
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci., 05 January 2022
Sec. Animal Nutrition and Metabolism
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.806105
This article is part of the Research TopicThe Interaction between Digestive Tract Microbes and Hosts in PoultryView all 25 articles
The purpose of this experiment was to study the effects of Plotytarya strohilacea Sieb. et Zuce tannin on broilers growth performance, antioxidant function, intestinal development, intestinal morphology and the cecal microbial composition. In this experiment, a total of 360 1-day-old Arbor Acres male broilers were randomly divided into 4 treatment groups, with 6 replicates in each group and 15 broilers in each replicate. The control group (Control) was fed the basal diet, and the broilers were fed a basal diet supplemented with 0 (Control), 100 (PT1), 400 (PT2), and 800 (PT3) mg/kg Plotytarya strohilacea Sieb. et Zuce tannins for 42 days, respectively. The results showed that the average daily feed intake (ADFI) of the PT1 group was significantly lower than that of the control group, and there was a significant quadratic relationship between the ADFI and the concentration of tannin (P < 0.05). Compared with the control group, the F/G of broilers during the 22–42 days phase in the PT1 group showed a decreasing trend (P = 0.063). The serum catalase (CAT) activity in the PT1 group was significantly higher than those of the other three groups, and the effect was significantly quadratically related to the dosage (P < 0.05). The glutathione peroxidase (GSH-Px) activity in the PT1, PT2 and control groups were significantly higher than that of the PT3 group, and the effect was significantly quadratically related to the addition amount (P < 0.05). The serum total antioxidant capacity (T-AOC) activity in the PT1 group was significantly higher than that in the control group, and the effect was significantly quadratically related to the addition amount (P < 0.05). Compared to the control group, the villus height of jejunum in the PT1, PT2 and PT3 groups were significantly higher, and there was a significant quadratic relationship between the villus height of jejunum and the addition amount (P < 0.05). In addition, adding tannins to diets significantly increased Parabacteroides in the dominant genus (P < 0.05). In conclusion, dietary supplementation with Plotytarya strohilacea Sieb. et Zuce tannin improved the growth performance, antioxidant function, and intestinal morphology along with an increased abundance of Parabacteroides in the cecum, and the recommended dosage of tannin in broiler diets was 100 mg/kg.
With the rapid development of livestock and poultry, long-term and low-dose preventive drugs or excessive use and abuse of antibiotics and other factors result in the presence of antibiotic residues in livestock and poultry products, the resistance of pathogens to antibiotics, the imbalance of normal microbial flora, the decline of animal immunity, and related effects (1–3). With the increasing food safety awareness of the population and the implementation of the policy of antibiotic prohibition in various countries worldwide to control the use of antibiotics, the cost of poultry production will increase (4). The search for antibiotic alternatives with natural, inexpensive, non-toxic and residual characteristics has become a study focal point in the poultry industry (5).
Platycarya strobilacea Sieb. Et Zucc, a walnut plant, is widely distributed in northwest China, east China, central China, south China and other places. Platycarya strobilacea Sieb. Et Zucc is widely used in traditional medicine in China with a long history. It can dispel wind, dispel phlegm, relieve dryness and kill insects (6, 7). Allspice fruit extract is derived from the fruit sequence of Platycarya strobilacea Sieb. Et Zucc and is a natural product that includes plant polyphenols, flavonoids, ellagic acid, vitamins, tannins and other bioactive substances (8, 9). The tannins are ellagic tannins, and the main ingredient is oak ellagin equine, which can be obtained by the hydrolysis of ester ellagic tannins formed by two hexahydroxybiphenyl diacyl groups and glucose. Tannins have special biological and pharmacological activities, and previous studies have mainly focused on the inhibition of cell toxicity, harmful intestinal bacteria, and oxidation and the prevention of cancer and anti-aging effects (10, 11). Tannins have also been used as a compound Chinese medicine in the treatment of acute chronic rhinitis and sinusitis (12). Faraha et al. (13) have studied that grape seed extracts which contain tannins significantly decreased serum total cholesterol, low-density lipoprotein cholesterol and meat malondialdehyde level, and increased the antibody titer against Newcastle disease virus vaccine of broilers. However, few studies have compared and analyzed the effects of Platycarya strobilacea Sieb. Et Zucc tannins on the growth performance, intestinal development and intestinal microbial community of poultry based on microbial sequencing technology.
In this study, Platycarya strobilacea Sieb. Et Zucc tannins were used as an antibiotic substitution to determine their effects on the growth performance of broilers. Additionally, 16S rDNA sequencing was used to compare the effects of tannins on the intestinal microbial community of these broilers, in combination with intestinal development and intestinal morphology, to better understand the impact of tannins on the growth performance of broilers.
In this experiment, a total of 360 1-day-old Arbor Acres male broilers were randomly divided into 4 treatment groups, with 6 replicates in each group and 15 broilers in each replicate. The broilers were fed a basal diet supplemented with 0 (Control), 100 (PT1), 400 (PT2), and 800 (PT3) mg/kg Platycarya strobilacea Sieb. Et Zucc tannins, respectively. The tannins with a content of ≥ 65% tannins were provided by the Research Group on the Chemical Utilization of Plant Tannins, Institute of Chemical Industry of Forest Products, CAF. The testing period was from 1 to 42 days of age. Two feeding phase diets were utilized: a starter diet from 1 to 21 days and a grower diet from 22 to 42 days (Table 1). The diets were formulated to meet the nutrient requirements recommended by the National Research Council (14). Birds had ad libitum access to feed and water and were reared with 23 h of light per day. Animal use and care were approved by the Academic Committee of Southwest Forestry University. Every effort was made to reduce animal stress. Daily, health status was observed and dead chickens and feed consumption were recorded.
The feed intake and body weight of birds were recorded on days 21 and 42. The average daily feed intake (ADFI), average daily gain (ADG), and feed:gain ratio (F/G) were calculated for days 1–21, days 22–42 and days 1–42.
On day 42, 6 birds per treatment group with similar weight per group were selected from 6 replicates and euthanized by severing the jugular vein after blood sampling collection from the wing vein with vacuum blood collection tubes. Approximately 2 ml of blood in coagulation-promoting tubes was collected and centrifuged at 4,000 × g for 10 min to obtain serum and was then stored at −80°C until analysis. The lengths of the duodenum and cecum (both sides) were measured, and the corrected lengths of the duodenum and cecum were calculated according to the formula: Duodenal/Cecum length (cm/kg) = Duodenal/Cecum length (cm)/live weight (kg). Then, cecal chyme was collected, immersed in liquid nitrogen, and stored at −80°C for DNA extraction and 16S rDNA amplicon sequencing analysis by Novogene Corporation (Beijing, China). Then, the weights of the duodenum and cecum (both sides) were measured (contents removed), and the duodenum and cecum indices were calculated according to the formula: Duodenal/Cecum index (%) = [Duodenal/Cecum weight (g)/live weight (kg)] * 100. Approximately 2 cm of intestinal tissue from the jejunum was excised, emptied of chyme, and then fixed with 4% paraformaldehyde solution.
Serum was used to test blood antioxidant indices. According to the kit instructions, the total superoxide dismutase (T-SOD), catalase (CAT), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) activities in the serum and liver were detected by colorimetry. All commercial antioxidant indicator kits were purchased from Nanjing Jiancheng Bioengineering Institute.
Intestinal segments (2 cm) in the middle of jejunum and ileum were taken and stained with hematoxylin and eosin (H&E). Consecutive sections of jejunum and ileum (5 m) were prepared for histomorphological observation. The well-oriented villi and the associated crypt of each sample were selected for morphological analysis using Leica Microsystems. The villus height and crypt depth were measured. Then the ratio of villus height to crypt depth (V/C) was then calculated.
A total of 500 ± 50 mg of cecal chyme was randomly weighed from each replicate using a DNA Kit (DP328, Tiangen Biotechnology Co., Ltd.) to extract the total genomic DNA, and the integrity and concentration of RNA were detected by a NanoDrop ND 2000 (Thermo, America). According to the target fragment, PCR amplification of the V4 region of 16S rDNA and 1.5% agarose gel electrophoresis were performed to extract 400–450 bp PCR products, which were purified using a GeneJET Gel Extraction Kit (Thermo, America). The library was constructed with an Ion Plus Fragment Library Kit (Thermo Fisher Scientific, Waltham, MA, USA). After Qubit quantification and library testing, the constructed library was sequenced using the IonS5TMXL sequencing platform at Novogene Bioinformatics Technology Co., Ltd. (Beijing, China).
Based on the IonS5TM XL sequencing platform, the raw tag quality was filtered by FLASH (V1.2.7), and effective tags were obtained. All effective tags were clustered into operational taxonomic units (OTUs) with 97% identity, and then the OTU sequences and Silva132 database were used for species annotation analysis. The dominant species (relative abundance > 2%) at the family and genus levels were analyzed according to species annotation results. For alpha diversity measurements, the alpha diversity indices were calculated based on the OTUs using the Shannon, Simpson and Chao1 indices. LEfSe analysis was employed to identify the biological differences between treatments.
All data are presented as means with pooled SEM values. Statistical analyses were carried out with SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, United States). One-way ANOVA followed by the LSD multiple comparison test was used to evaluate the differences among the treatment groups. P ≤ 0.05 was considered statistically significant. Probabilities of P > 0.05 but P < 0.10 were defined as tendencies.
Table 2 shows the effects of Plotytarya strohilacea Sieb. et Zuce tannin on the broilers' growth performance. The ADFI of broilers during the days 1–21 phase in the PT1 group was significantly lower than that in the control group, and there was a significant quadratic correlation between ADFI and the amount of tannin (P < 0.05). Compared with the control group, the F/G of broilers during the days 22–42 phase in the PT1 group showed a decreasing trend (P = 0.063).
Table 3 shows the effects of Plotytarya strohilacea Sieb. et Zuce tannin on antioxidant activity of broilers at 42 days of age. The serum CAT activity of broilers in the PT1 group was significantly higher than that of control, PT2 and PT3 groups, and there was a significant quadratic correlation between CAT activity and the amount of tannin (P < 0.05). The GSH-Px activity of broilers in the PT1, PT2 and control groups were significantly higher than that in the PT3 group. The activity of GSH-Px was significantly correlated with the addition amount of tannin (P < 0.05). The serum total antioxidant capacity (T-AOC) of broilers in the PT1 group was significantly higher than that in the control group. There were significant linear and quadratic correlations between T-AOC capacity and the amount of tannin (P < 0.05).
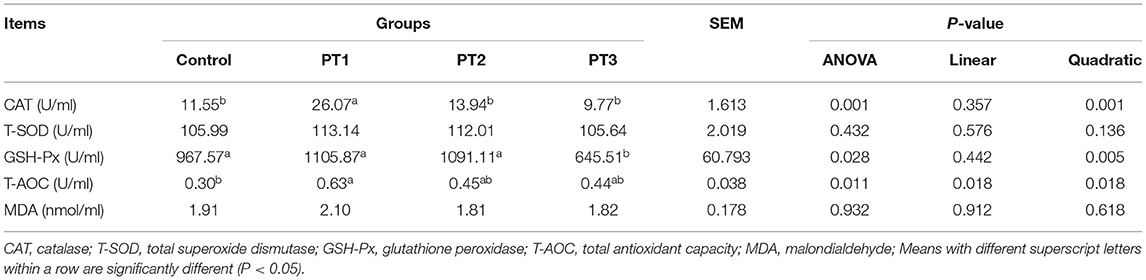
Table 3. Effects of Plotytarya strohilacea Sieb. et Zuce tannin on serum antioxidant activity of broilers at 42 days of age.
Table 4 shows the effects of Plotytarya strohilacea Sieb. et Zuce tannin on the correction length and index of broilers intestines at 42 days of age. There were no significant differences (P > 0.05) among any of the treatments for the corrected length and weight of broiler intestines at 42 days of age.
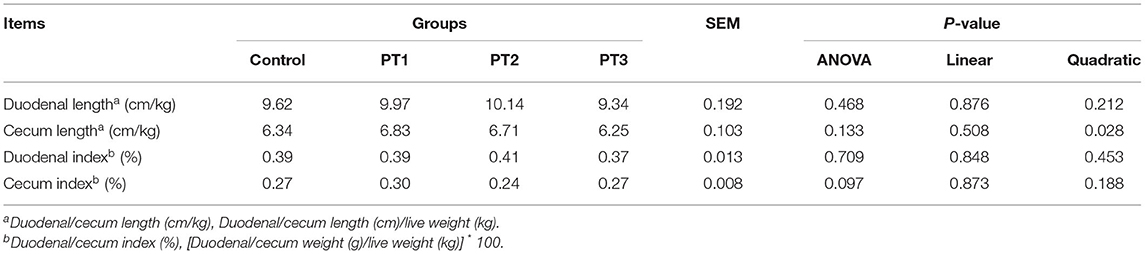
Table 4. Effects of Plotytarya strohilacea Sieb. et Zuce tannin on correction length and index of broilers intestines at 42 days of age.
Table 5 shows the effects of Plotytarya strohilacea Sieb. et Zuce tannin on intestinal morphology of the broilers at 42 days of age. The villus heights of the jejunum in the PT1, PT2 and PT3 groups were significantly higher than that in the control group, and there were significant linear and quadratic correlations between the villus height of the jejunum and the amount of tannin (P < 0.05). The V/C of the ileum in the control and PT1 groups were significantly higher than that in the PT2 group. There was a significant linear correlation between V/C and the amount of tannin (P < 0.05).
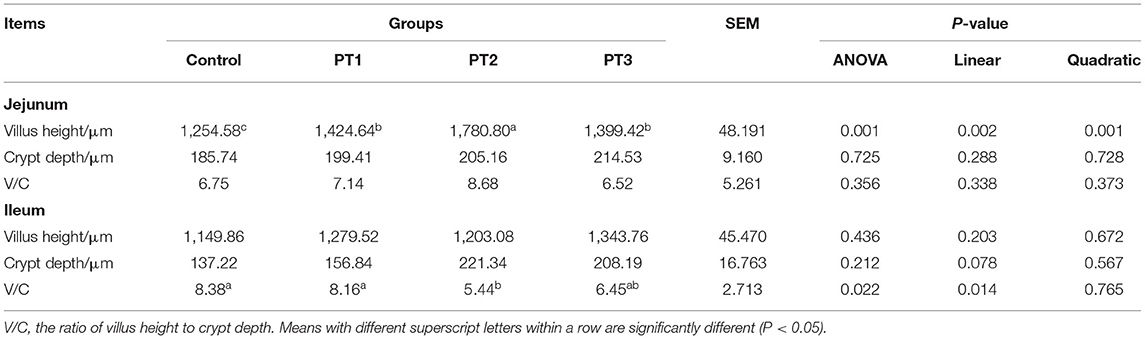
Table 5. Effects of Plotytarya strohilacea Sieb. et Zuce tannin on intestinal morphology of the broilers at 42 days of age.
Table 6 shows the alpha diversity of the cecal microbiota of the broilers at 42 days of age. There was no significant difference (P > 0.05) in the alpha diversity (including observed species, Shannon, Simpson, and Chao1 indices) of the cecal microbiota between the control and PT1 groups. Tables 7, 8 show the dominant flora with a relative abundance >2% at the family and genus levels. Table 7 shows that the dominant bacterial groups at the family level were Ruminococcaceae, Lachnospiraceae, Rikenelaceae, Tannerellaceae, Bacteroidaceae and Enterobacteriaceae. The average relative abundances were 35.22, 12.54, 9.58, 8.03, 4.41, and 2.47%, respectively. Among them, Tannerellaceae in the PT1 group was significantly higher than that in the control group (P = 0.048). Table 8 shows that the dominant bacteria at the genus level were Alistipes, Parabacteria, Akkermansia and Bacteroides. The average relative abundances were 9.58, 8.03, 4.68, and 4.41%, respectively. Parabacteria in the PT1 group was significantly higher than that in the control group (P = 0.039). In this experiment, linear discriminant analysis (LDA) was used to reduce dimensionality and to evaluate the impact of significantly different species. The LDA threshold was set to be >4. Table 8 shows that the dominant bacterium in the PT100 group was Parabacteroides, and its relative abundance was significantly higher than that in the control group (P = 0.039). LEfSe analysis (Figure 1) further identified the species with significant differences between the groups.
Growth performance is the most direct index to reflect the growth of broilers. Improving animal growth performance is the key to increasing economic benefits. The application and research of plant tannins in the production of monogastric animals are relatively limited. In the early stage, raw feed materials (sorghum) might be supplemented with a high dose or high content of plant tannins in the diet, resulting in negative effects such as decreases in feed intake, protein digestibility and production performance of monogastric animals. Therefore, this supplementation is regarded as an antinutritional factor (15, 16). However, several studies in recent years have shown that adding an appropriate amount of plant tannins to the diet can promote the growth performance, antioxidant function and immune performance of monogastric animals (17, 18). Starčević et al. (19) showed that adding an appropriate amount of plant tannins to the diet can improve the growth performance of broilers. Our results showed that the addition of an appropriate amount of Plotytarya strohilacea Sieb. et Zuce tannins in the diet tended to reduce the F/G, but it affected the ADFI of broilers from 1 to 21 days of age. This is not completely consistent with previous research results (19). It may be the bitter and astringent taste of tannin, which affects the palatability and reduces the feed intake of broilers in the early stage (20). However, combined with the results of ADG and F/G in the early stage, it was found that it had no negative impact on broilers. This indicates that the tannins may not inhibit digestive enzymes at multiple sites in the digestive tract and do not affect the digestion, absorption and utilization of nutrients in broilers. In our study, adding an appropriate amount of tannins to the diet had a certain beneficial effect on the growth performance of broilers.
Tannins can effectively remove oxygen free radicals in animals and can improve the activity of antioxidant enzymes in animals to reduce oxidative stress (21). CAT is one of the main antioxidant enzymes in animals. It can scavenge oxygen free radicals (ROS and NO) and protect the body from oxidative stress (22). GSH-Px can catalyze the decomposition of hydrogen peroxide in vivo. It is also one of the main antioxidant enzymes in the body and is conducive to the functional and structural integrity of the cell membrane. T-AOC is a comprehensive index reflecting the antioxidant system of the body. In our study, we found that tannins could significantly improve the serum CAT and T-AOC activities of broilers in a dose-dependent manner. In addition, the addition of vanillin tannin also showed a quadratic correlation with GSH-Px activity. GSH-Px activity was the highest when 100 mg/kg tannin was added, which is similar to previous studies (23). For example, studies have found that the addition of plant tannins is conducive to the enhancement of the antioxidant function of broilers to protect the excessive oxidation of lipids (23). López-Andrés et al. (24) found that adding tannins to the diet can improve the antioxidant function of serum. Hua et al. (25) showed that the addition of tannins can significantly improve the serum T-AOC of rabbit under heat stress and reduce the effect of heat stress on the body. Faraha et al. (13) added plant tannins to a broiler diet to reduce the content of MDA in muscle. Ye et al. (26) found that adding tannins to the diet tended to improve CAT activity in vole livers. In summary, under the test conditions, tannin has strong antioxidant capacity, and the antioxidant effect is the best when 100 mg/kg tannin is added.
The intestinal tract is the main organ for digestion and absorption of broilers. The length and weight of each intestinal segment reflect the growth and development degree of each intestinal segment, and the development degree can reflect the functions of digestion and absorption. In this experiment, the corrected length of intestinal weight and the intestinal index were used to evaluate intestinal growth and development. It has been found that adding an appropriate amount of tannins can protect and promote intestinal health by reducing oxidative stress, promoting the proliferation of beneficial bacteria and inhibiting the proliferation of harmful bacteria (27, 28). In our study, the results showed that the dietary addition of Plotytarya strohilacea Sieb. et Zuce tannins did not significantly affect the intestinal correction length or index of broilers, which was inconsistent with the results of previous studies (27, 28). This discrepancy may be caused by the different extraction sources of added tannins and the concentrations of the active components, and the specific mechanism needs to be further studied.
Intestinal villus height, crypt depth and V/C are important indices to measure intestinal digestion and absorption capacity. Liu et al. (29) found that adding chestnut tannins to the diet can significantly improve the villus height of the jejunum of broilers under heat stress, but the crypt depth showed no significant change. Brus et al. (28) found that the addition of low concentrations of tannins (0.05 and 0.1%) to the diet can stimulate the proliferation of intestinal epithelial cells and promote the intestinal development of broilers. The results of our experiment were similar with the results of study of Brus et al. (28). The addition of an appropriate amount of tannin to the diet could significantly increase the villus height of the jejunum of 42-day-old broilers, and there was a significant linear correlation between the villus height of the jejunum and tannin concentration. In addition, there was a significant linear correlation between the V/C of the ileum and the concentration of tannin. In agreement with the growth performance of this experiment, the ADFI of broilers decreased, but the ADG did not decrease. It is speculated that tannins can increase the area of nutrients absorbed by broilers' small intestine to reduce the negative impact of tannin's bitter taste. This may be because low-dose tannins can promote the proliferation of intestinal epithelial cells and protect DNA integrity in the process of cell proliferation (28) to promote the healthy development of the intestine and benefit production performance.
In this experiment, third-generation high-throughput sequencing technology was used. Third-generation full-length amplicon sequencing mainly amplified the full-length 16S sequence to construct the SMRT bell library. The community structure differences of different samples and groups were then obtained through analysis. According to the results of growth performance and antioxidant function, the differences in cecal microflora between the control and PT1 groups were further studied. It was found that the addition of 100 mg/kg tannins in the diet could significantly increase the relative abundance of Parabacteroides, which were also the dominant bacteria in broilers. Wang et al. (30) showed that Parabacteroides have anti-inflammatory, immune regulatory and metabolism effects, which are conducive to the health of the body. Kverka et al. (31) showed that Parabacteroides can significantly reduce the severity of intestinal inflammation in acute and chronic colitis model mice. Koh et al. (32) found that Parabacteroides can increase the expression of intestinal tight junction proteins, maintain the integrity of the intestinal barrier and be conducive to intestinal health. According to the results of growth performance, antioxidant and intestinal morphology, it is speculated that tannins can improve antioxidant function and promote the growth performance of broilers by increasing the abundance of Parabacteroides, maintaining the integrity of the intestinal barrier and improving blood biochemical indices.
The addition of an appropriate amount of tannin in the diet can improve the serum antioxidant function, intestinal morphology, and growth performance, which may be related to the increase of the abundance of intestinal Parabacteroides by tannin. Taking into account growth performance, oxidation resistance and intestinal morphology, the recommended dosage of tannin in broiler is 100 mg/kg.
The data presented in the study are deposited in the figshare repository, https://doi.org/10.6084/m9.figshare.17078465.v1, accession number 17078465.
The animal study was reviewed and approved by the Academic Committee of Southwest Forestry University.
ZT analyzed the data and drafted the manuscript. FL raised broilers and collected cecal samples. LL analyzed and interpreted the data. FW conceived and designed the manuscript. AG reviewed the manuscript and given critical suggestions and comments. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bogaard AE, London N, Stobberingh EE. Antimicrobial resistance in pig faecal samples from the Netherlands (five abattoirs) and Sweden. J Antimicrob Chemother. (2000) 45:663–71. doi: 10.1093/jac/45.5.663
2. Barton MD. Antibiotic use in animal feed and its impact on human health. Nutr Res Rev. (2000) 13:279–99. doi: 10.1079/095442200108729106
3. Sorum H, Sunde M. Resistance to antibiotics in the normal flora of animals. Vet Res. (2001) 32:227–41. doi: 10.1051/vetres:2001121
4. Shan Z, Guang Z, Dan S, Qiang W, Yan H, Tianxing W. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. (2021) 100:100935. doi: 10.1016/j.psj.2020.12.032
5. Yunqi X, Shan Z, Haibing T, Shourong S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother Res. (2018) 32:384–94. doi: 10.1002/ptr.5966
6. Zhang L, Wang Y, Xu M. Effect of the Platycarya Strobilacea Sieb. et Zucc extract on the growth performance of Epinephelus awoara. Feed Research. (2016) 13:35–40. doi: 10.13557/j.cnki.issn1002-2813.2016.13.009
7. Xu M, Wang YM, Zhang LL, Hu XY. In vitro antibacterial activity of extract from Platycarya strobilacea Sieb. et Zucc Biomass Chemical Engineering. (2020) 54:15–20. doi: 10.3969/j.issn.1673-5854.2020.02.003
8. Tanaka T, Kirihara S, Nonaka GI, Nishioka I. Tannins and Related Compounds. CXXIV Five New Ellagitannins, Platycaryanins A, B, C, and D, and Platycariin, and a New Complex Tannin, Strobilanin, from the Fruits and Bark of Platycarya strobilacea SIEB et ZUCC and Biomimetic Synthesis of C-Glycosidic. Chem Pharm Bull. (2008) 41:1708–16. doi: 10.1248/cpb.41.1708
9. Zhang L, Wang Y, Xu M. Acid hydrolysis of crude tannins from infructescence of Platycarya strobilacea Sieb. et Zucc to produce ellagic acid. Nat Prod Res. (2014) 28:1637–40. doi: 10.1080/14786419.2014.923998
10. Yen GC, Duh PD, Tsai HL. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. (2002) 79:307–13. doi: 10.1016/S0308-8146(02)00145-0
11. Hafsa SA, Ibrahim SA. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J Anim Physiol Anim Nutr. (2018) 102:268–75. doi: 10.1111/jpn.12688
12. Deng Y, Shao LiX. Analysis and antitumor activity in vitro of the essential oil from Fruit sequence of Schistoderma schistoderma by GC-MS. Guid J TCM. (2013) 11:80–2.
13. Faraha MH, Abdallah FM, Ali HA, Hernandez-Santana A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal. (2016) 11:771–7. doi: 10.1017/S1751731116002251
14. National Research Council. Nutrient Requirements of Poultry. 9th rev. edition. Washington, DC: National Academic Press (1994).
15. Treviño J, Ortiz L, Centeno C. Effect of tannins from faba beans (Vicia faba) on the digestion of starch by growing chicks. Anim Feed Sci Technol. (1992) 37:345–9. doi: 10.1016/0377-8401(92)90017-Z
16. Smulikowska S, Pastuszewska B. Świȩch E, Ochtabińska A, Mieczkowska A, Nguyen VC, et al. Tannin content affects negatively nutritive value of pea for monogastrics. Anim Feed Sci Technol. (2001) 10:511–23. doi: 10.22358/jafs/68004/2001
17. Özcan C, Bekir HK, Onur T, Ömer S, Umair A, Sacit F. Effect of dietary tannic acid supplementation in corn- or barley-based diets on growth performance, intestinal viscosity, litter quality, and incidence and severity of footpad dermatitis in broiler chickens. Livest Sci. (2017) 202:52–7. doi: 10.1016/j.livsci.2017.05.016
18. Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. (2018) 4:137–50. doi: 10.1016/j.aninu.2017.09.004
19. Starčević K, Krstulović L, Brozić D, Maljković MM, Stojević Z, Mikulec Ž. Production performance, meat composition and oxidative susceptibility in broiler chicken fed with different phenolic compounds. J Agric Sci Technol. (2015) 95:1172–8. doi: 10.1002/jsfa.6805
20. Provenza FD. Post-ingestive feedback as anelementary determinant of food preference and intake in ruminant. Range Manage. (1995) 48:2–17. doi: 10.2307/4002498
21. Gülcin I, Huyut Z, Elmasta M, Aboul-Enein H. Radical scavenging and antioxidant activity of tannic acid. Arab J Chem. (2010) 3:43–53. doi: 10.1016/j.arabjc.2009.12.008
22. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. (2010) 5:51–66. doi: 10.1038/nprot.2009.197
23. Chamorro S, Viveros A, Centeno C, Romero C, Arija I, Brenes A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal. (2013) 7:555–61. doi: 10.1017/S1751731112001851
24. López-Andrés P, Luciano G, Vasta V, Gibson TMM. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br J Nutr. (2013) 110:632–9. doi: 10.1017/S0007114512005703
25. Hua WL, Xiao FD, Jian MT, Qi Z. A comparative study of growth performance and antioxidant status of rabbits when fed with or without chestnut tannins under high ambient temperature. Anim Feed Sci Technol. (2011) 164:89–95. doi: 10.1016/j.anifeedsci.2010.09.020
26. Ye MH, Nan YL, Ding MM, Hu JB, Liu Q, Wei WH. Effects of dietary tannic acid on the growth, hepatic gene expression, and antioxidant enzyme activity in Brandt's voles (Microtus brandti). Comp Biochem Phys B. (2016) 19–26:196–197. doi: 10.1016/j.cbpb.2016.01.011
27. Vasta V, Pennisi P, Lanza M, Barbagallo D, Bella M, Priolo A. Intramuscular fatty acid composition of lambs given a tanniniferous diet with or without polyethylene glycol supplementation. Meat Sci. (2007) 76:739–45. doi: 10.1016/j.meatsci.2007.02.015
28. Brus M, Gradišnik L, Trapečar M, Škorjanc D, FrangeŽ R. Beneficial effects of water-soluble chestnut (Castanea sativa Mill.) tannin extract on chicken small intestinal epithelial cell culture. Poult Sci. (2018) 97, 1271–1282. doi: 10.3382/ps/pex424
29. Liu HW, Zhao LiK, Deng JS. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J Anim Physiol. (2018) 102:717–26. doi: 10.1111/jpn.12839
30. Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. (2019) 26:222–35. doi: 10.1016/j.celrep.2018.12.028
31. Kverka M, Zakostelska Z, Klimesova K, Sokol D, Hudcovic T, Hrncir T. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin Exp Immunol. (2011) 163:250–9. doi: 10.1111/j.1365-2249.2010.04286.x
Keywords: Plotytarya strohilacea Sieb. et Zuce tannin, growth performance, intestinal morphology, cecal microbial, broilers
Citation: Tong Z, Lei F, Liu L, Wang F and Guo A (2022) Effects of Plotytarya strohilacea Sieb. et Zuce Tannin on the Growth Performance, Oxidation Resistance, Intestinal Morphology and Cecal Microbial Composition of Broilers. Front. Vet. Sci. 8:806105. doi: 10.3389/fvets.2021.806105
Received: 31 October 2021; Accepted: 25 November 2021;
Published: 05 January 2022.
Edited by:
Shourong Shi, Poultry Institute, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Yuna Min, Northwest A and F University, ChinaCopyright © 2022 Tong, Lei, Liu, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiwei Guo, Zy5haXdlaS5zd2Z1QGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.