- Heilongjiang Provincial Key Laboratory of Prevention and Control of Bovine Diseases, College of Animal Science and Veterinary Medicine, Heilongjiang Bayi Agricultural University, Daqing, China
Inactive ovaries (IO) and ovarian (follicular or luteal) cysts (FC or LC) are two common ovarian diseases leading to infertility in dairy cattle. Both disorders are associated with altered metabolites and hormones. There are currently no known effective biomarkers that can be used for early diagnosis of ovarian diseases. The purpose of this study was to identify the plasma biomarkers of ovarian diseases in Holstein dairy cows that facilitate an early diagnosis of the diseases and control its progression. The experiment was performed from 3 weeks postpartum and last for 7 weeks. Seventy-six multiparous Holstein cows (mean age, 4.36 years; weight, 635.63 kg) were divided into healthy control group (HC, n = 22), FC group (n = 18), LC group (n = 18) and IO group (n = 18) by rectal palpation or ultrasonography during the last 2 weeks before trial end. Blood was collected via tail vein for measurement of plasma energy metabolites, liver function indicators, minerals, and hormones at 3 and 8 weeks postpartum. Data were analyzed by Mann-Whitney U, Kruskal-Wallis, Spearman correlation, binary logistic regression analysis and receiver operating characteristic analysis, where applicable. At 8 weeks postpartum, FC cows had a more severe body condition score loss and these had greater levels of non-esterified fatty acids (NEFA) and estradiol, and lesser levels of alanine aminotransferase (ALT), progesterone and insulin-like growth factor 1 (IGF-1) levels than HC cows (P < 0.05). LC cows had a lower milk yield, higher NEFA and progesterone levels, and lower calcium, phosphorus and magnesium levels than HC cows (P < 0.05). IO cows had a lower body condition score, higher NEFA levels, and lower ALT, calcium, phosphorus, magnesium, estradiol, progesterone and IGF-1 levels than HC cows (P < 0.05). At 3 weeks postpartum, cows with ovarian diseases had greater (P < 0.05) concentrations of NEFA, and lesser concentrations of ALT, calcium, phosphorus and IGF-1 than HC cows. Early warning values for ovarian diseases were plasma NEFA concentrations >0.50 mmol/L, or calcium concentrations <2.02 mmol/L. Therefore, plasma NEFA and calcium could be used as early-warning indicators for ovarian diseases in dairy cows.
Introduction
Selection for milk yield in dairy cows in the last 70 years has resulted in a significant increase in milk yield and concomitant reduction in fertility (1). Inactive ovaries (IO) and ovarian cysts are two major causes of infertility in dairy cows (2, 3). Historically, IO has been defined as growth of follicles only to the stage of follicular wave emergence, that is, up to ~8 mm diameter, after which, growth stops (4), while ovarian cysts (follicular or luteal) are defined as anovulatory ovarian structures with a cavity >20 mm in diameter in the absence of a corpus luteum (5). Notably, the difference between follicular and luteal cysts (LC) is that the wall is <3 mm in follicular cyst (FC) and >3 mm in LC (6).
About 20% of dairy cows in anestrus by the start of breeding programs (7) or by 63 days after calving (8) were involved in the IO. Around the same time, the incidence of ovarian cysts may vary from 2.7 to 15.1% (9, 10) or from 6 to 30% (11, 12). Both ovarian diseases may increase calving to conception interval, calving interval, calving to first service interval and days open. In addition, cows affected may be at greater risk of being culled because of poor reproductive performance (13), thereby causing vast economic losses to the dairy industry (2). Therefore, it is crucial to discover novel biomarkers for early detection of ovarian diseases and monitoring of disease progression.
The transition period is one of the most challenging periods in dairy cows and encompasses the 3 wk prior to and 3 wk after parturition (14, 15). During this period, cows undergo drastic adaptations as regards the metabolism of glucose, fatty acids and minerals (14, 16). Despite this, parturition and the onset of lactation put an enormous physiological stress on the cow's homeostatic processes (17). Further, pregnancy and lactation are recognized as inducing remarkable physiological and metabolic adaptations in dairy cows essential for a good reproductive and productive performance (18–20). Despite the action of homeostatic mechanisms to maintain blood parameters within physiologic levels, changes in metabolites and hormones occur as a result of increased metabolic demands in lactating animals. These changes are not necessarily indicative of diseases but make animals physiologically unstable and more susceptible to a number of metabolic diseases at this stage than during other life periods compromising productivity (21).
High producing dairy cows often undergo a period of negative energy balance (NEB) during the first weeks of lactation (22). The NEB status in early lactation is characterized by alterations in blood metabolite and hormone profile, which is of critical importance to subsequent health and fertility (14, 23). Altered patterns of blood biomarkers have been used as diagnostic indicators of some diseases for a few decades. For example, elevated blood β-hydroxybutyrate prepartum (24–26), serum non-esterified fatty acids (NEFA) prepartum (26–28), serum calcium the week before and soon after calving (26–29) have been used to predict postpartum diseases.
However, it should be noted that there are currently no effective early markers available for early diagnosis of ovarian diseases in dairy cows. Although an reliable diagnosis of ovarian diseases currently employs a combination of trans-rectal palpation, trans-rectal ultrasonography and plasma progesterone assay (30). Unfortunately, once diagnosed, it is too late to intervene effectively. Thus, the objective of this study was to identify the plasma biomarkers of the ovarian diseases in Holstein dairy cows that facilitate an early diagnosis of the diseases and control its progression.
Materials and Methods
Ethics
The study protocol was approved by the Ethics Committee (SY201909005) on the Use and Care of Animals of Heilongjiang Bayi Agricultural University (Daqing, China).
Animals
This prospective observational experiment was conducted on a commercial farm in Heilongjiang Province, China, from September 2019 to January 2020. The experiment was performed from week 3 postpartum and last for 7 weeks. Seventy-six multiparous Holstein cows (parity 2–5) without oestrus synchronization were divided into healthy control group (HC, n = 22; mean age, 4.25 ± 0.94 years; mean weight, 634.50 ± 22.90 kg), FC group (n = 18; mean age, 4.63 ± 0.19 years; mean weight, 652.28 ± 36.25 kg), LC group (n = 18; mean age, 4.03 ± 0.59 years; mean weight, 635.34 ± 12.95 kg) and IO group (n = 18; mean age, 4.54 ± 0.87 years; mean weight, 620.64 ± 34.41 kg) by rectal palpation or ultrasonography during the last 2-week period. Inactive ovaries and ovarian cysts were recorded as ovarian disorders. All cows were suffering no other clinical disorders. The subject animals were maintained in free-stall housing with continuous access to fresh water and were milked three times per day. Total mixed rations during early lactation were formulated in accordance with the 2001 US National Research Council standards. The total mixed rations consisted of 8–9 kg of concentrate, 19 kg of silage, 3.5–4.0 kg of hay, and 350 g of fat. Feed analysis showed 55.60% of dry matter, 16% of crude protein, 7.322 MJ·kg−1 net lactation production, 5.60% of fat, 39.10% of neutral detergent fiber, 20.30% of acid detergent fiber, 180 g of calcium, and 116 g of phosphorus.
Ovarian Ultrasonography
Ovarian ultrasonographic examinations were performed in all cows by using a DP-2,200 VET ultrasonograph (Mindray Biomedical Electronics) as described previously. Briefly, Healthy control group were selected from cows with normal corpus luteum and/or large follicles (>10 mm) in two examinations (10 days apart). Inactive ovaries group were selected from cows without ovarian cysts, persisted corpus luteum or follicle, and normal corpus luteum and/or large follicles (>10 mm) (2). Two ultrasonographic examinations of the ovaries, approximately 7 d apart, reveal no substantial changes in the follicular structures (31). Follicular cysts were characterized by a size of 3–5 cm, a thin wall ≤ 0.3 cm, duration of maintenance of at least 10 days, and progesterone concentration < 1 ng/mL, whereas luteal cysts were typified by a diameter of 3–5 cm, a cavity filled with liquid, a thick luteinized wall with a thickness >0.3 cm that persisted for at least 14 days, and progesterone concentration >1 ng/mL (32, 33).
Data Collection
Age, parity, weight and milk yield were collected from the Afitag pedometer (Afimilk 0418A09QPDX, Kibbutz Afikim, Israel) of the cattle farm. Body condition score (BCS) was weekly done by 2 trained farm veterinarian using the established 5-point method ranged from 1 to 5 with 0.25 unit intervals (34). Body condition score loss was calculated as the difference between the BCS on wk 3 and wk 8.
Sample Collection
At 3 wk and 8 wk postpartum, before milking and fasting in the morning, 10 mL of blood was collected from the jugular vein into an anticoagulant tube (BD, Franklin Lakes, NJ) containing sodium heparin. Anticoagulated blood was centrifuged at 1,500 × g for 5 min and the supernatant was placed into a 1.5 mL Eppendorf tube. Then, the supernatant was centrifuged at 12,000 × g for 5 min and 500 μL of plasma was transferred into a 1.5 mL Eppendorf tube and stored at −80°C for biochemical analysis, radioimmunoassay and ELISA.
Plasma Analysis
β-hydroxybutyrate, NEFA, glucose, triglycerides, total cholesterol, urea nitrogen, aspartate aminotransferase, alanine aminotransferase (ALT), γ-glutamyltransferase, total protein, albumin, globulin, total bilirubin, calcium, phosphorus and magnesium in plasma were analyzed using commercial biochemical assay kits (Mindray Biomedical Electronics Co. Ltd, Shenzhen, China). All metabolite concentrations were quantified using a Mindray BS-830S fully automatic biochemistry analyzer (Mindray Biomedical Electronics Co. Ltd, Shenzhen, China). All measurements were executed according to the manufacturer's instructions.
Estradiol, progesterone, insulin and growth hormone concentrations were measured using four commercial kits with the same lot number (Xinfan Biotechnology Co. Ltd, Shanghai, China) and the kit manufacturer's validated radioimmunoassay procedures. Assay sensitivities were 2 pg/ml (estradiol), 0.2 ng/ml (progesterone), 2 μIU/ml (insulin) and 10 ng/ml (growth hormone). Intra-assay coefficients of variation is >10%, inter-assay coefficients of variation is >15%.
The quantities of insulin-like growth factor 1 (IGF-1) were detected using a bovine-specific ELISA kit purchased from Xinfan Biotechnology Co., Ltd. (Shanghai, China), in accordance with the manufacturer's instructions.
Statistical Analysis
Statistical analyses were performed using SPSS 26.0 (IBM, New York, NY) software. Since the data did not fit a normal distribution, non-parametric tests (Kruskal-Wallis rank test or Mann-Whitney U-test) were used. Group differences were first tested with the Kruskal-Wallis rank test and if significant they were analyzed further with the Mann-Whitney U-test. Data were expressed as mean ± SD. Differences between HC and ovarian diseases groups were compared by the Mann-Whitney U-test. Data were presented as average ± SEM. Correlation between plasma biomarkers and ovarian diseases were explored using Spearman rank correlation coefficients. To predict diseases, binary logistic regression models were created. We finally used the Relative Operating Characteristic (ROC) curve to test the significance of the logistic regression models. Statistical significance is set as P < 0.05.
Results
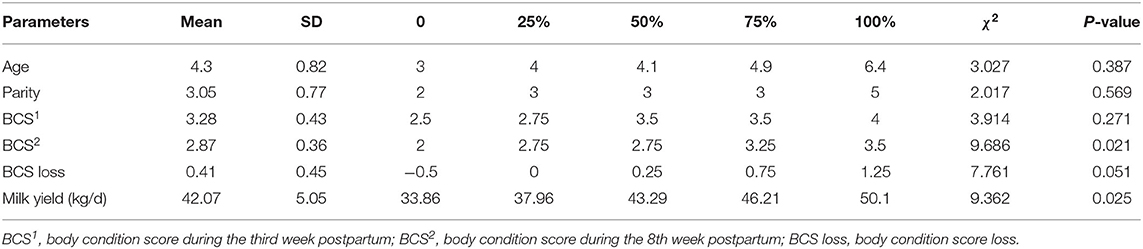
Table 1 shows the distribution of age, parity, BCS, and milk yield for all 76 cows. There are significant differences in the BCS2 and milk yield among the four groups (P < 0.05). Body condition score loss showed a marginal significant difference (P = 0.051). The results were further analyzed by comparing the different groups of diseased and healthy animals (Figure 1). The mean BCS2 was significantly lower in the IO group compared to the HC group (2.38 ± 0.22 vs. 3.02 ± 0.07, P < 0.01) (Figure 1A). Body condition loss was significantly higher in FC compared with HC groups (0.83 ± 0.26 vs. 0.23 ± 0.08, P < 0.01) (Figure 1B). Milk yield was significantly lower in LC than in HC groups (37.77 ± 1.79 kg/d vs. 45.45 ± 0.44 kg/d, P < 0.05) (Figure 1C).
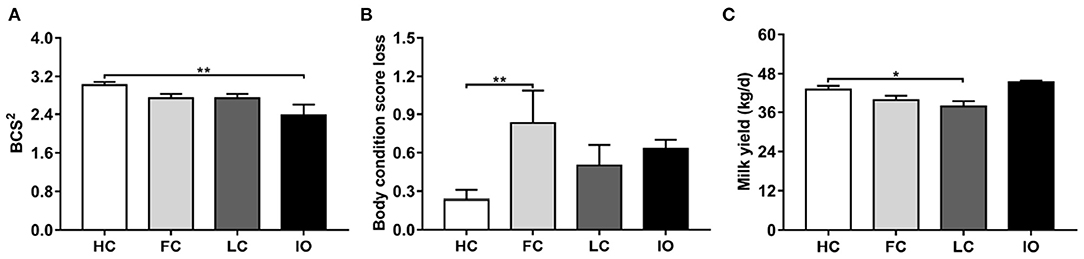
Figure 1. (A–C) Comparison of general data between the follicular cysts (FC, n = 18) group, luteal cysts (LC, n = 18) group, and inactive ovaries (IO, n = 18) group and healthy control (HC, n = 22) group, respectively. *P < 0.05, **P < 0.01 vs. healthy control group. BCS2, body condition score during the 8th week postpartum.
The same analysis was also performed on plasma biochemical indexes. There were significant differences (P < 0.05) in plasma NEFA, ALT, calcium, phosphorus, magnesium, estradiol, progesterone, and IGF-1 levels among groups (Table 2). Figure 2 shows the results of the subgroup analyses. Plasma NEFA levels were significantly higher in FC, LC, and IO than in HC cows (0.73 ± 0.07, 0.53 ± 0.05, and 0.57 ± 0.01 vs. 0.42 ± 0.02 mmol/L, P < 0.05) (Figure 2A). Plasma ALT levels were significantly lower in FC and IO than in HC cows (12.67 ± 1.38, and 12.00 ± 1.15 vs. 19.77 ± 1.60 U/L, P < 0.05) (Figure 2B). Plasma calcium levels were significantly lower in LC and IO than in HC cows (1.91 ± 0.11, and 1.82 ± 0.01 vs. 2.13 ± 0.02 mmol/L, P < 0.01) (Figure 2C). Plasma phosphorus levels were significantly lower in LC and IO than in HC cows (1.73 ± 0.06, and 1.37 ± 0.03 vs. 1.97 ± 0.05 mmol/L, P < 0.05) (Figure 2D). Plasma magnesium levels were significantly lower in LC than in HC cows (1.08 ± 0.03 vs. 1.18 ± 0.02 mmol/L, P < 0.01) (Figure 2E). Plasma estradiol levels were significantly higher in FC cows and lower in IO cows than in HC cows (21.45 ± 1.25, and 7.63 ± 0.61 vs. 15.14 ± 1.21 pg/mL, P < 0.01) (Figure 2F). Plasma progesterone levels were significantly higher in LC cows and lower in FC and IO cows than in HC cows (8.30 ± 2.08, 0.33 ± 0.14, and 0.41 ± 0.18 vs. 5.36 ± 0.58 ng/mL, P < 0.05) (Figure 2G). Plasma IGF-1 levels were significantly lower in FC and IO than in HC cows (79.37 ± 13.37, and 28.25 ± 1.47 vs. 104.74 ± 4.65 ng/mL, P < 0.05) (Figure 2H).
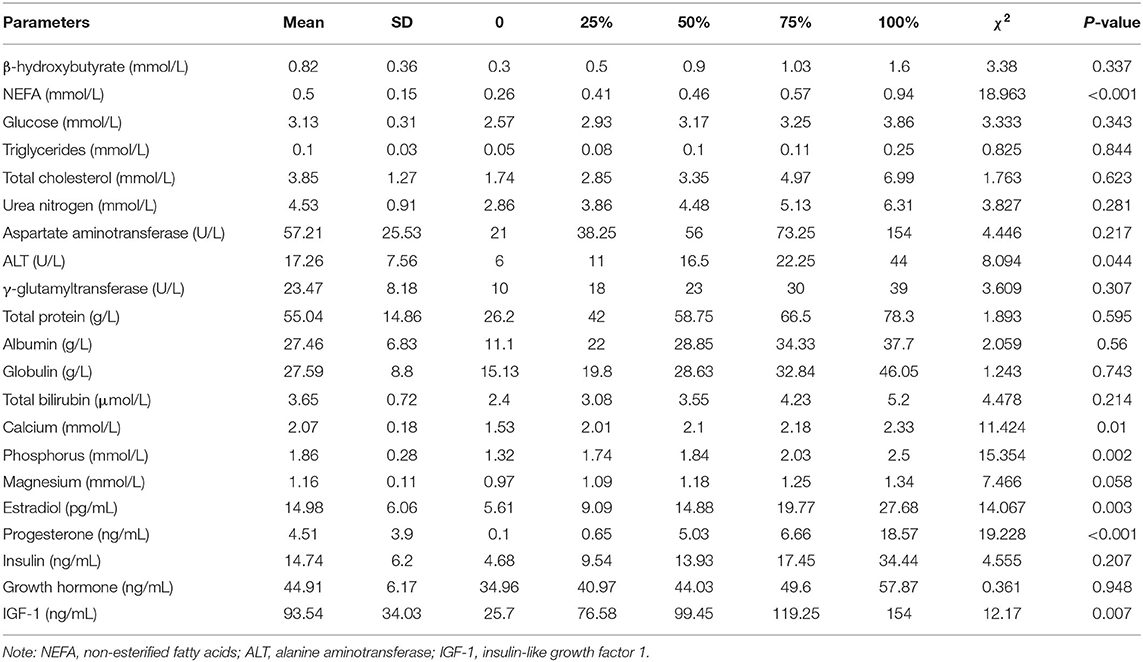
Table 2. Kruskal-Wallis test of plasma energy metabolites, liver function indicators, minerals and hormones among the four groups (n = 76).
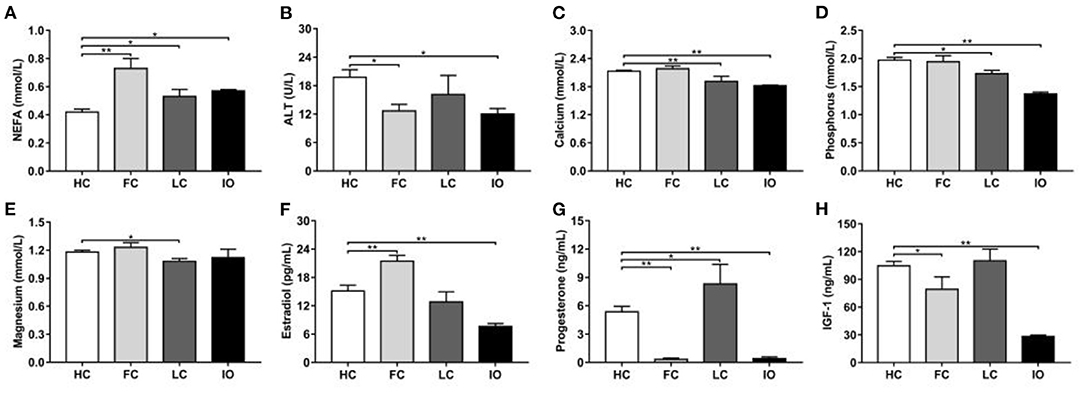
Figure 2. (A–H) Comparison of plasma biochemical indicators between the follicular cysts (FC, n = 18) group, luteal cysts (LC, n = 18) group, and inactive ovaries (IO, n = 18) group and healthy control (HC, n = 22) group, respectively. Note: *P < 0.05, **P < 0.01 vs. healthy control group. NEFA, non-esterified fatty acids; ALT, alanine aminotransferase; IGF-1, insulin-like growth factor 1.
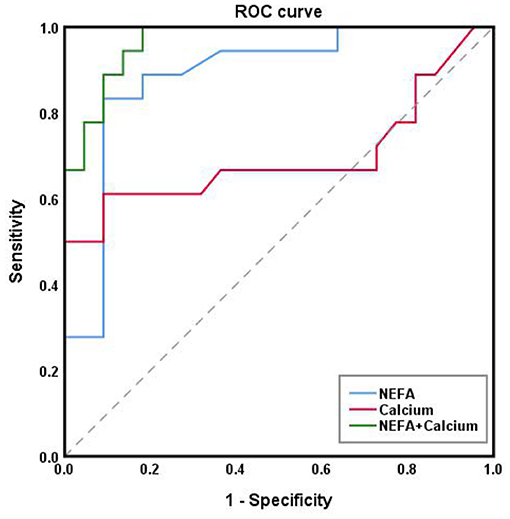
From here on, we refer to the three diseases collectively as “ovarian diseases.” Statistical comparison between two groups was performed using the Mann–Whitney U-test. Compared to healthy controls, cows with ovarian diseases had higher (p < 0.01) NEFA and lower (P < 0.05) ALT, calcium, phosphorus and IGF-1 concentrations (Figure 3). Spearman correlation analysis showed that plasma NEFA level was positively correlated (P < 0.01) with ovarian diseases, while plasma ALT, calcium, phosphorus, progesterone, and IGF-1 levels were negatively correlated (Table 3, P < 0.01). With each of them, a binary logistic regression analysis was performed. Notably, only two of these parameters (NEFA and calcium) correlated with ovarian diseases. To obtain the early warning indicators of ovarian diseases, we applied ROC curve analysis to evaluate the accuracy of our test. ROC curve analysis indicated that a NEFA value of 0.50 mmol/L discriminated diseased from healthy cows with an area under the curve of 0.886, sensitivity of 83.3% and specificity of 90.9% (Figure 4 and Table 4). Similarly, the sensitivity and specificity of calcium in predicting ovarian diseases were 61.1 and 90.9%, respectively; when the cutoff value of calcium was settled at 2.02 mmol/L, the area under the curve was 0.697 (Figure 4 and Table 4). Furthermore, this analysis also showed that NEFA combined with calcium for the diagnosis was prior than the single indicator. Specifically, this model performance increased as calcium was added, with an area under the curve of 0.967, sensitivity of 100%, and specificity of 81.8% (Figure 4 and Table 4).
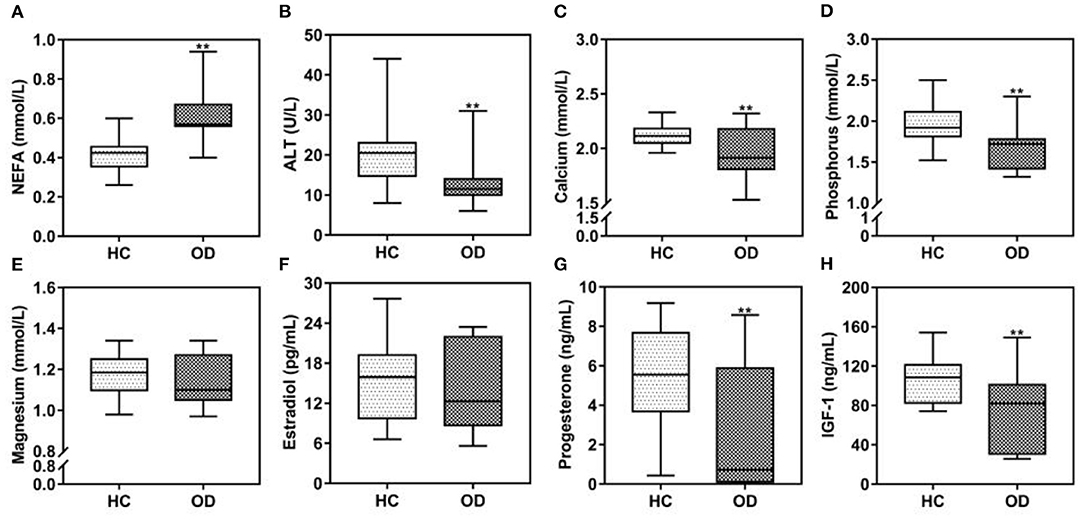
Figure 3. (A–H) Comparison of plasma biochemical indicators between the ovarian diseases (OD, n = 54) group and healthy control (HC, n = 22) group at 3 weeks postpartum. Note: **P < 0.01 vs. healthy control group. NEFA, non-esterified fatty acids; ALT, alanine aminotransferase; IGF-1, insulin-like growth factor 1.
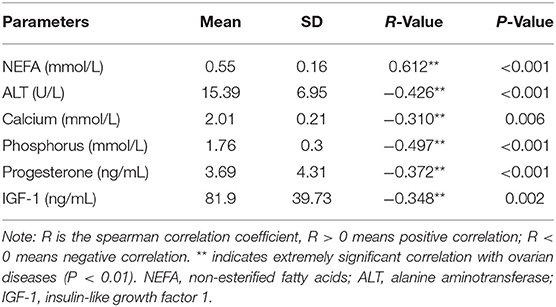
Table 3. Correlation between plasma biochemical indexes and ovarian diseases in dairy cows (n = 76).

Table 4. The cutoff point, sensitivity, specificity, standard error and area under the ROC curve of NEFA and Calcium diagnosed by ovarian diseases in dairy cows.
Discussion
Ovarian diseases are one of the most common ovarian dysfunctions in dairy cattle, which can lead to a considerable economic loss through its high incidence and can reduce the reproductive performance (6). Although several studies have reported that ovarian diseases are related to alterations in metabolites and hormonal factors (35), the mechanisms involved in the development of ovarian diseases are yet to be elucidated along with the contribution of other possible pathogenic determinates. We therefore compared the plasma energy metabolic, liver function, mineral and hormone levels (8 wk postpartum) between the FC cows, LC cows, and IO cows and HC cows, respectively. Furthermore, earlier diagnosis, especially before 3 weeks postpartum, may facilitate access to interventions that improve ovarian diseases. To obtain biomarkers for early diagnosis of the diseases, we evaluated the early warning effects of plasma biochemical indicators (3 wk postpartum) on cows with ovarian diseases, including FC, LC and IO.
Follicular cysts are anovulatory follicular structures (11). Its etiology is not fully understood, but hormonal and metabolic abnormalities are recognized as important causes of FC (36). Cows diagnosed with FC have a dysfunctional hypothalamic-pituitary-ovarian axis signature that includes parabasal concentration of progesterone (<1 ng/mL), increased peripheral estradiol levels, increased lutenizing hormone pulse frequency and amplitude, and reduced lutenizing hormone and follicle-stimulating hormone receptors that translate into a lack of lutenizing hormone surge and ovulation (36). Consistent with this, we observed that, as expected, FC cows in the current study had higher peripheral estradiol levels and lower progesterone levels (0.33 ± 0.14 vs. 5.36 ± 0.58 ng/mL) compared with HC cows. Moreover, we also observed that FC cows had higher plasma levels of NEFA and BCS loss in early lactation than HC cows. These observations are consistent with several previous studies (36–38). The increased plasma NEFA levels and higher BCS loss in FC cows suggests greater adipose tissue mobilization during the NEB in early lactation (39). Numerous studies showed the adverse effects of NEFA for granulosa cell proliferation and survival, steroidogenesis, and follicular development and oocyte maturation (40–43). Therefore, it is reasonable to speculate that the increased plasma NEFA concentrations found in FC cows in the current study may damage the granulosa cell function and steroidogenesis. Although the pathogenesis and mechanism of cyst formation are not fully understood, it has been proposed that the IGF system could play an essential role, as it is a key intraovarian regulator (44). Beam et al. (45) reported that follicular competence early postpartum was associated with higher plasma IGF-1. Our findings for IGF-1 in FC and HC cows were consistent with a previous study that reported reduced plasma levels of IGF-1 in FC cows when compared to HC cows (37). It is well-known that ALT is the most widely used biomarker to detect liver disease in clinical practice (46). Increased levels of circulating ALT may reflect the underlying liver pathology. A recent study that revealed half of FC cows with liver disorders, such as fatty liver and hepatitis (47). This finding suggests that liver disorders are well-connected to development of FC in dairy cows and that steroid hormone metabolism is delayed, resulting in the formation of FC (47). On the contrary, we observed that FC cows had lower plasma ALT levels, and we postulate that an enhanced hepatic lipid mobilization accounts for this phenomenon (48). Altogether, these previous findings along with ours, indicate that the formation of FC is associated with an increased fat mobilization and the disorder of steroidogenesis caused by NEB in early lactation.
The formation of LC is attributed to luteinnization of unovulated follicles, its pathogenesis still remains unclear. It is generally believed that LC is related to abnormal corpus luteum function. Previous research suggested that cows with LC might tend to be in anoestrus as the higher amount of progesterone secreted by this luteinized structure may change the pattern of gonadotrophin's secretion (13). Douthwaite and Dobson reported that the mean plasma progesterone concentration was higher in the cows with LC than in those with HC (49). Furthermore, the mean plasma progesterone concentration of a cow with a LC has been reported to be 3.6 ng/mL (50), with a range from 3.0 to 10.4 ng/mL (51). These values are similar to values reported in our current study. Cows that had delayed commencement of luteal activity had high NEFA values at 1-week postpartum (52). In addition, Both the areas occupied by endothelial and by steroidogenic cells were negatively correlated with the blood concentration of NEFA (53). These findings are similar ours and lend further support to a causal role of NEFA in LC development. High concentration of plasma NEFA and low milk production in LC cows are related to NEB in early lactation (54). An early study by Davis et al., suggested that Ca2+ and phospholipid enhanced phosphorylation of endogenous luteal cytosol protein, while low concentration of Mg2+ inhibited the phosphorylation of target protein (55). The same study also showed that a phospholipid-sensitive, Ca2+-dependent protein kinase might provide an important link between hormonally-induced changes in phospholipid metabolism and corpus luteum function. In the present study, we found that LC cows had lower plasma levels of calcium, phosphorus, and magnesium than HC cows, suggesting phosphorylation disorder was present in LC cows. Altogether, these previous findings along with ours, suggest that the formation of LC is related to the disorder of steroidogenesis caused by NEB in early lactation and the disorder of phosphorylation caused by mineral deficiency. Importantly, both mechanisms would lead to abnormal corpus luteum function.
Inactive ovaries are driven by a loss of periodic follicular activity and transient ovarian dysfunction (56). Nutritional status is generally recognized as a significant contributing factor to inactive ovaries, due to a close link between postpartum ovarian activity and energy balance (57, 58). Our current result shows that cows with inactive ovaries have a significant lower BCS than healthy control cows. This is consistent with another report showing that low BCS is associated with inactive ovaries (59). Low levels of estradiol and progesterone in plasma were also observed in cows with inactive ovaries but not in healthy controls. This fits the known hormonal characteristics of inactive ovaries (60). The plasma concentration of NEFA characterizes the magnitude of fat mobilization (61). Meanwhile, an increase in circulating NEFA is often an accurate indicator of NEB in dairy cows (62). IGF-1 plays an important role in gonadotropin-induced folliculogenesis, ovarian steroidogenesis and corpus luteum function. It also modulates pituitary and hypothalamus function (63). Zulu et al. reported negative association of serum IGF-1 with NEFA but positive association with estradiol (37). The same study also reported that NEFA was higher and IGF-1 was lower in inactive ovary than in normal cows. These results is consistent with our observations. Interestingly, cows with inactive ovaries had low ALT levels similar to that observed in FC cows. Our isolated finding should be interpreted with caution. The direct role of calcium and phosphorus in the inactive ovaries of cows was undescribed so far, but we observed that the plasma calcium and phosphorus levels were significantly lower in cows with inactive ovaries compared with healthy control cows. Notably, a recent study demonstrated that hypocalcemic and hypophosphatemic female mice developed infertility accompanied by decreased estradiol and progesterone levels, elevated follicle-stimulating hormone and luteinizing hormone levels, defects in follicular development and corpus luteum formation, uterine hypoplasia, and decreased ovarian expression of angiogenic factors (64). It is reasonable to speculate that calcium and phosphorus play a role in the development of inactive ovaries. Collectively, these results indicate that the onset of inactive ovaries may not only be relevant for an increased fat mobilization caused by NEB in early lactation, but also for the low levels of plasma calcium and phosphorus. However, the synergy mechanisms underlying the both in inactive ovaries remain to be further investigated.
As mentioned in the introduction, many biomarkers of energy status such as β-hydroxybutyrate, NEFA, calcium and BCS have been used to evaluate postpartum diseases in dairy cows (65). However, little is known about the early warning and assessment of ovarian diseases in dairy cows. Although blood biomarkers at the time of ovarian diseases can be used to monitor diseases themself, it is too late to implement appropriate interventions. Previous studies have addressed this same question. Ospina et al. reported lower sensitivity and specificity of NEFA measured pre-partum (threshold of ≥0.39 mmol/L) compared with post-partum (threshold of ≥0.57 mmol/L) for detection of any of the following diseases: displaced abomasum, clinical ketosis, metritis, or retained placenta (66). Pre-partum sensitivity and specificity were 48 and 69%, while post-partum sensitivity and specificity were 75 and 61% (66). Calcium concentrations can be used to identify hypocalcemia, which occurs when calcium levels are below the normal reference range of 8.5–10 mg/dl (67). Calcium serum levels below 9.4 mg/dl measured 1-week pre-partum had very low sensitivity at 35% for displaced abomasum, while specificity was 71% (25). In this study, we found that NEFA and calcium also predict ovarian diseases in dairy cows. A higher sensitivity and specificity of NEFA measured (threshold of ≥0.50 mmol/L) compared with calcium (threshold of <2.02 mmol/L) for the prediction of ovarian diseases. NEFA sensitivity and specificity were 83.3 and 90.9%, while calcium sensitivity and specificity were 61.1 and 90.9%. Furthermore, the sensitivity (100%) and specificity (81.8%) were greater when a combined NEFA and calcium approach was applied. Similarly, the area under the ROC curve for the prediction of ovarian diseases was 0.967, which was higher than those regarding NEFA (0.886) and calcium (0.697). The risk of ovarian diseases onset increases for concentrations above the NEFA thresholds and/or below the calcium thresholds. Since most ovarian diseases incidence occurs at 8 weeks postpartum, this allows for approximately 30 days to implement interventions to prevent disease development in at-risk cattle. Interventions may include improving environmental factors, nutrition, or transitional treatments.
Conclusion
In summary, we found that ovarian diseases were associated with an increased fat mobilization and the disorder of steroidogenesis caused by early postpartum NEB and the disorder of phosphorylation duo to mineral deficiency. Importantly, higher plasma NEFA levels and lower calcium levels could be used to predict an increased risk of ovarian diseases in dairy cows. In particular, NEFA could be applied jointly with calcium to achieve a higher prediction performance. To the best of our knowledge, this is the first study that has reported models predictive of early lactation disease incidence at the cohort level using aggregate biomarkers measured at 3 wk postpartum. Future work could include validating the models in studies with larger sample sizes and building separate models for different ovarian diseases or groups of ovarian diseases. Our results have important practical implications. Earlier and more accurate disease prediction will result in the ability to implement earlier interventions, thereby potentially reducing the incidence of ovarian diseases.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Ethics Committee on the Use and Care of Animals of Heilongjiang Bayi Agricultural University.
Author Contributions
YS: analyzed the data and wrote the manuscript. CXia and CXu: designed the study and revised the manuscript. ZW and YB: participated in the acquisition of the data. JC and HY: performed the laboratory analysis. All authors contributed to the article and approved the submitted version.
Funding
The Key Project of Natural Science Foundation of Heilongjiang Province of China (ZD2021C006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The study was supported by the National Natural Science Foundation of China (31772804; 31873028) and the Scientific Research Start-up Funding for Introducing Talents in Heilongjiang Bayi Agricultural University (XBY202101).
References
1. Hailay T, Hoelker M, Poirier M, Gebremedhn S, Rings F, Saeed-Zidane M, et al. Extracellular vesicle-coupled miRNA profiles in follicular fluid of cows with divergent post-calving metabolic status. Sci Rep. (2019) 9:12851. doi: 10.1038/s41598-019-49029-9
2. Azarbayejani R, Mohammadsadegh M. Glucose, insulin, and cortisol concentrations and glucose tolerance test in Holstein cows with inactive ovaries. Trop Anim Health Prod. (2021) 53:41. doi: 10.1007/s11250-020-02448-7
3. Rodríguez FM, Gareis NC, Hein GJ, Salvetti NR, Amweg AN, Huber E, et al. Role of components of the insulin-like growth factor system in the early stages of ovarian follicular persistence in cattle. J Comp Pathol. (2017) 157:201–14. doi: 10.1016/j.jcpa.2017.07.010
4. Xu C, Xia C, Sun Y, Xiao X, Wang G, Fan Z, et al. Metabolic profiles using 1H-nuclear magnetic resonance spectroscopy in postpartum dairy cows with ovarian inactivity. Theriogenology. (2016) 86:1475–81. doi: 10.1016/j.theriogenology.2016.05.005
5. Borş SI, Ibănescu I, Creangă Ş, Borş A. Reproductive performance in dairy cows with cystic ovarian disease after single treatment with buserelin acetate or dinoprost. J Vet Med Sci. (2018) 80:1190–94. doi: 10.1292/jvms.17-0690
6. Borş SI, Bor? A. Ovarian cysts, an anovulatory condition in dairy cattle. J Vet Med Sci. (2020) 82:1515–22. doi: 10.1292/jvms.20-0381
7. Rhodes FM, McDougall S, Burke CR, Verkerk GA, Macmillan KL. Invited review: treatment of cows with an extended postpartum anestrous interval. J Dairy Sci. (2003) 86:1876–94. doi: 10.3168/jds.S0022-0302(03)73775-8
8. Santos JE, Juchem SO, Cerri RL, Galvão KN, Chebel RC, Thatcher WW, et al. Effect of bST and reproductive management on reproductive performance of holstein dairy cows. J Dairy Sci. (2004) 87:868–81. doi: 10.3168/jds.S0022-0302(04)73231-2
9. Borsberry S, Dobson H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec. (1989) 124:217–9. doi: 10.1136/vr.124.9.217
10. Cattaneo L, Signorini ML, Bertoli J, Bartolomé JA, Gareis NC, Díaz PU, et al. Epidemiological description of cystic ovarian disease in argentine dairy herds: risk factors and effects on the reproductive performance of lactating cows. Reprod Domest Anim. (2014) 49:1028–33. doi: 10.1111/rda.12432
11. Garverick HA. Ovarian follicular cysts in dairy cows. J Dairy Sci. (1997) 80:995–1004. doi: 10.3168/jds.S0022-0302(97)76025-9
12. Kesler DJ, Garverick HA. Ovarian cysts in dairy cattle: a review. J Anim Sci. (1982) 55:1147–59. doi: 10.2527/jas1982.5551147x
13. Peter AT. An update on cystic ovarian degeneration in cattle. Reprod Domest Anim. (2004) 39:1–7. doi: 10.1046/j.0936-6768.2003.00466.x
14. Fiore E, Piccione G, Rizzo M, Morgante M, Barberio A, Giudice E, et al. Adaptation of some energetic parameters during transition period in dairy cows. J Applied Anim Res. (2018) 46:402–5. doi: 10.1080/09712119.2017.1313742
15. Tessari R, Berlanda M, Morgante M, Badon T, Gianesella M, Mazzotta E, et al. Changes of plasma fatty acids in four lipid classes to understand energy metabolism at different levels of non-esterified fatty acid (NEFA) in dairy cows. Animals. (2020) 10:1410. doi: 10.3390/ani10081410
16. Fiore E, Blasi F, Morgante M, Cossignani L, Badon T, Gianesella M, et al. Changes of milk fatty acid composition in four lipid classes as biomarkers for the diagnosis of bovine ketosis using bioanalytical thin layer chromatography and gas chromatographic techniques (TLC-GC). J Pharm Biomed Anal. (2020) 188:113372. doi: 10.1016/j.jpba.2020.113372
17. Giannuzzi D, Tessari R, Pegolo S, Fiore E, Gianesella M, Trevisi E, et al. Associations between ultrasound measurements and hematochemical parameters for the assessment of liver metabolic status in Holstein–Friesian cows. Sci Rep. (2021) 11:16314. doi: 10.1038/s41598-021-95538-x
18. Piccione G, Messina V, Schembari A, Casella S, Giannetto C, Alberghina D. Pattern of serum protein fractions in dairy cows during different stages of gestation and lactation. J Dairy Res. (2011) 78:421–5. doi: 10.1017/S0022029911000562
19. Arfuso F, Fazio F, Levanti M, Rizzo M, Pietro1 SD, Giudice E, et al. Lipid and lipoprotein profile changes in dairy cows in response to late pregnancy and the early postpartum period. Arch Anim Breed. (2016) 59:429–34. doi: 10.5194/aab-59-429-2016
20. Fiore E, Arfuso F, Colitti M, Gianesella M, Giudice E, Piccione G, et al. Expression of selected genes related to energy mobilisation and insulin resistance in dairy cows. Anim Prod Sci. (2017) 57:1007–13. doi: 10.1071/AN15376
21. Fiore E, Arfuso F, Gianesella M, Vecchio D, Morgante M, Mazzotta E, et al. Metabolic and hormonal adaptation in Bubalus bubalis around calving and early lactation. PLoS ONE. (2018) 13:e0193803. doi: 10.1371/journal.pone.0193803
22. Steeneveld W, Amuta P, van Soest FJS, Jorritsma R, Hogeveen H. Estimating the combined costs of clinical and subclinical ketosis in dairy cows. PLoS ONE. (2020) 15:e0230448. doi: 10.1371/journal.pone.0230448
23. Xu W, Vervoort J, Saccenti E, Kemp B, van Hoeij RJ, van Knegsel ATM. Relationship between energy balance and metabolic profiles in plasma and milk of dairy cows in early lactation. J Dairy Sci. (2020) 103:4795–805. doi: 10.3168/jds.2019-17777
24. Leblanc SJ, Leslie KE, Duffield TF. Metabolic predictors of displaced abomasum in dairy cattle. J Dairy Sci. (2005) 88:159–70. doi: 10.3168/jds.S0022-0302(05)72674-6
25. Chapinal N, Carson M, Duffield TF, Capel M, Godden S, Overton M. The association of serum metabolites with clinical disease during the transition period. J Dairy Sci. (2011) 94:4897–903. doi: 10.3168/jds.2010-4075
26. Roberts T, Chapinal N, Leblanc SJ, Kelton DF, Dubuc J, Duffield TF. Metabolic parameters in transition cows as indicators for early-lactation culling risk. J Dairy Sci. (2012) 95:3057–63. doi: 10.3168/jds.2011-4937
27. LeBlanc S. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Dev. (2010) 56:S29–35. doi: 10.1262/jrd.1056S29
28. Ospina PA, Nydam DV, Stokol T, Overton TR. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: critical thresholds for prediction of clinical diseases. J Dairy Sci. (2010) 93:546–54. doi: 10.3168/jds.2009-2277
29. Seifi HA, Leblanc SJ, Leslie KE, Duffield TF. Metabolic predictors of post-partum disease and culling risk in dairy cattle. Vet J. (2011) 188:216–20. doi: 10.1016/j.tvjl.2010.04.007
30. Jeengar K, Chaudhary V, Kumar A, Raiya S, Purohit GN. Ovarian cysts in dairy cows: old and new concepts for definition, diagnosis and therapy. Anim Reprod. (2014) 11:63–73.
31. Peter AT, Vos PL, Ambrose DJ. Postpartum anestrus in dairy cattle. Theriogenology. (2009) 71:1333–42. doi: 10.1016/j.theriogenology.2008.11.012
32. Nelson ST, Martin AD, Østerås O. Risk factors associated with cystic ovarian disease in Norwegian dairy cattle. Acta Vet Scand. (2010) 52:60. doi: 10.1186/1751-0147-52-60
33. Probo M, Comin A, Mollo A, Cairoli F, Stradaioli G, Veronesi MC. Reproductive performance of dairy cows with luteal or follicular ovarian cysts after treatment with buserelin. Anim Reprod Sci. (2011) 127:135–9. doi: 10.1016/j.anireprosci.2011.07.019
34. Edmonson AJ, Lean IJ, Weaver LD, Farver T, Webster G. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. (1989) 72:68–78. doi: 10.3168/jds.S0022-0302(89)79081-0
35. Gareis NC, Angeli E, Huber E, Salvetti NR, Rodríguez FM, Ortega HH, et al. Alterations in key metabolic sensors involved in bovine cystic ovarian disease. Theriogenology. (2018) 120:138–46. doi: 10.1016/j.theriogenology.2018.07.045
36. Lima FS, Acosta DAV, Egan TR, Skenandore C, Sulzberger S, French DD, et al. Steroidogenic, metabolic, and immunological markers in dairy cows diagnosed with cystic ovarian follicles at early and mid-late lactation. Front Vet Sci. (2019) 6:324. doi: 10.3389/fvets.2019.00324
37. Zulu VC, Sawamukai Y, Nakada K, Kida K, Moriyoshi M. Relationship among insulin-like growth factor-I, blood metabolites and postpartum ovarian function in dairy cows. J Vet Med Sci. (2002) 64:879–85. doi: 10.1292/jvms.64.879
38. Wathes DC, Taylor VJ, Cheng Z, Mann GE. Follicle growth, corpus luteum function and their effects on embryo development in postpartum dairy cows. Reprod Suppl. (2003) 61:219–37.
39. Guyot H, Detilleux J, Lebreton P, Garnier C, Bonvoisin M, Rollin F, et al. Comparison of various indices of energy metabolism in recumbent and healthy dairy cows. PLoS ONE. (2017) 12:e0169716. doi: 10.1371/journal.pone.0169716
40. Leroy JL, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, et al. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction. (2005) 130:485–95. doi: 10.1530/rep.1.00735
41. Vanholder T, Lmr Leroy J, Van Soom A, Maes D, Coryn M, Fiers T, et al. Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Anim Reprod Sci. (2006) 92:51–63. doi: 10.1016/j.anireprosci.2005.05.014
42. Leroy JL, Rizos D, Sturmey R, Bossaert P, Gutierrez-Adan A, Van Hoeck V, et al. Intrafollicular conditions as a major link between maternal metabolism and oocyte quality: a focus on dairy cow fertility. Reprod Fertil Dev. (2011) 24:1–12. doi: 10.1071/RD11901
43. Van Hoeck V, Leroy JL, Arias Alvarez M, Rizos D, Gutierrez-Adan A, Schnorbusch K, et al. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: mechanistic insights. Reproduction. (2013) 145:33–44. doi: 10.1530/REP-12-0174
44. Rodríguez FM, Salvetti NR, Colombero M, Stangaferro ML, Barbeito CG, Ortega HH, et al. Interaction between IGF1 and IGFBPs in bovine cystic ovarian disease. Anim Reprod Sci. (2013) 140:14–25. doi: 10.1016/j.anireprosci.2013.04.012
45. Beam SW, Butler WR. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol Reprod. (1997) 56:133–42. doi: 10.1095/biolreprod56.1.133
46. Sung KC, Lee MY, Lee JY, Lee SH, Kim SH, Kim SH. Utility of ALT concentration in men and women with nonalcoholic fatty liver disease: cohort study. J Clin Med. (2019) 8:445. doi: 10.3390/jcm8040445
47. Tanemura K, Ohtaki T, Kuwahara Y, Tsumagari S. Association between liver failure and hepatic UDP-glucuronosyltransferase activity in dairy cows with follicular cysts. J Vet Med Sci. (2017) 79:86–91. doi: 10.1292/jvms.15-0674
48. Ferro Y, Montalcini T, Mazza E, Foti D, Angotti E, Gliozzi M, et al. Randomized clinical trial: bergamot citrus and wild cardoon reduce liver steatosis and body weight in non-diabetic individuals aged over 50 years. Front Endocrinol (Lausanne). (2020) 11:494. doi: 10.3389/fendo.2020.00494
49. Douthwaite R, Dobson H. Comparison of different methods of diagnosis of cystic ovarian disease in cattle and an assessment of its treatment with a progesterone-releasing intravaginal device. Vet Rec. (2000) 147:355–9. doi: 10.1136/vr.147.13.355
50. Ribadu AY, Ward WR, Dobson H. Comparative evaluation of ovarian structures in cattle by palpation per rectum, ultrasonography and plasma progesterone concentration. Vet Rec. (1994) 135:452–7. doi: 10.1136/vr.135.19.452
51. Leslie KE, Bosu WT. Plasma progesterone concentrations in dairy cows with cystic ovaries and clinical responses following treatment with fenprostalene. Can Vet J. (1983) 24:352–6.
52. Jackson RA, Wills JR, Kendall NR, Green MJ, Murray RD, Dobson H. Energy metabolites in pre- and postpartum dairy cattle as predictors of reproductive disorders. Vet Rec. (2011) 168:562. doi: 10.1136/vr.d1565
53. Cools S, Van den Broeck W, Bossaert P, Hostens M, Opsomer GA. field study to unravel factors that are significantly associated with the secretory activity of the corpus luteum during the first three postpartum cycles in high yielding dairy cows, based on the amount of steroidogenic and endothelial cells present in the luteal tissue. Reprod Domest Anim. (2014) 49:881–93. doi: 10.1111/rda.12348
54. Bouvier-Muller J, Allain C, Enjalbert F, Tabouret G, Portes D, Caubet C, et al. Response to dietary-induced energy restriction in dairy sheep divergently selected for resistance or susceptibility to mastitis. J Dairy Sci. (2016) 99:480–92. doi: 10.3168/jds.2015-9785
55. Davis JS, Clark MR. Activation of protein kinase in the bovine corpus luteum by phospholipid and Ca2+. Biochem J. (1983) 214:569–74. doi: 10.1042/bj2140569
56. Zhao C, Bai Y, Fu S, Wu L, Xia C, Xu C. Comparison of metabolic alterations in serum and milk whey between inactive ovaries and estrus dairy cows. Front Vet Sci. (2021) 7:609391. doi: 10.3389/fvets.2020.609391
57. Montiel F, Ahuja C. Body condition and suckling as factors influencing the duration of postpartum anestrus in cattle: a review. Anim Reprod Sci. (2005) 85:1–26. doi: 10.1016/j.anireprosci.2003.11.001
58. Castro N, Kawashima C, van Dorland HA, Morel I, Miyamoto A, Bruckmaier RM. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J Dairy Sci. (2012) 95:5804–12. doi: 10.3168/jds.2012-5666
59. Gentry LR, Thompson DL Jr, Gentry GT Jr, Davis KA, Godke RA. High versus low body condition in mares: interactions with responses to somatotropin, GnRH analog, and dexamethasone. J Anim Sci. (2002) 80:3277–85. doi: 10.2527/2002.80123277x
60. Viana JH, Dorea MD, Siqueira LG, Arashiro EK, Camargo LS, Fernandes CA, et al. Occurrence and characteristics of residual follicles formed after transvaginal ultrasound-guided follicle aspiration in cattle. Theriogenology. (2013) 79:267–73. doi: 10.1016/j.theriogenology.2012.08.015
61. Contreras GA, O'Boyle NJ, Herdt TH, Sordillo LM. Lipomobilization in periparturient dairy cows influences the composition of plasma nonesterified fatty acids and leukocyte phospholipid fatty acids. J Dairy Sci. (2010) 93:2508–16. doi: 10.3168/jds.2009-2876
62. Busato S, Bionaz M. The interplay between non-esterified fatty acids and bovine peroxisome proliferator-activated receptors: results of an in vitro hybrid approach. J Anim Sci Biotechnol. (2020) 11:91. doi: 10.1186/s40104-020-00481-y
63. Zulu VC, Nakao T, Sawamukai Y. Insulin-like growth factor-I as a possible hormonal mediator of nutritional regulation of reproduction in cattle. J Vet Med Sci. (2002) 64:657–65. doi: 10.1292/jvms.64.657
64. Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1, 25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol Endocrinol Metab. (2010) 299:E928–35. doi: 10.1152/ajpendo.00378.2010
65. Wisnieski L, Norby B, Pierce SJ, Becker T, Gandy JC, Sordillo LM. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev Vet Med. (2019) 163:68–78. doi: 10.1016/j.prevetmed.2018.12.014
66. Ospina PA, Nydam DV, Stokol T, Overton TR. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J Dairy Sci. (2010) 93:1596–603. doi: 10.3168/jds.2009-2852
Keywords: dairy cows, inactive ovaries, follicular cysts, luteal cysts, early warning
Citation: Song Y, Cheng J, Yu H, Wang Z, Bai Y, Xia C and Xu C (2021) Early Warning for Ovarian Diseases Based on Plasma Non-esterified Fatty Acid and Calcium Concentrations in Dairy Cows. Front. Vet. Sci. 8:792498. doi: 10.3389/fvets.2021.792498
Received: 10 October 2021; Accepted: 17 November 2021;
Published: 08 December 2021.
Edited by:
Giuseppe Piccione, University of Messina, ItalyReviewed by:
Francesca Arfuso, University of Messina, ItalyEnrico Fiore, University of Padua, Italy
Copyright © 2021 Song, Cheng, Yu, Wang, Bai, Xia and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Xia, eGN3bHh5ZjIwMTRAMTYzLmNvbQ==; Chuang Xu, eHVjaHVhbmc3MTc1QDE2My5jb20=
 Yuxi Song
Yuxi Song Jiaxin Cheng
Jiaxin Cheng Cheng Xia
Cheng Xia Chuang Xu
Chuang Xu