
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 12 October 2022
Sec. Animal Reproduction - Theriogenology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.1013533
This article is part of the Research Topic Advanced Cryogenic Tools To Preserve Gametes, Embryos, And Gonadal Tissues View all 7 articles
The effects of adding mixed chicken and Japanese quail egg yolks (EYs) to the cryodiluent on the quality of ram semen before freezing and post-thawing were evaluated. Additionally, the composition of chicken and quail egg EYs and their mixture were analyzed for results explanation. The semen was collected from rams (n = 5) and extended with cryodiluent containing the EY of chicken, quail or their mixture (1:1). The extended semen was chilled slowly to 5 °C within 2 h and equilibrated for 2 h, before frozen on the liquid nitrogen vapor and cryopreserved at −196 °C. The straws were evaluated before freezing and post-thawing for sperm motility, vitality and abnormality besides plasma-membrane and DNA integrities. The moisture, ash, protein, and fatty acid (FA) contents of chicken EY, quail EY and their mixture were analyzed. Sperm vitality, plasma membrane integrity and DNA integrity before freezing were significantly (P < 0.05) higher in quail EY than chicken EY and mixed EYs cryodiluent. The chicken EY extender significantly improved the vitality, plasma membrane and DNA integrities of post-thawed ram semen in comparison with quail EY or mixed EYs extenders. While, the post-thawing sperm abnormalities was lower (P ≤ 0.05) in quail EY than chicken EY and mixed EYs cryodiluent. The post-thawing sperm motion kinetics parameters were higher in quail EY than chicken EY and mixed EYs cryodiluent. The highest percentages of moisture, ash, saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) were detected in quail EY had. While, the highest percentages of fat, protein and polyunsaturated fatty acids (PUFAs) were detected in chicken EY. In conclusion, using of chicken EY can improve total motility, vitality, plasma membrane integrity and DNA integrity of cryopreserved ram semen. While, using of quail EY can improve sperm abnormalities and kinetic motion parameters of cryopreserved ram semen. Mixing chicken and quail EYs added no value for post-thawing ram semen parameters.
Cryopreservation of semen is one of the essential technologies in animal research for enhancing productive and reproductive efficiencies (1). Semen cryopreservation helps to prolong sperm viability by slowing metabolism and preventing bacterial development, reducing the accumulation of metabolic byproducts (2–4). Cryopreservation of mammalian sperm is a complex procedure involving balancing several elements to produce the best results. Compared to other species, ram sperm has a low intramembrane cholesterol-to-phospholipid ratio. Therefore, ram sperms are more sensitive to cold-shock than sperms of other species (5).
Diluents, dilution, cooling, freezing, and thawing procedures all have a vital role in the success of ram semen cryopreservation (6, 7). The acrosome, nucleus, mitochondria, axoneme and plasma membrane, are all affected by rapid temperature fluctuations(cold shock) and the creation and dissolution of ice during the freezing-thawing process (3, 8). Cold shock damage occurs when sperm is rapidly cooled from 30 to 4 degrees Celsius (9). Temperature changes during cooling cause stress on sperm membranes, resulting in lipid phase shifts and a difference in the functional condition of sperm membranes. Therefore, semen must be diluted with a cryoprotectant extender to avoid sperm damage during freezing (10).
Egg yolk (EY) has a good effect as a non-permeable cryoprotectant at both cooling and sub-zero temperatures (11, 12). It has traditionally been used in commercial sperm extenders to protect the sperm membrane (13). The efficiency of using EY based diluents in the cryopreservation of animal semen including bull (14), camels (15), buck (16), fish (17) and boar (18) has been well demonstrated.
The mechanism by which EY protects sperm cells during cooling and freezing remains un fully understood. In 1939, the protective effects of EY on sperm during freezing were discovered for the first time (19). Low-density lipoproteins (LDLs) in the EY fraction have been identified as the active components (20). However, LDLs from sources other than EY did not provide the same protection level as EY (21, 22), which was likely because the LDL component of yolk contains numerous other substances, including proteins, which function in tandem to protect the sperm cells (20).
The chemical composition of the EYs of various avian species differs, particularly in terms of their cholesterol, fatty acid, and phospholipid contents (23, 24), which determine EY protection ability during cooling, freezing, and thawing (18). Conventionally, chicken EY has been used commonly as a non-permeable semen cryoprotectant, likely due to its ubiquitous availability (18). Nevertheless, EYs of other avian species improve the post-thaw quality of bull (25), ram (26–30), equine (31–35), buffalo (36–38) and boar (18) sperms.
Comparison among the efficacy of EYs of different avian species has started gaining momentum. Chicken and quail EYs have been reported to be better among many other avian species for cryopreservation of ram semen. Because of the relatively lower saturated fatty acids (SFA) ratio to polyunsaturated fatty acids (PUSFAs) in quail yolk (18), quail EY can result in better post-thaw quality or at least similar sperm protection as chicken EY in ram (28–30), jackass (31), buffalo (13) and Spanish Ibex (39). The information regarding the efficacy of using mixture of EYs of different avian species for semen cryopreservation is absent or scarce in the literature. To the best of our knowledge, the use of chicken and quail EYs mixture in cryodiluent for cryopreservation of ram semen has not been reported. Therefore, the objective of this study was to compare the efficacies of using chicken and quail EYs and their mixture in cryodiluent for cryopreservation of ram semen via semen evaluation before freezing and post-thawing. Additionally, the composition of chicken and quail egg EYs and their mixture were analyzed for results explanation.
This experiment was performed in the autumn on seven mature, fertile Najdi rams aged 2–4 years with an average body condition score of 3 (0 = extremely thin, 5 = obese). At the experimental farm, Department of Animal Production, King Saud University, Riyadh, Saudi Arabia (latitude 24° 48′ N and longitude 46° 31′ E), rams were sheltered in a covered yard within an open-sided barn. The daily energy and protein requirements of rams were met with commercial mixed pellets (14.5 % crude protein and 2.78 Mcal metabolizable energy kg−1 dry matter, respectively). All rams were healthy and passed the breeding soundness evaluation test including semen evaluation using computer-assisted sperm analysis (CASA) (Sperm Class Analyzer® version 4.0.0.5, Microptic S.L., Barcelona, Spain). The King Saud University Research Ethics Committee (REC) authorized the current project (Ethical Reference No: KSU-SE-21-33). This authorization is based on the advice of the Research Ethics Sub-Committee (minute number 7 and date 22/04/2021), as well as a suitable risk-to-benefit ratio and a study design that minimizes risks.
Unless otherwise stated, all materials were obtained from Sigma (Sigma-Aldrich Corp., St. Louis, MO, USA). Tris-based buffer was prepared by dissolving 250 mM (3.028 g) Tris, 88.5 mM (1.78 g) citric acid, and 69.38 mM (1.25 g) fructose in 100 mL of distilled water (16). The buffer was then supplemented with 18 percent (v/v) EY and 8 percent (v/v) glycerol. Gentamicin (13.3 mg mL−1) was added. Three different diluents were prepared based on the type of egg yolk (EY): Tris chicken EY (C), Tris Japanese quail EY (Q), Tris chicken and Japanese quail EYs mixture (1:1) (CQ).
Semen samples were collected twice weekly from each ram using an artificial vagina. The color, density, volume, mass activity, sperm motility, and sperm cell concentration of each ejaculate were evaluated macroscopically and microscopically. Sperm motility and sperm cell concentration were automatically determined using a Sperm Class Analyzer® (SCA® version 4.0.0.5, Microptic S.L., Barcelona, Spain). The yellow and milky ejaculates that met the conventional parameters of 1.5 mL volume, 4 mass activity score, 80% sperm progressive motility, and 2 × 109 sperm mL−1 concentration were selected for further processing. The ejaculates were pooled to eliminate individual ram variations. Seven pooled ejaculates were utilized in this study.
In a water bath, various types of diluents were used to dilute the semen gradually in a dilution rate 1:4 (semen: C, Q, or CQ). The diluted semen was gradually chilled within 2 h from 37 to 5 °C. The diluted cooled semen was loaded into 0.25 mL straws using a semiautomatic filling and sealing machine (minitube GmBH, Tiefenbach, Germany) and left at 5 °C for 2 h to allow for glycerol equilibration. After achieving equilibrium, the straws were frozen in liquid nitrogen vapor (5 cm above liquid nitrogen surface) for 15 min. The straws were then preserved at 196 °C via submerging in liquid nitrogen.
The semen samples were evaluated after achieving glycerol equilibration (before freezing) and post-thawing. The frozen semen was examined after at least 2 weeks of cryopreservation. Thirty seconds were spent thawing the frozen straws in a 37 °C water bath. The semen evaluation was done mainly using SCA® (version 4.0.0.5, Microptic S.L., Barcelona, Spain) which is computer-assisted sperm analysis (CASA) system for semen analysis allows the accurate, repetitive and automatic assessment of the following sperm parameters: motility, concentration, morphology, DNA fragmentation and vitality (16, 40). The details of CASA system was presented in Supplementary material.
SCA® was used to evaluate sperm velocity in three ways (linear, curvilinear, and straight-line) for each sperm. The average pathway velocity (VAP), straight-line velocity (VSL), and curvilinear velocity (VCL) of sperm movement were measured. For evaluating progressiveness on a relative scale, the linearity (LIN = VSL/VCL), straightness (STR = VSL/VAP), and wobble (WOB = VAP/VCL) metrics were utilized and reported as a percentage. The amplitude of lateral head displacement (ALH) and beat cross frequency (BCF) were measured. The results were categorized according to velocity as rapid (VCL >75 μm/s), medium (45 < VCL < 75 μm/s), slow (10 < VCL < 45 μm/s) or static (VCL < 10 μm/s). The sperm presenting movement with a STR index ≥80% was considered progressive motile. The results were categorized as rapid progressive, slow progressive, non-progressive or static according to WHO 4th edition. The results were categorized as total progressive (summation of rapid and slow progressive), non-progressive or immotile according to WHO 5th edition.
Using a FluoVit kit and protocol (Microptic S.L., Barcelona, Spain), the live/dead sperms were determined. Briefly, 10 μL of sperm sample was placed in a 1.5-ml Eppendorf tube, and 1 μL of warmed (37 °C) Hoechst trihydrochloride trihydrate stain was added. Five min were spent incubating this mixture at 37 °C. Then, 1 μL of warmed (37 °C) propidium iodide was added and stirred gently using a micropipette. Five min later, 10 μL of the stained sample was placed on a standard microscope slide with a cover glass and examined using fluorescence microscopy. A dead sperm displayed a fluorescent red hue, while a live sperm displayed a luminous blue hue. SCA vitality software was used to perform an automatic analysis. Each sample was tested with at least 200 sperms.
The semen samples were placed on glass slides, and a conventional sperm smear was performed in duplicate and left to dry in the air. The slides containing sperm smears were placed vertically for 1–2 min on a staining tray with New Rapid SpermBlue® fixative/stain (Microptic S.L., Barcelona, Spain), and the excess stain was drained. The slides were then dipped once in distilled water for approximately 3 sec, put at an angle of 60–80 degrees to remove excess fluid, and allowed to air dry. All of the sperm components (acrosome, head, midpiece, and main piece of tail) were stained with varying blue intensities. Sperm abnormalities were automatically determined and recorded as head length (m), head width (m), head area (m2), head perimeter (m), acrosome (percent), elongation, ellipticity, regularity, rugosity, midpiece width (m), midpiece area (m2), distance (m), and angle (°). The stained smears were examined with SCA® abnormality software under a microscope at 600x magnification.
A hypo-osmotic swelling test (HOST) was utilized as a supplemental test to the viability assessment technique to determine the functional integrity of the sperm plasma membrane. The experiment was carried out by incubating 10 μL of sperm with 100 μL of a 190 mOsm hypo-osmotic solution for 40 min at 37 °C in a 1.5-ml Eppendorf tube. On a warm slide, 100 μL of the sample was distributed with a cover slip. At least five distinct fields were utilized to count 200 sperms. The percentages of sperm with inflated and coiled tails were determined.
Halomax® kit (Halotech, Madrid, Spain) was an in vitro diagnostic solution that allows the measurement of sperm DNA fragmentation for ram (Ovis aries). Halomax® offers a better evaluation of semen quality compared to a traditional method which ignore the most important valuable parameter for a breeder, which is the quality of the genetic material that the sperm cell will transmit to the oocyte. Elevated sperm DNA fragmentation has a negative impact on pregnancy rates and litter size. The evaluation was done according to the manufacturer's instructions. Briefly, the samples of sperm were diluted to a concentration between 5 and 10 × 106 mL1. The agarose gel from the kit was incubated at 90–100 °C for 5 min to fuse the agarose and then at 37 °C for 5 min in a water bath. Twenty-five microliters of the semen sample were added to the Eppendorf tube along with the gel and thoroughly mixed. Twenty microliters of the solution were deposited on a super-coated slide, then placed on a cool surface and covered with a 22x22-mm coverslip. Slides were refrigerated for 5 min at 4 °C to produce a micro-gel containing an implanted sperm. Carefully removing the coverslips, the slides were immersed for 7 min in the previously prepared acid solution (80 μL HCl in 10 mL of distilled water). The slides were then moved to a tray containing the lysing solution and incubated for 25 min, followed by rinsing with distilled water and dehydration in increasing ethanol concentrations for 2 min (70, 90, and 100 percent). The slides were stained with Giemsa dye or Wright stain, washed with tap water, and dried at 25 °C. Each slide was examined at 100 magnifications with a light microscope, and 200 sperms were scored. A sperm without fragmented DNA was placed in an agarose matrix and treated with lysing solutions to deproteinize the nucleus and generate the halos of dispersed DNA. The halos relate to the DNA loops loosely connected to the remaining nuclear structure (core). The sperm nuclei with fragmented DNA produce tiny or no halos of dispersed DNA, whereas the nuclei without fragmented DNA release DNA, forming large halos.
The moisture, ash, and protein content of three EYs group were evaluated. The fatty acids (FAs) of EY were quantified using gas chromatography. FA methyl esters were separated utilizing a GC-9A equipped with a flame ionization detector and a silica capillary column. At 35 mL min−1, nitrogen was used as a carrier gas. The pressure of hydrogen and air was established at 0.5 kg cm−1. The injector and detector were both kept at 250 degrees Celsius. A comparison of retention durations and standards was used to identify the FA methyl esters. The fatty acid composition was expressed as a percentage of the total FAs (41). For trace elements, EY samples from several avian species were digested in 69 percent nitric acid and 30 percent hydrogen peroxide utilizing a microwave acid digestion device (Ultrawave, Milestone, Monroe, CT, USA). With deionized water, the solutions were diluted to 25 mL. Cu (Copper), Zn (Zinc), Fe (Iron), Se (Selenium) and Mn (Manganese) contents in samples were determined using inductively coupled plasma–mass spectrometry (7700X ICP-MS, Agilent, Palo Alto, CA, USA). The concentrations of elements were given in milligrams per kilogram (ppm) of dry weight (42).
All data were subjected to one-way analysis of variance (ANOVA) to compare the various types of extenders. The following model was used: “Yjk = μ + Ak + ejk” where Yjki is the target variable, μ is the overall mean, Ak is the fixed effect of the treatment (avian egg yolk extender), and ejki is the residual error. Duncan's multiple range test was used to compare between the means of treatment to identify the statistically significant differences. The data was represented as mean ± standard error. Differences were considered significant at P < 0.05.
Quail EY had higher percentages of moisture and ash; while, chicken EY had higher percentages of fat and protein. Quail EY had higher percentages of SFAs and MUFAs. In comparison, chicken EY had a higher percentage of PUFAs; quail EY had higher percentages of myristic, palmitoleic, and stearic. While, chicken EY had higher percentages of linoleic, linolenic, gondoic and lignoceric. No significant (P > 0.05) difference was observed between the three groups regarding percentages of oleic and palmitic FAs. Margaric and heneicosylic FAs were detected in chicken EY and were not detected in quail EY (Figure 1). Cu, Zn, Fe, Se and Mn concentrations were significantly higher in quail EY than in the mixture of chicken and quail EYs and chicken EY. Cu, Zn, Fe, Se and Mn concentrations were significantly higher in the chicken quail EYs mixture than in chicken EY (Figure 2).
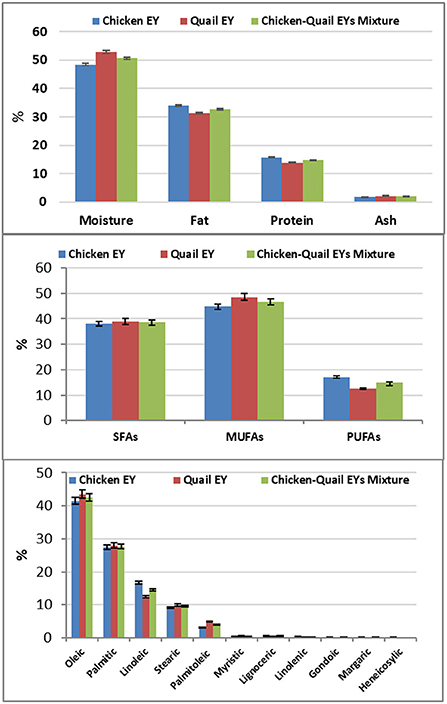
Figure 1. The component and fatty acids of chicken and Japanese quail egg yolk (EY) and their mixture (1:1).
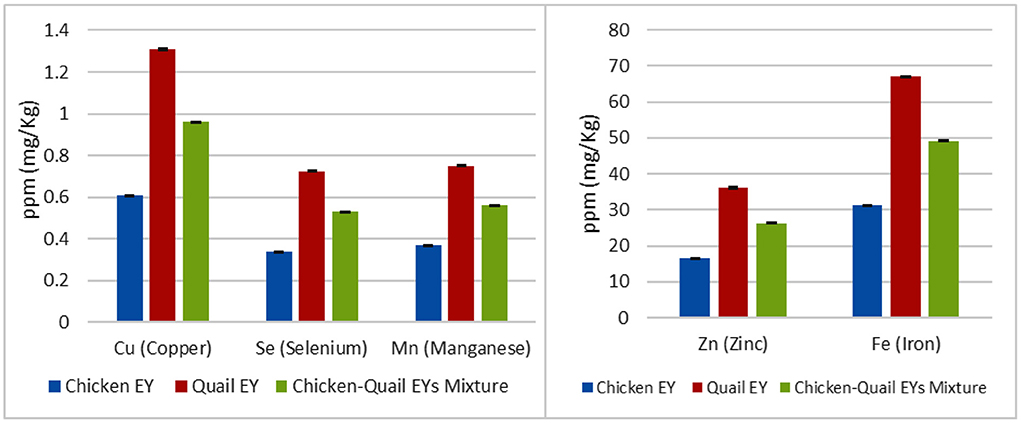
Figure 2. The Cu (Copper), Zn (Zinc), Fe (Iron), Se (Selenium) and Mn (Manganese) concentrations (mg/kg) in the chicken, Japanese quail egg yolk (EY) and their mixture.
The rapid motility before freezing was significantly (P < 0.05) lower in CQ diluent than in the C and Q diluents. However, slow progressive motility before freezing was significantly (P < 0.05) lower in the CQ diluent than in C and Q diluents (Figure 3).
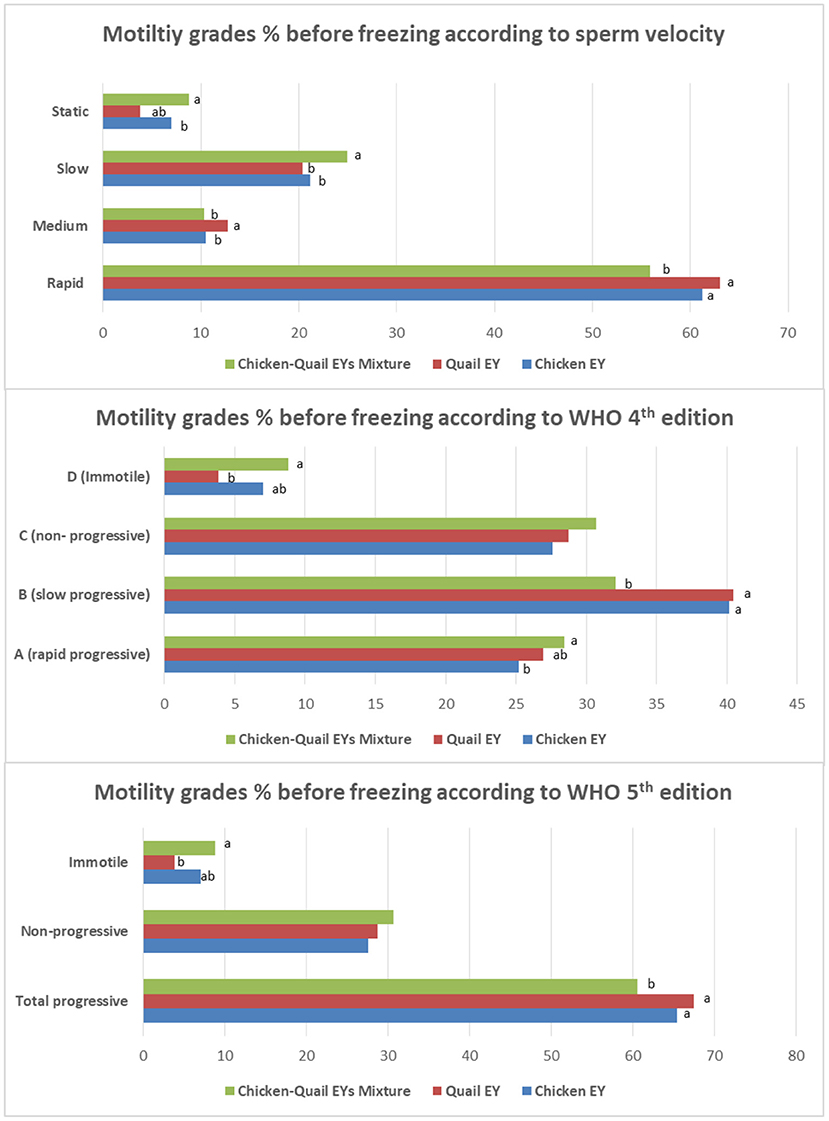
Figure 3. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motility grades of ram sperm before freezing. a,b,c, different letters indicate significant differences among the different egg yolks for each evaluated parameter; Progressive motility, Percentage of sperm presenting movement with a STR index ≥80% within the sample; Statics, VCL < 10 μm/s; Slow, 10 < VCL < 45 μm/s; Medium, 45 < VCL < 75 μm/s; Rapid, VCL >75 μm/s. In WHO 4th edition, the more details of progressive motility were added including slow or rapid progressive. While, in WHO 5th edition, the total progressive motility was presented as a one category regardless sperm velocity.
Total progressive motilities before freezing were significantly lower in CQ than C and Q diluents (Table 1). Total motility was significantly (P < 0.05) lower in the CQ diluent than in the Q diluent. Vitality, plasma membrane integrity and DNA integrity before freezing were significantly (P < 0.05) higher in Q diluents than in C and CQ diluents. Vitality and DNA integrity before freezing were significantly (P < 0.05) higher in C diluent than in CQ diluent. There was not significantly (P > 0.05) different between CQ and C diluents regarding plasma membrane integrity before freezing. However, the sperm abnormality before freezing was significantly (P < 0.05) lower in Q diluent than in C and CQ diluents.
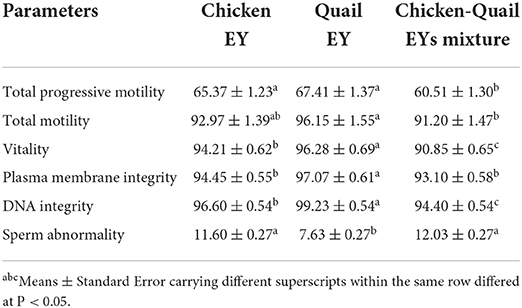
Table 1. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motility, vitality, plasma membrane integrity, DNA integrity and abnormalities of ram sperm before freezing.
The sperm VCL, VSL, VAP, WOB and ALH were significantly (P < 0.05) higher in C diluent than in Q and CQ diluents. The sperm VCL and ALH were significantly (P < 0.05) higher in Q diluent than in CQ diluent. While, the sperm VSL and WOB were significantly (P < 0.05) higher in CQ diluent than in Q diluent. LIN of sperm in C and CQ diluents was significantly (P < 0.05) higher than Q diluent. The STR of sperm was not significantly differed between three diluents. The BCF in Q diluent was significantly (P < 0.05) higher than C and CQ (Table 2).
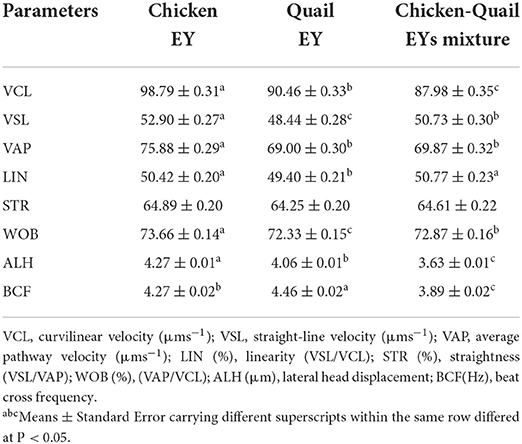
Table 2. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motion kinetics parameters of ram sperm before freezing.
The sperm head width in diluent CQ was higher than Q. The sperm head area and midpiece area before freezing were significantly (P < 0.05) higher in diluent CQ than C and Q. The percentage of sperm acrosome before freezing was significantly (P < 0.05) higher in C diluent than in Q diluent. No significant differences were observed between three diluents regarding head length, head perimeter, elongation, ellipticity, regularity, distance and angle (Table 3).
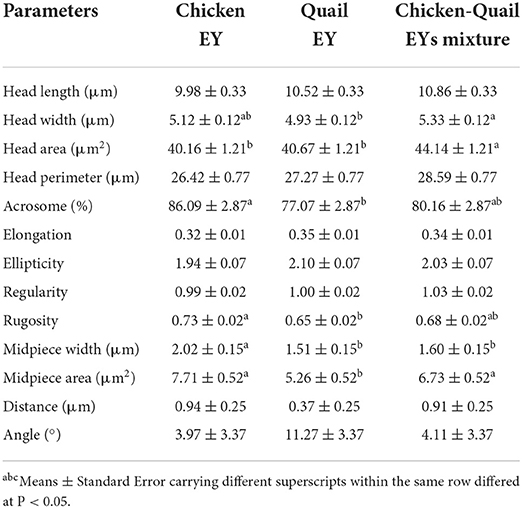
Table 3. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on morphometry of ram sperm before freezing.
The fast and rapid sperm motilities in the three diluents were not significantly (P > 0.05) different after freezing. There was no significant (P > 0.05) difference between the three diluents regarding medium and slow sperm motility. The static motility sperm in diluent C was significantly (P < 0.05) lower than in diluents in Q and CQ (Figure 4).
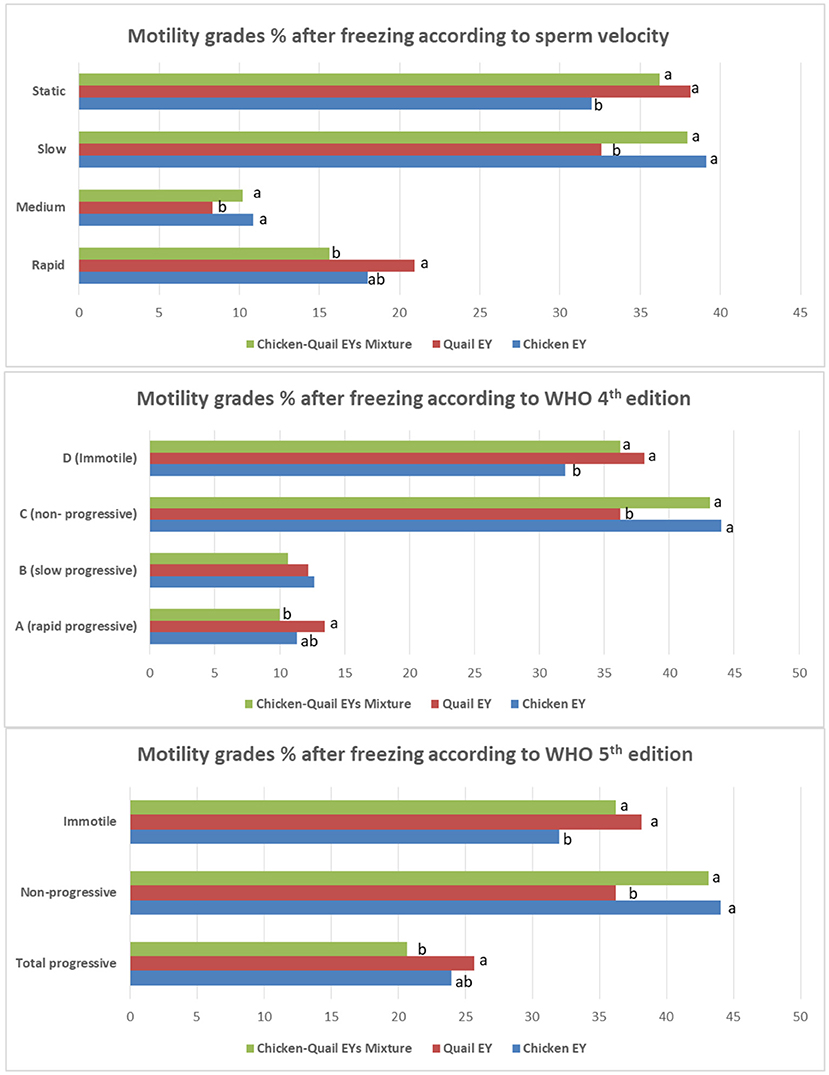
Figure 4. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motility grades of ram sperm post-thawing. a,b,c, different letters indicate significant differences among the different egg yolks for each evaluated parameter; Progressive motility, Percentage of sperm presenting movement with a STR index ≥80% within the sample; Statics, VCL < 10 μm/s; Slow, 10 < VCL < 45 μm/s; Medium, 45 < VCL < 75 μm/s; Rapid, VCL >75 μm/s. In WHO 4th edition, the more details of progressive motility were added including slow or rapid progressive. While, in WHO 5th edition, the total progressive motility was presented as a one category regardless sperm velocity.
The total progressive motility of sperm after freezing was significantly higher in Q diluent than in CQ diluent (Table 4). The total motility, vitality, plasma membrane integrity and DNA integrity were significantly (P < 0.05) higher in C diluent than in Q and CQ diluents. The percentage of sperm abnormality was significantly (P < 0.05) lower in diluent Q than in C and CQ diluents. All sperm kinetic parameters were significantly higher in Q diluent than in C and CQ diluents (Table 5). All morphometric parameters except head length and regularity did not differ between the three diluents (Table 6).
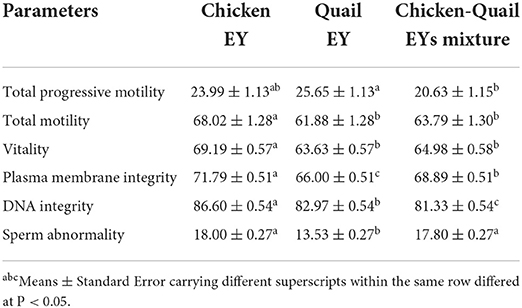
Table 4. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motility, vitality, plasma membrane integrity, DNA integrity and abnormalities of ram sperm post-thawing.
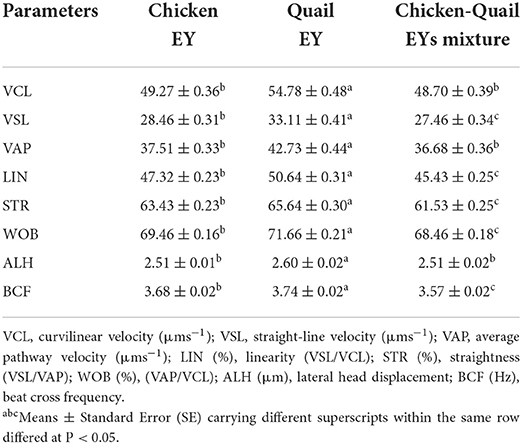
Table 5. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on motion kinetics parameters of ram sperm post-thawing.
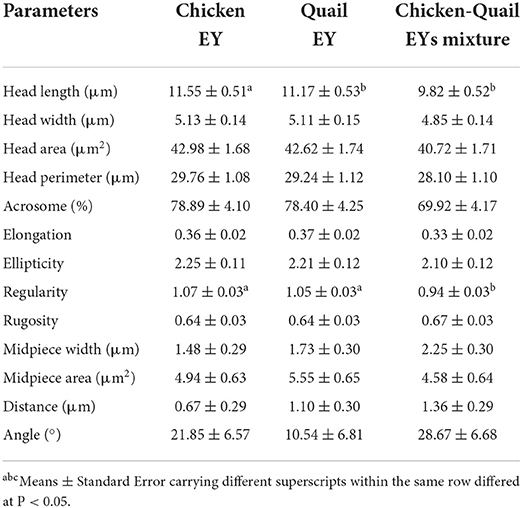
Table 6. Effects of adding and combining egg yolks (EY) of chicken and/or quail to the Tris glycerol extender on morphometry of ram sperm post-thawing.
Egg yolk and other non-penetrating cryoprotectants can protect sperm motility, vitality, and acrosome integrity during cooling and cryopreservation (5, 43, 44). Egg yolk is added to semen extenders to prevent early cold shock (16, 45). Egg yolk LDLs are the main active components (46). However, LDLs from sources other than EY did not provide the same protection as yolk (21, 22). This is likely because the LDL component of yolk contains various other chemicals, including proteins, that work in unison to protect sperm cells (46). Egg yolk LDL protects sperm membranes by binding and neutralizing seminal plasma proteins, which degrade sperm rapidly (47, 48). Therefore, LDL can improve sperm quality and fertility (43, 48). Due to its availability, chicken EY is the main avian EY used to cryopreserve mammalian sperm. However, EYs of other avian species succeed to cryopreserve semen of different animal species (25–38).
Our results after freezing revealed that the Q diluent was superior in the sperm abnormality, total progressive motility and all sperm kinetic parameters. While, C diluent was superior in the total motility, vitality, plasma membrane integrity and DNA integrity. Our findings contradict Kulaksiz et al. (28), who found that ram semen extended in other avian species EYs produced higher-quality sperm than ram sperm extended in chicken EY. Among chucker, chicken, goose, turkey, duck, and quail EYs utilized in ram semen cryodiluents, chucker EY was employed as a substitute to chicken EY and resulted in the highest sperm motility (54%) and vitality (59%) and the lowest sperm acrosomal abnormalities (28). Using of goose EY in ram cryodiluent was better than chicken and duck EYs; goose EY reduced acrosome defects (28). While, quail EY diluent improved sperm membrane integrity (28). Pigeon, duck, and turkey EYs were superior to chicken EYs for cryopreserving ram sperm in tris glycerol extender (26). Using turkey, ostrich, and duck EYs as ram semen diluent showed similar fertilization and embryonic development (27). In a recent experiment using chicken, quail, turkey, and duck EYs for ram semen cryopreservation, quail EY was an acceptable substitute for chicken EY because of it resulted in the lowest DNA fragmentation (8.8%) and the highest motility, sperm membrane integrity and vitality (29). The quail and turkey EYs extenders were superior to chicken and duck EYs in diluting epididymal semen after 4°C storage in Awassi rams (30). In ram and boar, chicken EY extender demonstrated better sperm parameters than quail EY extender (18, 28).
Our results are in agreement with the results of Trimeche, et al. (31), who found quail EY to be a viable replacement for chicken EY in frozen stallion sperm extenders. Using duck and chukar (Alectoris chukar) EYs to freeze stallion sperm improved post-thaw longevity and/or motility in comparison with chicken EY (32, 33).
In another experiment investigated the protective effects of EYs from five avian species (chicken, duck, goose, quail, and pigeon) on bull semen cryopreservation, the pigeon EY was suggested as a good alternative to chicken EY because it increased post-thawed vitality and progressive motility (25). For cryopreservation of buck sperm, chicken EY cannot be replaced by duck, goose, pigeon, quail, or turkey EY (16).
In another study comparing usage of quail and chicken EYs for cryopreservation of Spanish Ibex sperm, quail EY offered no advantages over chicken EY (39). The authors reported that sperm frozen with chicken EY resulted in higher motility, membrane integrity, and vitality in addition to lower abnormality than those frozen with quail EY. However, using quail EY improved acrosome intactness (39).
In buffalo bulls, duck EY extender has superior post-thaw sperm quality than chicken EY (45). In a similar experiment, quail EY at 5% and turkey EY at 10% were superior to chicken EY at 20% for in vitro post-thaw semen quality and in vivo buffalo fertility.
In the current study, the significant differences in evaluating parameters between quail and chicken EYs diluents and their mixture can be explained by the results of EY analysis which showed that quail EY had more SFAs and MUFAs and less PUFAs than chicken EY. Quail EY had more myristic, palmitoleic and stearic FAs and less linoleic, linolenic, gondoic, and lignoceric FAs than chicken EY. This explanation is in agreement with the finding of other researches who reported that the difference in post-thaw semen quality is due to the difference in the biochemical composition of EYs (18, 31). Varied avian EYs have different levels of phospholipids, FAs, and cholesterol (25, 28, 31). The significant difference in post-thaw ram semen quality cannot be explained only by FAs profile but also by other EY component as elements.
The lipid concentration and composition of the sperm plasma membrane determine its cryotolerance. Due to its lipid and FA contents, ovine sperm is particularly vulnerable to cold shock (49, 50). Ram sperm membrane has a low cholesterol/phospholipid ratio, low cardiolipin, phosphatidylserine, ethanolamine plasmalogen, and phosphatidylethanolamine concentrations, and a high content of phospholipids with PUFA, making them highly susceptible to cryodamage (49–53). Ram sperm and seminal plasma have 1.8 and 1.8% total lipids, respectively (54). SFA was 66.6 and 49.9% in seminal plasma and ram sperm, respectively (54). Butyric FA (C4:0) and Margaric FA (C16:0) are the most abundant SFA in ram sperm and seminal plasma (54). Docosahexaenoic acid was the highest abundant UFA in ram sperm and seminal plasma (54).
Our results revealed that all evaluating parameters significantly affected by freezing when comparing these parameters before and after freezing. Generally, post-thaw sperm motility, mitochondrial function, membrane integrity, and vitality decreased after cryopreservation and thawing. This drop in the post thawing parameters can be explained by the effect of cryopreservation on lipids. Cryopreservation of ram sperm alters choline glycerophospholipids with or without EY (55, 56). After cryopreservation, glycerophospholipids containing 22:6n-3 disappear (57). Cryopreservation impairs mitochondrial activity and damages mitochondria by removing two essential lipids (cardiolipin with 18:2n-6 and phosphatidylethanolamine with 20:4n-6) (57). After cryopreservation, sperm membrane lipids lose sterols, and sphingomyelin species with long chain PUFA decrease (57). These lipid alterations influence plasma membrane integrity, mitochondrial activity, and post-thawing characteristics. Any change in ram sperm lipid profile affects post-thawing sperm quality and cryopreservation outcomes (57). Therefore, preservation and protection of the lipid profile of ram sperm is the basis of any cryopreservation protocol to succeed. During cryopreservation, diluent lipids transfer to sperm membranes to protect it. Therefore, selection of FAs profile of diluent is important for obtaining best cryoprotectant effect.
FAs can be added to semen diluent to replace and/or replenish phospholipids lost during cryopreservation and thawing, increasing sperm osmotic and cryo-tolerances (58, 59). FAs improve sperm vitality, plasma membrane integrity, and livability during cryopreservation and reduces ice-crystal formation (60–62). Oleic and palmitic FA are the main sperm SFAs (63, 64). Palmitic or oleic FA increased bull sperm quality (65). Adding linolenic FA to BioXcell® extender improved chilled and frozen bull sperm (60). Low oleic FA increased rooster semen quality during chilled storage (66). Boar sperm can utilize oleic and palmitic FA as ATP substrates via mitochondrial -oxidation (67). Therefore, oleic or palmitic FAs increased boar sperm quality during cold storage (67). Adding linolenic FA to buffalo diluent improved post-thaw semen quality and fertility (68). Adding n-3 linolenic FA can raise PUFA levels in the sperm membrane (69), improving membrane fluidity and sperm motility. Adding PUFAs to EY can extend shelf life of preserved ram semen (70). PUFAs increase the fluidity and cryo-tolerance of sperm membrane (71). As previously established, normal ram sperm morphology in chicken and fowl EYs diluents may be due to FA absorption (72).
Free FA toxicity affects sperm motility and aggregation (73). Linolenic FA (18:3) was more hazardous than linoleic FA (18:2) for human sperm (73). Oleic FA kills goat sperm (74). Palmitic FA (16:0) and stearic FA (18:0) were non-toxic to human sperm at 100 mg/dl.
Lipid peroxidation and profile alterations cause impaired sperm function (75, 76). During semen freeze-thaw, lipid peroxidation rises (77, 78). After abrupt temperature changes, mitochondria and sperm plasma membranes peroxidase (79, 80). Lipid peroxidation weakens sperm membranes and mitochondria (81, 82).
Disagreements in employing EYs from different avian species in semen extenders for cryopreservation of other animal species are related to differences in sperm membrane composition and EY components, which may result in species-specific interactions (39).
In conclusion, mixing chicken and quail EYs added no value for post-thawing ram semen parameters. Chicken EY is superior in most post-thawing parameters, while quail EY is superior in sperm abnormalities and kinetic motion parameters. Therefore, chicken EY and quail EY can be used separately and without mixing in the ram semen cryodiluent. Further studies are needed to evaluate the fertility rate after using and mixing EYs from avian species.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the King Saud University Research Ethics Committee (REC) authorized the current project (Ethical Reference No: KSU-SE-21-33). This authorization is based on the advice of the Research Ethics Sub-Committee (minute number 7 and date 22/04/2021), as well as a suitable risk-to-benefit ratio and a study design that minimizes risks.
AS contributed to conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, review and editing. HB-A contributed to investigation and methodology. IO and IS contributed to writing—review and editing. AA contributed to funding acquisition, resources, software, supervision, validation, and visualization. All authors contributed to the article and approved the submitted version.
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (13-BIO2462-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1013533/full#supplementary-material
1. Benson EJW, Walters EM, Critser JK. The cryobiology of spermatozoa. Theriogenology. (2012) 78:1682–99. doi: 10.1016/j.theriogenology.2012.06.007
2. Iaffaldano AM, Rosato MP. The preservability of turkey semen quality during liquid storage in relation to strain and age of males. Animal Reprod Sci. (2008) 109:266–73. doi: 10.1016/j.anireprosci.2007.11.024
3. Nur BZi, Ustuner B, Sagirkaya H, Ozguden CG. Effects of different cryoprotective agents on ram sperm morphology and DNAintegrity. Theriogenology. (2010) 73:1267–75. doi: 10.1016/j.theriogenology.2009.12.007
4. Wishart GJ. Metabolism of fowl and turkey spermatozoa at low temperatures. Reproduction. (1984) 70:145–149. doi: 10.1530/jrf.0.0700145
5. Salamon S, Maxwell WMC. Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination. Animal Reproduct Sci. (1995) 37:185–249. doi: 10.1016/0378-4320(94)01327-I
6. Chelucci S, Pasciu V, Succu S, Addis D, Leoni GG, Manca ME, et al. Soybean lecithin–based extender preserves spermatozoa membrane integrity and fertilizing potential during goat semen cryopreservation. Theriogenology. (2015) 83:1064–1074. doi: 10.1016/j.theriogenology.2014.12.012
7. Pontbriand D, Howard JG, Schiewe MC, Stuart LD, Wildt DE. Effect of cryoprotective diluent and method of freeze-thawing on survival and acrosomal integrity of ram spermatozoa. Cryobiology. (1989) 26:341–54. doi: 10.1016/0011-2240(89)90058-8
8. O'Connell M, McClure N, Lewis SE. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Human Reproduct. (2002) 17:704–709. doi: 10.1093/humrep/17.3.704
9. Watson PF. The causes of reduced fertility with cryopreserved semen. Animal Reprod Sci. (2000) 60:481–492. doi: 10.1016/S0378-4320(00)00099-3
10. Saha MA, Bari FY. Cryopreservation techniques for ram sperm. Vet Med Int. (2022) 2022:e7378379. doi: 10.1155/2022/7378379
11. Amirat L, et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: a comparison with Optidyl®, a commercial egg yolk extender. Theriogenology. (2004) 61:895–907. doi: 10.1016/S0093-691X(03)00259-0
12. Moussa M, Marinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen–thawed bull semen. Theriogenology. (2002) 57:1695–706. doi: 10.1016/S0093-691X(02)00682-9
13. Akhter S, Rakha BA, Andrabi SMH, Ansari MS. Comparison of egg yolks from three avian species in extender for cryopreservation of Sahiwal bull epididymal spermatozoa. Animal Sci Papers Rep. (2010) 29:131–38.
14. Fleisch A, Malama E, Witschi U, Leiding C, Siuda M, Janett F, et al. Effect of extender on the quality and incubation resilience of cryopreserved Holstein bull semen. Czech J Animal Sci. (2022) 67:75–86 doi: 10.17221/196/2021-CJAS
15. Swelum AA, M Saadeldin I, Ba-Awadh H, G Al-Mutary M, F Moumen A, N Alowaimer A, et al. Efficiency of commercial egg yolk-free and egg yolk-supplemented tris-based extenders for dromedary camel semen cryopreservation. Animals. (2019) 9:11. doi: 10.3390/ani9110999
16. Swelum AA, Saadeldin IM, Alanazi MB, Ba-Awadh H, Afifi M, Alowaimer AN. Effects of adding egg yolks of different avian species to Tris glycerol extender on the post-thawing quality of buck semen. Animal Reproduct Sci. (2018) 195:345–54. doi: 10.1016/j.anireprosci.2018.06.016
17. Muchlisin ZA, et al. Effect of Dimethyl sulfoxide (DMSO) and egg yolk on sperm motility, fertility and hatching rates of depik Rasbora tawarensis (Pisces: Cyprinidae) eggs after short-term cryopreservation. Aquaculture Res. (2020) 51:1700–705. doi: 10.1111/are.14516
18. Bathgate R, Maxwell WM, Evans G. Studies on the effect of supplementing boar semen cryopreservation media with different avian egg yolk types on in vitro post-thaw sperm quality. Reprod Domest Anim. (2006) 41:68–73. doi: 10.1111/j.1439-0531.2006.00623.x
19. Phillips PH. Preservation of bull semen. J Bio Chemistry. (1939) 130:415 doi: 10.1016/S0021-9258(18)73593-X
20. Watson PF. The roles of lipid and protein in the protection of ram spermatozoa at 5 C by egg-yolk lipoprotein. Reproduction. (1981) 62:483–92. doi: 10.1530/jrf.0.0620483
21. Gebauer MR, Pickett BW, Komarek RJ, Gaunya WS. Motility of bovine spermatozoa extended in 'defined' diluents. J Dairy Sci. (1970) 3:817–23. doi: 10.3168/jds.S0022-0302(70)86297-X
22. Miller LD, Mayer DT. Survival of bovine spermatozoa in media containing a lipoprotein complex isolated from spermatozoa and other sources. University of Missouri, College of Agriculture, Agricultural Experiment Station. (1960).
23. Bair W, Marion WW. Yolk cholesterol in eggs from various avian species. Poultry Sci. (1978) 57:1260–65. doi: 10.3382/ps.0571260
24. Surai PF, Speake BK, Noble RC, Mezes M. Species-specific differences in the fatty acid profiles of the lipids of the yolk and of the liver of the chick. J Sci Food Agricult. (1993) 79:733–6. doi: 10.1002/(SICI)1097-0010(199904)79:5<733::AID-JSFA244>3.0.CO;2-M
25. Su L, Li X, Quan J, Yang S, Li Y, He X, et al. A comparison of the protective action of added egg yolks from five avian species to the cryopreservation of bull sperm. Anim Reprod Sci. (2008) 104:212–19. doi: 10.1016/j.anireprosci.2007.06.019
26. Gholami M, Faraji Z, Zamiri MJ. Effect of egg yolk of four avian species on the cryopreserved ram spermatozoa. Iranian J Vet Res Shiraz Univer. (2012) 13:38
27. Ali BT, Bomboi G, Floris B. Replacing chicken yolk with yolks from other sources in ram semen diluents and their effects on fertility in vitro. Small Ruminant Res. (2013) 113:405–10. doi: 10.1016/j.smallrumres.2013.01.017
28. Kulaksiz R, Çebi C, Akçay E, Daşkin A. The protective effect of egg yolk from different avian species during the cryopreservation of Karayaka ram semen. Small Ruminant Res. (2010) 88:2010. doi: 10.1016/j.smallrumres.2009.11.014
29. Saieed Y, Mahdi ZA, Ibrahim AA. Effect of addition from egg yolk of different avian to Tris extender on freezing semen traits of Awassi rams. Iraqi J Agricultural Sci. (2018) 49:663
30. Mustafa FF, Talat Naoman U. Comparative study of using four types of avian egg yolk for epididymal sperms chilled storage in awassi rams (Ovis aries). J Applied Vet Sci. (2021) 6:46–50. doi: 10.21608/javs.2021.88532.1096
31. Trimeche A, Anton M, Renard P, Gandemer G, Tainturier D. Quail egg yolk: a novel cryoprotectant for the freeze preservation of Poitou jackass sperm. Cryobiology. (1997) 34:385–93. doi: 10.1006/cryo.1997.2009
32. J Clulow JR, Maxwell WM, Evans G, Morris LH. A comparison of duck and chicken egg yolk for cryopreservation of stallion sperm. Aust Vet J. (2007) 85:232–35. doi: 10.1111/j.1751-0813.2007.00151.x
33. Humes R, Webb G. Use of chicken or chukar egg yolk with two cryoprotectants for preservation of stallion semen. Anim Reprod Sci. (2006) 94:62–3. doi: 10.1016/j.anireprosci.2006.03.023
34. Burris C, Webb G. Effects of egg yolk source on the cryopreservation of stallion semen. J Equine Vet Sci. (2009) 29:336–7. doi: 10.1016/j.jevs.2009.04.044
35. Webb GW, Burris CL, Harmon SE, Baker RH. Effects of egg yolk source on the cryopreservation of stallion spermatozoa. J Equine Vet Sci. (2011) 31:166–73. doi: 10.1016/j.jevs.2011.02.003
36. Andrabi SM, Ansari MS, Ullah N, Anwar M, Mehmood A, Akhter S. Duck egg yolk in extender improves the freezability of buffalo bull spermatozoa. Animal Reprod Sci. (2008) 104:427–33. (2008). doi: 10.1016/j.anireprosci.2007.07.003
37. Waheed S, Ahmad N, Najib-ur-Rahman, Jamil-ur-Rahman H, Younis M, Iqbal S. Evaluation of duck egg yolk for the cryopreservation of Nili-Ravi buffalo bull semen. Animal Reprod Sci. (2012) 195–99. doi: 10.1016/j.anireprosci.2012.02.011
38. Akhter S, Rakha BA, Ansari MS, Husna AU, Iqbal S, Khalid M. Evaluation of quail and turkey egg yolk for cryopreservation of Nili-Ravi buffalo bull semen. Theriogenology. (2017) 87:259–65. doi: 10.1016/j.theriogenology.2016.09.002
39. Santiago-Moreno J, Coloma MA, Toledano-Díaz A, Gómez-Brunet A, Pulido-Pastor A, Zamora-Soria A, et al. A comparison of the protective action of chicken and quail egg yolk in the cryopreservation of Spanish ibex epididymal spermatozoa. Cryobiology. (2008) 57:25–9. doi: 10.1016/j.cryobiol.2008.05.001
40. Swelum A, Saadeldin IM, Bahadi M, Afifi M, Al-Mutary M, Alowaimer AN. The effect of heterologous seminal plasma from ram, buck or camel on the freezability of ram semen. Vet Med. (2018) 63:500–12. doi: 10.17221/52/2018-VETMED
41. Ding X, Yu Y, Su Z, Zhang K. Effects of essential oils on performance, egg quality, nutrient digestibility and yolk fatty acid profile in laying hens. Animal Nutr. (2017) 3:127–31. doi: 10.1016/j.aninu.2017.03.005
42. Van Dyke JU, Beck ML, Jackson BP, Hopkins WA. Interspecific differences in egg production affect egg trace element concentrations after a coal fly ash spill. Environ Sci Technol. (2013) 47:13763–771. doi: 10.1021/es401406c
43. Rekha BF, Zohara FY, Alam MGS. Comparisons of commercial Triladyl and locally manufactured extenders for the chilling of semen and their effects on pregnancy rates after transcervical AI in Bangladeshi Indigenous (Ovis aries) sheep. Animal Reprod. (2018) 13:735–42. doi: 10.21451/1984-3143-AR733
44. Zohara F, Bari F, Alam MGS. Effects of proportion of egg yolk and preservation time on chilled semen from indigenous rams. GSTF J Vet Sci. (2014) 1:1. doi: 10.5176/2345-7880_1.1.3
45. Anzar M, Rajapaksha K, Boswall L. Egg yolk-free cryopreservation of bull semen. Plos ONE. (2019) 14:e0223977. doi: 10.1371/journal.pone.0223977
46. Morris GJ, Clarke A. The Effects of Low Temperatures on Biological Membranes. London: Academic Press (1981). p. 189–218.
47. Bergeron A, Manjunath P. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev. (2006) 73:1338–44. doi: 10.1002/mrd.20565
48. Mehdipour M, Kia HD, Moghaddam G, Hamishehkar H. Effect of egg yolk plasma and soybean lecithin on rooster frozen-thawed sperm quality and fertility. Theriogenology. (2018) 116:89–94. doi: 10.1016/j.theriogenology.2018.05.013
49. Darin-Bennett A, White IG. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology. (1997) 14:466–70. doi: 10.1016/0011-2240(77)90008-6
50. Poulos A, Darin-Bennett A, White IG. The phospholipid-bound fatty acids and aldehydes of mammalian spermatozoa. Comp Biochem Physiol B. (1973) 46:541–49. doi: 10.1016/0305-0491(73)90094-1
51. Parks JE, Graham JK. Effects of cryopreservation procedures on sperm membranes. Theriogenology. (1992) 38:209–22. doi: 10.1016/0093-691X(92)90231-F
52. Quinn PJ, White IG. Phospholipid and cholesterol content of epididymal and jaculated ram spermatozoa and seminal plasma in elation to cold shock. Aus J Biol Sci. (1967) 20:1205–16. doi: 10.1071/BI9671205
53. Scott TW, Voglmayr JK, Setchell BP. Lipid composition and metabolism in testicular and ejaculated ram spermatozoa. Biochemical J. (1967) 102:456. doi: 10.1042/bj1020456
54. Díaz R, Torres MA, Bravo S, Sanchez R, Sepúlveda N. Determination of fatty acid profile in ram spermatozoa and seminal plasma. Andrologia. (2016) 48:723–26. doi: 10.1111/and.12506
55. V. Hinkovska-Galcheva D., Petkova, K. Koumanov. Changes in the phospholipid composition and phospholipid asymmetry of ram sperm plasma membranes after cryopreservation. Cryobiology. (1989) 26:70–5. doi: 10.1016/0011-2240(89)90034-5
56. Hinkovska-Galcheva V, Peeva D, Momchilova-Pankova A, Petkova D, Koumanov K. Phosphatidylcholine and phosphatidylethanolamine derivatives, membrane fluidity and changes in the lipolytic activity of ram spermatozoa plasma membranes during cryoconservation. Int J Biochem. (1998) 20:867–71. doi: 10.1016/0020-711X(88)90076-6
57. Carro M, Luquez JM, Peñalva DA, Buschiazzo J, Hozbor FA, Furland NE. PUFA-rich phospholipid classes and subclasses of ram spermatozoa are unevenly affected by cryopreservation with a soybean lecithin-based extender. Theriogenology. (2022) 186:122–34. doi: 10.1016/j.theriogenology.2022.03.035
58. Glazar AI, Mullen SF, Liu J, Benson JD, Critser JK, Squires EL, et al. Osmotic tolerance limits and membrane permeability characteristics of stallion spermatozoa treated with cholesterol. Cryobiology. (2009) 59:201–6. doi: 10.1016/j.cryobiol.2009.07.009
59. Lessard C, Parent S, Leclerc P, Bailey JL, Sullivan R. Cryopreservation alters the levels of the bull sperm surface protein P25b. J Androl. (2000) 21:700–7. doi: 10.1002/j.1939-4640.2000.tb02138.x
60. Kaka A, Wahid H, Rosnina Y, Yimer N, Khumran AM, Sarsaifi K, et al. α-Linolenic acid supplementation in BioXcell® extender can improve the quality of post-cooling and frozen-thawed bovine sperm. Anim Reprod Sci. (2015) 153:1. doi: 10.1016/j.anireprosci.2014.12.001
61. Nasiri AH, Towhidi A, Zeinoaldini S. Combined effect of DHA and α-tocopherol supplementation during bull semen cryopreservation on sperm characteristics and fatty acid composition. Andrologia. (2012) 44:550–55. doi: 10.1111/j.1439-0272.2011.01225.x
62. Wathes DC, Abayasekara DR, Aitken RJ. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. (2007) 77:190–201. doi: 10.1095/biolreprod.107.060558
63. Kelso KA, Redpath A, Noble RC, Speake BK. Lipid and antioxidant changes in spermatozoa and seminal plasma throughout the reproductive period of bulls. Reproduction. (1997) 109:1–6. doi: 10.1530/jrf.0.1090001
64. LenzI . Fatty acid composition of spermatozoa and immature germ cells. Molecular Human Reprodu. (2000) 6:226–31. doi: 10.1093/molehr/6.3.226
65. Kiernan M, Fahey AG, Fair S. The effect of the in vitro supplementation of exogenous long-chain fatty acids on bovine sperm cell function. Reprod Fertil Dev. (2013) 25:947–54. doi: 10.1071/RD12204
66. Eslami M, Ghaniei A, Rad HM. Effect of the rooster semen enrichment with oleic acid on the quality of semen during chilled storage. Poultry Sci. (2016) 95:1418–24. doi: 10.3382/ps/pew041
67. Zhu Z, Li R, Feng C, Liu R, Zheng Y, Hoque SAM, et al. Exogenous oleic acid and palmitic acid improve boar sperm motility via enhancing mitochondrial b-oxidation for ATP generation. Animals. (2020) 10:591. doi: 10.3390/ani10040591
68. Ejaz R, Ansari MS, Rakha BA, Qadeer S, Husna AU, Akhter S. Evaluation of α-linolenic acid for freezability and in vivo fertility of Nili Ravi (Bubalus bubalis) buffalo semen. Theriogenology. (2017) 104:1–6. doi: 10.1016/j.theriogenology.2017.07.019
69. Towhidi A, Parks JE. Effect of n-3 fatty acids and α-tocopherol on post-thaw parameters and fatty acid composition of bovine sperm. J Assist Reprod Genet. (2012) 29:1051–6. doi: 10.1007/s10815-012-9834-7
70. Zarei M, et al. Egg yolk enriched with polyunsaturated fatty acids (PUFAs) improves the shelf life of ram semen in liquid storage. Small Ruminant Res. (2018) 166:2018. doi: 10.1016/j.smallrumres.2018.05.002
71. Royere D, Hamamah S, Nicolle JC, Barthelemy C, Lansac J. Freezing and thawing alter chromatin stability of ejaculated human spermatozoa: fluorescence acridine orange staining and Feulgen-DNA cytophotometric studies. Gamete Res. (1998) 21:51–7. doi: 10.1002/mrd.1120210107
72. Cerolini S, Maldjian A, Pizzi F, Gliozzi TM. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction. (2001) 12:395–401. doi: 10.1530/rep.0.1210395
73. Siegel I, Dudkiewicz AB, Friberg J, Suarez M, Gleicher N. Inhibition of sperm motility and agglutination of sperm cells by free fatty acids in whole semen. Fertil Steril. (1986) 45:273–9. doi: 10.1016/S0015-0282(16)49167-3
74. Pellicer-Rubio MT, Combarnous Y. Deterioration of goat spermatozoa in skimmed milk-based extenders as a result of oleic acid released by the bulbourethral lipase BUSgp60. J Reprod Fertil. (1998) 112:95–105. doi: 10.1530/jrf.0.1120095
75. Aitken RJ, Wingate JK, De Iuliis GN, Koppers AJ, McLaughlin EA. Cis-unsaturated fatty acids stimulate reactive oxygen species generation and lipid peroxidation in human spermatozoa. J Clin Endocrinol Metab. (2006) 91:4154–63. doi: 10.1210/jc.2006-1309R.
76. Aitken RJ, Buckingham DW, Carreras A, Irvine DS. Irvine. Superoxide dismutase in human sperm suspensions: relationship with cellular composition, oxidative stress, and sperm function. Free Radic Biol Med. (1996) 21:495–504. doi: 10.1016/0891-5849(96)00119-0
77. Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev. (2001) 59:451–58. doi: 10.1002/mrd.1052
78. Park NC, Park HJ, Lee KM, Shin DG. Free radical scavenger effect of rebamipide in sperm processing and cryopreservation. Asian J Androl. (2003) 5:195–202.
79. Agarwal A, Prabakaran SA, Said TM. Prevention of oxidative stress injury to sperm. J Androl. (2205) 26:654–60. doi: 10.2164/jandrol.05016
80. Brouwers F, Gadella BM. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic Biol Med. (2004) 35:1382–91. doi: 10.1016/j.freeradbiomed.2003.08.010
81. Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. (1995) 7:659–68. 1995. doi: 10.1071/RD9950659
Keywords: quail, chicken, preservation, egg yolk, fatty acids, ram, semen
Citation: Swelum AA, Ba-Awadh HA, Olarinre IO, Saadeldin IM and Alowaimer AN (2022) Effects of adding mixed chicken and quail egg yolks to the cryodiluent on the quality of ram semen before and after cryopreservation. Front. Vet. Sci. 9:1013533. doi: 10.3389/fvets.2022.1013533
Received: 07 August 2022; Accepted: 16 September 2022;
Published: 12 October 2022.
Edited by:
Barbara Merlo, University of Bologna, ItalyReviewed by:
Mahdieh Mehdipour, University of Tabriz, IranCopyright © 2022 Swelum, Ba-Awadh, Olarinre, Saadeldin and Alowaimer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ayman A. Swelum, YXN3ZWx1bUBrc3UuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.