
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 16 November 2022
Sec. Comparative and Clinical Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1047497
This article is part of the Research TopicRising Stars in Comparative and Clinical Medicine: 2022View all 29 articles
 Muhammad Rashid1*†
Muhammad Rashid1*† Naveed Zahra2†
Naveed Zahra2† Amna Chudhary3
Amna Chudhary3 Tauseef Ur Rehman1
Tauseef Ur Rehman1 Muhammad Tahir Aleem4
Muhammad Tahir Aleem4 Abdulaziz Alouffi5
Abdulaziz Alouffi5 Aymen Mohammed6
Aymen Mohammed6 Muhammad Imran Rashid7
Muhammad Imran Rashid7 Muhammad Ehsan1
Muhammad Ehsan1 Muhammad Irfan Malik1
Muhammad Irfan Malik1 Ghulam Hussain Dilber8
Ghulam Hussain Dilber8 Amir Bakhsh9
Amir Bakhsh9 Mashal M. Almutairi10*
Mashal M. Almutairi10*Intestinal parasitic infection is one of the major challenges in obtaining optimal production and maintaining the health and welfare of all animals including cattle and buffaloes. Anti-parasitic treatments appear to be a reliable countermeasure. However, the effectiveness and selection of suitable anthelmintics require situational assessments in a given locality. In the current study, the efficacy and impact of benzimidazole (albendazole) were assessed in a total of 400 (100 each) on the performance of buffaloes, buffalo-heifer, cattle, and cattle-heifers at two commercial dairy farms in the Province of Punjab, Pakistan. Additionally, the cost-benefit ratio was calculated by assessing the inputs (medication, feed, and labor cost) and outputs (milk and weight gain). The qualitative and quantitative examination of helminth eggs in each type of animal indicated a prevalence of 73.3, 78.3, 76.6, and 85.0% in cattle, cattle-heifers, buffaloes, and buffaloes-heifers, respectively. Specifically, a highest rate (10.0–13.3%) of Haemonchus sp. infection was only observed in cattle and heifers, while Fasciola sp. infections (10.0–11.6%) were the most often found species in buffaloes and heifers. The highest anthelmintic impacts (egg per gram of feces, p < 0.001) were observed on day 14 post-medication. Until 60 days of post-anthelmintic treatment, an average increase of 0.8 and 0.7 L in milk production per day in cattle and buffaloes, respectively while a total of 11.45 and 9.45 kg body weight were noticed in cattle-heifer and buffaloes-heifer, respectively. Cumulative cost-benefit analysis indicated a positive correlation between treated and non-treated animals. These findings reiterate the importance of anthelmintic drugs in reducing the impacts of parasites on the productivity, health, and well-being of an animal under high infection challenges.
Livestock plays a major role in the economy of Pakistan; it contributes to 61.9% of the total agriculture revenue and shares 14.0% of Pakistan's gross domestic product (1). To supply cheap, high-quality and surplus milk, meat, and meat products for human consumption, farmers are favoring exotic highly productive breeds over local animal breeds (2). As low as 30–35 million people are engaged in the livestock sector in Pakistan, highlighting the potential and dependency of the economy on livestock (3). The major constraint to sustainable animal production comes from several diseases (4–13). Helminth infections not only reduce productivity but also compromise the quality of food. Parasitized animals reduce live weight gain, increased age of puberty, low productivity, and higher susceptibility to other pathogens; collectively contributing to severe economic losses to stakeholders (14, 15). Infection with various species of parasites in cattle and buffaloes is a growing global problem (16–29). The most prevalent cattle helminths include Strongylus sp., Paramphistome sp., Strongyloides sp., Moniezia sp., Toxocara vitulorum, Trichuris sp., Fasciola sp., and Bunostomum sp. (30). Depending upon animal rearing standards, resistance against parasites, and biosecurity measures, the prevalence of helminth infections is variable and ranges in the world from 0.78 to 84.1% (31). This prevalence ranges in Pakistan from 33.68 to 51.0% (32). As a countermeasure, broad-spectrum anthelmintic drugs such as albendazole and Ivermectin were used to significantly improve the health and well-being of animals which then favorably impact the productivity and standards of animal-origin food and food products (33). The recorded increment of milk production upon post-anthelmintic medication was estimated to be 0.42 L per day (34). The efficacy of albendazole against Ostertagia ostertagi ranges from 84.9 to 99% depending upon the developmental life stages of the parasite (35). Basically, anthelmintic drugs are effective in controlling parasitic infections, but excessive use of anthelmintics (under doses and narrow spectrum drugs) induced an increase in the resistant parasite population (36).
The present study was conducted to evaluate the effect of anthelmintic treatment on milk production and live weight gain in cattle, buffaloes, and their heifers. Moreover, the efficacy of the drug was monitored to ascertain existing anthelmintic resistance and a cost-benefit ratio was performed.
The study was carried out during the spring season on two private dairy farms, consisting of buffaloes from the Sheikhupura district and cattle from the Rajanpur district of Punjab Province, Pakistan. A total of 400 samples (100 each) from cattle, cattle-heifer, buffaloes, and buffalo-heifer were collected for this study.
Animals were selected based on the following criteria: (i) Adult cattle and buffaloes were in the first trimester of lactation. (ii) Age of cattle-heifers and buffalo-heifers ranged from 5 to 8 months. (iii) Animals were not dewormed during the last 3 months prior to the study. (iv) Animals' eggs per gram (EPG) ≥ 200 in feces were included in the treated and control group (37). A total of 60 animals for each group of cattle and cattle-heifer (Holstein Friesian); and buffaloes and buffalo-heifers (Nili Ravi breed) were included for anthelmintic treatment to proceed with the present study. Live body weight was estimated by taking the girth circumference in centimeters (cm) with measuring tape and compared it with tables (http://bairnsley.com/Weight%20by%20Girth.htm) (37). The average adult buffalo and cattle weights were 500 and 550 kg, and buffalo-heifer and cattle-heifers were 90 and 150 kg, respectively. All animals at each farm were kept approximately under the same managemental conditions.
Fecal samples were collected from the rectum of each animal and placed in labeled Ziplock® plastic bags. The samples were shipped in iceboxes to the Laboratory for microscopic examination. These samples were processed by both qualitative (floatation and sedimentation) and quantitative (modified McMaster egg counting) techniques as previously described (38–40).
All experimental animals were kept under the same environmental conditions with a slight difference in the types of concentrate and fodder. Briefly, cattle and cattle-heifers were fed with a concentrate of Dairy Pellet (crude protein: 18–21%) of Maxim International Pvt. Ltd. and silage, while buffaloes and buffalo-heifers were fed with manually prepared concentrate (No recorded crude protein) containing a mixture of different food ingredients (cotton seed cake, rice bran, wheat bran, bread pieces, and mustard oil) and seasonal fodders.
The average EPG of all animal groups before medication (day 0) is shown in Tables 2, 3. Following these findings, animals were classified into control and treatment groups. The animals of treatment groups were medicated with albendazole (Valbazen 113.6 mg/ml; Pfizer Pharma, New York) at the rate of 10 mg/kg body weight. The efficacy was calculated upon average variation in egg reduction compared with day 0. Fecal egg count reduction testing (FECRT) was used to identify the helminths' resistance against albendazole. This test was applied till 60 days post-medication with 2 weeks intervals compared with the EPG count of infected animals before/after treatment (41). The weight gain of treated groups was subtracted from control groups to estimate the actual weight gain upon the anthelmintic appliance. The anthelmintic efficacy was estimated using the following formula:
For the cost-benefit ratio, the considered input variables were the cost for treatment, feed, and labor while output variables were milk production and estimated live weight gain (42). Local market prices of these variables in each studied area were considered according to their region for this study. Briefly, the treatment cost of anthelmintic for adult cattle and buffalo was estimated to be US$ 1.2/animal, while for cattle-heifer and buffalo-heifer it was US$ 0.4 and 0.2/animal, respectively. The price and details of feed costs were estimated as previously described by Rashid et al. (2). Cost of input parameters including feed [concentrate (US$ 0.4/kg for cattle and 0.27 US$ 0.4/kg for buffalo) and fodder (silage of US$ 0.048/kg for cattle and seasonal fodder of 0.029 US$/kg for buffalo)], treatment and labor were encountered. On dairy farms, one labor was engaged for 10 adults or 20 heifers with a pay package of US$ 114.59/month (https://www.reference.com/geography/average-salary-pakistan-d487909030150b6f). Income consists of milk production (cattle for US$0.54/L and buffalo for 0.76/L) and lives weight (US$ 3.82/kg) gain was taken for the cost-benefit ratio (CBR). Live weight gain/loss of heifers was measured at days 0, 7, 15, 30, 45, and 60 according to the formula.
Descriptive statistical and two-way ANOVA analysis was performed on EPG and body weight gain in control and treated groups using GraphPad Prism 7 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). The percentage of EPG reduction within 2 weeks interval was calculated with reference to pre-medication (days 0) while the increment in milk production was calculated from the control group and analyzed by Student t-test. Moreover, the value of CBR was calculated from the total income and cost. The threshold value of 5% was considered for all the statistical tests.
Samples were collected according to instructions and guidelines approved by animal Ethics committee No. DR 1112.
A total number of eight types of helminth species were present in the studied animals. The most prevalent helminths were Ostertagia sp. (8.3, 5.0, 8.3, and 8.3%), Strongyloides sp. (8.3, 10.0, 8.3, and 8.3%) followed by Oesophagostomum sp., Haemonchus sp., Trichostongylus sp., Moniezia sp., Toxocara vitulorum, and Fasciola in cattle, cattle-heifer, buffaloes, and buffaloes-heifers, respectively. The overall prevalence of helminth infection in cattle and cattle-heifers was recorded to be 73.3 and 78.3%, respectively, whereas in buffaloes and buffalo-heifers it was 76.6 and 85.0%, respectively (p > 0.05). Specifically, a higher prevalence of Haemonchus sp. (10.0–13.3%) was observed in cattle and cattle-heifers, followed by Fasciola (10.0–11.6%) in buffaloes and buffalo-heifers, respectively while a lower prevalence of Toxocara vitulorum was observed in both animal species (cattle and buffaloes) (Table 1).
The reduction of EPG on day 7 in treated cattle and cattle-heifers was 88.1 and 91.9% whereas in buffaloes and buffalo-heifers it was 92.0 and 93.8%, respectively. The highest EPG reduction rate observed on day 14 in treated cattle and cattle-heifers was 93.8 and 94.4% respectively. In buffaloes and buffalo heifers, the maximum reduction in EPG count was 95.2 and 97.8%, respectively on day 14. The reduction in EPG reached 73.9 and 70.9% in cattle and cattle-heifers, respectively while in buffaloes and buffalo-heifer reduction was 71.6 and 75.5% on 60 days of post-anthelmintic treatment. The anthelmintic was highly significant (p < 0.001) in helminth reduction with respect to the control group (Tables 2, 3).
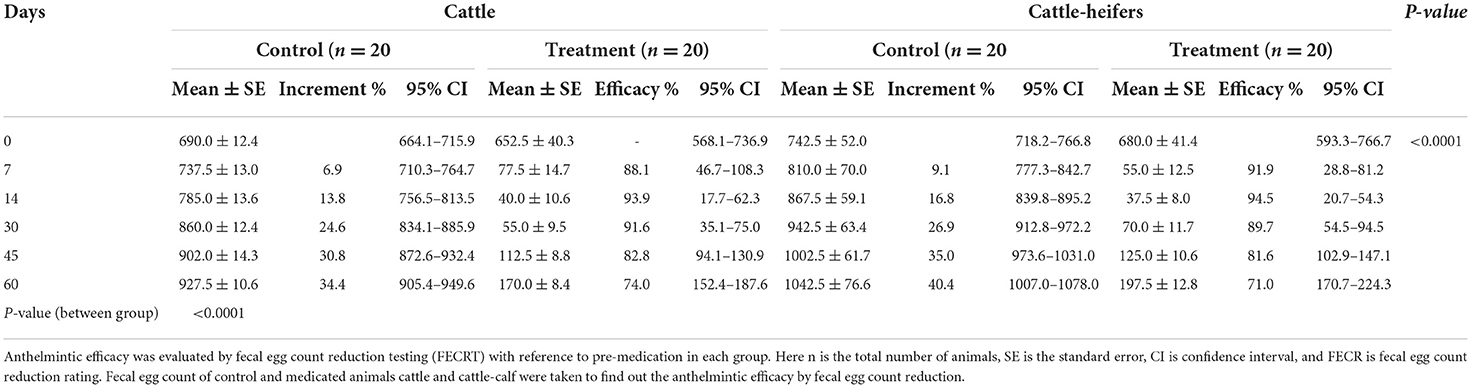
Table 2. Descriptive analysis [mean EPG and standard error (SE)] with 95% confidence interval (CI) in control and medicated groups of cattle and cattle-heifers.
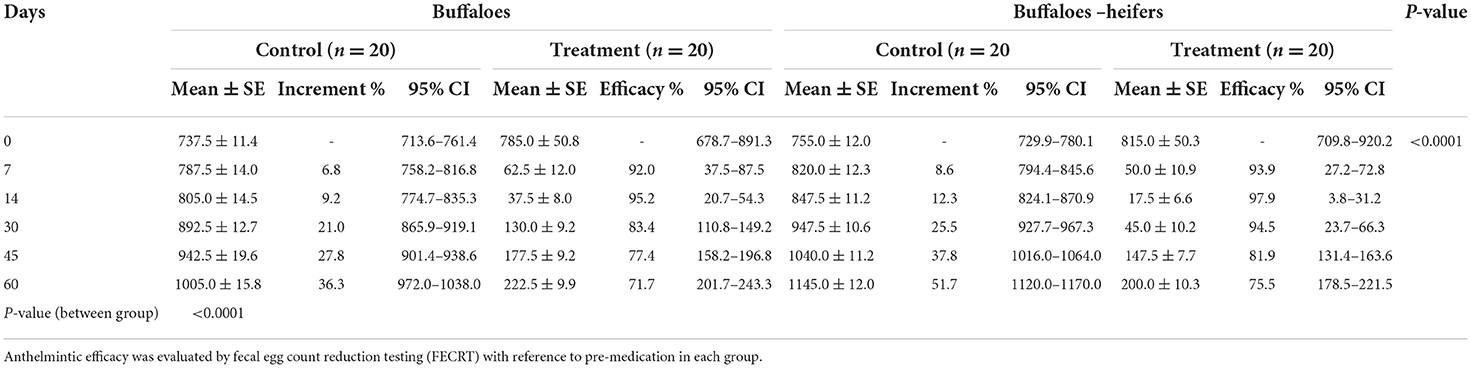
Table 3. Descriptive analysis [mean EPG and standard error (SE)] with 95% confidence interval (CI) in control and medicated groups of buffaloes and buffalo-heifers.
The average milk yield of control and treated cattle was 18.5 ± 0.36 and 19.3 ± 0.32 L/day, respectively (p > 0.05) with differences of 0.8L/day whereas in buffaloes it was 7.5 ± 0.22 and 8.2 ± 0.23 L/day (p > 0.05) with differences of 0.7 L/day (buffalo). The mean weight of cattle-heifers ranged from 151.4 to 153.0 kg/heifer while buffalo-heifers ranged from 89.9 to 92.8 kg/heifer at pre-medication (day 0). The weight gain in treated heifers after 60 days was significant (p < 0.05) compared with the control group. After 60 days of post-anthelmintic treatment, the mean weight gains in treated cattle-heifer and buffalo-heifers were recorded on days 0, 7, 15, 30, 45, and 60 with a total gain of 11.5 and 9.5 kg, respectively (Figure 1).
The CBR values indicate the ratio of income/loss per unit cost. The highest CBR value was recorded for the animal groups of treated adult cattle (2.515) followed by control adult cattle (2.423), treated adult buffalo (1.759), control adult buffalo (1.618), treated buffalo-heifer (1.351), treated cattle-heifer (1.056), control buffalo-heifers (0.935), and control cattle-heifers (0.748). Higher CBR values indicate farm profitability while lesser value shows a loss in the rearing of those animals (Table 4).
The current study provides the data on the most prevalent helminth species (Haemonchus sp. in cattle and Fasciola sp. in buffaloes), mixed parasitic infection, AE, and CBR. In the field condition, a higher prevalence of fasciolosis (20%) in cattle and buffaloes in Toba Tek Singh, Pakistan was observed (43). A higher prevalence of 23.3, 26.67, 25 and 33.33% of mix infection was observed in cattle, cattle-heifer, buffaloes, and buffalo-heifers in the studied animals. Similarly, the prevalence of mixed infection was found at 7, 18, 9, and 23% in cattle, cattle-heifer, buffaloes, and buffalo-heifers, respectively in a study conducted in the same province similar to the current study. This previous study's finding (0.00–3.27) for the prevalence of Oesophagostomum was slightly different (3.3–8.3) from the current study (15). The similarity (mixed infections) and difference (Oesophagostomum) were due to the same study area and different feeding systems. The distribution of these parasites at dairy farms are mainly attributed to the contaminated fodder (44) and intermediate host. Moreover, since feed and water are available ad libitum to all free-moving animals at dairy farms (45), the incidence is likely to occur frequently. Generally, the mean EPG in heifers was higher than in adult animals pertaining to the higher susceptibility of heifers than immune-compromised adult animals (46). Moreover, the mean EPG in the buffaloes breed was higher than cattle breed representing their susceptibility or no previous anthelmintic treatment. The EPG in control groups increased with the passage of time due to the persistent and continuous expansion of intestinal parasites as already described by Saqib et al. (37). The maximum reduction in EPG was on the 14th day post-medication. However, a higher reduction in percentage was observed in buffaloes and buffalo-heifers indicating a reduced anthelmintic resistance as compared to cattle breeds (47) that are treated regularly favoring the establishment of anthelmintic resistance. The efficacy of anthelmintic was not 100% in any animal species/breed which might be the problem of drug resistance. It might be due to the regular or under-dose usage of the same group of medicine. Therefore, it is recommended to apply alternative treatment, and a combination of two groups (37). Generally, investors consider exotic cattle breeds over the buffaloes at dairy farms mainly due to a higher milk yield (2). In this study, the average increase in milk production in cattle and buffalo was 0.8 and 0.7 L/ day, respectively, which confirms previous studies undertaken in the Netherlands (48). This increase in milk production in cattle was lower than in buffaloes and this can be associated with less reduction of EPG in cattle in the local production system. The average weight of cattle-heifers was higher than buffalo-heifers at the same age which might be due to the improved genetic potential in the cattle breed. Owing to the increasing genetic profile, the first conception rate of cattle heifers is earlier at 14–16 months of age than buffalo heifers which are at 22 months of age (49, 50). The weight gain of treated buffalo and cattle-heifers was 500 and 650 g/day while in the control group it was 350 and 460 g/day, respectively, which is in accordance with the results of a previous study on a dairy farm in Pakistan and the Manawatu region of New Zealand (49, 51). Additionally, the CBR of treated animals was higher than that of the non-treated ones (37) in adult cattle followed by adult buffaloes, buffalo-heifers, and cattle-heifers. It is noteworthy, that infrastructure cost to control environmental conditions and auto-machine, which is necessary for exotic animal dairies was not considered. On the other hand, native buffaloes do not require extra management as they are fully acclimatized and adapted to the native environment. Considering this difference in managemental cost, the CBR of cattle will be considerably lower than buffaloes. It was also observed in this study that non-treated cattle-heifers and buffalo-heifers were not profitable, mainly due to their susceptibility to parasitic infections and extra requirements of feed at this stage of development, which can be interfered with by the existing parasites.
Taken together, these studies indicate that anthelmintic treatment may positively impact EPG reduction and can cause an increase in milk production and gain in body weight in both cattle and buffaloes at studied dairies. Collectively, this improved efficiency of production favors the direct and positive cost-benefit ratio for dairy farmers.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by DR 1112. Written informed consent was obtained from the owners for the participation of their animals in this study.
MR and AC performed experiments. MR, AC, and NZ drafted MS. TR and MTA did proofreading and correction. MIR did data analysis. AB provided sources for sampling and data history of animals. AA, AM, and MMA provide the funding and proofreading of MS. ME, MM, and GH did data curation. All authors contributed to the article and approved the submitted version.
The work was financially supported by the researchers supporting project number (RSP2022R494), King Saud University, Riyadh, Saudi Arabia.
The authors are grateful to the researchers supporting project number (RSP2022R494), King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KL declared a shared affiliation with the author MTA to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Anonymous. Economic Survey of Pakistan. Ministry of Finance, Islamabad: Government of Pakistan. Fed Sec. (2021) 2015:1–22. doi: 10.55603/jes.v1i1.a2
2. Rashid M, Haroon A, Rashid MI, Khalid S, Liaquat A, Saghir A, et al. Economic significance of tropical theileriosis on a Holstein Friesian dairy farm in Pakistan. J Parasitol. (2018) 104:310–2. doi: 10.1645/16-179
3. Durrani A, Kamal N, Khan M. Incidence of theileriosis and estimation of packed cell volume, total erythrocyte count and haemoglobin in buffaloes. J Anim Plant Sci. (2006) 16:85–8. Available online at: http://thejaps.org.pk/docs/16_3-4_2006/07-701.pdf
4. Asif M, Shah N, Zeeshan AB, Muhammad Fakhar-e-Alam, Kulyar, Rashid M, et al. Viral outbreaks: a real threat to the world. Adv Life Sci. (2020) 8:08–19. Available online at: https://als-journal.com/submission/index.php/ALS/article/view/999/462
5. Daniel A, Awan ZA, Imran A, Khan RM. Empirical Analysis of Farmers Preferences and Willingness towards Organic Farming in Gilgit-Baltistan, Pakistan. Adv Life Sci. (2021) 8:262–6. Available online at: https://submission.als-journal.com/index.php/ALS/article/view/1077/561
6. Du XX, Sherein SA, Liu P, Haque MA, Khan A. Bovine Mastitis: Behavioral Changes, Treatment and Control. Continental Vet J. (2022) 2:15–23.
7. Haque MA, Quan H, Zuo Z, Khan A, Siddique N, He C, et al. Pathogenicity of feed-borne Bacillus cereus and its implication on food safety. Agrobiol Records. (2021) 3:1–16. doi: 10.47278/journal.abr/2020.015
8. Khalaf DD, Soliman MMH, Mansour AS. Conventional and molecular identification of mycotic mastitis caused by Candida in farm animals. Int J Vet Science. (2021) 10:64–8. doi: 10.47278/journal.ijvs/2020.011
9. Osman SA, Tharwat M, Saeed EM. An outbreak of ovine listeriosis in Qassim region, Saudi Arabia: Epidemiological, clinical and treatment outcomes. Int J Vet Sci. (2021) 10:312–6. doi: 10.47278/journal.ijvs/2021.060
10. Sajjad MM, Rasheed M, Farooq W, Yasmin F, Niaz S, Yaqub T, et al. Recent updates on molecular detection of H9N2 as low pathogenic strain of avian influenza virus from poultry farms of Lahore, Pakistan. Agrobiol Records. (2021) 5:15–20. doi: 10.47278/journal.abr/2020.029
11. Sulieman AM, Dafallah FE, Abdel-Rahman EH, Alshammari NI, Shommo SA, Ibrahim SE. Isolation, Identification and Characterization of Salmonella spp. from chicken purchased at Wad Madani City, Gezira State, Sudan. Adv Life Sci. (2020). 8:98–102. Available online at: https://als-journal.com/submission/index.php/ALS/article/view/1050/477
12. Tahir A, Khan MA, Bibi K, Bibi S, Rauf F, Ayaz F. Prevalence of colibacillosis in young broiler chicks and antibiogram of escherichia coli in different areas of Hazara Region. Adv Life Sci. (2021) 8:238–40. Available online at: http://www.als-journal.com/articles/vol8issue3/835.21/837.pdf
13. Abbas RZ, Zaman MA, Sindhu D, Sharif M, Rafique A, Saeed Z, et al. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pak Vet J. (2020) 40:83. doi: 10.29261/pakvetj/2020.083
14. Yadav A, Khajuria J, Raina A. Gastrointestinal parasitic infestation profile of bovines at RS Pura. J Vet Parasitol. (2004) 18:167–9. Available online at: https://www.indianjournals.com/ijor.aspx?target=ijor:jvp&volume=19&issue=2&article=007
15. Raza MA, Iqbal Z, Jabbar A, Yaseen M. Point prevalence of gastrointestinal helminthiasis in ruminants in southern Punjab, Pakistan. J. Helminth. (2007) 81:323–8. doi: 10.1017/S0022149X07818554
16. Regassa F, Sori T, Dhuguma R, Kiros Y. Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. Int J Appl Res Vet Med. (2006) 4:51.
17. Zaman MA, Sajid M, Sikandar A, Awais MM. Point prevalence of gastrointestinal helminths and their association with sex and age of the buffaloes in lower Punjab, Pakistan. Int J Agric Biol. (2014) 16:1229–31.
18. Marskole P, Verma Y, Dixit AK, Swamy M. Prevalence and burden of gastrointestinal parasites in cattle and buffaloes in Jabalpur, India. Vet World. (2016) 9:1214. doi: 10.14202/vetworld.2016.1214-1217
19. Rehman T, Khan MN, Abbas RZ, Babar W, Sikandar A, Zaman MA. Serological and coprological analyses for the diagnosis of Fasciola gigantica infections in bovine hosts from Sargodha, Pak. J Helminth. (2016) 90:494–502. doi: 10.1017/S0022149X15000711
20. Akram MZ, Zaman MA, Jalal H, Yousaf S, Khan AY, Farooq MZ, et al. Prevalence of gastrointestinal parasites of captive birds in Punjab, Pakistan. Pak Vet J. (2019) 39:132–4. doi: 10.29261/pakvetj/2018.123
21. Shaukat A, Mehmood K, Shaukat I, Naeem MA, Mehfooz A, Saleem MI, et al. Prevalence, haematological alterations, and chemotherapy of bovine anaplasmosis in sahiwal and crossbred cattle of District Faisalabad, Punjab, Pakistan. Pak J Zool. (2019) 51:2023–32. doi: 10.17582/journal.pjz/2019.51.6.2023.2032
22. AbouLaila M, AbdEl-Aziz AR, Menshawy S, Yokoyama N, Igarashi I, Al-Wabel M, et al. Evaluation of the inhibitory effects of coumermycin A1 on the growth of Theileria and Babesia parasites in vitro and in vivo. Pak Vet J. (2021) 41:469–74. doi: 10.29261/pakvetj/2021.064
23. Ceylan O, Uslu A, Ceylan C, Sevinc F. Predominancy of Rhipicephalus turanicus in tick infested sheep from turkey: a large-scale survey. Pak Vet J. (2021) 41:429–33. doi: 10.29261/pakvetj/2021.036
24. Esmaeilnejad B, Tavassoli M, Samiei A, Hajipour N, Imani-Baran A, Farhang-Pajuh F. Evaluation of hematological, antioxidant enzymes and oxidative stress parameters in buffaloes infected with babesiosis. Continental Vet J. (2022) 2:29–34.
25. Lan Y, Li K, Mehmood K. Molecular investigation of important protozoal infections in yaks. Pak Vet J. (2021) 41:557–561. doi: 10.29261/pakvetj/2020.048
26. Rehman TU, Saeed Z, Zaman MA, Sikandar A, Ali HM. Factors influencing the incidence of Eimeria leuckarti in horses. Agrobiol Rec. (2021) 6:13–7. doi: 10.47278/journal.abr/2021.004
27. Sobhy H, AboElnaga TR, Behour TS, Razin EA. In vitro trypanocidal activity of essential oils of some plants against Trypanosoma evansi. Int J Vet Sci. (2021) 10:191–5. doi: 10.47278/journal.ijvs/2021.043
28. Strbac F, Bosco A, Amadesi A, Rinaldi L, Stojanović D, Simin N, et al. Ovicidal potential of five different essential oils to control gastrointestinal nematodes of sheep. Pak Vet J. (2021) 41:353–8. doi: 10.29261/pakvetj/2021.026
29. Yawoz M, Jaafar SE, Alali F, Babur C. Seroprevalence of camels listeriosis, brucellosis and toxoplasmosis from Kirkuk Province, Iraq. Pak Vet J. (2021) 41:335–40. doi: 10.29261/pakvetj/2021.030
30. Das M, Deka DK, Sarmah AK, Sarmah PC, Islam S. Gastrointestinal parasitic infections in cattle and swamp buffalo of Guwahati, Assam, India. Indian J Anim Res. (2018) B-2427:1–7. doi: 10.18805/ijar.B-3427
31. Bundy DA, Golden MH. The impact of host nutrition on gastrointestinal helminth populations. Parasitol. (1987) 95:623–35. doi: 10.1017/S0031182000058042
32. Khan MN, Sajid MS, Khan MK, Iqbal Z, Hussain A. Gastrointestinal helminthiasis: prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol Res. (2010) 107:787–94. doi: 10.1007/s00436-010-1931-x
33. Pankaj P, Raina V, Pourouchottamane R, Venkatsubramanian V, Mohanty T, Muzamil S, et al. Effect of deworming on seminal characteristics of Karan fries bulls. Appl Biol Res. (2013) 15:85–90. Available oline at: https://www.indianjournals.com/ijor.aspx?target=ijor:abr&volume=15&issue=2&article=001
34. Nødtvedt A, Dohoo I, Sanchez J, Conboy G, DesCôteaux L, Keefe G. Increase in milk yield following eprinomectin treatment at calving in pastured dairy cattle. Vet Parasitol. (2002) 105:191–206. doi: 10.1016/S0304-4017(02)00024-9
35. Williams J, Broussard S. Comparative efficacy of levamisole, thiabendazole and fenbendazole against cattle gastrointestinal nematodes. Vet Parasitol. (1995) 58:83–90. doi: 10.1016/0304-4017(94)00701-D
36. Waller PJ. Anthelmintic resistance. Vet Parasitol. (1997) 72:391–412. doi: 10.1016/S0304-4017(97)00107-6
37. Saqib AM, Saeed K, Rashid I, Ijaz M, Akbar H, Rashid M, et al. Anthelmintic drugs: their efficacy and cost-effectiveness in different parity cattle. J Parasitol. (2022) 104:79–85. doi: 10.1645/17-4
38. Roberts FH, O'sullivan PJ. Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Aust J Agric Res. (1950) 1:99–102. doi: 10.1071/AR9500099
39. Hansen J, Perry B. The Epidemiology, Diagnosis, and Control of Helminth Parasites of Ruminants. A Handbook. Nairobi: The International Laboratory for Research on Animal Diseases (1994).
40. Dryden MW, Payne PA, Ridley R, Smith V. Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther. (2005) 6:15–28.
41. Cabaret J, Berrag B. (2004). Faecal egg count reduction test for assessing anthelmintic efficacy: average versus individually based estimations. Vet Parasitol. (2004) 121:105–13. doi: 10.1016/j.vetpar.2004.01.020
42. Rashid M, Rashid MI, Akbar H, Ahmad L, Hassan MA, Ashraf K, et al. A systematic review on modelling approaches for economic losses studies caused by parasites and their associated diseases in cattle. Parasitol. (2018) 146:1–13. doi: 10.1017/S0031182018001282
43. Athar LA, Khan MN, Sajid MS, Khan IA. Cost benefits analysis of anthelmintic treatment of cattle and buffaloes. Pak Vet J. (2011) 31:14–9.
44. Gadberry S, Powell J. Internal parasites in beef and dairy cattle. (2012). Available online at: https://articles.extension.org/pages/11022/internal-parasites-in-beef-and-dairy-cattle (accessed June 18, 2018).
45. Meyer U, Everinghoff M, Gädeken D, Flachowsky G. Investigations on the water intake of lactating dairy cows. Livest Prod Sci. (2004) 90:117–21. doi: 10.1016/j.livprodsci.2004.03.005
46. Melancon JJ. Parasites of Dairy Cattle. Merial Veterinary Bulletin TSB-9- 99013-FTB. (1999). Available online at: http://us.merial.com/pdf/page_pdf/parasites_of_dairy_cattle.pdf (accessed June 25, 2018).
47. Anziani OS, Suarez V, Guglielmone AA, Warnke O, Grande H, Coles GC. Resistance to benzimidazole and macrocyclic lactone anthelmintics in cattle nematodes in Argentina. Vet Parasitol. (2004) 122:303–6. doi: 10.1016/j.vetpar.2004.05.018
48. Ploeger HW, Schoenmaker GJ, Kloosterman A, Borgsteede FH. Effect of anthelmintic treatment of dairy cattle on milk production related to some parameters estimating nematode infection. Vet Parasitol. (1989) 34:239–53. doi: 10.1016/0304-4017(89)90054-X
49. Bhatti S, Sarwar M, Khan M, Hussain S. Reducing the age at first calving through nutritional manipulations in dairy buffaloes and cows: a review. Pak Vet J. (2007) 27:42. Available online at: http://www.pvj.com.pk/pdf-files/27_1/page%2042-47.pdf
50. Novaković Ž, Sretenović L, Aleksić S, Petrović MM, Pantelić V, Ostojić Andrić D. Age at first conception of high yielding cows. Biotechnol Anim Husb. (2011) 27:1043–50. doi: 10.2298/BAH1103043N
Keywords: anthelmintic, efficacy, cattle, buffaloes, heifers, cost-benefit ratio
Citation: Rashid M, Zahra N, Chudhary A, Rehman TU, Aleem MT, Alouffi A, Mohammed A, Rashid MI, Ehsan M, Malik MI, Hussain Dilber G, Bakhsh A and Almutairi MM (2022) Cost-benefit ratio of anthelmintic treatment and its comparative efficacy in commercial dairy farms. Front. Vet. Sci. 9:1047497. doi: 10.3389/fvets.2022.1047497
Received: 18 September 2022; Accepted: 25 October 2022;
Published: 16 November 2022.
Edited by:
Isa Ozaydin, Kafkas University, TurkeyReviewed by:
Kun Li, Nanjing Agricultural University, ChinaCopyright © 2022 Rashid, Zahra, Chudhary, Rehman, Aleem, Alouffi, Mohammed, Rashid, Ehsan, Malik, Hussain Dilber, Bakhsh and Almutairi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Rashid, cmFzaGlkLmxhZ2hhcmlAaXViLmVkdS5waw==; Mashal M. Almutairi, bW1hbG11dGFpcmlAa3N1LmVkdS5zYQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.