- 1Investigación en Técnicas de Reproducción Asistida – Universidad de León, Instituto de Desarrollo Ganadero y Sanidad Animal, University of León, León, Spain
- 2Animal Reproduction and Obstetrics, Department of Veterinary Medicine, Surgery and Anatomy, University of León, León, Spain
- 3Celular Biology, Department of Molecular Biology, University of León, León, Spain
- 4Anatomy, Department of Veterinary Medicine, Surgery and Anatomy, University of León, León, Spain
- 5Laboratory of Equine Reproduction and Equine Spermatology, Veterinary Teaching Hospital, University of Extremadura, Cáceres, Spain
- 6Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
The frequency of semen collection is a crucial factor to consider in the rams performance inside breeding centers workout. To evaluate this factor, ram Breeding Soundness Evaluation could include sperm quality evaluation and new predictive and non-invasive tools such as ultrasound technique. In this work, an advanced ultrasonography technology, analyzing the testicular volume, echotexture, and vascular function, was used in three different frequencies of semen collection (abstinence frequency, AF; standard frequency, SF; and intensive frequency, IF). Semen samples were cooled (15◦C, 6 h) and evaluated in terms of production, motility, viability, apoptosis, and content of reactive oxygen species. Correlation coefficients were calculated between ultrasonography measurements of echotexture and blood flow and sperm quality parameters. Our results showed an increase in the testicular echotexture when the frequency of semen collection was intensified. Doppler parameters (PSV, RI, PI, TABF) increased (P ≤ 0.05) when the frequency of semen collection was intensified. The sperm motility and functionality decreased in the samples of IF (P ≤ 0.05), evidencing the frequency of semen collection's influence. Moreover, moderate positive correlations were established among echotexture and different Doppler parameters with motility parameters in SF. Furthermore, the influence of abstinence days on AI success was analyzed in a field assay. The highest fertility rates were obtained when males had two to five abstinence days. To conclude, frequency of semen collection could be influenced in terms of semen quantity and sperm quality, showing changes in parenchyma echotexture and testicular vascularization. The standard semen collection frequency was the most adequate option. In addition, ultrasonography may be a predictive tool for estimating variations in the sperm quality of donor rams subjected to different frequencies of semen collection in reproduction centers.
Introduction
Reproductive performance is the most important parameter affecting flock profitability (1), and the reproductive capacity of the rams plays a key role (2). MacLaren (3) suggests that 50% of the reproductive potential of a flock is provided by the ram (3). Testis evaluations are performed to assess the overall potential capacity of rams to serve and impregnate ewes, also known as the Breeding Soundness Evaluation (BSE) (2). A BSE may include anatomical and structural examinations and assessment for health status, body condition score (BCS), testicular measurements, sperm quality, and libido (4). In this context, sexual behavior and semen characteristics are the main parameters limiting male reproductive efficiency, and both of them are greatly influenced by the frequency of semen collection. Variations in ejaculation frequency induce changes in the sperm maturation process, sperm functional characteristics, ionic composition, and enzymatic activity of the seminal plasma (5, 6). In a previous study, Ollero et al. (5) documented that different periods of abstinence could affect sperm quality in terms of viability, motility, and acrosome integrity in the ovine species. To our knowledge, there are no studies in a large number of rams assessing the effect of semen collection frequency in male reproductive capacity, semen production, and quality employing innovative and predictive techniques.
Traditional methods, such as measurement of scrotal circumference and libido or palpation and manipulation of the genital organs, have been used to evaluate the potential reproductive capacity of rams (3, 7, 8). In recent years, several new tools, including ultrasonography, have been used to predict variations in semen characteristics and the ram's reproductive capacity in reproduction centers and flocks (9, 10). Specifically, ultrasonography is a non-invasive, non-ionizing, and non-damaging technique and an indispensable tool in reproductive clinics that provides real-time and sequential information on male reproductive performance (11, 12). The B-mode ultrasound has been used in different domestic animals species as a valid tool to estimate the testicular volume (13–15), estimate the parenchyma echotexture (16–18), and identify uncertain clinical findings, such as early stages of macroscopic pathological processes or monitoring changes in lesions (12). Color and Pulse Doppler ultrasonography has been employed to characterize and quantify testes' blood flow in different species, such as stallions (19) and dogs (20). This technology has also been used to evaluate scrotal disorders in dogs (14) and camelids (21) and correlate them with sperm quality. Several studies have assessed using B-mode ultrasound in physiological and pathological conditions (12, 22–24), and the relationship between the puberty and the changes in the echogenicity of the parenchyma (25–28) in ovine andrology. In addition, several studies on the use of Doppler ultrasonography for the evaluation of testicular blood flow in rams are available (9, 10, 26, 29) that consider the influence of this parameter in testicular function. Testicular blood flow is the main route through which all the required nutrients, oxygen, regulatory hormones, and secretory products are regulated and exchanged to and from the testes (30). In this respect, different studies have evidenced an association between testicular blood flow and sperm quality in several species such as humans (31), stallions (13, 32), or rams (9, 10), and used Doppler parameters to diagnose fertility rates in camelids (21) or dogs (14). Because of this, we hypothesize that semen collection frequency could be a crucial factor causing testicular changes in those parameters detected by ultrasound and in sperm quality.
Considering the importance of rams management in terms of performance within a breeding center, the first objective of this study is to investigate the effects of the frequency of semen collection on male reproductive performance including testicular function, and semen quantity and quality employing a multiparametric approach based on testicular morphometry, echotexture and vascularization, blood testosterone level, libido, and sperm motility and functionality parameters. Secondly, the study aims to approach the possible association between sperm quality parameters and ultrasonography measurements in the different semen collection frequencies. As a third aim, we study the direct consequences of different semen extraction frequencies in terms of sperm quality and fertility in a field assay.
Materials and methods
Animals
Twent-five sexually mature (age range 2–7 years) Assaf rams were used during the breeding season in the current study. All the rams were previously examined, and they did not have any disease. Animals were housed grouped (five animals per each group) in closed pens with access to an open area at the Animal Selection and Reproduction Center of the Junta de Castilla y León (CENSYRA) (Villaquilambre, León, Spain), where they were fed on a standard balanced diet. The current study was performed in accordance with the Guidelines of the European Union Council (2010/63/EU), following Spanish regulations (RD/1201/2005, abrogated by RD/53/2013) for the use of laboratory animals. All the experiments were approved by the Institutional Animal Care and Use Committee at the University of León (ÉTICA-ULE-013-2018).
Experimental design
Experiment 1: Evaluation of different semen collection frequencies
Sexually mature Assaf rams were used during the breeding season. All rams were enrolled in the abstinence semen collection frequency (AF) where the males were sexually rested and semen was not collected for 1 month. At the end of this period, testicular volume, testicular echotexture, and Doppler parameters were assessed. After the animal's evaluation, two consecutive ejaculates per ram were collected and mixed, measuring the following parameters: ejaculate volume, sperm concentration, and total sperm production. Ejaculates were analyzed including motility and sperm physiology parameters (detailed in section “Sperm evaluation”). Then, all rams were enrolled in the standard semen collection frequency (SF) for 1 month: two consecutive ejaculates per day/two collection days per week. At the end of this period, all ultrasonographic measurements were repeated, and, after the animal's evaluation, two consecutive ejaculates per ram were collected, mixed, and analyzed. To conclude this experiment, the 25 rams were enrolled in the intensive semen collection frequency (IF): two consecutive ejaculates per day/five collection days per week (Monday–Friday). Again, 1 month later, all ultrasonographic measurements were repeated. In this scenario, after 2 days of abstinence by the weekend, two consecutive ejaculates per ram were collected and analyzed on Monday (IFM) and, after five consecutive days of semen collection, two consecutive ejaculates per ram were collected and analyzed on Friday (IFF). The experimental design is shown in Figure 1.
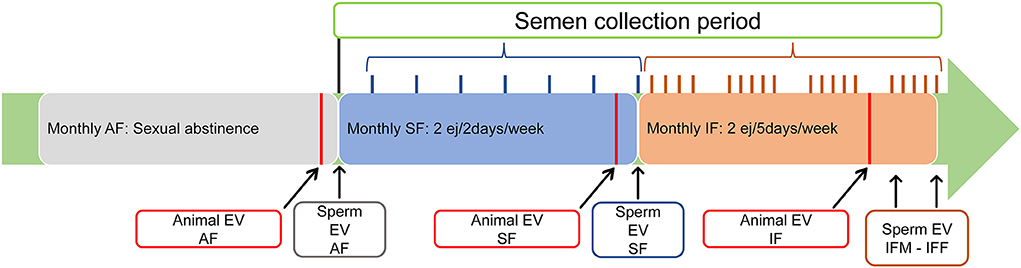
Figure 1. Experimental design. All three semen collection frequencies were extended for 1 month. Animal evaluation (Animal EV): clinical examination (inspection and palpation of external genitalia, libido, and blood collection) and ultrasonography evaluation (testicular volume, echotexture, and blood flow) were carried out at least 24 h before experimental semen collection). Sperm evaluation (Sperm EV): sperm production, motility, and physiology were performed at the end of the evaluated semen collection frequency. AF, abstinence semen collection frequency; SF, standard semen collection frequency; IFM, intensive semen collection frequency on Monday; IFF, intensive semen collection frequency on Friday.
Experiment 2: Ram's performance and sperm yield: A field assay
In this part of the experiment, we tried to extrapolate our findings to field assay. In a reproduction center simulation, ejaculates obtained from our experimental rams (25 males) in experiment 1 were classified following the reproduction center criteria in valid (ejaculate volume > 0.5 ml, mass motility > 3, sperm concentration > 3,000 × 106 sperm/ml) and non-valid ejaculates (some of the criteria below the minimum value) as previously described Neila-Montero et al. (33). Then, sperm quality, including motility and cytometry, was assessed in both experimental groups.
For the fertility trial, the experimental groups were classified by male abstinence period before semen collection for insemination: the fertility standard interval (SI), when seminal doses were obtained from males with two–five abstinence days before semen collection for AI; the fertility high interval (HI), when seminal doses were obtained from males with at least 10 abstinence days before semen collection for AI; the fertility low interval (LI), when seminal doses were obtained from males with semen collection the previous day for AI; the annual fertility (AN), annual average fertility from the National Association of Breeders of the Assaf Sheep Breed (ASSAF.E). A descriptive assay was performed to assess the effect of the abstinence period of semen collection on fertility AI.
For this purpose, 357 seminal doses (400 × 106 sperm/dose) from 10 mature Assaf rams housed in the Sheep and Goat Selection and Genetic Improvement Center of Castilla y León (Ovigén; Villalazán, Junta de Castilla y León, Spain) were used during the breeding season. Ejaculates were diluted to 1,600 × 106 sperm/ml with INRA 96 and cooled using a rate of −0.5°C/min from 30°C down to 15°C using a programmable water bath (CC-K8, Huber, Germany). A total of 357 Assaf adult ewes from six commercial farms were inseminated 6–8 h after semen collection. Females were subjected to treatment for estrus induction and synchronization using intravaginal sponges with 20 mg fluorogestone acetate (Chronogest®, MSD, Kenilworth, NJ, USA) over 14 days. The sponges were removed, and ewes were treated with 500 IU of eCG intramuscular (Folligon®, MSD, Bogotá, Colombia). Cervical artificial inseminations (54 ± 1 h post sponges removal) were performed by two experienced veterinarians of ASSAF.E. Reproductive success was evaluated in terms of fertility (lambing ewes/inseminated ewes (%) according to the births registered at 137–154 days post-insemination).
Previous clinical examination
Before being included in the study, every single male underwent a general clinical examination, visual inspection of the scrotum, and testicular palpation for the evaluation of consistency, symmetry, mobility, and sensitivity of testes. Epididymis and pampiniform plexus were also palped in order to ensure that no observable gross pathology was resent on the external genitalia. Then, the rams were mildly sedated with xylazine (0.05 mg/kg), administered intramuscularly, and restrained in the standing position using a containment rack. The wool on both sides of the scrotum was shaved.
Testosterone levels
After all clinical measures, blood samples were collected into a vacutainer tube without anticoagulant from the jugular vein. The samples were refrigerated at 5°C, and the blood serum was collected and stored at −20°C until assayed. A commercial ELISA kit using the Immulite 2000 XPi Immunoassay System (Siemens, Eschborn, Germany) was used to determine the serum testosterone. According to the manufacturer's instructions, the sensitivity was 0.15 ng/ml, and the intra- and inter-assay coefficients of variation were 5.1 and 7.2% when the average samples were 9.91 ng/ml.
Libido evaluation
Libido was subjectively categorized (zero to 10 score). We calculated time spent from contact with a female decoy to ejaculation in both daily semen collections. Time intervals were used to qualify the rams. If ejaculation occurred in 1 min or less, the male obtained the maximum score (10). If ejaculation occurred in 1–2 min, the male obtained a score of nine. If ejaculation occurred in 2–3 min, the male obtained a score of eight, etc. When ejaculation occurred after more than 10 min, the male obtained a score of zero. The average score of the two ejaculations was calculated.
Testicular volume
All the ultrasonographic measurements were carried out by the same technician. All examinations were performed using a real-time ultrasound scanner, EXAPAD (IMV, France), equipped with a 7.5 MHz linear array. The transducer was covered with a copious amount of gel to facilitate ultrasonographic imaging. Scanning was performed without pressure to avoid a distortion of the testicular shape. Images of the caudocranial, lateral-lateral, and ventrodorsal axis of the testes were obtained. The testicular width, height, and length were measured using electronic calipers integrated into the ultrasound machine. Cursors were set at the borders of the tunica albuginea. The measurements were performed in triplicate of three different images (technical replicates). The echogenicity, homogeneity, and surface of the scrotal contents were also assessed. The testicular volume was calculated using the formula described by Hedia et al. (9): L × H × W × 0.71.
Initial semen handling
Semen collection was performed during the breeding season. At all the sampling times, the ejaculate volume, sperm concentration, and total sperm output (ejaculate volume × sperm concentration) were calculated immediately after collection. Ejaculates were collected by artificial vagina at 40°C (IMV Technologies, L'Aigle, France) in the presence of a female decoy, and the tubes were maintained at 30°C before cooling. All the semen collections were carried out by the same investigator. The ejaculate volume was estimated by collecting them in Falcon® type graduated semen collection tubes. Sperm concentration was assessed by a cell counter (NucleoCounter SP-100, ChemoMetec, Allerod, Denmark). Ejaculates were diluted 1:1 (v/v) with INRA 96. The samples were then refrigerated using a rate of −0.5°C/min from 30°C down to 15°C. After that, the final concentration was adjusted to 1,600 × 106 sperm/ml and the samples were packed into 0.25 ml plastic straws. The seminal doses were stored at 15°C. Sperm evaluation was performed 6–8 h after semen collection.
Ultrasonography evaluation of testicular function
For testicular evaluation function, all examinations were carried out by the same technician. Measurements were performed using the same real-time ultrasound scanner equipped with a 7.5 MHz linear array and 10 MHz high-frequency linear array transducers to evaluate the testicular echotexture and testicular blood flow, respectively.
Testicular echotexture
The probe was positioned by pressing on the center of the testicle transversely, and at least three clips per testicle were measured (technical replicates). The software Ecotext® (HUMECO; Huesca, Spain) was used for analyzing the parenchyma echotexture. The following parameters were measured: Ecotext 1 (EC1: black pixels number), Ecotext 2 (EC2: white pixels number), Ecotext 3 (EC3: mean gray level of pixels), the tubular density (density of tubules/cm2), the tubular diameter [mean diameter (μm) of the lumen of the seminiferous tubules] and the tubular area [proportion (%) of the total area that was occupied by the lumen of the tubules in the parenchyma].
Testicular blood flow
Doppler parameters were measured in the supratesticular artery located in the spermatic cord region, and the transducer was positioned at a midway point between the inguinal ring and the testicle (34). For distinguishing between a testicular artery and vein by Doppler analysis, an artery, for example, will have a waveform on the spectral graph that reflects the arterial pulse in each cardiac cycle (systole and diastole). However, the flow in a vein is almost constant, that is, without a pulse. At least three consecutive waveforms were measured per testicle (technical replicates), and the Doppler parameters were automatically calculated by the software package provided with the ultrasound machine. Peak systolic velocity (PSV) was measured, and the Doppler indices studied were resistive index [RI = (maximum velocity-minimum velocity)/maximum velocity] and pulsatility index [PI = (maximum velocity-minimum velocity)/mean velocity]. Furthermore, the total artery blood flow (TABF) was calculated [mean velocity × A; A (cross-section of the artery): πr2]. Based on previous studies (11, 19, 35), the angle between the long axis of the vessel and the Doppler beam was from 20 to 60 degrees in the direction of the blood flow. Additionally, the Doppler gate was kept constant at 1 mm. To minimize variations in measurements, the ultrasound settings (focus, gains, brightness, and contrast) were standardized, fixed, and used equally for all examinations like others authors (9).
Sperm evaluation
Motility and kinetic parameters by CASA
The motility and kinetic parameters were assessed using the Assisted Sperm Analysis (CASA) (Sperm Class Analyzer -SCA- software V 6.3.0.59; Microptic S.L., Barcelona, Spain). The parameters setting was set to capture at 100 frames/s a total of 50 frames, and particles with an area of 20–70 μm2 were considered compatible with the head area. Aliquots of each ejaculate were diluted to 25 × 106 sperm/ml in an extender (TES-Tris-fructose and 1% egg yolk) and tempered on a 37°C plate for 5 min. After that, a 5 μl drop was placed in a Makler counting cell chamber (10 μm depth; Sefi Medical Instruments, Haifa, Israel). Samples were examined with an × 10 negative phase contrast objective in a microscope equipped with a warmed stage at 38°C (Eclipse E400, Nikon, Tokyo, Japan). At least 400 sperm from four different randomly selected fields were captured and analyzed. The reported kinetic parameters were the velocity according to the straight path (VSL, μm/s); the amplitude of the lateral displacement of the sperm head (ALH, μm); the head beat-cross frequency (BCF, Hz); the total motility (TM), defined as the percentage of sperm with VCL (curvilinear velocity) >15 μm/s; the progressive motility (PM), defined as the percentage of sperm with VCL >45 μm/s; and the rapid progressive motility (RAP PM), defined as the percentage of sperm with VCL >75 μm/s. All parameters were previously described by Palacin-Martinez et al. (36).
Sperm functionality by flow cytometry
Staining for determination of viability, caspases 3 and 7 activity, and mitochondrial functionality
Fluorescence probe Zombie Violet™ Fixable Viability Kit was acquired from BioLegend (San Diego, CA, USA), and CellEvent™ Caspase-3/7 Green Detection Reagent and CellROX™ Deep Red Reagent were supplied from ThermoFisher (Invitrogen, Eugene, Oregon, USA).
For staining, a protocol previously described by Riesco et al. (37) was used. Sperm samples were diluted in PBS to a concentration of 2 × 106 sperm/ml to wash the cells by short centrifugation (15”; MiniSpin plus, Eppendorf, Hamburg, Germany) with the removal of the supernatant. Then, cells were incubated at room temperature and in the dark for 30 min with 96 μl Zombie Violet™ (1:1,000 final dilution), 2 μl CellEvent™ Caspase-3/7 (4 μM final concentration), and 2 μl CellROX™ (5 μM final concentration). After that, a new wash was performed to stop cell staining and avoid an over-staining effect, and the pellet was resuspended in 1 ml PBS, immediately conducting the analysis by flow cytometry.
The combination Zombie Violet™ Fixable Viability Kit, CellEvent™ Caspase-3/7 Green Detection Reagent, and CellROX™ Deep Red was used to simultaneously determine the viability through plasma membrane integrity, caspases 3 and 7 activity as a marker of apoptosis, and mitochondrial function through reactive oxygen species (ROS) content, respectively.
Flow cytometry analyses
Flow cytometry analyses were conducted in the flow cytometer MACSQuant Analyzer 10 (Miltenyi Biotech, Bergisch Gladbach, Germany) equipped with three lasers emitting at 405, 488, and 635 nm (violet, blue, and red, respectively) and 10 photomultiplier tubes. Violet fluorescence was detected in V1 (excitation 405 nm, emission 450/50 nm), green fluorescence was detected in B1 (excitation 488 nm, emission 525/50 nm), and red fluorescence was detected in R1 [excitation 635 nm, emission 655–730 nm (655 LP + split 730)]. Samples were acquired using MACS Quantify software (Miltenyi Biotech, Bergisch Gladbach, Germany), recording a total of 40,000 cells per sample at a flow rate of 200–300 cells/s. Data were analyzed using FlowJo V 10.2 (Ashland, Wilmington, DE, USA). The interest sperm subpopulations assessed were plotted as follows: viable sperm (Zombie Violet™ low intensity -alive-), apoptotic sperm (CellEvent™ Caspase 3/7 positive), and sperm with high mitochondrial activity (CellROX™ positive).
Statistical analyses
Data were analyzed with SAS/STAT® version 9.1 statistical package (SAS Institute, Cary, NC, USA). Data were analyzed by a mixed linear model (MIXED procedure), considering the male effect as a random factor. Libido was analyzed by Kruskal–Wallis test (NPAR1WAY procedure). Relations between sperm quality parameters and ultrasonography measurements were studied by Pearson's correlation. The results are displayed as the mean ± standard error of the mean (SEM). Differences were statistically significant at P ≤ 0.05.
Results
Testicular yield
Significant differences (P ≤ 0.05) were found among all the frequencies of semen collection in the serum testosterone levels (Figure 2A), with the highest levels in the IF. Libido was significantly (P ≤ 0.05) higher in intensive semen collections (IFM and IFF; Figure 2B) compared to AF and SF. Concerning testicular measurements, a significant increase in volume was observed in IF compared to AF (P ≤ 0.05; Figure 2C). According to sperm production, the ejaculate volume was significantly decreased (P ≤ 0.05) in SF and IFF in comparison with AF, but non-significant differences (P > 0.05) between both were found (Figure 2D). Sperm concentration decreased in both sperm evaluations of IF (Figure 2E), and sperm production was gradually lower (P ≤ 0.05) with increasing intensity of semen collection frequency. However, non-significant differences concerning sperm production were revealed between SF and IFM (P > 0.05; Figure 2F).
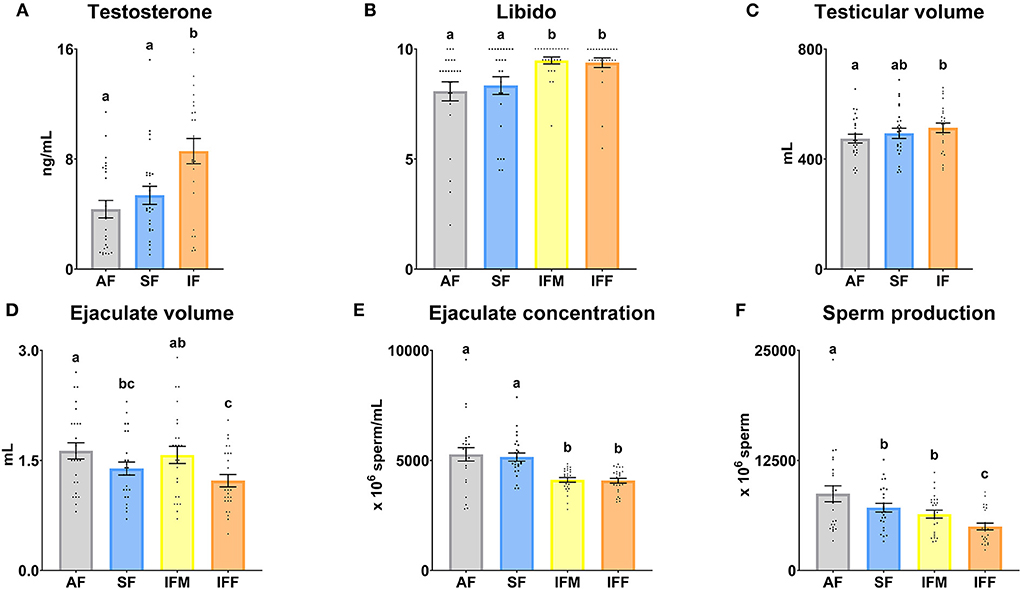
Figure 2. Ram reproductive values according to semen collection frequencies. (A) Testosterone, serum testosterone levels (ng/ml); (B) Libido, subjective evaluation (zero to 10); (C) Testicular volume (ml); (D) Ejaculate volume (ml); (E) Ejaculate concentration (× 106 sperm/ml); (F) Sperm production (× 106 sperm). The same 25 males were analyzed in each experimental group (AF, abstinence semen collection frequency; SF, standard semen collection frequency; IFM, intensive semen collection frequency on Monday; and IFF, intensive semen collection frequency on Friday). Graph dots represent individual male values [graphs (A–C)] and ejaculates [graphs (D–F)]. Means (±SEM) are shown. Different lowercase superscripts letters (a–c) indicate differences (P ≤ 0.05) among the semen collection frequencies.
Ultrasonography evaluation of testicular function
Right and left testis did not show significant differences with respect to echotexture and Doppler parameters (data not shown). Thus, the means of the right and lefts testis were used for further analysis.
The testicular echotexture results are shown in Figure 3. EC1, tubular area and tubular diameter decreased significantly (P ≤ 0.05) when the frequency of semen collection increased. However, EC2 and EC3 increased significantly (P ≤ 0.05) under the same conditions. The tubular density was similar among the semen collection frequencies (P > 0.05).
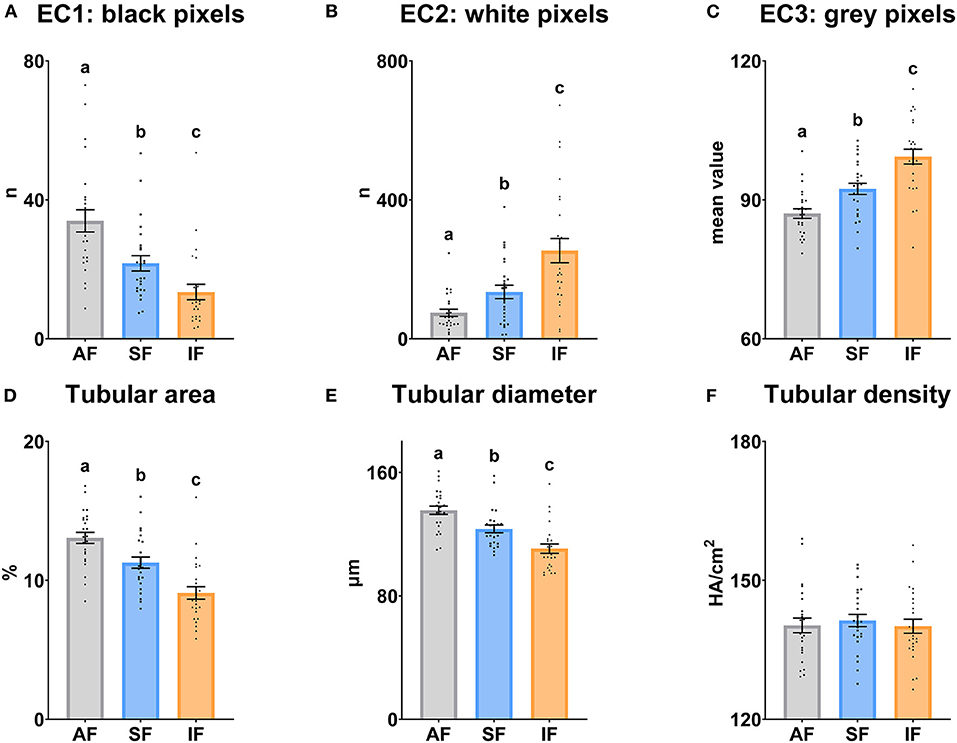
Figure 3. Echotexture characteristics of the ram testes according to semen collection frequencies. (A) EC1, Ecotext 1 (black pixels); (B) EC2, Ecotext 2 (white pixels); (C) EC3, Ecotext 3 (mean value of gray pixels); (D) Tubular area, proportion (%) of the total area corresponding with the lumen of the seminiferous tubules; (E) Tubular diameter, mean diameter (μm) of the lumen of the seminiferous tubules; (F) Tubular density, the density of hypoechogenic areas per cm2 corresponding with the seminiferous tubules. The same 25 males were analyzed in each experimental group (AF, abstinence semen collection frequency; SF, standard semen collection frequency; IF, intensive semen collection frequency). Graph dots represent individual male values. Means (±SEM) are shown. Different lowercase superscripts letters (a–c) indicate differences (P ≤ 0.05) among the semen collection frequencies.
The testicular vascularization results are shown in Figures 4, 5. The PSV parameter was significantly higher (P ≤ 0.05) in SF and IF with respect to AF. In relation to RI and TABF parameters, both increased significantly (P ≤ 0.05) in IF in comparison to AF and SF. In addition, there were significant differences among all semen collection frequencies in PI (P ≤ 0.05).
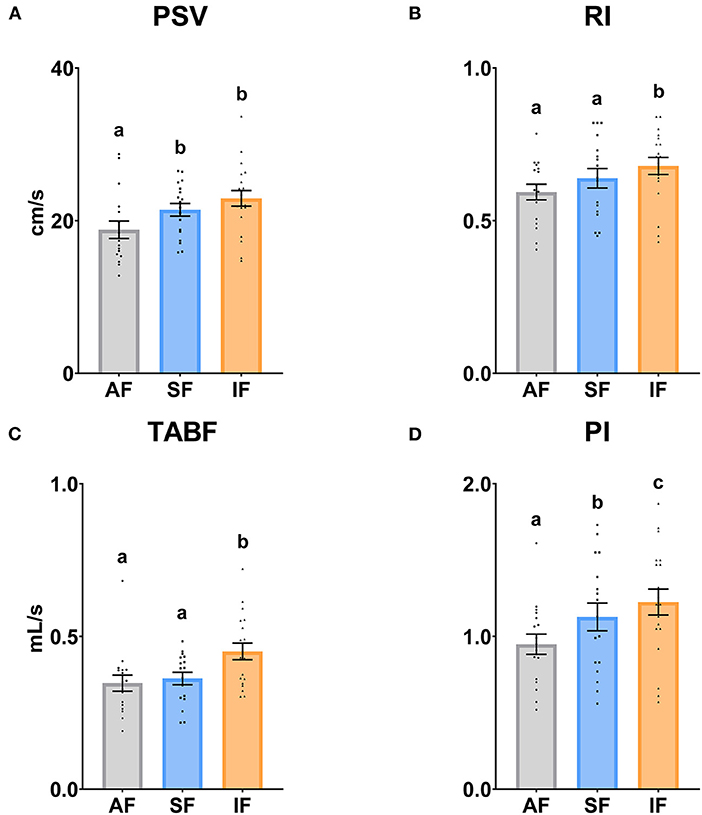
Figure 4. Ram supratesticular artery Doppler parameters according to semen collection frequencies. (A) PSV, peak systolic velocity (cm/s); (B) RI, resistive index; (C) TABF, total artery blood flow (ml/min); (D) PI, pulsatility index. The same 17 males were analyzed in each experimental group (AF, abstinence semen collection frequency; SF, standard semen collection frequency; IF, intensive semen collection frequency). Graph dots represent individual male values. Means (±SEM) are shown. Different lowercase superscripts letters (a–c) indicate differences (P ≤ 0.05) among the semen collection frequencies.
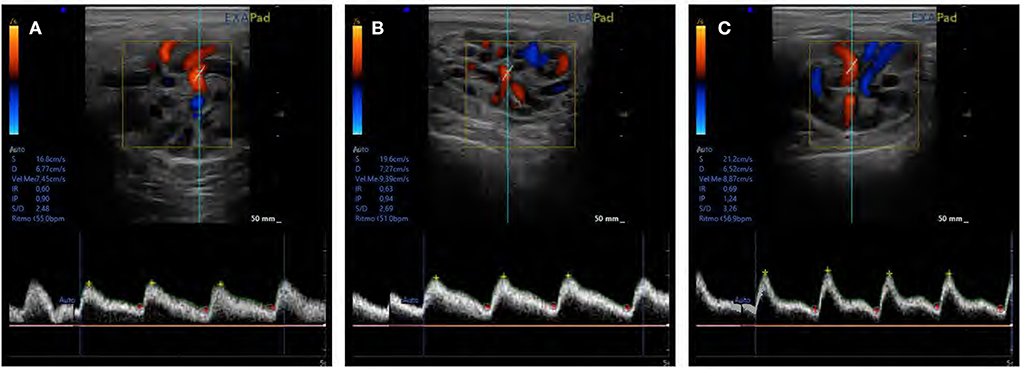
Figure 5. An assessment of testicular blood flow in supratesticular artery using pulse Doppler ultrasonography in (A) abstinence, (B) standard, and (C) intensive semen collection frequencies.
Sperm quality evaluation
Motility and kinetic parameters
The sperm motility parameters are shown in Figure 6. There were significant differences between AF and IFF in TM (P ≤ 0.05), decreasing when the semen collection intensity increased. In the same way, the TM was significantly higher (P ≤ 0.05) in SF in comparison with IFF (Figure 6A). Regarding RAP PM, the highest percentage was observed in AF (P ≤ 0.05; Figure 6C). PM, VSL, and ALH were significantly higher (P ≤ 0.05) in AF and SF in comparison with IFM and IFF (Figures 6B,D,E). BCF was significantly higher (P ≤ 0.05) in AF in comparison with IFM and IFF. In the same way, SF was significantly higher (P ≤ 0.05) than IFF (Figure 6F).
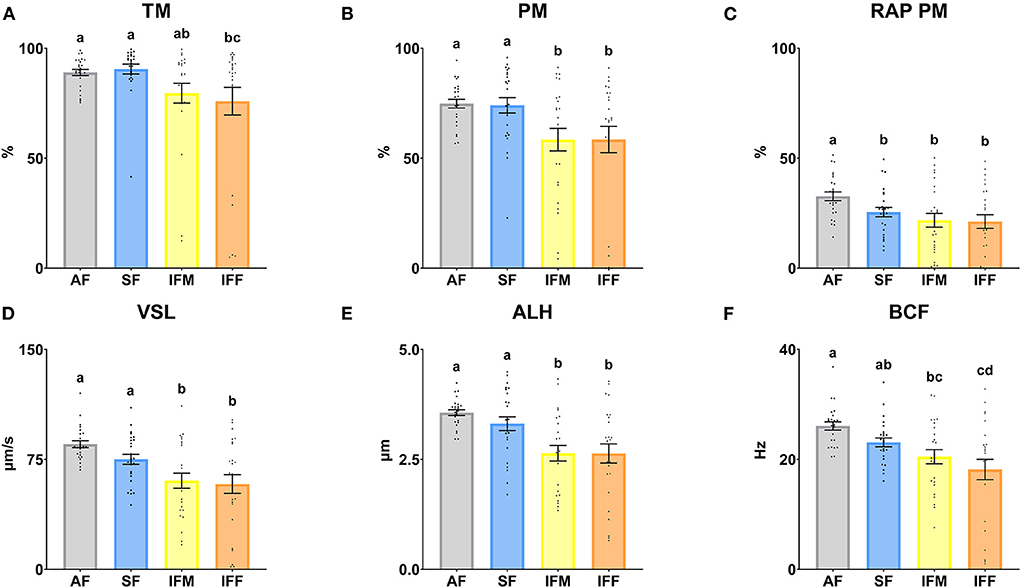
Figure 6. Ram sperm motility according to ejaculate collection frequencies. (A) TM, total motility (%); (B) PM, progressive motility (%); (C) RAP PM, rapid progressive motility (%); (D) VSL, straight-line velocity (μm/s); (E) ALH, head lateral amplitude (μm); (F) BCF, beat frequency (Hz). The same 25 males were analyzed in each experimental group (AF, abstinence semen collection frequency; SF, standard semen collection frequency; IFM, intensive semen collection frequency on Monday; and IFF, intensive semen collection frequency on Friday). Graph dots represent individual male values. Means (±SEM) are shown. Different lowercase superscripts letters (a–d) indicate differences (P ≤ 0.05) among the semen collection frequencies.
Sperm functionality
Attending to the flow cytometry analysis, total viability was significantly lower (P ≤ 0.05) in IFM with respect to AF and SF (Figure 7A). The percentage of apoptotic sperm, with active caspases 3 and 7, was significantly higher (P ≤ 0.05) in IFF compared with the other experimental groups. Moreover, this parameter also was significantly higher (P ≤ 0.05) in SF with respect to AF (Figure 7B). Finally, the percentage of sperm with high mitochondrial activity showed the same statistically significant differences as the previous parameter analyzed (Figure 7C) but with an opposite trend.
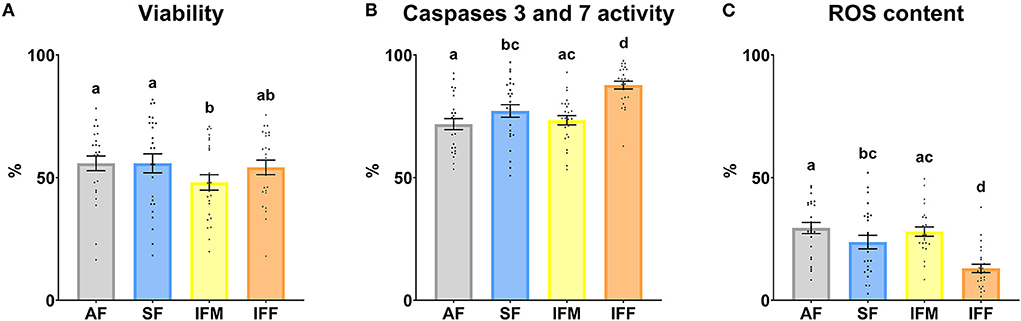
Figure 7. Ram sperm functionality according to ejaculate collection frequencies. (A) Viability, Zombie Violet™ (%); (B) Caspases 3 and 7 activity, CellEvent™ Caspase-3/7 Green (%); (C) ROS content, CellROX™ Deep Red (%). The same 25 males were analyzed in each experimental group (AF, abstinence semen collection frequency; SF, standard semen collection frequency; IFM, intensive semen collection frequency on Monday; and IFF, intensive semen collection frequency on Friday). Graph dots represent individual male values. Means (±SEM) are shown. Different lowercase superscripts letters (a–d) indicate differences (P ≤ 0.05) among the semen collection frequencies.
Correlations between ultrasonography parameters and sperm quality
Correlations between ultrasonography parameters and sperm quality are shown in Figures 8–10, related to AF, SF, and IF, respectively. The highest correlation between ultrasonography and sperm quality in AF was found between Tubular density and RAP PM (R2 = −0.408; Figure 8). Concerning SF, the pulsatility index presented the highest correlation with RAP PM (R2 = 0.637). Also, RI, TABF, Area, and Diameter showed significant moderate positive correlations with motility parameters (Figure 9). Attending to IF, Doppler indexes (RI and PI) correlated strongly and positively with C3&7A. However, the same Doppler indexes showed strong negative correlations with ROS (P ≤ 0.05). All the correlations studied are included in a correlation matrix (Figure 10).
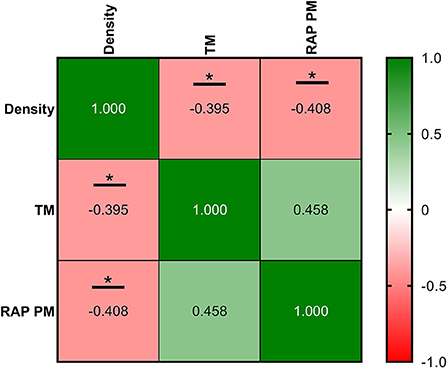
Figure 8. Correlation coefficients between ultrasonography measurements and sperm quality parameters in abstinence semen collection frequency (AF). Density, the density of hypoechogenic areas per cm2 corresponding with the seminiferous tubules; TM, total motility (%); RAP PM, rapid progressive motility (%). The same 25 males were analyzed in each parameter. The R squared value between two parameters is represented in each cell and graph. In the correlation matrix, green color indicates positive correlations, and red color indicates negative relationships. The color intensity represents the strength of the correlation between two parameters. Asterisks show significant correlations (P ≤ 0.05) between ultrasonography measurements and sperm quality parameters.
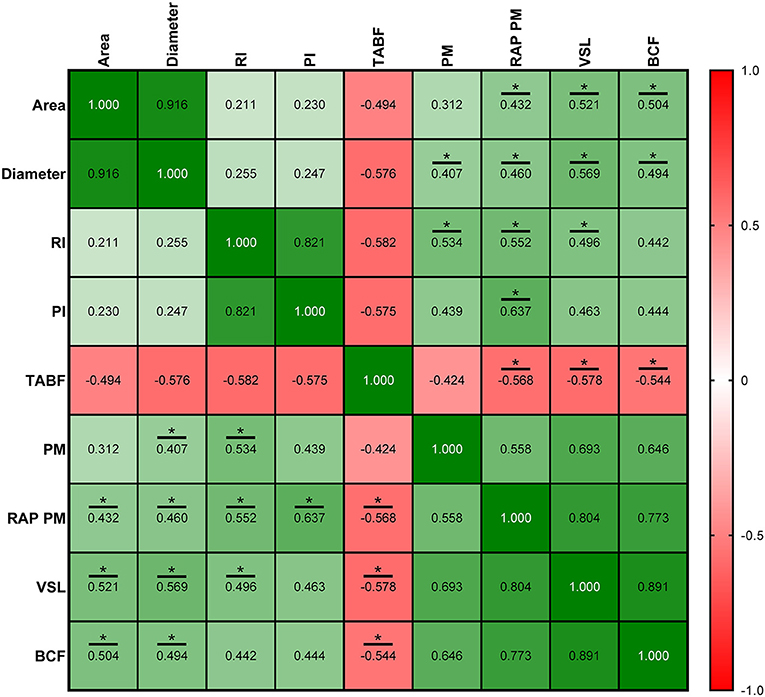
Figure 9. Correlation coefficients between ultrasonography measurements and sperm quality parameters in standard semen collection frequency (SF). Area, proportion (%) of the total area corresponding with the lumen of the seminiferous tubules; Diameter, mean diameter (μm) of the lumen of the seminiferous tubules; RI, resistive index; PI, pulsatility index; TABF, total artery blood flow (ml/min); PM, progressive motility (%); RAP PM, rapid progressive motility (%); VSL, straight-line velocity (μm/s); BCF, beat frequency (Hz). The same 25 males were analyzed in each parameter. The R squared value between two parameters is represented in each cell and graph. In the correlation matrix, green color indicates positive correlations, and red color indicates negative relationships. The color intensity represents the strength of the correlation between two parameters. Asterisks show significant correlations (P ≤ 0.05) between ultrasonography measurements and sperm quality parameters.
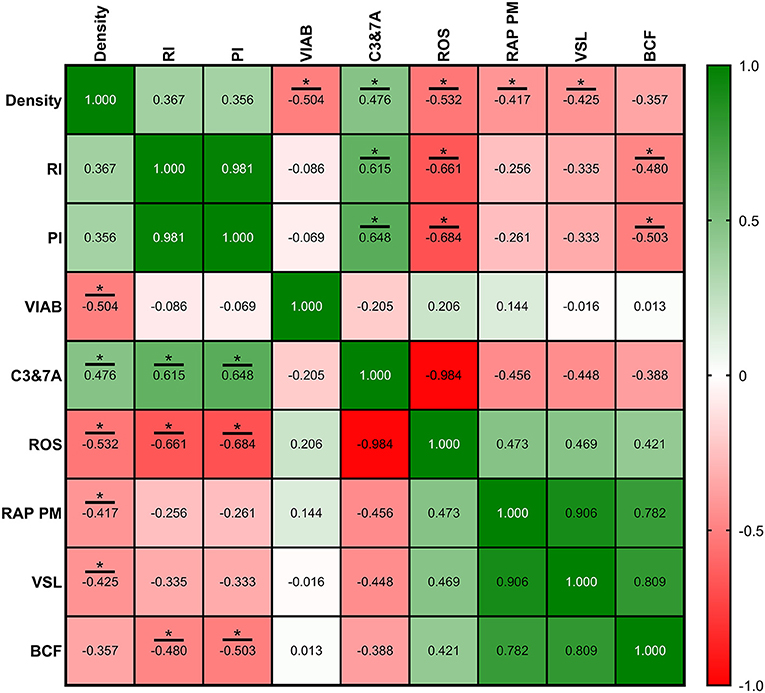
Figure 10. Correlation coefficients between ultrasonography measurements and sperm quality parameters in intensive semen collection frequency (IF). Sperm quality parameters used were measured on Friday (corresponding with IRF). Density, density of hypoechogenic areas per cm2 corresponding with the seminiferous tubules; RI, resistive index; PI, pulsatility index; VIAB, viability (%); C3&7A, caspases 3 and 7 activity (%); ROS, ROS content (%); RAP PM, rapid progressive motility (%); VSL, straight-line velocity (μm/s); BCF, beat frequency (Hz). The same 25 males were analyzed in each parameter. The R squared value between two parameters is represented in each cell and graph. In the correlation matrix, green color indicates positive correlations, and red color indicates negative relationships. The color intensity represents the strength of the correlation between two parameters. Asterisks show significant correlations (P ≤ 0.05) between ultrasonography measurements and sperm quality parameters.
Field results: Semen yield, sperm quality and fertility trials
Concerning ejaculate yield, a higher percentage of valid ejaculates was recorded in SF (76%) compared with IF (60%) (Figure 11A). In terms of ejaculation yield, the percentage of valid sperm numbers and doses were higher in SF in comparison with IF (Figure 11C). Motility parameters registered significant differences (P ≤ 0.05) between SF and IF in valid ejaculates in terms of TM, PM, and RAP PM, being lower in IF. On the other hand, significant differences were found between valid and non-valid ejaculates in IF with respect to TM and PM (P ≤ 0.05; Figure 11B). Cytometry analyses showed that apoptosis was significantly lower in valid ejaculates in SF compared to valid ejaculates in IF (P ≤ 0.05). Contrary to this, mitochondrial activity was higher in valid ejaculates in SF with respect to valid ejaculates in IF (P ≤ 0.05). Regarding the valid ejaculates comparison, there were significant differences (P ≤ 0.05) in viability, apoptosis, and mitochondrial activity in SF (Figure 11D).
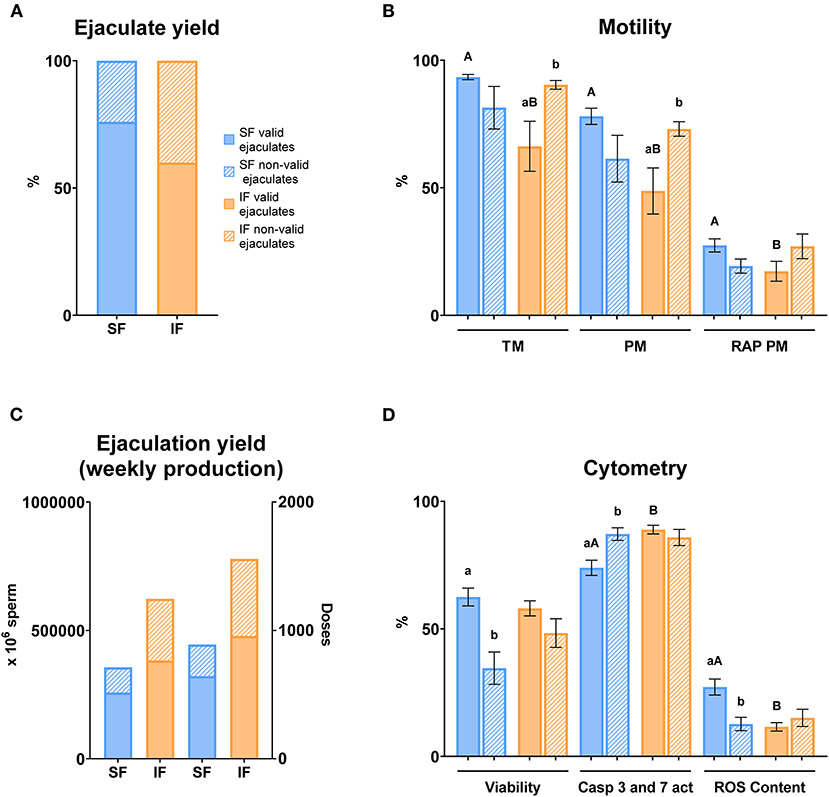
Figure 11. Semen yield and sperm quality in valid and non-valid ejaculates. Criteria of valid ejaculate: ejaculate volume >0.5 ml, mass motility >3, sperm concentration > 3,000 × 106 sperm/ml. Non-valid ejaculates: some of the criteria previously described below the minimum value. (A) Ejaculate yield: % of valid and non-valid ejaculates in two semen collection frequencies (SF, standard semen collection frequency; IF, intensive semen collection frequency corresponding with IFF; Friday); (B) Motility: sperm samples of SF (blue columns) and IF (orange columns); TM, total motility (%); PM, progressive motility (%); RAP PM, rapid progressive motility (%); (C) Ejaculation yield (weekly production): number of sperm and doses produced weekly in two semen collection frequencies (SF and IF); (D) Cytometry: sperm samples of SF (blue columns) and IF (orange columns); Viability, Zombie Violet™ (%); Caspases 3 and 7 activity, CellEvent™ Caspase-3/7 Green (%); ROS content, CellROX™ Deep Red (%). The same 25 males were analyzed in each experimental group. Different lowercase superscripts letters (a,b) indicate differences (P ≤ 0.05) for each assessment point between valid and non-valid ejaculates. Different lowercase superscripts capital letters (A,B) indicate differences (P ≤ 0.05) for each assessment point between semen collection frequencies.
A fertility trial was carried out considering an abstinence period before semen collection for AI (Figure 12A). According to this, the highest fertility rates were obtained with SI of semen collection (P ≤ 0.05). However, when males were submitted both high or low intervals (HI and LI, respectively), fertility rates decreased significantly (P ≤ 0.05), following the same trend with respect to SI and AN. Additionally, there was no identification of differences between them. In addition, the annual fertility was significantly lower than SI (P ≤ 0.05). With respect to the descriptive study per male, we observed the same trend in most of them.
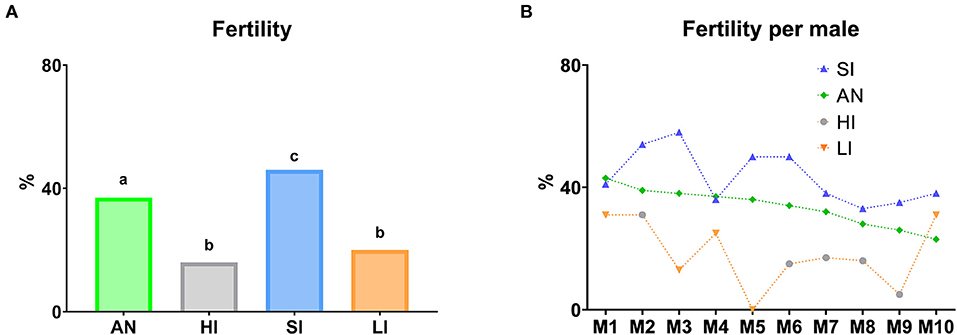
Figure 12. Fertility according to different semen collection frequencies. (A) Fertility per group according to abstinence days before semen collection for AI. (B) Descriptive study of the fertility per male according to abstinence days before semen collection for AI. AN (Annual fertility), annual average of lambing ewes/inseminated ewes (%); HI (Fertility high interval), fertility with seminal doses obtained from males with at least 10 abstinence days; SI (Fertility standard interval), fertility with seminal doses obtained from males with two to five abstinence days; LI (Fertility low interval), fertility with seminal doses obtained from males with semen collection the previous day. In graphic (A), different lowercase superscripts letters (a–c) indicate differences (P ≤ 0.05) for each assessment point among semen collection ratios due to abstinence days before semen collection for AI. In graphic (B), graph dots represent individual males.
According to this, most males presented higher fertility rates with SI of semen collection. However, when males were submitted both high or low intervals (HI or LI, respectively), fertility rates decreased following the same trend (Figure 12B).
Discussion
The improvement of semen donor rams in terms of sperm quality and yield in reproduction centers is a feasible alternative to increase the results of artificial insemination (AI). In this context, semen collection frequency is a common factor studied in several species such as ram (38), boar (39), stallion (40), pigeon (41) or human (42). In our study, sperm production and quality parameters decreased when the semen collection frequency increased. These evidences are in accordance with findings from other studies carried out in rams (6) or humans (43), where it was also reported that increased semen collection frequency had a negative impact on sperm count. Our current findings were consistent with those in a report from Ollero et al. (5), where about 27% less ejaculate volume was obtained after 1 or 2 days of abstinence than after abstinence for 3 days, and sperm concentration decreased significantly as the abstinence period decreased. In our study, 72 h of sexual abstinence (weekend) were enough for a partial recuperation of ejaculate volume and sperm production, allowing us to discard a loss of testicular functionality phenomenon. Linked to our findings on sperm quantity, sperm motility, which is used in the routine evaluation of sperm (5), showed a significant (P ≤ 0.05) decrease in IF, demonstrating the influence of semen collection frequency in sperm motility parameters. Our findings in ovine species are consistent with those reported for rams (6) and boars (39). Concerning the sperm functionality, there were no significant differences (P > 0.05) in viability. In contrast, more advanced cytometry parameters in sperm preservation showed interesting changes. Caspases 3 and 7 activity presented the highest value in IF. These caspases are specific cysteinyl aspartate proteases that execute the breakdown of structural proteins and DNA (44). For this reason, this finding was related to apoptotic changes, which could compromise the ability to fertilize the oocyte (45, 46). Moreover, the lowest percentage of ROS content was found in IF. The ROS content measured by the CellROX probe in sperm mainly reflects intense mitochondrial activity rather than oxidative stress (47–50). Thus, a high frequency of semen collection could reduce the mitochondrial activity of ram sperm. Contrary to our findings, cytometry parameters in humans were not significantly affected after a 2-week period of daily ejaculation, although a decreasing trend in intracellular ROS production was also observed (43). More interestingly, after a short recovery period (3 days of abstinence during the weekend) in the IF model, apoptosis and mitochondrial activity were significantly improved. The current findings obtained in sperm quality are in accordance with Ihukwumere and Okere observations (51) and could occur because sperm cells may need a minimum storage time in the cauda epididymis. Several epididymal components have collaborated in fertilizing capacity and motility of sperm cells due to biochemical and biophysical changes and interactions (52). For instance, clusterin, which is the most abundant protein of the cauda epididymal fluid in rams (53), participates in sperm maturation, lipid transport (54), and sperm membrane remodeling; acts as chaperone (55); and prevents peroxidative damage (56).
Traditional methods such as libido and clinical examinations (3, 7, 8) or basic ultrasound evaluation including testicular volume (26) have been used to complete the BSE. In our study, a testicular volume increase (P ≤ 0.05) was detected in IF in comparison with AF, which could be explained by a high demand for sperm production that provokes a temporal and compensatory testicular hypertrophy (57). Another possible partial explanation to the observed increase of testicular volume is the advance of the breeding season (9) since the duration of the experiment was 2 months. As expected, serum testosterone and libido were higher (P ≤ 0.05) in IF in relation to the other experimental groups. This could be related to the season and the testicular overexertion, which could trigger the activation of different pathways of the hypothalamus-hypophysis axis, provoking the testosterone increase. Some authors demonstrated this effect when they applied several treatments to improve the reproduction performance, such as buserelin (58) or eCG (59).
In these types of studies, new parameters and integrative studies on ultrasonography assessment could be more predictive and reinforce the optimization of the current ram reproductive handling to obtain high sperm quality and fertility. Consequently, we performed a testicular echotexture test using Ecotext® and a testicular vascularization evaluation using Doppler parameters in combination with some sperm quality analyses, including motility and sperm physiology, to analyze their possible correlations. According to the published literature, this is the first time that a male factor (frequency of semen collection) was used in an integrative way within the ram BSE: from ultrasonographic evaluation, such as ram reproductive ability predictor, to measure to sperm quality analyses.
The echotexture parameters revealed changes in the parenchyma structure, increasing echogenicity as the frequency of semen collection intensifies. We observed a significant decrease in black pixels number (EC1), tubular area, and tubular diameter with the intensification of semen collection; all of them could be related to the lumen of seminiferous tubules. We also observed a significant increase in white pixels number (EC2) and mean gray level of pixels (EC3); both could be due to different lumen cell types, which was demonstrated by Giffin et al. (25). These researchers correlated the testicular echotextural attributes with the predominant cell type (the lower echotexture with the higher cell differentiation degree) in the lumen of the seminiferous tubules in the ovine species. Thus, our findings could be explained by the alteration suffered in the composition of the lumen cells under different semen collection frequencies. Moreover, in a study conducted by Camela et al. (26), peripubertal rams showed lower seminiferous tubules lumen and, therefore, greater testicular echotexture than postpubertal rams. These findings in peripubertal rams could be in accordance with the echotextural changes when the intensity of the semen collection frequency increased, showing less hypoechogenic areas related to seminiferous tubules lumen. On the other hand, the density of hypoechogenic areas per cm2 did not show changes among regimes. This could be because, in adult males, the relative seminiferous tubule quantity remains stable when males achieve sexual maturity (60). In spite of this, in AF, negative correlations were found between Density and TM and RAP PM. This could suggest that males with more seminiferous tubules have poor sperm motility in an abstinence semen collection frequency. Although a large amount of sperm could be stored in the epididymis in males with more seminiferous tubules, during prolonged abstinence periods, sperm are exposed to several sperm motility inhibiting factors [acidic pH and a high potassium to sodium ratio in epididymal fluid (61, 62)], which may negatively impact their future motility after ejaculation (63).
Following the integral assessment of the reproductive capacity of rams based on ultrasound evaluation, the Pulse-Doppler mode was described as an indicator of testicular functionality in standard conditions in ram (9, 10), dog (14), stallion (32), or human (64). However, Doppler parameters had not been investigated in different semen collection frequencies correlating these analyses with sperm quality assays. Firstly, PSV and PI increased significantly (P ≤ 0.05), and RI and TABF did not vary significantly (P > 0.05) in SF compared to AF. This could be explained due to PSV and PI may be early predictors of testicular blood perfusion changes, as Jolly et al. (65) described. Moreover, RI is altered when more severe disorders occur (64). Infertile dogs had lower PSV than fertile dogs without varying RI because vascular bed resistance depends on multiple factors such as diameter and tortuosity of the vessels (66). Although it has not been previously described, we observed positive correlations between some ultrasonography (Area, Diameter, RI, and PI) and sperm motility parameters (PM, RAP PM, VSL, and BCF). In this sense, other sperm quality parameters such as live sperm and sperm concentration correlated positively with some seminal plasma antioxidants such as SOD, GPx, and GSH (67). Moreover, Hedia et al. (67) confirmed positive correlations between seminal plasma antioxidants presented in high-quality spermatozoa samples and Doppler indexes, connecting with our positive correlations in SF. On the other hand, all indexes (RI, PI, and TABF) increased significantly in IF with respect to SF. This finding could indicate an increase in resistance to blood flow, pulsatility in the oscillations of the waveform, and blood flow per minute as a consequence of the testicular stress by the intensive semen collection frequency. Low oxygen tension in the seminiferous tubules is essential for spermatogenesis (68); thus, the poor-quality sperm in IF could be justified by increased blood flow with higher oxygen tension. Recently, Ntemka et al. (29) correlated Doppler indexes (RI and PI) negatively with sperm abnormalities. In our work, we found, for the first time, that these Doppler indexes correlated negatively with functionality sperm parameters measured by flow cytometry in consonance with Hedia et al. (9) and Ntemka et al. (29). Nevertheless, to find these correlations, we hypothesized that it is necessary to overexert the testis. A recent study carried out by Brito et al. (69) comparing young and senile dogs revealed a lesser sperm quality in senile dogs and did not detect significant differences in ultrasonographic B-mode evaluation (70). In this sense, vascular characteristics of the testes may represent the causal factors underlying changes in spermatogenesis and, as a consequence, affecting the sperm quality of donor rams negatively in an intensive semen collection frequency. Based on our results, sophisticated studies of testicular echotexture and vascular evaluation measured by specific software and Doppler mode, respectively, are crucial in the reproductive ultrasonography evaluation of males.
In the second part of the paper, we carried out a field trial to demonstrate the importance of semen collection frequencies in AI success. It has been demonstrated that fertility is affected by many factors (intrinsic and extrinsic) related to the female such as the age of the ewe, the lambing-AI interval, or the cumulative number of AI/ewe; the farm such as environmental conditions, the sanitary status, or reproductive handling; the technique itself; and the male such as seasonality, sperm quality, or sperm conservation (71–73). Within the male factors, the frequency of semen collection has not been previously related to fertility rates. Although the ejaculates were considered valid according to the criteria of the reproduction centers (33), when we carried out more advanced analyses such as sperm motility and functionality, we detected significant differences in several parameters (TM, PM, RAP PM, Caspases 3 and 7 activity, and ROS content) and, therefore, we think that the fertility could be altered. In our field results, the standard interval of sexual abstinence, from 2 to 5 days, represented the highest fertility rates. Moreover, in a descriptive assay, we could observe the same trend in fertility rates in most evaluated males comparing different abstinence days before semen collection for AI. As we mentioned before, this fact could be due to the influence of the storage time in the cauda epididymis (53). This work paves the way to know what would be the optimal frequency of semen collection for each ram. It would be very beneficial to group males into different frequencies of semen collection to obtain the maximum reproductive performance from each ram and increase their fertility. To achieve this, a complex ultrasonographic evaluation should be included in the ram's BSE to predict the individual ram's reproductive capacity and optimize the reproductive handling of males.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RM-G: conceptualization, methodology, formal analysis, investigation, writing—original draft, data curation, and visualization. MR: conceptualization, methodology, formal analysis, investigation, supervision, data curation, writing—review and editing, and visualization. LA-L and MA: conceptualization, methodology, investigation, resources, data curation, writing—review and editing, visualization, and funding acquisition. MN-M, CP-M, CO-F, and MH: conceptualization, methodology, and investigation. PP: formal analysis, investigation, resources, data curation, writing—review and editing, visualization, supervision, and funding acquisition. LA: conceptualization, resources, data curation, writing—review and editing, visualization, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by Ministerio de Ciencia e Innovación (PID2021-122470OB-I00), MINECO (AGL2017-83098-R) and Junta de Castilla y León (LE253P18). RM-G was supported by Junta de Castilla y León (through the Consejería de Educación and FSE PO 1420 – CyL, fellowship ORDEN EDU/556/2019), MN-M was supported by MEC (fellowship FPU17/04142), and CP-M was supported by MINECO (PRE2018-086400).
Acknowledgments
The authors acknowledge Ainoa Jordán Esteban, Cecilia Pérez Caso, and Pedro de Vega Álvarez for their help in the acquisition and analysis of the samples, and ASSAF.E, and the staff from Ovigén and CENSYRA of León.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pardos L, Maza MT, Fantova E, Sepúlveda W. The diversity of sheep production systems in Aragón (Spain): characterisation and typification of meat sheep farms. Spanish J Agric Res. (2008) 6:497–507. doi: 10.5424/sjar/2008064-344
2. Mozo R, Galeote AI, Alabart JL, Fantova E, Folch J. Evaluating the reproductive ability of breeding rams in North-Eastern Spain using clinical examination of the body and external genitalia. BMC Vet Res. (2015) 11:1–11. doi: 10.1186/s12917-015-0600-9
3. MacLaren APC. Ram fertility in South-West Scotland. Br Vet J. (1988) 144:45–54. doi: 10.1016/0007-1935(88)90151-0
4. Kimberling CV, Parsons GA. Breeding soundness evaluation and surgical sterilization of the ram. In:Youngquist RS, Threlfall, WR, , editors. Current Therapy in Large Animal Theriogenology, 2nd ed. Amsterdam: Elsevier Inc. (2006). p. 620–8. doi: 10.1016/B978-072169323-1.50088-X
5. Ollero M, Muiño-Blanco T, López-Pérez MJ, Cebrián-Pérez JA. Viability of ram spermatozoa in relation to the abstinence period and successive ejaculations. Int J Androl. (1996) 19:287–92. doi: 10.1111/j.1365-2605.1996.tb00477.x
6. Kaya A, Aksoy M, Tekeli T. Influence of ejaculation frequency on sperm characteristics, ionic composition and enzymatic activity of seminal plasma in rams. Small Rumin Res. (2002) 44:153–8. doi: 10.1016/S0921-4488(02)00051-2
7. Tulley D, Burfening PJ. Libido and scrotal circumference of rams as affected by season of the year and altered photoperiod. Theriogenology. (1983) 20:435–48. doi: 10.1016/0093-691X(83)90203-0
8. Fthenakis GC, Karagiannidis A, Alexopoulos C, Brozos C, Saratsis P, Kyriakis S. Clinical and epidemiological findings during ram examination in 47 flocks in southern Greece. Prev Vet Med. (2001) 52:43–52. doi: 10.1016/S0167-5877(01)00238-0
9. Hedia MG, El-Belely MS, Ismail ST, Abo El-Maaty AM. Monthly changes in testicular blood flow dynamics and their association with testicular volume, plasma steroid hormones profile and semen characteristics in rams. Theriogenology. (2019) 123:68–73. doi: 10.1016/j.theriogenology.2018.09.032
10. Batissaco L, Celeghini ECC, Pinaffi FLV, de Oliveira BMM, de Andrade AFC, Recalde ECS, et al. Correlations between testicular hemodynamic and sperm characteristics in rams. Brazilian J Vet Res Anim Sci. (2014) 50:384. doi: 10.11606/issn.2318-3659.v50i5p384-395
11. Ortega-Ferrusola C, Gracia-Calvo L, Ezquerra J, Pena F. Use of colour and spectral Doppler ultrasonography in stallion andrology. Reprod Domest Anim. (2014) 49:88–96. doi: 10.1111/rda.12363
12. Gouletsou PG. Ultrasonographic examination of the scrotal contents in rams. Small Rumin Res. (2017) 152:100–6. doi: 10.1016/j.smallrumres.2016.12.022
13. Pozor MA, Nolin M, Roser J, Runyon S, Macpherson ML, Kelleman A. Doppler indices of vascular impedance as indicators of testicular dysfunction in stallions. J Equine Vet Sci. (2014) 34:38–9. doi: 10.1016/j.jevs.2013.10.021
14. Zelli R, Troisi A, Elad Ngonput A, Cardinali L, Polisca A. Evaluation of testicular artery blood flow by Doppler ultrasonography as a predictor of spermatogenesis in the dog. Res Vet Sci. (2013) 95:632–7. doi: 10.1016/j.rvsc.2013.04.023
15. Brito LF, Silva AED, Barbosa RT, Kastelic JP. Testicular thermoregulation in Bos indicus, crossbred and Bos taurus bulls: relationship with scrotal, testicular vascular cone and testicular morphology, and effects on semen quality and sperm production. Theriogenology. (2004) 61:511–28. doi: 10.1016/S0093-691X(03)00231-0
16. Abecia JA, Carvajal-Serna M, Casao A, Palacios C, Pulinas L, Keller M, et al. The continuous presence of ewes in estrus in spring influences testicular volume, testicular echogenicity and testosterone concentration, but not LH pulsatility in rams. Animal. (2020) 14:2554–61. doi: 10.1017/S1751731120001330
17. Giffin JL, Franks SE, Rodriguez-Sosa JR, Hahnel A, Bartlewski PM, A. study of morphological and haemodynamic determinants of testicular echotexture characteristics in the ram. Exp Biol Med. (2009) 234:794–801. doi: 10.3181/0812-RM-364
18. Brito LFC, Silva AEDF, Barbosa RT, Unanian MM, Kastelic JP. Effects of scrotal insulation on sperm production, semen quality, and testicular echotexture in Bos indicus and Bos indicus x Bos taurus bulls. Anim Reprod Sci. (2003) 79:1–15. doi: 10.1016/S0378-4320(03)00082-4
19. Pozor MA, McDonnell SM. Color Doppler ultrasound evaluation of testicular blood flow in stallions. Theriogenology. (2004) 61:799–810. doi: 10.1016/S0093-691X(03)00227-9
20. Bumin A, Kaya M, Kaya Ü, Kibar M, Alkan Z. Gray-scale, colour and power Doppler sonography of scrotal disorders in dogs. Revue Méd Vét. (2007) 158:128–33.
21. Kutzler M, Tyson R, Grimes M, Timm K. Determination of testicular blood flow in camelids using vascular casting and color pulsed-wave Doppler ultrasonography. Vet Med Int. (2011) 2011:1–7. doi: 10.4061/2011/638602
22. Gouletsou PG, Fthenakis GC. Clinical evaluation of reproductive ability of rams. Small Rumin Res. (2010) 92:45–51. doi: 10.1016/j.smallrumres.2010.04.005
23. Gouletsou PG, Amiridis GS, Cripps PJ, Lainas T, Deligiannis K, Saratsis P, et al. Ultrasonographic appearance of clinically healthy testicles and epididymides of rams. Theriogenology. (2003) 59:1959–72. doi: 10.1016/S0093-691X(02)01259-1
24. Andrade AKG, Soares AT, Freitas FF, Silva S V, Peña-Alfaro CE, Batista AM, et al. Testicular and epididymal ultrasonography in Santa Inês lambs raised in Brazil. Anim Reprod. (2014) 11:110–8.
25. Giffin JL, Bartlewski PM, Hahnel AC. Correlations among ultrasonographic and microscopic characteristics of prepubescent ram lamb testes. Exp Biol Med. (2014) 239:1606–18. doi: 10.1177/1535370214543063
26. Camela ESC, Nociti RP, Santos VJC, Macente BI, Murawski M, Vicente WRR, et al. Changes in testicular size, echotexture, and arterial blood flow associated with the attainment of puberty in Dorper rams raised in a subtropical climate. Reprod Domest Anim. (2019) 54:131–7. doi: 10.1111/rda.13213
27. Bartlewski PM, Giffin JL, Oluwole OA, Hahnel AC. Prospective ultrasonographic and endocrine predictors of spermatogenic onset in ram lambs. Anim Reprod Sci. (2017) 179:44–8. doi: 10.1016/j.anireprosci.2017.01.015
28. Chandolia RK, Bartlewski PM, Omeke BC, Beard AP, Rawlings NC, Pierson RA. Ultrasonography of the developing reproductive tract in ram lambs: effects of a GnRH agonist. Theriogenology. (1997) 48:99–117. doi: 10.1016/S0093-691X(97)00194-5
29. Ntemka A, Kiossis E, Boscos C, Theodoridis A, Kourousekos G, Tsakmakidis I. Effects of testicular hemodynamic and echogenicity changes on ram semen characteristics. Reprod Domest Anim. (2018) 53:50–5. doi: 10.1111/rda.13279
30. Samir H, Radwan F, Watanabe G. Advances in applications of color Doppler ultrasonography in the andrological assessment of domestic animals: a review. Theriogenology. (2021) 161:252–61. doi: 10.1016/j.theriogenology.2020.12.002
31. Biagiotti G, Cavallini G, Modenini F, Vitali G, Gianaroli L. Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int. (2002) 90:903–8. doi: 10.1046/j.1464-410X.2002.03033.x
32. Ortiz-Rodriguez JM, Anel-Lopez L, Martin-Munõz P, Lvarez M, Gaitskell-Phillips G, Anel L, et al. Pulse Doppler ultrasound as a tool for the diagnosis of chronic testicular dysfunction in stallions. PLoS ONE. (2017) 12:e0175878. doi: 10.1371/journal.pone.0175878
33. Neila-Montero M, Riesco MF, Montes-Garrido R, Palacin-Martinez C, Chamorro C, de Paz P, et al. An optimized centrifugation protocol for ram sperm ensuring high sample yield, quality and fertility. Theriogenology. (2022) 191:179–91. doi: 10.1016/j.theriogenology.2022.08.006
34. Hassan MAA, Sayed RKA, Abdelsabour-Khalaf M, Abd-Elhafez EA, Anel-Lopez L, Riesco MF, et al. Morphological and ultrasonographic characterization of the three zones of supratesticular region of testicular artery in Assaf rams. Sci Rep. (2022) 12:1–12. doi: 10.1038/s41598-022-12243-z
35. Samir H, Sasaki K, Ahmed E, Karen A, Nagaoka K, El Sayed M, et al. Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats. J Vet Med Sci. (2015) 77:549–56. doi: 10.1292/jvms.14-0633
36. Palacin-Martinez C, Alvarez M, Montes-Garrido R, Neila-Montero M, Anel-Lopez L, de Paz P, et al. Frequency of semen collection affects ram sperm cryoresistance. Animals. (2022) 12:1492. doi: 10.3390/ani12121492
37. Riesco MF, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Montes-Garrido R, Alvarez M, et al. ProAKAP4 as novel molecular marker of sperm quality in ram: an integrative study in fresh, cooled and cryopreserved sperm. Biomolecules. (2020) 10:1046. doi: 10.3390/biom10071046
38. Aguirre V, Orihuela A, Vázquez R. Effect of semen collection frequency on seasonal variation in sexual behaviour, testosterone, testicular size and semen characteristics of tropical hair rams (Ovis aries). Trop Anim Health Prod. (2007) 39:271–7. doi: 10.1007/s11250-007-9010-8
39. Frangeî R, Gider T, Kosec M, FrangeŽ R, Gider T, Kosec M. Frequency of boar ejaculate collection and its influence on semen quality, pregnancy rate and litter size. Acta Vet Brno. (2005) 74:265–73. doi: 10.2754/avb200574020265
40. Gamboa S, Francisco M, Gomes P, Mendes C, Machado-Faria M, Ramalho-Santos J. Level of glycolyzable substrates in stallion semen: effect of ejaculation frequency on sperm survival after cool storage during the nonbreeding season. J Equine Vet Sci. (2011) 31:109–15. doi: 10.1016/j.jevs.2010.12.011
41. Klimowicz M, Łukaszewicz E, Dubiel A. Effect of collection frequency on quantitative and qualitative characteristics of pigeon (Columba livia) semen. Br Poult Sci. (2005) 46:361–5. doi: 10.1080/00071660500098210
42. AlAwlaqi A, Hammadeh ME. Sexual abstinence and sperm quality. Int J Women's Heal Reprod Sci. (2017) 5:11–7. doi: 10.15296/ijwhr.2017.03
43. Mayorga-Torres BJM, Camargo M, Agarwal A, du Plessis SS, Cadavid ÁP, Cardona Maya WD. Influence of ejaculation frequency on seminal parameters. Reprod Biol Endocrinol. (2015) 13:1–7. doi: 10.1186/s12958-015-0045-9
44. Martí E, Pérez-Pé R, Muiño-Blanco T, Cebrián-Pérez JA. Comparative study of four different sperm washing methods using apoptotic markers in ram spermatozoa. J Androl. (2006) 27:746–53. doi: 10.2164/jandrol.106.000109
45. Said T, Agarwal A, Grunewald S, Rasch M, Baumann T, Kriegel C, et al. Selection of nonapoptotic spermatozoa as a new tool for enhancing assisted reproduction outcomes: an in vitro model. Biol Reprod. (2006) 74:530–7. doi: 10.1095/biolreprod.105.046607
46. Dogan S, Mason MC, Govindaraju A, Belser L, Kaya A, Stokes J, et al. Interrelationships between apoptosis and fertility in bull sperm. J Reprod Dev. (2013) 59:18–26. doi: 10.1262/jrd.2012-068
47. Plaza Davila M, Martin Muñoz P, Tapia JA, Ortega Ferrusola C, Balao da Silva CC, Peña FJ, et al. Inhibition of mitochondrial complex I leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE. (2015) 10:e0138777. doi: 10.1371/journal.pone.0138777
48. Riesco MF, Alvarez M, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Montes-Garrido R, et al. Multiparametric study of antioxidant effect on ram sperm cryopreservation—from field trials to research bench. Animals. (2021) 11:283. doi: 10.3390/ani11020283
49. Anel-Lopez L, Riesco MF, Montes-Garrido R, Neila-Montero M, Boixo JC, Chamorro C, et al. Comparing the effect of different antibiotics in frozen-thawed ram sperm: is it possible to avoid their addition? Front Vet Sci. (2021) 8:656937. doi: 10.3389/fvets.2021.656937
50. Martin Muñoz P, Ortega-Ferrusola C, Anel-Lopez L, del Petre C, Alvarez M, de Paz P, et al. Caspase 3 activity and lipoperoxidative status in raw semen predict the outcome of cryopreservation of stallion spermatozoa. Biol Reprod. (2016) 95:53. doi: 10.1095/biolreprod.116.139444
51. Ihukwumere FC, Okere C. Effects of frequent ejaculations on semen characteristics of Nigerian Yankasa rams. Small Rumin Res. (1990) 3:77–83. doi: 10.1016/0921-4488(90)90034-4
52. Dacheux J-L, Gatti JL, Dacheux F. Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech. (2003) 61:7–17. doi: 10.1002/jemt.10312
53. van Tilburg M, Sousa S, Lobo MDP, Monteiro-Azevedo ACOM, Azevedo RA, Araújo AA, et al. Mapping the major proteome of reproductive fluids and sperm membranes of rams: from the cauda epididymis to ejaculation. Theriogenology. (2021) 159:98–107. doi: 10.1016/j.theriogenology.2020.10.003
54. Tenniswood MP, Guenette RS, Lakins J, Mooibroek M, Wong P, Welsh JE. Active cell death in hormone-dependent tissues. Cancer Metastasis Rev. (1992) 11:197–220. doi: 10.1007/BF00048064
55. Humphreys DT, Carver JA, Easterbrook-Smith SB, Wilson MR. Clusterin has chaperone-like activity similar to that of small heat shock proteins. J Biol Chem. (1999) 274:6875–81. doi: 10.1074/jbc.274.11.6875
56. Reyes-Moreno C, Boilard M, Sullivan R, Sirard M-A. Characterization and identification of epididymal factors that protect ejaculated bovine sperm during in vitro storage. Biol Reprod. (2002) 66:159–66. doi: 10.1095/biolreprod66.1.159
57. Gaudino R, Cavarzere P, Camilot M, Teofoli F, Zampieri N, Tatò L. Prepubertal serum inhibin B in cryptorchid infants and in monorchid boys with compensatory testicular hypertrophy. Fertil Steril. (2008) 90:2217–21. doi: 10.1016/j.fertnstert.2007.10.017
58. El-Shalofy AS, Hedia MG. Effects of buserelin administration on testicular blood flow and plasma concentrations of testosterone and estradiol-17β in rams. Domest Anim Endocrinol. (2021) 77:106646. doi: 10.1016/j.domaniend.2021.106646
59. Beracochea F, Manes J, Viera MN, Santiago-Moreno J, Ungerfeld R. Administration of equine Chorionic Gonadotrophin (eCG) to rams to improve the reproductive performance during the non-breeding season. Livest Sci. (2020) 240:104125. doi: 10.1016/j.livsci.2020.104125
60. Steger K, Wrobel KH. Postnatal development of ovine seminiferous tubules: an electron microscopical and morphometric study. Ann Anat. (1996) 178:201–13. doi: 10.1016/S0940-9602(96)80048-3
61. Iwamoto T, Gagnon C. A human seminal plasma protein blocks the motility of human spermatozoa. J Urol. 1988;140(5 PART I):1045–8. doi: 10.1016/S0022-5347(17)41923-9
62. Skandhan KP. Hypothesis: epididymis inhibits sperm motility inside male reproductive tract. Med Hypotheses. (2004) 62:146–50. doi: 10.1016/S0306-9877(03)00238-X
63. Ayad BM, Van der Horst G, du Plessis SS. Short abstinence: a potential strategy for the improvement of sperm quality. Middle East Fertil Soc J. (2018) 23:37–43. doi: 10.1016/j.mefs.2017.07.005
64. Pinggera G-M, Mitterberger M, Bartsch G, Strasser H, Gradl J, Aigner F, et al. Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int. (2008) 101:722–6. doi: 10.1111/j.1464-410X.2007.07343.x
65. Jolly TAD, Bateman GA, Levi CR, Parsons MW, Michie PT, Karayanidis F. Early detection of microstructural white matter changes associated with arterial pulsatility. Front Hum Neurosci. (2013) 7:782. doi: 10.3389/fnhum.2013.00782
66. de Souza MB, England GCW, Mota Filho AC, Ackermann CL, Sousa CVS, de Carvalho GG, et al. Semen quality, testicular B-mode and Doppler ultrasound, and serum testosterone concentrations in dogs with established infertility. Theriogenology. (2015) 84:805–10. doi: 10.1016/j.theriogenology.2015.05.015
67. Hedia MG, El-Belely MS, Ismail ST, El-Maaty AMA. Seasonal variation in testicular blood flow dynamics and their relation to systemic and testicular oxidant/antioxidant biomarkers and androgens in rams. Reprod Domest Anim. (2020) 55:861–9. doi: 10.1111/rda.13696
68. Bergh A, Damber J-E. Vascular controls in testicular physiology. In:de Kretse D, , editor. Molecular Biology of the Male Reproductive System. Amsterdam: Elsevier (1993). p. 439–68. doi: 10.1016/B978-0-08-091764-1.50017-9
69. Brito MM, da Rosa Filho RR, Losano JDA, Vannucchi CI. Ageing changes testes and epididymis blood flow without altering biometry and echodensity in dogs. Anim Reprod Sci. (2021) 228:106745. doi: 10.1016/j.anireprosci.2021.106745
70. Brito MM, Angrimani DSR, Rui BR, Kawai GKV, Losano JDA, Vannucchi CI. Effect of senescence on morphological, functional and oxidative features of fresh and cryopreserved canine sperm. Aging Male. (2020) 23:279–86. doi: 10.1080/13685538.2018.1487931
71. Anel L, Kaabi M, Abroug B, Alvarez M, Anel E, Boixo JC, et al. Factors influencing the success of vaginal and laparoscopic artificial insemination in churra ewes: a field assay. Theriogenology. (2005) 63:1235–47. doi: 10.1016/j.theriogenology.2004.07.001
72. Anel L, Alvarez M, Martinez-Pastor F, Garcia-Macias V, Anel E, De Paz P. Improvement strategies in ovine artificial insemination. Reprod Domest Anim. (2006) 41:30–42. doi: 10.1111/j.1439-0531.2006.00767.x
Keywords: Doppler, fertility, ovine, semen collection frequency, sperm quality, ultrasonography
Citation: Montes-Garrido R, Riesco MF, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Boixo JC, de Paz P, Ortega-Ferrusola C, Hassan MAA, Anel L and Alvarez M (2022) Application of ultrasound technique to evaluate the testicular function and its correlation to the sperm quality after different collection frequency in rams. Front. Vet. Sci. 9:1035036. doi: 10.3389/fvets.2022.1035036
Received: 02 September 2022; Accepted: 11 November 2022;
Published: 25 November 2022.
Edited by:
Federica Turri, Institute of Agricultural Biology and Biotechnology (CNR), ItalyReviewed by:
Jordi Miro, Universitat Autónoma de Barcelona, SpainHassan Hussein, Assiut University, Egypt
Copyright © 2022 Montes-Garrido, Riesco, Anel-Lopez, Neila-Montero, Palacin-Martinez, Boixo, de Paz, Ortega-Ferrusola, Hassan, Anel and Alvarez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Anel-Lopez, bGFuZWxAdW5pbGVvbi5lcw==
†These authors have contributed equally to this work
 Rafael Montes-Garrido
Rafael Montes-Garrido Marta F. Riesco
Marta F. Riesco Luis Anel-Lopez
Luis Anel-Lopez Marta Neila-Montero1,2
Marta Neila-Montero1,2 Mohamed A. A. Hassan
Mohamed A. A. Hassan Luis Anel
Luis Anel Mercedes Alvarez
Mercedes Alvarez