
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 15 December 2022
Sec. Animal Nutrition and Metabolism
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1025677
This article is part of the Research Topic Technological Strategies to Improve Animal Health and Production View all 34 articles
 Peng Li1†
Peng Li1† Liyun Zheng1†
Liyun Zheng1† Ya Qi1
Ya Qi1 Zhipeng Liu1
Zhipeng Liu1 Encun Du2
Encun Du2 Jintao Wei2
Jintao Wei2 Zhengfan Zhang1
Zhengfan Zhang1 Shuangshuang Guo1*‡
Shuangshuang Guo1*‡ Binying Ding1*‡
Binying Ding1*‡Necrotic enteritis (NE) is a great threat to the intestinal health of broilers, resulting in decreased growth performance and significant economic losses. Lactobacillus fermentum (LF) and Lactobacillus paracasei (LP) exert beneficial effects on intestinal health. The aim of the present study was to investigate the effects of dietary LF and LP on the intestinal health and growth performance of broilers challenged with coccidia and Clostridium perfringens (CCP). The animal trial was carried out using 336 broilers (Ross 308) for 35 days with a completely randomized design. The broilers were divided into 4 groups based on treatment as follows: the control (CTR) group was fed the basal diet and without CCP challenge and the CCP group was fed the basal diet and with CCP challenge. The broilers in the CCP+LF and CCP+LP groups were challenged by CCP, and meanwhile, LF (1 × 109 CFU/g) and LP (1 × 109 CFU/g) were supplemented into the basal diets, respectively. The results showed that the growth performance and the intestinal morphology were negatively affected by the CCP challenge. In addition, the number of coccidia in the intestinal digesta and the relative abundance of Escherichia coli in the cecal digesta were increased. Besides, the mRNA level of IgA in the jejunum was downregulated, and the transcript level of IL-8 was upregulated by the CCP challenge. Dietary LF and LP failed to improve the growth performance of broilers with the CCP challenge. However, they were beneficial for intestinal barrier function. In addition, dietary LF was able to alleviate the downregulation of TGF-β mRNA level in the spleen with CCP challenge and decreased the lesion scores compared with the CCP group. Furthermore, dietary LP alleviated the upregulation of the IL-8 mRNA level in the jejunum with CCP challenge and reduced the number of coccidia in the ileal digesta. In conclusion, dietary LF and LP failed to mitigate the negative effects of CCP infection on growth performance; however, they were able to improve the intestinal health of broilers challenged with CCP by strengthening the intestinal barrier and alleviating inflammation.
Necrotic enteritis (NE) in broilers caused by C. perfringens is an enterotoxemic disease that shapes the impaired intestinal barrier, unstable intestinal flora, and weakened immunity of birds (1). In fact, NE results in significant economic losses. A study suggested that a loss of $0.05 per bird was caused by NE (2). Previously, antibiotics were used to treat NE in poultry production. However, antibiotics are banned in feed in many countries, and in recent years, the incidence of NE has been increasing. At present, nutrition-based interventions to address this problem have attracted the attention of many scholars. Probiotics have great potential to improve intestinal health, and dietary probiotics continue to be a good antibiotic substitute to alleviate NE in broilers. It was reported that Lactobacillus fermentum (LF) could produce short-chain fatty acids (SCFAs), which can inhibit the proliferation of Escherichia coli (3). Some studies also suggested that dietary LF was beneficial to the intestinal barrier and immunity of weaned piglets (4, 5). Another study demonstrated that Lactobacillus paracasei (LP) improved intestinal flora, which contributed to the growth performance and immunity of birds (6, 7).
Although some biological effects of LF and LP had been investigated, they were not fully characterized in poultry. The reported beneficial effects of LF and LP on intestinal health suggest their potential to alleviate NE. Hence, it was necessary to study the effects of dietary LP and LF supplementation in NE broilers. However, the lack of a reliable NE model might leave us stranded. A stable NE model in broilers should be of great importance. In our previous study, C. perfringens could be used in establishing the NE model (8, 9). However, due to differences in individual birds or dietary factors, the infection of C. perfringens was also not always successful to establish the NE model. A study suggested that the coinfection of coccidia and C. perfringens might make a more stable NE model (10). In the present study, coccidia and C. perfringens were used to establish a NE model, and dietary LP and LF were employed to prevent NE. This study aimed at investigating the effects of dietary LF and LP on the intestinal health and growth performance of broilers infected with NE.
The animal study was carried out at the Wuhan Polytechnic University (Hubei, China). A total of 336 1-day-old healthy Ross 308 broilers with uniform weight were randomly assigned to 4 treatments. There were 6 replicates in each group and 14 broilers (7 males and 7 females) in each replicate. The broilers in the control (CTR) group were fed the basal diet and without coccidia and were affected by the C. perfringens (CCP) challenge. The basal diet was formulated according to the recommendation of the NRC (1994). The formula and nutrient levels of the basal diet are shown in Table 1. The broilers in the CCP group were fed the basal diet and challenged by CCP. The C. perfringens (CVCC2030) was purchased from the China Veterinary Microbial Culture Collection and Management Center (Beijing, China), and the coccidia were purchased from Foshan Zhengdian Biotechnology Co., Ltd. (Guangdong, China). A quadrivalent anti-coccidiosis vaccine consisted of 5 × 104 oocysts of E. tenella strain PTMZ, E. necatrix strain PNHZ, and E. maxima strain PMHY, as well as 1 × 105 oocysts of E. acervuline strain PAHY. The recommended dose of the vaccine was 1,100 ± 110 sporulated oocysts per bird. In the present study, the number of coccidia oocysts inoculating each bird was 30 times the recommended dose. Birds in the CCP+LF and CCP+LP groups were also challenged with CCP, and the diets of the CCP+LF and CCP+LP groups were supplemented with 1 × 109 CFU/g LF and 1 × 109 CFU/g LP, respectively. The diet for each group was formulated one time a week. A 35-day trial was performed, and an overview of the trial is shown in Figure 1. On day 9 of the trial, the anti-coccidiosis vaccine was administered into the crop of broilers in the CCP, CCP+LF, and CCP+LP groups. The birds in the CTR group were treated with an equal volume of saline. From days 13 to 18, the broilers in the CCP, CCP+LF, and CCP+LP groups were challenged with C. perfringens. Specifically, 64 ml of C. perfringens broth (1 × 108 CFU/mL) was well mixed into 1,200 g feed of each group, which was then equally distributed to each replicate. Importantly, these feeds should be consumed within 2 h, and the birds in the CTR group were fed a diet with an equal volume of sterile broth. On days 13 and 19, two birds in each replicate were selected to collect blood from the wing vein and slaughtered for sample collection. On days 1, 13, 19, and 35, all broilers and feed were weighed for the calculation of growth performance. All broilers were raised in wire cages with free access to water and feed. During the first 3 days of the trial, the room temperature was controlled at 35°C, and then, the temperature was decreased by 1°C per day until it was maintained at 25°C. A 24-h light regime was implemented throughout the animal trial.
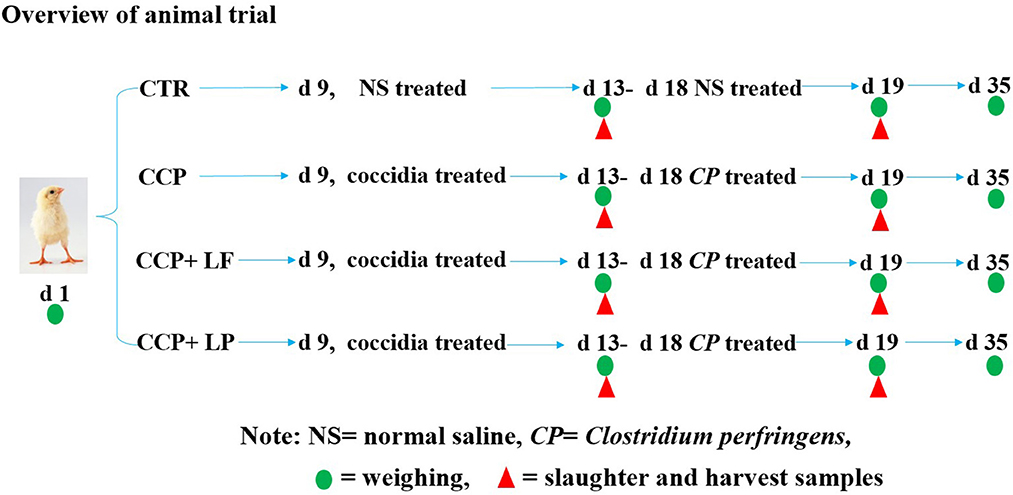
Figure 1. The schematic diagram of the overview of the trial. All broilers were weighed to investigate the growth performance on days 1, 13, 19, and 35. Some broilers were selected to be slaughtered, and samples were collected for laboratory analysis. Among them, green circles indicate that all chickens were weighed and recorded on that day, and red triangles indicate the broiler chickens that were selected for slaughter and sampling on that day.
The body weight of the broilers and the feed consumption were weighed on days 1, 13, 19, and 35. The average daily gain (ADG), average daily feed intake (ADFI), and the feed conversion ratio (FCR) from days 1–13, days 14–19, days 20–35, and days 1–35 were calculated. The indexes of the spleen, the bursa of Fabricius, and the thymus on days 13 and 19 were also analyzed. The formula for calculating the immune organ index is as follows: immune organ index = the weight of organ (g) /the body weight of broiler (kg).
The blood was collected from the underwing vein and centrifuged at 3,000 r/min for 15 min at 4°C, and the serum was separated from the supernatant. The kits purchased from Nanjing Jiancheng Biotechnology Co., Ltd. (Jiangsu, China) were used to determine the levels of lysozyme, inductible nitric oxide synthase (i-NOS), and lactate dehydrogenase (LDH) in the serum. An automatic biochemical analyzer (Unicel DXC800, Beckman Coulter, USA) was used to analyze the levels of glucose (GLU), calcium (Ca), and phosphorus (P) in the serum.
Approximately 1 cm of mid-segments of the duodenum, jejunum, and ileum were collected and fixed in 4% paraformaldehyde. Then, these intestinal segments were embedded in wax blocks and sectioned at 4 μm, and the sections were stained with eosin-hematoxylin. Intestinal villus height (VH) and crypt depth (CD) were measured as described in the previous study (11). Briefly, 10 straight and intact intestinal villi were randomly selected in each sample, and then the VH and CD were measured by an image analysis system using the Olympus BX-41TF microscope. The vertical distance from the tip of the villus to the crypt opening was considered as the villus height, and the vertical distance from the crypt opening to the ending was the crypt depth. The average values were calculated from the ten measurements, and the ratio of VH to CD was also calculated. In addition, the duodenum, jejunum, and ileum were cut lengthwise, and the digesta were rinsed. Then, a 6-point scoring system (12) was employed to score the intestinal lesion. The specific rules for this 6-point scoring were as follows: an intestine without any abnormalities should be a zero. The intestine marked 1 should be described as having a thin wall and diffuse fibrin attached to the mucosal surface of the intestine. There were 1–5 necrotic or ulcerated spots on the intestinal mucosa or deposits of fibrin that could not be removed, which should be marked as 2 points. The more severe the bowel lesion, the more spots there were. For example, 3 points for a number of lesions between 6 and 15, and 4 points for more than 16 lesions. The presence of a 2–3 cm patch of necrotic plaque on the intestine should be marked as 5 points. Beyond that, once the necrotic plaque penetrated from the intestinal mucosa into the lamina propria and muscle layers of the intestine, it was considered as 6 points.
The digesta of the jejunum, ileum, and cecum as well as the feces were collected on days 13 and 19, and then stored in a refrigerator at −20°C. A total of 2 g of samples were mixed with 10 ml of saline, and then 50 ml of saline was continuously added with stirring until there was no obvious fecal mass. A 60-mesh nylon was used to filter the mixture, and then the filtrate was collected and pipetted into the two counting chambers of the M. mcswelli counting plate. The chambers were filled with filtrate, and air bubbles should be removed. The counting plate was placed for 3 min before being counted using a microscope. The formula for calculating the data was as follows: number of coccidial oocysts per gram of feces (OPG) = [(n1 + n2)] × 60 × dilution factor/(2 × 0.15) × 2. Among them, n1 and n2 were the numbers of the coccidial oocysts in each chamber, and the volume of the counting chamber was 0.15 ml. The dilution factor was the number of times the mixture was diluted. The total volume of the mixture was 60 ml, and the weight of the digesta or feces was 2 g.
The jejunum and the spleen were collected and placed in an RNase-free centrifuge tube and then snap-frozen in liquid nitrogen before being transferred to a refrigerator at −80°C. Extraction of total RNA, preparation of cDNA, and PCR were performed as previously described (13). The TRIzol reagent (Takara, Dalian, China) was used to obtain the total RNA, and the purity was checked with the following criteria: an OD260/OD280 ratio of ~2.0 and a 28 S/18 S rRNA ratio of > 1.8. Then, the gDNA Eraser (Takara, Dalian, China) was used to prepare the cDNA. An Applied Biosystems 7500 Fast Real-Time PCR System (Foster City, CA) was used to perform the qPCR. The 2−ΔΔCT method (14) was used to analyze the relative expression of each gene, and the β-actin was used as the reference gene. Primers of the genes in the present study are listed in Table 2.
The cecal digesta was collected on day 19. QIAamp fast DNA stool mini kits (Qiagen, Hilden, Germany) were used to extract the genomic DNA of the cecal microflora. A NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) was used to measure the concentration of DNA. The steps of qPCR and the calculation method of genes were the same as described above. The total bacteria (16s rRNA) were used as the reference gene, and the specific 16S rRNA genes were targeted for E. coli, Lactobacillus, and C. perfringens. The primers of these bacteria were as follows: total bacteria: F-ACTCCTACGGGAGGCAGCAGT and R-GTATTACCGCGGCTGCTGGCAC; E. coli: F-GTTAATACCTTTGCTCATTGA and R-ACCAGGGTATCTAATCCTGT; Lactobacillus: F-AGCAGTAGGGAATCTTCCA and R-CACCGCTACACATGGAG; and C. perfringens: F-AAAGATGGCATCATCATTCAAC and R-TACCGTCATTATCTTCCCCAAA.
A one-way ANOVA program in SPSS 23.0 software (SPSS Inc., Chicago, IL) was used to analyze the data, and then, Tukey's multiple comparisons were used to investigate the differences between groups. The Kolmogorov-Smirnov test was used to analyze the data that did not comply with normal distribution, and a non-parameter test and pairwise comparisons were used to analyze the data. The Mann-Whitney U test was used to analyze lesion scores between treatments. The data of the lesion score was expressed in the median and interquartile range, and data other than the lesion score were presented as mean ± standard error. A P-value of < 0.05 was considered to be significantly different between groups, and the Graphpad prism 8.0 software was used for creating graphs.
The FCR during days 1–13 and 14–19 was negatively affected by the CCP challenge, as well as the ADG and ADFI during days 14–19 (P < 0.05) (Table 3). The growth performance in other periods was not significantly affected by CCP treatment. In addition, dietary LF and LP failed to alleviate the negative effects of CCP on growth performance.
The index of the thymus on day 13 tended to be reduced (P = 0.091), and the spleen index on day 19 tended to be increased by CCP challenge (P = 0.093) (Table 4). Dietary LF and LP were not able to improve immune organ indices. The content of serum glucose on day 13 was decreased (P < 0.05), and serum lysozyme on day 19 tended to be increased by CCP challenge (P = 0.059) (Table 5). Additionally, compared with the CCP group, the levels of serum i-NOS on day 13 were increased in the CCP+LF group (P < 0.05). The contents of serum calcium on days 13 and 19 were elevated in the broilers of the CCP+LP group (P < 0.001).
The crypt depths of the duodenum, the jejunum, and the ileum on days 13 and 19 were increased by CCP challenge (P < 0.05) (Figure 2). In addition, the ratios of VH to CD in the duodenum, the jejunum, and the ileum on day 13 and in the jejunum and the ileum on day 19 decreased. Dietary LF and LP elevated the ratios of VH to CD in the duodenum and the jejunum on day 13 and in the jejunum on day 19 of broilers with CCP challenge. Moreover, dietary LF increased the ratio of VH to CD in the ileum on day 13, and dietary LP improved it in the duodenum on day 19 with the CCP challenge.
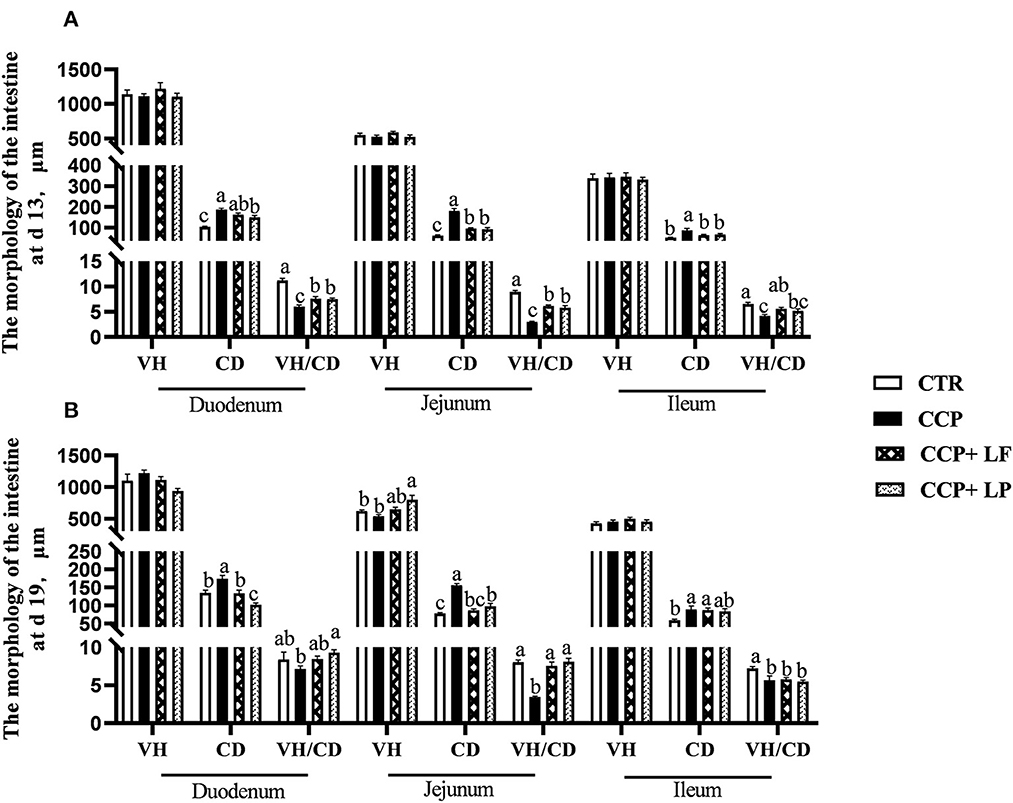
Figure 2. The intestinal villus height, crypt depth, and the ratio of villus height to crypt depth. (A) the data were collected on day 13, and (B) the data were collected on day 19. Among them, a,b, and c means in the different pillars without common superscripts differ significantly (P < 0.05). VH, villus height; CD, crypt depth; VH/CD, the ratio of VH to CD. n = 12. Data were presented as mean ± standard error. CTR, the control group; CCP, the group co-infected with coccidia and C. perfringens (CCP), CCP+LF and CCP+LP denote the groups fed Lactobacillus fermentum- and Lactobacillus paracasei-supplemented diets with CCP challenge.
The CCP+LF group had a lower lesion score in the jejunum than that of the CCP group (P < 0.05) (Figure 3A). The relative abundance of E. coli in the cecal digesta was upregulated by CCP challenge (P < 0.05) (Figure 3B), while dietary LP decreased it. Although we did not observe a statistical difference in the relative abundance of C. perfringens, it was numerically raised by the CCP challenge. Unexpectedly, dietary LP upregulated the relative abundance of C. perfringens in the cecal digesta. Compared with the CCP group, the number of coccidial oocysts in the ileal digesta of the CCP+LP group on day 13 was decreased (P < 0.05) (Figure 3C). It was concluded that dietary LF and LP exerted beneficial effects in CCP-challenged birds.
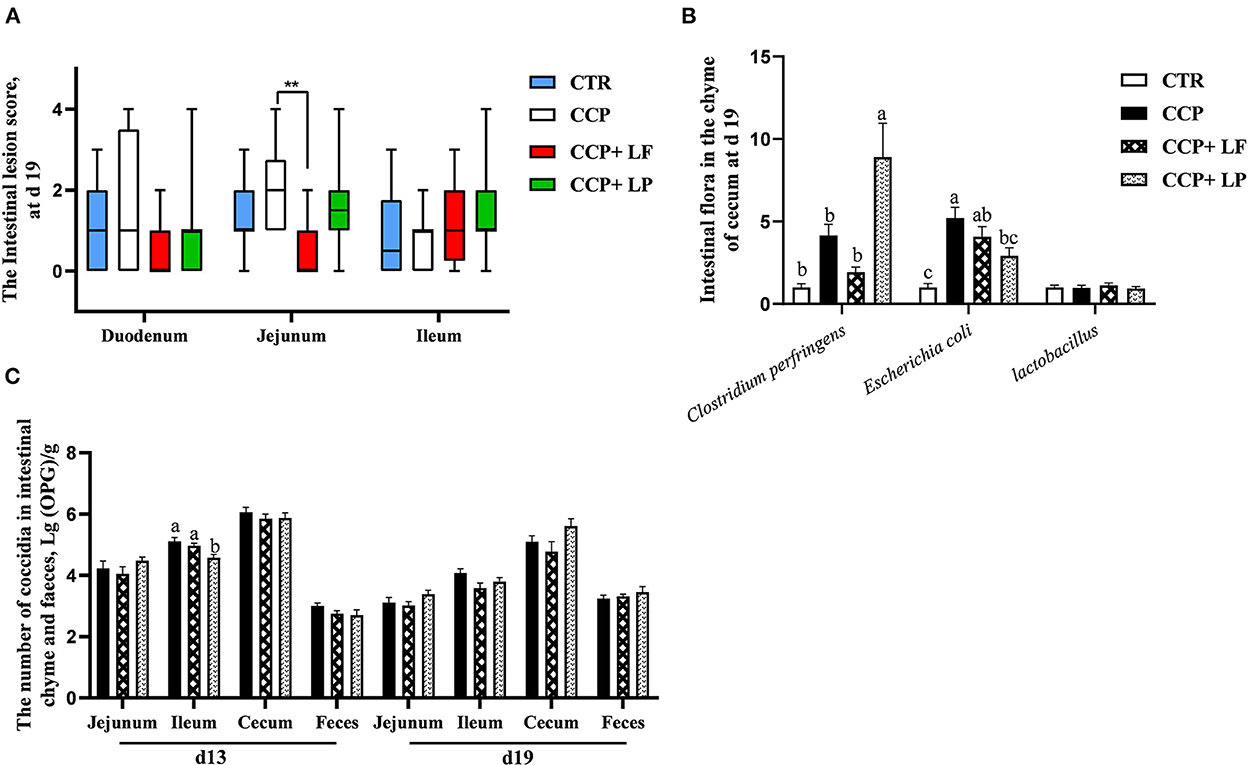
Figure 3. The intestinal lesion score, the number of cecal bacteria, and intestinal coccidia. The data of the intestinal lesion score is arranged in (A) (n = 12), the levels of some bacteria in the cecal chyme are shown in (B) (n = 10), and the numbers of coccidia in the chyme are arranged in (C) (n = 12 in the chyme of jejunum, ileum, and chyme. n = 6 in the feces). The data in (A) were expressed in the median and interquartile range, and data in (B,C) were presented as mean ± standard error. CTR, the control group; CCP, the group coinfected with coccidia and C. perfringens (CCP), CCP+LF and CCP+LP denote the groups fed Lactobacillus fermentum- and Lactobacillus paracasei-supplemented diets with CCP challenge. In (A), ** = 0.001 < P < 0.01, among (B,C), a,b, and c indicate that the different pillars without common superscripts differ significantly (P < 0.05).
The mRNA levels of ZO-1, Mucin-2, and Occludin in the jejunum on day 13 were downregulated by CCP challenge, as well as the transcript level of Mucin-2 in the jejunum on day 19 (P < 0.05) (Figures 4A,B). These results were logically consistent with the findings that CCP challenge weakened intestinal morphology. Dietary LF upregulated the mRNA levels of ZO-1 and Mucin-2 in the jejunum of broilers with CCP challenge on day 13 (P < 0.05). Dietary LP elevated the mRNA level of Claudin-1 in the jejunum of broilers with CCP challenge on day 19 (P < 0.05). Besides, the results of immune-related genes showed that the mRNA level of IL-8 was upregulated on day 13, and the transcript level IgA in the jejunum was downregulated by CCP challenge (P < 0.05), as well as the TGF-β in the spleen (P = 0.094) (Figure 4C). Compared with the CCP group, the mRNA level of IL-8 in the jejunum on day 13 was downregulated, and the mRNA level of IFN-γ in the spleen on day 19 was upregulated with LP supplementation (P < 0.05). Additionally, dietary LF upregulated the transcript level of TGF-β in the spleen of the birds with CCP challenge on day 13 (P < 0.05).
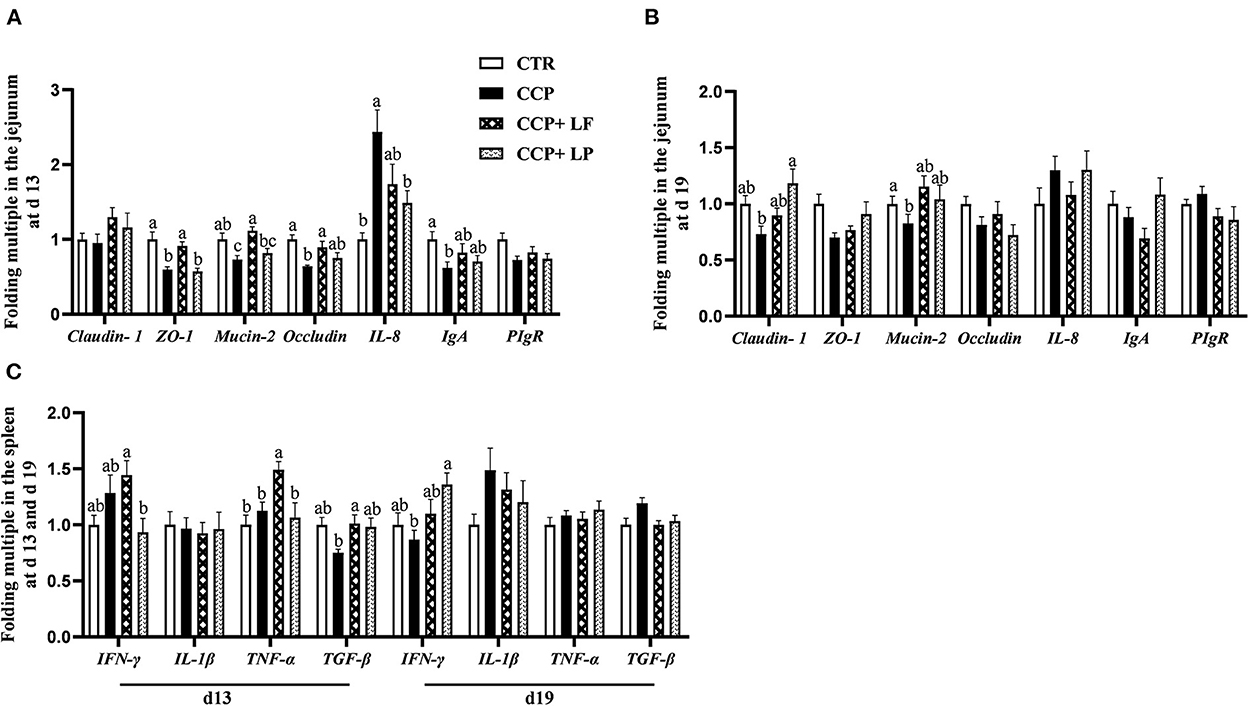
Figure 4. The mRNA transcript levels of some genes in the jejunum and spleen. (A) The data of the gene mRNA levels in the jejunum on day 13, (B) the data of the gene mRNA levels in the jejunum on day 19, and (C) the data of the gene mRNA levels in the spleen. Among them, a,b, and c means in the different pillars without common superscripts differ significantly (P < 0.05). n = 12. Data were presented as mean ± standard error. CTR, the control group; CCP, the group coinfected with coccidia and C. perfringens (CCP), CCP+LF and CCP+LP denote the groups fed Lactobacillus fermentum- and Lactobacillus paracasei-supplemented diets with CCP challenge.
A wheat-based diet was proven to help establish the NE model with coccidia and C. perfringens challenge (1). We presented an undated report based on this idea in this study. Liu et al. (15) suggested that the growth performance of broilers with CCP challenge was negatively affected, and it might be recorded only during CCP infection. In the present study, we observed consistent outcomes. Specifically, combined infection with coccidia and C. perfringens reduced the growth performance of broilers, which might only be observed during the infection period. There was no difference in growth performance between CCP-challenged and unchallenged broilers in the period after infection. A possible explanation was that the immune system of broilers was used to maintain immune homeostasis during the CCP challenge and that numerous nutrients needed to be ingested for immune responses. Since then, the compensatory growth mechanism (16) has been activated to eliminate the difference in growth performance. Some probiotics were found to be useful for the growth performance and immunity of broilers (17, 18), which opened a new window for relieving the NE in broilers. Unexpectedly, we observed that LF and LP were ineffective in the growth performance of CCP-infected broilers in this study. It was speculated that LF and LP might not be able to colonize in the intestine of broilers, as we found in the present study that dietary LF and LP failed to elevate the relative abundance of Lactobacillus. This transient probiotic might only be beneficial to the intestine, and this effect was not sufficient to improve the growth performance. To investigate our conjecture, we would conduct further studies on the intestines of broilers.
A healthy intestine should be equipped with a complete morphological structure and a stable microbial environment (19), and the tight junctions underlie the molecular basis of the intestinal barrier (20). Additionally, the mucins secreted by goblet cells of the intestine are regarded as the first guardian of intestinal health (21). In the present study, the mRNA levels of Occludin, ZO-1, and Mucin-2 were downregulated by the CCP challenge. It was demonstrated that the CCP challenge weakened the intestinal barrier, which was consistent with previous reports (1). Besides, the ratio of villi height to crypt depth decreased with the CCP challenge. It was generally known that the longer the intestinal villi, the stronger the intestinal absorption function. Additionally, the intestinal crypts were rich in immature cells that were not capable of absorption, and the increase in crypt depth might be detrimental to the absorption function of the intestine. The ratio of intestinal VH to CD was considered a reliable indicator for assessing intestinal absorptive function (22). The negative effects of the CCP challenge on growth performance might be attributed to its disruption of the intestinal barrier. Interestingly, dietary LF and LP contributed to improving the intestinal morphology of broilers with CCP challenge in the present study. This was demonstrated in a number of studies that short-chain fatty acids produced by Lactobacillus in the intestine were beneficial for intestinal development and immunity (23, 24), which might be the evidence that dietary LF and LP improved the intestinal barrier function in this study. Although LF and LP did not improve the growth performance of CCP-challenged broilers, they strengthened the intestinal barrier. It showed that a combination of probiotics and other additives might improve the growth performance. This deserved an in-depth investigation.
The large quantity of microbes in the intestine is involved in shaping intestinal physiology (19), and a stable microflora is especially important. A study suggested that the stability of intestinal flora was perturbed by the CCP challenge and that some pathogenic bacteria might be active (1, 8). Pathogenic bacteria, such as E. coli, contain components of the cell wall like lipopolysaccharides that cause inflammation in the body by activating the toll-like receptor signaling pathways (25). In the present study, the relative abundance of E. coli in the cecal digesta was raised with CCP challenge, and C. perfringens was also numerically increased. Additionally, the number of coccidial oocysts was elevated. Dietary LP was able to alleviate the upregulation of E. coli. However, the relative abundance of C. perfringens was upregulated with LP supplementation. This was a somewhat surprising result since it was expected that dietary LP might inhibit the proliferation of C. perfringens in the intestine. A possible explanation was that, although dietary LP inhibited the proliferation of E. coli, in fact, it also disturbed the intestinal flora. The unstable flora structure provided an opportunity for the colonization of C. perfringens in the intestine. However, it did not aggravate the negative effects of the CCP challenge on broilers. Dietary LP decreased the number of coccidial oocysts with CCP challenge. It seemed that dietary LF and LP might have the potential to improve the intestinal environment. A report demonstrated that almost 80% of the immune response was mediated by gut microbes (26). Hence, it could be interesting to investigate the immunity in broilers.
It was accepted that calcium contributed to the performance and immunity of poultry (27, 28). In the present study, dietary LP elevated the level of calcium in the serum. Additionally, the level of serum i-NOS was also raised with LF and LP treatments. Based on those findings, it might be concluded that dietary LF and LP were beneficial for the immunity of broilers. A study suggested that CCP challenge caused inflammatory responses in broilers (1, 8), and IL-8 was considered a chemokine that was essential for angiogenesis and inflammation (29). Additionally, IL-8 has attracted considerable attention as an immunomodulator in inflammatory responses (29). In the present study, the mRNA level of IL-8 in the jejunum was upregulated with CCP challenge, and dietary LP could alleviate its upregulation. In addition, the transcript level of TGF-β in the spleen was downregulated with CCP challenge, and dietary LF also reversed this result. Pro-inflammatory and anti-inflammatory cytokines were involved in maintaining immune homeostasis. TGF-β was a pivotal member of the anti-inflammatory factor family (30). The present findings demonstrated that the CCP challenge disrupted immune homeostasis and promoted inflammatory responses. This could be one of the reasons for the abnormal immune organ indices in broilers challenged by CCP.
Combined infection with coccidia and C. perfringens impaired the intestinal barrier function by disrupting the intestinal morphology and tight junctions. In addition, coinfection led to inflammation in broilers. All these adverse factors negatively affect the growth performance of broilers. Dietary LF and LP improved the intestinal health of broilers challenged with CCP by strengthening the intestinal barrier and alleviating inflammation. Dietary supplementation of LF and LP has great potential to alleviate NE in broilers. Here, a detailed investigation of the mechanisms of the regulatory effects of dietary LF and LP on intestinal barrier function and immunity needs to be implemented.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Wuhan Polytechnic University.
BD and SG designed the study. PL wrote the manuscript. PL, LZ, YQ, ZL, ED, JW, and ZZ helped to collect and analyze experimental results. BD, SG, and PL participated in the writing and revision of the manuscript. All authors contributed to the data interpretation and approved the final version of the manuscript.
This study was supported by the Hubei Provincial Key Project for Scientific and Technical Innovation (2020BBA054), the Natural Science Foundation of Hubei Province (2021CFB559), and the Open Project of Key Laboratory of Animal Embryo Engineering and Molecular Breeding of Hubei Province (KLAEMB-2021-04).
All authors would like to thank all volunteers for their commitment and patience during the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Daneshmand A, Kermanshahi H, Mohammed J, Sekhavati MH, Javadmanesh A, Ahmadian M, et al. Intestinal changes and immune responses during Clostridium perfringens-induced necrotic enteritis in broiler chickens. Poult Sci. (2022) 101:101652. doi: 10.1016/j.psj.2021.101652
2. Jayaraman S, Thangavel G, Kurian H, Mani R, Mukkalil R, Chirakkal H. Bacillus subtilis PB6 improves intestinal health of broiler chickens challenged with Clostridium perfringens-induced necrotic enteritis. Poult Sci. (2013) 92:370–4. doi: 10.3382/ps.2012-02528
3. Blomberg L, Henriksson A, Conway PL. Inhibition of adhesion of Escherichia coli K88 to piglet ileal mucus by Lactobacillus spp. Appl Environ Microbiol. (1993) 59:34–9. doi: 10.1128/aem.59.1.34-39.1993
4. Wang A, Yu H, Gao X, Li X, Qiao S. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie Van Leeuwenhoek. (2009) 96:89–98. doi: 10.1007/s10482-009-9339-2
5. Wang X, Yang F, Liu C, Zhou H, Wu G, Qiao S, et al. Dietary supplementation with the probiotic Lactobacillus fermentum I5007 and the antibiotic aureomycin differentially affects the small intestinal proteomes of weanling piglets. J NUTR. (2012) 142:7–13. doi: 10.3945/jn.111.147074
6. Zhang H, Sun J, Liu X, Hong C, Zhu Y, Liu A, et al. Lactobacillus paracasei subsp. paracasei LC01 positively modulates intestinal microflora in healthy young adults. J Microbiol. (2013) 51:777–82. doi: 10.1007/s12275-013-3279-2
7. Xu Y, Tian Y, Cao Y, Li J, Guo H, Su Y, et al. Probiotic Properties of Lactobacillus paracasei subsp. paracasei L1 and Its Growth Performance-Promotion in Chicken by Improving the Intestinal Microflora. Front Physiol. (2019) 10:937. doi: 10.3389/fphys.2019.00937
8. Guo S, Xi Y, Xia Y, Wu T, Zhao D, Zhang Z, et al. Dietary lactobacillus fermentum and bacillus coagulans supplementation modulates intestinal immunity and microbiota of broiler chickens challenged by clostridium perfringens. Front Vet Sci. (2021) 8:680742. doi: 10.3389/fvets.2021.680742
9. Zhang B, Lv Z, Li H, Guo S, Liu D, Guo Y. Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Br J Nutr. (2017) 118:321–32. doi: 10.1017/S0007114517002094
10. Belote BL, Tujimoto-Silva A, Hummelgen PH, Sanches A, Wammes J, Hayashi RM, et al. Histological parameters to evaluate intestinal health on broilers challenged with Eimeria and Clostridium perfringens with or without enramycin as growth promoter. Poult Sci. (2018) 97:2287–94. doi: 10.3382/ps/pey064
11. Li P, Gao M, Fu J, Yan S, Liu Y, Mahmood T, et al. Dietary soya saponin improves the lipid metabolism and intestinal health of laying hens. Poult Sci. (2022) 101:101663. doi: 10.1016/j.psj.2021.101663
12. Shojadoost B, Vince AR, Prescott JF. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: a critical review. Vet Res. (2012) 43:74. doi: 10.1186/1297-9716-43-74
13. Wu M, Yi D, Zhang Q, Wu T, Yu K, Peng M, et al. Puerarin enhances intestinal function in piglets infected with porcine epidemic diarrhea virus. Sci Rep. (2021) 11:6552. doi: 10.1038/s41598-021-85880-5
14. Fu WJ, Stromberg AJ, Viele K, Carroll RJ, Wu G. Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem. (2010) 21:561–72. doi: 10.1016/j.jnutbio.2009.11.007
15. Liu D, Guo Y, Wang Z, Yuan J. Exogenous lysozyme influences Clostridium perfringens colonization and intestinal barrier function in broiler chickens. Avian Pathol. (2010) 39:17–24. doi: 10.1080/03079450903447404
16. Leeson S, Caston L, Summers JD. Broiler response to energy or energy and protein dilution in the finisher diet. Poult Sci. (1996) 75:522–8. doi: 10.3382/ps.0750522
17. Sandvang D, Skjoet-Rasmussen L, Cantor MD, Mathis GF, Lumpkins BS, Blanch A. Effects of feed supplementation with 3 different probiotic Bacillus strains and their combination on the performance of broiler chickens challenged with Clostridium perfringens. Poult Sci. (2021) 100:100982. doi: 10.1016/j.psj.2021.01.005
18. Whelan RA, Doranalli K, Rinttila T, Vienola K, Jurgens G, Apajalahti J. The impact of Bacillus subtilis DSM 32315 on the pathology, performance, and intestinal microbiome of broiler chickens in a necrotic enteritis challenge. Poult Sci. (2019) 98:3450–63. doi: 10.3382/ps/pey500
19. Li P, Gao M, Song B, Liu Y, Yan S, Lei J, et al. Fecal Microbiota Transplantation Reshapes the Physiological Function of the Intestine in Antibiotic-Treated Specific Pathogen-Free Birds. Front Immunol. (2022) 13:884615. doi: 10.3389/fimmu.2022.884615
20. Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim Sci J. (2020) 91:e13357. doi: 10.1111/asj.13357
21. Chen Z, Luo J, Li J, Kim G, Chen ES, Xiao S, et al. Foxo1 controls gut homeostasis and commensalism by regulating mucus secretion. J Exp Med. (2021) 218:e20210324. doi: 10.1084/jem.20210324
22. Csernus B, Czegledi L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. ARCH ANIM BREED. (2020) 63:325–35. doi: 10.5194/aab-63-325-2020
23. Rodriguez-Nogales A, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, et al. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition. Mol Nutr Food Res. (2017) 61:1700144. doi: 10.1002/mnfr.201700144
24. Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, et al. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. (2019) 6:47. doi: 10.3389/fnut.2019.00047
25. Ohno M, Hasegawa M, Hayashi A, Caballero-Flores G, Alteri CJ, Lawley TD, et al. Lipopolysaccharide O structure of adherent and invasive Escherichia coli regulates intestinal inflammation via complement C3. Plos Pathog. (2020) 16:e1008928. doi: 10.1371/journal.ppat.1008928
26. Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. (2006) 312:1355–9. doi: 10.1126/science.1124234
27. Ghasemi P, Toghyani M, Landy N. Effects of dietary 1 alpha-hydroxycholecalciferol in calcium and phosphorous-deficient diets on growth performance, tibia related indices and immune responses in broiler chickens. Anim Nutr. (2019) 5:134–9. doi: 10.1016/j.aninu.2018.04.011
28. Hakami Z, Sulaiman A, Alharthi AS, Casserly R, Bouwhuis MA, Abudabos AM. Growth performance, carcass and meat quality, bone strength, and immune response of broilers fed low-calcium diets supplemented with marine mineral complex and phytase. Poult Sci. (2022) 101:101849. doi: 10.1016/j.psj.2022.101849
29. Kim JH. Interleukin-8 in the tumor immune niche: lessons from comparative oncology. Adv Exp Med Biol. (2020) 1240:25–33. doi: 10.1007/978-3-030-38315-2_2
Keywords: Lactobacillus fermentum, Lactobacillus paracasei, intestinal health, broiler, necrotic enteritis
Citation: Li P, Zheng L, Qi Y, Liu Z, Du E, Wei J, Zhang Z, Guo S and Ding B (2022) Dietary Lactobacillus fermentum and Lactobacillus paracasei improve the intestinal health of broilers challenged with coccidia and Clostridium perfringens. Front. Vet. Sci. 9:1025677. doi: 10.3389/fvets.2022.1025677
Received: 23 August 2022; Accepted: 22 November 2022;
Published: 15 December 2022.
Edited by:
Bruno Solis-Cruz, Universidad Nacional Autonóma de México, MexicoReviewed by:
Muhammad Zahoor Khan, University of Agriculture, Dera Ismail Khan, PakistanCopyright © 2022 Li, Zheng, Qi, Liu, Du, Wei, Zhang, Guo and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangshuang Guo, Z3VvMXNodWFuZ3NodWFuZ0AxNjMuY29t; Binying Ding, ZGJ5aW5nNzQ3MUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.