P < 0.05) the villi height in the jejunum compared with the control group. Supplemental 5 and 10 mg YSE significantly decreased (P < 0.05) the level of serum alanine aminotransferase and the crypt depth in the ileum compared with the control group. The beta diversity showed a distinct difference in the ileum microbial composition between the control and the 10 mg YES group. YSE supplementation enriched the bacterial genera Sulfurospirillum, Solobacterium, Desulfovibrio, Desulfobulbus, Lactococcus, Parabacteroides, Acidaminococcus, Acetobacter, and Streptococcus. Additionally, Enterococcus genus showed a significantly negative correlation with serum alanine aminotransferase (R = −0.618, P = 0.043). Actinomyces genus showed a significantly negative correlation with cholesterol (R = −0.633, P = 0.036). Turicibacter genus showed a significantly positive correlation with villi height in the jejunum (R = 0.751, P = 0.008).
Discussion: In conclusion, orally supplemental YSE could improve serum biochemistry, immunoglobulin contents, and intestinal morphology by regulating the composition of microbial community in the ileum of young pigeons.
Introduction
Currently, domestic pigeons (Columba livia) are raised as a sort of commercial meat-type poultry in China (1), with a yearly production of around 680 million squabs, accounting for 80% of worldwide production (2). However, pigeons are the reservoirs of Salmonella and Trichomonas gallinae, and young pigeons during 1–3 months old are very susceptible to infection because of the weaning stress (3, 4). Although antibiotics have played a critical role in preventing pathogen infection and promoting animal production, the food and drug security organizations have called for restricting the use of antibiotics and chemotherapies in poultry, livestock, and aquaculture because of their adverse consequence on the development of antimicrobial resistance, and thereby posing a potential threat to human health (5–7). Thus, it is urgent to find antibiotic alternatives to improve growth performance, enhance immunity, and reduce stress of young pigeons (8). Plant-derived extracts and their concentrates are natural bioactive compounds that are widely and effectively applied as the antibiotic alternatives in animal industries (9, 10). Our team previously conducted an in vitro study on the functions of eleven plant extracts, and finally found that Yucca schidigera extract (YSE) can effectively inhibit the number of Escherichia coli, Salmonella and Trichomonas, and promote the growth of Lactobacillus. Hence, we decided to further explore the potential role of YSE in young pigeons.
As a medicinal plant extract, YSE has been widely used as a natural additive in food industries. It has numerous health benefits, including hypocholesterolemic, hypoglycemic, anti-inflammatory, antioxidant, anticancer, and immunostimulatory properties (11). Besides, YSE application in aquatic animals could effectively improve feed utilization, growth rate, and immune responses (12). And, it has also been demonstrated that in the livestock and poultry industries, YSE plays a significant role in promoting growth and production performance and reducing ammonia emission (13, 14). It was reported that dietary supplemental YSE could improve intestinal barrier function and reduce nitrogen emission in sow and weaned piglets (15, 16). Furthermore, Yucca schidigera administration increased the digestion of essential minerals, improved intestinal health and reduced the growth of pathogenic bacteria in broilers (17–19). However, whether supplemental YSE has benefits on young pigeons' intestinal health and immune system function has not been examined. We hypothesized that adding an appropriate level of YSE could improve the intestinal health and immune function of young pigeons. The current study's objective was to assess the effects of supplemental YSE on growth performance, serum biochemical indices, immune system function, and intestinal health, which could provide a theoretical basis for determining the appropriate requirment of YSE for young pigeons.
Materials and methods
Animals and treatment procedure
The study was carried out in accordance with the guidelines set by the Animal Care and Use Committee (permit number: SYXK-2017-0005) of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (IAHVM-BAAFS), Beijing, China. The protocols were approved by the Animal Care and Use Committee of IAHVM-BAAFS.
Birds, diets, and experimental design
A total number of 120, 28-day-old healthy White King pigeons with similar weight and size were randomly divided into four treatments, each consisting of six replicate cages (five birds per cage). The four groups were the control group, 5 mg YSE addition group (YSE5), 10 mg YSE addition group (YSE10), and 15 mg YSE addition group (YSE15). The pigeons were fed a mixed grain diet, and the compound feed was composed of maize, pea, soybean meal, wheat, and sorghum. The feed, sand, and water were available ad libitum. The ingredients and nutrient levels are shown in Table 1. Birds were raised in a room under a 16 h light and 8 h darkness cycle, and the temperature was kept at 22 ± 6°C during the entire experimental period. After acclimatization for 1 week, the control group was orally administrated with 1mL/bird/day 0.9% NaCl solution, and the other groups were orally administrated with 1mL/bird/day YSE solution. All the birds were gavaged by a pipette with sterilized tips at 10 am once a day after feeding 3h throughout the entire experiment. The YSE was dissolved in 0.9% NaCl solution with the final concentration of 5, 10, and 15 mg/mL. We selected the tested levels of YSE according to the published studies about the roles of YSE in quails and hens (11, 20). Based on their dietary YSE levels (50, 100, and 150 mg/kg) and daily feed intakes (0.09 kg/d for hens), the levels of YSE (5, 10, and 15 mg/d per pigeon) were used in this study. The experiment lasted 14 days.
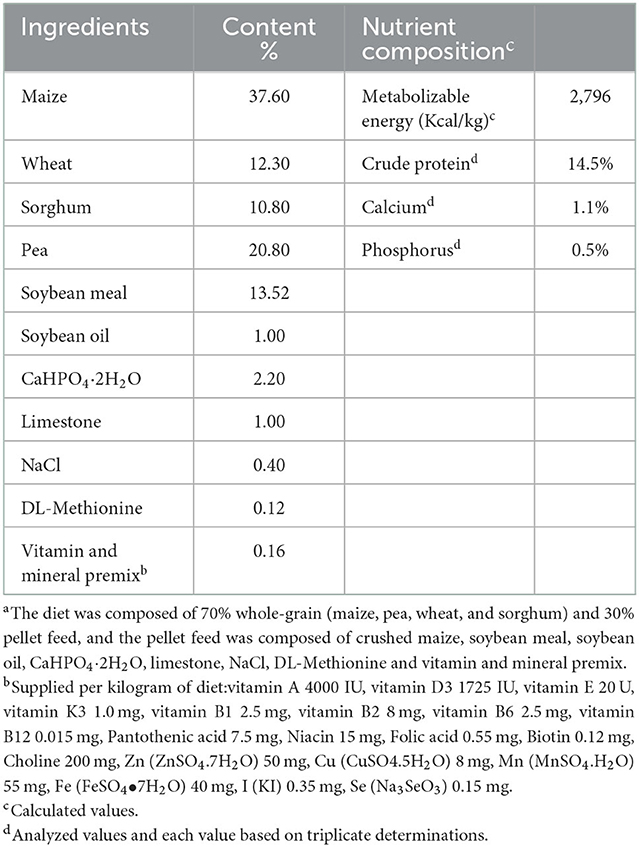
Table 1. Ingredient compositions and nutrient levels of basal diets for young pigeons (on as-fed basis)a.
YSE product and the main ingredients
The YSE (20.0% purity) was purchased from a commercial supplier (Xi'an Ruidi Biotechnology Co., Ltd., Xi'an, China). It is in the form of brown yellow powder with good water solubility. The main ingredients of YSE include the total flavonoids, phenols, polysaccharides, and saponins, and they were determined by the biochemicals kits (Norminkoda Biotechnology Co., Ltd. Wuhan, China). The contents of total flavonoids, phenols, polysaccharides, and saponins were 8.02 ± 0.058, 8.22 ± 0.240, 566.5 ± 7.071, 27.3 ± 0.853 mg/g, respectively based on triplicate assays.
Growth performance
Before weighing, the pigeons fasted for 12 h. Feed intake was recorded weekly. Pigeons, feed, and sand were weighed at 8 am on the first and last days of the experiment. Initial body weight (IBW), final body weight (FBW), average daily gain (ADG), average daily feed intake (ADFI), and the ratio of feed:gain (F/G) were measured and calculated at the end of the experiment.
Sample collection
On the 14th day of the experiment, six pigeons from each treatment (one bird from each replicate) were randomly selected. And, 7 mL of blood was collected aseptically from the wing portal vein and centrifuged at 3,000 × g for 10 min at 4°C, and the serum was obtained and stored at −20°C for analysis of serum biochemical indexes. After collecting the blood, pigeons were killed by jugular exsanguination. The immune organs including the spleen, bursa, and thymus were collected and weighed after stripping fat, and the immune organ index was expressed as the organ weight relative to the living body weight (g/kg). Next, ~2-cm long segments of duodenum (the midpoint), jejunum (preceding the Meckel's diverticulum) and ileum (preceding the ileocecal junction) were separated and washed in PBS, fixed in 4% paraformaldehyde solution and kept at 4°C for histological morphology analysis. The ileal contents were collected aseptically and stored at −80°C for DNA extraction.
Serum biochemical parameters and immunoglobulins contents
The levels of total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total triglyceride (TG), total cholesterol (T-CHO), albumin (ALB) and total protein (TP) in serum were determined by the commercial testing kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum immunoglobulins concentrations, including immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) were determined by ELISA tests (Bird IgA/IgG/IgM ELISA Quantitation kits, Shanghai Enzyme-linked Biotechnology Co., Ltd. Shanghai, China) following the manufacturer's protocol.
Intestinal histomorphology
Paraformaldehyde-fixed intestinal tissues were embedded in paraffin. Serial slices (5 μm) were prepared using haematoxylin and eosin staining and observed for histomorphology under an Olympus optical microscope using ProgRes CapturePro software, version 2.7 (Jenoptik, Germany). The villus height (VH) and crypt depth (CD) were measured from 10 randomly selected villi and associated crypts with one section per bird at 40 × magnification. The ratio of VH:CD was then calculated from the above measurements by dividing the villus height by the crypt depth.
DNA extraction and PCR amplification
The ileal contents were collected in sterilized plastic tubes and then immediately stored in liquid nitrogen to investigate the intestinal microflora. Total bacterial DNA was extracted from the collected digest samples using an E.Z.N.A. Stool DNA Kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer's protocol. The final DNA concentration and purity were determined by a NanoDrop 2000 UV–Vis spectrophotometer (Thermo Scientific, Wilmington, USA), and DNA quality was checked by 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacterial 16S rRNA gene were amplified with primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) (21, 22) using a thermocycler PCR system (GeneAmp 9700, ABI, Vernon, CA, USA). The PCR reactions were conducted with a high-fidelity polymerase using the following program: 3 min of denaturation at 95°C, 27 cycles of 30 s at 95°C, 30 s for annealing at 55°C, and 45 s for elongation at 72°C, and a final extension at 72°C for 10 min. PCR reactions were performed in triplicate according to previous studies (22, 23). The PCR products were visualized in a 2% agarose gel, and then purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA), and quantified using QuantiFluor-TMST (Promega, USA) according to the manufacturer's protocol (23).
Illumina MiSeq sequencing of 16S rRNA gene
Purified amplicons were pooled in equimolar amounts and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols (24) by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (accession number: PRJNA81814).
Processing of sequencing data
Raw fastq files were quality filtered by Trimmomatic software and merged by FLASH software (version 1.2, https://ccb.jhu.edu/sofware/FLASH/index.shtml) (22, 23). Operational taxonomic units (OTUs) were clustered with a 97% similarity cutoff using UPARSE (version 7.1, https://www.drive5.com/uparse/) analysis (25). The taxonomy of each 16S rRNA gene sequence was analyzed by the Ribosomal Database Project Classifier algorithm (version 11.1) against the Silva (SSU128) 16S rRNA database using a confidence threshold of 70% (The result of species annotation has the highest reliability using 70% confidence threshold) (26).
Statistical analyses
All data were analyzed using SPSS statistical software (version 20.0) and presented as means ± standard error. Differences were evaluated by one-way ANOVA, and comparisons between multiple groups were analyzed by post-hoc Duncan's multiple range test. Polynomial orthogonal contrasts were used to determine the linear and quadratic responses of dependent variables to YSE levels. The P < 0.05 were considered to be significant, and 0.05 ≤ P < 0.10 was defined as a tendency.
The beta diversity was analyzed by principal coordinate analysis diagram (PCoA) and hierarchical clustering by unweighted pair group method with arithmetic average (UPGMA). Kruskal-Wallis tests with Wilcoxon test were used to visualize the highly significant abundant taxa at phylum and genus levels among the groups.
Linear discriminant analysis for effect size (LEfSe) identifies features that are statistically different among biological classes (Kruskal-Wallis sum-rank test, P < 0.05). It then performs additional tests to assess whether these differences are consistent with respect to expected biological behavior (Wilcoxon ran-sum test, P < 0.05). Lastly, the effect relevance is estimated by the linear discriminant analysis (LDA) effect size. Pearson correlation coefficients between the top 20 abundant bacterial genera and serum biochemical indexes or intestinal morphology were displayed on the heat map.
Results
Growth performance
The data of growth performance were shown in Table 2. Supplementation of different levels of YSE had no significant effects (P > 0.05) on IBW, FBW, ADG, ADFI and F/G of the young pigeons.

Table 2. The effect of supplemental different levels of YSE on the growth performance of young pigeonsa.
Immune organ index
The immune organ indexes, including spleen, bursa, and thymus indexes were shown in Table 3. No significant differences were observed in spleen and thymus indexes among the four groups (P > 0.05).
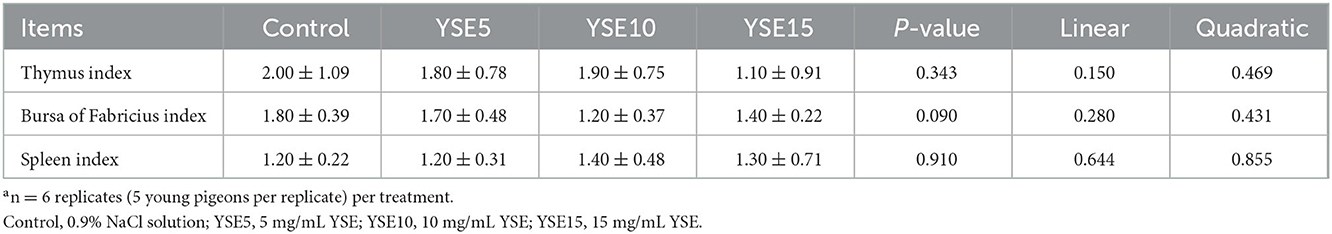
Table 3. Effects of supplemental different levels of YSE on immune organ index of young pigeons (g/kg)a.
Serum biochemical parameters and immunoglobulin contents
The comparison of serum biochemical parameters was shown in Table 4. The content of TP increased linearly and quadratically with the increase of YSE supplementation (P < 0.05), and pigeons gavaged with 10 mg YSE had a higher level of TP than the the control pigeons (P < 0.05). The level of T-CHO decreased significantly linearly with the increase of YSE addition (P < 0.05), and pigeons gavaged with 5 and 10 mg YSE had lower levels of T-CHO than the control pigeons (P < 0.05). The level of ALT decreased quadratically with the increase of YSE supplementation (P < 0.05), and compared with the control group, orally supplemental 10 and 15 mg YSE significantly decreased the levels of ALT (P < 0.05). There was no significant difference in the activities of T-SOD, T-AOC, ALB, TG, and AST among the four groups (P > 0.05).
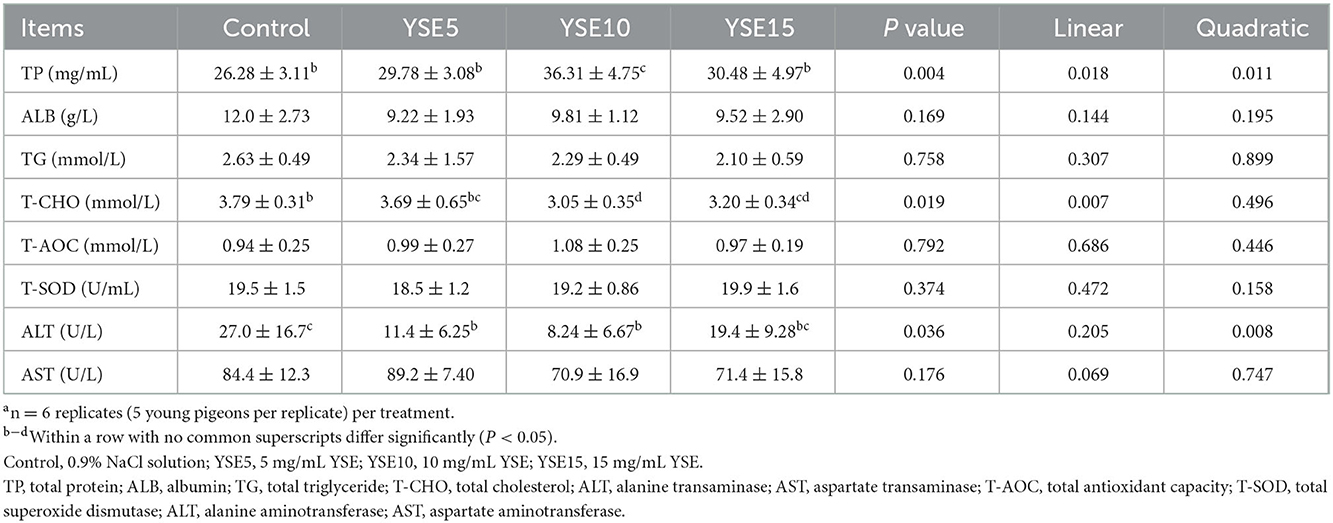
Table 4. Effects of supplemental different levels of YSE on serum biochemical parameters of young pigeonsa.
A comparison of serum IgA, IgG, and IgM activity was shown in Table 5. The content of IgM increased significantly linearly with the increase of YSE supplementation (P < 0.05), and pigeons gavaged with 10 mg YSE had a higher serum IgM content than the the control pigeons (P < 0.05). There was no significant difference in the serum IgG and IgA contents among the four groups (P > 0.05).
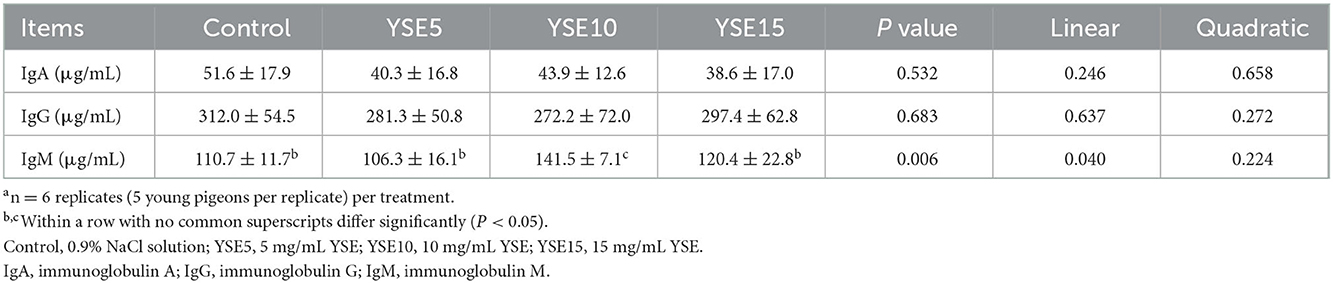
Table 5. Effects of supplemental different levels of YSE on serum immunoglobulins of young pigeonsa.
Histomorphological parameters in the small intestine
The duodenum, jejunum, and ileum morphology, including VH, CD, and VH/CD were shown in Table 6. In the duodenum, no significant differences were observed in the VH, CD, and VH/CD among groups (P > 0.05). The VH in the jejunum increased linearly with the increase of YSE supplementation (P < 0.05), and compared with the control group, orally supplemental 10 and 15 mg YSE increased significantly the VH (P < 0.05). There were no significant differences in the CD and VH/CD among groups (P > 0.05). In the ileum, the CD decreased quadratically the increase of YSE supplementation (P < 0.05), and pigeons gavaged with 5 mg and 10 mg YSE had lower CD than the the control pigeons (P < 0.05). No significant differences were observed between the VH and VH/CD among the four groups (P > 0.05).
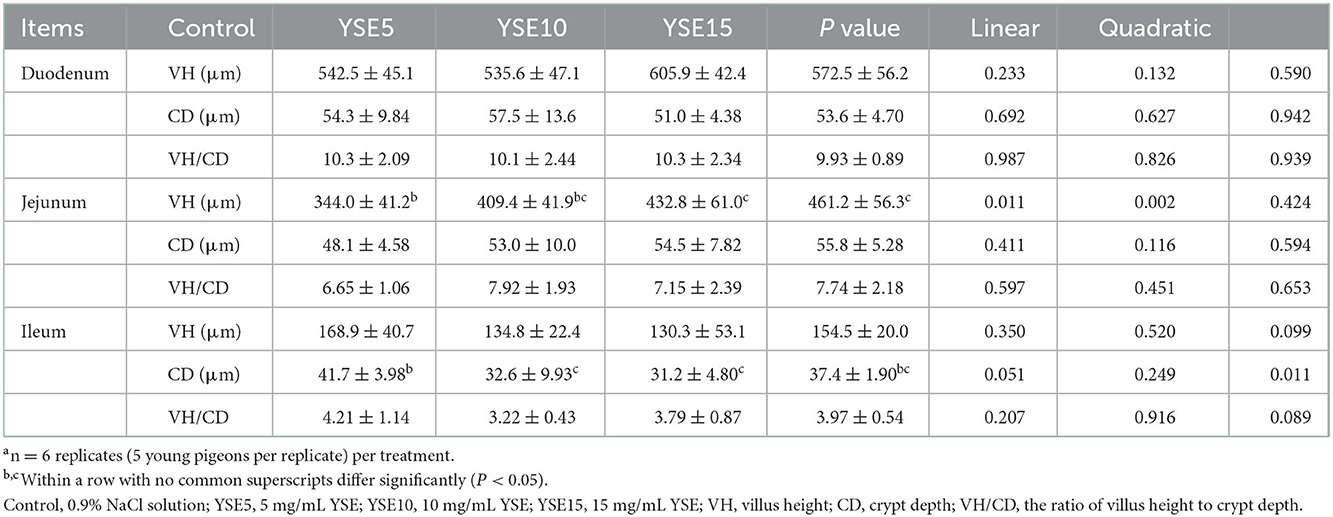
Table 6. Effect of supplemental different levels of YSE on the histomorphological parameters in the small intestine of young pigeonsa.
Intestinal microflora
According to the results of different levels of YSE on serum biochemical and intestinal morphology-related indicators, pigeons gavaged with 10 mg YSE had significantly higher levels of TP and IgM than other three groups. And, pigeons gavaged with 10 and 15 mg YSE had a lower level of T-CHO and a higher VH in jejunum than the control group. Besides, pigeons gavaged with 5 and 10 mg YSE had lower levels of ALT and CD in ileum than the control group. Thus, based on the beneficial effects of YSE on the serum biochemical parameters, immunoglobulin contents, and intestinal morphology, the level of 10 mg YSE was chosen for further experiment. In addition, the ileum is the longest segment among the three small intestinal segments in young pigeons, so the contents of the ileum were chosen to analyze the microbial diversity and composition.
Histogram analysis-phylum and genus level
As shown in Figure 1A, Firmicutes, Proteobacteria, and Actinobacteria were the main phyla in the ileum, and they accounted for 98.02% in the control group and 98.47% in the YSE10 group. At the phylum level, the analysis results showed that orally supplemental YSE increased the relative abundances of Patescibacteria and Desulfobacterota in the ileum of young pigeons (P < 0.05) (Figure 2A).
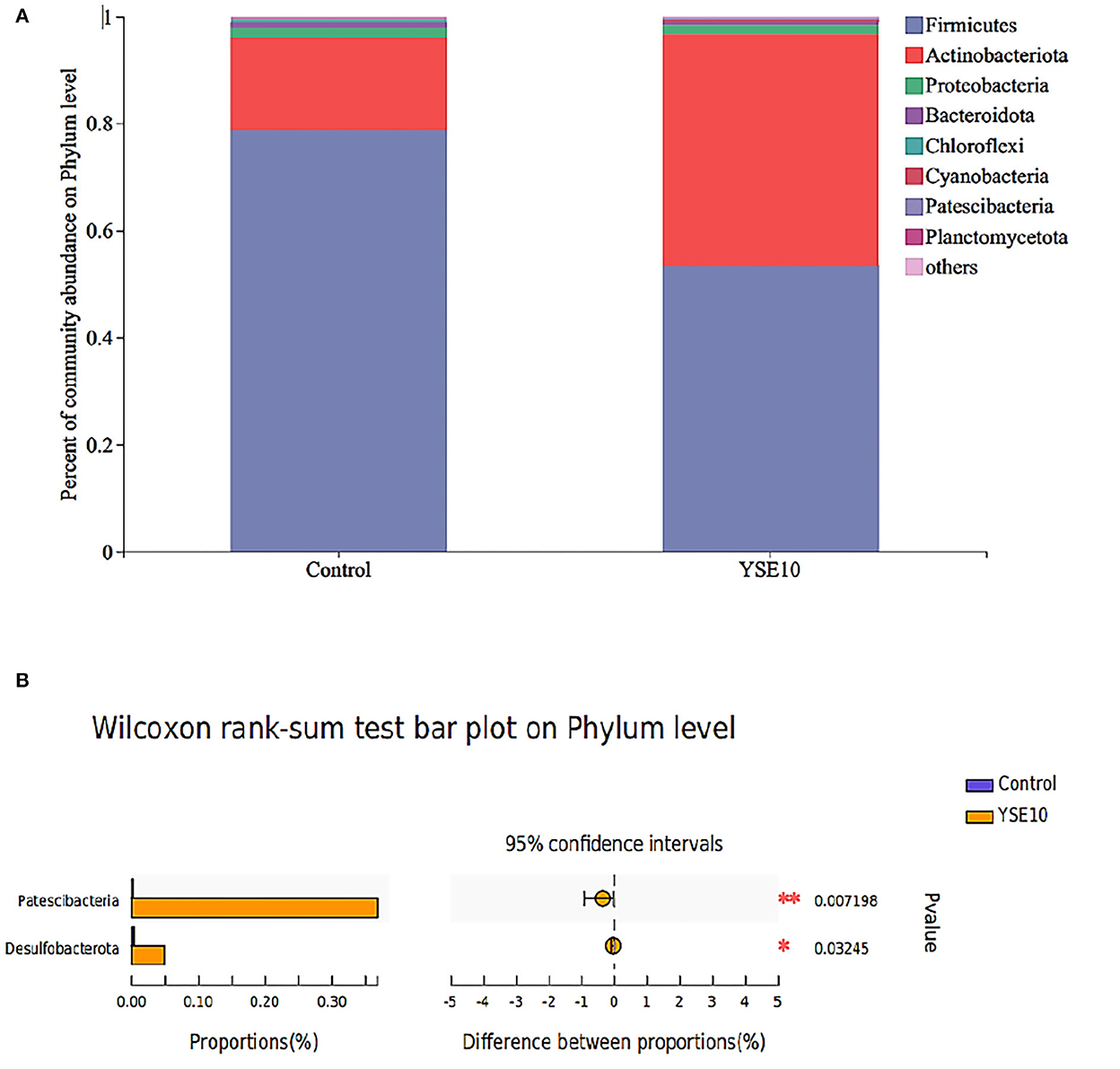
Figure 1. The composition of microbiota and the comparison of differences at the level of phylum in the ileum of young pigeons. (A) Percent of community abundance. (B) Wilcoxon rank-sum test bar plot. The relative abundances of phylum in all samples' sequence <0.01 are classified as “others”. Control, 0.9% NaCl solution; YSE10, 10 mg/mL YSE.
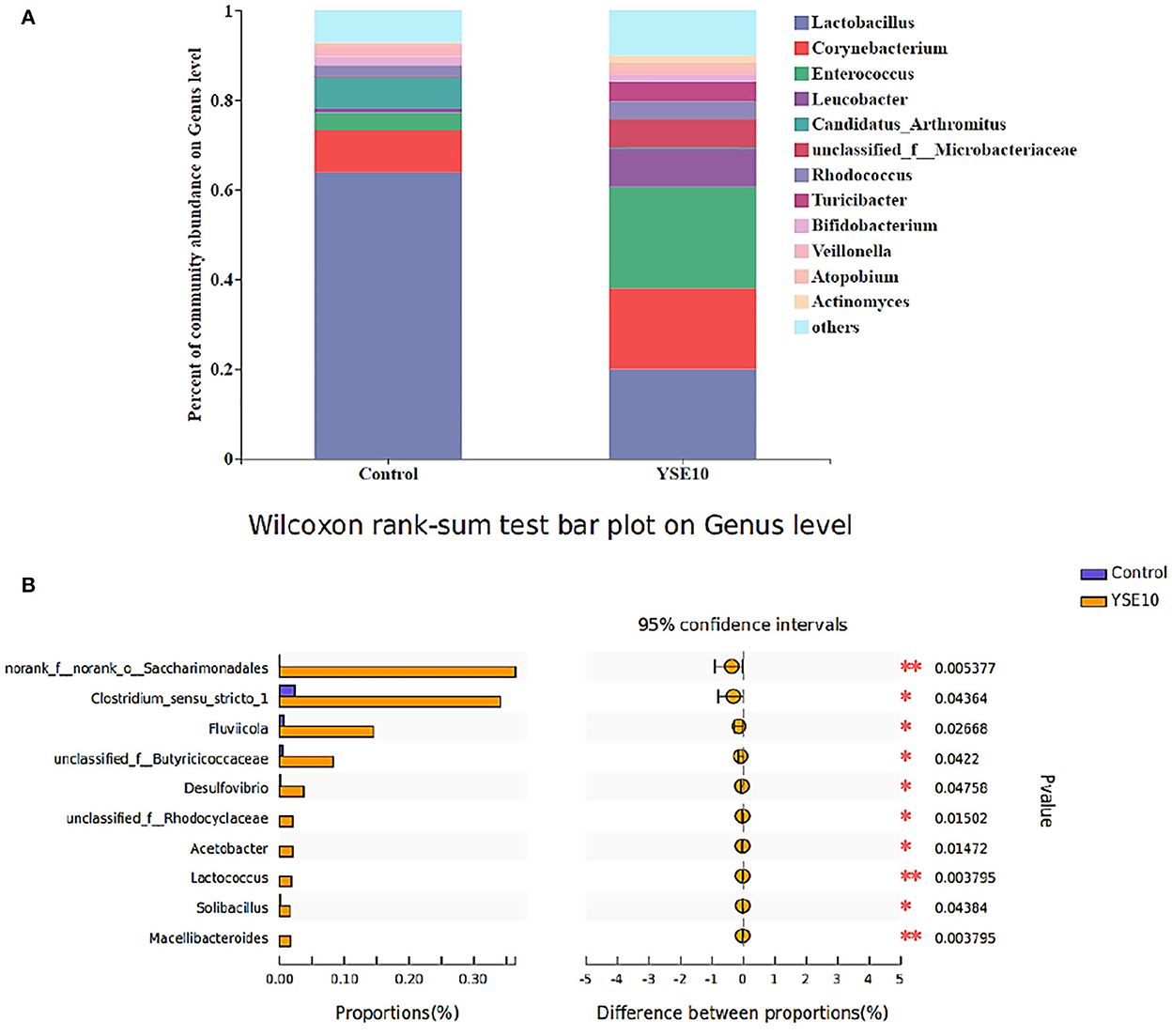
Figure 2. The composition of microbiota and the comparison of differences at the level of genus in the ileum of young pigeons. (A) Percent of community abundance. (B) Wilcoxon rank-sum test bar plot. The relative abundances of genus in all samples' sequence <0.01 are classified as “others”. Control, 0.9% NaCl solution; YSE10, 10 mg/mL YSE.
As shown in Figure 1, the dominant genera of the ileum were Entrococcus, Leucobacter, and unclassified_f_ _Microbacteriaceae. At the genus level, the analysis results showed that compared with the control group, the relative abundances of norank_f__norank_o__Saccharimonadales, Clostridium_sensu_stricto_1, unclassified_f__Butyricicoccaceae, Arcobacter, Desulfovibrio, Acetobacter, unclassified_f_ _Rhodocyclaceae, unclassified_f__Carnobacteriaceae, Solibacillus, and Lactococcus were increased in YSE10 group (P < 0.05, Figure 2B).
Alpha diversity of microbial community
The abundance-based coverage estimator (ACE) index, Chao index, Shannon index, and Simpson index were selected to analyze alpha diversity within a community (Figure 3). There were no significant differences observed among the groups in the ACE index, Chao index, Shannon index, and Simpson index (P > 0.05).
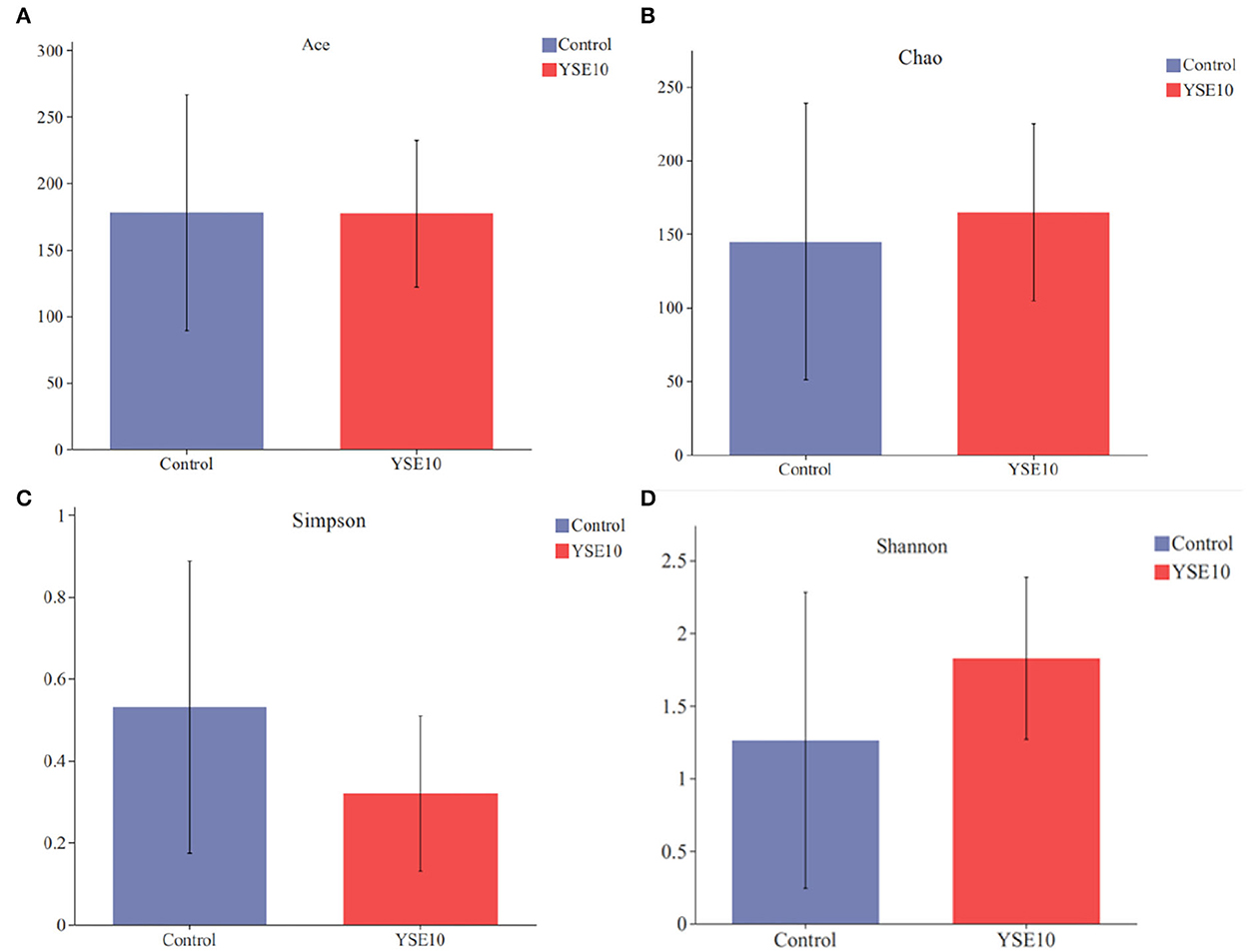
Figure 3. Alpha diversity of two groups. (A) Ace index; (B) Chao index; (C) Simpson index; (D) Shannan index. Control, 0.9% NaCl solution; YSE10, 10 mg/mL YSE.
Beta diversity of microbial community
Principal co-ordinates analysis (PCoA) was used to analyze the effects of YSE on the beta diversity of the microbial community based on the unweighted UniFrac metric, which was calculated based on the genus and relative abundance of the samples (Figure 4). The result displayed that a significant difference was observed in the diversity of the microbial communities between the control and the YSE10 group (P = 0.033).
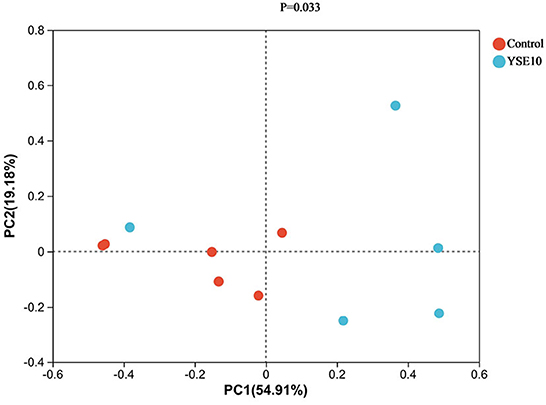
Figure 4. Principal component analysis of operational taxonomic units. PC1, principal coordinate (1) PC2, principal coordinate; (2) Control, 0.9% NaCl solution; YSE10, 10 mg/mL YSE.
We used the LDA to determine the taxa that most likely explain the differences between the YSE10 and control samples (Figure 5). Significant differences in microbiota were found when the LEfSe algorithm was used on genus-level OTU tables to determine taxa that characterize each biological class. The result confirmed the bacterial genera Sulfurospirillum, Solobacterium, Desulfovibrio, Desulfobulbus, Lactococcus, Parabacteroides, Acidaminococcus, Acetobacter, and Streptococcus were significantly enriched in the YSE10 group compared with those in the control.
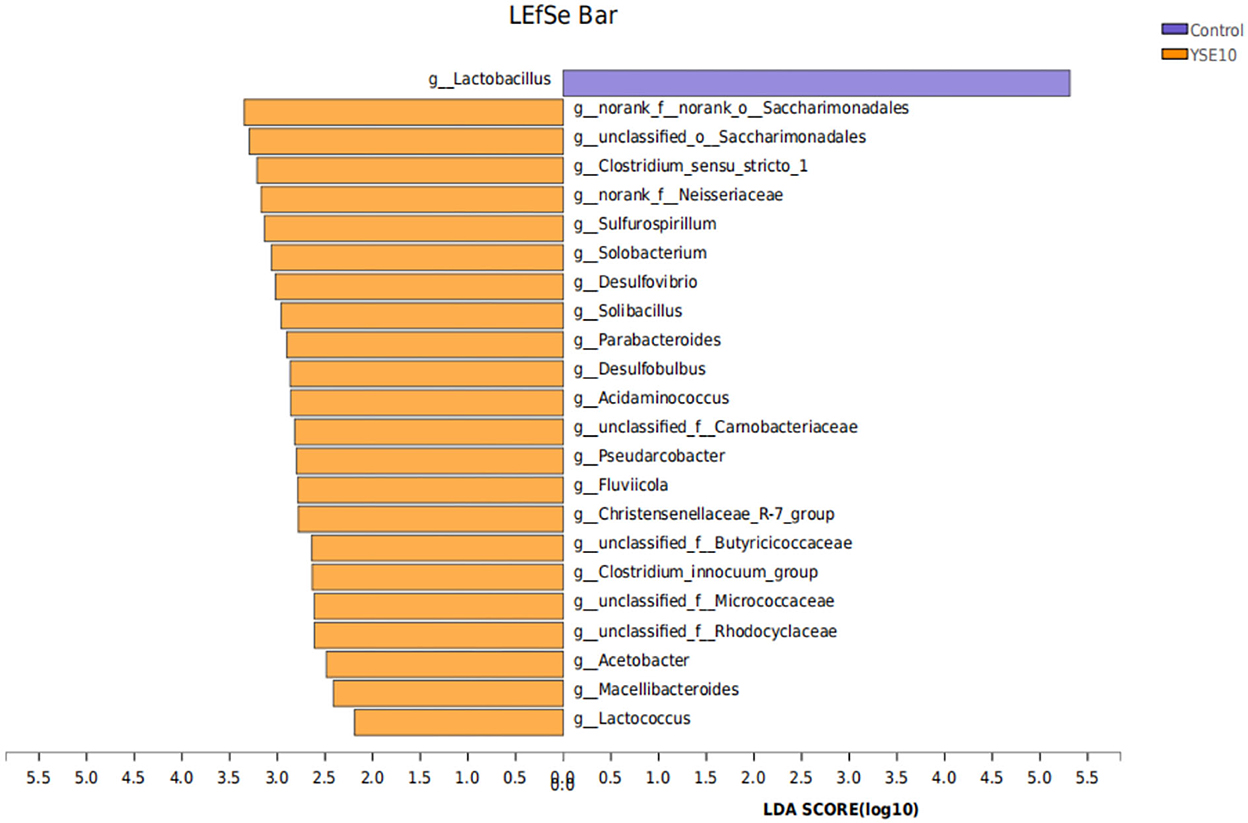
Figure 5. LDA Effect Size (LEfSe) algorithm was used on genus-level OTU tables to determine taxa that best characterize each biological class. Control, 0.9% NaCl solution; YSE10, 10 mg/mL YSE. LDA, linear discriminant analysis.
A heatmap was used to assess the correlations among the bacterial genera, and serum biochemical parameters, immunoglobulin, and intestinal morphological structure by color shades (Figure 6). The correlation heatmap showed that Enterococcus had a significantly negative correlation with ALT (R = −0.618, P = 0.043), but Lactobacillus had a significantly positive correlation with ALT (R = 0.682, P = 0.021). A significantly negative correlation was observed between Actinomyces and cholesterol (R = −0.633, P = 0.036), and a significantly positive correlation was observed between Turicibacter and the VH in jejunum (R = 0.751, P = 0.008).
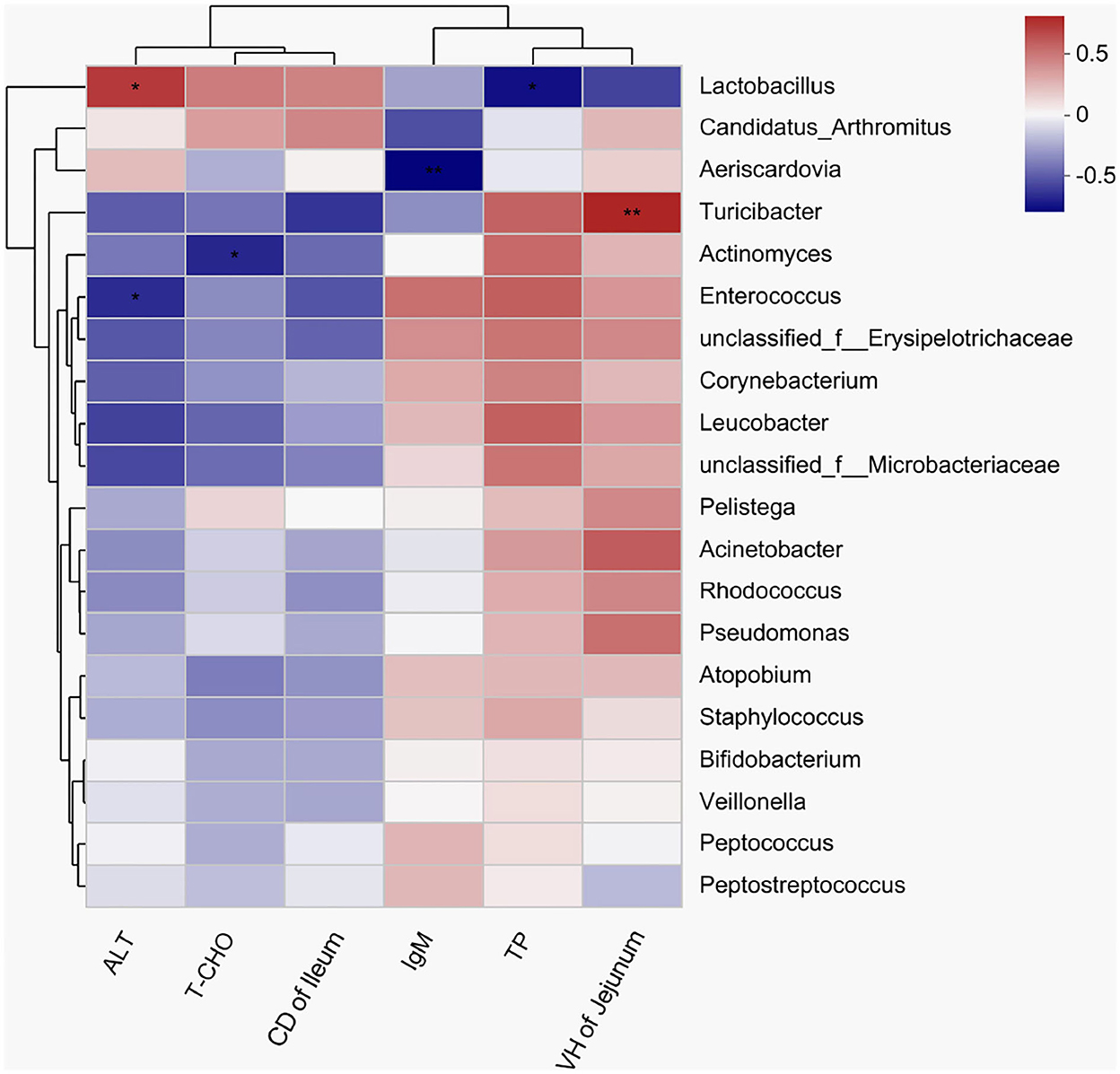
Figure 6. A heatmap showing correlations among the bacterial genera, serum biochemical indexes, and intestinal morphology. X and Y axes are environmental factors and genera, respectively. Red indicates a positive correlation; blue indicates a negative correlation. P < 0.05 is marked with “*”, and P < 0.001 is marked with “**”. ALT, alanine aminotransferase; T-CHO, total cholesterol; IgM, immunoglobulin M; TP, total protein.
Discussion
Different from other poultry, pigeons are fed crop milk and soaked grains by parents in a mouth-to-mouth manner for nearly 28 days after hatching (27, 28). Thereafter, they gradually learn to feed independently, and some pigeons with good performance were reserved as the breeding pigeons. However, the changes of feeding patterns and the separation from breeding parents could bring a “weaning” stress for the 28-day-old young pigeons. And at this time, young pigeons are vulnerable to pathogenic microorganisms such as Salmonella and Trichomonas, leading to a high mortality (29). Therefore, the young pigeons at 28 days reserved for breeding were focused on in the current study, so as to improve the health condition of young pigeons from the foundation. Previous studies demonstrated that dietary YSE supplementation improved the growth performance of broiler chickens during 1–42 days (30, 31). Unfortunately, the growth performance of young pigeons after adding YSE did not improve significantly in present study, and similar results had been reported in laying quails and hens (20, 32). The body weight of pigeons changed very little after 28 days, implying that the 28-day-old pigeons have reached the mature body weight (33). Perhaps YSE might be more effective in growth performance of the fast-growing broilers or squabs, not in the young pigeons. Besides, it may be caused by low levels of saponins (2.7%) and phenolic compounds (0.8%) in our YSE that could not improve digestion, absorption, and utilization of nutrients in the digestive tract (11).
In addition, the thymus, bursa of Fabricius and spleen are important immune organs of birds, and the development of immune organs has an important impact on the immune function of the body (34). However, in our study, YSE did not result in any discernable effects on the weight of these organs. It is possible that the experiment duration (14 days) is too short to assess the effects of YSE on immune organs. In addition to immune organs, immune molecules also play important roles in regulating immune reactions among the immune system (35). Serum IgG, IgM, and IgA contents are essential immune molecules to measure the functional status of the humoral immune system (36). In our study, the serum IgM level in the YSE10 group was significantly higher than in the control group. Dietary supplemental YSE significantly increased the IgG level observed in laying hens and broilers (11, 37). These results indicated that YSE seems to be more advantageous in production of immunoglobulin. The main task of immunoglobulin in the immune system is to neutralize microorganisms and toxins via the nonspecific defense system of body. The steroidal saponin is one of the main chemical constituents of Y. schidigera extract, which is most studied and has been shown to be capable of enhancing the natural immune response by stimulating the secretion of cytokines and triggering antibody production and humoral and cellular immune responses (38). Most notably, whether the high level of polysaccharides in our YSE could have functions in regulating immunity needs further demonstration. At present, there are few published studies on the role of polysaccharides from YSE. Therefore, the actual mechanisms involved in the protective effects of YSE remain undefined.
Many serum biochemical indicators can reflect the changes in nutrient metabolism and organ functions of animals (39). Serum glucose, TG and TP contents commonly reflect sugar, fat and protein metabolism in animals (40). In this study, orally supplemental 10 mg YSE increased the serum TP contents, indicating the optimum level of YSE may raise the utilization of TP and effectively inhibit the catabolism of reserve TP in young pigeons. The transaminases of ALT and AST are broadly present in mitochondria and have become the primary screening tool for detecting liver injury (41). The serum ALT level in the YSE10 group was significantly lower than that in the control group. The low level of liver enzymes may be attributable to the repair of hepatic injury in birds administered with the lemongrass essential oil (42). Lemongrass has a cytoprotective effect because of its phenolic components, and fortunately, YSE is also rich in phenolic components (43, 44). We inferred that YSE may have a positive effect on improving liver function. Further investigations are expected to confirm the speculation. The concentration of serum T-CHO was used to evaluate the serum lipid level, which could reflect the health status of the body. Saponins can combine with cholesterol to form water-insoluble complexes, accelerating the conversion rate of cholesterol in the liver to bile acid, and the content of cholesterol in the serum was reduced (45). Previous studies showed that dietary supplemental YSE decreased the serum T-CHO concentration in laying hens and quails (20, 46). In this study, orally supplemental 10 and 15 mg YSE decreased serum T-CHO concentration in young pigeons, which is consistent with previous studies. In addition, it was reported that dietary supplemental YSE could increase the villus height and the villus height/crypt depth ratio of the intestine in broiler chickens (30, 47). Our current study showed that orally supplemental YSE increased the villus height in the jejunum, and reduced the crypt depth in the ileum. It is concluded that YSE may promote intestinal development and enhance the integrity of the intestinal epithelium of young pigeons.
Gut microflora has important effects on the regulation of nutrient utilization, growth performance, energy homeostasis, and immune function by cooperating with the development of the gut system of the host (48, 49). It was reported that Yucca schidigera could reduce the diarrhea rate by the modification of the intestinal microbiota in weaned piglets (50). In this study, we found that oral supplementation of YSE enriched the abundance of Patescibacteria and Desulfobacterota phyla. In addition, the genera Sulfurospirillum, Solobacterium, Desulfovibrio, Desulfobulbus, Lactococcus, Parabacteroides, Acidaminococcus, and Acetobacter were markedly increased in the YSE group. Desulfococcus and Desulfovibrio are the main components of sulfate-reducing bacteria (SRB), and Desulfovibrio may possess a variety of enzymes acting together to resist oxidative stress (51). Meantime, the correlation results showed that Actinomyces was negatively correlated with cholesterol, and serum cholesterol in the YSE group decreased. The enzyme obtained from the mycelium of Actinomyces lavendula can effectively decrease cholesterol content (52). These results indicated that the YSE may reduce serum cholesterol levels by regulating the abundance of Actinomyces genus in the ileum. Actinobacteria can produce a wide range of secondary metabolites with structural diversity and are also the source of two-thirds of natural antibiotics and a series of anti-cancer, anti-worm, antifungal and immunosuppressive drugs (53). Moreover, various studies investigated that the Desulfobacterota that has been found in the environment, but the proportion in intestinal microorganisms is relatively small and its function is seldom reported (54). In our study, Enterococcus was negatively correlated with ALT. Dietary supplementation with Enterococcus faecium R1 significantly reduced the activity of AST in the blood, so as to attenuate liver injury in LPS-challenged piglets (55). Therefore, our study indicated that the YSE supplementation has important influences on the intestinal microbial composition, and the serum biochemical parameters may alter as the changes in microbial communities.
Conclusions
In conclusion, orally supplemental 10 mg YSE could enhance the humoral immune function via increasing IgM content, regulate the serum biochemical indexes by decreasing the contents of alanine aminotransferase and total cholesterol, and improve intestinal health by promoting intestinal morphology and altering the beta diversity and composition of ileal microbiota. Therefore, YSE can be developed as a feed additive to boost the immunity and intestinal healthy of young pigeons.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was reviewed and approved by Animal Care and Use Committee (Permit number: SYXK-2017-0005) of the Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences (IAHVM-BAAFS), Beijing, China; Animal Care and Use Committee of IAHVM-BAAFS.
Author contributions
XS drafted the manuscript and conducted the experiment. XL and SD assisted the experiment. ZW and DL conceived the idea. YS modified the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The present study was supported by the Pigeon Breeding Laboratory, Institute of Animal Husbandry and Veterinary Medicine, Beijing Academy of Agriculture and Forestry Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xu QQ, Zhang XY, Zou XT, Dong XY. Effects of in ovo injection of L-histidine on hatch performance and post-hatch development in domestic pigeons (Columba livia). Poult Sci. (2019) 98:3194–203. doi: 10.3382/ps/pez046
2. Jiang SG, Pan NX, Chen MJ, Wang XQ, Yan HC, Gao CQ. Effects of dietary supplementation with dl-methionine and dl-methionyl-dl-methionine in breeding pigeons on the carcass characteristics, meat quality and antioxidant activity of squabs. Antioxidants (Basel). (2019) 8:435. doi: 10.3390/antiox8100435
3. Ji F, Zhang D, Shao Y, Yu X, Liu X, Shan D, et al. Changes in the diversity and composition of gut microbiota in pigeon squabs infected with Trichomonas gallinae Sci Rep. (2020) 10:19978. doi: 10.1038/s41598-020-76821-9
4. Wang Z, Li X, Du S, Sun X, Huang J, Shao Y. Protective effects of Zinc on Salmonella invasion, intestinal morphology and immune response of young pigeons infected with Salmonella enterica serovar Typhimurium. Biol Trace Elem Res. (2022) 200:4817–27. doi: 10.1007/s12011-021-03057-7
5. El-Deep MH, Amber KA, Eid YZ, Alrashood ST, Khan HA, Sakr MS, et al. The influence of dietary chicken egg lysozyme on the growth performance, blood health, and resistance against Escherichia coli in the growing rabbits' cecum. Front Vet Sci. (2020) 7:579576. doi: 10.3389/fvets.2020.579576
6. El-Deep MH, Amber KA, Elgendy S, Dawood MAO, Elwakeel EM, Paray BA. Oxidative stress, hemato-immunological, and intestinal morphometry changes induced by ochratoxin A in APRI rabbits and the protective role of probiotics. Environ Sci Pollut Res Int. (2020) 27:35439–48. doi: 10.1007/s11356-020-09837-3
7. Saleh AA, Paray BA, Dawood MAO. Olive cake meal and bacillus licheniformis impacted the growth performance, muscle fatty acid content, and health status of broiler chickens. Animals (Basel). (2020) 10:695. doi: 10.3390/ani10040695
8. Dawood MAO, Koshio S, Esteban MÁ. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev Aquac. (2017) 10:950–74. doi: 10.1111/raq.12209
9. Makkar HP, Francis G, Becker K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal. (2007) 1:1371–91. doi: 10.1017/S1751731107000298
10. Lillehoj H, Liu Y, Calsamiglia S, Fernandez-Miyakawa ME, Chi F, Cravens RL, et al. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet Res. (2018) 49:76. doi: 10.1186/s13567-018-0562-6
11. Alagawany M, Abd El-Hack ME, El-Kholy MS. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ Sci Pollut Res Int. (2016) 23:6774–82. doi: 10.1007/s11356-015-5919-z
12. Elbialy ZI, Salah AS, Elsheshtawy A, Rizk M, Abualreesh MH, Abdel-Daim MM, et al. (2021). Exploring the multimodal role of Yucca schidigera extract in protection against chronic ammonia exposure targeting: growth, metabolic, stress and inflammatory responses in Nile tilapia (Oreochromis niloticus L). Animals (Basel). (2021) 11:2072. doi: 10.3390/ani11072072
13. Földešiová M, BaláŽi A, Chrastinová L, Pivko J, Kotwica J, Harrath AH, et al. Yucca schidigera can promote rabbit growth, fecundity, affect the release of hormones in vivo and in vitro, induce pathological changes in liver, and reduce ovarian resistance to benzene. Anim Reprod Sci. (2017) 183:66–76. doi: 10.1016/j.anireprosci.2017.06.001
14. Saeed M, Arain MA, Naveed M, Alagawany M, Abd El-Hack ME, Bhutto ZA, et al. Yucca schidigera can mitigate ammonia emissions from manure and promote poultry health and production. Environ Sci Pollut Res Int. (2018) 25:35027–33. doi: 10.1007/s11356-018-3546-1
15. Chen M, Wolin MJ. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. (1979) 38:72–7. doi: 10.1128/aem.38.1.72-77.1979
16. Fan X, Xiao X, Chen D, Yu B, He J, Yu J, et al. (2021). Yucca schidigera extract decreases nitrogen emission via improving nutrient utilisation and gut barrier function in weaned piglets. J Anim Physiol Anim Nutr (Berl). 106:1036–45. doi: 10.1111/jpn.13647
17. Ebru OE, Erdem E, Afsinya NU, Torlak E. Effect of Yucca schidigera spraying in different litter materials on some litter traits and breast burn of broilers at the fifth week of production. Kafkas Univ Vet Fak Derg. (2013) 19:749–753.
18. Patra AK, Stiverson J, Yu Z. Effects of quillaja and yucca saponins on communities and select populations of rumen bacteria and archaea, and fermentation in vitro. J Appl Microbiol. (2012) 113:1329–40. doi: 10.1111/j.1365-2672.2012.05440.x
19. Sun D, Jin X, Camerlink I, Tong M, Su J, Zhao F, et al. Effects of Yucca schidigera extract on growth performance and antioxidative function of small intestine in broilers. J Anim Physiol Anim Nutr (Berl). (2019) 103:738–46. doi: 10.1111/jpn.13067
20. Kaya S, Erdogan Z, Erdogan S. Effect of different dietary levels of Yucca schidigera powder on the performance, blood parameters and egg yolk cholesterol of laying quails. J Vet Med A Physiol Pathol Clin Med. (2003) 50:14–7. doi: 10.1046/j.1439-0442.2003.00487.x
21. Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. (2018) 8:568. doi: 10.1038/s41598-017-18904-8
22. Liu C, Wu H, Liu S, Chai S, Meng Q, Zhou Z. Dynamic alterations in yak rumen bacteria community and metabolome characteristics in response to feed type. Front Microbiol. (2019) 10:1116. doi: 10.3389/fmicb.2019.01116
23. Zhang L, Wu W, Lee YK, Xie J, Zhang H. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. (2018) 9:48. doi: 10.3389/fmicb.2018.00048
24. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. (2012) 6:1621–4. doi: 10.1038/ismej.2012.8
25. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. (2013) 10:996–8. doi: 10.1038/nmeth.2604
26. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. (2013) 41:D590–6. doi: 10.1093/nar/gks1219
27. Sales J, Janssens GPJ. Nutrition of the domestic pigeon (Columba livia domestica). Worlds Poult Sci J. (2003) 59:221–32. doi: 10.1079/WPS20030014
28. Xie WY, Fu Z, Pan NX, Yan HC, Wang XQ, Gao CQ. Leucine promotes the growth of squabs by increasing crop milk protein synthesis through the TOR signaling pathway in the domestic pigeon (Columba livia). Poult Sci. (2019) 98:5514–24. doi: 10.3382/ps/pez296
29. Kaczorek-Łukowska E, Sowińska P, Franaszek A, Dziewulska D, Małaczewska J, Stenzel T. Can domestic pigeon be a potential carrier of zoonotic Salmonella? Transbound Emerg Dis. (2021) 68:2321–33. doi: 10.1111/tbed.13891
30. Alfaro DM, Silva AVF, Borges SA, Maiorka FA, Vargas S, Santin E. Use of Yucca schidigera extract in broiler diets and its effects on performance results obtained with different coccidiosis control methods. J Appl Poult Res. (2007) 16:248–54. doi: 10.1093/japr/16.2.248
31. Sahoo SP, Kaur D, Sethi AP, Sharma A, Chandra M. Evaluation of Yucca schidigera extract as feed additive on performance of broiler chicks in winter season. Vet World. (2015) 8:556–60. doi: 10.14202/vetworld.2015.556-560
32. Chepete HJ, Xin H, Mendes LB Li H, Bailey TB. Ammonia emission and performance of laying hens as affected by different dosages of Yucca schidigera in the diet. J Appl Poultry Res. (2012) 21:522–30. doi: 10.3382/japr.2011-00420
33. Gao CQ, Yang JX, Chen MX, Yan HC, Wang XQ. Growth curves and age-related changes in carcass characteristics, organs, serum parameters, and intestinal transporter gene expression in domestic pigeon (Columba livia). Poult Sci. (2016) 95:867–77. doi: 10.3382/ps/pev443
34. Wang Y, Yi L, Zhao ML, Wu JQ, Wang MY, Cheng XC. Effects of zinc-methionine on growth performance, intestinal flora and immune function in pigeon squabs. Br Poult Sci. (2014) 55:403–8. doi: 10.1080/00071668.2014.919375
35. Work KA, Gibbs MA, Friedman EJ. The immune system game. Am Biol Teach. (2015) 77:382–90. doi: 10.1525/abt.2015.77.5.11
36. Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. (2004) 197:179–91. doi: 10.1111/j.0105-2896.2004.0109.x
37. Ayoub MM, Ahmed HA, Sadek KM, Alagawany M, Abd El-Hack ME, Othman S I, et al. Effects of liquid yucca supplementation on nitrogen excretion, intestinal bacteria, biochemical and performance parameters in broilers. Animals (Basel). (2019) 9:1097. doi: 10.3390/ani9121097
38. Gümüş R, Imik H. The effect of Yucca schidigera powder added to lamb feed on fattening performance, some blood parameters, the immune system, and the antioxidative metabolism of the hepatic tissue. Turk J Vet Anim Sci. (2016) 40:263–70. doi: 10.3906/vet-1504-92
39. Wang JP, Yoo JS, Kim HJ, Lee JH, Kim IH. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest Sci. (2009) 125:298–303. doi: 10.1016/j.livsci.2009.05.011
40. Ghasemi HA, Nari N. Effect of supplementary betaine on growth performance, blood biochemical profile, and immune response in heat-stressed broilers fed different dietary protein levels. J Appl Poult Res. (2020) 29:301–13. doi: 10.1016/j.japr.2019.11.004
41. Wei J, Guo W, Yang X, Chen F, Fan Q, Wang H, et al. Effects of dietary ramie level on growth performance, serum biochemical indices, and meat quality of Boer goats. Trop Anim Health Prod. (2019) 51:1935–41. doi: 10.1007/s11250-019-01891-5
42. Alagawany M, El-Saadony MT, Elnesr SS, Farahat M, Attia G, Madkour M, et al. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult Sci. (2021) 100:101172. doi: 10.1016/j.psj.2021.101172
43. Piacente S, Montoro P, Oleszek W, Pizza C. Yucca schidigera bark: phenolic constituents and antioxidant activity. J Nat Prod. (2004) 67:882–5. doi: 10.1021/np030369c
44. Bidinotto LT, Costa CA, Salvadori DM, Costa M, Rodrigues MA, Barbisan LF. Protective effects of lemongrass (Cymbopogon citratus STAPF) essential oil on DNA damage and carcinogenesis in female Balb/C mice. J Appl Toxicol. (2011) 31:536–44. doi: 10.1002/jat.1593
45. Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. (1986) 55:643–9. doi: 10.1079/BJN19860070
46. Wang JP, Kim IH. Effect of caprylic acid and Yucca schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br Poult Sci. (2011) 52:711–7. doi: 10.1080/00071668.2011.635638
47. Bafundo KW, Männer K, Duerr I. The combination of quillaja and yucca saponins in broilers: effects on performance, nutrient digestibility and ileal morphometrics. Br Poult Sci. (2021) 62:589–95. doi: 10.1080/00071668.2021.1891523
48. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. (2004) 101:15718–23. doi: 10.1073/pnas.0407076101
49. Willis WL, Isikhuemhen OS, Ibrahim SA. Performance assessment of broiler chickens given mushroom extract alone or in combination with probiotics. Poult Sci. (2007) 86:1856–60. doi: 10.1093/ps/86.9.1856
50. Yang Z, Wang Y, He T, Ziema Bumbie G, Wu L, Sun Z, et al. Effects of dietary Yucca schidigera extract and oral candida utilis on growth performance and intestinal health of weaned piglets. Front Nutr. (2021) 26:685540. doi: 10.3389/fnut.2021.685540
51. Dolla A, Fournier M, Dermoun Z. Oxygen defense in sulfate-reducing bacteria. J Biotechnol. (2006) 126:87–100. doi: 10.1016/j.jbiotec.2006.03.041
52. Imshenetskii AA, Nikitin LE, Nazarova TS, Shirshova GA, Muntian LN. Decomposition of human blood cholesterol by the enzyme from Actinomyces lavendulae. Prikl Biokhim Mikrobiol. (1979) 15:715–8.
53. Demain AL, Sanchez S. Microbial drug discovery: 80 years of progress. J Antibiot (Tokyo). (2009) 62:5–16. doi: 10.1038/ja.2008.16
54. Murphy CL, Biggerstaff J, Eichhorn A, Ewing E, Shahan R, Soriano D, et al. Genomic characterization of three novel Desulfobacterota classes expand the metabolic and phylogenetic diversity of the phylum. Environ Microbiol. (2021) 23:4326–43. doi: 10.1111/1462-2920.15614
Keywords: Yucca schidigera extract, young pigeons, serum indexes, immune function, intestinal flora
Citation: Sun X, Wang Z, Li X, Du S, Lin D and Shao Y (2023) Effects of Yucca schidigera extract on serum biochemical parameters, humoral immune response, and intestinal health in young pigeons. Front. Vet. Sci. 9:1077555. doi: 10.3389/fvets.2022.1077555
Received: 23 October 2022; Accepted: 28 December 2022;
Published: 12 January 2023.
Edited by:
Balamuralikrishnan Balasubramanian, Sejong University, South KoreaReviewed by:
Adham Al-Sagheer, Zagazig University, EgyptAli Daneshmand, University of New England, Australia
Utthapon Issara, Rajamangala University of Technology Thanyaburi, Thailand
Hyeun Bum Kim, Dankook University, South Korea
Copyright © 2023 Sun, Wang, Li, Du, Lin and Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongmei Lin,  MzE0NzI1MjU0QHFxLmNvbQ==; Yuxin Shao,
MzE0NzI1MjU0QHFxLmNvbQ==; Yuxin Shao,  c3l1MTIzQHNpbmEuY29t
c3l1MTIzQHNpbmEuY29t
†These authors have contributed equally to this work
 Xiaoshan Sun
Xiaoshan Sun Zheng Wang
Zheng Wang Xing Li1,2
Xing Li1,2 Yuxin Shao
Yuxin Shao