
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 08 November 2022
Sec. Veterinary Pharmacology and Toxicology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1045152
This article is part of the Research TopicTreatment of Animal Diseases with Veterinary PhytotherapyView all 14 articles
This study investigated the effect of Danggui Buxue decoction (DBD) on the immunity of an O-type foot-and-mouth disease (FMD) vaccine and intestinal mucosal immunity. SPF KM mice were continuously and orally administered DBD for 5 d and then inoculated with an O-type FMD vaccine. The contents of a specific IgG antibody and its isotypes IgG1, IgG2a, IgG2b, and IgG3 in serum and SIgA in duodenal mucosa were determined by ELISA at 1 and 3 W after the 2nd immunization. qRT-PCR was used to detect mRNA expression levels of IL-4, IL-10, IFN-γ, and IL-33 in the spleen, and mRNA expression levels of J-chain, pIgR, BAFF, APRIL, IL-10, IFN-γ and IL-33 in the duodenum. The results showed that compared with the control group, oral administration of DBD significantly increased levels of the anti-FMD virus (FMDV)-specific antibodies IgG, IgG1, and IgG2a in the serum of O-type FMD vaccine-immunized mice 1 W after the 2nd immunization (P < 0.05), upregulated mRNA expression levels of spleen lymphocyte cytokines IL-4 and IL-33 (P < 0.05), promoted the secretion of SIgA in duodenal mucosa (P < 0.05). The mRNA expression levels of J-chain, pIgR, BAFF, APRIL, IL-10, and IL-33 in duodenal tissues were upregulated (P < 0.05). This study indicates that DBD has a good promotion effect on the O-type FMD vaccine and the potential to be an oral immune booster.
Foot-and-mouth disease (FMD) is a highly contagious acute infectious disease caused by FMD virus (FMDV) and mainly affects cloven-hoofed animals such as pigs, cattle, goats, and camels (1). The incidence of FMD is extremely high and the transmission speed is extremely fast. FMDV is mainly transmitted through the digestive tract, respiratory tract, skin, and mucosa. Mucosal damage in the early stage of infection is highly susceptible to infection by other bacteria in the external environment (2). In today's intensive farming environment, once an individual gets sick, a group infection will soon break out and cause serious economic losses to the breeding industry (3). Presently, vaccination is the most important means for the prevention and control of FMD (4). Therefore, compulsory immunization with FMD vaccine was adopted in China to prevent and control the disease, which can control a possible pandemic of FMD to a certain extent (5). However, due to the uneven level of breeding health management in some areas, the phenomenon of poor immune effects of FMD vaccines has been reported from time to time (6). Therefore, it is necessary to develop an immune booster to improve the immune effect of the FMD vaccine.
Chinese herbal medicine comes from nature and has low toxicity and side effects. It also has a unique effect in coordinating the overall balance of the body and strengthening the disease-resistance ability. Recently, researchers have used modern science and technology to study traditional Chinese medicine and found that Chinese herbal medicine or effective active ingredients have significant immunomodulatory effects (7, 8). Danggui Buxue decoction (DBD) was created by Li Dongyuan, one of the four great scholars of the Jin and Yuan Dynasties. It is composed of Radix Astragali and Angelica sinensis and replenishes Qi and promotes the production of blood (9). Modern studies have confirmed that DBD can promote angiogenesis (10), improve bone marrow hematopoietic function (11), enhance non-specific immunity (12) and improve immune regulation in a pathological injury model (13). The intestinal tract is not only a place for digestion and absorption of nutrients but also has important immune functions. The intestinal mucosa is a key line of defense for the body to resist pathogen infection, and various defense mechanisms are conducted in the intestine, which play an important role in the establishment and maintenance of homeostasis between the host and the external environment (14). Chinese herbal medicine is a group of natural substances, which has the advantages of high safety, enhancing humoral and cellular immunity, non-toxic side effects, wide range of medication, etc. And it has been reported that a large number of herbal medicines or their extracts have been used as vaccine adjuvants, such as Angelica sinensis polysaccharide (15), ginsenoside Rg1 (16) and Qi-Wu Rheumatism Granule (17). Most Chinese herbal medicines are taken orally to exert effects and have a close relationship with the intestinal mucosal immune system. Therefore, this study was conducted to investigate the effect of oral DBD on the immune function of an O-type FMD vaccine and intestinal mucosal immunity in mice to provide a theoretical basis for the clinical application of traditional Chinese medicine.
Angelica sinensis and Radix astragali were purchased from Chongqing Rongchang Tongjunge pharmacy.
The O-type foot-and-mouth disease synthetic peptide vaccine (Peptide 2600+2700+2800) (20190408) was purchased from Shenlian Biomedicine (Shanghai, China) Co., Ltd. A mouse (FMDV-OIgG) ELISA kit (20190512), mouse FMDV-OIgG1, FMDV-OIgG2a, FMDV-OIgG2b, FMDV-OIgG3) ELISA kit (20190422), and mouse SIgA ELISA kit (20190612) were purchased from Shanghai Huzhen Industrial Co., Ltd. TRIzon Reagent (20190323), HiFi Script gDNA Removal RT Master Mix (30412), and 2xEs Taq Master Mix (50428) were purchased from Beijing Kangwei Century Co., Ltd. Eva GREEN (17E0.1-1075001) was purchased from Biotium, USA.
One hundred grams of Radix astragali and 20 grams of Angelica sinensis were accurately weighed, soaked in 8 times distilled water for 30 min with high heat until boiling, and then simmered gently for 1 h. The liquid was filtered and the residue was added to the same amount of distilled water for an additional 1 h. The filtrate was filtered again and combined. The final concentration of liquid was equivalent to 2 g/m L of the original medicine. The medicinal liquid was separated into 2, 1, 0.5, 0.25, 0.125, and 0.0625 g/mL DBD and stored at 4°C for later use.
A total of 94 SPF KM mice weighing 20 ± 2 g were purchased from Chongqing Ensiweier Biotechnology Co., Ltd. All experimental mice were raised in an environment with a temperature of 20–25°C and a relative humidity of 60–80% and were freely allowed to feed and drink. After 1 W of adaptive feeding, the formal experiment began. This study was approved by the Laboratory Animal Ethical Review Committee of Southwest University (permit number IACUC-20211020-01), and all experimental animals were euthanized at the end of the study.
In experiment 1, 70 KM mice, half male and half female, were randomly divided into 7 groups (n = 10), which were 2, 1, 0.5, 0.25, 0.125 and 0.0625 g/mL DBD dose groups and control group. The dose was 0.25 mL/each, which was given by gavage for 5 d. The control group was given the same amount of normal saline. Twenty-four hours after the last dose, all experimental mice were subcutaneously injected with 0.2 mL of O-type FMD vaccine in the groin followed by a second immunization at 2 W later. The mice were weighed before immunization and 1 and 3 W after immunization, and blood samples were collected under the jaw at 1 and 3 W after immunization. All mice were sacrificed by neck removal at 3 W after immunization, and the spleen and thymus were aseptically harvested (Figure 1).
In experiment 2, 24 KM mice, half male and half female, were randomly divided into two groups (n = 12), which were the optimal dose group of DBD and the control group, respectively. The mice were treated according to the method of Experiment 1. All experimental mice were sacrificed by neck stripping 3 W after the 2nd immunization, and the spleen, duodenum, and mucosal flushing fluid were aseptically extracted (Figure 1).
The spleen and thymus of all experimental mice were accurately harvested, and the blood stains on the surface were washed with sterilized saline. Then, excess water on the organ surface was aspirated with clean filter paper and weighed.
Calculation formula: Organ index = organ weight (mg)/mouse weight (g).
The serum and duodenal mucosa supernatant were obtained by separating the collected blood and duodenal mucosa rinse solution at 3,000 rpm/min for 10 min. The content of FMDV-specific antibody IgG in serum at 1 W and 3 W after the 2nd immunization, specific antibody isotypes IgG1, IgG2a, IgG2b, and IgG3 in serum, and SIgA in duodenal mucosal rinse solution 3 W after the 2nd immunization were detected according to ELISA kit instructions.
Total RNA was extracted from the spleen and duodenum using TRIzol Reagent and reverse transcribed into cDNA using HiFi Script gDNA Removal RT Master Mix and 2xEs Taq Master Mix. The mRNA expression levels of IL-4, IL-10, IFN-γ, and IL-33 in the spleen and APRIL, BAFF, pIgR, J-chain, IL-10, and IL-33 in the duodenum were detected by real-time PCR using Eva GREEN (Biotium17E0.1-1075001) with mouse β-actin as the internal reference. The results are expressed as 2−ΔΔct, and the primer sequences are shown in Table 1.
Data are expressed as the mean ± standard error (SE) and were analyzed by one-way ANOVA post-hoc Duncan's method and t-test with IBM SPSS Statistics 22.0 software. Differences with P < 0.05 were considered statistically significant.
As depicted in Tables 2, 3, the body weight (Table 2), spleen index, and thymus index (Table 3) of the experimental mice were not significantly affected by different doses of DBD compared with the control group (P > 0.05).
As shown in Figure 2, the serum anti-FMDV-specific antibody IgG content in each dose group was significantly increased at 1 W after the 2nd immunization (P < 0.05). The 0.5 g/mL and 2 g/mL dose groups significantly increased the content of IgG and IgG1 serum-specific antibodies (P < 0.05), and the 0.5 g/mL dose group simultaneously promoted the secretion of serum IgG2a (P < 0.05) 3 W after the 2nd immunization. There was no significant difference in antibody content among the other dose groups (P > 0.05).
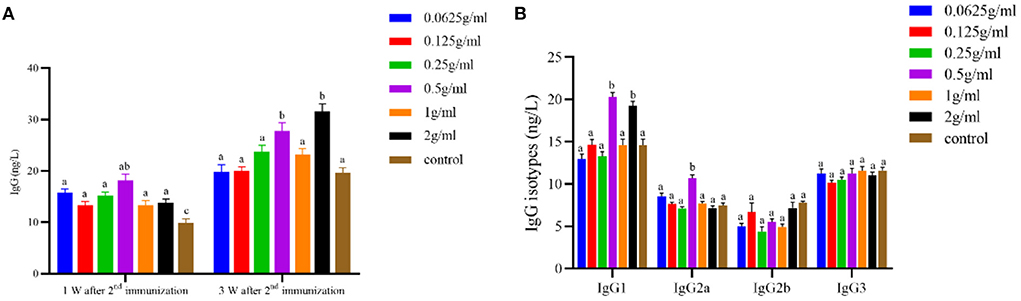
Figure 2. Effects of orally administered DBD on serum FMDV-specific antibody content in mice. (A) The concentration of IgG antibody. (B) The concentration of IgG isotypes antibody. a,bValues in the same column with different superscript letters differ significantly at P < 0.05.
As depicted in Figure 3 and compared with the control group, the mRNA expression levels of IL-4 and IL-33 in the spleen were significantly increased by oral DBD (P < 0.05), and the mRNA expression levels of IL-10 and IFN-γ were not different from those of the control group (P > 0.05).
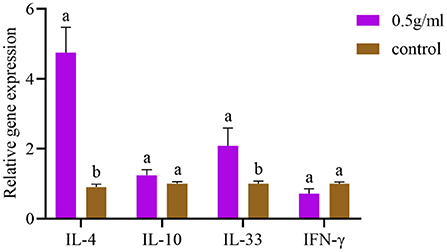
Figure 3. Effects of orally administered DBD on spleen cytokine mRNA expression in mice. a,bValues in the same column with different superscript letters differ significantly at P < 0.05.
The effect of DBD on the SIgA content of duodenal mucosal flushing fluid is shown in Figure 4. The results suggest that 0.5 g/mL DBD significantly increased the intestinal SIgA antibody content (P < 0.05).
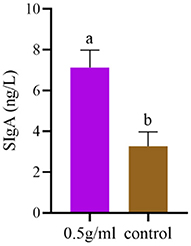
Figure 4. Effects of orally administered DBD on intestinal SIgA antibody content in the duodenum of mice. a,bValues in the same column with different superscript letters differ significantly at P < 0.05.
After oral administration of DBD, the mRNA expression levels of J-chain, pIgR, BAFF, APRIL, IL-10, and IL-33 in intestinal tissues were significantly increased compared with those in the control group (P < 0.05). Additionally, the expression of IFN-γ was decreased, but there was no significant difference compared with the control group (P > 0.05) (Figure 5).
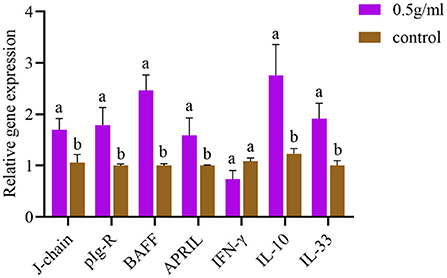
Figure 5. Effects of orally administered DBD on the mRNA expression of cytokines in the duodenum of mice. a,bValues in the same column with different superscript letters differ significantly at P < 0.05.
Most modern pharmacological studies on the immunomodulatory effect are conducted by regulating non-specific immunity or evaluating the therapeutic effect of pathological models, while there are few studies on specific immunity (18). Therefore, this trial explored the effects of gastric infusion DBD on specific immunity. The organ index is the ratio of an organ to its weight in a test animal, which can reflect the strength of its function to a certain extent. The spleen, thymus, and liver are the most important immune organs in mammals, and the quality of animal immune organs is increased by their proliferation caused by cell growth and division. Therefore, the weight is proportional to the immune function of the body (19, 20). In this study, it was found that the organ index of mice after gavage of different doses of DBD was not significantly different from that of the control group, but the 0.5 g/ml and 2 g/ml dose groups were superior to the control group. The humoral immune response of the body mainly includes IgG, IgM, IgA, IgD and IgE. And IgG is the main effector molecule of humoral immune response. The level of IgG antibody in serum can be used as an indicator of the success of vaccination. According to the differences in the antigenicity of the γ chain in IgG molecules, IgG in mice has four isotypes of antibodies, namely, IgG1, IgG2a, IgG2b, and IgG3. The results of this test showed that the oral administration of DBD could improve the antibody level of FMDV antigen, which was consistent with the results of Sun et al. (21).
When FMDV infects animals, both humoral and cellular immune functions resist the pathogen. This study found that DBD can increase the specific antibody level and specific antibody of the mouse class level, which suggests that after lavage DBD, the change in the antibodies may be related to the change in immune cells and immune active substances in the body. During the body's cellular immune response, immune cells affect the production of antibodies by secreting cytokines, which also inhibit the proliferation of related cells (22). Although the mechanism of antibody generation is extremely complex, numerous studies have shown that specific cytokines can promote certain types of antibody subclasses switching and further to inhibit other antibody subclasses (23). CD4+ cells are divided into Th1 and Th2 subsets. Th1 subsets mainly express IFN-γ and IL-2, which are involved in cellular immunity to endogenous cell infection, while Th2 subsets express IL-4 and IL-10, which participate in allergic reactions and humoral immunity against parasitic infection (24). IFN-γ is an important B cell type switching factor that can induce B cells to secrete antigen-specific IgG2a (25). IL-4 can induce antigen-dependent IgG2a and IgE production (26) and enhance IgG1-mediated humoral immunity and NK-cell killing (27). In this study, the mRNA expression of IL-4 in the spleen was significantly increased after oral administration of DBD, but IL-10 and IFN-γ were not significantly changed, which may be due to the balance regulation of Th1 and Th2 cytokines in mice by DBD. Thus, DBD affects the distribution of antibodies and the synergistic inhibitory effect of IL-4 on IFN-γ (28).
The intestinal mucosa is the body's first line of defense against infection, which can induce effective mucosal immunity and generate a systemic immune response. Many studies have confirmed that oral administration of Chinese herbal medicine can enhance intestinal mucosal immune function (29, 30). As an important effector factor of mucosal immune response, SIgA can block the adhesion of pathogens to the mucosa, neutralize toxins and other bioactive antigens, and prevent a variety of microorganisms and mucosal antigens from entering intestinal epithelial cells. It does not require complement to form immune complexes with viruses in the respiratory tract, digestive tract and other parts to be excreted. Therefore, the concentration of SIgA antibody is commonly used clinically to evaluate the mucosal immune effect. The concentration of the SIgA is commonly used to evaluate the mucosal immune effect (31, 32). Studies have examined the immune-enhancing effect of atractylodes polysaccharide (33) and found that after gavage of atractylodes polysaccharide, the serum-specific IgG antibody and intestinal total SIgA content of mice vaccinated with FMD vaccine were significantly increased, which was similar to the results of this experiment. This suggests that the enhancement of the serum IgG response by Chinese herbal medicine is related to the enhancement of intestinal mucosal immunity. The IgA monomer produced by IgA+ plasma cells and the 15 kDa disulfide bond (J-chain) are connected in the cytoplasm and constantly aggregate to form the polymeric immunoglobulin receptor (pIgA). pIgA and the 80 kDa polymeric immunoglobulin receptor (pIgR) are combined and translocated to the surface of epithelial cells to form SIgA. However, PIgR can delay the degradation of SIgA without affecting the affinity of the antigen (34). Intestinal SIgA secretion is mediated by T cell-dependent and non-T cell-dependent immune regulation. In the T cell-dependent pathway, the interaction between antigen-presenting cells and Th2 cells releases Th2 cytokines, which promote the proliferation of IgA+ B cells and their differentiation into IgA-secreting plasma cells. Studies have shown that the dependence of T cells on mouse intestinal SIgA is also important (35) because mature B cells express IgA only after class switch recombination (CSR). In the T cell-independent response, B cells can be activated by triggering toll-like receptors, B cell receptor (BCR) antigen compounds or activated by DC-presented natural antigens, and the activation of the B cell activating factor of the TNF family (BAFF) and proliferation inducing ligand (APRIL) are important factors for IgA type switching and recombination (36, 37). IL-10 promotes the proliferation and differentiation of B cells into IgA-secreting plasma cells and also acts as an anti-inflammatory cytokine to inhibit NKT cell-mediated colitis and protect intestinal mucosa by regulating the expression of CD1d in intestinal epithelial cells (38, 39). The above discussion is involved, which needs to be further improved and explored its mechanism by our laboratory.
In conclusion, oral DBD can improve the immune function of mice immunized with the O-type FMD vaccine by improving serum-specific antibodies and the intestinal mucosal immune response, which could boost the immune system and improve the immune effect of the FMD vaccine.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Laboratory Animal Ethical Review Committee, Southwest University, Chongqing, China. Written informed consent was obtained from the owners for the participation of their animals in this study.
BZ and LC conceived and designed the experiments. BZ, SB, and HD performed the experiments and collected and analyzed the data. HH and FG wrote the article, while HH, FG, and LC revised the article. All authors read and approved the final manuscript.
This research was supported by Chongqing Research Program of Basic and Frontier Technology (cstc2020jcy-jmsxmX0418) and Fundamental Research Funds for the Central Universities (SWU120002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Diaz-San Segundo F, Medina GN, Stenfeldt C, Arzt J, de Los Santos T. Foot-and-mouth disease vaccines. Vet Microbiol. (2017) 206:102–12. doi: 10.1016/j.vetmic.2016.12.018
2. Pega J, Bucafusco D, Di Giacomo S, Schammas JM, Malacari D, Capozzo AV, et al. Early adaptive immune responses in the respiratory tract of foot-and-mouth disease virus-infected cattle. J Virol. (2013) 87:2489–95. doi: 10.1128/JVI.02879-12
3. Carpenter TE, O'Brien JM, Hagerman AD, McCarl BA. Epidemic and economic impacts of delayed detection of Foot-And-Mouth disease: a case study of a simulated outbreak in California. J Vet Diagn Invest. (2011) 23:26–33. doi: 10.1177/104063871102300104
4. Ren HR, Jin Z, Pei X, Li MT, Wang YM, Zhang J. Assessment of immunization procedures for foot-and-mouth disease in large-scale pig farms in China based on actual data and dynamics. Anim Dis. (2022) 2:45–60. doi: 10.1186/s44149-021-00035-3
5. Li W, Shan AT, Yuan FY, Zhou DN, Zhao HZ, Liu ZW, et al. Immune purification and effect evaluation of foot and mouth disease in large-scale breeding farms. Chin J Vet Sci. (2021) 41:2299–303. doi: 10.16303/j.cnki.1005-4545.2021.12.01
6. Tian Q, Zhou Y, Zhang MH, Zhou KG, Cheng ZT, et al. R and GAM analysis for environment factors influencing on the immune effect of FMD, PRRS and CSF in pigs in Guizhou province. Chinese J Preventive Vet. (2020) 42:690–695. doi: 10.3969/j.issn.1008-0589.201908030
7. Chen ZJ, Liu LJ, Gao CF, Chen WJ, Vong CT, Yao P, et al. Astragali Radix (Huangqi): a promising edible immunomodulatory herbal medicine. J Ethnopharmacol. (2020) 258:112895. doi: 10.1016/j.jep.2020.112895
8. Wang D, Liu Y, Zhao W. The adjuvant effects on vaccine and the immunomodulatory mechanisms of polysaccharides from traditional Chinese medicine. Front Mol Biosci. (2021) 8:655570. doi: 10.3389/fmolb.2021.655570
9. Yang FX, Wang Y, Xia PF, Yang RJ, Wang YX, Zhang J, et al. Review of chemical constituents, pharmacological effects and clinical applications of Danggui buxue decoction and prediction and analysis of its Q-markers. China J Chin Materia Med. (2021) 46:2677–85. doi: 10.19540/j.cnki.cjcmm.20200828.201
10. Shi XQ, Zhu ZH, Yue SJ, Tang P, Chen YY, Pu ZJ, et al. Integration of organ metabolomics and proteomics in exploring the blood enriching mechanism of Danggui Buxue Decoction in hemorrhagic anemia rats. J Ethnopharmacol. (2020) 261:113000. doi: 10.1016/j.jep.2020.113000
11. Wang X, Bei H, Du R, Chen Q, Wu F, Chen J, et al. Metabolomic analysis of serum reveals the potential effective ingredients and pathways of Danggui Buxue Tang in promoting erythropoiesis. Complement Ther Med. (2020) 48:102247. doi: 10.1016/j.ctim.2019.102247
12. Li C, Zhu F, Wang S, Wang J, Wu B. Danggui Buxue Decoction ameliorates inflammatory bowel disease by improving inflammation and rebuilding intestinal mucosal barrier. Evid Based Complement Alternat Med. (2021) 2021:8853141. doi: 10.1155/2021/8853141
13. Deng P, Li X, Wei Y, Liu J, Chen M, Xu Y, et al. The herbal decoction modified Danggui Buxue Tang attenuates immune-mediated bone marrow failure by regulating the differentiation of T lymphocytes in an immune-induced aplastic anemia mouse model. PLoS ONE. (2017) 12:e0180417. doi: 10.1371/journal.pone.0180417
14. McCright J, Ramirez A, Amosu M, Sinha A, Bogseth A, Maisel K. Targeting the gut mucosal immune system using nanomaterials. Pharmaceutics. (2021) 13:1755. doi: 10.3390/pharmaceutics13111755
15. Gu P, Wusiman A, Zhang Y, Cai G, Xu S, Zhu S, et al. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant for H9N2 vaccine to improve immune responses in chickens compared to Alum and oil-based adjuvants. Vet Microbiol. (2020) 251:108894. doi: 10.1016/j.vetmic.2020.108894
16. Yuan D, Yuan Q, Cui Q, Liu C, Zhou Z, Zhao H, et al. Vaccine adjuvant ginsenoside Rg1 enhances immune responses against hepatitis B surface antigen in mice. Can J Physiol Pharmacol. (2016) 94:676–81. doi: 10.1139/cjpp-2015-0528
17. Xu Q, Zhou Y, Zhang R, Sun Z, Cheng LF. Antiarthritic activity of Qi-Wu Rheumatism Granule (a Chinese Herbal Compound) on complete Freund's adjuvant-induced arthritis in rats. Evid Based complement Alternat Med. (2017) 2017:1960517. doi: 10.1155/2017/1960517
18. Zeng Y, Zhang SY, Hu GY. Research progress of Danggui Buxue Decotion. Lishizhen Med Materia Medica Res. (2016) 27:422–4. doi: 10.3969/j.issn.1008-0805.2016.02.062
19. Fu YF, Jiang LH, Zhao WD, Meng XN, Huang SQ, Yang J, et al. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci Rep. (2017) 7:8676. doi: 10.1038/s41598-017-09340-9
20. Li ZH, Zhang JQ, Wang T, Zhang JF, Zhang LL, Wang T. Effects of capsaicin on growth performance, meat quality, digestive enzyme activities, intestinal morphology, and organ indexes of broilers. Front Vet Sci. (2022) 9:841231. doi: 10.3389/fvets.2022.841231
21. Sun YX. Immunological adjuvant effect of a water-soluble polysaccharide, CPP, from the roots of Codonopsis pilosula on the immune responses to ovalbumin in mice. Chem Biodivers. (2009) 6:890–6. doi: 10.1002/cbdv.200800154
22. Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. (2003) 17:112–8. doi: 10.1016/S0889-1591(02)00077-6
23. Stavnezer J. Antibody class switching. Adv Immun. (1996) 61:79–146. doi: 10.1016/S0065-2776(08)60866-4
24. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. definition according to profiles of lymphokine activities and secreted proteins. J Immunol. (1986) 136:2348–57.
25. Coutelier JP, van der Logt JT, Heessen FW, Vink A, van Snick J. Virally induced modulation of murine IgG antibody subclasse. J Exp Med. (1988) 168:2373–8. doi: 10.1084/jem.168.6.2373
26. Faquim-Mauro EL, Coffman RL, Abrahamsohn IA, Macedo MS. Cutting edge: mouse IgG1 antibodies comprise two functionally distinct types that are differentially regulated by IL-4 and IL-12. J Immunol. (1999) 163:3572–6. doi: 10.1093/intimm/12.12.1733
27. Stavnezer J. Immunoglobulin class switching. Curr Opin Immunol. (1996) 8:199–205. doi: 10.1016/S0952-7915(96)80058-6
28. Wurster AL, Rodgers VL, Satoskar AR, Whitters MJ, Young DA, Collins M, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon γ-producing Th1 cells. J Exp Med. (2002) 196:969–77. doi: 10.1084/jem.20020620
29. Shi YX, Yan JK, Wang XM, Zhang SL, Yu JJ. Effect of the Chinese herbal extract on the number of intraepithelial lymphocytes and goblet cells and the level of IL-2 in the intestine of stressed mice. Chin Vet Sci. (2011) 41:85–8. doi: 10.16656/j.issn.1673-4696.2011.01.015
30. Zhai L, Li Y, Wang W, Wang Y, Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. (2011) 29:5007–14. doi: 10.1016/j.vaccine.2011.04.097
31. Li Y, Jin L, Chen T. The Effects of secretory IgA in the mucosal immune system. Biomed Res Int. (2020) 2020:2032057. doi: 10.1155/2020/2032057
32. Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. (1986) 46:613–21. doi: 10.1016/0092-8674(86)90887-1
33. Xie F, Sakwiwatkul K, Zhang C, Wang Y, Zhai L, Hu S. Atractylodis macrocephalae Koidz. polysaccharides enhance both serum IgG response and gut mucosal immunity. Carbohydr Polym. (2013) 91:68–73. doi: 10.1016/j.carbpol.2012.07.083
34. Woof JM, Kerr MA. The function of immunoglobulin A in immunity. J Pathol. (2006) 208:270–82. doi: 10.1002/path.1877
35. Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5(J). Nat Immunol. (2008) 9:769–76. doi: 10.1038/ni.1622
36. Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. (2001) 293:2111–4. doi: 10.1126/science.1061964
37. Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. (2007) 8:294–303. doi: 10.1038/ni1434
38. Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. (2014) 509:497–502. doi: 10.1038/nature13150
Keywords: Danggui Buxue decoction, foot-and-mouth disease vaccine, humoral immunity, intestinal mucosal immunity, immune enhancement
Citation: Zhou B, Huang H, Gui F, Bi S, Du H and Cao L (2022) Enhancement of intestinal mucosal immunity and immune response to the foot-and-mouth disease vaccine by oral administration of danggui buxue decoction. Front. Vet. Sci. 9:1045152. doi: 10.3389/fvets.2022.1045152
Received: 15 September 2022; Accepted: 13 October 2022;
Published: 08 November 2022.
Edited by:
Dayou Shi, South China Agricultural University, ChinaReviewed by:
Wei Xu, Zhejiang University, ChinaCopyright © 2022 Zhou, Huang, Gui, Bi, Du and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liting Cao, Y2FvbGl0aW5nQHN3dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.