
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 18 January 2022
Sec. Veterinary Pharmacology and Toxicology
Volume 8 - 2021 | https://doi.org/10.3389/fvets.2021.799773
This article is part of the Research Topic Insights in Veterinary Pharmacology and Toxicology: 2021 View all 9 articles
 Liye Wang1,2
Liye Wang1,2 Lihua Wen1
Lihua Wen1 Yuanhu Pan1,3,4
Yuanhu Pan1,3,4 Zhenzhen Wang2
Zhenzhen Wang2 Kaixiang Zhou1,3,4
Kaixiang Zhou1,3,4 Kun Mi1,3,4
Kun Mi1,3,4 Zhenli Liu1,3,4
Zhenli Liu1,3,4 Wei Qu1,3,4*
Wei Qu1,3,4* Lingli Huang1,3,4*
Lingli Huang1,3,4*Diaveridine (DVD) is widely used for the prevention and treatment of coccidiosis and leucocytozoonosis infections in food-producing animals. To gain a better understanding of DVD metabolism and pharmacokinetics in healthy Landrace/Doric Cross castrated male pigs and both female and male Cobb 500 broiler chickens, a method involving radioactive tracing coupled with LC/MS-IT-TOF was developed for the identification and quantitation of DVD and its metabolites in pig and chicken plasma, and then was applied to investigate DVD pharmacokinetics. A simple MCX solid phase extraction procedure was adopted for sample preparation. After a single oral administration of 3H-DVD (10 mg/kg BW), three radioactive compounds (D0: DVD; D1: 3′-desmethyl-DVD; and D2: monoglucuronide of 3′-desmethyl-DVD) were identified in pig plasma, while only two radioactive compounds (D0 and D2) were identified in chicken plasma. In both species, the Cmax values for all detected compounds were reached at 2 h after dosing. The Cmax order was D2 (1.38 μg/ml) > D0 (0.49 μg/ml) > D1 (0.24 μg/ml) in pigs and D0 (1.55 μg/ml) > D2 (0.27 μg/ml) in chickens. The longer t1/2 (elimination half-life) of D0 contributed to the slow elimination of DVD-related compounds. The t1/2β of D0 in pigs (66.41 h) was significantly longer than that in chickens (48.30 h), but the t1/2 of total DVD-related metabolites in pigs (42.86 h) was lower than that in chickens (56.11 h). These findings suggested that the metabolism and pharmacokinetics of DVD in pigs and chickens were significantly different, and that this would affect its effectiveness, toxicology, and food safety in these animals.
Diaveridine {DVD; 5-[(3′,4′-dimethoxyphenyl)methyl]-2,4-pyrimidinediamine; Figure 1} is a synthetic inhibitor of dihydrofolate reductase (DHFR), an essential enzyme in bacterial folate synthesis (1). When combined with sulfonamides or other antimicrobial agents, DVD blocks the metabolism of folic acid in bacteria by two different mechanisms thereby leading to antibacterial synergistic effects that result in a reduction in resistant strain generation (2). DVD exhibits in vitro antibacterial efficacy against most Gram-negative and Gram-positive bacteria, including Escherichia coli, Clostridium spp., Salmonella spp., Staphylococcus aureus, and Bacillus anthracis (3, 4). It is also noteworthy that DVD has remarkable activity against coccidia and other protozoa. Therefore, it is usually used in combination with sulfaguanidine and sulfamonomethoxine to prevent intestinal infections in the clinic (5). DVD was first considered by the Australian National Drugs and Poisons Schedule Committee in 1969 and was exempted from scheduling on the basis that it exhibited low toxicity. At the February 2003 meeting, DVD was reinstated in the Standard for the Uniform Scheduling of Drugs and Poisons (SUSDP) on the grounds that it was only used in the poultry industry (6). At present, DVD has not been licensed in the FDA and European Medicines Agency. In China, DVD has been currently permitted and extensively used for prevention and treatment of coccidiosis in poultry and rabbits and intestinal infection in livestock and poultry. For instance, sulfamethoxydiazine (SMD) and DVD premix (containing SMD and DVD 200 g and 40 g per 1000 g) was approved for prevention of intestinal bacterial infection and coccidiosis in pigs and poultry, but prohibited in laying hens. The SMD and DVD tablet (30 mg, SMD and DVD 25:5) has been approved for intestinal bacterial infection in livestock by orally administered 0.8–2 tablets at an interval of 12 h for 3–5 days. The sulfaquinoxaline and DVD premix (containing sulfaquinoxaline and DVD 200 g and 40 g per 1,000 g) was allowed to protect poultry from coccidiosis, but prohibited in laying hens (7, 8).
Toxicology studies have demonstrated that DVD is not mutagenic in either tester strain TA100 and TA98 in the presence and absence of rat S9 mix, but it was found to be mutagenic to strain TA100 after metabolic activation with hamster S9 mix (9, 10). This suggests that its metabolites may play an important role in its toxicity or activity. However, the metabolism and pharmacokinetics of DVD in food animals have only been reported in a few studies. In pig microsomes, six metabolites of DVD involving O-demethylation, α-hydroxylation, and N-oxidation were characterized using high-performance liquid chromatography combined with ion trap/time-of-flight mass spectrometry (11). In chicken, 14 metabolites of DVD involving O-demethylation, α-hydroxylation, benzene ring-hydroxylation, N-oxidation, 4′-methylation, NH2-glucuronidation O-demethylation-glucuronidation, and N-oxidation glucuronidation were identified in the plasma after a single oral administration of DVD by high-performance liquid chromatography linear ion trap orbitrap (LC-LTQ-Orbitrap) (12). Although mass spectrometry is well known as a method for powerful structural elucidation of drug metabolites (13), owing to the complexity of a biological sample matrix, false negative or positive results are often observed because characterization of metabolites by mass spectrometry relies only on the ion information. Metabolism studies reported for DVD indicate it can undergo extensive biotransformation in livestock, although its precise metabolism still requires further research. Our previous study identified DVD metabolites in pigs at 6 h after administration by coupling radiotracing with a LC/MS-IT-TOF method. The results showed that the method was sensitive and reliable, and a total of four radiolabeled compounds were identified from this in vivo experiment, of which two were new metabolites (14). Recently, a comparative pharmacokinetic study of DVD in pigs and chickens was reported following a single intravenous or oral administration (15). However, only the parent drug DVD was detected, and the data did not reveal the pharmacokinetic behaviors of any DVD-related compounds or the individual pharmacokinetic characteristics of its metabolites. It is therefore important to investigate the pharmacokinetic profiles of DVD and its metabolites to understand its disposition process and characteristics in animals.
Recently, isotopic tracing combined with online radioactivity detection and ion trap/time-of-flight mass spectrometry (MS-IT-TOF) has been shown to be a reliable analytical tool for the study of the metabolic pharmacokinetics of target compounds in an organism (16). Online radioactivity detection can be used to recognize drug-related metabolites in complex matrices and provide radiochromatographic profiles for quantitative analysis. Furthermore, MS-IT-TOF can be used to provide accurate mass spectral information for the structural elucidation of drug metabolites (17). Consequently, radiotracing combined with modern mass spectrometry technology is regarded as the gold standard for metabolic investigations by the International Cooperation on Harmonization of Technical Requirements for the Registration of Veterinary Medicinal Products (VICH) (18, 19).
In the present study, a method that included radioactive tracing coupled with LC/MS-IT-TOF was developed to identify the major metabolites of DVD in pig and chicken plasma. The method was then applied to investigate the pharmacokinetic features of DVD and its metabolites in pigs and chickens after an oral dose of 3H-DVD. This work should assist in revealing the pharmacokinetic parameters of DVD and its major metabolites in target animals. Moreover, these results should further enhance interpretation of the dose-effect relationship and species differences regarding DVD, in addition to providing a basis for the design of optimal doses of DVD for the treatment of livestock.
Tritium-labeled DVD (3H-DVD) was synthesized by the Shanghai Institute of Applied Physics, Chinese Academy of Sciences (Shanghai, China). Its chemical purity, radiochemical purity, and specific activity were ≥98%, 98.2%, and 22.08 Ci/g, respectively. The resulting solution was stably stored at −20°C for 6 months. An oral dosing formulation was prepared by accurately mixing 3H-DVD and non-labeled DVD at a mass ratio of 1:96 in a vehicle of 0.5% methylcellulose with a specific activity of 0.23Ci/g.
DVD reference standards (purity ≥98%, C13H16N4O2, Molecular Weight: 260.29, CAS: 5355-16-8) was supplied by Wuhan Yuancheng Science and Technology Development Co., Ltd. (Wuhan, China). Ultima GoldTM was purchased from PerkinElmer Life and Analytical Sciences (Groningen, The Netherlands; Waltham, MA, USA). 3H liquid scintillation cocktail for online v.ARC radioactive detection was purchased from XenoBiotic Laboratories, Inc. (Nanjing, China). Methanol, HPLC grade was purchased from Fisher Chemicals Co., (Waltham, MA, USA). Water was purifified by a Milli-Q water purifification system (Millipore, Billerica, MA, USA). Oasis MCX (3 ml, 60 mg) cartridges were purchased from Waters Co. (Milford, MA, USA). All other chemicals and reagents were commercially obtained and of analytical grade or higher purity.
Four healthy castrated male pigs (Landrace/Doric Cross, 60-d-old, 30 ± 2 kg) were supplied by the China Breeding Pig Testing Center of Huazhong Agricultural University (Wuhan, China). Six healthy Cobb 500 broiler chickens (three female and three male, 40-d-old, 2.5 ± 0.1 kg) were purchased from Wuhan Chia Tai chicken farm (Wuhan, China). All animals were maintained under standard environmental conditions by using normal husbandry practices and were acclimatized for 1 week. The animals were fed a commercial standard diet and water ad libitum, but were fasted overnight before dose administration and up to 4 h after dose administration. All animal care and experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of Hubei Provincial Laboratory Animal Public Service Center (permit number SYXK 2013-0044) and approved by the Ethics Committee of Huazhong Agricultural University.
Pigs (n = 4) and chickens (n = 6) were weighed individually and given a single dose of 10 mg/kg BW 3H-DVD with a specific activity of 0.23 Ci/g. The 3H-DVD solution was prepared by accurately mixing 3H-DVD and non-labeled DVD at a mass ratio of 1:96 and diluting it with 0.5% methylcellulose to make a homogeneous suspension with a concentration of 25 mg/ml. The suspension was administered by gastric gavage using a soft stomach tube connected to an injection syringe. About 1.5 ml of blood was collected into heparinized tubes from the anterior venae cavae of pig and the wing veins of chicken at different time points. For pigs, blood was collected before administration and 0.17, 0.33, 0.5, 0.67, 1, 1.5, 2, 4, 6, 8, 12, 24, 48, 72, 168, and 336 h after oral treatment. For chickens, blood was collected before administration and 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, 36, 48, 72, 168, and 336 h after oral dosing. Plasma samples were collected after centrifugation at 4°C and 2,254 × g for 10 min and were stored at −20°C until analysis.
Plasma samples were completely thawed at room temperature. Each sample was divided into two parts, one was used for the assay of total radioactivity, and the other was subjected to a MCX Solid Phase Extraction (SPE) procedure for the qualitative and quantitative analysis of DVD-related metabolites.
The total radioactivity assay was conducted in accordance with a previous study with a few modifications (20). Briefly, 50 μl of plasma was mixed with 50 μl of 0.1 mol/L EDTA-Na and 50 μl of 30% H2O2 at 50°C in a shaking water bath for 2 h for bleaching. The obtained transparent liquid was mixed with 10 ml of Ultima Gold™ scintillation fluid and was analyzed directly using a Packard Tri-Carb 2900 TR liquid scintillation counter (PerkinElmer Life and Analytical Sciences, Waltham, MA, USA). The scintillation counter data were automatically corrected for counting efficiency using an instrument-stored quench curve that was drawn with a series of sealed quenched 14C standards. All the samples were analyzed in duplicate and the actual radioactivity of each sample was calculated by subtracting the background value from the mean radioactivity in two blank samples.
A 500 μl plasma sample was adjusted to pH 2.8–3.2 with 1 ml of 1 M hydrochloric acid (HCl), and a 2 ml aliquot of the mixture was placed in a MCX cartridge preconditioned with 3 ml of methanol followed by 3 ml of 0.1 mol/L HCl water. The cartridge was washed with 3 ml of 0.1 mol/L HCl water and then eluted with 5 ml NH4OH/methanol (8:92, v:v). The effluent was concentrated to dryness under a stream of nitrogen at 40°C. The residue was reconstituted in 250 μl of methanol-water (1:9, v:v) before injection onto the LC-v.ARC/MS-IT-TOF system for metabolite identification and quantitation.
Measurement of total radioactivity in each sample was conducted using a Packard Tri-Carb 2900 TR liquid scintillation counter. The detection time was 10 min for two cycles. The scintillation counter data were automatically corrected for counting efficiency using an instrument-stored quench curve that was drawn with a series of sealed quenched 14C standards. The actual radioactivity was calculated by subtracting the background value from the mean radioactivity in three blank samples.
The assay of DVD-related metabolites was conducted using a high-performance liquid chromatography system (Shimadzu Corp., Kyoto, Japan) coupled with an online isotope detector system (v.ARC, XenoBiotic Laboratories, Inc., Nanjing, China) and a hybrid IT/TOF-MS (Shimadzu Corp., Kyoto, Japan). The liquid chromatography system was equipped with a solvent delivery pump (LC-20AD), an auto-sampler (SIL-20AC), a DGU-20A 3 degasser, a photodiode array detector (SPD-M20A), a communication base module (CBM-20A), and a column oven (CTO-20AC). The chromatographic separation was performed using an Agilent Extend-C18 column [(2.1 mm ×150 mm) i.d.; 5 mm particle size; Agilent Technologies, Santa Clara, CA, USA] at 35°C, and an autosampler set at 4°C. The mobile phases consisted of solvent A (5 mM ammonium acetate containing 0.01% ammonia) and solvent B (100% methyl alcohol) delivered at a flow rate of 0.2 ml/min, with the following optimized gradient program: 0–20 min, 10–40% (B); 20–47 min, 40–90% (B); 47–49 min, 90% (B); and 49–55 min, 10% (B). The injection volume ranged from 10 to 30 μl.
Mass spectrometric detection was performed using an electrospray ionization (ESI) source operated in positive mode. Mass spectrometric analyses were carried out by scanning over the range of 100–1000 Da using data-dependent MS/MS acquisition on the suspected metabolite ions. Liquid nitrogen was used as the nebulizing gas at a flow rate of 1.5 L/min. The capillary and skimmer voltages were set at 4.5 kV and 1.6 kV, respectively. The CDL and heat block temperatures were both maintained at 200°C. MS2 spectra were produced using the CID of the selected precursor ions with argon as the collision gas at a relative energy of 50%. The ion accumulation time was set to 30 ms and the precursor ion isolation width was set to 1 Da. External mass calibration was carried out prior to data acquisition using direct infusion of a reference standard from 50 to 1000 Da. The reference standard consisted of 0.25 ml/L trifluoroacetic acid and 0.1 g/L sodium hydrate. The flow rate of the infusion pump was 5 ml/min. All calculated mass errors were <5 ppm after mass calibration with the reference standard. The identification of unknown metabolites was performed by comparing changes in their molecular mass, chromatographic retention time, full-scan MS/MS spectra, and accurate mass measurement with those of the parent drug. An accuracy error threshold of ± 5 mDa was set as a limit for the calculation of possible elemental compositions. Fully characterized metabolites were designated with the letter D followed by a number.
The developed method was validated in terms of its 3H-H exchange efficiency, linearity of LSC and LC-v.ARC, limits of quantification (LOQ), accuracy, precision, the correlation curve between LSC and LC/LSC, column efficiency, and stability according to VICH GL 46 and GL 47.
When tritium is used as a trace element, it easily undergoes an exchange with the protons in water under physiological conditions to produce tritiated water (HTO). HTO generation from 3H-H exchange causes the loss of radiotracer capacity in the test compound and/or its metabolites and creates ambiguous metabolism data. To assess the 3H-H exchange efficiency, triplicate/nonlyophilized volumetric aliquots (0.2 ml) were taken, mixed with 10 ml of scintillation fluid, and were analyzed directly by LSC. In parallel, triplicate volumetric aliquots (0.2 ml) were frozen, lyophilized in a benchtop vacuum freeze dry system (Labconco Corp., Kansas City, MO, USA), reconstituted in 0.2 ml Milli-Q H2O (EMD Millipore, Billerica, MA, USA), processed as previously indicated, and analyzed by LSC for total radioactivity. The observed differences in total radioactivity for each sample before and after lyophilization were attributed to the 3H-H exchange efficiency.
Recovery is an important parameter for method validation. Radioactive labeling is steadily retained in the metabolic transformation, so the extraction recovery of drug-related metabolites can easily be confirmed by comparing changes in radioactivity before and after sample preparation. In this study, the extraction recovery of total DVD-related metabolites was estimated by determining the radioactivity in the samples before and after MCX purification.
Standard curves were plotted by measuring the serial volumes of 3H-DVD working solution (109 × dilution of 3H-DVD standard solution) on five different days and at five concentration levels (5.13, 20.52, 82.08, 256.50, and 769.50 μg/kg) for LSC and v.ARC. Analyses were performed in triplicate. Correlation coefficients were used to evaluate the linearity of the calibration curve. Unknown concentrations were calculated from the calibration curve equation.
Aliquots of 10 μl deionized water and 1 (262 dpm, 2.56 μg/kg), 2 (524 dpm, 5.13 μg/kg), 3 (786 dpm, 7.70 μg/kg), 4 (1048 dpm, 10.26 μg/kg), 5 (1310 dpm, 12.83 μg/kg), 6 (1572 dpm, 15.39μg/kg), 7 (1834 dpm, 17.96 μg/kg), 8 (2096 dpm, 20.52 μg/kg), 9 (2358 dpm, 23.09 μg/kg), 10 (2620 dpm, 25.65 μg/kg), and 15 μL (3930 dpm, 38.48 μg/kg) of 109× diluted 3H-DVD working solution were mixed with the scintillation liquid and the radioactivity was determined by LSC. Each sample was analyzed for three replicates. Limits of detection (LOD) and limits of quantification (LOQ) were calculated using triple blank activity and the lowest activity with a deviation <5% (M), respectively. The calculation equation was as follows:
The prepared blank samples were fortified with 3H-DVD at three different concentrations [5.13 μg/kg (524 dpm), 10.26 μg/kg (1048 dpm), and 20.52 μg/kg (2096 dpm)] and the radioactivity was determined by LSC. Five sets of each concentration were used in the intraday experiment. Five replicates of three concentrations were analyzed on five different days for the interday experiment. Accuracy was defined as the mean absolute recovery and was calculated by comparing the analytical results of the extracted sample with those of the standard working solution. Precision was defined by the relative standard deviation (RSD).
Radioactivity in the HPLC eluates was quantified using the v.ARC detector. The linear correlations between cpm (X) from the v.ARC detector and dpm (Y) from the Tri-Carb 2900TR analyzer were determined by analyzing the radioactivity of a series of 3H-DVD working solutions via LSC and LC-v.ARC. The calibration curve was plotted by linear regression.
For each matrix, LSC was conducted to measure the radioactivity of the prepared sample aliquots before (R1) and after (R2) chromatographic elution. Column efficiency (X) was calculated as follows:
The stability of DVD and its metabolites was determined in pig plasma at 2 h after the single oral administration of 3H-DVD (10 mg/kg BW), and stored at −20°C for 90 d. The samples were thawed, prepared, and analyzed monthly. The measured values were compared in triplicate against freshly acquired samples.
Plasma concentration vs. time data were sequentially fitted to a non-compartmental model, using the computer program Phoenix (Version 8.2; Pharsight Corporation, Mountain View, CA, USA). The model was determined for best fit on the basis of linear trapezoidal linear interpolation. The compartment model was the best fit for both pigs and chickens. Plasma curves of DVD and its metabolites, after an oral administration of DVD, were obtained for each pig and chicken. Cmax and the time to reach Cmax (Tmax) were determined directly from the concentration vs. time curve. t1/2, AUC0−tk, AUC0−∞, AUMC, MRT, ClB, and Vd were calculated with the following equation:
Where the terminal elimination rate constant (Ke) was calculated from the log-linear portion of the elimination curve using a linear regression analysis. Clast is the last observed concentration.
Differences in pharmacokinetic parameters from the two species were performed by independent sample t-test using IBM SPSS Statistics 25 software. P < 0.05 was considered as statistically significant.
To quantitatively ascertain the “3H-H exchange risk” of radioactive samples, the total radioactivity in pig and chicken plasma was measured before and after freeze-drying using the Tri-Carb 2900TR. No significant difference (<5%) was observed among all the samples before and after lyophilization in terms of their radioactivity suggesting that the “3H-H exchange risk” could be ignored (Figure 2A) (21). The extraction efficiencies of MCX sample preparation for pig and chicken plasma were 95.58 ± 3.51 and 92.18 ± 5.16%, respectively. 3H-DVD was serially diluted into different volumes and then was detected by LCS and LC-v.ARC. There was a strong linear correlation between radioactivity and response (R2 = 0.9998 and 0.9999, respectively; Figure 2B). The triple blank activity and lowest quantitative LSC limit were 270 and 524 dpm, which corresponded to a analyte concentration of 2.64 and 5.13 μg/kg, respectively. The recovery rates for the blank samples with added 1 ×, 2 ×, and 4 × LOQ radioactivity were in the range of 97.6–103.3%. The coefficient of variation was <5%. The curve (Figure 2C) delineating the correlation between LSC and LC-v.ARC was y = 3.6469x –59.784 (R2 = 0.9999). The liquid column recovery rate was 98.5 ± 1.7%. Hence, there was negligible chromatographic column sorption of DVD-related metabolites. The degradation rates of fresh pig plasma stored at −20 °C were <1% after 1 month, <2% after 2 months, and 2–6% after 3 months (Figure 2D). Therefore, samples must be analyzed within 2 months. The methodology and data met the requirements of VICH GL 46 and GL 47 (18, 19).
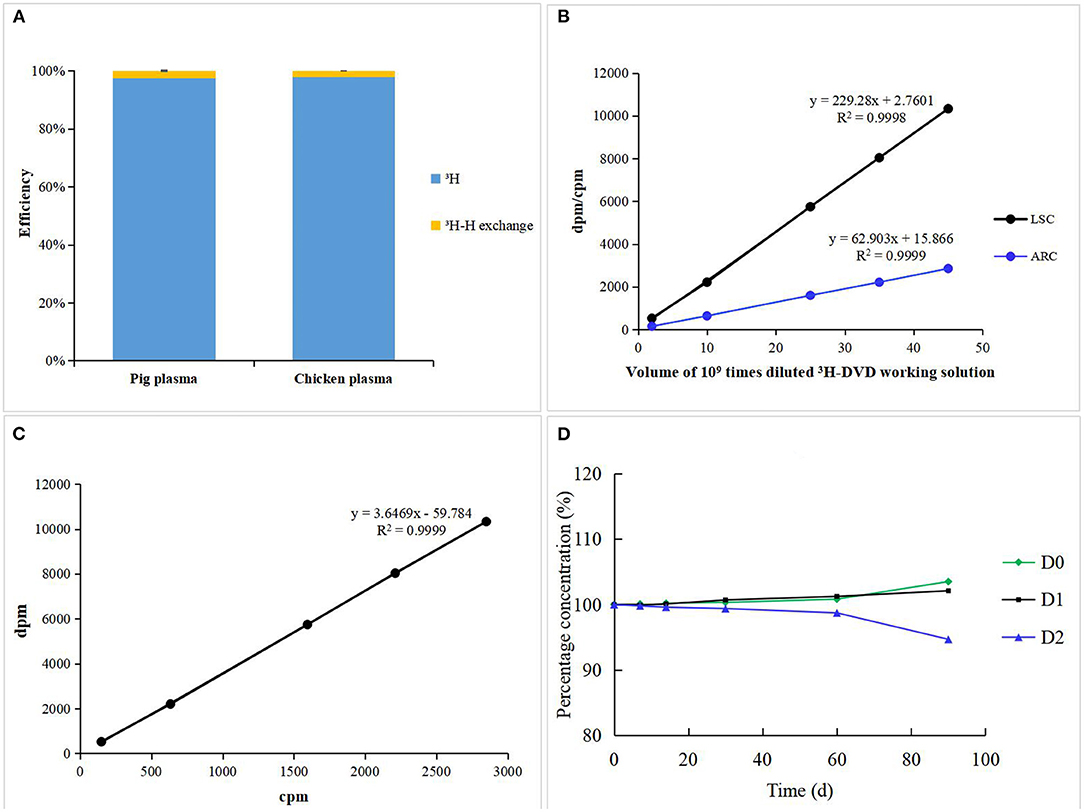
Figure 2. (A) 3H-H exchange efficiency of incurred pig and chicken plasma. (B) Standard curves and Linearity of LSC and LC-v.ARC. (C) Correlation curve between LSC and LC-v.ARC. (D) Stability of DVD and its metabolites in incurred pig plasma stored at −20°C.
The metabolite profiles of DVD in pig and chicken plasma were qualitatively identified using the LC-v.ARC/MS-IT-TOF system. In pig plasma, three radioactive substances were detected, named D0, D1, and D2 (Figure 3). Their chemical structures were characterized by comparing changes in their molecular mass, accurate mass measurement, and chromatographic retention time with those of the parent drug. The accurate MS/MS spectra for DVD and its metabolites are shown in Figure 4.
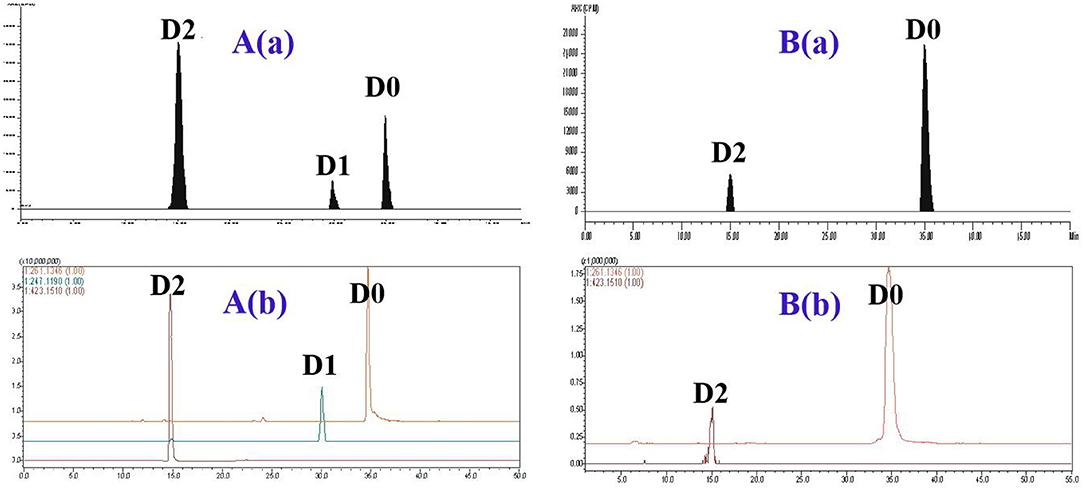
Figure 3. The representative HPLC radiochromatogram (RAC) and corresponding accurate extracted ion chromatogram (EIC) of pig plasma (A) and chicken plasma (B) at 2 h from a single oral administration of 3H-DVD at a dose of 10 mg/kg body weight: (a) RAC and (b) EIC.
DVD (D0) and D2 were detected in pig plasma at 0.17 h and 0.33 h after a single oral administration (Table 1). D0, D1, and D2 were detected at 0.5 h, and were continuously observed until 48 h after administration. D2 was the predominant metabolite, followed by D0. Maximum plasma concentrations of D0, D1, and D2 were observed at 2 h after dosing at 0.49±0.02 μg/ml, 0.24±0.06 μg/ml, and 1.38±0.04 μg/ml, respectively. D2 declined rapidly over time and only D0 and D1 were observed over the period of 72–168 h; and after that, no metabolites were detected in the plasma.
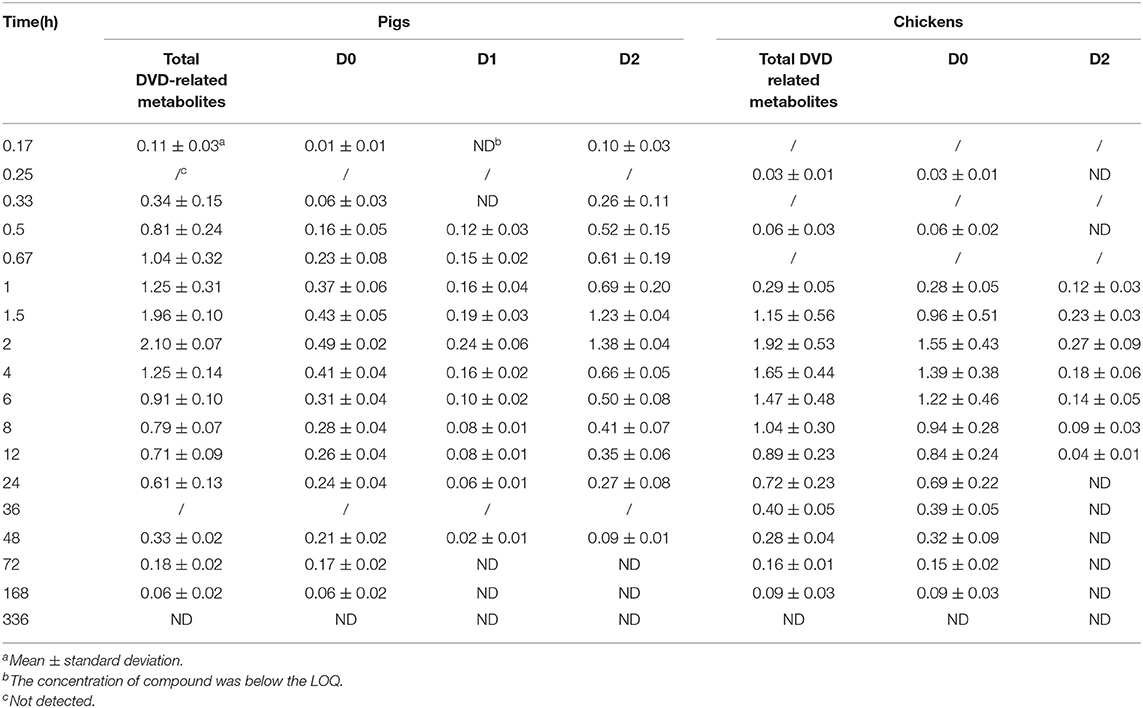
Table 1. Concentrations (μg/ml) of DVD and its metabolites in the plasma of pigs and chickens at different time points after a single oral administration of 3H-DVD at a dose of 10 mg/kg body weight (n = 5).
D0. D0 showed a protonated molecular ion signal at m/z 261 (Supplementary Figure 1). The MS2 spectrum of D0 generated fragment ions at m/z 245, which were formed by the loss of a methyl radical from m/z 261. The fragment ion at m/z 217 resulted from the loss of 28 Da at m/z 245 and corresponded to a CO loss. The formation of m/z 123 occurred by cleavage of a methylene carbon from the m/z 217 species. These results were compared with those of the reference substance. D0 was inferred to be DVD.
D1. D1 had a protonated molecule ion signal at m/z 247, which was 14 Da lower than that of D0, suggesting that D1 is an O-demethylation metabolite of DVD (Supplementary Figure 2). Protonated D1 fragmented to yield an abundant product ion at m/z 232 through the loss of a methyl group from the parent ion. Considering the DVD structure, methane loss may have occurred at the C-3′ or C-4′ methoxy groups. This pattern was similar to that of the analog trimethoprim (TMP) (22). The 3′-demethylated TMP content (>30%) was considerably higher than the 4′-demethylated TMP content (1%) in urine at 8 h after a single oral administration dose of 10 mg/kg BW TMP. A three-dimensional structure simulation analysis showed that the stability of the 4′-hydroxymethyl group was higher than that of the 3′-hydroxymethyl group. Therefore, we assumed that D1 most likely corresponded to 3′-OH-DVD.
D2. Metabolite D2 exhibited a protonated molecular ion signal at m/z 423, which was 176 Da higher than that of D1, implying that D2 was a glucuronide metabolite of D1 (Supplementary Figure 3). However, the position of glucuronidation could not be defined owing to limited mass spectrum information. Compared with the DVD structural analog brodimoprim, the compound resulting from glucuronidation of demethyl-brodimoprim was identified as demethyl-brodimoprim-O-glucuronide owing to the fact that the hydroxyl in the benzene ring was more reactive toward glucuronidation than the amino groups in the pyrimidine ring (23). We proposed that demethylation took place first, followed by formation of a hydroxyl, which was then believed to conjugate with glucuronic acid. Therefore, D2 was tentatively identified as demethyl-diaveridine-O-glucuronide.
In chicken plasma, two radioactive substances were detected and identified to be D0 and D2 (Figure 3). Only D0 was detected in the plasma 0.25 h and 0.5 h after the dose administration. From 1 to 12 h after dose administration, D0 and D2 were detected while only D0 was observed from 24 to 168 h. After that, the concentration of D0 was lower than the quantifiable level. Overall, D0 was the primary radioactive component. Maximum plasma concentrations of D0 and D2 were observed at 2 h after dosing at 1.55 ± 0.43 and 0.27 ± 0.09 μg/ml, respectively (Table 1).
The mean plasma concentration–time curves for DVD and its major metabolites in pig and chicken plasma after an oral dose are shown in Figure 5. Table 2 displays the primary pharmacokinetic parameters obtained using non-compartment model analysis. After a single oral dosing in pig, the concentrations of DVD and its three related metabolites D0, D1, and D2 reached a peak (Cmax) at 2 h, at levels 2.10, 0.49, 0.24, and 1.38 μg/ml, respectively. Although the concentration of D2 was significantly higher than that of D0, D2 was rapidly eliminated. The t1/2 values of total DVD and its three related metabolites were 42.86, 66.41, 17.86, and 18.26 h, respectively. The AUC0− ∞ of total DVD and its three related metabolites were 53.77, 33.94, 3.72, and 17.58 μg·h/L, respectively; the MRT values were 54.98, 92.96, 22.93, and 23.01 h, respectively.
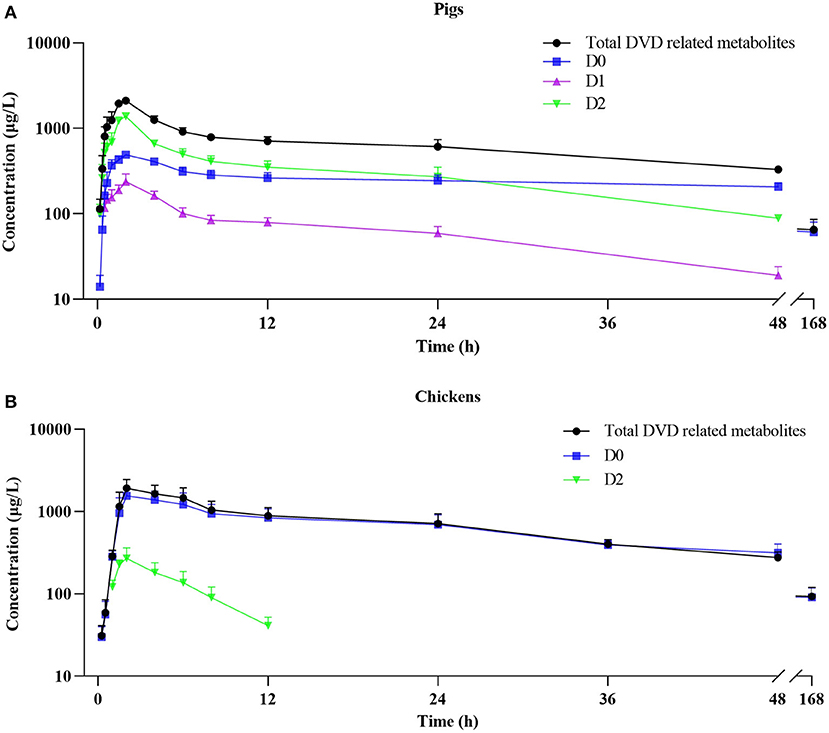
Figure 5. Plasma concentration-time curve of DVD related-metabolites after a single oral administration of 3H-DVD at a dose of 10 mg/kg body weight: (A) pigs and (B) chickens.
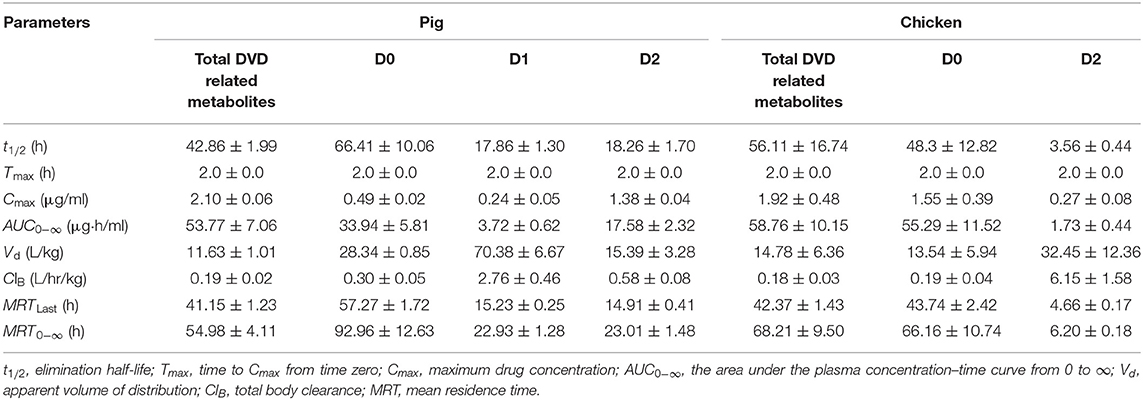
Table 2. Pharmacokinetic parameters of DVD and its metabolites in the plasma of pigs and chickens after oral administration of 3H-DVD at a dose of 10 mg/kg body weight.
Following a single oral dosing, D0 and D2 were detected in chicken plasma. Similar to those of the pig, the Cmax of DVD and its related metabolites D0 and D2 were observed at 2 h after the administration with average concentrations of 1.92±0.53, 1.55±0.43, and 0.27±0.09 μg/ml, respectively. Compared with the quick elimination of D2 (t1/2 = 3.56 h), the elimination of D0 from the plasma occurred slowly with a t1/2 of 48.30 h. The AUC0−∞ of total DVD and its related metabolites D0 and D2 were 58.76, 55.29, and 1.73 μg·h/L, respectively; and the MRT values were 68.21, 66.16, and 6.20 h, respectively.
Statistical analysis was used to evaluate differences in pharmacokinetics parameters between the total DVD related-metabolite and the same metabolites in the plasma of the two species. Results showed that the MRT0−∞ value of total DVD-related metabolites in chickens was markedly longer than that in pigs (P < 0.05), while other observed pharmacokinetic parameters were without notable differences between the two species. For the metabolites of D0 and D2, there were no significant difference for t1/2 of D0 between the two species. However, there were significant differences for other observed pharmacokinetic parameters. These results suggested that DVD underwent extensive metabolism and presented different pharmacokinetic characteristics in pigs and chickens.
Pharmacokinetic studies are critical steps for understanding dynamic characteristics and optimizing dosage regimens for systemically acting antimicrobial drugs (24). Because drug metabolism is often unknown, general pharmacokinetic studies usually focus on the drug prototype and ignore the pharmacokinetic behavior of drug-related metabolites that may have some pharmacological or toxicological activity (25). Landrace/Doric Cross castrated male pigs and Cobb 500 broiler chickens, as the main commercial breeds used in animal husbandry in China, were selected in this study. Metabolites of DVD in pig and chicken plasma were identified and their pharmacokinetic behaviors were studied by a method that included radioactive tracing coupled with LC/MS-IT-TOF. Because of the traceable characteristics of the radioactive label, DVD-related metabolites can be identified and quantified by the LC-v.ARC system, and LC/MS-IT-TOF can provide accurate mass spectra information for the metabolic illustration (17). Oasis MCX, a strong cation-exchange cartridge, is preferred for the extraction of basic analytes in complex matrices, and is the most commonly employed sorbent for the clean-up of DVD structural analogs such as TMP, DVD, and ADP (26–28). In the present study, a routine MCX purification procedure was performed for plasma samples, and extraction efficiency was evaluated by comparing total radioactivity before and after sample preparation. The results suggested that the extraction recoveries of DVD-related metabolites in pig and chicken plasma were more than 90%.
Metabolism plays an important role in the kinetic elimination of DVD in pigs and chickens. In pig plasma, in addition to unmodified DVD, two radioactive metabolites, 3-desmethyl-DVD (D1), and the monoglucuronide of 3-desmethyl-DVD (D2) were detected. The metabolism of brodimoprim (BMP), an analog of DVD, has been studied in rats using NMR and mass spectrometry. Unchanged BMP, methylated BMP, and the glucuronide of BMP were found in plasma after a single oral dose of 300 mg/kg BW (23). Similarly, the metabolism of 14C-trimethoprim (14C-TMP) has been investigated in pigs of various ages after i.v. administration. TMP, demethylated TMP, and the glucuronide of TMP were detected in the plasma; D0 was the most prominent component in 1-day-old pig plasma, while the TMP glucuronide conjugate was the major component in 8-day-old pig plasma (29). These results were consistent with the findings in the current study. In the in vitro metabolism study of DVD using pig liver microsomes, D1 was detected, but no D2 was found (11). This suggests that the liver is a vital organ for the demethylation of DVD, and glucuronide conjugation may occur in the extrahepatic metabolic system. In chicken plasma, only D0 and D1 were detectable; D2 was not observed. Differences in the metabolites between pigs and chickens were probably caused by different species-related expression in the enzymes responsible for the biotransformation of DVD (30); this requires further study.
The disposition curves were described by a noncompartmental model based on SMT. The detailed pharmacokinetic parameters of DVD and its metabolites in pigs and chickens were investigated in this study using an LC-v.ARC/MS-IT-TOF-based method. Following oral administration, DVD was well absorbed and reached Cmax at 2 h with average concentrations of 0.49 and 1.55 μg/ml in pig and chicken plasma, respectively. Using an HPLC-UV method, the Cmax values for DVD were similar (0.43 and 1.45 μg/ml in pigs and chickens, respectively, in previous studies), but the Tmax values were slightly different (1.04 and 3.25 h in pigs and chickens in previous studies). Moreover, in the present study, the t1/2 values for DVD in the two species were significantly longer than those of a previous study (15). These distinctions were probably related to the different sample collection time points as well as the different types of analytical methods used in the two investigations. Detection ability of the high-performance liquid chromatography–ultraviolet (HPLC-UV) method is limited. For example, DVD was detected at 168 h in pig and chicken plasma in our study, whereas it was only detected at 6 h in pigs and at 24 h in chickens using a HPLC-UV by Li et al. (15). Although radioactive tracing has sufficient sensitivity for metabolite discovery and quantification, the accuracy of quantification is questionable owing to the absence of reference standards. Slight errors in quantitative concentrations will lead to significant differences in pharmacokinetic parameters. Thus, it is necessary to prepare metabolite references of DVD for further investigation.
Notably in pigs, D1 was not modified from its basic 2,4-diaminopyrimidine structure and would therefore be expected to have some antibacterial activity (31). Consequently, it may be necessary to consider the metabolite D1 for calculation of the appropriate dosage of DVD. The identification of D0 combined with the potentially active metabolite D1 in plasma suggested that although DVD is usually used as an intestinal infection drug (32), it will have a better therapeutic effect as a systemic drug. The elimination half-life differed significantly among the detected compounds; this was characterized by the rapid elimination of metabolites D1 and D2 and the slow elimination of D0. The differences may be attributed to the biotransformation, which could increase the hydrophilicity of the parent drug, facilitating its removal from organs and tissues (33). Our results showed that the t1/2 of DVD in chicken was slightly longer than that in pig after oral dosing. This finding is consistent with those found for TMP (34). The slow elimination of DVD in chicken plasma indicates a longer residue in tissues and organs. Li and Bu (3) confirmed that withdrawal period of DVD in muscle, liver, kidney and skin with fat were 4.77, 4.94, 6.74. and 4.58 d, respectively (3). Compared with muscle and skin with fat, the kidney has a longer elimination half-life of DVD, followed by the liver. This could be explained by the kidneys and liver having a greater blood supply than muscle and skin with fat (35).
Similar to aditoprim (ADP), the types and concentrations of metabolites in pigs were significantly higher than those in chickens, indicating that the metabolic activity in pigs regarding 2,4-diaminopyrimidines was stronger than that of chickens. The concentration of total DVD related metabolites in pig plasma was significantly higher than that in chickens, which may be attributed to the weak absorption of DVD by the short intestinal structure of chickens. However, due to the weak metabolic ability of DVD in chickens, the concentration of parent D0 in plasma was significantly higher than that found in pigs. This observation could be associated with the long elimination half-time of DVD in chickens, which is caused by the stronger lipotropy of the parent drug. These studies demonstrate that there are significant interspecies differences in the metabolic and kinetic characteristics of DVD in pigs and chickens, that will affect the pharmacological, toxicological, and residue behavior of DVD in the two species (36).
In the current work, reliable radioactive tracing coupled with a LC/MS-IT-TOF method was developed for the identification and determination of DVD and its metabolites in pig and chicken plasma and was successfully applied to a pharmacokinetic study where 3H-DVD was administered as a single oral dose. DVD was rapidly metabolized in both pigs and chickens, and three DVD-related (D0, D1, D2) and two DVD-related metabolites (D0, D2) were detected in the plasma of pigs and chickens, respectively. The metabolic capacity of pigs toward DVD was stronger than that of chickens. D0 contributes to the long half-life of the total DVD-related compounds in the two species. The plasma results revealed the dynamic process of DVD metabolism in pig and chicken and should help to improve the theoretical basis for dosage optimization of DVD in these animals. Evaluation of the complete metabolism, distribution, and depletion of DVD in different species needs to be further investigated in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by all animal care and experimental protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of Hubei Provincial Laboratory Animal Public Service Center (Permit No. SYXK 2013-0044) and approved by the Ethics Committee of Huazhong Agricultural University.
LWa contributed manuscript preparation. ZW revised the manuscript. KZ and KM analyzed the data. LWe and ZL performed all the experiments. WQ revised the manuscript and interpreted the statistical analysis. LH and YP contributed to the conception and designed the studies. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was partly financially supported by the Coordinated Research Project of International Atomic Energy Agency (2257/D52043) and the Key Scientific and Technological Project of Henan Province Department of China (No. 202102110103).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Charlesworth Group's author services for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.799773/full#supplementary-material
3H-DVD, tritium-labeled diaveridine; AUC0−∞, the area under the plasma concentration–time curve from 0 to ∞; BW, body weigh; CID, collision-induced dissociation; CDL, curved desorption line; ClB, total body clearance; Cmax, maximum drug concentration; D1, 3′-desmethyl-DVD; D2, monoglucuronide of 3′-desmethyl-DVD; DVD/D0, diaveridine; HPLC, high-performance liquid chromatography; LC/MS-IT-TOF, liquid chromatography combined with hybrid ion trap/time-of-flight mass spectrometry; LC-LTQ-Orbitrap, high-performance liquid chromatography/linear ion trapped orbitrap; LSC, liquid scintillation counter; MRT, mean residence time; SPE, solid phase extraction; t1/2, elimination half-life; Tmax, time to Cmax from time zero; v.ARC, online isotope detector system; Vd, apparent volume of distribution; VICH, International Cooperation on Harmonization of Technical Requirements for the Registration of Veterinary Medicinal Products.
1. Yang YJ, Liu XW Li B, Li SH, Kong XJ, Qin Z, et al. Simultaneous determination of diaveridine, trimethoprim and ormetoprim in feed using high performance liquid chromatography tandem mass spectrometry. Food Chem. (2016) 212:358–66. doi: 10.1016/j.foodchem.2016.05.184
2. Prescott JF. Sulfonamides, Diaminopyrimidines, and Their Combinations. (eds). Hoboken, NJ: John Wiley and Sons, Inc. (2013). p 279–94.
3. Li YJ, Bu SJ. Determination of sulphachloropyrazine-diaveridine residues by high performance liquid chromatography in broiler edible tissues. J Vet Med Sci. (2015) 77:1555–63. doi: 10.1292/jvms.14-0605
4. Carroll PT, Robb CA, Tippett LO, Langston JB. In vivo antibacterial activity of diaveridine-sulfonamide combinations. Invest Urol. (1971) 9:21–4. doi: 10.7164/antibiotics.24.620
5. Noack S, Chapman HD, Selzer PM. Anticoccidial drugs of the livestock industry. Parasitol Res. (2019) 118:2009–26. doi: 10.1007/s00436-019-06343-5
6. Australian National Drugs and Poisons Schedule Committee. In: Record of Reasons 40th Meeting. (2004). p. 74–5.
7. China Agriculture Ministry Application Application specification of feed medicine additive Regulation No. 168. Beijing (2001).
8. China Veterinary Pharmacopoeia Commission Guidelines Guidelines of China veterinary pharmacopoeia for chemical medicine (2010 edition). Beijing: China Agricultural Press (2011).
9. Yoshimura H. Mutagenicity of the coccidiostat diaveridine in the Salmonella/mammalian microsome assay. Mutat Res. (1991) 261:149–52. doi: 10.1016/0165-1218(91)90061-P
10. Wang JZ, Sun FF, Tang SS, Zhang SX, Cao XY. Acute, mutagenicity, teratogenicity and subchronic oral toxicity studies of diaveridine in rodents. Environ Toxicol Phar. (2015) 40:660–70. doi: 10.1016/j.etap.2015.08.022
11. Liu ZY, Wu Y, Sun ZL, Wan LR. Characterization of in vitro metabolites of trimethoprim and diaveridine in pig liver microsomes by liquid chromatography combined with hybrid ion trap/time-of-flight mass spectrometry. Biomed Chromatogr. (2012) 26:1101–8. doi: 10.1002/bmc.1754
12. Wang H, Yuan B, Zeng Z, He L, Ding H, Guo C, et al. Identification and elucidation of the structure of in vivo metabolites of diaveridine in chicken. J Chromatogr B Analyt Technol Biomed Life Sci. (2014) 965:91–9. doi: 10.1016/j.jchromb.2014.06.010
13. Meyer MR, Maurer HH. Current applications of high-resolution mass spectrometry in drug metabolism studies. Anal Bioanal Chem. (2012) 403:1221–31. doi: 10.1007/s00216-012-5807-z
14. Wang LY, Wen LH, Pan YH, Liu ZL, Zhang CY, Yuan ZH, et al. Development of radioactive tracing coupled with LC/MS-IT-TOF methodology for the discovery and identification of diaveridine metabolites in pigs. Food Chem. (2021) 363:130200. doi: 10.1016/j.foodchem.2021.130200
15. Li YF, Guo HY, Yang F, Zhou LG, Huang XH, Ding HZ, et al. Comparative pharmacokinetics of diaveridine in pigs and chickens following single intravenous and oral administration. J Vet Pharmacol Ther. (2017) 40:500–4. doi: 10.1111/jvp.12384
16. Dalvie D. Recent advances in the applications of radioisotopes in drug metabolism, toxicology and pharmacokinetics. Curr Pharm Design. (2000) 6:1009–28. doi: 10.2174/1381612003399941
17. Isin EM, Elmore CS, Nilsson GN, Thompson RA, Weidolf L. Use of radiolabeled compounds in drug metabolism and pharmacokinetic studies. Chem Res Toxicol. (2012) 25:532–42. doi: 10.1021/tx2005212
18. International Cooperation on Harmonization of Technical Requirements for the Registration of Veterinary Medicinal Products. Studies to evaluate the metabolism and residue kinetics of veterinary drugs in food-producing animals: Metabolism study to determine the quantity and identify the nature of residues VICH GL46 (MRK) (2012). Available online at: https://vichsec.org/en/guidelines/pharmaceuticals/pharma-safety/metabolism-and-residue-kinetics (accessed June 7, 2021).
19. International Cooperation on Harmonization of Technical Requirements for the Registration of Veterinary Medicinal Products. Studies to evaluate the metabolism and residue kinetics of veterinary drugs in food-producing animals: Comparative metabolism studies in laboratory animals VICH GL47 (MRK) (2012). Available online at: https://vichsec.org/en/guidelines/pharmaceuticals/pharma-safety/metabolism-and-residue-kinetics (accessed June 7, 2021).
20. Wang LY, Huang LL, Pan YH, Kuča K, Klímová B, Wu QH, et al. Metabolism and disposition of aditoprim in swine, broilers, carp and rats. Sci Rep. (2016) 6:20370. doi: 10.1038/srep20370
21. Shaffer CL, Gunduz M, Thornburgh BA, Fate GD. Using a tritiated compound to elucidate its preclinical metabolic and excretory pathways in vivo: exploring tritium exchange risk. Drug Metab Dispos. (2006) 34:1615–23. doi: 10.1124/dmd.106.010934
22. Meshi T, Sato Y. Studies on sulfamethoxazole/trimethoprim. Absorption, distribution, excretion and metabolism of trimethoprim in rat. Chem Pharm Bull. (1972) 20:2079–90. doi: 10.1248/cpb.20.2079
23. Lin YP Si DY, Liu CX. Detecting and identifying in vivo metabolites of brodimoprim via LC/ESI-MS with data-dependent scanning. Chem Res Chinese U. (2008) 24:430–6. doi: 10.1016/S1005-9040(08)60090-2
24. Fan JH, de Lannoy IAM. Pharmacokinetics. Biochem Pharmacol. (2014) 87:93–120. doi: 10.1016/j.bcp.2013.09.007
25. Sun J, Zhang L, Zhang LC, Liu QW, A. validated UHPLC–MS/MS method for simultaneous determination of lumiracoxib and its hydroxylation and acyl glucuronidation metabolites in rat plasma: Application to a pharmacokinetic study. J Pharmaceut Biomed. (2021) 201:114105–114105. doi: 10.1016/j.jpba.2021.114105
26. Economou A, Petraki O, Tsipi D, Botitsi E. Development of a liquid chromatography-tandem mass spectrometry method for the determination of sulfonamides, trimethoprim and dapsone in honey and validation according to Commission Decision 2002/657/EC for banned compounds [corrected]. Talanta. (2012) 97:32–41. doi: 10.1016/j.talanta.2012.03.058
27. Luo HS, Zhang LF, Xue FQ Li Y, Wang XY, Fei CZ, et al. Simultaneous determination of trimethoprim and diaveridine in tissues of chicken, porcine, and fish by hydrophilic interaction liquid chromatography–tandem mass spectrometry. Food Anal Method. (2014) 7:308–17. doi: 10.1007/s12161-013-9628-2
28. Wang LY, Huang LL, Pan YH, Wu QH, Xie SY, Yuan ZH. Simultaneous determination of aditoprim and its three major metabolites in pigs, broilers and carp tissues, and its application in tissue distribution and depletion studies. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. (2016) 33:1299–311. doi: 10.1080/19440049.2016.1200751
29. Gyrd-Hansen N, Friis C, Nielsen P. RasmussenF. Metabolism of trimethoprim in neonatal and young pigs: comparative in vivo and in vitro studies. Acta pharmacologica et toxicologica. (1984) 55:402–9. doi: 10.1111/j.1600-0773.1984.tb02002.x
30. Li ZS, Lin CH, Xu JJ, Wu HF, Feng JH, Huang HG. The relations between metabolic variations and genetic evolution of different species. Anal Biochem. (2015) 477:105–14. doi: 10.1016/j.ab.2015.02.024
31. European Medicines Agency. Technical report: Committee for veterinary medicinal products trimethoprim summary report (2) (EMEA/MRL/255/97-FINAL) (1997). Available online at: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500015681.pdf (accessed June 7, 2021).
32. Kang YP Yu J, Huh Y, Oh JH, Kwon CH, Lee SJ, et al. Development of high performance liquid chromatography-ultraviolet detection method for screening mebendazole, clorsulon, diaveridine, and tolfenamic acid in animal-based food samples. Drug Test Anal. (2014) 6:246–56. doi: 10.1002/dta.1467
33. Ludwig B. Use of pharmacokinetics when dealing with the drug residue problem in food-producing animals. DTW. Deutsche tierarztliche Wochenschrift. (1989) 96: 243–248.
34. Atef M, Al-Khayyat AA, Fahd K. Pharmacokinetics and tissue distribution of trimethoprim in sheep. J Vet Med A. (1978) 25:579–84. doi: 10.1111/j.1439-0442.1978.tb00959.x
35. Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. (2014) 27:68–88. doi: 10.1128/CMR.00046-13
Keywords: diaveridine, radioactive tracing, methodology, metabolites, pharmacokinetics
Citation: Wang L, Wen L, Pan Y, Wang Z, Zhou K, Mi K, Liu Z, Qu W and Huang L (2022) Metabolite Identification and Pharmacokinetic Behavior of Diaveridine in the Plasma of Pigs and Chickens Based on Radioactive Tracing Coupled With LC/MS-IT-TOF Assay. Front. Vet. Sci. 8:799773. doi: 10.3389/fvets.2021.799773
Received: 22 October 2021; Accepted: 13 December 2021;
Published: 18 January 2022.
Edited by:
Arturo Anadón, Complutense University of Madrid, SpainCopyright © 2022 Wang, Wen, Pan, Wang, Zhou, Mi, Liu, Qu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Qu, cXdAbWFpbC5oemF1LmVkdS5jbg==; Lingli Huang, aHVhbmdsaW5nbGlAbWFpbC5oemF1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.